Abstract
Background:
Decompressive craniectomy (DC) is an aggressive life-saving surgical intervention for patients with malignant cerebral infarction (MCI). However, DC remains inconsistently and infrequently utilized, primarily due to enduring concern that increased survival occurs only at the cost of poor functional outcome. Our aim was to clarify the role of DC performed within 48 hours (early DC) for patients with MCI, including patients aged >60 years.
Methods:
We performed a meta-analysis of all available randomized controlled trials comparing early DC to best medical care for MCI. Studies were identified through literature searches of electronic databases including PubMed, EMBASE, and Scopus. We employed a Mantel-Haenszel fixed effects model to assess treatment effect on dichotomized modified Rankin Scale (mRS) outcomes at 12 months.
Results:
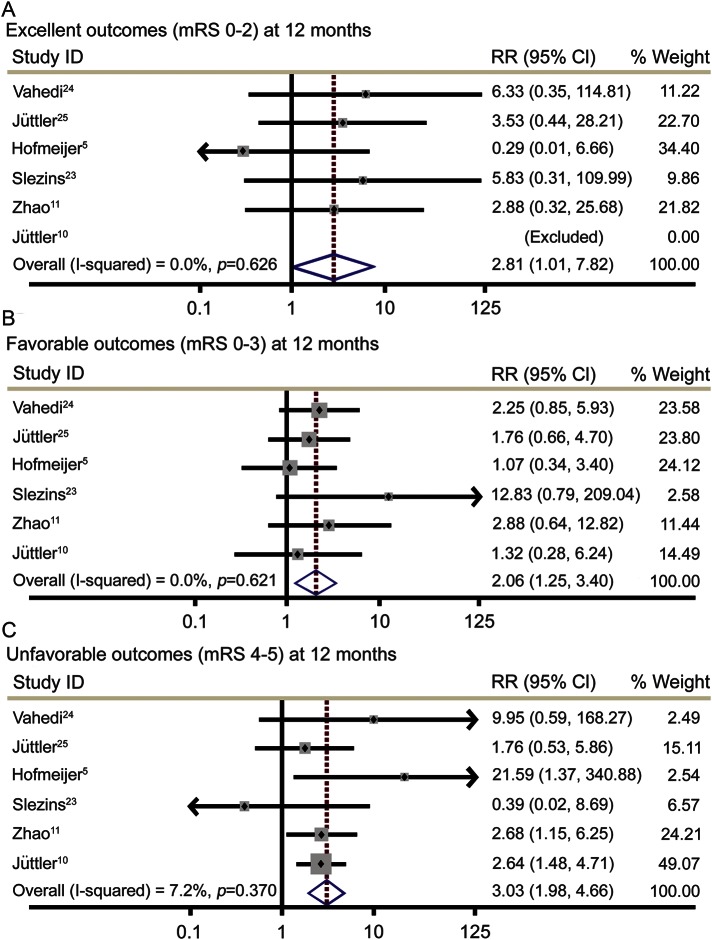
A total of 289 patients from 6 randomized controlled trials comparing early DC to best medical care were included. Early DC resulted in an increased rate of excellent outcomes, defined as mRS ≤2 (relative risk [RR] 2.81, 95% confidence interval [CI] 1.01–7.82, p = 0.047), and favorable outcomes, defined as mRS ≤3 (RR 2.06, 95% CI 1.25–3.40, p = 0.005). Early DC also increased the rate of survival with unfavorable outcomes, defined as mRS 4–5 (RR 3.03, 95% CI 1.98–4.65, p < 0.001).
Conclusions:
Early DC increases the rate of excellent outcomes, i.e., functional independence, in addition to favorable and unfavorable outcomes; however, these findings must be interpreted within the context of patients' goals of care. We have developed a clinical decision algorithm that incorporates goals of care, which may guide consideration of early DC for MCI in clinical practice.
