Abstract
An investigation into the use of Lewis base catalysis for the enantioselective chlorolactonization of 1,2-disubstituted alkenoic acids is described. Two mechanistically distinct reaction pathways for catalytic chlorolactonization have been identified. Mechanistic investigation revealed that tertiary amines predominately operate as Brønsted rather than Lewis bases. Two potential modes of activation have been identified that involve donation of electron density of the carboxylate to the C=C bond as well hydrogen bonding to the chlorinating agent. Sulfur- and selenium-based additives operate under Lewis base catalysis; however, due to the instability of the intermediate benzylic chloriranium ion, chlorolactonization suffers from low chemo-, diastereo-, and enantioselectivities. Independent generation of the benzylic chloriranium ion shows that it is in equilibrium with an open cation, which leads to low specificities in the nucleophilic capture of the intermediate.
Introduction
The lactone function is an important structural motif that is found in a variety of natural products and pharmaceutical agents.1 It is also frequently used as a versatile building block en route to other oxygen-containing heterocycles and carboxylic acid derivatives.2 Among the many methods of forming common-ring lactones,3 halogen-initiated cyclization of unsaturated carboxylic acids remains an attractive and direct approach to the synthesis of functionalized lactones, providing a heterocycle with up to two stereogenic centers and a useful functional group.4
Among the common halogens, halolactonization initiated by chlorine (or other electrophilic chlorine equivalents) is much less common, particularly for enantioselective variants, than the corresponding transformations with bromine and iodine.5 This deficiency is particularly striking when one considers the greater abundance of chlorine6 and the large number of chlorine-containing natural products.7 Several important reasons for this discrepancy exist; the low stability of the intermediate chloriranium ions often leads to lower yielding reactions, and chlorides are less useful for further manipulation than are bromides or iodides, for example.8 Of these, the latter is balanced by the need to prepare chlorinated products. Recent interest in the synthesis of chlorinated natural products has further highlighted the need for additional methods for the stereoselective installation of chlorine into complex molecules.9 The results reported herein are an account of our work in this area to use Lewis base catalysis10 as a means to catalyze and control the enantioselectivity of chlorolactonization of 1,2-disubstituted alkenoic acids.
Background
Current Methods for Enantioselective Chlorolactonization
The first halolactonization reactions were reported by Frost in the Fittig School in Strassbourg in 1884,11 followed by more detailed studies by Stobbe around the turn of the 20th century.12 Since then, it has been widely used in organic synthesis. However, for quite some time, the only way to obtain enantiopure lactones was by starting from chiral alkenoic acids containing a stereogenic element.13 Reagent-controlled, enantioselective halocyclizations started to appear only relatively recently. In 1992, Taguchi and co-workers reported the first enantioselective halolactonization, promoted by stoichiometric amounts of chiral titanium complex, affording enantioenriched iodo lactones in 65% ee.14 A number of other methods employing stoichiometric reagents such as chiral amines,15 pyridines,16 and dimeric iodonium salts17 have been reported.
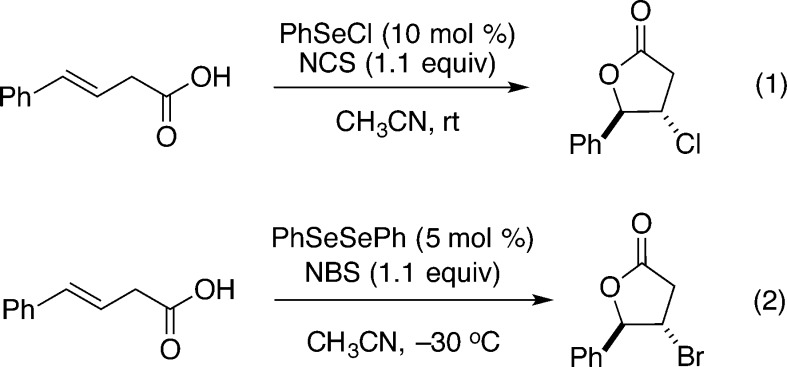
Remarkably, methods for catalytic halolactonization were absent until 2004 when Tunge and co-workers reported chlorolactonization catalyzed by phenylselenyl chloride using N-chlorosuccinimide as a chlorine source; in the same report, catalytic bromolactonization under similar conditions is disclosed (Scheme 1).18
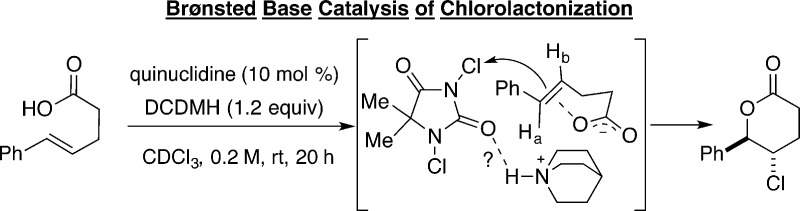
Scheme 1.
