Abstract
Background:
In multiple sclerosis (MS), comorbidities have been associated with disability progression and an increased risk of mortality. We investigated the association between comorbidities and mortality in MS after accounting for disability and health behaviors.
Methods:
We followed North American Research Committee on Multiple Sclerosis (NARCOMS) Registry participants who completed the Fall 2006 survey on comorbidities until death (reported or matched in the National Death Index) or date of last follow-up in 2014. We used proportional hazards regression to investigate the association between comorbidities and mortality, controlling for demographic, clinical, health behavior, and disability factors.
Results:
Of 9,496 participants meeting the inclusion criteria, 502 (5.3%) were deceased. Most participants reported having ≤3 comorbid conditions (70.9% survivors, 76.9% decedents). In individual regression models, vascular, visual, and mental comorbidities were associated with increased mortality risk after adjustment for factors associated with survival. When combined into a single model, vascular (hazard ratio 1.269; 1.041–1.547), visual (1.490; 1.199–1.852), and mental comorbidities (excluding anxiety, 1.239; 1.024–1.499) remained independently associated with an increased risk of mortality.
Conclusions:
Presence of comorbidities was independently associated with an increased risk of mortality as compared to absence of comorbidities after adjusting for factors associated with survival. Specifically, vascular, visual, and mental comorbidities increased the risk of mortality. This highlights the need for clinicians to attend to these comorbidities, which can be modified by treatments or other interventions, and potentially reduce the risk of mortality in persons with MS who have these conditions.
While understanding about mortality in multiple sclerosis (MS) has been increasing, many questions still remain.1 Studies have used data from multiple sources to compare the risk of mortality between the MS and general populations. Several studies from around the world indicate that survival has improved in people with MS over time, but these improvements appear to parallel improvements in life expectancy attained in the general population.2–5 Therefore, survival remains 6–7 years shorter for individuals with MS than expected when compared to the general population.6,7 Strategies to mitigate this survival gap would benefit from a better understanding of what factors contribute to reduced survival, including potentially modifiable factors.
Factors reported to be associated with shorter survival (early mortality) include disability, comorbid health conditions, and health behaviors such as smoking and low levels of physical activity. However, most studies have not evaluated the joint role of all of these factors on mortality. Two population-based studies have examined the effect of specific comorbidities on mortality. One of the studies examined the effect of neurologic comorbidities on mortality and found an increased risk of mortality associated with stroke, but did not control for health behaviors.8 Using population-based administrative data, the other study found comorbidity was associated with an increased risk of mortality among persons with MS, but the risk conferred by comorbidities to persons with MS was similar to that conferred to controls, and information regarding disability and health behaviors was not available.6 Therefore, we aimed to evaluate the association of different comorbidities on mortality after accounting for factors already reported to be associated with shorter survival.
METHODS
North American Research Committee on Multiple Sclerosis registry
The North American Research Committee on Multiple Sclerosis (NARCOMS) Registry is a voluntary self-report registry for persons with MS developed by the Consortium of MS Centers.9 Participants may enroll by completing a questionnaire online or by mailing in a questionnaire.9 After enrollment, participants are asked to complete surveys semiannually, on paper or online per their preference. The current study was restricted to those registry participants who completed the Fall 2006 update special section on comorbidities. Additionally, the respondents had to reside in the United States and have reported a year of birth and age at symptom onset.
Standard protocol approvals, registrations, and patient consents
Participants agree to the use of their information for research purposes. The registry is approved by the Institutional Review Board at the University of Alabama at Birmingham.
Clinical and demographic characteristics
The Patient Determined Disease Steps (PDDS) is a validated measure that correlates highly with a physician-scored Expanded Disability Status Scale (EDSS).10 It is scored ordinally from 0 (normal) to 8 (bedridden), where a score of 0 approximates an EDSS score of 0, a score of 3 represents early gait disability without needing an assistive device and approximates an EDSS score of 4.0–4.5, and scores of 4, 5, and 6 represent EDSS scores of 6–6.5. The cognitive subscale (Cognition Performance Scale [CPS]) is a single item that is a part of the Performance Scales and has been shown to have adequate convergent and divergent validity.11,12 The CPS is used to assess cognition by having participants evaluate their cognitive status from 1 of 6 levels: 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), 4 (severe), or 5 (total cognitive disability). The CPS correlates strongly with the Perceived Deficits Questionnaire (r = 0.71, p < 0.0001), a 20-item self-report questionnaire for cognition incorporated in the Multiple Sclerosis Quality of Life Inventory.13 Disease-modifying therapy (DMT) use was identified by searching enrollment and update survey immunotherapy records for self-reported current or past use of any of the following drugs: alemtuzumab, azathioprine, cyclophosphamide, daclizumab, dimethyl fumarate, fingolimod, IV immunoglobulin, glatiramer acetate, interferon-β-1a (IM), interferon-β-1a (SC), interferon-β-1b, laquinimod, methotrexate, mitoxantrone, mycophenolate mofetil, natalizumab, peginterferon-β-1a, rituximab, and teriflunomide. Education was included as a measure of socioeconomic status with categories of <12 years, high school diploma, associate's degree or technical degree, bachelor's degree, and postgraduate degree. Smoking status was assessed using validated questions from the Behavioral Risk Factor Surveillance System.14
Mortality
Briefly, for NARCOMS participants, death was determined by using the National Death Index (NDI) to ascertain whether that person matched a decedent in the database of US deaths, or was based on notification of death and date of death from the family of the deceased participant. The NDI was searched through December 31, 2009, and deaths after that date were solely from reported registry status. More details on the NDI matching of participants are reported elsewhere.15 The date of death was required for the deceased participant to be included in the analysis. Respondents were considered to be alive if not reported as deceased, and were censored at the date of their last follow-up through 2014.
