Abstract
Objective:
To evaluate the use and tolerability of noninvasive positive pressure ventilation (NIV) in patients with amyotrophic lateral sclerosis (ALS) early in their disease by comparing active NIV and sham NIV in patients not yet eligible for NIV use as recommended by practice guidelines.
Methods:
This was a single-center, prospective, double-blind, randomized, placebo (sham)–controlled pilot trial. Patients with ALS were randomized to receive either sham NIV or active NIV and underwent active surveillance approximately every 3 months until they reached a forced vital capacity (FVC) <50% or required NIV for clinical symptom management.
Results:
In total, 54 participants were randomized. The mean NIV use was 2.0 hours (95% confidence interval [CI] 1.1–3.0) per day in the sham NIV treatment group and 3.3 hours (CI 2.0–4.6) per day in the active NIV group, which did not differ by treatment group (p = 0.347). The majority of sham NIV participants (88%) and active NIV participants (73%) reported only mild or no problem with NIV use. Difference of change in FVC through the treatment period by group (0.44 per month) favored active NIV (p = 0.049). Survival and changes in maximal inspiratory or expiratory pressure did not differ between treatment groups.
Conclusions:
The efficacy of early NIV in ALS should be tested in randomized, placebo-controlled trials. The trial is registered on clinicaltrials.gov (NCT00580593).
Classification of evidence:
This study provides Class II evidence that for patients with ALS, adherence with NIV and sham NIV are similar.
Noninvasive positive pressure ventilation (NIV) is recommended to treat respiratory insufficiency in amyotrophic lateral sclerosis (ALS) and improves survival and quality of life in this setting.1,2 However, the optimal time to initiate NIV is unknown. Initiation of NIV prior to development of respiratory symptoms has been postulated to slow the course of respiratory weakness through reduction in respiratory muscle overload, among other factors.3 We hypothesized that initiation of NIV as early as possible may prevent pulmonary function decline and extend survival. However, whether patients with ALS will tolerate NIV early in the disease course is unknown. Therefore, in order to assess the feasibility of the design, we conducted a randomized, double-blind pilot clinical trial to compare active NIV to sham NIV in patients with ALS who were not yet eligible for routine clinical NIV use.
METHODS
Study design overview and eligibility.
This study was a single-center, randomized, double-blind, sham-controlled parallel-group pilot trial of NIV compared with sham NIV in patients with ALS who had not yet met clinical criteria for NIV use. The purpose was to assess the feasibility of the study design. The study aimed to address 2 main questions: (1) Would patients with ALS use NIV while they still had adequate respiratory function? (2) Is sham positive airway pressure a reasonable placebo for patients with ALS? The study also sought to explore whether use of NIV slowed the deterioration in pulmonary function and functional status during treatment, or overall time to death. Eligible participants were 18 years of age or older and diagnosed with probable or definite ALS by El Escorial criteria.4 Exclusion criteria were forced vital capacity (FVC) <50% predicted, current involvement in a clinical treatment trial, previous or current use of positive pressure ventilation equipment (as this could unmask participants), oxygen therapy, previous pneumothorax, and bullous emphysema. The study was conducted at the University of Michigan Motor Neuron Disease Center from July 2007 through November 2011.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the University of Michigan Institutional Review Board and participants provided written informed consent. The trial was registered on clinicaltrials.gov (NCT00580593).
Intervention during treatment phase: Active and sham NIV.
