Abstract
Objective:
To identify predictors of early lobar intracerebral hemorrhage (ICH) recurrence, defined as a new ICH within 6 months of the index event, in patients with cerebral amyloid angiopathy (CAA).
Methods:
Participants were consecutive survivors (age ≥55 years) of spontaneous symptomatic probable or possible CAA-related lobar ICH according to the Boston criteria, drawn from an ongoing single-center cohort study. Neuroimaging markers ascertained in CT or MRI included focal (≤3 sulci) or disseminated (>3 sulci) cortical superficial siderosis (cSS), acute convexity subarachnoid hemorrhage (cSAH), cerebral microbleeds, white matter hyperintensities burden and location, and baseline ICH volume. Participants were followed prospectively for recurrent symptomatic ICH. Cox proportional hazards models were used to identify predictors of early recurrent ICH adjusting for potential confounders.
Results:
A total of 292 patients were enrolled. Twenty-one patients (7%) had early recurrent ICH. Of these, 24% had disseminated cSS on MRI and 19% had cSAH on CT scan. In univariable analysis, the presence of disseminated cSS, cSAH, and history of previous ICH were predictors of early recurrent ICH (p < 0.05 for all comparisons). After adjusting for age and history of previous ICH, disseminated cSS on MRI and cSAH on CT were independent predictors of early recurrent ICH (hazard ratio [HR] 3.92, 95% confidence interval [CI] 1.38–11.17, p = 0.011, and HR 3.48, 95% CI 1.13–10.73, p = 0.030, respectively).
Conclusions:
Disseminated cSS on MRI and cSAH on CT are independent imaging markers of increased risk for early recurrent ICH. These markers may provide additional insights into the mechanisms of ICH recurrence in patients with CAA.
Cerebral amyloid angiopathy (CAA) is characterized by progressive deposition of β-amyloid in the media and adventitia of small cortical and leptomeningeal vessels, and is a major cause of intracerebral hemorrhage (ICH) in elderly.1 CAA-related ICH typically occurs in cortical and cortico-subcortical (i.e., lobar) brain regions2 and has a high rate of recurrence associated with considerable morbidity and mortality.3,4 Although the distribution of recurrent ICH risk over time after a CAA-related lobar ICH is not well-characterized, a subgroup of patients with CAA appear to have early recurrence, weeks after a lobar ICH, while others might have a recurrent ICH years after their baseline ICH.5–7
Cortical superficial siderosis (cSS) reflects linear blood breakdown products (including hemosiderin) that occur over the superficial layers of the cerebral cortex or in the subarachnoid space.8–10 It has recently emerged as another key hemorrhagic imaging marker for CAA.9 Moreover, cSS is associated with a high risk of incident and recurrent ICH in patients with CAA, independent of other putative markers of the disease, like multiple lobar microbleeds.10–12 It has been suggested that cSS is caused by recurrent episodes of convexity subarachnoid hemorrhage (cSAH) from brittle, CAA-affected leptomeningeal vessels.13 In addition, there is evidence suggesting that patients with cSAH are at risk of future lobar ICH and cSAH recurrence.14 We hypothesized that cSS and cSAH, as markers of focally active or more severe CAA, might also be independent predictors of recurrent lobar ICH occurring early after an initial symptomatic lobar ICH. To test this hypothesis, we examined early recurrent ICH in a longitudinal cohort study of consecutive CAA-ICH survivors.
METHODS
Study design.
This was a prospective cohort study of consecutive survivors of CAA-related lobar ICH.
Standard protocol approvals, registrations, and patient consents.
This study was performed with approval of the institutional review board of Massachusetts General Hospital (MGH) with informed consent from all participants or family members.
Participants.
Study participants were consecutive survivors drawn from an ongoing longitudinal cohort study of spontaneous lobar ICH as previously described in detail.15 Participants were recruited from patients ≥55 years old admitted to MGH from July 1994 to October 2012 for lobar ICH. Lobar ICH was defined as symptomatic intraparenchymal hemorrhage involving the cerebral cortex and underlying subcortical white matter without extending into deep brain regions (e.g., basal ganglia, thalamus). Our inclusion criteria for this study were (1) patients diagnosed with CAA according to the classic Boston criteria (i.e., including lobar cerebral microbleeds [CMBs], but not including cSS in the diagnostic criteria)2; (2) available MRI including T2*-weighted gradient-recalled echo (T2*GRE) or susceptibility-weighted imaging (SWI) sequences, fluid-attenuated inversion recovery (FLAIR), and brain CT scan; (3) survival beyond index hospital stay; and (4) availability of follow-up data, including information on recurrent symptomatic ICH events. Participants were excluded if MRI or CT scans were of insufficient quality for rating or performed after a surgical procedure including craniotomy or ventriculostomy.
Clinical and genotype data.