Malignant cerebral infarction (MCI) refers to large-volume ischemic strokes involving the middle cerebral artery territory, which are complicated by life-threatening cerebral edema.1 Managed medically, MCI carries a mortality rate of 80%.1,2 Decompressive craniectomy (DC), the surgical removal of a portion of the skull allowing outward herniation of infarcted brain tissue, may reduce MCI mortality to ∼20%.2–4 Both nonrandomized and randomized controlled trials (RCTs) suggest that DC is most effective within 48 hours of stroke onset (early DC),2,3,5,6 but despite decreased mortality, survivors often have poor functional outcomes secondary to stroke severity.2,7
In 2007, a landmark interim pooled analysis of 3 RCTs demonstrated improved functional outcomes, defined as modified Rankin Scale (mRS) scores 0–3, for MCI patients treated with early DC.6 However, this morbidity benefit was not present upon completion of the trials5,8,9; nor has it been demonstrated in subsequent RCTs,10,11 which employed a less stringent definition of acceptable functional outcome (mRS 0–4) with poor generalizability.12–17 Consequently, there is enduring concern that early DC increases survival only at the cost of poor quality of life.8,9,12,15 Lack of clarity is evidenced by wide variations in clinical practice with infrequent and inconsistent utilization of early DC for MCI patients in the United States and other countries.15,18–20
In light of these considerations, we performed a meta-analysis of all available RCTs comparing 12-month functional outcomes in adult MCI patients treated with early DC vs best medical care. Our aim was to generate more robust outcome data to better inform clinical decision-making.
METHODS
Literature search
Studies were identified through electronic database searches of PubMed, EMBASE, Scopus, and Cochrane Library. Conference presentations were identified in PubMed, BIOSIS Previews, Proceedings, and EMBASE. The search was developed and conducted by L.M.H. For full details of our literature search, see appendix e-1 at Neurology.org/cp. We performed our final literature search on January 9, 2015.
Study selection and data extraction
We determined a priori to include all RCTs of early DC for MCI in patients ≥18 years of age that reported functional outcomes at 12 months. Early DC was defined as enrollment with the intent to perform DC within 48 hours from stroke onset; this definition was derived from clinical trial data demonstrating increased treatment effect within this time period.2,3,5,6 Cohort studies and case series were not eligible due to high risk of bias.
All retrieved RCTs were reviewed independently by 2 authors (C.D.S. and B.J.M.). Quality control and risk of bias were assessed using a 7-point scoring system recommended by the Cochrane Collaboration.21 Accordingly, studies were assigned an overall risk of bias score: low, unclear, or high. Two authors (C.D.S. and B.J.M.) independently abstracted patient ages (dichotomized: ≤60 years or >60 years) and mRS scores at 12 months from each eligible trial. Patient ages from Slezins et al.23 were obtained via correspondence with the author. Any discrepancies between the authors were resolved by review and consensus.
Statistical analysis
All 6 trials reported mRS scores at 12 months. We defined outcomes as excellent (mRS 0–2), favorable (mRS 0–3), or survival with unfavorable outcome (mRS 4–5) in best accordance with both clinical convention and public perception.6,12–15,17 Under this stratification, excellent outcomes (mRS 0–2) represent patients with only mild disability and preserved functional independence; favorable outcomes (mRS 0–3) represent patients with preserved functional independence, plus moderately disabled nonindependent patients who retain the ability to ambulate unassisted; unfavorable outcomes (mRS 4–5) comprise surviving patients who are unable to ambulate unassisted or attend to their own bodily needs, plus patients who are bedbound, requiring constant nursing care.
To assess treatment effect, we employed a Mantel-Haenszel fixed-effects model for its statistical properties when handling infrequent outcomes and small sample sizes, and in order to report our findings in terms of relative risk (RR).22 All studies that met our inclusion criteria were included in the primary analysis. In order to assess the robustness of our findings, we conducted a prespecified sensitivity analysis excluding any studies assigned an unclear or high risk of bias rating. Heterogeneity between studies was investigated by calculating I2. Clinically significant heterogeneity requiring further investigation was conservatively defined as I2 > 40%.22 We assessed publication bias and small study effects visually through funnel plots and statistically via Begg and Egger tests. Statistical significance was predefined as an α level <0.05. A preplanned quantitative subgroup analysis of outcomes for patients older than 60 years was undertaken to explore the effect of age on treatment outcomes. All statistical analyses were conducted using STATA 13.1 (StataCorp, College Station, TX). The analysis plan was approved by the University of Pittsburgh Institutional Review Board. The study protocol was not published a priori.
