Abstract
Objective:
To assess whether multifocal, high-frequency repetitive transcranial magnetic stimulation (rTMS) of motor and prefrontal cortex benefits motor and mood symptoms in patients with Parkinson disease (PD).
Methods:
Patients with PD and depression were enrolled in this multicenter, double-blind, sham-controlled, parallel-group study of real or realistic (electric) sham rTMS. Patients were randomized to 1 of 4 groups: bilateral M1 ( + sham dorsolateral prefrontal cortex [DLPFC]), DLPFC ( + sham M1), M1 + DLPFC, or double sham. The TMS course consisted of 10 daily sessions of 2,000 stimuli for the left DLPFC and 1,000 stimuli for each M1 (50 × 4-second trains of 40 stimuli at 10 Hz). Patients were evaluated at baseline, at 1 week, and at 1, 3, and 6 months after treatment. Primary endpoints were changes in motor function assessed with the Unified Parkinson's Disease Rating Scale-III and in mood with the Hamilton Depression Rating Scale at 1 month.
Results:
Of the 160 patients planned for recruitment, 85 were screened, 61 were randomized, and 50 completed all study visits. Real M1 rTMS resulted in greater improvement in motor function than sham at the primary endpoint (p < 0.05). There was no improvement in mood in the DLPFC group compared to the double-sham group, as well as no benefit to combining M1 and DLPFC stimulation for either motor or mood symptoms.
Conclusions:
In patients with PD with depression, M1 rTMS is an effective treatment of motor symptoms, while mood benefit after 2 weeks of DLPFC rTMS is not better than sham. Targeting both M1 and DLPFC in each rTMS session showed no evidence of synergistic effects.
ClinicalTrials.gov identifier:
Classification of evidence:
This study provides Class I evidence that in patients with PD with depression, M1 rTMS leads to improvement in motor function while DLPFC rTMS does not lead to improvement in depression compared to sham rTMS.
Parkinson disease (PD) presents with both motor and nonmotor features. Motor symptoms can respond to pharmacologic and other therapies such as deep brain stimulation,1 but these treatments are often ineffective for nonmotor symptoms. Depression is particularly common, with a prevalence ranging from 40% to 70%.2 Not infrequently, depression in PD is resistant to medication and affects patients’ quality of life.3
One treatment with the potential to improve both motor and mood symptoms in PD is repetitive transcranial magnetic stimulation (rTMS), a noninvasive brain stimulation modality that changes cortical excitability that persists beyond the stimulation session itself.4–6 High-frequency (HF) rTMS to the bilateral primary motor cortex (M1) has been shown to improve motor symptoms in PD,7–10 while HF rTMS to the left dorsolateral prefrontal cortex (DLPFC) is an effective treatment for medication-refractory depression,4,11,12 including depression in PD.13–16
Despite the frequent comorbidity of mood and motor symptoms in PD, most rTMS studies have focused on one or the other symptom, usually targeting one stimulation site (e.g., DLPFC or motor cortex). In addition, studies have had limited sample size and used sham controls that may not offer true blinding. The present study was designed to address these issues.
METHODS
This study was designed to answer the following questions with Class I level of evidence in patients with PD with comorbid depression: Is M1 HF rTMS superior to realistic sham for motor symptoms as assessed by Unified Parkinson’s Disease Rating Scale (UPDRS) part III? Is DLPFC HF rTMS superior to realistic sham for mood symptoms as assessed by Hamilton Depression Rating Scale (HAM-D)? Is combined M1 and DLPFC HF rTMS superior to realistic sham for both motor and mood symptoms, and is there any evidence of synergistic effects? The primary endpoint for all measures was 1 month after the end of rTMS sessions.
Participants.
