Abstract
Objective:
To determine whether cognitive impairment in Parkinson disease (PD) and Alzheimer disease (AD) derives from the same network pathology.
Methods:
We analyzed 18F-fluorodeoxyglucose PET scans from 40 patients with AD and 40 age-matched healthy controls from the Alzheimer’s Disease Neuroimaging Initiative and scanned an additional 10 patients with AD and 10 healthy controls at The Feinstein Institute for Medical Research to derive an AD-related metabolic pattern (ADRP) analogous to our previously established PD cognition-related pattern (PDCP) and PD motor-related pattern (PDRP). We computed individual subject expression values for ADRP and PDCP in 89 patients with PD and correlated summary scores for cognitive functioning with network expression. We also evaluated changes in ADRP and PDCP expression in a separate group of 15 patients with PD scanned serially over a 4-year period.
Results:
Analysis revealed a significant AD-related metabolic topography characterized by covarying metabolic reductions in the hippocampus, parahippocampal gyrus, and parietal and temporal association regions. Expression of ADRP, but not PDCP, was elevated in both AD groups and correlated with worse cognitive summary scores. Patients with PD showed slight ADRP expression, due to topographic overlap with the network underlying PD motor-related pattern degeneration, but only their PDCP expression values increased as cognitive function and executive performance declined. Longitudinal data in PD disclosed an analogous dissociation of network expression.
Conclusions:
Cognitive dysfunction in PD is associated with a specific brain network that is largely spatially and functionally distinct from that seen in relation to AD.
Cognitive impairment is commonly observed in patients with Parkinson disease (PD), even early in the clinical course,1,2 but its cause remains unclear. The postmortem observation of amyloid-β plaques and tau neurofibrilliary tangles, pathologic hallmarks of Alzheimer disease (AD), in individuals with PD and dementia has led to the hypothesis that the cognitive changes in PD are caused by comorbid AD.3–5 Many patients with PD have substantial cognitive loss without forming plaques and tangles, however, and the severity of neuropsychological deficits in patients with PD with coexisting cortical Lewy body and AD-like pathology correlates only with the former.6 Whether AD contributes to the cognitive deficits in PD thus remains an unsettled question.
The fact that neuronal dysfunction in neurodegenerative diseases is propagated along discrete networks makes such questions amenable to neuroimaging approaches. We and others have used 18F-fluorodeoxyglucose (FDG)–PET and fMRI to identify disease-specific networks in PD, AD, Huntington disease, and other conditions.7–13 We have shown that akinesia-rigidity in PD correlates with the expression of a PD motor-related pattern (PDRP)10,11 such that more severe symptoms are reflected in higher PDRP scores; tremor is mediated by a distinct cerebello-thalamo-cortical network14; and the expression of a distinct PD cognition-related pattern (PDCP), characterized by diminished metabolism in the medial frontal and parietal regions, rises as cognitive function deteriorates.15–18 Similarly, in AD, neuropsychological test performance correlates with the expression of an AD-related pattern (ADRP) characterized by covarying reductions in hippocampal, superior temporal, and parieto-occipital resting-state activity.7 To determine whether patients with PD, with or without cognitive deficits, express ADRP, we compared ADRP and PDCP expression in patients with AD and those with PD. We also analyzed changes in ADRP and PDCP over time in a separate longitudinal cohort of patients with PD.
METHODS
Participants.
Patients with AD.
To ensure the most rigorous comparison, we used the same spatial covariance analysis approach we previously used to characterize PDRP10,19 to identify and validate a reliable ADRP topography for prospective use. We analyzed metabolic images from 40 patients with AD (23 men and 17 women, mean ± SD age 75.9 ± 5.6 years, Mini-Mental State Examination [MMSE] score 22.6 ± 3.3) and 40 age-matched healthy controls (23 men and 17 women, age 76.0 ± 4.7 years) who had been scanned with FDG-PET as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI; see table e-1 at Neurology.org for demographic and neuropsychological data).
Using a random number generator (http://www.r-project.org/), we assigned the patients with AD and controls to 1 of 2 groups. The first group comprised 20 patients with AD (AD1; 13 men and 7 women, age 76.0 ± 6.2 years, MMSE score 23.6 ± 2.4) and 20 normal controls (NL1; 10 men and 10 women, age 76.6 ± 5.2 years). We used scans from these AD1 and NL1 participants to identify a significant ADRP topography and scans from a second group of 20 patients with AD (AD2; 10 men and 10 women, age 75.8 ± 5.2 years, MMSE score 21.6 ± 3.8) and 20 normal controls (NL2; 13 men and 7 women, age 75.5 ± 4.2 years) for testing. A third group constituting an additional validation set, consisting of 10 additional patients with AD (AD3; 6 men and 4 women, age 74.5 ± 5.3 years, MMSE score 23.9 ± 4.2) and 10 age-matched healthy volunteers (NL3; 4 men and 6 women, age 73.4 ± 4.8 years), were scanned with FDG-PET on the GE Advance tomograph (General Electric, Milwaukee, WI) at The Feinstein Institute for Medical Research (FIMR; Manhasset, NY) as described elsewhere.19,20 One of the investigators (M.L.G.) made the diagnosis of AD in the AD3 cohort on the basis of published criteria.21
Patients with PD.