Clinical data were systematically and prospectively collected and recorded at the time of index symptomatic lobar ICH presentation at MGH and included demographic data, history of hypertension, diabetes mellitus, anticoagulant use, aspirin use, statin use, and history of ICH, as described.15 Blood pressure (BP) measurements were obtained from medical records after discharge from the hospital until the last follow-up or before recurrent symptomatic lobar ICH or death.16 APOE genotype was determined from all patients who donated blood samples for genetic analysis and provided informed consent as previously described.17
Brain CT analysis.
All admission CT scans were reviewed by a trained investigator (D.R.) blinded to clinical, MRI, and follow-up data. Acute cSAH was defined as acute blood in the subarachnoid space identified as a linear hyperdensity in one or several cortical sulci at the superficial surface of cerebral cortex without extension into the interhemispheric fissure, sylvian fissure, basal cistern, or ventricles.18 Acute cSAH directly adjacent to any ICH were not considered. Volume of ICH and CT-defined white matter hypodensity (CT-WMH) were calculated and assessed as previously described.19,20 The interrater agreement (D.R. and A.C.) for the presence of acute cSAH was 100% (Cohen κ = 1.00) in 20 randomly selected patients.
MRI acquisition and analysis.
MRI with axial gradient-echo images was performed as previous described.1,19 All images were reviewed by a trained investigator (D.R.) blinded to all clinical and follow-up data. cSS was defined as curvilinear chronic blood products in the subarachnoid space or in the superficial layer of the cerebral cortex detected as linear hypointensities on T2*GRE images or SWI and without corresponding hyperintense signal on T1-weighted or FLAIR images.11 Severity of cSS was classified as focal (involving ≤3 sulci) or disseminated (>3 sulci).9 The interrater agreement for cSS presence and severity was 90% (Cohen κ = 0.79) and 85% (Cohen κ = 0.74), respectively, as described previously.21 cSAH was defined as evidence of acute blood in the subarachnoid space localized in the superficial layer of the cerebral cortex, which showed linear hyperintensity on FLAIR images with corresponding linear hypointensity on T2*GRE images22 or SWI. We did not include cSS and cSAH adjacent to any ICH or due to ICH extension into the interhemispheric fissure, sylvian fissure, basal cistern, or ventricles. CMBs were defined as small round signal voids visualized on T2*GRE images23 and categorized into 0, 1, 2–4, or ≥5.4 White matter hyperintensities including periventricular hyperintensities and deep white matter hyperintensities were evaluated using the Fazekas rating scale.24
Follow-up.
The clinical data collected during follow-up including medication use, appearance of new neurologic symptoms, recurrent lobar ICH, and death were obtained from consenting survivors and their caregivers by systematic telephone interview at 6-month intervals after ICH through December 2014.15 All recurrent ICH reports were confirmed by direct review of all available relevant medical records and brain CT scans by a study investigation blinded to other clinical data to assess the presence or absence of recurrent ICH. The number of subsequent symptomatic recurrent ICH events after index ICH was also recorded. Early symptomatic recurrent CAA-related lobar ICH was defined as symptomatic lobar ICH within 6 months after index ICH. The cutoff value at 6 months was defined based on observed recurrence patterns seen in a large cohort of patients with CAA-related ICH. The Kaplan-Meier plots showed the steepest increase in recurrence risk within 6 months after index ICH, suggesting a skewed distribution of recurrence.6
Statistical analyses.
Discrete variables are presented as count (%) and continuous variables as mean (SD) or median (interquartile range [IQR]) as appropriate. Unadjusted, univariable analysis between participants with (i.e., ICH within 6 months after index event) and without early symptomatic recurrent CAA-related lobar ICH (including both patients without any recurrent symptomatic lobar ICH and late recurrent systematic lobar ICH, i.e., occurring more than 6 months after index event) was completed by using the Pearson χ2 and the Fisher exact test for discrete variables, and the independent-sample t test or the Mann-Whitney U test for continuous variables. The Pearson correlation and the χ2 test were used to investigate the correlation between the presence of cSS and potential risk factors. Kaplan-Meier plots with significance testing by the log-rank test were used to determine univariable predictors for early recurrent symptomatic ICH. For patients experiencing multiple recurrent ICH events (>1 recurrent event) during follow-up, data were censored after the first recurrent ICH event.
Multivariable analyses were subsequently pursued to account for potential confounders. Multivariable Cox proportional hazards models were used to identify independent predictors of early recurrent symptomatic ICH, including variables with p < 0.1 in univariable analysis. Survival time was calculated from date of index ICH until the date of early recurrent ICH, the last follow-up date, the last known date without the outcome of interest, or date of death. Backward elimination was used to generate the best minimal model by eliminating nonsignificant variables (p > 0.05). Multicollinearity was assessed using variance inflation factors (VIF) and predictors with VIF >5 were removed from the model.
All analyses were performed with SPSS statistics version 17.0. Significance tests were 2-tailed with p value <0.05.