Both treatment groups used the Phillips Respironics, Inc. (Murrysville, PA), NIV Harmony bilevel positive airway pressure machine. Participants were randomized to receive either active NIV or sham NIV. Active NIV was set to a pressure of 8 centimeters of water inspiratory positive airway pressure (IPAP) and 4 centimeters of water expiratory positive airway pressure (EPAP).5 These settings were selected because they are the mean settings used by ALS clinics in the United States,6 and no studies have been done to assess the optimal NIV starting pressure. No upward adjustments in pressure were performed during the randomized treatment phase of the study because of the study population's presymptomatic state and because higher pressures were thought likely to be poorly tolerated. For the sham NIV, the device manufacturer's (Phillips Respironics, Inc.) engineers altered the internal NIV device software such that the sham device only emitted 4 centimeters of water during both IPAP and EPAP. The sham NIV was paired with a sham NIV ComfortGel nasal or full face mask (Phillips Respironics, Inc.) or Puritan Bennett Breeze SleepGear nasal pillows (Coviden, Mansfield, MA), where the plastic elbow or exhalation port was increased in size to create a leak such that the pressure measured at the mask was always <1 centimeter of water. Sham NIV produced a similar sound to active NIV, and the machine display cycled between 8 centimeters of water with inspiration and 4 centimeters of water during expiration, as did the active NIV. Study personnel fitted the participants with the appropriate mask size/interface and provided instructions on device use. Participants were asked to use NIV while they slept and were encouraged to use it for at least 4 hours each night. All participants received phone calls from study personnel at 7, 14, 30, and 60 days after NIV initiation to encourage use and troubleshoot problems with the device.
Baseline data collection.
At the time of enrollment, FVC%, maximum inspiratory and expiratory pressures (MIP and MEP), body mass index, date of ALS symptom onset, and site of ALS onset (bulbar or limb) were recorded in addition to baseline demographics. In addition, the ALS Functional Rating Scale–revised (ALSFRS-R) and baseline dyspnea index were performed.
Longitudinal assessments.
Participants were followed in person in clinic approximately every 3 months. Any participant who completed at least one follow-up assessment contributed to the longitudinal outcome analyses. At the study visits, active and sham NIV objective usage hours were downloaded from the device. Pulmonary function tests (FVC%, MIP, and MEP) were measured by a respiratory therapist or nurse practitioner using a hand-held spirometer or pressure transducer with an analog dial. The analog dial maximal value was 60 centimeters of water for both inspiration and expiration. For patients unable to adequately use a hand-held spirometer, measurements were made in the University of Michigan pulmonary function laboratory. ALSFRS-R and the transitional dyspnea index (TDI) were also assessed. When a participant reached an FVC% <50% or any respiratory insufficiency symptoms were elicited by the clinical team, study treatment (treatment phase) concluded and standard NIV for clinical purposes was begun (observational phase). The decision to switch the participant from study treatment to standard NIV was made by a masked health care provider. At the conclusion of study treatment, participants were queried about their belief in the intervention assigned. Responses included definitely real, probably real, uncertain, probably pretend, and definitely pretend, and dichotomized into definitely and probably real vs other responses. Participants continued to be followed with pulmonary function tests (FVC, MIP, and MEP), ALSFRS-R, and vital status every 3 months after study treatment conclusion until death.
Outcomes.
The first primary outcome was NIV use measured by objective mean usage hours over the study treatment period. The primary question addressed with level II evidence was whether those randomized to active and sham had different hours of NIV use. The second primary outcome was perceived treatment allocation dichotomized into definitely real and probably real vs all others compared. The primary research question addressed with level II evidence was whether perceived treatment allocation differed between those randomized to active and sham NIV. Secondary outcomes included survival, rate of change of FVC%, and ALSFRS-R and TDI over time between the 2 groups.
Sample size, randomization, and blinding.
A sample size of 60 participants total was selected a priori because it would yield a standard error for the primary outcome, mean hours of NIV use, that would not exceed 6.5%. Participants were randomized 1:1 in blocks of 4 (SAS version 7; SAS Institute, Cary, NC). An unmasked investigator (T.F.) accessed the randomization schedule to assign the active or sham NIV and then trained the participant on the NIV device. All other study investigators, the clinical team, and the participants were masked to treatment allocation. Participants were considered randomized at the time they agreed to take their NIV device home from the clinic.
Statistical methods.
Primary outcome analysis.