We quantified ADRP expression values and subject scores for the previously characterized PDCP network topography in a cross-sectional sample of 89 patients (61 men and 28 women, age 61.6 ± 9.0 years, MMSE score 28.0 ± 2.3) who were recruited at the Movement Disorders Center of Northwell Health (Great Neck, NY) and diagnosed according to the UK Brain Bank criteria for idiopathic PD.22 These patients had pure parkinsonism without a history of known causative factors (such as encephalitis or neuroleptic treatment and without supranuclear gaze abnormalities or ataxia); patients with Lewy body dementia were excluded. Table e-2 summarizes the demographic and neuropsychological data for these participants.
We classified patients with PD as having dementia or not having dementia according to the Dementia Rating Scale23: 11 had scores <127, classifying them as having PD dementia (PDD); the remaining 78 were classified as not having dementia. Using the neuropsychological battery (see e-Methods), we assigned patients with PD without dementia to 3 previously defined cognitive categories: cognitively intact [PD-MCI(−); n = 18], mild cognitive impairment (MCI) involving a single cognitive domain [PD-MCI(s); n = 30], and MCI involving multiple cognitive domains [PD-MCI(m); n = 30]. The neuropsychological testing criteria for MCI in patients with PD are described elsewhere.2,18
In addition, we compared the progression of ADRP and PDCP in a previously published longitudinal imaging cohort of 15 patients with PD (11 men and 4 women, age 58.0 ± 10.2 years) who were scanned with FDG-PET at baseline (within 2 years of diagnosis) and again 24 and 48 months later.24 All participants in the cross-sectional and longitudinal PD cohorts underwent FDG-PET at FIMR according to the same protocol used for the AD3 group.
Standard protocol approvals, registrations, and patient consents.
For the patients with AD and healthy volunteers participating in ADNI protocols, written informed consent was obtained after approval was granted by the Institutional Review Board of the collaborating institutions. For participants scanned at FIMR, we obtained ethics permission from the Institutional Review Board of Northwell Health and written consent from each participant after a detailed explanation of the procedures.
Image analysis.
FDG-PET scans were preprocessed with SPM5 (Wellcome Trust Centre for Neuroimaging, Institute of Neurology, London, UK) running on Matlab 6.0 (Mathworks Inc, Natick, MA). Scans were spatially normalized to a standard PET brain template and smoothed with a 3-dimensional gaussian kernel (full width at half maximum = 10 mm).
Spatial covariance analysis10,19,20 identified a distinct ADRP topography in the combined AD1 (n = 20) and NL1 (n = 20) ADNI derivation sample. (The computational procedures used to identify and validate this pattern are detailed in e-Methods.) We computed ADRP expression values (subject scores) for each member of the ADNI (AD2/NL2, n = 40) and FIMR (AD3/NL3, n = 20; PD, n = 89) testing samples using an automated algorithm available at http://www.feinsteinneuroscience.org. The resulting measures were z scored with respect to corresponding measures from the NL1 normal reference sample used in pattern identification. We used analogous procedures to quantify PDCP expression in the same scans in each of the testing cases. To accord with previously published results, PDCP expression values were z scored with respect to values from the group of normal controls (NL4; 8 men and 7 women, age 56.7 ± 12.3 years) used for reference in prior studies.18,25,26
Network correlates of neuropsychological test performance.
We evaluated the relationship between network expression and cognitive performance in the combined ADNI (AD1/AD2) patient cohort and the FIMR PD sample. For the ADNI participants, we used the summary score data for memory and executive functioning provided in the database.27 Because the ADNI database does not provide a summary score for language function, we constructed a language index score based on the measures that were available for each participant, standardized (z scored) with respect to age-corrected normative values, and averaged individual scores to form a language summary index. We used a similar approach to compute summary scores for memory, executive function, and language in members of the cross-sectional PD sample.
Data analysis.
We assessed differences in ADRP expression between patients with AD and controls separately for the ADNI (AD2/NL2) and FIMR (AD3/NL3) testing sets and for the PD and age-matched normal reference (NL4) groups scanned at FIMR. Subject scores computed in each of the subgroups within this PD sample, PD-MCI(−), PD-MCI(s), PD-MCI(m), and PDD, were separately compared to corresponding control values. Differences in ADRP expression between patients and controls in each testing sample were assessed with Student t tests, and differences in ADRP expression across the PD subgroups were assessed with 1-way analysis of variance (ANOVA), followed by post-hoc Dunnett tests for multiple comparisons. We used identical statistical procedures to assess group differences in PDCP expression in each testing sample. Potential network × subgroup interaction effects in the PD data were evaluated with 2-way repeated-measures ANOVA (RMANOVA), with network as the within-subject variable and subgroup as the between-subject variable.