Comorbidities
The Fall 2006 survey asked NARCOMS participants to report their comorbidity status (presence/absence) using the following question format: “Has a doctor ever told you that you have…?.”16 Respondents indicated the presence or absence of a comorbidity and, if present, the year of diagnosis. The comorbidities were grouped into categories: vascular (e.g., high cholesterol, hypertension, heart trouble, peripheral vascular disease, diabetes), autoimmune (thyroid, rheumatoid arthritis, lupus, inflammatory bowel disease [IBD], Sjögren), gastrointestinal (irritable bowel, IBD, liver, peptic ulcer), cancer (breast, lung, skin, colon, rectal), visual (cataracts, glaucoma, uveitis), musculoskeletal (arthritis, fibromyalgia, hip replacement, knee replacement, rheumatoid arthritis), and other (lung trouble, anemia, kidney, HIV) as described previously,17 and to be consistent with other studies in MS.18,19 Comorbidity status was updated in subsequent surveys through 2014 in which respondents could indicate the presence or absence of the comorbidity. If the comorbidity was newly reported in a survey after Fall 2006, then the year of comorbidity diagnosis was assumed to be the year of the survey. Additionally, for categories that had overlapping diseases, due to initially using the categories independently, the participant was considered to fall into each comorbidity group.
Statistical analysis
The association of each comorbidity group with mortality was evaluated using univariate analysis followed by multivariable proportional hazards regression using time from MS symptom onset as the time scale. The models accounted for left truncation of the data using the age at entry into the NARCOMS registry. Time-independent covariates adjusted for in the model included sex, smoking status in 2006 (never, current, or former), education (less than bachelor's vs bachelor's or higher), and age at symptom onset. Time-varying covariates used in the models were the PDDS, CPS, and each comorbidity group as described above (yes/no). The PDDS and CPS used for each respondent began with their enrollment survey and then any subsequent responses through their last collected update survey (up to 2014).
Sensitivity analyses
To assess the effect of using NDI data for only 3 of the 8 years of the study period, we conducted sensitivity analyses by repeating our analyses using only NDI data and follow-up through 2009. Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
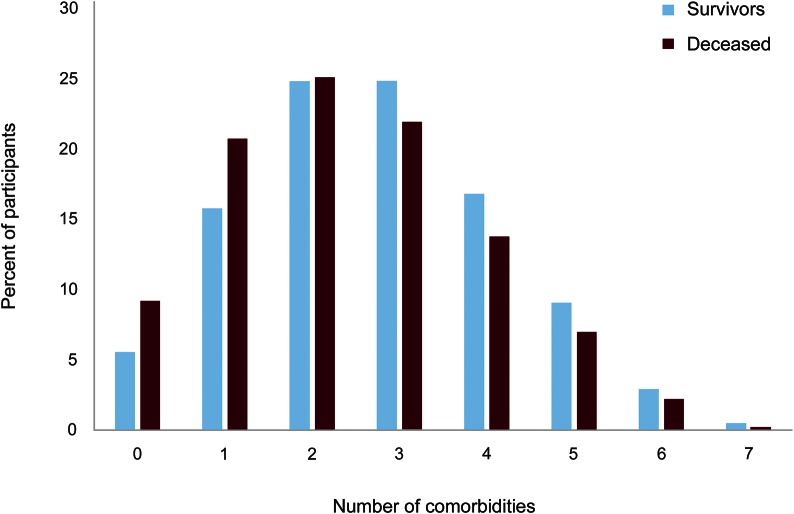
Of 9,927 participants who completed the comorbidity questions in the Fall 2006 survey, 9,639 reported a physician diagnosis of MS, resided in the United States, and had a date of birth and symptom onset age for the time calculations. We identified 645 deaths, but 143 of these did not have a date of death reported, leaving 502 (5.3%) deaths in the cohort. The decedents (n = 502) were more likely to be male, to be older at enrollment, and to have more disability (table 1) than survivors (n = 8,994). The proportion reporting autoimmune, musculoskeletal, mental, and other comorbidities (table 1) was lower for the decedents. Only 5.5% of survivors and 9.2% of decedents did not report a comorbidity (figure); therefore, we did not compare the characteristics of participants with and without any comorbidity. Differences in the demographic and clinical characteristics of participants with and without comorbidity by comorbidity group are shown in table e-1 at Neurology.org/cp.
