Abstract
Objective:
We investigated sex differences in verbal memory across different levels of neural dysfunction, measured by temporal lobe glucose metabolic rates (TLGluMR).
Methods:
Three hundred ninety controls and 672 participants with amnestic mild cognitive impairment (aMCI) and 254 with Alzheimer disease (AD) dementia from the Alzheimer's Disease Neuroimaging Initiative completed the Rey Auditory Verbal Learning Test (RAVLT) and [18F]-fluorodeoxyglucose–PET. Cross-sectional analyses were conducted using linear regression to examine the sex by TLGluMR interaction on RAVLT performance in the overall sample and within diagnostic groups adjusting for age, education, and APOE ε4 genotype.
Results:
Across groups, female sex and higher TLGluMR and their interaction were associated with better verbal memory (p values ≤ 0.005). The female advantage in verbal memory varied by TLGluMR such that the advantage was greatest among individuals with moderate to high TLGluMR and minimal or absent among individuals with lower TLGluMR. Diagnosis-stratified analyses revealed that this interaction was driven by the aMCI group (p values = 0.009). The interaction was not significant in control and AD dementia groups.
Conclusions:
Women show better verbal memory than men in aMCI despite similar levels of brain hypometabolism. The lifelong advantage that females show over males in verbal memory might represent a form of cognitive reserve that delays verbal memory decline until more advanced pathology, as indexed by TLGluMR. This issue is clinically important because verbal memory scores are used in diagnosing aMCI and AD dementia.
The “cognitive reserve” theory proposes that persons with favorable premorbid factors (e.g., higher education, IQ) maintain normal cognitive function longer as Alzheimer-related brain pathology accumulates.1,2 In those with higher cognitive reserve, the onset of accelerated cognitive decline is delayed until time points closer to dementia diagnosis; however, once decline begins, persons with high reserve have more rapid decline because pathology is more advanced.1–5
Throughout life, females outperform males on verbal memory tests.6–8 This female advantage may reflect a sex-specific form of cognitive reserve,9 masking brain pathology and an amnestic mild cognitive impairment (aMCI) diagnosis in the early stages of dementia. Consistent with the cognitive reserve theory, this advantage persists during preclinical stages of Alzheimer disease (AD) including aMCI,10 but wanes during AD dementia suggesting accelerated decline in women vs men.10,11 This sex difference is clinically relevant because cut scores on verbal memory tests used in diagnosing aMCI and AD dementia are typically not sex-adjusted.
The medial temporal lobe mediates verbal memory12,13 and is an initial brain region to exhibit AD-related neuropathology.14 Temporal lobe glucose metabolic rates (TLGluMR), measured by [18F]-fluorodeoxyglucose–PET (FDG-PET), provide an in vivo measure of neural dysfunction.15,16 We examined how the association between verbal memory and TLGluMR differs in women and men within and across diagnostic groups (control, aMCI, AD dementia). We hypothesized that the magnitude of the female advantage in verbal memory would vary by TLGluMR. Based on the cognitive reserve theory, we predicted that women would outperform men on verbal memory at moderate to high TLGluMR but that female advantage would not be evident at low TLGluMR.
METHODS
Participants and data source.
Cross-sectional data were extracted from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) in June 2014. Detailed information about ADNI can be found at www.adni-info.org. ADNI began in 2004 as a longitudinal, multisite cohort study that recruited healthy older adults, and individuals with early or late MCI and early AD. See www.loni.ucla.edu/ADNI for recruitment procedures17 and www.adni-info.org/Scientists/ADNIGrant/ProtocolSummary.aspx for eligibility criteria. About 50% of participants underwent FDG-PET imaging at their baseline ADNI visit. A total of 1,334 participants had concurrent diagnostic, TLGluMR, and verbal memory data from one visit cycle as required for this study. Among the 1,334 participants, we excluded 18 individuals, including 9 individuals missing APOE genotype, 6 individuals with evidence of brain infection, infarction, or other focal lesions at the screening/baseline MRI, and 3 individuals with an MCI diagnosis that did not meet standard criteria for aMCI including objective memory impairment and a subjective memory complaint.18 Our final sample comprised 1,316 participants (399 from ADNI1 and 917 from ADNIGO/2).
Standard protocol approvals, registrations, and patient consents.
ADNI was approved by the institutional review board at each site and was compliant with the Health Insurance Portability and Accountability Act. Written consent was obtained from all participants.
Neuropsychological outcomes.