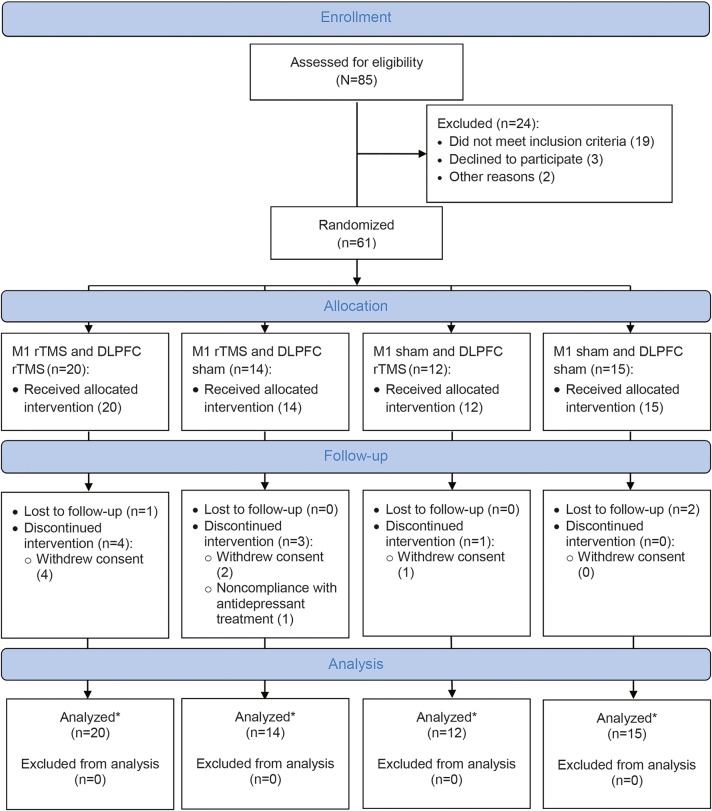
Patients with idiopathic PD (according to UK Parkinson’s Disease Brain Bank criteria), bilateral motor symptoms (Hoehn and Yahr stage II–IV), and comorbid major depression (according to DSM-IV) were eligible. Depression inclusion criteria included HAM-D score >7 despite current use of antidepressant(s) for a minimum of 90 days, adequate past trial of antidepressants (6 weeks on an optimized dose), or documented intolerability to antidepressants. Patients with ferromagnetic implants, a history of seizures, major head trauma, dementia (Montreal Cognitive Assessment [MoCA] scale score <26), or depression with psychotic symptoms were excluded. Participants were prospectively recruited from 7 clinical centers from May 1, 2011, to June 30, 2014: Beth Israel Deaconess Medical Center, Harvard Medical School (Boston, MA), New York University School of Medicine (New York, NY), Toronto Western Research Institute (Toronto, ON, Canada), University of California School of Medicine (Los Angeles, CA), Cleveland Clinic (Cleveland, OH), University of Florida (Gainesville, FL), and University of North Dakota School of Medicine (Grand Forks, ND; figure 1).
Figure 1. Repetitive Transcranial Magnetic Stimulation (rTMS) for Motor and Mood Symptoms of Parkinson's Disease (MASTER-PD) study flow diagram.
*The analysis included all participants who completed the primary study end point visit and were not excluded from analysis. DLPFC = dorsolateral prefrontal cortex.
Study design.
This was a double-blind, sham-controlled, randomized, parallel-group study of fixed-dose, HF rTMS in patients with PD with depressive symptoms. Participants were randomized in a 1:1:1:1 fashion to receive rTMS over the bilateral M1, left DLPFC, both, or neither (sham rTMS). Primary outcome measures were a change in UPDRS-III and HAM-D at 1 month after the completion of rTMS treatment compared with baseline (pretreatment) scores. Secondary outcome measures included the Beck Depression Inventory-II, Clinical Anxiety Scale, MoCA, and Clinical Global Impression scale, UPDRS total score and subscales (parts I, II, and IV), and Parkinson’s Disease Questionnaire (PDQ-39). Scores were assessed at baseline, at 1 week after treatment (±3 days), and after 1 month (±7 days), 3 months (±7 days), and 6 months (±7 days). All motor and mood scores were assessed in the medication “off” state (≥12 hours after the last dose). An investigator blinded to rTMS conditions performed all neurologic and psychiatric evaluations. Throughout the study, participants continued their antidepressant and antiparkinsonian medications without dose adjustments. To assess the efficacy of the realistic sham, at the end of the treatment period, participants were asked to guess the stimulation received (forced choice of any real vs sham).
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Board of each participating site. Written informed consent was obtained from each participant. The study was registered with ClinicalTrials.gov with the identifier NCT01080794.
Treatment regimen.
Participants received rTMS always in the “on” state (≈1 hour after anti-PD medications) while seated in a chair with EMG electrodes over the abductor pollicis brevis (APB) muscle to record motor evoked potentials. A Magstim Super-Rapid stimulator (Magstim Co, Ltd, Carmarthenshire, Wales, UK) was connected to a 70-mm-diameter figure-of-eight coil (or equivalent sham coil). The left and right M1 sites were determined as the scalp location from which TMS evoked motor evoked potentials of maximal amplitude in the contralateral APB. Resting motor threshold was determined with the left M1. The left prefrontal site was located 5 cm anterior to the optimal left M1 location for the APB.4
The rTMS course consisted of daily sessions of 2,000 stimuli for the left DLPFC and 1,000 stimuli for each M1 (50 trains of 40 stimuli at 10 Hz for 10 days). All centers used the same method for targeting the motor cortex and the left DLPFC. In each session, real or sham rTMS was delivered over the left DLPFC and left and right M1 sequentially. At one site (treating 9 patients, 3 of whom were in the M1 + DLPFC group), the stimulation order was reversed (left M1 stimulation was followed by right M1 and then left DLPFC for all participants). For sham rTMS, we replicated the look, sound, and feel of active stimulation, in the absence of a significant magnetic field, using a matched air-cooled sham coil. Scalp muscle stimulation associated with rTMS was replicated with rubber electrodes placed over the center of the coil connected to a constant current stimulator (Digitimer DS7AH) with a pulse duration of 200 microseconds at 120 V and current intensity set to 10 mA. If stimulation at 10 mA was not perceived, it was adjusted upward; if it was uncomfortable, it was lowered.