RESULTS
Study selection and study characteristics
In total, we identified 1,229 nonduplicate articles. After screening each article for eligibility, we reviewed 7 full-text publications (figure 1). Six RCTs comprising 289 patients met our inclusion criteria: 4 completed trials of early DC, the published preliminary results from a trial of early DC, and a subgroup analysis comprised of patients randomized within 48 hours from Hemicraniectomy After Middle Cerebral Artery Infarction With Life-threatening Edema Trial (HAMLET), a DC trial which enrolled patients up to 96 hours from onset (table).5,10,11,23–25 One study was excluded, Hemicraniectomy and Durotomy Upon Deterioration From Infarction-Related Swelling Trial (HeADDFIRST), which enrolled patients up to 96 hours from onset and, notably, intentionally delayed DC until patients had severe clinical or radiographic deterioration.26
Figure 1. Literature review flowchart.
DC = decompressive craniectomy.
Table.
Study inclusion characteristics

Inclusion criteria for the individual trials were similar, with exception of the prespecified age ranges (table). Baseline patient characteristics of the treatment groups were well-matched with exception of Jüttler et al.25 and Slezins et al.,23 which reported higher mean NIH Stroke Scale scores in the medically managed group. Slezins et al.23 also reported a lower mean age in the patients undergoing DC.
Risk of bias for independent studies
The 4 completed RCTs of early DC, Jüttler et al.,25 Vahedi et al.,24 Zhao et al.,11 and Jüttler et al.,10 received a low risk of bias rating. Slezins et al.23 is a brief preliminary report that does not comprehensively detail study methodology. Hofmeijer et al.5 reported a post hoc analysis of 39 patients enrolled in HAMLET within 48 hours of stroke onset, but did not report baseline patient characteristics or whether their initial randomization stratification was maintained in this subgroup.5,27 Secondary to these concerns, Slezins et al.23 and Hofmeijer et al.5 both received an unclear risk of bias rating. A full risk of bias rating table is included in figure e-1.
Primary analysis and sensitivity analysis
At 12 months, patients with MCI who were treated with early DC had an increased rate of excellent outcomes, defined as mRS 0–2 (RR 2.81, 95% confidence interval [CI] 1.01–7.82, p = 0.047), and favorable outcomes, defined as mRS 0–3 (RR 2.06, 95% CI 1.25–3.40, p = 0.005). Treatment with early DC was also associated with an increased number of survivors with unfavorable functional outcome, defined as mRS 4–5 (RR 3.03, 95% CI 1.98–4.65, p < 0.001) (figure 2). There was an absolute risk increase (ARI) of 6.6% for excellent functional outcome with early DC, which corresponds to a number needed to treat (NNT) of 15.2. For favorable outcome, the ARI was 15.0% and NNT 6.7, whereas for unfavorable functional outcome ARI was 29.5% and NNT 3.4. There was no clinical heterogeneity in any of the analyses (I2 = 0–7.2% for primary outcome analyses) or evidence of publication bias or small study effects.
Figure 2. Functional outcomes for patients treated with early decompressive craniectomy (DC) vs best medical care.
(A) Excellent outcomes. (B) Favorable outcomes. (C) Unfavorable outcomes. CI = confidence interval; mRS = modified Rankin Scale; RR = relative risk.
In our sensitivity analysis, the magnitude of treatment effect and statistical significance of early DC remained fundamentally unaltered across all outcome measures. Excellent outcomes (RR 3.84, 95% CI 1.01–14.52, p = 0.048), favorable outcomes (RR 2.01, 95% CI 1.12–3.59, p = 0.019), and unfavorable outcomes (RR 2.71, 95% CI 1.74–4.20, p < 0.001) were increased by early DC. There was no clinical heterogeneity (I2 = 0% for all sensitivity analyses). For excellent functional outcomes, ARI was 7.6% and NNT 13.2, for favorable functional outcomes ARI was 14.0% and NNT 7.1, and for unfavorable outcomes ARI was 26.9% and NNT 3.7.