Cognitive assessments in ADNI included the Mini-Mental State Examination (MMSE)19 to assess global cognitive function and the Clinical Dementia Rating (CDR)20 to assess dementia severity. Verbal memory measures were the Wechsler Memory Scale Logical Memory II (LM-II), a paragraph recall test, and the Rey Auditory Verbal Learning Test (RAVLT), a list-learning and memory test. A female advantage is observed for both verbal memory tests in ADNI; however, we used the RAVLT as our verbal memory outcome because it is independent of diagnostic criteria. In the RAVLT, the participant is read a list of 15 unrelated words and is instructed to recall aloud as many words as possible.21 This process is repeated for 5 learning trials (“immediate recall score,” range 0–75). Then, an interference list of 15 words unrelated to the first list and to each other is read aloud and the participant is asked to recall aloud as many of these words as possible. The participant is then asked to recall the first word list. After a 30-minute delay in which only nonverbal tasks are administered, the participant is again asked to recall as many words from the first list as possible (“delayed recall score,” range 0–15). The immediate and delayed recall scores were our primary score outcomes.
Diagnostic criteria.
An AD dementia diagnosis in ADNI required an MMSE score between 20 and 26, a CDR of 0.5 or 1, and a probable diagnosis of AD dementia by the NINCDS/ADRDA (National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association)22 criteria. An aMCI diagnosis required an MMSE score between 24 and 30, a CDR of 0.5, a subjective memory complaint, and objective memory loss as measured by education-adjusted scores on the LM-II, without significant impairment in other cognitive domains or functional impairment. A diagnosis of early vs late aMCI differentiates modest from advanced impairment on delayed recall of LM-II.23 Classification as “normal” required an MMSE score between 24 and 30 and a CDR of 0.
FDG-PET.
FDG data were collected as 6 × 5–minute frames 30 minutes after injection of 5 mCi of FDG. Images were preprocessed at the University of Michigan, following a standard procedure described at the following website: http://adni.loni.usc.edu/methods/pet-analysis/pre-processing/. Fully processed images were downloaded from ADNI (http://adni.loni.ucla.edu/). ADNI investigators at the University of California, Berkeley, established FDG-PET regions of interest (ROIs) based on a meta-analysis of studies identifying brain regions most frequently demonstrating metabolic changes in AD or correlated with cognitive performance.24,25 Five ROIs were established, labeled “MetaROIs,” that were located in bilateral posterior cingulate gyrus, bilateral angular gyri, and middle/inferior temporal gyrus. For our primary analysis, we used data from the middle/inferior temporal ROI denoted here as TLGluMR because this region mediates verbal memory12,13 and temporal hypometabolism is associated with poorer verbal memory performance.26 For our secondary analysis, we used MetaROI data to determine whether effects are specific to the temporal lobe or generalize to other regions that demonstrate AD-associated metabolic change. The protocol for image analysis is described at the following website: http://www.adni-info.org/Scientists/ADNIStudyProcedures.aspx. FDG uptake measures were normalized to a reference region including the pons and cerebellum.24,25
Statistical analysis.
Differences between sexes in demographic characteristics and variables of interest (RAVLT scores and TLGluMR) were examined in the overall sample and within diagnostic group using independent t tests for continuous variables and χ2 tests for categorical variables. In the overall sample, we used multivariable linear regression to test the independent and interactive associations of sex and TLGluMR for both RAVLT outcomes (immediate and delayed recall). Analyses covaried for age, education, APOE status, and diagnostic group. APOE status was dichotomized as APOE ε4 carriers vs noncarriers. In the initial model, we examined the independent effects of sex and TLGluMR on RAVLT outcomes. In the second model, a sex by TLGluMR interaction term was added, but was excluded if not significant (p > 0.05). In secondary analyses, we used the same statistical approach to examine the independent and interactive associations of sex and TLGluMR on RAVLT immediate and delayed recall but we stratified by diagnostic group instead of covarying for diagnostic group. Given our previous finding of a stronger relationship between verbal memory and hippocampal volume ratio (HpVR: hippocampal volume/intracranial volume) in women vs men, we repeated analyses adjusting for HpVR to determine whether HpVR accounted for the TLGluMR × sex interactions on RAVLT. Analyses were repeated substituting the MetaROI FDG-PET data for TLGluMR to determine whether effects generalize to other AD-related regions.
RESULTS
Sample characteristics.
Among 1,316 participants, there were 390 controls, 672 with aMCI (299 early aMCI, 373 late aMCI), and 254 with AD dementia (tables 1 and 2). In the overall sample, women were younger, less educated, and less likely to be white compared to men (p values < 0.05). Within all diagnostic groups, women were younger and less educated than men (p values < 0.05). In analyses adjusting for age, education, and APOE ε4, women outperformed men on RAVLT immediate recall in the overall sample and within each group (p values < 0.01). Women performed better on delayed recall compared to men (p < 0.001) in the overall sample; however, in diagnosis-stratified analyses, the female advantage was evident only in controls and in the aMCI group (p values ≤ 0.001) but not the AD dementia group. Overall, TLGluMR was higher in women vs men; however, this sex difference in TLGluMR was not significant within any diagnostic group.