Duration of treatment.
Participants received real or sham rTMS for 25 minutes for the left DLPFC and 12.5 minutes for each M1, with no pauses between. Each participant received 10 rTMS sessions over 2 weeks. The treatment duration was same for both rTMS targets and was chosen on the basis of prior rTMS studies in PD.14,15
Statistical analysis.
Demographic and clinical variables were compared by use of analysis of variance (ANOVA) with Tukey post hoc and χ2 tests (for proportions). Significance of primary endpoints (change in UPDRS-III and HAM-D from baseline to month 1 after treatment) was assessed with independent-samples t test for absolute change in a measured score. To ensure that results at the primary endpoint were not driven by individual outliers, we tested any significant findings for individuals >2 SDs from the mean and, if present, repeated the analysis with those participants excluded. In additional analyses incorporating all study time points, we conducted repeated-measures ANOVA (rANOVA) for all study groups with Dunnett post hoc tests (with the double-sham rTMS group as a reference). Study data were captured with the REDCap database.17
Sample size estimation and interim analyses.
Initial study design called for enrollment of 160 participants on the basis of predicted motor effects of 0.9 in the M1 + DLPFC group and 0.6 in the other 2 rTMS groups compared to double sham (mean change of 9 vs 6 vs 1 point in UPDRS-III with an SD of 10).9 Effect sizes for mood were predicted to be larger.9,14 Controlling for 2 primary outcome measures across 3 rTMS groups (Dunnett 2-sided multiple-comparison test with control procedure), this sample size provided 81.7% power to claim that at least one of the active groups was significantly different from the double sham in the mean change of UPDRS-III.
An interim analysis was planned a priori at the study midpoint (80 participants enrolled) to potentially eliminate ≥1 of the 4 subgroups, thus increasing power in the remaining groups. This interim analysis was conducted early (61 participants enrolled) because of slower-than-expected recruitment.
RESULTS
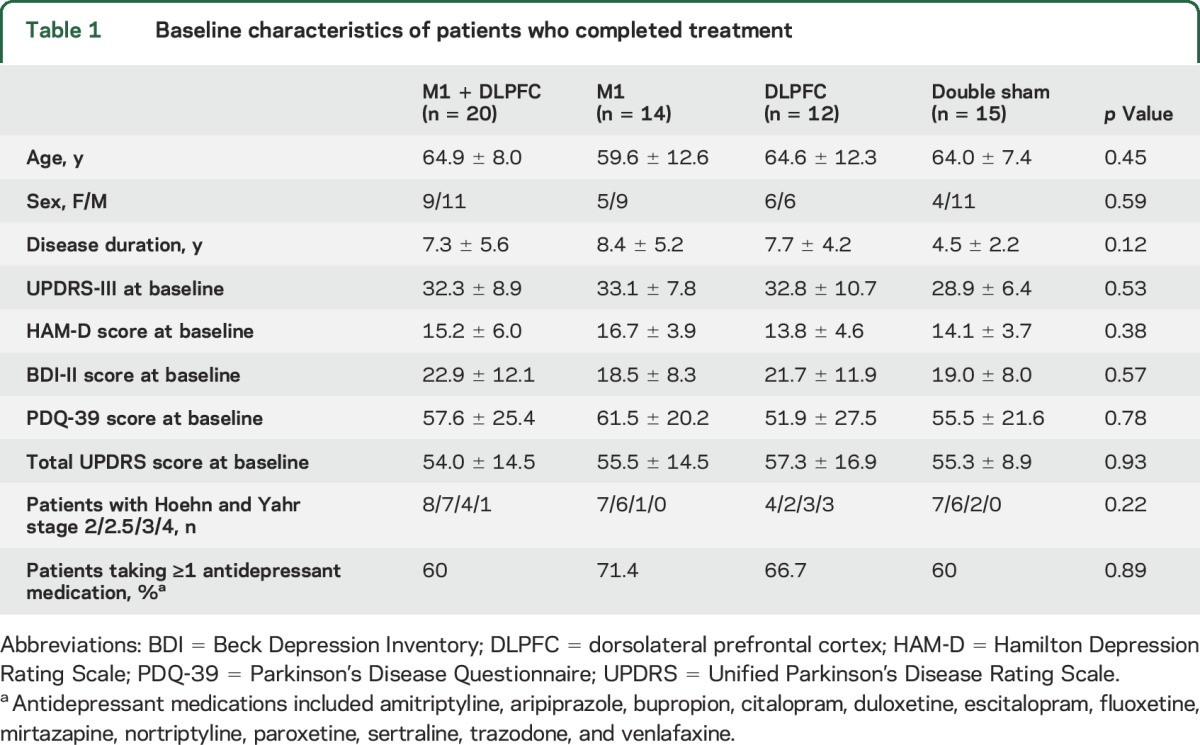
Of the 85 participants who completed screening (figure 1 and figure e-1 at Neurology.org), 61 were randomized to receive rTMS: M1 + DLPFC rTMS (n = 20); M1 rTMS ( + sham DLPFC; n = 14); DLPFC rTMS ( + sham M1; n = 12); and double sham (n = 15). Of these, 60 (98%) were assessed at the primary endpoint, and 50 (82%) completed all study visits. Patients’ demographics are summarized in table 1. No differences in demographic or clinical variables were found between study completers (n = 50) and noncompleters (n = 11). Similarly, no differences were found in the distribution of participants with different stages (severity) of the disease across study groups (p > 0.05, χ2, table 1).