We used a similar approach to evaluate the neuropsychological summary scores for the 2 disease groups. We constructed separate 1-way ANOVA models for each cognitive domain to identify differences across the PD subgroups; p values were adjusted for multiple comparisons with the Dunnett correction. Relationships between individual subject ADRP and PDCP expression values and the neuropsychological summary scores were evaluated separately in the AD and PD groups by calculating the Pearson product-moment correlations.
To compare changes in ADRP and PDCP expression over time in the longitudinal PD cohort, we analyzed the subject scores using 2-way RMANOVA with network and time as within-subject variables. If a significant network × time interaction effect was evident in the data, we used 1-way RMANOVA to assess changes over time in the expression of each network. All statistical analyses were performed with SAS 9.3 for Windows (SAS Institute, Inc, Cary, NC) and considered significant at p < 0.05 (2-tailed).
RESULTS
ADRP, but not PDCP, expression was consistently elevated in patients with AD.
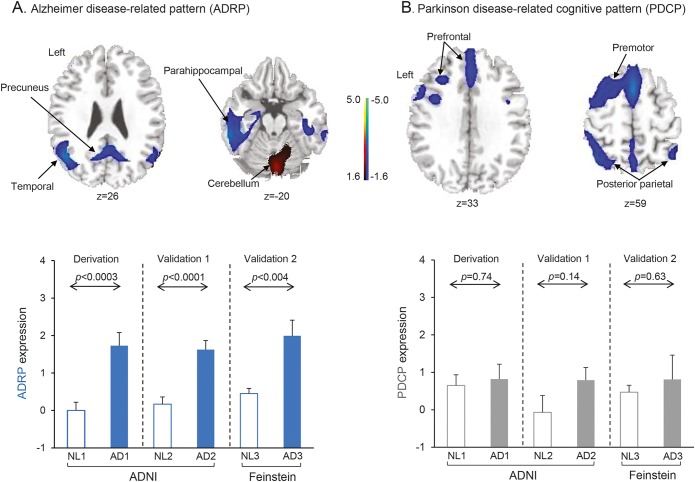
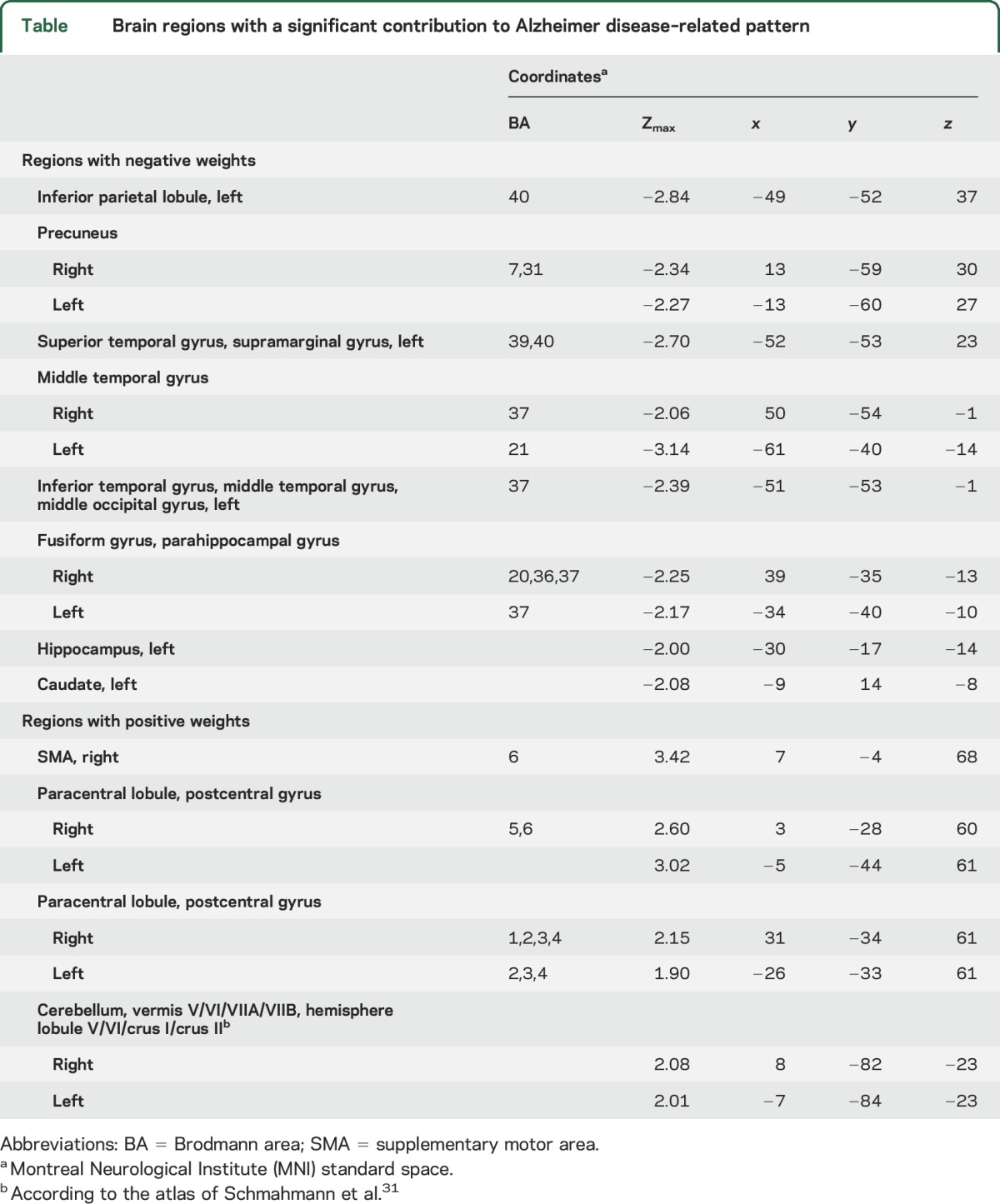
Spatial covariance analysis of metabolic scans from the combined AD1/NL1 derivation sample revealed a distinct ADRP topography characterized by metabolic reductions in the hippocampus, parahippocampal gyrus, and temporal and parietal association regions, along with an increase in activity in the sensorimotor cortex and cerebellum (figure 1A, top and table). The voxel weights on the pattern, reflecting local contributions to overall network activity, were found to be stable by bootstrap estimation (absolute value of the inverse coefficient of variation range = [−2.822 to 2.814], p < 0.005, 1,000 iterations). The ADRP was topographically unrelated to the PDCP (r2 = 0.013, voxel weight correlation, figure 1B, top). It is worth noting that the ADRP exhibited some correlation with the PDRP, although the relationship did not reach significance (r2 = 0.123, voxel-weight correlation); there are shared regional metabolic reductions involving the inferior parietal lobule bilaterally, which are more salient for ADRP than PDRP11 (see e-Methods). ADRP subject scores (figure 1A, bottom), which measure pattern expression in individual cases, were consistently elevated in patients with AD relative to their healthy counterparts (p < 0.0003, permutation test).
Figure 1. Alzheimer disease (AD)–related pattern (ADRP) expression, but not Parkinson disease (PD) cognition-related pattern (PDCP) expression, is increased in AD.