Table 1.
Demographics and clinical characteristics of respondents
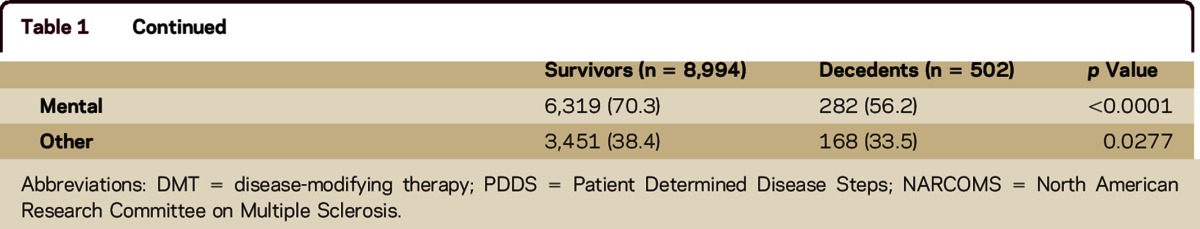
Figure. Comorbidities reported by survivors and decedents (excluding the other comorbid conditions category).
To evaluate the effect of the covariates on mortality, separate proportional hazards models were constructed with and without the inclusion of disability measures (table 2). Men had an increased risk of mortality, but some of this risk was attenuated when disability status was included in the model, indicating that some of the risk of mortality for male participants was accounted for by having higher levels of disability. As the age at symptom onset increased, there was a slightly increased risk of mortality with and without the presence of disability. There was no significant association between education level, DMT use, and smoking status and the risk of mortality regardless of whether disability was included in the model. Increased disability was associated with an increased risk of mortality, but this association was partially attenuated after accounting for other covariates in the model.
Table 2.
Effect of different covariate models on mortality
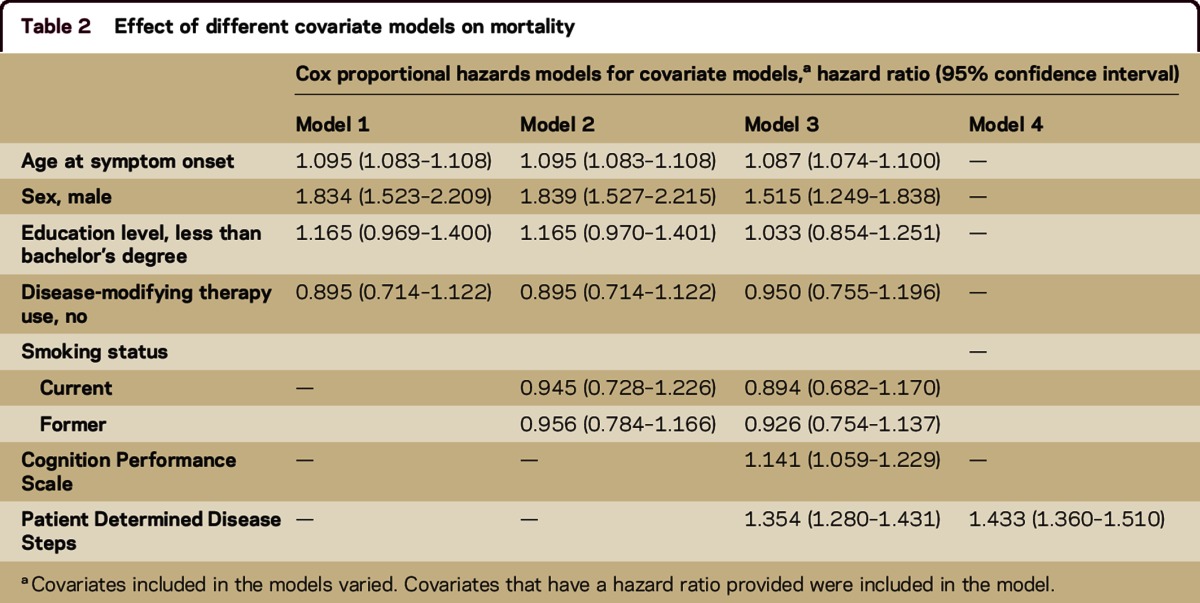
As a whole, the model with all covariates excluding comorbid health conditions showed an increased risk of mortality as the age at symptom onset increased (hazard ratio [HR] 1.087; 95% confidence interval [CI] 1.074–1.100), for male participants (HR 1.515; 95% CI 1.249–1.838), for those with greater self-reported cognitive impairment (HR 1.141; 95% CI 1.059–1.229), and for those with greater physical disability (HR 1.354; 95% CI 1.280–1.431). Smoking status was not independently associated with mortality (data not shown).