To explore the difference in NIV use by treatment groups, we tested whether reasons for the conclusion of study treatment (i.e., percent who concluded study treatment because they became eligible for clinical NIV) differed by treatment groups using the Fisher exact test. Because time intervals (approximately 3 months) between study visits differed within and between participants, we calculated total NIV use per day (total hours of use for that interval divided by total cumulative hours for the interval between study visits). We fitted a linear regression model to estimate average hours of NIV use by group, adjusting for treatment assignment and disease duration (time from symptom onset) because the disease duration differed by treatment group despite randomization (table 1). To test whether rate of change for average hours of NIV use (per 4 weeks) differed by group, we further included weeks from baseline and an interaction term for treatment assignment by weeks from baseline in the fitted model. We also estimated the percent of days NIV was used between study visits and the average time NIV was used during days that included any NIV use and tested whether those estimates differed by treatment group. Because the outcomes did not follow a normal distribution and due to possible correlation within participants, a block bootstrap method was further adapted to provide robust confidence intervals (CIs).7,8 The p values for treatment group differences were calculated using a t distribution of 5,000 bootstrapped estimates.
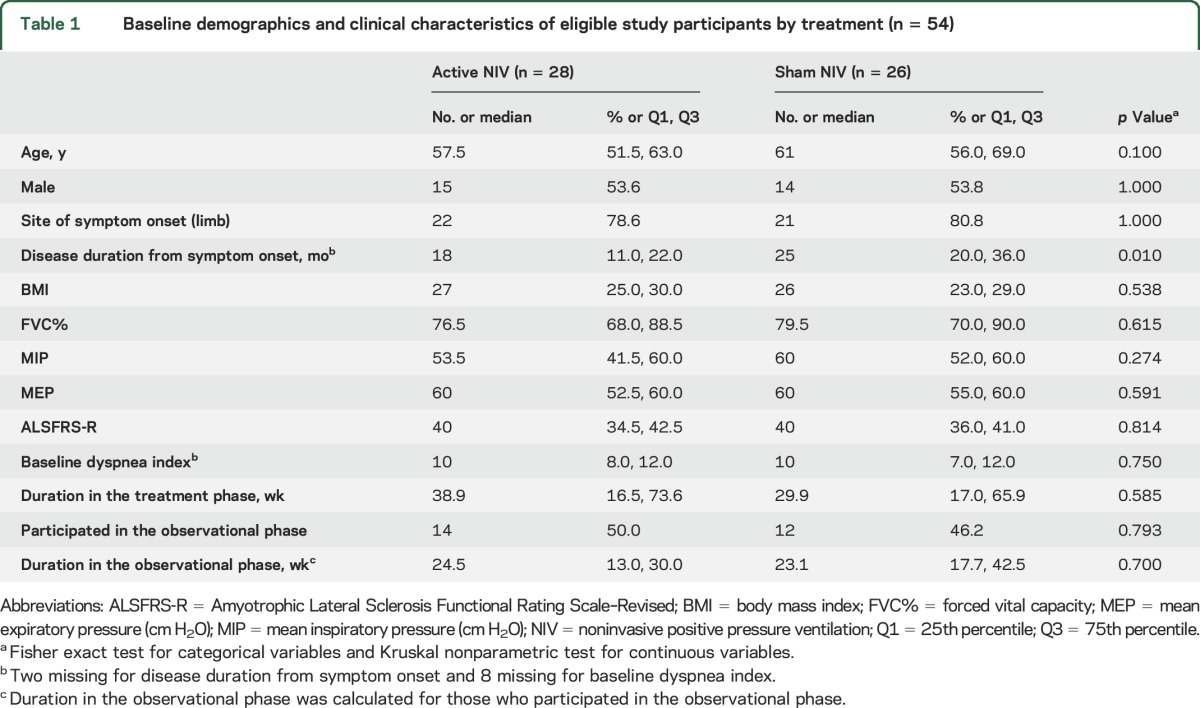
Table 1.
Baseline demographics and clinical characteristics of eligible study participants by treatment (n = 54)
Secondary outcome analysis.