Subgroup analysis: Patients older than 60 years
Three RCTs of MCI included patients older than 60 years, with a total of 66 patients randomized to early DC and 83 to best medical care.10,11,23 Twelve-month outcomes were assessed quantitatively rather than by meta-analysis due to the limited number of trials. In contrast to younger patients, no older patients attained an excellent outcome (mRS 0–2) irrespective of treatment arm, and markedly fewer patients reached mRS 3. A total of 7.6% of older patients who underwent early DC achieved mRS 3 compared with 3.6% of older patients who received best medical care, corresponding to an ARI of 4.0% and NNT 25.0 for favorable outcomes. For unfavorable outcomes, ARI was 32.5% and NNT 3.1. A total of 75.9% of older patients in the medical treatment arm died in comparison with 39.4% of older patients who underwent early DC, absolute risk reduction 36.5% and NNT 2.7 to prevent mortality (figure 3).
Figure 3. Modified Rankin Scale (mRS) score shift analysis at 12 months.

(A) All ages. (B) Age ≤60 years. (C) Age >60 years. Percentages may not sum to 100 because of rounding. Early DC = decompressive craniectomy performed within 48 hours; medical = best medical care. a The mRS is a validated functional outcome scale commonly used in clinical stroke trials. Scores are defined as 0, no symptoms; 1, no significant disability, able to carry out all usual activities despite some symptoms; 2, slight disability, remains independent, but unable to carry out all previous activities; 3, moderate disability, requires some assistance, but able to walk unassisted; 4, moderately severe disability, unable to attend to own bodily needs or walk unassisted; 5, severe disability, bedridden, requiring constant nursing attention; 6, dead.32 b No patients attained mRS score 0 or 1 due to stroke severity.
DISCUSSION
Our meta-analysis demonstrates that early DC increases excellent outcomes, i.e., functional independence (mRS 0–2), and favorable outcomes (mRS 0–3) in patients with MCI. Better functional outcomes in early DC patients are likely not only due to preventing lethal brain herniation, but also improving cerebral hemodynamics, thereby limiting secondary injury and increasing potential for long-term recovery.28,29 However, we also found that early DC in MCI increases the risk of survival with unfavorable functional outcome (mRS 4–5). These somewhat paradoxical findings, in which both favorable and unfavorable functional outcomes are increased, are driven by the large reduction in MCI-related mortality in patients treated with early DC.5,6,10,11
Nevertheless, our findings clarify and guide decision-making for MCI patients who are considered good candidates for early DC. Goals of care are driven primarily by (1) maximizing functional outcome or survival, (2) minimizing the likelihood of survival with disability, or (3) quality of life. As we have demonstrated, if the priority is to maximize functional outcome or survival, patients should undergo early DC. Conversely, due to stroke severity, MCI patients who wish to avoid survival with considerable disability should be managed medically and, when appropriate, referred to palliative care. Finally, quality of life determinations following early DC may be derived from the pooled functional outcomes of all 6 RCTs and further refined by age to account for differences between younger and older patients (figure 3). For example, an otherwise healthy 50-year-old MCI patient who finds mRS 0–3 acceptable and mRS 4–5 unacceptable has a 43% chance of good quality of life, a 35% chance of poor quality of life, and a 22% chance of death 12 months after early DC. If that same patient found mRS 0–4 acceptable, the probabilities shift to a 69% chance of good quality of life, a 9% chance of poor quality of life, and a 22% chance of death. This clinical decision algorithm is illustrated in figure 4.
Figure 4. Clinical decision algorithm for consideration of early decompressive craniectomy (DC) in malignant cerebral infarction (MCI).
Early DC = DC performed within 48 hours; mRS = modified Rankin Scale. a Twelve-month outcomes.