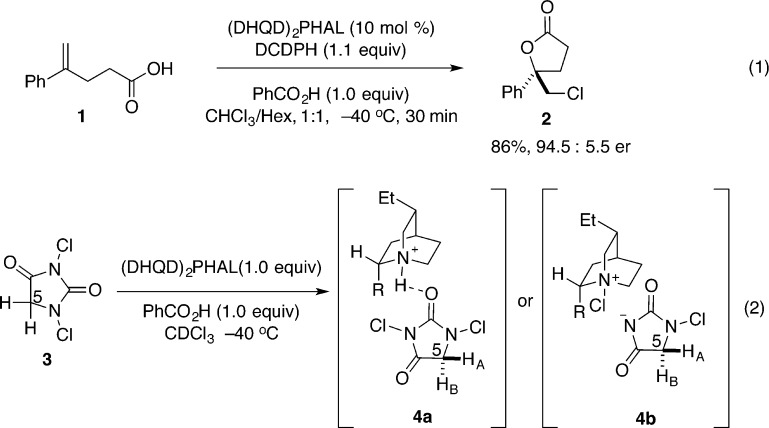
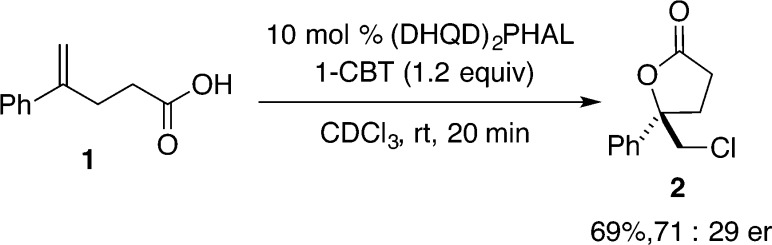
The first catalytic, highly enantioselective chlorolactonization was disclosed by Borhan and co-workers in 2010 using a dimeric cinchona alkaloid based catalyst (Scheme 2, eq 1).19 Chlorolactonization of 1,1-disubstituted alkene 1 with 1,3-dichlorodiphenylhydantoin (DCDPH) as a source of chlorine could be successfully catalyzed by (DHQD)2PHAL in the presence of a stoichiometric amount of benzoic acid. Butyrolactone 2 was obtained in synthetically useful yield and enantioselectivity; lower selectivity was observed when electron-rich styrenes and 1,1-disubstituted aliphatic alkenes were used as substrates. A series of experiments were conducted to shed light on the origin of enantioselectivity of this transformation. In a low temperature experiment using stoichiometric amounts of reactants, close association of chlorinating agent 3 with (DHQD)2PHAL was observed by 1H NMR spectroscopy (Scheme 2, eq 2). On the basis of the observed splitting of the HC(5) signal, two different complexes were proposed: 4a invoking a hydrogen bond association between the protonated catalyst and the chlorenium source and complex 4b involving a tight ion pair between a chlorinated quinuclidine and the conjugate base of monochlorohydantoin. Given the need for a stoichiometric amount of benzoic acid, the authors conclude that 4a is a relevant intermediate but recognize that they cannot unambiguously exclude the involvement of 4b since the reaction also proceeds in the absence of benzoic acid, albeit with lower selectivity. Further studies using a series of modified, N-chlorinated hydantoins (both chiral and achiral) supported the hypothesis of participation of complex between the chlorenium source and (DHQD)2PHAL in the enantiodetermining step.20
Scheme 2.
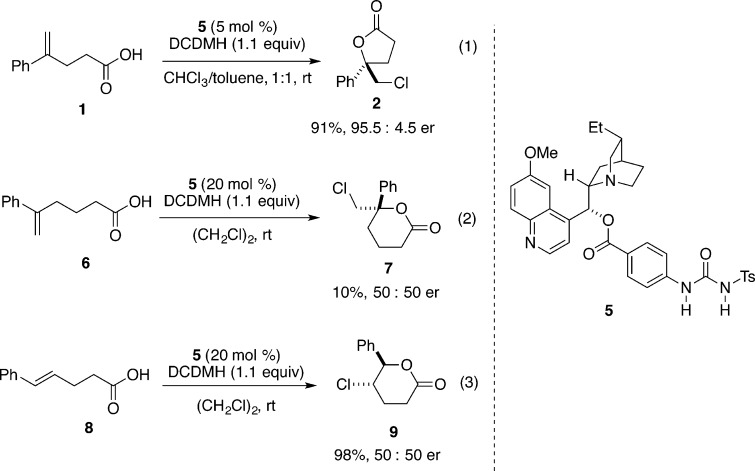
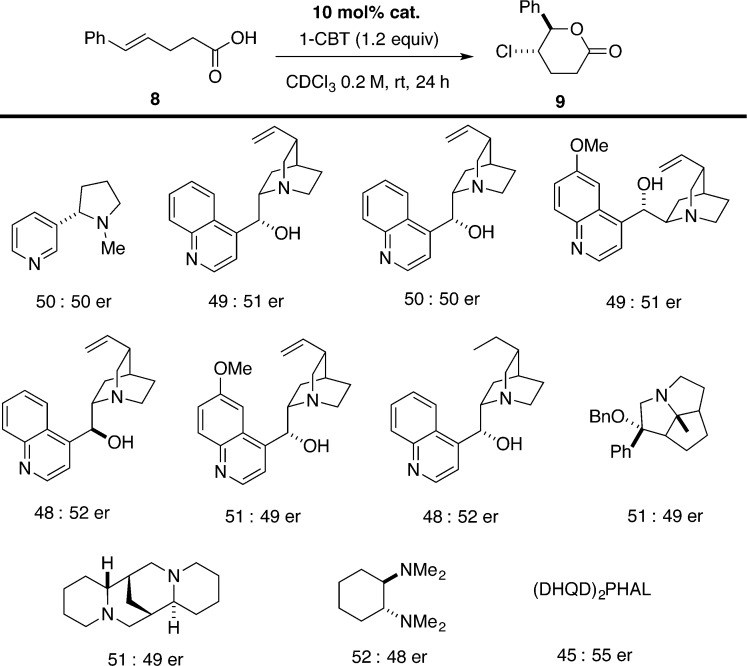
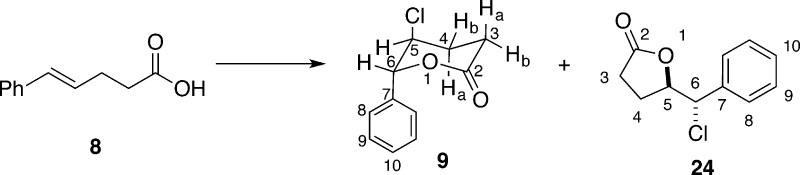
Enantioselective chlorolactonization of 1,1-disubstituted styrenyl carboxylic acids has also been reported by Tang and co-workers who used a cinchona-derived urea catalyst 5.21 Substrate 1 undergoes highly selective chlorolactonization at room temperature with only 5 mol % catalyst loading using 1,3-dichlorodimethylhydantoin (DCDMH) as a chlorenium source (Scheme 3, eq 1). The authors propose an activation of halogenating reagent by hydrogen bonding with the catalyst. Unfortunately, this catalyst promotes the chlorolactonization on only a very limited number of substrates, mainly 4-arylpent-4-enoic acids. Extension of the substrate scope to homologue 6 was not successful; lactone 7 is obtained as a racemic mixture in low yield (Scheme 3, eq 2). Finally, isomeric 1,2-disubstituted styrenyl carboxylic acid 8 was fully converted into corresponding lactone 9; however, the product was also racemic (Scheme 3, eq 3).
Scheme 3.
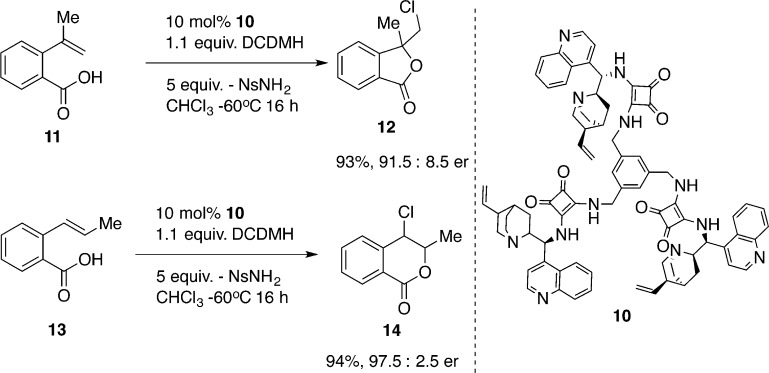