Table 1.
Baseline characteristics of patients who completed treatment
Only 49% of participants correctly guessed the stimulation status (real vs sham), confirming the efficacy of the blinding method used. rTMS was well tolerated by all participants, although 34 (68% of completers) reported adverse events, most commonly headache and neck pain, which were mild and transient. One serious adverse event (ischemic stroke) occurred in a patient receiving active rTMS (deemed unrelated to the study). The distribution of adverse events was similar in the active TMS groups (25 of 46) and the double-sham group (9 of 15).
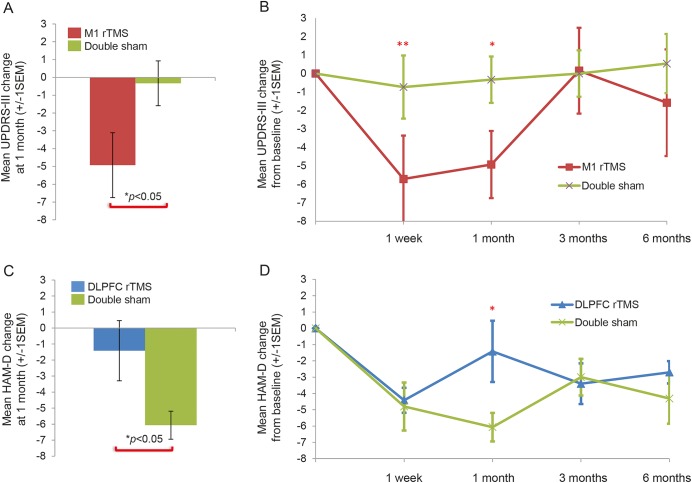
At the primary study time point, UPDRS-III absolute change was greater in the M1 group (−4.9 points) than in the double-sham group (−0.3 points; mean difference = −4.6, 95% confidence interval [CI] −0.1 to −9.1, t = −2.1, p < 0.05), indicating improvement in motor symptoms with real stimulation (table 2 and figure 2A). There were no significant outliers. rANOVA comparing UPDRS-III at all study time points between those 2 groups was not significant (figure 2B). Exploratory post hoc analysis comparing the M1 and double-sham groups at the primary study endpoint showed improvement in the M1 group in UPDRS-III subscores of rigidity (mean difference = −0.5, 95% CI −1.4 to −0.8, t = 3.0, p < 0.01) and bradykinesia (mean difference = 0.3, 95% CI −0.6 to −0.01, t = 2.1, p < 0.05) but not tremor, gait, or axial symptoms.
Table 2.
Unified Parkinson’s Disease Rating Scale (UPDRS) part III and Hamilton Depression Rating Scale (HAM-D) score (±SD) at baseline and primary study time point by study group
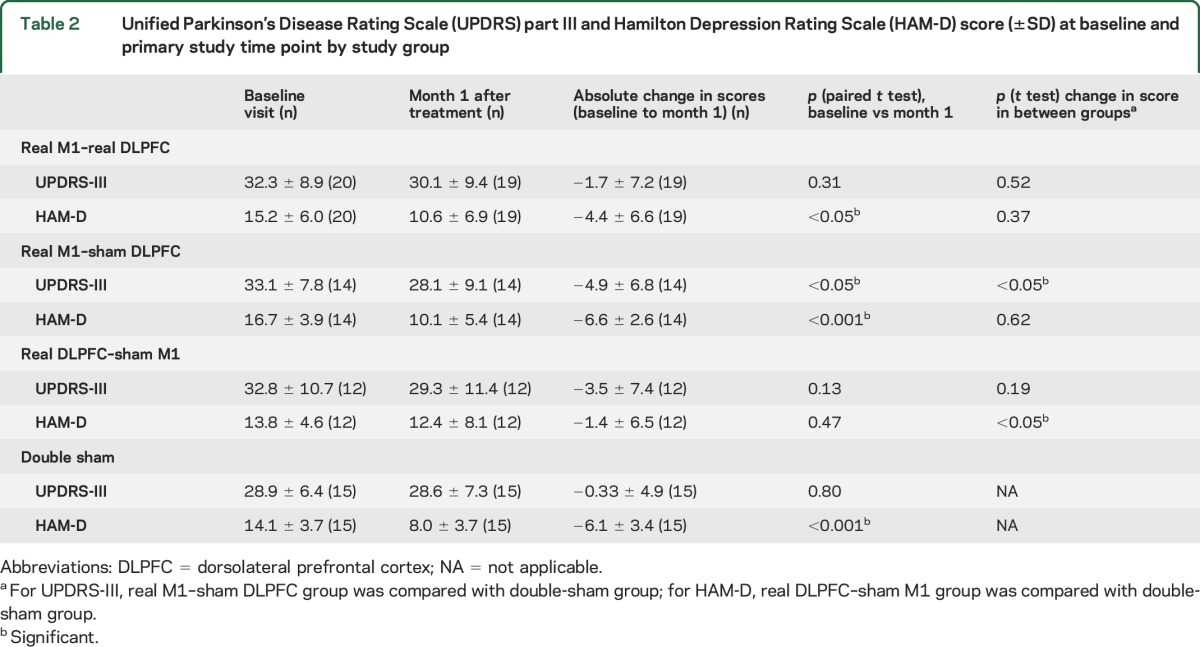
Figure 2. Hamilton Depression Rating Scale (HAM-D) scores at study time points in dorsolateral prefrontal cortex (DLPFC) repetitive transcranial magnetic stimulation (rTMS) group and Unified Parkinson’s Disease Rating Scale (UPDRS) part III scores at study time points in M1 rTMS group compared to double-sham group.