Table 1.
Overall sample characteristics by sex
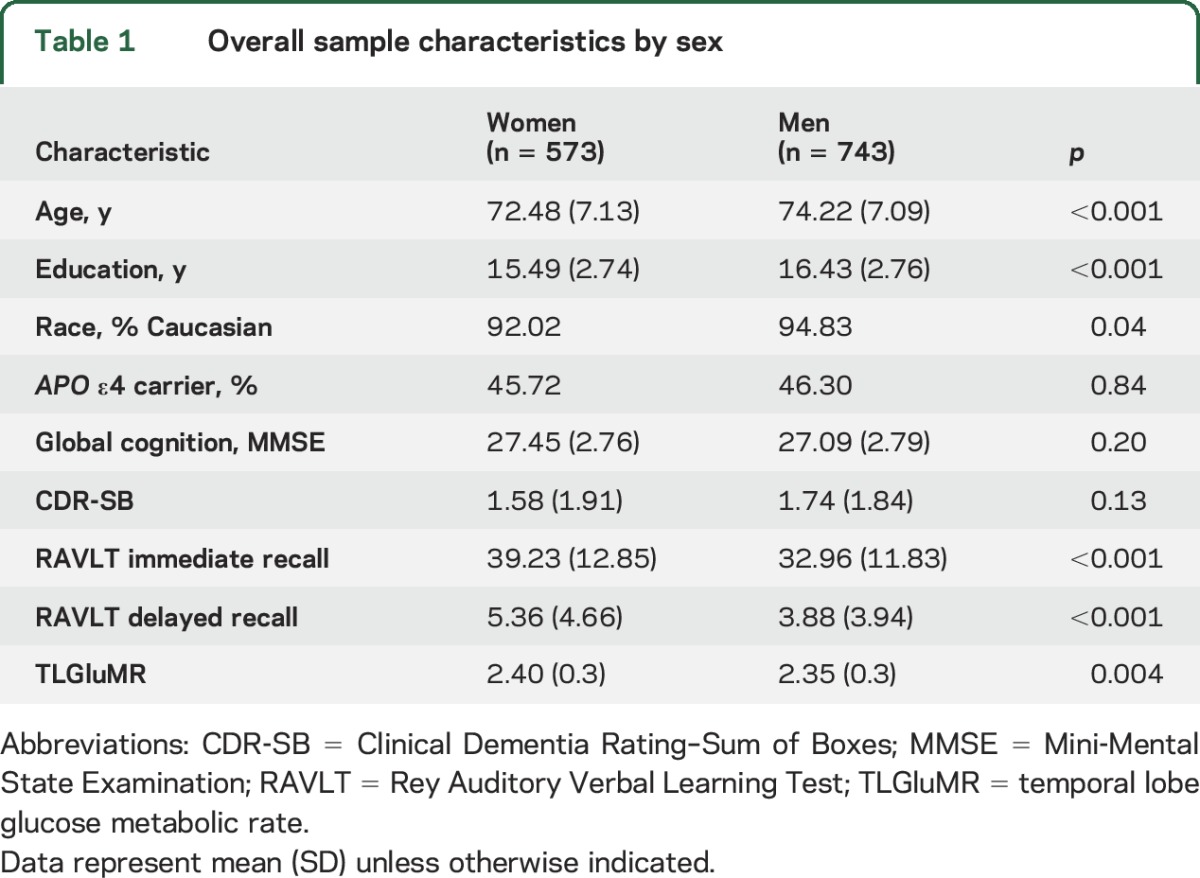
Table 2.
Sample characteristics by sex and diagnostic group

Linear regression results: Overall sample.