The most recent report of enantioselective chlorolactonization by Zhou and co-workers involves vinylbenzoic acids using a C3-symmetric, cinchonine-squaramide catalyst 10 (Scheme 4).22 With catalyst 10 in the presence of an excess of 4-nitrobenzenesulfonamide and DCDMH, α-methylstyrene derivative 11 is converted to γ-lactone 12 in good yield and enantioselectivity. Although the role of the additive is not clear, the reaction presumably is operating through hydrogen bond activation of halogenating agent. Under identical reaction conditions, isomer 13 affords isochromanone 14 in highly enantiopure form.23 To our knowledge, this example represents the only case of enantioselective chlorolactonization of 1,2-disubstituted alkenoic acids.
Scheme 4.
A recent mechanistic investigation by Borhan and co-workers performed on deuterium-labeled substrates established that chlorolactonization of 1,1-disubstituted alkenoic acids occurs predominantly as a syn addition of chlorenium and the nucleophile across the double bond, which precludes the formation of chloriranium ion as an intermediate.24 It was concluded that the reaction operates via an open tertiary carbocation intermediate which then undergoes ring closing under the stereocontrol of the catalyst. This mechanism of operation explains the lack of selectivity for 1,2-disubstituted olefinic acids in the chlorolactonization with cinchona-based catalysts; these substrates should form less stable secondary carbocations and would be more prone to be captured as chloriranium ions or tight ion pairs in an anti addition process.
Objectives
The primary objective of this study was to develop a catalytic, enantioselective chlorolactonization of 1,2-disubstituted styrenyl carboxylic acids. Halolactonizations of this class of substrates are particularly attractive since the resulting lactones contain two consecutive stereogenic centers. Enantioselective halolactonization of 1,2-disubstituted alkenoic acids remains rare.25
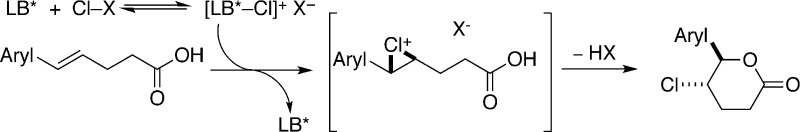
As a part of our ongoing program on enantioselective Lewis base catalyzed difunctionalization of alkenes,26 we sought to identify a chiral Lewis base that could induce the formation of an enantioenriched chloriranium ion which could be further captured by a carboxylic acid to afford an anti-configured lactone (Scheme 5). A previous study from these laboratories demonstrated that chloriranium ions are configurationally stable and do not undergo racemization by olefin-to-olefin transfer.27 Thus, chlorofunctionalization reactions may be more amenable to enantioselective catalysis than, e.g., bromo-26a−26c or selenofunctionalization.26d On the basis of this hypothesis, the following goals were set for this investigation: (1) identify a chlorinating agent that would have a slow background (uncatalyzed) reaction, (2) identify a Lewis base that would activate it and promote chlorolactonization, and (3) evaluate chiral, nonracemic Lewis basic catalysts that afford high enantioselectivities with a range of alkenes.