It should be noted that age is a key determinant of functional outcome. Goals of care for MCI patients older than 60 years must take into account the low likelihood of attaining an mRS score ≤3 following early DC. In fact, while only a small number of patients achieved mRS 2 (2% in the medical arm compared to 8.6% in the DC arm), none of them were older than 60 years. On the other hand, the large mortality benefit from DC persists for patients over age 60 years; therefore families and clinicians must weigh the survival benefit against the possibility of considerable neurologic disability and functional dependence. Additional prognostic considerations should include preexisting radiographic and neurologic signs of herniation and total infarct volume.7,23 There is no evidence that laterality (dominant vs nondominant hemispheric infarction) affects long-term outcome, although severe language deficits may be considered unacceptable quality of life.30,31
This meta-analysis has multiple limitations. The first limitation relates to our definitions of excellent (mRS 0–2), favorable (mRS 0–3), and survival with unfavorable (mRS 4–5) functional outcomes. The mRS is a widely used, validated functional outcome scale that measures a person's degree of disability or dependence.32 At extremes of the mRS spectrum, there is general consensus that scores 0–2 represent good outcomes and scores 5–6 represent poor outcomes.12–15 mRS 3 is usually regarded as favorable, but perception of mRS 4 (which represents moderately severe disability with the inability to attend to one's bodily needs or walk unassisted) varies widely.12–16 Our own stratification was informed by multiple independent studies of physicians, nurses, and the general public in which 6%–38% of respondents considered mRS 4 a quality of life worth living.12,13,15,16 Nonetheless, retrospective studies have shown that most MCI patients who have undergone DC have a reasonable quality of life and are satisfied with their treatment, even those with an mRS of 4,33–35 creating an inherent difficulty in defining acceptable outcomes. Therefore, our findings should not be applied rigidly, but rather serve as a framework for clinical decision-making based upon individual patient and family goals of care. Although the most common interpretations of acceptable and unacceptable outcome are presented in figure 4, these probabilities can simply be recalculated to reflect alternative valuations of mRS outcome strata when necessary.
A second limitation is that there are only 6 RCTs of early DC eligible for our analysis and each trial was relatively small. We could have expanded the number of eligible patients by including DC trials that enrolled patients past 48 hours; however, there is sufficient evidence to suggest that patients treated later experience a different clinical course.5,26 We prespecified exclusion of patients undergoing late DC in order to maintain a pure sample population, which is reflected in the consistency of treatment effect across our studies as calculated by I2. For similar reasons, we excluded nonrandomized trials, whose results are potentially subject to substantial bias.
Another limitation is the timing of our outcome assessment. Although existing data support the use of 12-month functional outcomes rather than earlier time points, it is recognized that some patients continue to regain function after 1 year, which could lead to an underestimation of treatment effect.27,36 Only one RCT of DC has reported outcomes past 12 months.27 When making outcome assessments, some of the studies attempted to blind the assessors to treatment assignment,6,11 while others did not.10,23,25 A further limitation is that the dichotomization of mRS outcomes leads to a loss of information and less precise valuations. For example, defining unfavorable outcome as mRS 4 and 5 does not account for the fact that mRS 4 is both more frequent and more desirable than mRS 5. Finally, the RCTs included in our meta-analysis restricted eligibility to patients who were otherwise healthy and functionally independent at baseline. Therefore our results cannot be generalized to MCI patients with notable medical comorbidities or those who were previously functionally dependent; however, such patients are less commonly considered for DC in clinical practice.
Strengths of our study include consistency in the magnitude of treatment effect and statistical significance observed across the primary analysis and sensitivity analysis. Additionally, our data were derived entirely from RCTs with an overall low risk of bias. We did not find any evidence of heterogeneity of treatment effect, publication bias, or small study effects. Unlike previous publications, we address the effect of early DC on achieving functional independence and also include the largest reported cohort of randomized MCI patients older than 60 years. Most importantly, despite multiple RCTs and previously published meta-analyses,8,37,38 early DC for MCI remains inconsistently and infrequently utilized.12,13,18–20
Our specific aim was to generate outcome data with direct clinical applicability. Our study design and clinical decision algorithm incorporates individual patient or surrogate decision-maker perceptions regarding quality of life and therefore may be applied on a case-by-case basis when considering early DC for patients with MCI.
Our meta-analysis demonstrates that early DC improves excellent outcomes, defined as functional independence, at 12 months for patients with MCI. Additionally, early DC for MCI led to increased survival with both favorable and unfavorable functional outcomes at 12 months—a finding dependent upon the patient's valuation of mRS outcome strata. Our findings and clinical decision algorithm add clarity to the role of early DC in MCI and may help guide management of patients considered good candidates for decompressive surgery.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Daniel G. Winger of the University of Pittsburgh Clinical and Translational Science Institute for reviewing the statistical analysis.