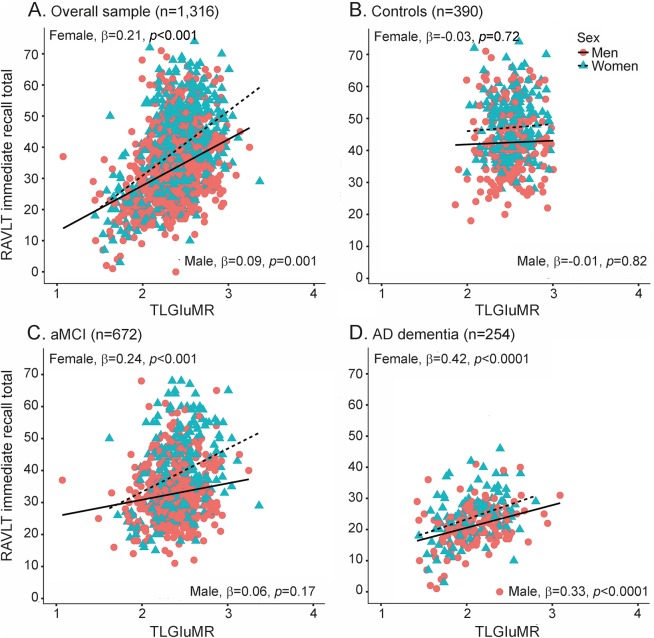
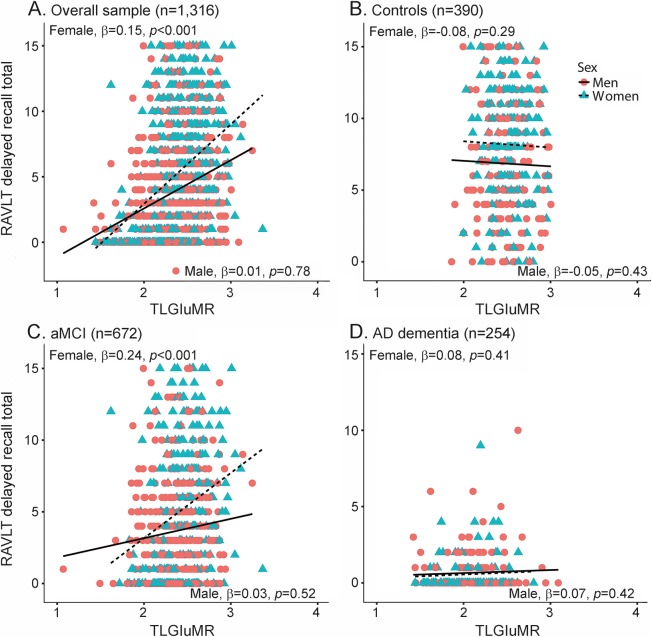
Our hypothesis that the magnitude of the female advantage in verbal memory would vary by TLGluMR was supported by a significant TLGluMR by sex interaction for both immediate (p = 0.005) and delayed recall (p = 0.002; table 3) in the overall sample (figures 1A and 2A). Better immediate recall was associated with higher TLGluMR in both men and women; however, this association was stronger in women compared to men for immediate recall (B [unstandardized coefficient] = 8.89, β [standardized coefficient] = 0.21, SE = 1.40, p < 0.0001 for women vs B = 4.01, β = 0.09, SE = 1.24, p = 0.001 for men). This is reflected in the greater slope of immediate recall across TLGluMR in women vs men (figure 1A). Better delayed recall was associated with higher TLGluMR in women only (B = 2.19, β = 0.15, SE = 0.53, p < 0.001 for women vs B = 0.01, β = 0.05, SE = 0.46, p = 0.78 for men). This is reflected in the female-specific slope of delayed recall across TLGluMR (figure 2A). The female advantage on immediate and delayed recall was most apparent in the medium to high range of TLGluMR (right side of the x-axis), whereas verbal memory performance converges for men and women with lower TLGluMR (left side of the x-axis). When we added HpVR as a covariate to analyses in the overall sample, HpVR was a significant predictor of both outcomes (p values < 0.001), and the TLGluMR × sex interactions remained significant for both immediate (B = 5.93, SE = 1.95, p = 0.002) and delayed recall (B = 2.21, SE = 0.72, p = 0.002).
Table 3.