Scheme 5.
Results and Discussion
Survey of Achiral Lewis Bases
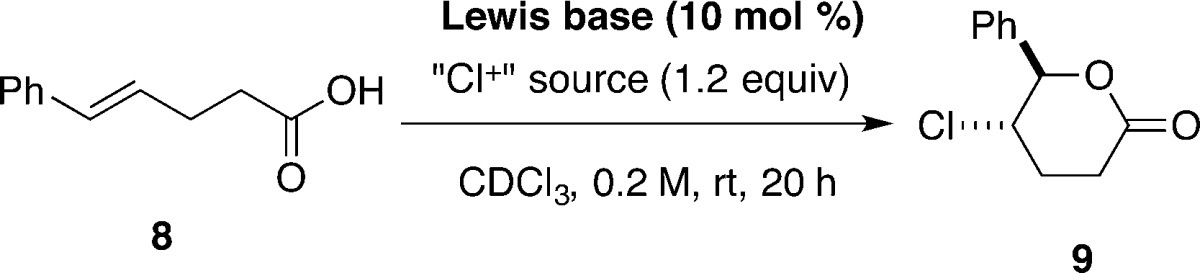
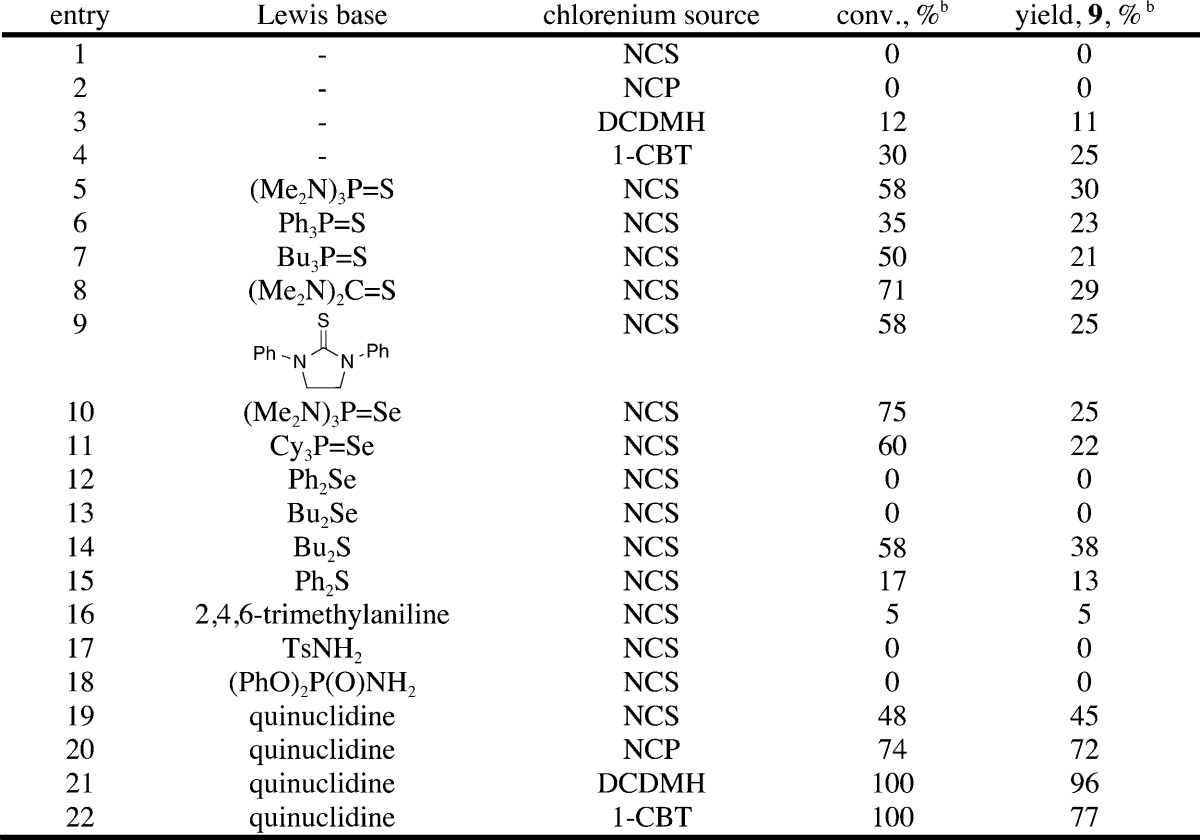
An initial survey of chlorolactonization conditions using unsaturated acid 8 as a representative substrate was carried out in deuterated chloroform and monitored by 1H NMR spectroscopy. The survey of suitable chlorinating agents is summarized in Table 1. No background reaction was observed with N-chlorosuccinimide (NCS) and N-chlorophthalimide (NCP), whereas DCDMH gave a very slow reaction; 11% of lactone 9 was formed after 20 h (entries 1–3). The most reactive reagent, 1-chlorobenzotriazole (1-CBT), gave a higher rate of uncatalyzed reaction, resulting in a 25% yield of 9 after 20 h (entry 4).