To assess if active NIV significantly affected rate of change for each secondary outcome during the treatment phase, a linear regression model was fitted. In each fitted model, we included time from symptom onset, treatment assignment, time (weeks) from the baseline (weeks = 0), and an interaction term between the treatment assignment and time. Again, because most of the secondary outcomes did not follow a normal distribution and correlation within participants was possible, a block bootstrap method was further adapted to provide robust CI estimates and p values were calculated using a t distribution of 5,000 bootstrap estimates.7,8
Finally, we performed a log-rank test to assess whether marginal survival distributions between treatment groups differed. In addition, after adjustment for disease duration, we tested if hazard ratios between the treatment groups differed. Survival duration was based on time from the study start date until the date of death, or censored at the conclusion of data collection (November 11, 2011) or time of loss to follow-up. Analyses were conducted in R 3.1.1.9
RESULTS
Of the 60 participants consented, 54 participants (28 active NIV and 26 sham NIV) were randomized (figure e-1 at Neurology.org). The 3 who subsequently withdrew did so prior to the first follow-up visit and thus were not able to contribute any outcome data. Baseline demographics and clinical characteristics of the active and sham NIV groups are presented in table 1, and were similar with the exception that sham participants were a little longer into their disease course. The overall mean of FVC% at initiation was 79.2% with no between-group difference (active NIV minus sham NIV = −2.1%, p = 0.62).
Difference in reason for study intervention conclusion.
Of the 54 participants randomized, 35 (13 in sham NIV and 22 in active NIV, p = 0.09) continued with study treatment until the conclusion of the study treatment phase. There was no difference in median time to transition from randomized treatment to clinically indicated NIV (p = 0.59) (table 1). The mean FVC was 48% (46%) in the active NIV (n = 22) and 51% in the sham NIV (n = 13) group (p = 0.26) when study treatment ended. Among the remaining 19 who concluded study treatment for other reasons, 1 sham participant and no active participants died due to other cause, 2 sham participants and 1 active participant went to hospice and were lost to follow-up, 8 sham participants and 1 active participant were unable to return due to other reasons (e.g., moving), and 1 from each group was missing a reason. In addition, 6 participants, 2 sham and 4 active, ended study treatment because data collection ended (November 2011) (figure e-1).
Active and sham NIV use.
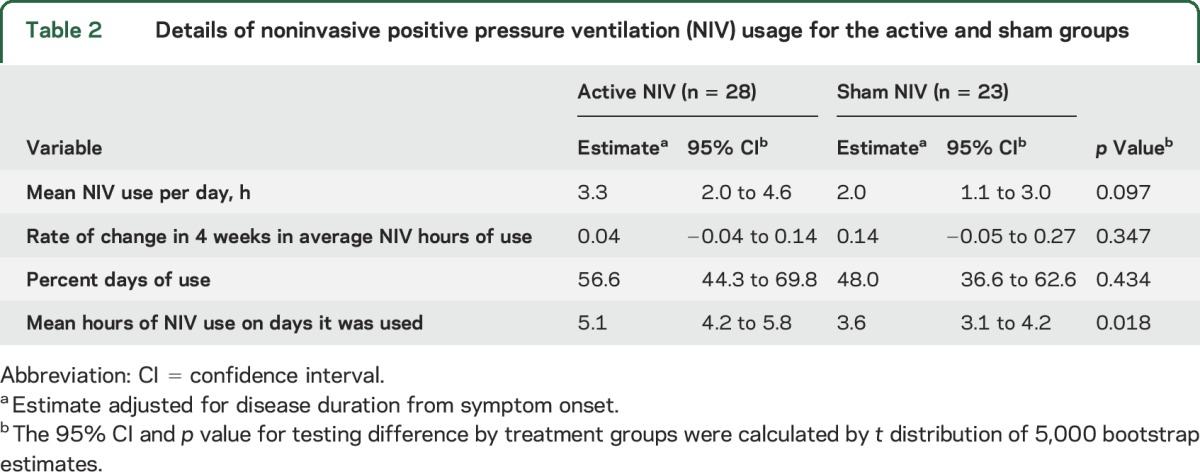
The mean NIV use per day was 3.3 hours (95% CI 2.0–4.6) for active NIV treatment and 2.0 hours (95% CI 1.1–3.0) for sham NIV treatment (p = 0.1). Rate of change per 4 weeks of average NIV hours of use was stable through time in each group and did not differ by treatment group (p = 0.347) (table 2). Although no significant difference was found in the percent of days NIV was used by treatment group (p = 0.434), patients in the active NIV group used NIV 1.5 hours longer (95% CI 0.2–2.5) on days during which it was used (p = 0.018) (table 2).