(A) Top, ADRP, identified by spatial covariance analysis of 18F-fluorodeoxyglucose–PET scans of 20 patients with AD (AD1) and 20 normal controls (NL1) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), was characterized by reduced activity in the hippocampus, parahippocampal gyrus, and parietal and temporal association regions, with relative increases in the cerebellum, sensorimotor cortex, and supplementary motor area (represented by the second principal component, which accounted for 12.8% of the subject × voxel variation in the data). Voxel weights on the pattern, reflecting local contributions to overall network activity, were found to be stable by bootstrap estimation (absolute value of the inverse coefficient of variation range = [−2.822 to 2.814], p < 0.005, 1,000 iterations). Bottom, In the derivation sample, which comprised 20 patients with AD (AD1) and 20 normal controls (NL1) selected randomly from the ADNI database (see supplemental data), ADRP expression values (subject scores) accurately discriminated patients from healthy controls (p < 0.0003, permutation test). Prospectively computed ADRP subject scores achieved comparable group separation (p < 0.004, Student t test) in 2 separate testing samples. The first testing set comprised the 20 patients with AD (AD2) and 20 normal ADNI participants (NL2) not used for pattern derivation. The second testing set comprised 10 patients with AD (AD3) and 10 normal controls (NL3) scanned separately at the Feinstein Institute. (B) Top, PDCP was previously identified by spatial covariance analysis of metabolic images from 15 patients with PD with varying levels of cognitive dysfunction.15 PDCP is characterized by reduced activity in the presupplementary motor area, premotor, and prefrontal regions and in parietal associative cortex, with relative increases in the cerebellar vermis and dentate nuclei. Bottom, PDCP expression did not differ (p > 0.14) between patients with AD and controls in the derivation sample or in the ADNI and Feinstein validation samples. (The covariance maps shown on the top in A and B were overlaid on T1-weighted magnetic resonance template images. For each pattern, the display was thresholded at |Z| = 1.64 [p < 0.05]. Voxels with positive region weights [relative increases] are color-coded red; those with negative region weights [relative decreases] are color-coded blue. Error bars shown on the bottom of A and B represent 1 SEM.)
Table.
Brain regions with a significant contribution to Alzheimer disease–related pattern
For validation, we used an automated algorithm to quantify ADRP expression in the ADNI (AD2 and NL2) and FIMR (AD3 and NL3) testing sets on a prospective single-scan basis. As in the derivation sample, ADRP subject scores (figure 1A, bottom) were markedly elevated in patients relative to controls in both AD testing sets (AD2, p < 0.0001; AD3, p < 0.004, Student t tests). In contrast to the ADRP, expression values for the PDCP (figure 1B, bottom) did not differ from normal in the derivation set (p = 0.74) or in either of the testing sets (AD2, p = 0.14; AD3, p = 0.63). There was no discernible correlation between ADRP and PDCP expression values in the AD patient sample (p > 0.34) or between ADRP or PDCP expression and subject age (p > 0.18).