Table 1. Optimization of Chlorolactonization Conditionsa.
Reactions were performed at rt by the addition of 0.2 mmol of 8, 0.24 mmol of chlorinating agent, and 1.0 mL of CDCl3 in a 5 mm NMR tube, followed by the addition of 0.02 mmol of catalyst.
Determined by integration of 1H NMR signals against tetramethylsilane internal standard.
The next stage of the optimization involved the evaluation of various Lewis bases to activate NCS for chlorolactonization. Recent work on the activation of NCS with phosphine sulfides for electrophilic halogenation of arenes suggested the use of various sulfur-containing Lewis bases.28 Hexamethylthiophosphoramide, triphenyl- and tributylphosphine sulfide, and thiourea derivatives showed moderate activity as catalysts; however, they were more active in catalyzing the unproductive consumption of 8 than in catalyzing the formation of 9 (entries 5–9). Selenium-based donors such as hexamethylselenophosphoramide and tricyclohexylphosphine selenide were also inefficient catalysts for chlorolactonization (entries 10 and 11). Selenoethers were unreactive with NCS (entries 12 and 13), and thioethers gave complex mixtures of products without full consumption of the starting material (entries 14 and 15).
A recent report from Yamamoto describes the aniline-promoted, catalytic chlorination of electron-rich aromatic compounds and stimulated a survey of nitrogen-containing catalysts.29 2,4,6-Trimethylaniline, tosylamide, and diphenyl phosphoramide were tested; however, they were completely unreactive with NCS (entries 16–18). Remarkably, however, quinuclidine catalyzed the chlorolactonization; even though full conversion was not achieved, the reaction was clean and did not show any observable side products (entry 19). Other chlorinating agents were tested with quinuclidine, and to our delight, DCDMH resulted in a very fast and clean reaction. 1-Chlorobenzotriazole also gave good conversions, but side products were observed (entries 20–22). Notably, Yamamoto reports that arylamines generate an N-chloroarylamine intermediate that acts as a highly reactive but selective electrophilic halogen source. Moreover, N-chloroammonium salts (including quinuclidinium salts) have been used for selective chlorination of electron-rich aromatic compounds.30 Thus, it appeared plausible that under the optimized reaction conditions N-chloroquinuclidine is formed catalytically.
Synthesis of Chiral Quinuclidines
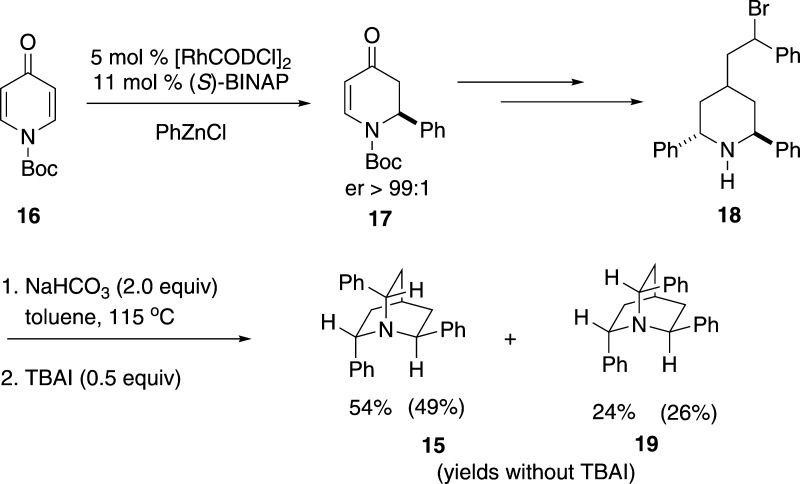
The third stage of the method development involved the synthesis of chiral, nonracemic quinuclidines for enantioselective chlorolactonization. The first structure targeted was the C3-symmetric triphenyl quinuclidine 15 reported several years ago by Corey and co-workers.31 Quinuclidine 15 was synthesized from N-(tert-butoxycarbonyl)-4-pyridone 16 according to the route developed by Corey (Scheme 6).
Scheme 6.