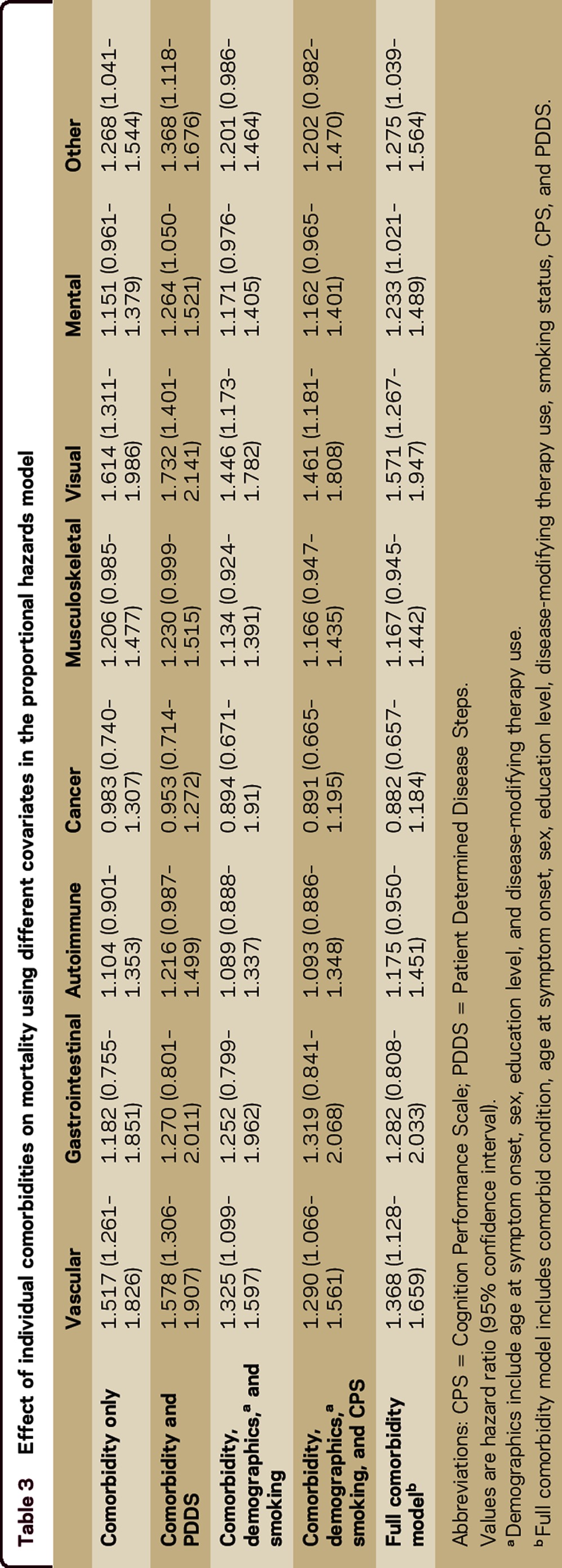
The proportional hazards models examining the effect of the individual comorbidities on mortality had HRs consistent in magnitude and significance with the effects seen in the fully adjusted covariate model describe above (table e-2). In individual models for each comorbidity, adjusting for all covariates, the vascular (HR 1.368; 95% CI 1.128–1.659, p = 0.0015), visual (HR 1.571; 95% CI 1.267–1.947, p < 0.0001), mental (HR 1.233; 95% CI 1.021–1.489, p = 0.0293), and other (HR 1.275; 95% CI 1.039–1.564, p = 0.0198) comorbidities were associated with an increased risk of mortality (table 3, full comorbidity model). Table 3 also shows how the risk of mortality is affected by adjusting for different covariates for the individual comorbidities. After including comorbidity and disability in the model, the risk of mortality conferred by comorbidity increased, but after adjusting for the remaining covariates, the effect of the comorbidity on the risk of mortality was reduced.
Table 3.