In patients with AD, neuropsychological scores correlated with ADRP but not PDCP expression levels.
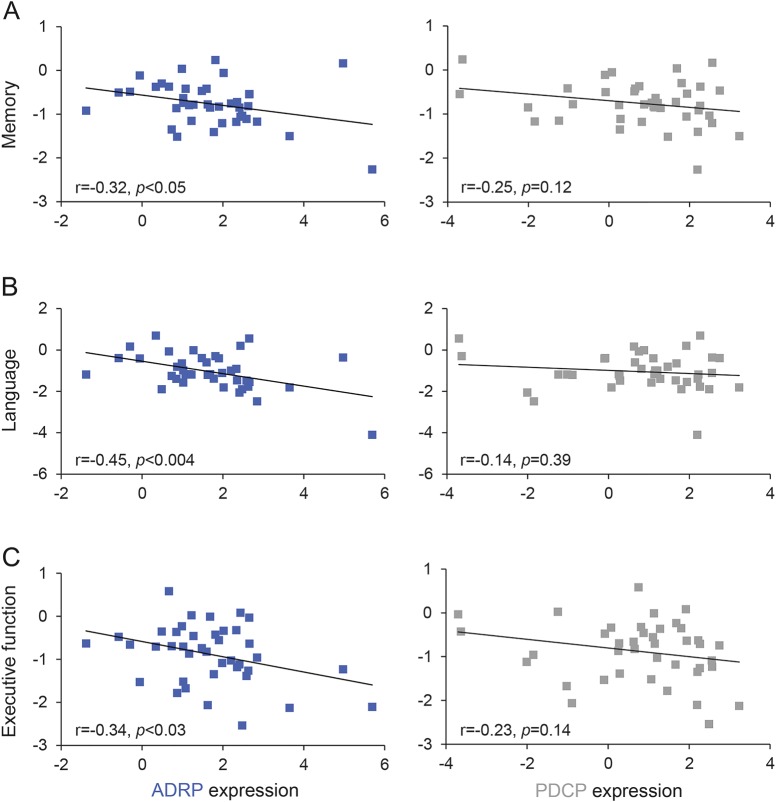
We used the summary score data for memory, language, and executive functioning provided by the ADNI database,27 which included data from a variety of measures, depending on which tests were administered (e-Methods). Summary scores for each of the 3 cognitive domains correlated with ADRP expression in the combined AD cohorts: the worse the summary score, the more elevated the expression of ADRP (memory: r = −0.32, p < 0.05; language: r = −0.45, p < 0.004; executive function: r = −0.34, p < 0.03, Pearson correlations, figure 2, left). There was no such correlation between neuropsychological summary scores and PDCP expression (p > 0.12, figure 2, right).
Figure 2. Alzheimer disease (AD)–related pattern (ADRP) expression, but not Parkinson disease cognition-related pattern (PDCP) expression, correlates with neuropsychological summary scores in AD.
Network correlations with neuropsychological summary scores in the patients with AD from the AD1 and AD2 cohorts (see text). Significant negative correlations (r < −0.32, p < 0.05) were seen between ADRP expression and summary scores for memory (A, left), language (B, left), and executive function (C, left). By contrast, no cognitive correlations (p > 0.12) were observed with PDCP expression values (A–C, right) in the same AD cohort.
Cognitive impairment in patients with PD correlates with expression of PDCP but not ADRP.
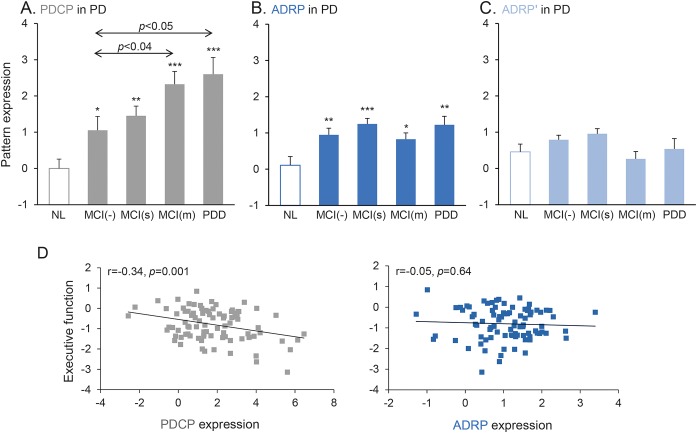
As expected,18 the PD cohort showed PDCP expression (p < 0.0005) that increased as cognitive impairment worsened (F3,85 = 3.42, p < 0.03, 1-way ANOVA, figure 3A). Patients with PD also expressed ADRP (p < 0.05, Student t tests), but there was no association with the degree of cognitive impairment (F3,85 = 1.61, p = 0.19, 1-way ANOVA). Indeed, ADRP levels were similarly elevated (p < 0.02, Student t test) in all patients with PD (figure 3B), including the cognitively intact PD-MCI(−) group, which showed modest elevations in both PDCP and ADRP expression relative to healthy controls (ADRP, p < 0.01; PDCP, p < 0.04, Student t tests); the level of pattern expression in these participants (subject scores ≈1.0) was similar for the 2 topographies (p = 0.76 for comparison of ADRP and PDCP subject scores, paired Student t test). In the cognitively impaired PD-MCI(m) and PDD subgroups, PDCP expression was greater than ADRP [PD-MCI(m), p = 0.001; PDD, p < 0.04, paired Student t tests]. The entire PD data set showed a clear network × subgroup interaction (F3,85 = 3.78, p = 0.01, 2 × 4 RMANOVA) such that stepwise increases in PDCP expression corresponded with more severe cognitive dysfunction but without corresponding differences in ADRP. PDCP and ADRP expression values did not correlate in the PD subgroups with dementia (p = 0.67) or without dementia (p = 0.66).