The last step in the reaction sequence is the cyclization of diastereomeric mixture of bromides 17. In this two-step protocol, 17 was exposed to sodium bicarbonate in refluxing toluene for 12 h followed by addition of 0.5 equiv of tetrabutylammonium iodide (TBAI) and further heating at 115 °C for 6 h. Presumably, the iodide displaces the bromide ion from the less reactive diastereomer of 17 to produce an intermediate benzyl iodide of the correct configuration that undergoes ring closure to the desired quinuclidine 15. According to the original report, benzyl bromides 17 gave exclusively 15 in 76% yield; however, in our hands, a minor product of the isomeric quinuclidine 19 was obtained (Scheme 6). The outcome of the reaction did not change with or without the added TBAI. Fortunately, the diastereomeric quinuclidines were separable by silica gel column chromatography, thus providing the opportunity to explore the use of 19 for enantioselective chlorofunctionalization reactions as well. In previous work, Corey also described a synthesis and resolution of diphenyl quinuclidine 20, which was also prepared and was tested for enantioselective chlorolactonization.32
Synthesis of N-Chloroquinuclidines
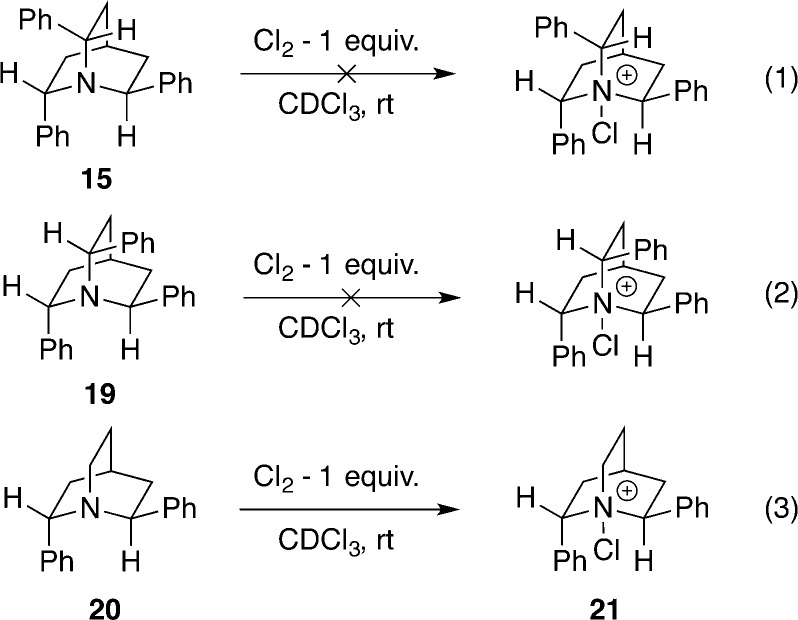
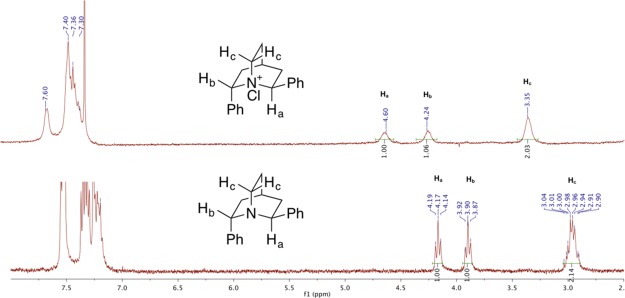
Attempts to prepare N-chloroquinuclidinium salts from 15 and 19 failed with chlorinating agents such as iodosobenzene dichloride, NCS, and DCDMH. The targeted species were not formed even when molecular chlorine was used! Quinuclidines 15 and 19 were resistant to the action of 1 equiv of chlorine at 25 °C; however, upon exposure to an excess of chlorine, the quinuclidines decomposed (Scheme 7). The low reactivity of 15 and 19 can be attributed to the inductive electron-withdrawing effect and the steric congestion caused by the phenyl groups around nitrogen atom. Fortunately, diphenyl quinuclidine 20 did react with chlorine to form a new species, 21. All three proton signals adjacent to the nitrogen center were broadened and shifted downfield (Figure 1).
Scheme 7.
Figure 1.
1H NMR spectrum of 22.
Survey of Enantiomerically Enriched Lewis Bases
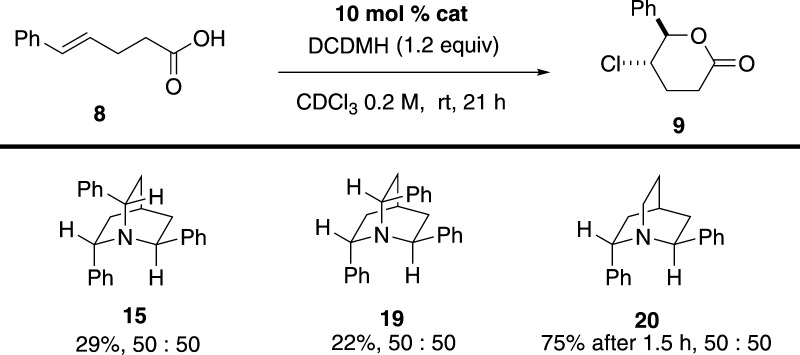
Chiral quinuclidines 15 and 19 were tested in the reaction of interest with DCDMH as a chlorinating agent (Scheme 8). Not surprisingly, both amines only slightly accelerated the rate of the reaction, providing lactone 9 in 50:50 er. The inability of triaryl-substituted quinuclidines to promote chlorolactonization can clearly be attributed to the decreased nucleophilicity of the nitrogen atom, thus inhibiting formation of the chlorinating reagent. In support of this hypothesis, the more electron-rich quinuclidine 20 efficiently promoted chlorolactonization of 8 to afford 9 in 75% yield in 90 min; however, the resulting product was still racemic. Other chlorinating agents such as NCS or NCP did not improve the enantioselectivity.
Scheme 8.
A survey of other chiral tertiary amines was carried out using a stronger chlorinating agent 1-CBT (Scheme 9). Disappointingly, the best catalyst, (DHQD)2PHAL, provided 9 in only 45:55 er. Previously, (DHQD)2PHAL had been shown to catalyze the chlorolactonization of 1 with high enantioselectivity using DCDPH as a chlorine source.19
Scheme 9.
To demonstrate that enantioselective cyclizations are possible using 1-CBT as a chlorine source, alkene 1 was subjected to chlorination with (DHQD)2PHAL as the catalyst, which afforded 2 in moderate yield and enantioselectiveity (Scheme 10). Thus, although this combination of reagents is less selective than the optimized procedure developed by Borhan, the failure to effect enantioselective cyclization of 8 clearly shows the extreme substrate dependence of this process.
Scheme 10.