Effect of individual comorbidities on mortality using different covariates in the proportional hazards model
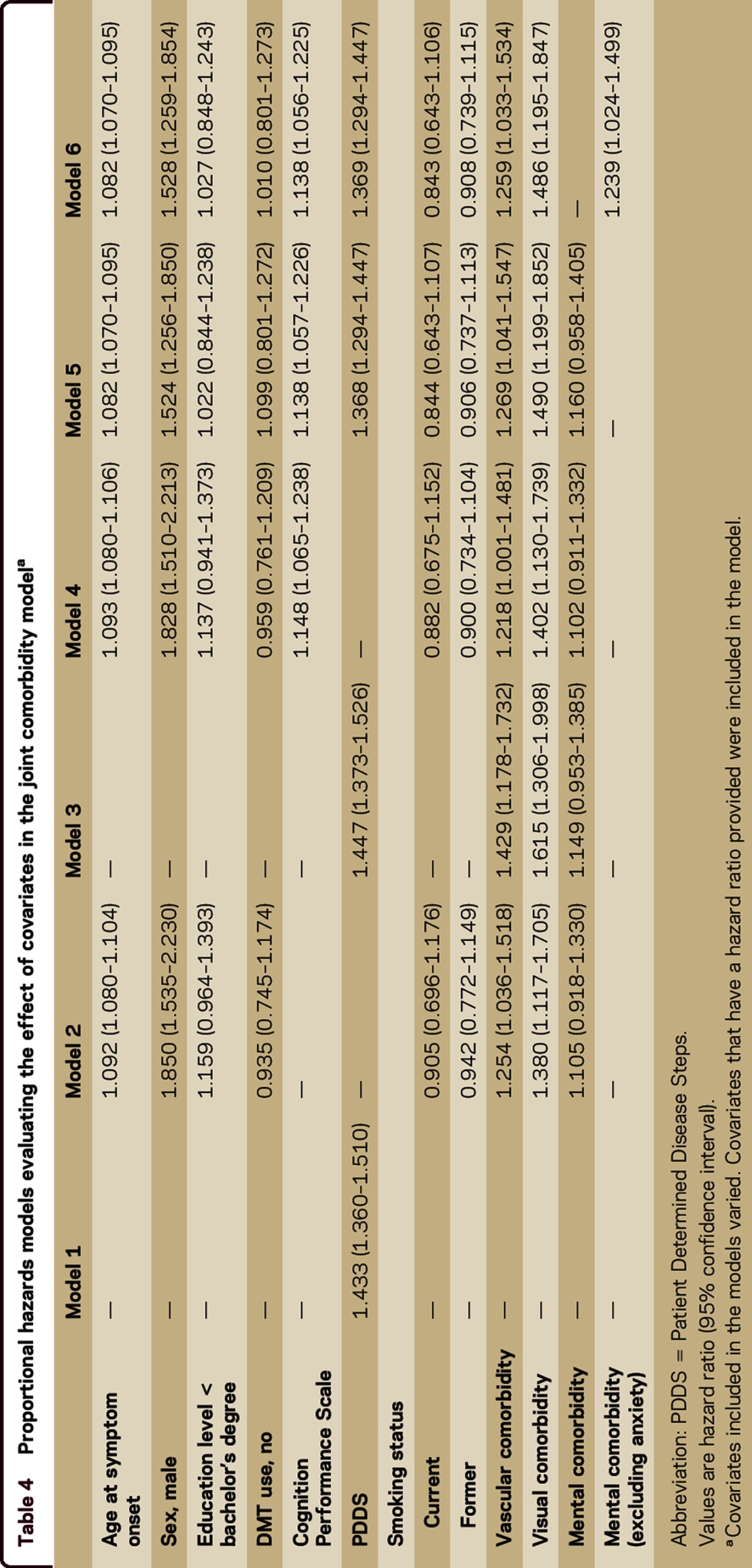
When the comorbidities associated with an increased risk of mortality were analyzed together along with covariates, the HRs for the comorbidities were attenuated (table 4). While the associations between vascular and visual comorbidities with mortality remained significant, the association between mental comorbidity and mortality did not. However, removing anxiety from the mental comorbidity category resulted in the mental comorbidity continuing to be associated with an increased risk of mortality in the joint comorbidity model (table 4, model 6). Older age at onset, male sex, greater physical disability, and cognitive impairment remained associated with an increased risk of death. Socioeconomic status and smoking status were not associated with mortality.
Table 4.
Proportional hazards models evaluating the effect of covariates in the joint comorbidity modela
When we repeated our analyses using only NDI data and follow-up through 2009, our findings were similar for the multivariate analysis in the full comorbidity model for each comorbidity group with the exception of the autoimmune and other comorbidity group (data not shown). The autoimmune comorbidity group was significant (HR 1.574; 95% CI 1.154–2.072, p = 0.0035), but was not when considered in the joint comorbidity model (HR 1.261; 95% CI 0.930–1.710, p = 0.1361). The other comorbidity category was not significant in the sensitivity analyses (HR 1.335; 95% CI 0.999–1.784, p = 0.0509). Additionally, in the joint comorbidity model, the mental comorbidity group that included anxiety was significant (HR 1.328; 95% CI 1.028–1.716, p = 0.0298) and the risk of mortality was increased for the mental comorbidity group when anxiety was included (HR 1.403; 95% CI 1.086–1.814, p = 0.0097).
DISCUSSION
In this study of over 9,000 persons with MS, we evaluated the joint effects of physical and cognitive disability, health behaviors, and comorbidity on mortality, going beyond earlier studies, which have tended to look at many of these factors in isolation. We found that male sex, older age at MS onset, increasing physical or cognitive disability, and comorbidity were independently associated with mortality. Smoking, use of DMT, and socioeconomic status, as measured by education, were not associated with mortality.