Figure 3. Parkinson disease (PD) cognition-related pattern (PDCP) expression, but not Alzheimer disease (AD)–related pattern (ADRP) expression, is associated with cognitive impairment in PD.
Mean PDCP (A) and ADRP (B) expression values are displayed for patients with PD with no evidence of mild cognitive impairment [MCI(−); n = 18], single-domain MCI [MCI(s); n = 30], multiple-domain MCI [MCI(m); n = 30], and PD with dementia (PDD; n = 11); values for normal controls (NL; n = 15) are provided for reference (see text). Arrows indicate post hoc Dunnett test relative to MCI(−) group. (C) ADRP' subject scores, reflecting the expression of this pattern after the exclusion of ADRP/PDRP overlap regions (see text), did not differ significantly from normal in any of the PD subgroups, regardless of cognitive status. (D) PDCP expression in this PD cohort (left) correlated significantly (r = −0.34, p = 0.001) with executive function, whereas ADRP did not correlate with summary scores for executive functioning (right) in these patients with PD (*p < 0.05, **p < 0.01, ***p < 0.001, Student t tests compared to normal controls).
If ADRP expression is present in patients with PD but does not correlate with cognitive deficits, what does it represent? Given the aforementioned overlap in the parietal association cortex between ADRP and PDRP topographies, we computed expression values for a modified ADRP topography, called ADRP', which was defined by masking the overlapping regions in each brain before forward application of the native ADRP pattern (figure e-1A). ADRP' expression (figure 3C) did not differ from normal in any of the PD subgroups (p > 0.05, Student t tests) but remained elevated in each of the AD patient samples (p < 0.01, figure e-1B). ADRP expression in patients with PD thus largely reflects changes in PDRP due to PD rather than an AD-associated disease process.
PDCP, but not ADRP, expression levels correlate with neuropsychological measures in patients with PD.
We chose the cognitive measures for our PD cohorts to match the ADNI composite measures as closely as possible, and we used a similar approach to compute the analogous summary scores (e-Methods). Consistent with MCI classification, neuropsychological summary scores (table e-2) declined with worsening cognitive performance in all 3 domains across the PD patient subgroups. Summary scores for memory differed across the PD subgroups (F3,81 = 13.7, p < 0.0001, 1-way ANOVA), with worse performance in the PD-MCI and PDD subgroups [p < 0.001 for each subgroup relative to PD-MCI(−); post hoc Dunnett tests]. Executive function (F3,83 = 5.4, p < 0.002) and language (F3,82 = 5.3, p < 0.003) showed similar trends. PD-MCI(m) and patients with PDD exhibited clear deficits in executive performance compared with PD-MCI(−) (p < 0.01); language deficits were more pronounced in the PDD group [p < 0.005 relative to PD-MCI(−), post hoc Dunnett test].
Higher PDCP expression values reflected worse summary scores for executive performance (r = −0.34, p = 0.001, figure 3D, left). There was a weak correlation between PDCP expression and memory impairment (r = −0.22, p < 0.04) but not with language deficits (r = −0.09, p = 0.42, data not shown). ADRP values, in contrast, did not correlate with cognitive performance in any of the 3 domains (p > 0.56, figure 3D, right). Thus, in patients with AD, individual differences in memory, language, and executive function were reflected in ADRP but not PDCP expression. In patients with PD, in contrast, executive function had a clear relationship with PDCP expression but not with ADRP expression.
PDCP, but not ADRP, expression increases over time in patients with PD.
In the longitudinal cohort of early patients with PD (figure 4), a significant network × time interaction effect (F2,23 = 4.34, p < 0.03, 2-way RMANOVA) revealed different progression rates for the 2 networks over the course of disease. PDCP expression in this early PD cohort increased with time (F2,23 = 7.16, p < 0.004, 1-way RMANOVA), and subject scores for this pattern measured at 4 years were higher than at baseline (p < 0.003, post hoc Dunnett test). Expression values for concurrently measured ADRP, however, did not change over the 4 years of follow-up (ADRP: F2,23 = 2.21, p = 0.13, 1-way RMANOVA), nor did it correlate with age (p > 0.31). Moreover, although ADRP expression in these patients was elevated relative to control values (p < 0.02, Student t test) at all 3 time points, the expression of ADRP' did not reach significance at any time point (p > 0.05, Student t test).