Mechanistic Experiments
Lewis vs Brønsted Base Catalysis
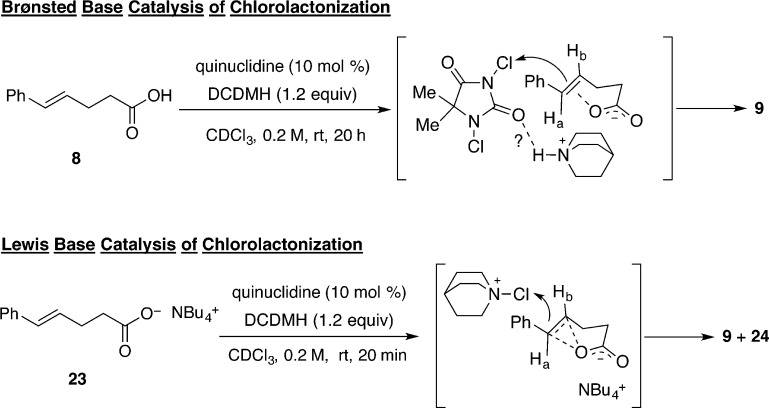
The working hypothesis for this reaction proposes that the chlorinating agent transfers a chlorenium ion to the nitrogen atom of a chiral quinuclidine. The generated N-chloroquinuclidinium salt reacts with an alkene to generate a chloriranium ion, which is then opened with the carboxylate (formed by deprotonation by the conjugate base of the chlorinating agent). The only observed product was the trans-configured lactone, thus supporting the hypothesis of anti-addition via chloriranium ion formation.
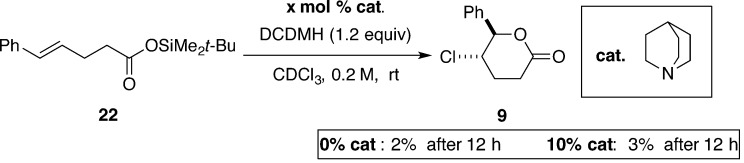
To further probe the validity of this hypothesis, additional experiments were performed to test some of the assumptions implicit in the proposed mechanism. To test the involvement of the carboxylate ion, substrate 22 (the TBDMS ester of 8) was prepared and subjected to the reaction conditions with and without quinuclidine as the catalyst. Compound 22 was unreactive under both conditions, suggesting that formation of the chloriranium ion, if involved, requires the free carboxylate (Scheme 11).
Scheme 11.
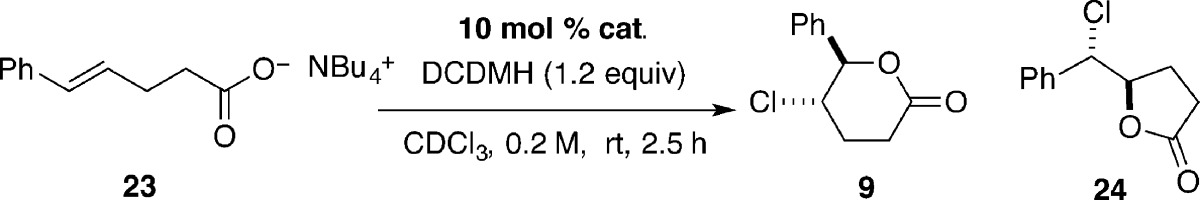
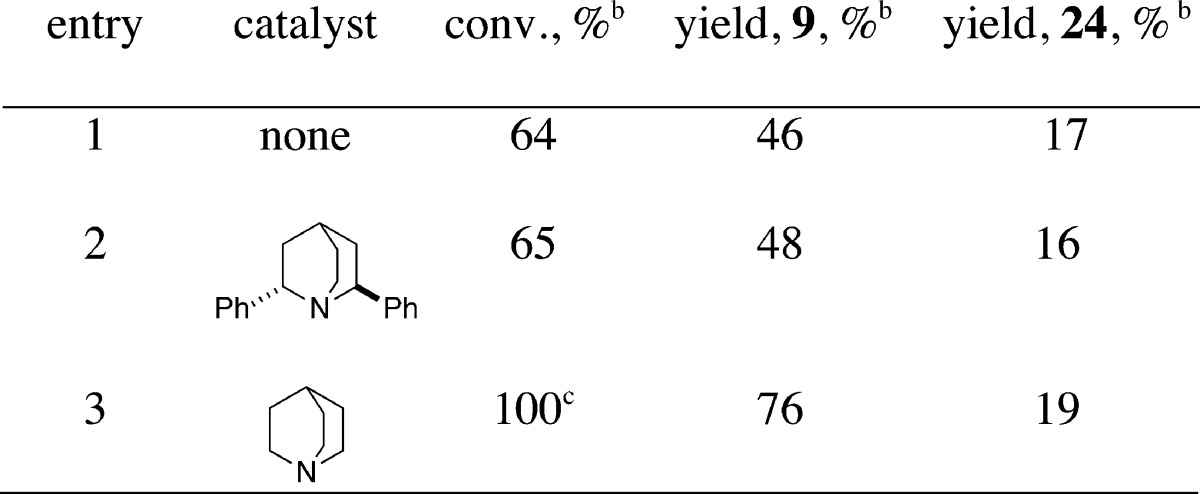
To test the involvement of a carboxylate salt in the chlorolactonization, substrate 23, the tetrabutylammonium salt of 8 was prepared and tested for its competence under different reaction conditions. Remarkably, in the presence of DCDMH alone (i.e., no catalyst), 23 reacted with a significant background rate to afford a mixture of δ- and γ-lactone products, 9 and 24, respectively (Table 2, entry 1). Addition of 10 mol % of 20 to the reaction mixture had no effect (Table 2, entry 2). During the course of the reaction, N-chloroquinuclidinium salt, 21, was not observed by 1H NMR analysis; in fact, the chemical shifts of the catalyst 20 did not change. However, with 10 mol % of quinuclidine itself, cyclization was complete in 20 min (Table 2, entry 3).