Results of multivariable linear regression analyses modeling the independent and interactive associations of sex and TLGluMR with verbal memory performance
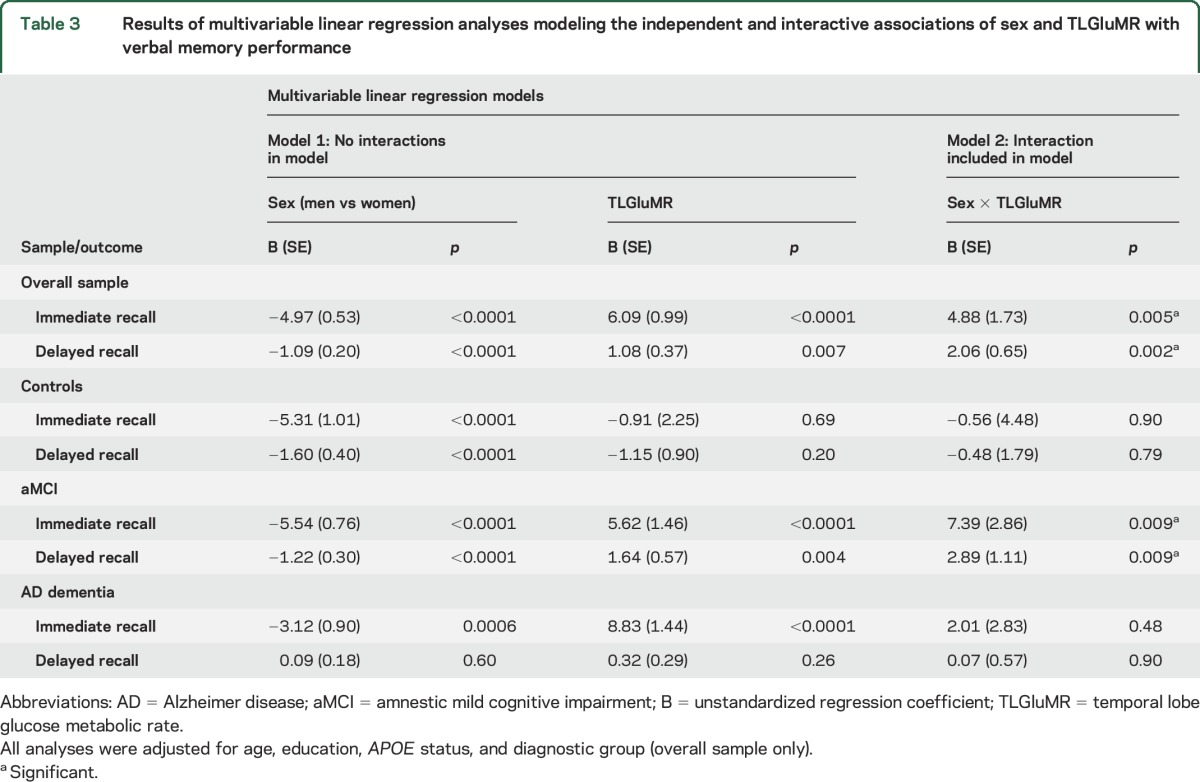
Figure 1. Relationship between TLGluMR and RAVLT immediate recall scores in men and women.
RAVLT immediate recall scores as a function of TLGluMR and sex in the (A) overall group, (B) controls, (C) aMCI, and (D) AD dementia. Note that sex × TLGluMR interaction was significant in the overall sample (A, p = 0.005) and in aMCI (C, p = 0.009), but not in controls (B, p > 0.05) and AD (D, p > 0.05). AD = Alzheimer disease; aMCI = amnestic mild cognitive impairment; β = sex-specific standardized regression coefficient of the relationship between RAVLT scores and TLGluMR controlling for age, education, APO ε4, and diagnosis (overall sample only); RAVLT = Rey Auditory Verbal Learning Test; TLGluMR = temporal lobe glucose metabolic rate.
Figure 2. Relationship between TLGluMR and RAVLT delayed recall scores in men and women.
RAVLT delayed recall scores as a function of TLGluMR and sex in the (A) overall group, (B) controls, (C) aMCI, and (D) AD dementia. Note that sex × TLGluMR interaction was significant in the overall sample (A, p = 0.002) and in aMCI (C, p = 0.009), but not in controls (B, p > 0.05) and AD (D, p > 0.05). AD = Alzheimer disease; aMCI = amnestic mild cognitive impairment; β = sex-specific standardized regression coefficient of the relationship between RAVLT scores and TLGluMR controlling for age, education, APO ε4, and diagnosis (overall sample only); RAVLT = Rey Auditory Verbal Learning Test; TLGluMR = temporal lobe glucose metabolic rate.
Linear regression analyses: Diagnosis-stratified.
In diagnosis-stratified analyses, the TLGluMR by sex interaction was significant in aMCI for both immediate (p = 0.009) and delayed (p = 0.009) recall (figures 1C and 2C), but not AD dementia (figures 1D and 2D) or control groups (figures 1B and 2B). Consistent with the overall sample, the association between TLGluMR and RAVLT was stronger in women with aMCI compared to men with aMCI for immediate recall (B = 9.98, β = 0.24, SE = 2.23, p < 0.0001 for women vs B = 2.59, β = 0.06, SE = 1.86, p = 0.17 for men) and for delayed recall (B = 3.35, β = 0.22, SE = 0.87, p < 0.001 for women vs B = 0.46, β = 0.03, SE = 0.73, p = 0.52 for men). Figures 1C and 2C show that the female advantage on immediate and delayed recall in aMCI is most apparent in the medium to high range of TLGluMR (right side of the x-axis), whereas performance converges for men and women with lower TLGluMR (left side of the x-axis). Conversely, in controls, women outperformed men on immediate (p < 0.0001) and delayed (p < 0.0001) recall irrespective of TLGluMR and TLGluMR was not associated with immediate recall (p = 0.69) or delayed recall (p = 0.20) (figures 1B and 2B). In AD dementia, women outperformed men in immediate recall irrespective of TLGluMR (p = 0.0006; figure 1D), but not delayed recall (p = 0.60; figure 2D). In AD dementia, higher TLGluMR was associated with better immediate recall irrespective of sex (p < 0.0001), but not with delayed recall (p = 0.26).