(A and B) Improvement in motor symptoms after rTMS to primary motor cortex vs sham. Consistent with prior studies, there was a significant improvement in motor scores after M1 rTMS compared to double sham at the primary endpoint (A) that returned to baseline by 3 months (B). (C and D) Lack of improvement in mood symptoms after rTMS to DLPFC vs sham. In contrast to prior reports, rTMS to left DLPFC resulted in less antidepressant response at the primary 1-month endpoint than double sham (C). This difference was specific to this single time point (D) and driven in part by a single outlier (see text). *p < 0.05, **p < 0.001.
Absolute change in HAM-D was unexpectedly less in the DLPFC group (−1.4) than in the double-sham group (−6.1) at the primary endpoint (mean difference = −4.7, 95% CI 0.7–8.7, t = 2.4, p < 0.05, table 2 and figure 2C). When a single outlier was removed, the difference was no longer significant. rANOVA comparing HAM-D score at all study time points between those 2 groups was not significant (figure 2D).
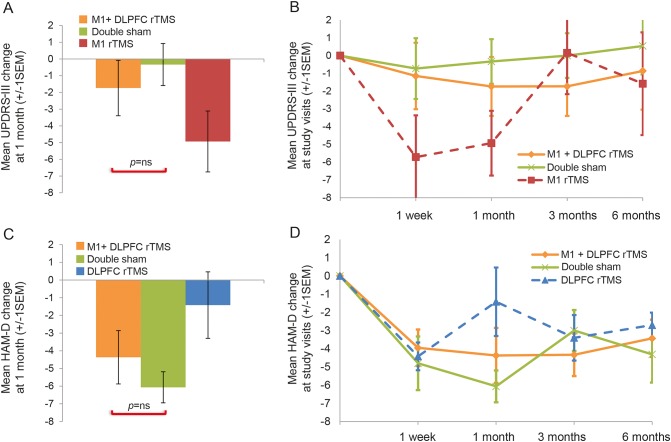
In the M1 + DLPFC group, there was no significant improvement in either motor or mood symptoms at the primary endpoint compared to the double-sham group (table 2 and figure 3, A and C). Similarly, rANOVA comparing UPDRS-III and HAM-D scores at all study time points between those 2 groups was not significant (table e-1 and figure 3, B and D). There was no added benefit of M1 + DLPFC stimulation on motor symptoms compared to M1 stimulation alone (figure 3, A and B), as well as no benefit of M1 + DLPFC stimulation on mood symptoms compared to DLPFC alone (figure 3, C and D).
Figure 3. Time course of Unified Parkinson’s Disease Rating Scale (UPDRS) part III and Hamilton Depression Rating Scale (HAM-D) scores by treatment group.
Lack of improvement in motor or mood symptoms after combined M1 + DLPFC rTMS vs sham. There was no significant improvement in motor (A and B) or mood (C and D) symptoms after combined M1 + dorsolateral prefrontal cortex (DLPFC)repetitive transcranial magnetic stimulation (rTMS) vs sham. There was also no added benefit of M1 + DLPFC stimulation on motor symptoms compared to M1 stimulation alone (A and B) or mood benefit compared to DLPFC alone (C and D).