Table 2.
Details of noninvasive positive pressure ventilation (NIV) usage for the active and sham groups
Assessment of masking.
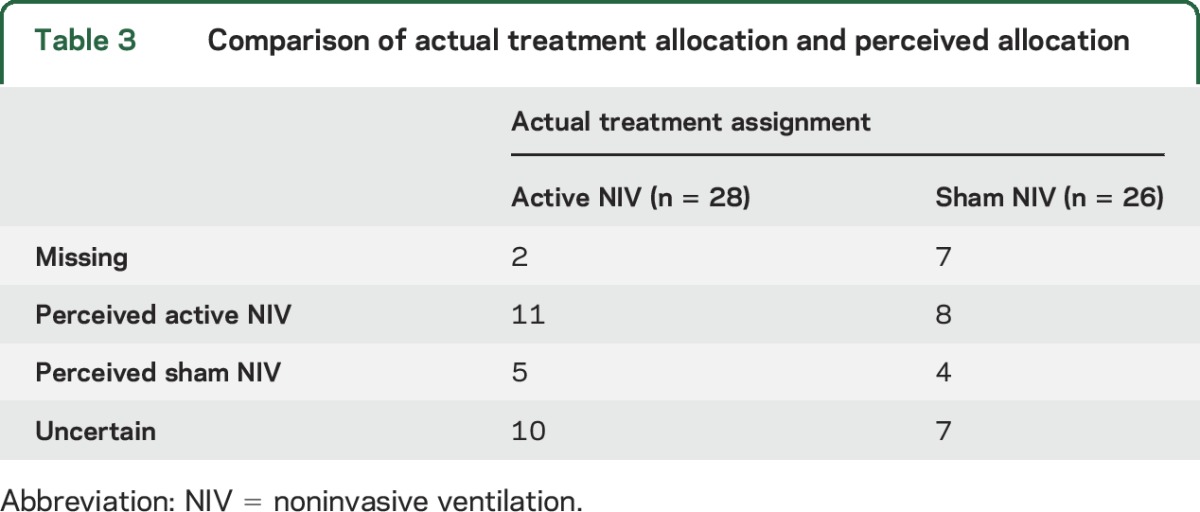
The belief of participants in their intervention allocation by actual treatment assignment at the conclusion of the treatment phase is summarized in table 3. Study participants' perception of treatment assignment did not differ between active NIV and sham NIV (Fisher exact test of independence, p = 0.7).
Table 3.
Comparison of actual treatment allocation and perceived allocation
Problems with NIV use.