In secondary analyses that substituted the MetaROI for TLGluMR, results were similar. In the overall group, the MetaROI by sex interaction was significant for both immediate (p = 0.0006) and delayed (p = 0.002) recall, whereby the association between MetaROI and RAVLT performance was stronger in women compared to men for immediate (B = 4.29, β = 0.26, SE = 0.55, p < 0.001 for women vs B = 1.95, β = 0.12, SE = 0.50, p < 0.001 for men) and delayed recall (B = 1.24, β = 0.22, SE = 0.21, p < 0.001 for women vs B = 0.26, β = 0.05, SE = 0.18, p = 0.16 for men). Similar to the TLGluMR analysis, the MetaROI by sex interaction was significant in aMCI but not controls or AD dementia for immediate (p = 0.009) and delayed (p = 0.009) recall in diagnosis-stratified analyses. Again, the association between MetaROI and RAVLT performance was stronger in women with aMCI compared to men with aMCI for immediate (B = 3.48, β = 0.21, SE = 0.57, p < 0.001 for women vs B = 1.92, β = 0.12, SE = 0.75, p = 0.01 for men) and delayed recall (B = 2.01, β = 0.34, SE = 0.32, p < 0.001 for women vs B = 0.52, β = 0.09, SE = 0.29, p = 0.08 for men).
DISCUSSION
We examined the association of sex, TLGluMR, and their interaction with verbal memory performance to evaluate whether the female advantage in verbal memory might represent a form of cognitive reserve. Consistent with the broader literature, women outperformed men on immediate and delayed recall in the overall sample.6–8 Consistent with previous studies,26,27 higher TLGluMR was related to better memory performance in the overall sample; however, the association between TLGluMR and verbal memory significantly differed by sex in the overall sample. Specifically, the female advantage was most apparent among individuals with medium to high TLGluMR, indicating neural dysfunction of none to moderate. The advantage was attenuated among individuals with lower TLGluMR, indicating more advanced neural dysfunction.
Diagnosis-stratified analyses revealed that results in the overall sample were driven by the significant sex by TLGluMR interaction in the aMCI group where the female advantage was most apparent among individuals with medium to high TLGluMR, but not among individuals with lower TLGluMR. Results suggest that women with aMCI outperform men with aMCI on verbal memory tasks despite similar levels of brain hypometabolism. Consistent with the current results, we previously showed that women have a verbal memory advantage over men despite moderate hippocampal volume loss across diagnostic groups and within the aMCI group.9 Together, the results suggest that the female advantage in verbal memory is sustained despite hippocampal atrophy and metabolic deficits in the aMCI stage of AD, but is eliminated when hippocampal atrophy and metabolic deficits become more severe.
We found similar results when we used FDG-PET data from a MetaROI that characterizes AD-associated metabolic change. As TLGluMR and MetaROI were highly correlated (R = 0.95, p < 0.001), this result is expected. The similar results between analyses using TLGluMR or MetaROI suggest that TLGluMR is more a marker for AD-associated hypometabolism than a region-specific marker for temporal dysfunction.
Among controls, the female advantage in verbal memory was evident regardless of TLGluMR, and memory performance was not related to TLGluMR. The lack of a relationship between TLGluMR and memory performance in healthy, older adults is consistent with previous studies28 and may reflect limited variability in TLGluMR among controls (SD = 0.21) compared to aMCI (SD = 0.26) or AD (SD = 0.30) groups or a threshold effect, whereby TLGluMR is not associated with memory if above a certain level.
In AD dementia, our hypothesis that the female advantage in verbal memory would be eliminated among individuals with low TLGluMR was partly supported. The advantage was eliminated in delayed recall; however, a floor effect limits interpretation. Counter to hypotheses, female patients with AD dementia significantly outperformed male patients with AD dementia on immediate recall (p = 0.0008); however, the sex difference was smaller (mean difference = 2.8) compared to control (mean difference = 4.8) and aMCI (mean difference = 6.3) groups. Our results suggest an attenuation of the female advantage in verbal memory in AD dementia and not an elimination or reversal as some previous studies have indicated.10,11 Poorer immediate recall scores were significantly associated with lower TLGluMR among patients with AD dementia; however, delayed recall scores were not associated, likely because of the floor effect among delayed recall scores in AD dementia.