Subgroup analysis of mild PD (baseline UPDRS-III < 32, median score for the study population) or more advanced PD (UPDRS-III ≥ 32) showed no difference in change in UPDRS-III or HAM-D (from baseline to the primary study time point) between each subgroup and the double-sham group. Similarly, no mood effects of rTMS were observed when the study population was divided into 2 subgroups by median HAM-D score.
No study group differences were found in the secondary outcome scores, including Beck Depression Inventory-II, Apathy Evaluation Scale, Clinical Anxiety Scale, MoCA, and PDQ-39; in subscales of the UPDRS (parts I, II IV) and UPDRS total score; and in the Clinical Global Impression scale completed by a blinded physician and study participants.
Given our above finding of improved motor function in the M1 rTMS group, we performed additional analyses to better understand this result. First, we looked to see whether improvement in UPDRS-III translated into improvement in quality of life (measured by PDQ-39) in the M1 rTMS group, but no such correlation was found. Similarly, there was no difference in change of the PDQ-39 between the M1 and double-sham groups. Next, although disease duration was not significantly different across all study groups, there was a difference between the M1 and double-sham subgroups (table 1), so we tested how this factor influenced results. Across all participants, there was no significant relationship between disease duration and UPDRS improvement at 1 month (r = −0.05, p = 0.71). When disease duration was entered as a covariate, the difference in UPDRS improvement between the M1 and double-sham group persisted (−5.0 in the M1 group vs −0.3 in the double-sham group), but statistical significance was reduced (p = 0.07).
DISCUSSION
In this clinical trial, we found benefit of M1 rTMS for motor symptoms of PD but no benefit of left DLPFC rTMS for mood symptoms and no benefit of combined M1 + DLPFC rTMS for motor or mood symptoms.
HF rTMS to bilateral M1 was beneficial for motor symptoms, and the effect persisted for at least 1 month. This result is consistent with prior studies5,10,18,19 and confirms the conclusions of 2 meta-analyses.7,9 Our study shows motor benefit compared to realistic sham, with documentation that participants were unable to reliably distinguish the 2 interventions. The magnitude of the improvement (−4.9 points in the UPDRS-III) was equal to a minimal clinically important change on the UPDRS-III20 but slightly below that found in recent meta-analyses (−6.4 and −6.3 points7,21). We believe that the lower magnitude of improvement seen here is most likely secondary to the use of realistic sham (which might have mitigated expectations and improved blinding) and to smaller sample sizes in other studies. Another possible reason is the enrollment of patients with PD with depression.
Consistent with prior reports,22,23 post hoc analysis of the M1 group revealed benefits of rTMS for measures of bradykinesia and rigidity but not tremor or axial symptoms, for which alternative rTMS targets may be better.24 Nevertheless, the benefit of M1 rTMS on these symptoms did not translate to an improvement in quality of life, as measured by the PDQ-39 scale.
Contrary to prior reports,14,25 we failed to find an improvement in PD-associated depression after 2 weeks of left DLPFC rTMS. The magnitude of our antidepressant effect, while similar to that seen in prior studies in PD (≈30% improvement in HAM-D),14,25,26 was greater in the double-sham group than in the real DLPFC rTMS group, likely the result of a pronounced placebo response. We doubt this difference is meaningful because, unlike the motor effect, it was restricted to a single time point and driven by an outlier. Nevertheless, real DLPFC rTMS did not afford any advantage over realistic sham. As opposed to other rTMS studies in patients with PD with depression, our participants continued rather than withheld antidepressants, which eliminated possible confounding effects of antidepressant withdrawal but could have amplified placebo response. Additionally, we included patients with PD with HAM-D score of ≥8, which is low compared to prior studies. However, our subgroup analysis of mild and severe depression showed no difference of rTMS effects.
The duration of the rTMS course in our study (and in prior studies of PD-associated depression) was only 10 days, while in the major multisite, double-blind clinical trials for primary (non-PD) depression, it was 20 to 30 days,27 and it often took 15 days for patients to experience a significant antidepressant response beyond sham.28 Therefore, a longer course of rTMS could still be beneficial for PD-associated depression.