Footnotes
Editorial, page 381
Supplemental data at Neurology.org/cp
AUTHOR CONTRIBUTIONS
C. Streib: study concept and design, data extraction, statistical analysis/interpretation of the data, drafting/revising the manuscript. L. Hartman: study concept and design, developed and conducted literature search. B. Molyneaux: study concept and design, data extraction, statistical analysis/interpretation of the data, drafting/revising the manuscript.
STUDY FUNDING
C. Streib was an NIH StrokeNET fellow supported by 1U01NS086489-02 (P.I. Tudor Jovin and Lawrence Wechsler). This project was supported by the NIH through UL1-TR-000005 and UL1-RR-024153.
DISCLOSURES
C.D. Streib and L.M. Hartman report no disclosures. B.J. Molyneaux serves on a scientific advisory board for and has received compensation for travel expenses from Remedy Pharmaceuticals. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996;53:309–315. [DOI] [PubMed] [Google Scholar]
- 2.Schwab S, Steiner T, Aschoff A, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke 1998;29:1888–1893. [DOI] [PubMed] [Google Scholar]
- 3.Cho DY, Chen TC, Lee HC. Ultra-early decompressive craniectomy for malignant middle cerebral artery infarction. Surg Neurol 2003;60:227–232; discussion 232–233. [DOI] [PubMed] [Google Scholar]
- 4.Malm J, Bergenheim AT, Enblad P, et al. The Swedish malignant middle cerebral artery infarction study: long-term results from a prospective study of hemicraniectomy combined with standardized neurointensive care. Acta Neurol Scand 2006;113:25–30. [DOI] [PubMed] [Google Scholar]
- 5.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery Infarction With Life-Threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 2009;8:326–333. [DOI] [PubMed] [Google Scholar]
- 6.Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 2007;6:215–222. [DOI] [PubMed] [Google Scholar]
- 7.Kilincer C, Asil T, Utku U, et al. Factors affecting the outcome of decompressive craniectomy for large hemispheric infarctions: a prospective cohort study. Acta Neurochir 2005;147:587–594. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Flores S, Berge E, Whittle IR. Surgical decompression for cerebral oedema in acute ischaemic stroke. Cochrane Database Syst Rev 2012:CD003435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell P, Gregson BA, Crossman J, et al. Reassessment of the HAMLET study. Lancet Neurol 2009;8:602–603; author reply 603–604. [DOI] [PubMed] [Google Scholar]
- 10.Jüttler E, Unterberg A, Woitzik J, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med 2014;370:1091–1100. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Su YY, Zhang Y, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care 2012;17:161–171. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz S, Kuhner C. Prognosis and quality of life after decompressive hemicraniectomy: a nationwide survey in Germany on the attitudes held by doctors and nurses. Nervenarzt 2012;83:731–740. [DOI] [PubMed] [Google Scholar]
- 13.Klein A, Kuehner C, Schwarz S. Attitudes in the general population towards hemi-craniectomy for middle cerebral artery (MCA) infarction: a population-based survey. Neurocrit Care 2012;16:456–461. [DOI] [PubMed] [Google Scholar]
- 14.Chaisinanunkul N, Saver J, Jovin T, et al. A utility-weighted modified Rankin Scale: derivation and application to completed stroke trials (P5.008). Neurology 2014;82:P5.008. [Google Scholar]
- 15.Neugebauer H, Creutzfeldt CJ, Hemphill JC III, Heuschmann PU, Jüttler E. DESTINY-S: attitudes of physicians toward disability and treatment in malignant MCA infarction. Neurocrit Care 2014;21:27–34. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa K, Bianchi MT, Nakagawa SS, Sorond FA. Aggressive care after a massive stroke in young patients: is that what they want? Neurocrit Care 2010;13:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong KS, Saver JL. Quantifying the value of stroke disability outcomes: who global burden of disease project disability weights for each level of the modified Rankin scale. Stroke 2009;40:3828–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suyama K, Horie N, Hayashi K, Nagata I. Nationwide survey of decompressive hemicraniectomy for malignant middle cerebral artery infarction in Japan. World Neurosurg 2014;82:1158–1163. [DOI] [PubMed] [Google Scholar]