Increasing physical disability was associated with increased mortality. This is consistent with findings by previous reports.20–22 We also found that greater cognitive impairment was associated with increased mortality, independent of the effects of physical disability. The association between cognitive impairment and mortality has been little studied in MS. A study looked at the effect of cognitive impairment in MS and did not find an independent association with mortality,20 but the sample size in that study was small. The literature on aging suggests that cognitive impairment confers an increased risk of mortality even among those with cognitive impairment that does not reach the threshold for dementia.23–25 Another study found an increased risk of mortality (HR 1.70; 95% CI 1.32–2.19) in moderately to severely cognitively impaired older primary care adults after controlling for demographic and clinical characteristics.24 This issue warrants further study.
In multivariable models that adjusted for demographics and disability status, vascular, visual, and mental comorbidity were associated with mortality. When the comorbidities were included in the same model, mental comorbidity was no longer significant. Excluding anxiety from the mental comorbidity changed this result. This observation appears to be in line with another study, which found that while depression and bipolar disorder were associated with increased risk of mortality, anxiety was protective of mortality.6 While prior studies consistently suggest an adverse association of comorbidity with mortality, findings regarding individual comorbidities have been inconsistent, possibly due to differences in the study populations examined, types of data sources used, comorbidities considered, and covariates. In a cohort of 2,994 US veterans, comorbidity, as measured using the Seattle Index of Comorbidity, was associated with an increased risk of mortality; the effects of individual comorbidities were not considered. In an incident cohort of 1,713 persons with MS in the UK Clinical Practice Research Database, in which data are drawn from primary care records, heart disease and cancer were associated with mortality after adjustment for health behaviors, sex, year of birth, and MS treatment, but depression, lung disease, diabetes, and hypertension were not. In a population-based study of a prevalent MS cohort in Manitoba, Canada, that used administrative (claims) data, diabetes (HR 1.47; 1.25–1.73), ischemic heart disease (HR 1.50; 1.28–1.75), depression (HR 1.62; 1.39–1.88), and chronic lung disease (HR 1.21; 1.03–1.42) were associated with increased mortality in the MS population, while the effects of visual comorbidities were not evaluated.6 Further, health behaviors and disability status were not considered. In a northern Finnish cohort, the authors evaluated the effect of neurologic comorbidities on mortality in MS, and reported that stroke was associated with shorter survival.8
Information on the effect of smoking on mortality in patients with MS is limited. Four studies to date have examined this issue, 3 in the United Kingdom and one in a US Veterans population.26–29 Previous reports showed an association of current smoking with an increased risk of mortality.26–28 However, 2 of them did not include data on comorbid health conditions while another27 did not control for disability status. While a fourth study did control for disability and comorbid health conditions, a summary index was used to measure comorbidities, thus the effects of individual comorbidities were not elucidated.29 The large proportion of male and nonwhite participants in this Veterans cohort makes its observations less generalizable to the overall MS population. Our study may have had a limited ability to detect an effect of smoking status on mortality because we used smoking status from only one point in time or because the registry had a low proportion of respondents who are current smokers.
This study had several strengths. We investigated mortality in a large sociodemographically diverse cohort with a median of 12 years of longitudinal data. This allowed us to account for changes in disability status over time, and the acquisition of comorbidities over time. Limitations of the study should also be recognized. Participants are volunteers who may not fully represent the MS population, although the characteristics of our study population are similar to those reported elsewhere.5,30,31 Participants self-reported their disability and comorbidities, but the measures used have been validated previously.10,12,16 The ascertainment of death using both the NDI data and the information reported by relations of the deceased participant could be a source of ascertainment bias in this study. However, sensitivity analysis showed that the conclusions were consistent for the comorbidity models using only the NDI death data compared to those using all reported death data. While some deaths may have been missed in the later time of follow-up, this would tend to bias our findings toward the null.
Comorbidity and physical and cognitive disability are independently associated with mortality in MS. Comorbidities may be prevented or treated, including all of those associated with mortality in our models. Future studies should evaluate whether changing health behaviors, such as quitting smoking, achieving a healthy weight, and being physically active, may reduce disability progression by reducing the risk of comorbidity. While effective pharmacotherapy is lacking for cognitive impairment in MS, nonpharmacologic therapies are emerging.32–34 Although we did not identify an effect of use of DMT on mortality, we did not take into account when the DMT use occurred and this may have reduced our ability to detect a benefit in our study population. Other studies have suggested such effects, however35; this indicates the need for further research in this area.
Consideration of the effect of multiple comorbidities on MS is needed. A large proportion of our study participants reported having multiple comorbidities and we showed that risk of mortality is further increased in a person with both vascular and visual comorbidities. It is important for clinicians to address these issues especially for those conditions that can be treated or benefit from healthier lifestyle choices.
ACKNOWLEDGMENT
The NARCOMS Registry is supported in part by the Consortium of Multiple Sclerosis Centers. The Cognition Performance Scale has a filed copyright assigned to DeltaQuest Foundation, Inc., effective October 1, 2005. US Copyright law governs terms of use (TXu000743629/1996-04-04).