Figure 4. Parkinson disease (PD) cognition-related pattern (PDCP) expression, but not Alzheimer disease–related pattern (ADRP) expression, increases in PD over time.
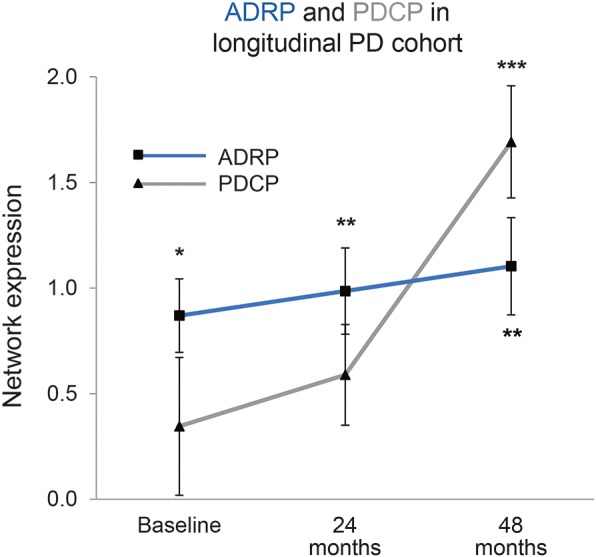
Mean ADRP (squares) and PDCP (triangles) expression in a longitudinal early-stage PD cohort (see text). PDCP expression values (gray line) increased over time in this group (p < 0.004, repeated-measures analysis of variance [RMANOVA]), while concurrent ADRP changes (blue line) did not reach significance (p = 0.13, RMANOVA). ADRP expression in these patients was elevated relative to control values (p < 0.02, Student t test) at all time points; PDCP expression, in contrast, reached abnormal levels (p < 0.001) only at the final time point. (Error bars represent 1 SE at each time point. *p < 0.05, **p < 0.01, ***p < 0.001, Student t tests compared to normal controls.)
DISCUSSION
In this study, we used the distinct network topographies of AD and PD to investigate whether the cognitive deficits that commonly occur in patients with PD are more likely attributable to PD itself or to comorbidity with AD. Our analyses indicate that, despite overlapping regions of pathology, cognitive dysfunction in PD is largely distinct from that which occurs in AD.
This is not to say, however, that some patients with PD may not also have AD. Although most of the 11 patients with PD with the most severe disease (PDD) had ADRP expression levels tightly clustered around the mean, 2 participants showed relatively high ADRP expression that remained elevated even after masking regions of ADRP/PDRP overlap in the parietal association cortex (ADRP: 2.63 and 2.00, subject score = 0.98 ± 0.76 for the remaining 9 PDD cases; ADRP': 1.40 and 1.85, subject score = 0.29 ± 0.86 for the remaining 9 cases). Even more strikingly, the patient with PDD with the highest ADRP' expression was also exceptional in expressing almost normal PDCP levels; this patient most likely has true comorbid AD. It is worth noting that our metabolic network findings complement structural imaging data showing AD-like volumetric changes in the cerebral cortex of patients with PD.28 The pattern of volume loss seen with structural MRI in patients with AD29 exhibits some topographic homology with ADRP but not with PDCP; it is possible that Lewy- and Alzheimer-type pathologies affect cognition differently in individuals with PD.6
From a clinical standpoint, it is important for physicians and patients to recognize that cognitive dysfunction in PD is likely not caused by comorbid AD. Furthermore, the disease specificity of ADRP and PDCP provides a distinct contrast with resting-state networks found in healthy controls such as the default mode network that show progressive attrition in both disease populations.30 To fully articulate the relationship of the observed imaging changes with the underlying disease process will require multimodal imaging in conjunction with molecular genetic analyses and thorough postmortem assessments. In the meanwhile, having objective imaging biomarkers to differentiate between underlying pathologies will help with prognosis, selection of participants for clinical trials, and choice of treatments when they become available.
Supplementary Material
ACKNOWLEDGMENT
The authors wish to thank Ms. Ivana De Lucia for her valuable technical assistance and Ms. Yoon Young Choi and Ms. Toni Fitzpatrick for manuscript preparation/copyediting.
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- ADRP
Alzheimer disease–related pattern
- ANOVA
analysis of variance
- FDG
18F-fluorodeoxyglucose
- FIMR
Feinstein Institute for Medical Research
- MCI
mild cognitive impairment
- MCI(−)
cognitively intact
- MCI(m)
mild cognitive impairment involving multiple cognitive domains
- MCI(s)
mild cognitive impairment involving a single cognitive domain
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- PDCP
Parkinson disease cognition-related pattern
- PDD
Parkinson disease dementia
- PDRP
Parkinson disease motor-related pattern
- RMANOVA
repeated-measures analysis of variance
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Mattis: study design, data acquisition, statistical analysis, data analysis and interpretation, and drafting and revising of the manuscript for intellectual content. Dr. Niethammer: data interpretation and revising of the manuscript for intellectual content. Dr. Sako: data analysis and drafting of the manuscript for intellectual content. Dr. Tang: statistical analysis, data analysis and interpretation, and drafting and revising of the manuscript for intellectual content. Dr. Nazem: data analysis and drafting of the manuscript for intellectual content. Dr. Gordon: data acquisition and revising of the manuscript for intellectual content. V. Brandt: revising of the manuscript for intellectual content. Dr. Dhawan: data acquisition, data interpretation, study supervision, and revising of the manuscript for intellectual content. Dr. Eidelberg: study design, statistical analysis, data interpretation, study supervision, obtaining funding, and drafting and revising of the manuscript for intellectual content.