- 19.Bar M, Mikulik R, Skoloudik D, et al. Decompressive surgery for malignant supratentorial infarction remains underutilized after guideline publication. J Neurol 2011;258:1689–1694. [DOI] [PubMed] [Google Scholar]
- 20.Adeoye O, Hornung R, Khatri P, Ringer A, Kleindorfer D. The rate of hemicraniectomy for acute ischemic stroke is increasing in the United States. J Stroke Cerebrovasc Dis 2011;20:251–254. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [Updated September 2008]. The Cochrane Collaboration. Chichester, UK: Wiley-Blackwell; 2008:xxi, 649. [Google Scholar]
- 23.Slezins J, Keris V, Bricis R, et al. Preliminary results of randomized controlled study on decompressive craniectomy in treatment of malignant middle cerebral artery stroke. Medicina 2012;48:521–524. [PubMed] [Google Scholar]
- 24.Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 2007;38:2506–2517. [DOI] [PubMed] [Google Scholar]
- 25.Jüttler E, Schwab S, Schmiedek P, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke 2007;38:2518–2525. [DOI] [PubMed] [Google Scholar]
- 26.Frank JI, Schumm LP, Wroblewski K, et al. Hemicraniectomy and durotomy upon deterioration from infarction-related swelling trial: randomized pilot clinical trial. Stroke 2014;45:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geurts M, van der Worp HB, Kappelle LJ, Amelink GJ, Algra A, Hofmeijer J. Surgical decompression for space-occupying cerebral infarction: outcomes at 3 years in the randomized HAMLET trial. Stroke 2013;44:2506–2508. [DOI] [PubMed] [Google Scholar]
- 28.Amorim RL, de Andrade AF, Gattas GS, et al. Improved hemodynamic parameters in middle cerebral artery infarction after decompressive craniectomy. Stroke 2014;45:1375–1380. [DOI] [PubMed] [Google Scholar]
- 29.Jaeger M, Soehle M, Meixensberger J. Improvement of brain tissue oxygen and intracranial pressure during and after surgical decompression for diffuse brain oedema and space occupying infarction. In: Poon WS, ed. Acta Neurochirurgica Supplementa. Vienna: Springer-Verlag Wien; 2005:117–118. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia R, Rai V, Singh S, et al. Outcome after decompressive hemicraniectomy in right versus left hemispheric malignant middle cerebral artery infarction. Cerebrovasc Dis 2013;35:114. [Google Scholar]
- 31.Sundseth J, Sundseth A, Thommessen B, et al. Long-term outcome and quality of life after craniectomy in speech-dominant swollen middle cerebral artery infarction. Neurocrit Care 2015;22:6–14. [DOI] [PubMed] [Google Scholar]
- 32.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 33.van Middelaar T, Nederkoorn PJ, van der Worp HB, Stam J, Richard E. Quality of life after surgical decompression for space-occupying middle cerebral artery infarction: systematic review. Int J Stroke 2014;10:170–176. [DOI] [PubMed] [Google Scholar]
- 34.Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg 2012;117:749–754. [DOI] [PubMed] [Google Scholar]
- 35.Kiphuth IC, Kohrmann M, Lichy C, Schwab S, Huttner HB. Hemicraniectomy for malignant middle cerebral artery infarction: retrospective consent to decompressive surgery depends on functional long-term outcome. Neurocrit Care 2010;13:380–384. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia R, Rai V, Singh S, et al. Longterm assessment is important in patients undergoing decompressive craniectomy for malignant middle cerebral artery infarction. Stroke 2014;44. [Google Scholar]
- 37.Yang MH, Lin HY, Fu J, Roodrajeetsing G, Shi SL, Xiao SW. Decompressive hemicraniectomy in patients with malignant middle cerebral artery infarction: a systematic review and meta-analysis. Surgeon 2015;13:230–240. [DOI] [PubMed] [Google Scholar]
- 38.Lu X, Huang B, Zheng J, et al. Decompressive craniectomy for the treatment of malignant infarction of the middle cerebral artery. Sci Rep 2014;4:7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.