RESULTS
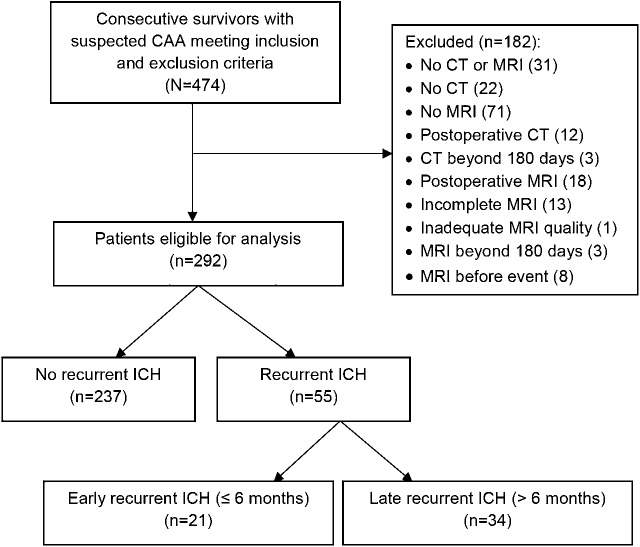
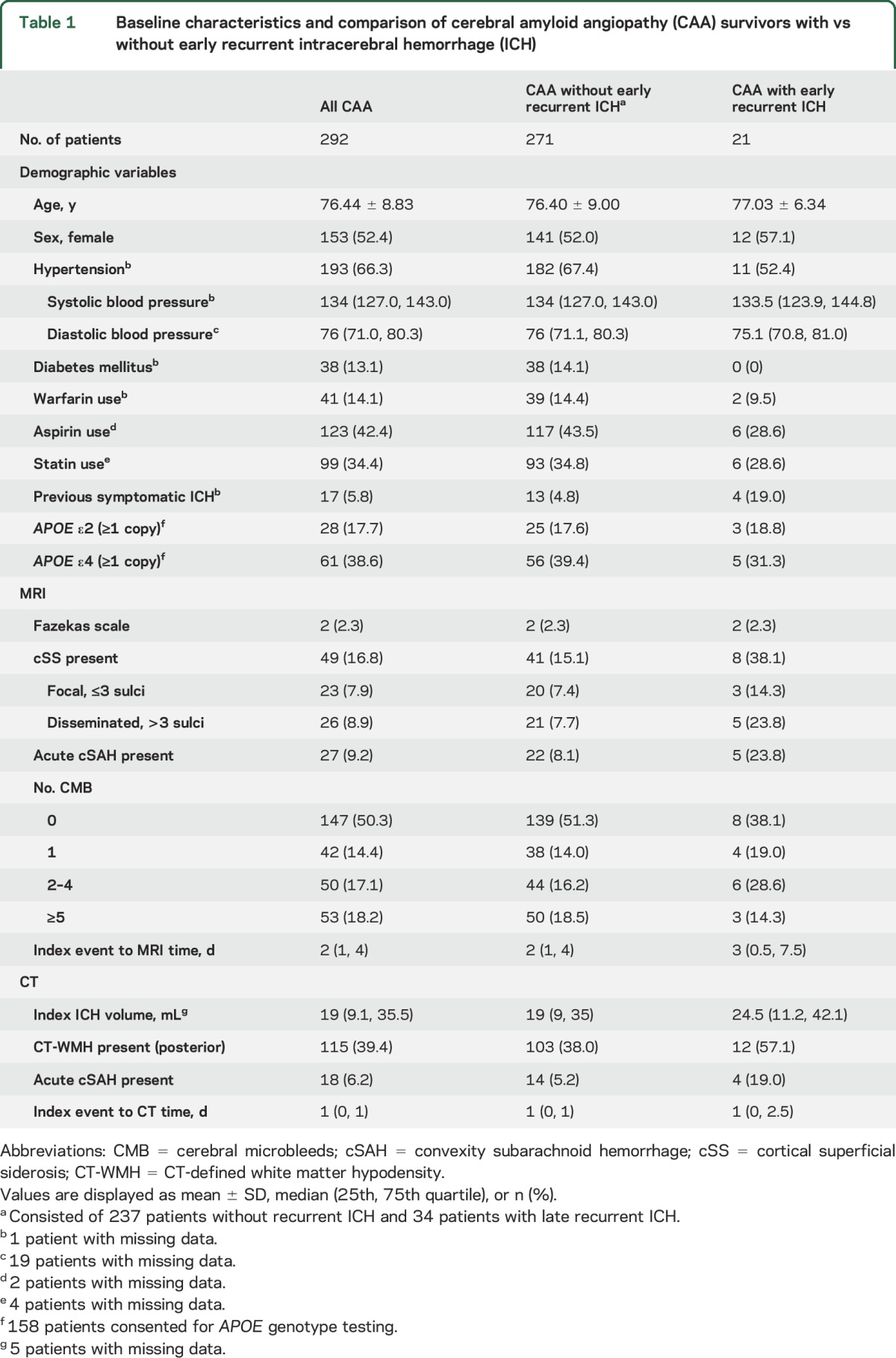
The final cohort consisted of 292 patients. A flow diagram of patient identification and inclusion in the current analysis is presented in figure 1. Five patients were diagnosed with definite CAA (autopsy-confirmed), 5 patients were diagnosed with probable CAA with supporting pathology, 166 patients were diagnosed with probable CAA, and 116 patients were diagnosed with possible CAA according to Boston criteria.2 Baseline characteristics of the patients are detailed in table 1.