Regarding the main objective of this study, multifocal stimulation, concurrent rTMS of the left DLPFC and bilateral M1 was not better than sham for either motor or mood symptoms. Furthermore, there was no evidence of synergistic effects compared with left DLPFC or M1 alone. The neurophysiology of concomitant TMS stimulation over different sites is unknown, and it is possible that instead of a summation, a cancellation occurred. Three prior studies have combined M1 and DLPFC rTMS in PD, and all reported motor benefit, but they differed from the current study in important ways.29–31 Two of these studies lacked a sham control, used low-frequency rather than HF rTMS to M1, and used a different type of TMS coil. The third study30 was the most similar to the present study but included rTMS to the right DLPFC, a different type of sham, no M1 stimulation condition, and different TMS frequency and duration.30
The divergent placebo response with regard to mood and motor outcomes deserves mention. While the double-sham group had a robust antidepressant response that persisted for the study duration, there was no improvement in motor scores in this group, despite participants being told that the study was designed to improve both. The fact that one can observe a pronounced placebo-induced improvement in one domain and no improvement in another provides insights into the selectivity of placebo effects and is of great relevance for appropriate design of PD therapeutic trials.32
The study has several important limitations. First, it was terminated prematurely due to difficulty with recruitment and an interim analysis showing lack of efficacy in the M1 + DLPFC group. Thus, statistical power (compared to the initial study design) was reduced, and results were not corrected for multiple comparisons. Although one can argue that such correction is not necessary given clear a priori hypotheses supported by the literature, this increases the chance of type II error. Second, we enrolled a heterogeneous patient population with mild and advanced disease and with variable years of drug exposure, which may have obscured effects in specific subgroups. Third, although there were no significant differences in disease duration across groups, we cannot exclude the possibility that this may have influenced motor improvement in our M1 subgroup. Finally, the details of the PD medication regimen and presence of motor fluctuations were not collected in a systematic manner. This could affect both mood and motor outcome measures assessed exclusively during the “off” state.
The study failed to demonstrate a beneficial effect of multifocal rTMS for the treatment of motor and mood symptoms in PD, although it provided several valuable outcomes that can guide future work. First, M1 rTMS appears promising for improving motor symptoms in PD. Second, there appears to be a pronounced placebo response to rTMS for mood symptoms in PD, and future work should consider longer-duration trials consistent with studies in primary depression. Finally, the combined M1 + DLPFC rTMS protocol used here does not appear to be a promising therapeutic avenue, and future work should consider alternative protocols.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Rebecca A. Betensky (Harvard School of Public Health; Harvard Catalyst) for advice regarding statistical analysis and the following people for help with various aspects of the study: Ann Connor, RN, and Andrea Vatulas (Harvard Medical School); Drs. Choi Deblieck, Alexander Bystritsky, and Emad Farag (UCLA); Drs. Peter Giacobbe, Nicolas Phielipp, Kaviraj Udupa, Behzad Elahi, Soumya Ghosh, and Tasnuva Hoque, Puja Bhakta, and Utpal Saha (Toronto Western Research Institute); and Jamika Singleton-Garvin and Dr. Alexandra Lloyd-Smith (NYU).
GLOSSARY
- ANOVA
analysis of variance
- APB
abductor pollicis brevis
- CI
confidence interval
- DLPFC
dorsolateral prefrontal cortex
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HAM-D
Hamilton Depression Rating Scale
- HF
high-frequency
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson disease
- PDQ-39
Parkinson's Disease Questionnaire
- rANOVA
repeated-measures analysis of variance
- rTMS
repetitive transcranial magnetic stimulation
- TMS
transcranial magnetic stimulation
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept/design: Dr. Pascual-Leone, Dr. Chen, Dr. Wu, Dr. Fernandez, and Dr. Simon. Data analysis/interpretation: Dr. Brys, Dr. Fox, Dr. Agarwal, Dr. Biagioni, Geraldine Dacpano, Dr. Pawan Kumar, Dr. Chen, Dr. Wu, Dr. Fernandez, Dr. Wagle-Shukla, Dr. Jau-Shin Lou, Zachary Gray, Dr. Di Rocco, and Dr. Pascual-Leone. Drafting/revising of the manuscript: Dr. Brys, Dr. Fox, Dr. Agarwal, Dr. Simon, Dr. Wu, Dr. Di Rocco, and Dr. Pascual-Leone. Statistical analysis: Elizabeth Pirraglia. Obtained funding: Dr. Pascual-Leone, Dr. Chen, Dr. Wu, and Dr. Fernandez.
STUDY FUNDING
The study was sponsored by The Michael J. Fox Foundation for Parkinson Research and NIH grants K23 NS083741 and KL2 TR000065s. Dr. A. Pascual-Leone was also partly supported by the Sidney R. Baer Jr. Foundation, the NIH (R01 HD069776, R01 NS073601, R21 NS082870, R21 MH099196, R21 NS085491, R21 HD07616), the Football Players Health Study at Harvard University, Harvard Catalyst, and the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, UL1 RR025758).