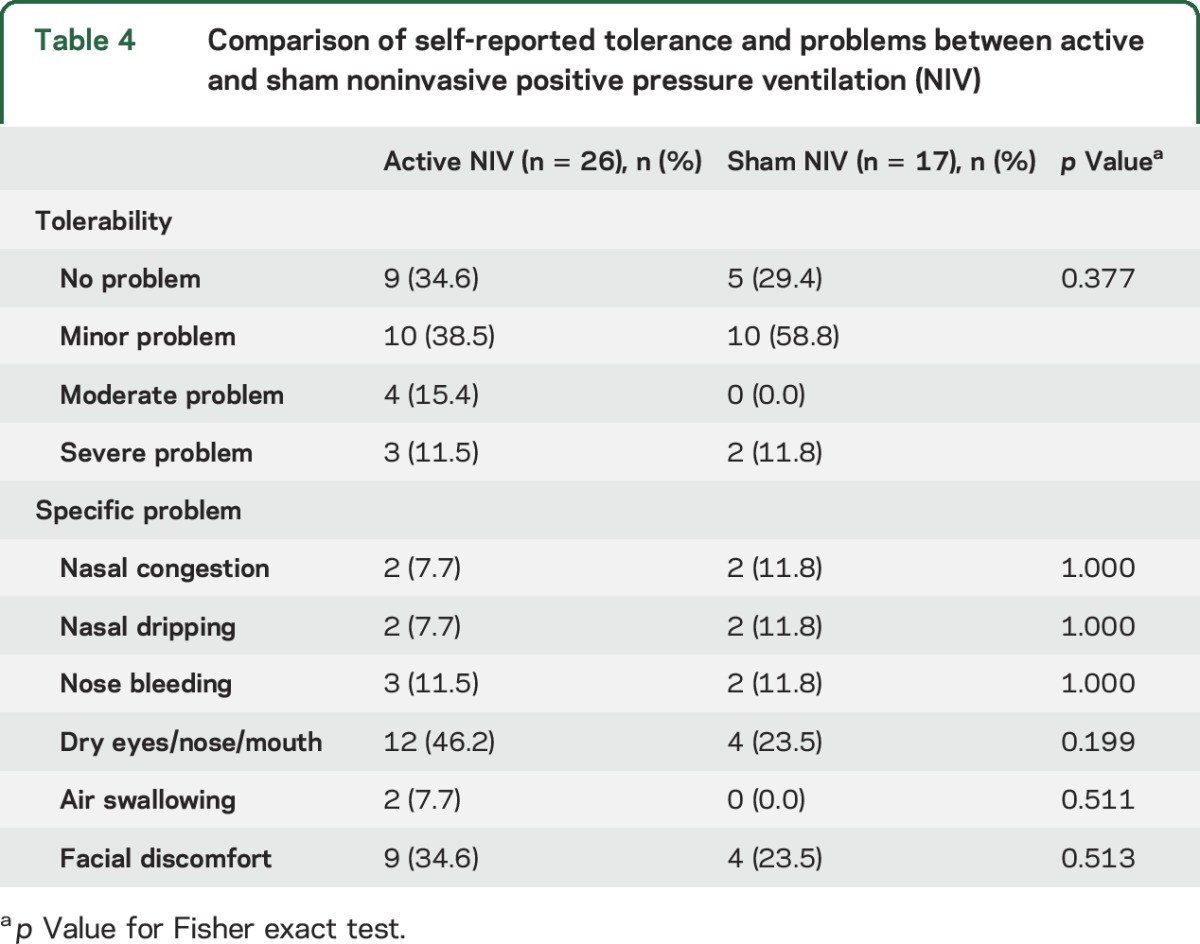
Table 4 compares the active and sham NIV participants' self-report of NIV tolerance and specific problems experienced with NIV collected at the end of the treatment phase. The majority of active NIV (73%) and sham NIV (88%) participants reported no or only mild problem with NIV use. Dry eyes, nose, or mouth and facial discomfort were the most commonly reported problems in both treatment groups.
Table 4.
Comparison of self-reported tolerance and problems between active and sham noninvasive positive pressure ventilation (NIV)
Change in pulmonary function tests and reported dyspnea during the treatment phase.