Figure 1. Flow diagram of patient identification, inclusion into the study.
CAA = cerebral amyloid angiopathy; ICH = intracerebral hemorrhage.
Table 1.
Baseline characteristics and comparison of cerebral amyloid angiopathy (CAA) survivors with vs without early recurrent intracerebral hemorrhage (ICH)
During a median follow-up period of 28.1 months (IQR 6.59–56.03 months), 55 recurrent symptomatic lobar ICH events occurred in 292 patients: 21 patients (7.2%) had early recurrent ICH. Among patients with early recurrent ICH, 5 patients (23.8%) had disseminated cSS, 5 patients (23.8%) had cSAH on MRI, and 4 patients (19%) had cSAH on CT scan (figure 2). There appeared to be no association between the location of cSS/cSAH at baseline and the location of recurrent ICH in the early recurrence group (data not shown).
Figure 2. Disseminated cortical superficial siderosis and early recurrent lobar intracerebral haemorrhage (ICH).
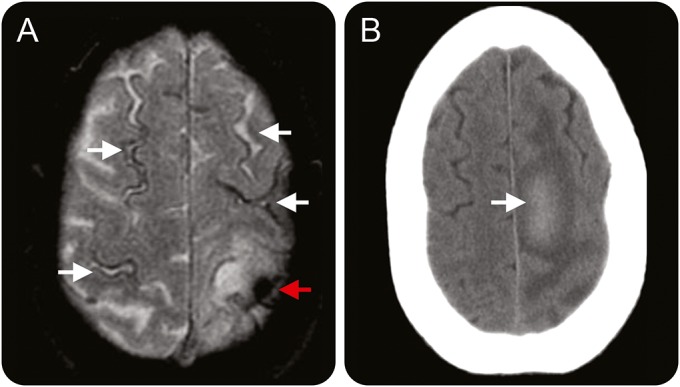
Baseline brain MRI scan and follow-up CT scan of a 79-year-old man who had recurrent symptomatic lobar ICH at 2 months after index ICH. (A) Axial T2*-weighted gradient-recalled echo at baseline demonstrates disseminated cortical superficial siderosis (white arrows) and index ICH in the left posterior frontal parietal area (red arrow). (B) Follow-up axial noncontrast CT scan at 2 months after index ICH demonstrates new subacute lobar intracerebral hemorrhage in the posterior aspect of the left superior frontal gyrus with surrounding edema.
In the whole cohort, 49 patients (16.8%) had cSS at baseline; cSS was associated with history of symptomatic ICH (p < 0.001), multiple (≥2) CMBs (p = 0.001), and older age (p = 0.017). cSAH on CT and MRI was associated with history of symptomatic ICH (p = 0.02 and p < 0.001, respectively). History of warfarin or aspirin use were not associated with the presence of cSS or cSAH on CT or MRI scans (p > 0.05).
In Kaplan-Meier analysis, the presence of cSS, cSAH-CT, cSAH-MRI, and history of symptomatic ICH prior to index event were predictors for early recurrent ICH (log rank p value = 0.002, p = 0.004, p = 0.009, and 0.007 respectively). Disseminated cSS (p = 0.001), but not focal cSS (p = 0.104), was associated with early recurrent ICH compared to individuals without cSS (figure 3). No other factors including age, hypertension, history of warfarin use, history of aspirin use, APOE ε2 or ε4 alleles, multiple (≥2) CMBs, the presence of posterior CT-WMH, or ICH volume were associated with early recurrence (p > 0.05).
Figure 3. Cortical superficial siderosis (cSS), convexity subarachnoid hemorrhage (cSAH), and early recurrent lobar intracerebral hemorrhage (ICH).