Footnotes
Supplemental data at Neurology.org/cp
AUTHOR CONTRIBUTIONS
Amber R. Salter conducted the analyses and drafted the manuscript. Ruth Ann Marrie conceived of the idea and drafted the manuscript. Gary R. Cutter, Robert Fox, Guoqiao Wang, and Tuula Tyry edited the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
A. Salter serves on a DSMB for GlaxoSmithKline. T. Tyry reports no disclosures. G. Wang receives support from a North American Research Committee on Multiple Sclerosis (NARCOMS) Research Fellowship, which is supported in part by the Foundation of the CMSC and an unrestricted educational grant by Biogen. R.J. Fox serves on scientific advisory boards for Biogen Idec and Novartis; serves on the editorial boards of Neurology and Multiple Sclerosis Journal; receives publishing royalties for Multiple Sclerosis and Related Disorders (Demos Medical, 2013); serves as a consultant for Actelion, Biogen Idec, MedDay, Genentech, Novartis, Mallinckrodt, Teva, and XenoPort; and receives research support from Novartis, National MS Society, NIH, and Consortium of MS Centers. G.R. Cutter is employed by the University of Alabama at Birmingham and is president of Pythagoras, Inc., a private consulting company located in Birmingham Alabama; serves/has served on scientific advisory boards for Apotek, Biogen-Idec, Cleveland Clinic (Vivus), GlaxoSmithKline Pharmaceuticals, Gilead Pharmaceuticals, Horizon Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Merck/Pfizer, Opko Biologics, Neuren, Sanofi-Aventis, Teva, NHLBI, NINDS, and NICHD; has received speaker honoraria from funding for travel or speaker honoraria from Consortium of MS Centers and Teva; serves on the editorial boards of Multiple Sclerosis and Alzheimer's & Dementia: Translational Research & Clinical Interventions, as Contributing Editor for Neurology: Clinical Practice, and as statistical consulting reviewer for Journal of the American Society of Nephrology; serves as a consultant for Argenx, Consortium of MS Centers, Genzyme, Genentech, Janssen Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Nivalis, Novartis, Opexa Therapeutics, Receptos, Roche, EMD Serono, Somahlution, TG Therapeutics Inc., Teva Pharmaceuticals, and Transparency Life Sciences; receives/has received research support from NIH (NINDS, NIAID, NHLBI, NICHD, NIA, NIDDK), Consortium of MS Centers (CMSC), North American Research Consortium on Multiple Sclerosis (NARCOMS), US Department of Defense, Patient Centered Outcomes, Research Institute, Children's Hospital (Boston), State of Alabama, and Myasthenia Gravis Foundation of America; owns stock/stock options in Pythagoras, Inc.; and has reviewed statistical data in a medico-legal case. R.A. Marrie serves on the editorial boards of Neurology® and Multiple Sclerosis Journal and receives/has received research support from Sanofi-Aventis, Canadian Institutes of Health Research, the Public Health Agency of Canada, Research Manitoba, Multiple Sclerosis Society of Canada, National Multiple Sclerosis Society, Multiple Sclerosis Scientific Foundation, Consortium of Multiple Sclerosis Centers, and Rx & D Health Research Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Scalfari A, Knappertz V, Cutter G, Goodin DS, Ashton R, Ebers GC. Views & reviews: mortality in patients with multiple sclerosis. Neurology 2013;81:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004;127:844–850. [DOI] [PubMed] [Google Scholar]
- 3.Llorca J, Guerrero-Alonso P, Prieto-Salceda D. Mortality trends of multiple sclerosis in Spain, 1951–1997: an age-period-cohort analysis. Neuroepidemiology 2005;24:129–134. [DOI] [PubMed] [Google Scholar]
- 4.Ekestern E, Lebhart G. Mortality from multiple sclerosis in Austria 1970–2001: dynamics, trends, and prospects. Eur J Neurol 2004;11:511–520. [DOI] [PubMed] [Google Scholar]
- 5.Leray E, Vukusic S, Debouverie M, et al. Excess mortality in patients with multiple sclerosis starts at 20 years from clinical onset: data from a large-scale French observational study. PLoS One 2015;10:e0132033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology 2015;85:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manouchehrinia A, Tanasescu R, Tench CR, Constantinescu CS. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J Neurol Neurosurg Psychiatry 2016;87:324–331. [DOI] [PubMed] [Google Scholar]
- 8.Krökki O, Bloigu R, Ansakorpi H, Reunanen M, Remes AM. Neurological comorbidity and survival in multiple sclerosis. Mult Scler Relat Disord 2013;3:72–77. [DOI] [PubMed] [Google Scholar]
- 9.The Consortium of Multiple Sclerosis Centers (CMSC). Available at: http://www.mscare.org/?page=about_the_cmsc. Accessed November 4, 2015.