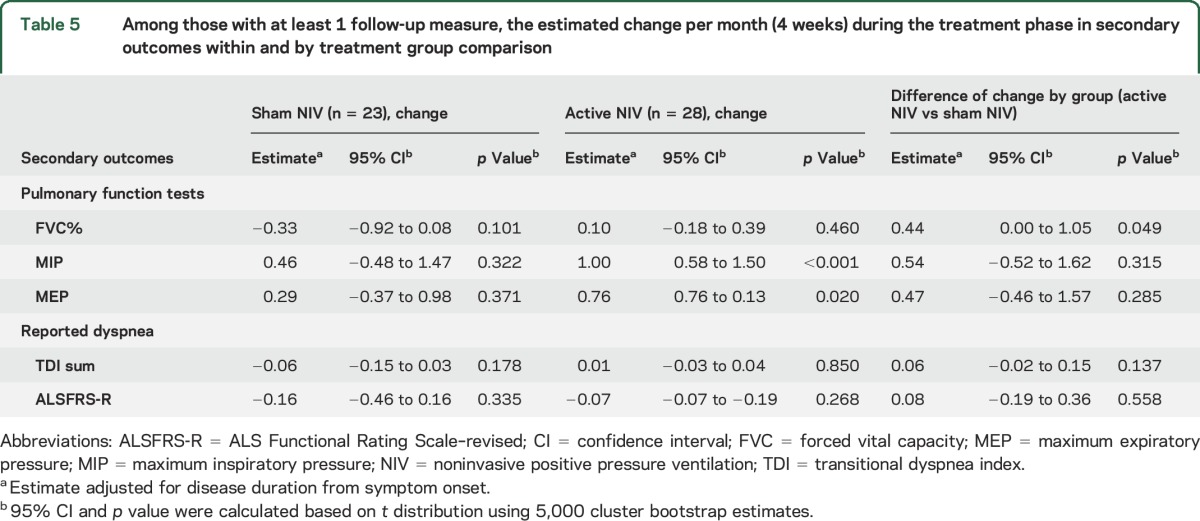
Among those with at least one follow-up assessment, the secondary outcomes did not change in the sham group across time; MIP and MEP improved in the active NIV group (table 5). However, of the secondary outcomes, only comparison of the mean difference in FVC% per month differed by treatment group, favoring the active NIV group (0.44 higher, p = 0.049) (table 5), after adjustment for disease duration. The change in FVC% over time by treatment group from this adjusted model is shown in figure e-2.
Table 5.
Among those with at least 1 follow-up measure, the estimated change per month (4 weeks) during the treatment phase in secondary outcomes within and by treatment group comparison
Survival.
Median survival times were 29 months in the sham and 27 months in the active NIV group; 47.8% of sham NIV patients and 46.3% of active NIV patients were censored. As shown in figure e-3, marginal survival distributions between treatment groups were not different (p = 0.64). After adjustment for disease duration, the only baseline characteristic that differed by treatment group, survival remained similar (hazard ratio = 0.90 [95% CI 0.41–1.97]) between treatment groups.
DISCUSSION
This double-blind, randomized controlled pilot trial of active vs sham NIV in patients with ALS with an FVC ≥50% demonstrates the feasibility of the study design. Participants in both the active and sham groups used NIV about half the days, and on those days, they used it for several hours. This quantity of NIV use is potentially sufficient to provide a treatment effect given that a similar amount of use was previously associated with a survival benefit when initiated later in the disease course.10 The majority of participants described no or only a mild problem with NIV use, supporting the tolerability of both sham and active NIV in this study population. Thus, given the general tolerability and high use on days used, future studies that remind participants to use NIV in order to increase number of days used may be helpful.
This study used a placebo NIV as a control in patients with ALS. Subtherapeutic positive airway pressure has been used before in multiple sleep apnea trials,11,12 but is uncommon in ALS. Participants were unaware of their treatment group assignment and found sham NIV to be a tolerable treatment. Participants did tend to use it for fewer hours than active NIV, but used it for a similar proportion of days. Our findings indicate that sham NIV is a reasonable placebo control for future trials of early NIV for patients with ALS.
While our study was not powered to detect differences in clinical outcome by treatment group, better respiratory function outcomes in the active NIV group were suggested, specifically with a significant attenuation of the monthly decline in FVC% for the active NIV group compared to sham NIV group. This lends support to our therapeutic hypothesis that NIV usage can attenuate respiratory function decline early in the disease course, evidenced by a highly clinically relevant variable, FVC%, the decline of which is associated with ALS patient survival.13 However, alternative explanations exist to a therapeutic effect. No difference in subjective dyspnea or functional status during the treatment phase was detected, and no overall difference in mortality was found. Future studies of early NIV should consider adjustments in positive pressure such as titration to specific nocturnal respiratory parameters or perhaps use of autotitration devices.