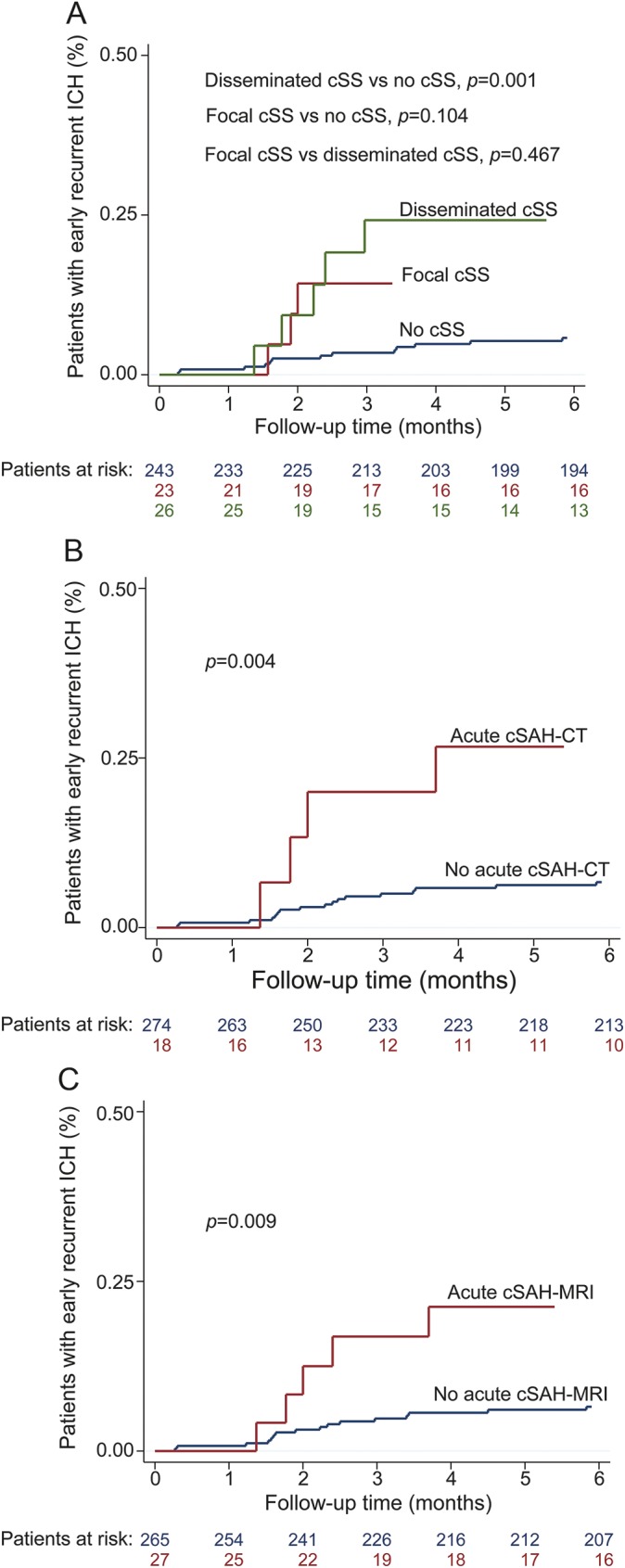
Kaplan-Meier plot estimates the progression of the early recurrent lobar ICH in all patients with cerebral amyloid angiopathy with the presence of (A) focal and disseminated cSS, (B) cSAH detected by CT scan, and (C) cSAH detected by MRI scan.
Multivariable Cox regression analysis was used to build 2 models: a model for both MRI and CT scans (main analysis) and a model for MRI only (secondary analysis). In the model for CT and MRI combined, both disseminated cSS and cSAH on CT scan were independently associated with early recurrent ICH. These effect sizes remained consistent even when age and history of previous symptomatic ICH were forced into the final model (table 2). In a model for MRI, disseminated cSS and cSAH-MRI were independently associated with increased risk for early recurrent ICH; other significant variables from univariable analysis (table e-1 at Neurology.org) including age did not remain significant in the final model. After forcing age and history of symptomatic ICH into the final model, only disseminated cSS was independently associated with increased risk for early recurrent ICH. In patients who had both cSAH and cSS in the same MRI (n = 5), the risk of early recurrent ICH was increased (hazard ratio 15.69; 95% confidence interval 4.53–54.38; p < 0.001). The effect sizes of the variables in both models remained consistent after additionally controlling for hypertension and multiple CMBs in further sensitivity analyses. Disseminated cSS has a predictive role in early and overall ICH recurrence but not in late ICH recurrence (data not shown). Based on CAA diagnosis, disseminated cSS had a predictive role for early recurrent ICH in probable or definite CAA (table 2; baseline characteristics shown in table e-2), but not in the possible CAA group although the number of events in this group (n = 1) likely limits our ability to find predictors (data not shown).
Table 2.