DISCLOSURE
M. Brys is currently an employee of Biogen. M. Fox is listed on submitted patents using brain imaging to guide brain stimulation. S. Agarwal, M. Biagioni, G. Dacpano, P. Kumar, E. Pirraglia, R. Chen, A. Wu, H. Fernandez, A. Shukla, J. Lou, Z. Gray, D. Simon, and A. Di Rocco report no disclosures relevant to the manuscript. A. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc, and Neosync, and is listed as an inventor on several issued and pending patents on the real-time integration of TMS with EEG and MRI. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the NIH, or the Sidney R. Baer Jr. Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson's disease. Mov Disord 2006;21(suppl 1):S284–S289. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL. Depression and Parkinson's disease: a review. Am J Psychiatry 1992;149:443–454. [DOI] [PubMed] [Google Scholar]
- 3.Ziemssen T, Reichmann H. Non-motor dysfunction in Parkinson's disease. Parkinsonism Relat Disord 2007;13:323–332. [DOI] [PubMed] [Google Scholar]
- 4.Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Early report rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996;348:233–237. [DOI] [PubMed] [Google Scholar]
- 5.Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol 2002;113:101–107. [DOI] [PubMed] [Google Scholar]
- 6.Peinemann A, Reimer B, Löer C, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol 2004;115:1519–1526. [DOI] [PubMed] [Google Scholar]
- 7.Chou YH, Hickey PT, Sundman M, Song AW, Chen NK. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol 2015;72:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R. Repetitive transcranial magnetic stimulation as treatment for depression in Parkinson's disease. Mov Disord 2010;25:2272–2273. [DOI] [PubMed] [Google Scholar]
- 9.Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for Parkinson's disease: a systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry 2005;76:1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elahi BB, Elahi BB, Chen R. Effect of transcranial magnetic stimulation on parkinson motor function: systematic review of controlled clinical trials. Mov Disord 2009;24:357–363. [DOI] [PubMed] [Google Scholar]
- 11.George MS, Wassermann EM, Kimbrell TA, et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997;154:1752–1756. [DOI] [PubMed] [Google Scholar]
- 12.Avery DH, Holtzheimer PE, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry 2006;59:187–194. [DOI] [PubMed] [Google Scholar]
- 13.Epstein CM, Evatt ML, Funk A, et al. An open study of repetitive transcranial magnetic stimulation in treatment-resistant depression with Parkinson's disease. Clin Neurophysiol 2012;127:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fregni F, Santos CM, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2004;75:1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dragasevic N, Potrebić A, Damjanović A, Stefanova E, Kostić VS. Therapeutic efficacy of bilateral prefrontal slow repetitive transcranial magnetic stimulation in depressed patients with Parkinson's disease: an open study. Mov Disord 2002;17:528–532. [DOI] [PubMed] [Google Scholar]
- 16.Epstein CM, Evatt ML, Funk A, et al. An open study of repetitive transcranial magnetic stimulation in treatment-resistant depression with Parkinson's disease. Clin Neurophysiol 2007;118:2189–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefaucheur JP. Repetitive transcranial magnetic stimulation (rTMS): insights into the treatment of Parkinson's disease by cortical stimulation. Neurophysiol Clin 2006;36:125–133. [DOI] [PubMed] [Google Scholar]
- 19.Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson's disease. Mov Disord 2006;21:6–10. [DOI] [PubMed] [Google Scholar]
- 20.Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the Unified Parkinson's Disease Rating Scale. Mov Disord 2006;21:1200–1207. [DOI] [PubMed] [Google Scholar]
- 21.Zanjani A, Zakzanis KK, Daskalakis ZJ, Chen R. Repetitive transcranial magnetic stimulation of the primary motor cortex in the treatment of motor signs in Parkinson's disease: a quantitative review of the literature. Mov Disord 2015;30:750–758. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Leone A, Valls-Solé J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. Akinesia in Parkinson's disease, II: effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology 1994;44:892–898. [DOI] [PubMed] [Google Scholar]
- 23.González-García N, Armony JL, Soto J, Trejo D, Alegría MA, Drucker-Colín R. Effects of rTMS on Parkinson's disease: a longitudinal fMRI study. J Neurol 2011;258:1268–1280. [DOI] [PubMed] [Google Scholar]
- 24.Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y. Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology 2013;80:1400–1405. [DOI] [PubMed] [Google Scholar]
- 25.Pal E, Nagy F, Aschermann Z, Balazs E, Kovacs N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson's disease: a randomized, double-blind, placebo-controlled study. Mov Disord 2010;25:2311–2317. [DOI] [PubMed] [Google Scholar]
- 26.Benninger DH, Berman BD, Houdayer E, et al. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology 2011;76:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007;62:1208–1216. [DOI] [PubMed] [Google Scholar]
- 28.Loo CK, Mitchell PB. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. J Affect Disord 2005;88:255–267. [DOI] [PubMed] [Google Scholar]
- 29.Cohen OS, Orlev Y, Yahalom G, et al. Repetitive deep transcranial magnetic stimulation for motor symptoms in Parkinson's disease: a feasibility study. Clin Neurol Neurosurg 2016;140:73–78. [DOI] [PubMed] [Google Scholar]
- 30.Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson's disease. Mov Disord 2006;21:325–331. [DOI] [PubMed] [Google Scholar]
- 31.Torres F, Villalon E, Poblete P, et al. Retrospective evaluation of deep transcranial magnetic stimulation as add-on treatment for Parkinson's disease. Front Neurol 2015;6:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetz CG, Wuu J, McDermott MP, et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov Disord 2008;23:690–699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.