Strengths of this study include the rigorous randomized, placebo-controlled design, application of sham NIV to an ALS trial, and assessment of the adequacy of blinding of participants. Limitations include the small sample size, from which clinical efficacy cannot be determined, and the single-center nature of the study, which may limit its generalizability.
This pilot trial demonstrates the feasibility of randomized, sham NIV-controlled trials of early NIV for patients with ALS. Further studies are needed to determine the efficacy of early NIV in ALS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Noah Lechtzin, MD, MHS, Associate Professor of Medicine, Johns Hopkins University, who served as the medical monitor for the trial.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale–revised
- CI
confidence interval
- EPAP
expiratory positive airway pressure
- FVC
forced vital capacity
- IPAP
inspiratory positive airway pressure
- MEP
maximum expiratory pressure
- MIP
maximum inspiratory pressure
- NIV
noninvasive positive pressure ventilation
- TDI
transitional dyspnea index
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Teresa L. Jacobs, MD: drafting of the manuscript, interpretation of the data. Devin L. Brown, MD: study concept and design, drafting of the manuscript. Jonggyu Baek, PhD: analysis and interpretation of data, drafting of the manuscript. Erin M. Migda, RN, BSN: acquisition of data, critical revision of manuscript for intellectual content. Timothy Funckes, RN: acquisition of data, critical revision of manuscript for intellectual content. Kirsten L. Gruis, MD, MS: study concept and design, study supervision, drafting of the manuscript, obtained funding.
STUDY FUNDING
This study was funded by the NIH (K23NS055200).
DISCLOSURE
T. Jacobs reports no disclosures relevant to the manuscript. D. Brown serves as an editorial board member of Neurology®, is funded by NIH grants R01HL123379, R01 NS070941, R01 HL126700, U10NS086526, and R01 DC012760, and received research support from the Blue Cross Blue Shield of Michigan Foundation, Michigan Department of Community Health, and the University of Michigan for stroke-related research. J. Baek was supported in part by U10NS086526. E. Migda and T. Funckes report no disclosures relevant to the manuscript. K. Gruis reports salary support from Idera Pharmaceuticals and prior salary support from Pfizer, Inc., and Alnylam Pharmaceuticals. She has received no commercial support for projects related to ALS. She was supported by K23NS055200. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation: a consensus conference report. Chest 1999;116:521–534. [DOI] [PubMed] [Google Scholar]
- 3.Aboussouan LS, Mireles-Cabodevila E. Respiratory support in patients with amyotrophic lateral sclerosis. Respir Care 2013;58:1555–1558. [DOI] [PubMed] [Google Scholar]
- 4.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis: subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the World Federation of Neurology Research Group on neuromuscular diseases and the El Escorial “clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 1994(124 suppl):96–107. [DOI] [PubMed] [Google Scholar]
- 5.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med 2001;163:540–577. [DOI] [PubMed] [Google Scholar]
- 6.Melo J, Homma A, Iturriaga E, et al. Pulmonary evaluation and prevalence of non-invasive ventilation in patients with amyotrophic lateral sclerosis: a multicenter survey and proposal of a pulmonary protocol. J Neurol Sci 1999;169:114–117. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G, Yu Z, Huang JZ. The cluster bootstrap consistency in generalized estimating equations. J Multivar Anal 2013;115:33–47. [Google Scholar]
- 8.Sherman M, Cessie S. A comparison between bootstrap methods and generalized estimating equations for correlated outcomes in generalized linear models. Commun Stat Simul Comput 1997;26:901–925. [Google Scholar]
- 9.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 10.Kleopa KA, Sherman M, Neal B, Romano GJ, Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci 1999;164:82–88. [DOI] [PubMed] [Google Scholar]
- 11.Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep 2010;33:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DL, Chervin RD, Kalbfleisch JD, et al. Sleep apnea treatment after stroke (SATS) trial: is it feasible? J Stroke Cerebrovasc Dis 2013;22:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traynor BJ, Zhang H, Shefner JM, et al. Functional outcome measures as clinical trial endpoints in ALS. Neurology 2004;63:1933–1935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