Multivariable analysis of predictors of early recurrent intracerebral hemorrhage (ICH) in patients with cerebral amyloid angiopathy
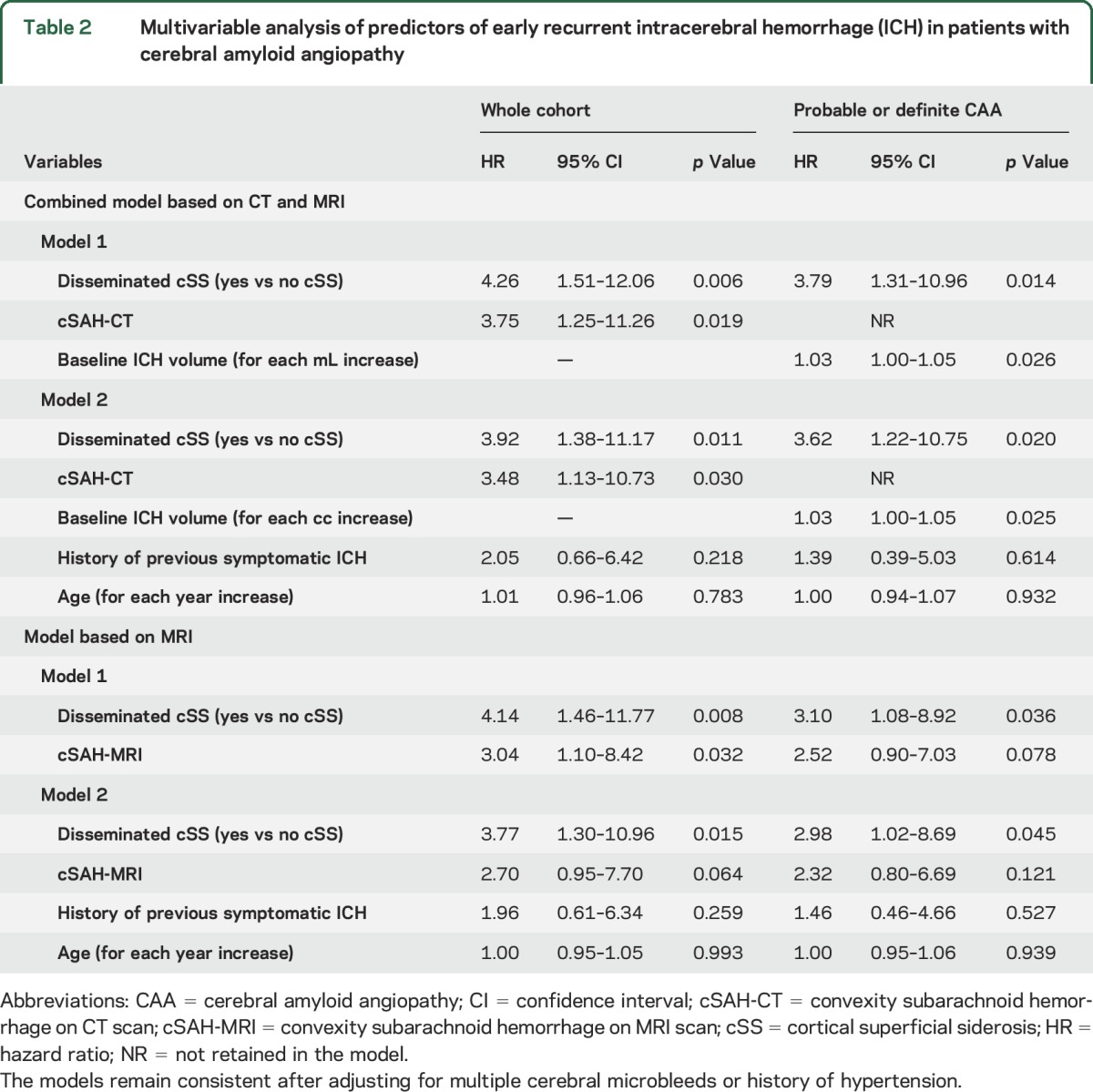
All results including Kaplan-Meier and Cox regression analysis remained consistent in sensitivity analyses, which included CAA patients with only a CT scan and patients with only an MRI scan (data not shown).
DISCUSSION
The main finding from this longitudinal cohort of patients with CAA-related ICH is that cSS, particularly disseminated cSS, and acute cSAH are strongly associated with early recurrent ICH risk during the first 6 months after the initial lobar ICH event.
cSS has emerged as another hemorrhagic imaging signature of CAA-related injury, along with strictly lobar CMBs and ICH.9 cSAH and cSS have also been suggested to be warning signs for future ICH. Case reports suggest that patients with CAA who have cSAH on initial MRI may be at imminent risk to develop lobar ICH (within 7–25 days).8,25,26 A longitudinal study reporting on 38 spontaneous cSAH cases with 1 autopsy-proved CAA-related cSAH suggested that patients with cSAH are at risk of recurrent lobar ICH or cSAH during a mean follow-up period of 24 ± 22 months.14 Furthermore, 3 retrospective cohort studies showed that cSS is a predictor of future lobar ICH in patients with CAA during a follow-up period of 24–35.3 months.10–12 Limited histopathologic data have suggested that the bleeding in CAA-related lobar ICH may in fact originate in cerebral sulci in amyloid-laden leptomeningeal vessels.26–28 In general, cerebrovascular amyloid appears to be more extensively deposited in leptomeningeal compared to cortical vessels in CAA.27 Our results suggest that disseminated cSS on MRI and cSAH on CT scan are independent predictors of early recurrent ICH even after controlling for confounding variables including age and history of previous ICH. Because of the study design, we may be lacking statistical power to determine the influence of other key factors such as APOE genotype15 and BP16 on early recurrent ICH risk. However, our data are in line with the current hypothesis that cSS and cSAH may be indicators of more severe or focally active disease in patients with CAA.13
The neuroimaging markers of cSS and cSAH likely represent a continuum of subarachnoid bleeding in patients with CAA: focal convexity linear subarachnoid hemorrhages (acute cSAH) marking acute bleed events and chronic sulcal blood degradation products marking episodes of prior subarachnoid bleeding (cSS). Interestingly, we found that patients who had both cSS and cSAH on the same MRI had a 16-fold increase in the risk of early recurrent ICH. We hypothesize that these 2 markers in conjunction may be indicators of particularly high disease activity, thus putting these patients at increased risk for early recurrent ICH and subsequent recurrent ICH in the future. Moreover, cSS had a predictive role in probable or definite CAA patients but not possible CAA patients. cSS was less frequent in patients with possible CAA, which might indicate less active disease. Further pathologic studies are required to examine these questions in more detail.