We suggest that the female advantage in verbal memory may represent a sex-specific form of cognitive reserve that allows women to better compensate for brain pathology and maintain normal cognitive performance. We show that the female advantage in verbal memory is maintained despite similar levels of temporal hypometabolism in women and men. The cognitive reserve theory further posits that the initiation of accelerated cognitive decline will occur at more advanced disease stages in those with greater reserve once neuropathology reaches a level that overwhelms compensation strategies.1,2 Using a previously employed cutoff for impairment on the RAVLT (<37 on immediate recall29 and <8 on delayed recall30), women in the present study reached this cutoff at a lower TLGluMR compared to men for both immediate (∼2.2 vs 2.6; figure 1A) and delayed recall (∼2.9 vs 3.7; figure 2A). Thus, consistent with the cognitive reserve theory, verbal memory impairment was evident at a greater degree of disease burden as measured by TLGluMR in women vs men.
Some, but not all, studies31,32 report that men are at higher risk of aMCI,33,34 whereas women are disproportionally affected by AD dementia.35,36 Our results may help to explain this paradoxical sex difference in aMCI and AD dementia rates. Verbal memory tests are used in diagnosing aMCI and AD dementia, and test norms are typically not sex-adjusted. Among individuals who transition from aMCI to AD dementia, the combination of a delay in the clinical manifestation of verbal memory impairment and more rapid decline thereafter in women vs men would lead to a shorter window of time for an aMCI diagnosis in women that may not be captured in longitudinal assessments given every 1 to 2 years. In ADNI, consistent with this view, among individuals with aMCI, cognitive decline occurs 2 times faster in women vs men.37 In addition, women in the Einstein Aging Study were less likely to transition from control to MCI but more likely to transition from normal to dementia than men.38
Our study has limitations. Our cross-sectional analysis precludes us from determining temporality in the relationship between verbal memory and TLGluMR. However, longitudinal studies indicate that brain hypometabolism is a marker for impending cognitive decline and incident MCI.16,39 In this cross-sectional design, we could not compare rates of decline between men and women; these rates would provide a more direct test of the cognitive reserve theory. Longitudinal analyses are under way that examine sex differences in the trajectory of verbal memory decline in the path to AD dementia. Lastly, because ADNI is based on a convenience sample of predominately white and well-educated volunteers, generalizability of results is limited.
We found that the magnitude of the female advantage in verbal memory varies across TLGluMR. Specifically, the female advantage in verbal memory was evident despite minimal to moderate temporal hypometabolism; however, the advantage was attenuated when hypometabolism was more severe. Given similar findings with hippocampal volume,9 we show that women show better verbal memory performance than men despite moderate levels of brain pathophysiology based on structural and functional neuroimaging outcomes. The female advantage in verbal memory may serve as a sex- and domain-specific form of cognitive reserve. If replicated, results suggest that aMCI may be clinically detected at a more advanced disease stage in women vs men because women are better able to compensate for underlying neuropathology. Implementing sex-adjusted norms in clinical verbal memory tests may improve the early detection of AD in women.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the ADNI study participants and investigators for their participation.
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- aMCI
amnestic mild cognitive impairment
- CDR
Clinical Dementia Rating
- FDG
[18F]-fluorodeoxyglucose
- HpVR
hippocampal volume ratio
- LM-II
Logical Memory II
- MMSE
Mini-Mental State Examination
- RAVLT
Rey Auditory Verbal Learning Test
- ROI
region of interest
- TLGluMR
temporal lobe glucose metabolic rates
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
E.S., A.B., P.M.: study concept. E.S., A.B., P.M., R.L., L.R., S.L.: study design. S.L., E.S.: data acquisition. L.R., E.S.: statistical analysis. E.S., A.B., P.M., R.L., S.L.: data interpretation. E.S.: initial manuscript preparation. All authors provided a critical review of the manuscript for important intellectual content and contributed to and approved the final manuscript.
STUDY FUNDING
ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from nonprofit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the US Food and Drug Administration and from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC; Johnson & Johnson Pharmaceutical Research and Development LLC; Medpace, Inc.; Merck and Co., Inc.; Meso Scale Diagnostics, LLC; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. Drs. Sundermann and Lipton's time was supported by funding for the Einstein Aging Study: National Institute on Aging grants AG003949, AG026728, TL1RR000087, T32-GM007288, the Leonard and Sylvia Marx Foundation, and the Czap Foundation.