- 10.Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of Patient Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz CE, Vollmer T, Lee H. Reliability and validity of two self-report measures of impairment and disability for MS. Neurology 1999;52:63–70. [DOI] [PubMed] [Google Scholar]
- 12.Marrie R, Goldman M. Validity of performance scales for disability. Mult Scler 2007;13:1176–1182. [DOI] [PubMed] [Google Scholar]
- 13.Ritvo P, Fischer J, Miller D, Andrews H, Paty DW, LaRocca N. Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 14.Arday DR, Tomar SL, Nelson DE, Merrit RK, Schooley MW, Mowery P. State smoking prevalence estimates: a comparison of the behavioral risk factor surveillance system and current population surveys. Am J Public Health 1997;87:1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutter GR, Zimmerman J, Salter AR, et al. Causes of death among persons with multiple sclerosis. Mult Scler Relat Disord 2015;4:484–490. [DOI] [PubMed] [Google Scholar]
- 16.Horton M, Rudick RA, Hara-Cleaver C, Marrie RA. Methods in neuroepidemiology: validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology 2010;35:83–90. [DOI] [PubMed] [Google Scholar]
- 17.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 2009;72:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tettey P, Simpson S, Taylor BV, van der Mei IAF. Vascular comorbidities in the onset and progression of multiple sclerosis. J Neurol Sci 2014;347:23–33. [DOI] [PubMed] [Google Scholar]
- 19.Moccia M, Lanzillo R, Palladino R, et al. The Framingham cardiovascular risk score in multiple sclerosis. Eur J Neurol 2015;22:1176–1183. [DOI] [PubMed] [Google Scholar]
- 20.Chruzander C, Johansson S, Gottberg K, et al. A 10-year follow-up of a population-based study of people with multiple sclerosis in Stockholm, Sweden: changes in disability and the value of different factors in predicting disability and mortality. J Neurol Sci 2013;332:121–127. [DOI] [PubMed] [Google Scholar]
- 21.Leray E, Morrissey SP, Yaouanq J, et al. Long-term survival of patients with multiple sclerosis in West France. Mult Scler 2007;13:865–874. [DOI] [PubMed] [Google Scholar]
- 22.Sadovnick AD, Ebers GC, Wilson RW, Paty DW. Life expectancy in patients attending multiple sclerosis clinics. Neurology 1992;42:991–994. [DOI] [PubMed] [Google Scholar]
- 23.Sachs GA, Carter R, Holtz LR, et al. Cognitive impairment: an independent predictor of excess mortality. Ann Inter Med 2011;155:300–308. [DOI] [PubMed] [Google Scholar]
- 24.Stump TE, Callahan CM, Hendrie HC. Care patients. J Am Geriatr Soc 2001;49:934–940. [DOI] [PubMed] [Google Scholar]
- 25.Perna L, Wahl H-W, Mons U, Saum KU, Holleczek B, Brenner H. Cognitive impairment, all-cause and cause-specific mortality among non-demented older adults. Age Ageing 2015;44:445–451. [DOI] [PubMed] [Google Scholar]
- 26.Lalmohamed A, Bazelier MT, Van Staa TP, et al. Causes of death in patients with multiple sclerosis and matched referent subjects: a population-based cohort study. Eur J Neurol 2012;19:1007–1014. [DOI] [PubMed] [Google Scholar]
- 27.Jick SS, Li L, Falcone GJ, Vassilev ZP, Wallander MA. Epidemiology of multiple sclerosis: results from a large observational study in the UK. J Neurol 2015;262:2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manouchehrinia A, Weston M, Tench CR, Britton J, Constantinescu CS. Tobacco smoking and excess mortality in multiple sclerosis: a cohort study. J Neurol Neurosurg Psychiatry 2014;85:1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil 2015;96:402–409. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs LD, Wende KE, Brownscheidle CM, et al. A profile of multiple sclerosis: the New York State Multiple Sclerosis Consortium. New York 1999;5:369–376. [DOI] [PubMed] [Google Scholar]
- 31.MSBase: baseline characteristics. Available at: https://www.msbase.org/cms/benchmarking.json. Accessed November 19, 2015.
- 32.He D, Zhou H, Guo D, Hao Z, Wu B. Pharmacologic treatment for memory disorder in multiple sclerosis. Cochrane Database Syst Rev 2011:CD008876. [DOI] [PubMed] [Google Scholar]
- 33.Carr SE, Nair R, Schwartz AF, et al. Group memory rehabilitation for people with multiple sclerosis: a feasibility randomized controlled trial. Clin Rehabil 2014;28:552–561. [DOI] [PubMed] [Google Scholar]
- 34.Ernst A, Blanc F, De Seze J, Manning L. Using mental visual imagery to improve autobiographical memory and episodic future thinking in relapsing-remitting multiple sclerosis patients: a randomised-controlled trial study. Restor Neurol Neurosci 2015;33:621–638. [DOI] [PubMed] [Google Scholar]
- 35.Goodin DS, Ebers GC, Cutter G, et al. Cause of death in MS: long-term follow-up of a randomised cohort, 21 years after the start of the pivotal IFNβ-1b study. Neurology 2012;2:e001972. [DOI] [PMC free article] [PubMed] [Google Scholar]