Previous studies have reported that hypertension and elevated BP may play an important role in recurrent lobar ICH.3,16 Perhaps surprisingly, the patients with early recurrent ICH appeared to have fewer cardiovascular risk factors, including hypertension, compared to those without recurrent ICH. It is possible that CAA patients with a diagnosis of hypertension may have had better control of BP, which consequently reduced their risk of CAA-related recurrent ICH.29 Further BP control studies in CAA patients with recurrent ICH events are needed.
Our study has several limitations. We could not fully evaluate BP variability or the adequacy of BP control16 in all patients the cohort due to the lack of multiple BP follow-up measurements, particularly in those individuals with early recurrence. APOE ε2 or ε4 alleles were not available for all patients in this cohort study; therefore we did not have enough power to identify an association with early recurrent ICH in this dataset. Although cSS and cSAH can result from an independent mechanism, unrelated to ICH, we cannot fully exclude the possibility that cSS and cSAH resulted from hematoma expansion into the subarachnoid space in some patients. However, it is unlikely that this would influence our results as we did not consider cSS/cSAH directly adjacent to areas of previous lobar ICH in our analysis. In addition, since our results are based on an analysis of 21 early recurrence events, to avoid overfitting, we adjusted models only for age and history of ICH. While we cannot rule out that our models were not fully adjusted for other potential confounders, based on the univariate analyses, our models took all candidate predictors into account. We acknowledge that the 6-month cutoff chosen is to a certain extent arbitrary; however, this cutoff seems to be reasonable based on previous data showing a skewed distribution of recurrence patterns after lobar ICH6 (figure e-1).
Despite these limitations, our results may have clinical relevance in the management of CAA survivors after lobar symptomatic ICH. Our demonstration that cSAH on noncontrast CT scans is a predictor for early recurrent ICH could potentially be applied broadly in clinical practice, as this imaging modality is widespread and routinely obtained in cases of ICH in most hospitals, even those with limited resources. Second, as patients with cSS or cSAH may be at high risk for rapidly recurrent symptomatic lobar ICH, our results may offer guidance to clinicians for counseling and close monitoring of these patients. In these cases, clinicians may wish to especially weigh the potential benefits of secondary cardiovascular prevention strategies like antithrombotics4 and statins30–33 vs the potential increased hemorrhagic risk incurred by these therapies. In addition, clinicians may consider recommending tighter BP control.16,29
Disseminated cSS on MRI and cSAH on CT scan are neuroimaging markers independently associated with an increased risk of early recurrent ICH in patients with CAA. Our data suggest that these markers may provide additional information on ICH recurrence risk assessment with relevance for clinical practice and management. Future prospective studies should attempt to replicate these findings and strive to provide additional insights on potential pathologic mechanisms.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Hui Zheng, PhD (the Biostatistics Center, Massachusetts General Hospital, Boston) for assistance with statistical analysis.
GLOSSARY
- BP
blood pressure
- CAA
cerebral amyloid angiopathy
- CMB
cerebral microbleed
- cSS
cortical superficial siderosis
- CT-WMH
CT-defined white matter hypodensity
- FLAIR
fluid-attenuated inversion recovery
- HTN
hypertension
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- MGH
Massachusetts General Hospital
- SWI
susceptibility-weighted imaging
- T2*GRE
T2*-weighted gradient-recalled echo
- VIF
variance inflation factor
Footnotes
Supplemental data at Neurology.org
Editorial, page 1854
AUTHOR CONTRIBUTIONS
Duangnapa Roongpiboonsopit: data collection, analysis and interpretation of data, and drafting and revising the manuscript. Andreas Charidimou: interpretation of data and revising the manuscript. Christopher M. William: data collection and revising the manuscript. Arne Lauer: data collection and revising the manuscript. Guido J. Falcone: data collection and revising the manuscript. Sergi Martinez-Ramirez: data collection and revising the manuscript. Alessandro Biffi: data collection and revising the manuscript. Alison Ayres: data collection and revising the manuscript. Anastasia Vashkevich: data collection and revising the manuscript. Oluwole O. Awosika: data collection and revising the manuscript. Jonathan Rosand: revising the manuscript. M. Edip Gurol: revising the manuscript. Scott B. Silverman: revising the manuscript. Steven M. Greenberg: revising the manuscript. Anand Viswanathan: study concept and design, analysis and interpretation of data, revising manuscript, and study supervision.