DISCLOSURE
E. Sundermann, P. Maki, and L. Rubin report no disclosures relevant to the manuscript. R. Lipton reports research support from the NIH: PO1 AG003949 (program director), PO1AG027734 (project leader), RO1AG025119 (investigator), RO1AG022374-06A2 (investigator), RO1AG034119 (investigator), RO1AG12101 (investigator), K23AG030857 (mentor), K23NS05140901A1 (mentor), and K23NS47256 (mentor), the National Headache Foundation, and the Migraine Research Fund; serves on the editorial boards of Neurology® and Cephalalgia and as senior advisor to Headache, has reviewed for the NIA and NINDS, holds stock options in eNeura Therapeutics (a company without commercial products); serves as consultant, advisory board member, or has received honoraria from the following: Alder, Allergan, American Headache Society, Autonomic Technologies, Avanir, Boston Scientific, Bristol-Myers Squibb, CoLucid, Dr. Reddy's, ElectroCore, Eli Lilly, Endo, eNeura Therapeutics, Informa, Labrys, Merck, Novartis, Teva, Vedanta. S. Landau has served as a paid consultant for Genentech, Synarc, Biogen, and Janssen. A. Biegon reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–1010. [PubMed] [Google Scholar]
- 2.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 3.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 1999;53:1942–1947. [DOI] [PubMed] [Google Scholar]
- 4.Le Carret N, Auriacombe S, Letenneur L, Bergua V, Dartigues JF, Fabrigoule C. Influence of education on the pattern of cognitive deterioration in AD patients: the cognitive reserve hypothesis. Brain Cogn 2005;57:120–126. [DOI] [PubMed] [Google Scholar]
- 5.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 2007;69:1657–1664. [DOI] [PubMed] [Google Scholar]
- 6.Kramer JH, Delis DC, Daniel MH. Sex differences in verbal learning. J Clin Psychol 1988;44:907–915. [Google Scholar]
- 7.Aartsen MJ, Martin M, Zimprich D. Gender differences in level and change in cognitive functioning: results from the Longitudinal Aging Study Amsterdam. Gerontology 2004;50:35–38. [DOI] [PubMed] [Google Scholar]
- 8.Pauls F, Petermann F, Lepach AC. Gender differences in episodic memory and visual working memory including the effects of age. Memory 2013;21:857–874. [DOI] [PubMed] [Google Scholar]
- 9.Sundermann EE, Biegon A, Rubin LH, et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 2016;86:1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beinhoff U, Tumani H, Brettschneider J, Bittner D, Riepe MW. Gender-specificities in Alzheimer's disease and mild cognitive impairment. J Neurol 2008;255:117–122. [DOI] [PubMed] [Google Scholar]
- 11.Chapman RM, Mapstone M, Gardner MN, et al. Women have farther to fall: gender differences between normal elderly and Alzheimer's disease in verbal memory engender better detection of Alzheimer's disease in women. J Int Neuropsychol Soc 2011;17:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 1991;253:1380–1386. [DOI] [PubMed] [Google Scholar]
- 13.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 1998;281:1188–1191. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 15.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 16.de Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci USA 2001;98:10966–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 23.Aisen PS, Petersen RC, Donohue MC, et al. Clinical core of the Alzheimer's Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement 2010;6:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 2010;6:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011;32:1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishi H, Sawamoto N, Namiki C, et al. Correlation between cognitive deficits and glucose hypometabolism in mild cognitive impairment. J Neuroimaging 2010;20:29–36. [DOI] [PubMed] [Google Scholar]
- 27.Didic M, Felician O, Gour N, et al. Rhinal hypometabolism on FDG PET in healthy APO-E4 carriers: impact on memory function and metabolic networks. Eur J Nucl Med Mol Imaging 2015;42:1512–1521. [DOI] [PubMed] [Google Scholar]
- 28.Habeck C, Risacher S, Lee GJ, et al. Relationship between baseline brain metabolism measured using [18F]FDG PET and memory and executive function in prodromal and early Alzheimer's disease. Brain Imaging Behav 2012;6:568–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia 2013;51:2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lines CR, McCarroll KA, Lipton RB, Block GA; Prevention of Alzheimer's in Society's Elderly Study Group. Telephone screening for amnestic mild cognitive impairment. Neurology 2003;60:261–266. [DOI] [PubMed] [Google Scholar]
- 31.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 2012;26:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:195–204. [DOI] [PubMed] [Google Scholar]
- 33.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 2012;78:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodaty H, Heffernan M, Kochan NA, et al. Mild cognitive impairment in a community sample: the Sydney Memory and Ageing Study. Alzheimers Dement 2013;9:310–317. [DOI] [PubMed] [Google Scholar]
- 35.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry 1998;55:809–815. [DOI] [PubMed] [Google Scholar]
- 36.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 1987;76:465–479. [DOI] [PubMed] [Google Scholar]
- 37.Lin KA, Choudhury KR, Rathakrishnan BG, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement 2015;1:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song C, Kuo L, Derby CA, et al. Multi-stage transitional models with random effects and their application to the Einstein Aging Study. Biom J 2011;53:938–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 2002;17:302–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.