STUDY FUNDING
This work was supported in part by the National Institute of Neurologic Disorders and Stroke Morris K. Udall Center of Excellence for Parkinson’s Disease Research at The Feinstein Institute for Medical Research (P50 NS071675 to D.E.) and the Thomas Hartman Foundation for Parkinson’s Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurologic Disorders and Stroke or the NIH. The sponsor did not play a role in study design, collection, analysis or interpretation of data, writing the report, or the decision to submit the paper for publication.
DISCLOSURE
P. Mattis, M. Niethammer, W. Sako, C. Tang, A. Nazem, V. Brandt, and V. Dhawan report no disclosures relevant to the manuscript. M. Gordon received research support, without direct compensation, from Merck, Lundbeck, Genentech, Accera, Baxter, Forest, and Eli Lilly; he has also participated in an advisory board for Lundbeck. D. Eidelberg serves on the scientific advisory board and has received honoraria from The Michael J. Fox Foundation for Parkinson's Research; is listed as coinventor of patents for markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same, without financial gain; and has received research support from the NIH (National Institute of Neurologic Disorders and Stroke, National Institute on Deafness and Other Communication Disorders, National Institute of Allergy and Infectious Diseases) and the Dana Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 2010;289:18–22. [DOI] [PubMed] [Google Scholar]
- 2.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord 2006;21:1343–1349. [DOI] [PubMed] [Google Scholar]
- 3.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 2012;72:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson's disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 2013;14:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J Neural Transm 2002;109:329–339. [DOI] [PubMed] [Google Scholar]
- 6.Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain 2011;134:1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habeck C, Foster N, Perneczky R, et al. Multivariate and univariate neuroimaging biomarkers of Alzheimer's disease. Neuroimage 2008;40:1503–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarmeas N, Habeck CG, Zarahn E, et al. Covariance PET patterns in early Alzheimer's disease and subjects with cognitive impairment but no dementia: utility in group discrimination and correlations with functional performance. Neuroimage 2004;23:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teune LK, Strijkert F, Renken RJ, et al. The Alzheimer's disease-related glucose metabolic brain pattern. Curr Alzheimer Res 2014;11:725–732. [DOI] [PubMed] [Google Scholar]
- 10.Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci 2009;32:548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niethammer M, Eidelberg D. Metabolic brain networks in translational neurology: concepts and applications. Ann Neurol 2012;72:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feigin A, Leenders KL, Moeller JR, et al. Metabolic network abnormalities in early Huntington's disease: an [(18)F]FDG PET study. J Nucl Med 2001;42:1591–1595. [PubMed] [Google Scholar]
- 13.Tang CC, Feigin A, Ma Y, et al. Metabolic network as a progression biomarker of premanifest Huntington's disease. J Clin Invest 2013;123:4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mure H, Hirano S, Tang CC, et al. Parkinson's disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 2011;54:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage 2007;34:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattis PJ, Tang CC, Ma Y, Dhawan V, Eidelberg D. Network correlates of the cognitive response to levodopa in Parkinson disease. Neurology 2011;77:858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meles SK, Tang CC, Teune LK, et al. Abnormal metabolic pattern associated with cognitive impairment in Parkinson's disease: a validation study. J Cereb Blood Flow Metab 2015;35:1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology 2008;70:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spetsieris P, Ma Y, Peng S, et al. Identification of disease-related spatial covariance patterns using neuroimaging data. J Vis Exp 2013;76:e50319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. Neuroimage 2011;54:2899–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 22.Hughes A, Daniel S, Ben-Shlomo Y, Lees A. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125:861–870. [DOI] [PubMed] [Google Scholar]
- 23.Mattis S. Dementia Rating Scale: Professional Manual. Odessa: Psychological Assessment Resources; 1988. [Google Scholar]
- 24.Tang C, Poston K, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J Neurosci 2010;30:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtbernd F, Ma Y, Peng S, et al. Dopaminergic correlates of metabolic network activity in Parkinson's disease. Hum Brain Mapp 2015;36:3575–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niethammer M, Tang CC, Ma Y, et al. Parkinson's disease cognitive network correlates with caudate dopamine. Neuroimage 2013;78:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 2012;6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weintraub D, Dietz N, Duda JE, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain 2012;135:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage 2001;14:1361–1369. [DOI] [PubMed] [Google Scholar]
- 30.Spetsieris PG, Ko JH, Tang CC, et al. Metabolic resting-state brain networks in health and disease. Proc Natl Acad Sci U S A 2015;112:2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI Atlas of the Human Cerebellum. San Diego: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.