STUDY FUNDING
Supported by NIH (R01AG047975, R01AG026484, P50AG005134, K23AG028726, K23 NS083711, and R01 NS070834).
DISCLOSURE
D. Roongpiboonsopit, A. Charidimou, C. William, A. Lauer, G. Falcone, S. Martinez-Ramirez, A. Biffi, A. Ayres, A. Vashkevich, and O. Awosika report no disclosures relevant to the manuscript. J. Rosand reports grants from National Institutes of Health outside the submitted work. M. Edip Gurol, S. Silverman, S. Greenberg, and A. Viswanathan report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 2004;35:1415–1420. [DOI] [PubMed] [Google Scholar]
- 2.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 3.Passero S, Burgalassi L, D'Andrea P, Battistini N. Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke 1995;26:1189–1192. [DOI] [PubMed] [Google Scholar]
- 4.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finelli PF, Kessimian N, Bernstein PW. Cerebral amyloid angiopathy manifesting as recurrent intracerebral hemorrhage. Arch Neurol 1984;41:330–333. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan A, Rakich SM, Engel C, et al. Antiplatelet use after intracerebral hemorrhage. Neurology 2006;66:206–209. [DOI] [PubMed] [Google Scholar]
- 7.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:660–667. [DOI] [PubMed] [Google Scholar]
- 8.Linn J, Herms J, Dichgans M, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol 2008;29:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linn J, Wollenweber FA, Lummel N, et al. Superficial siderosis is a warning sign for future intracranial hemorrhage. J Neurol 2013;260:176–181. [DOI] [PubMed] [Google Scholar]
- 11.Charidimou A, Peeters AP, Jager R, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology 2013;81:1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni J, Auriel E, Jindal J, et al. The characteristics of superficial siderosis and convexity subarachnoid hemorrhage and clinical relevance in suspected cerebral amyloid angiopathy. Cerebrovasc Dis 2015;39:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015;138:2126–2139. [DOI] [PubMed] [Google Scholar]
- 14.Beitzke M, Enzinger C, Wunsch G, Asslaber M, Gattringer T, Fazekas F. Contribution of convexal subarachnoid hemorrhage to disease progression in cerebral amyloid angiopathy. Stroke 2015;46:1533–1540. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med 2000;342:240–245. [DOI] [PubMed] [Google Scholar]
- 16.Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015;314:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 1995;38:254–259. [DOI] [PubMed] [Google Scholar]
- 18.Raposo N, Viguier A, Cuvinciuc V, et al. Cortical subarachnoid haemorrhage in the elderly: a recurrent event probably related to cerebral amyloid angiopathy. Eur J Neurol 2011;18:597–603. [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 2004;63:1606–1612. [DOI] [PubMed] [Google Scholar]
- 20.Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 21.Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, et al. Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology 2014;83:1838–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ertl L, Morhard D, Deckert-Schmitz M, Linn J, Schulte-Altedorneburg G. Focal subarachnoid haemorrhage mimicking transient ischaemic attack: do we really need MRI in the acute stage? BMC Neurol 2014;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi Y, Shibazaki K, Inoue T, Iguchi Y, Kimura K. A case of primary subarachnoid hemorrhage due to cerebral amyloid angiopathy [in Japanese]. Rinsho Shinkeigaku 2007;47:42–46. [PubMed] [Google Scholar]
- 26.Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007;68:457–460. [DOI] [PubMed] [Google Scholar]
- 27.Takeda S, Yamazaki K, Miyakawa T, et al. Subcortical hematoma caused by cerebral amyloid angiopathy: does the first evidence of hemorrhage occur in the subarachnoid space? Neuropathology 2003;23:254–261. [DOI] [PubMed] [Google Scholar]
- 28.Takeda S, Hinokuma K, Yamazaki K, et al. The hemorrhage caused by sporadic-type cerebral amyloid angiopathy occurs primarily in the cerebral sulci. Neuropathology 2012;32:38–43. [DOI] [PubMed] [Google Scholar]
- 29.Arima H, Tzourio C, Anderson C, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke 2010;41:394–396. [DOI] [PubMed] [Google Scholar]
- 30.Phuah CL, Raffeld MR, Ayres AM, et al. Subacute decline in serum lipids precedes the occurrence of primary intracerebral hemorrhage. Neurology 2016;86:2034–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amarenco P, Bogousslavsky J, Callahan A III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 32.Westover MB, Bianchi MT, Eckman MH, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol 2011;68:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woo D, Deka R, Falcone GJ, et al. Apolipoprotein E, statins, and risk of intracerebral hemorrhage. Stroke 2013;44:3013–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.