Table 2. Chlorolactonization of Tetrabutylammonium Carboxylate 23.a.
Reactions were performed at rt by the addition of 0.2 mmol of 23, 0.02 mmol of catalyst, and 1.0 mL of CDCl3 into a 5 mm NMR tube, followed by the addition of 0.24 mmol of DCDMH.
Determined by integration of 1H NMR signals against tetramethylsilane internal standard.
After 20 min.
The striking difference in reactivity between 22 and 23 implies a critical role for the carboxylate ion in the reaction mechanism. Several interpretations can be considered, including activation of the chlorinating reagent or activation of the substrate. Activation of the chlorinating agent could involve the carboxylate anion acting as a Lewis base, ultimately resulting in the formation of an acyl hypochlorite by chlorenium ion transfer to the carboxylate oxygen. From there, either intra- or intramolecular chlorenium ion transfer to the alkene could produce a chloriranium ion that suffers opening by the carboxylate.
Activation of the substrate could involve electronic polarization of the alkene by the pendant carboxylate to enhance nucleophilicity. Although not widely known, the rate of electrophilic addition reactions of alkenes is dependent on the nucleophile (AdE2 reactions).33 In the late 1960s, Shilov demonstrated that the rate of iodoetherification of allylphenols is dependent on the electron density at the oxygen (through ring substituents).34 Indeed, the entire spectrum of Ad type reactions from AdE2 to AdN2 (electrophile initiated, nucleophile initiated, and of course, synchronous in between) is treated in many advanced textbooks on physical organic chemistry.35,36
Spectroscopic Evidence for Activation of Alkene vs Activation of DCDMH
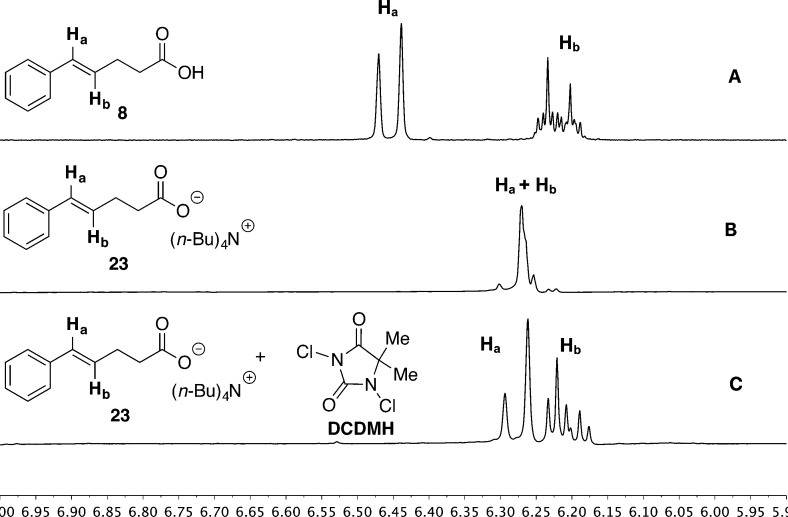
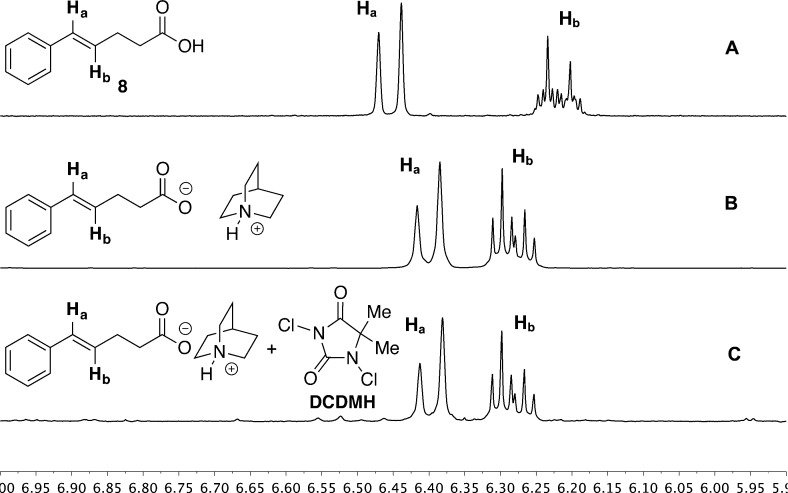
Insight into these possibilities was provided by inspection of the 1H NMR spectra of 23 as well as of reaction mixtures involving 23. The chemical shifts of the olefinic protons in free acid 8 appear at 6.455 ppm for Ha and 6.217 ppm for Hb (Figure 2A). However, carboxylate salt 23 shows a significant change such that these two protons appear as a merged multiplet centered at 6.270 ppm (Figure 2B). Clearly, Ha was shifted upfield, while Hb was shifted downfield.
Figure 2.
1H NMR spectra of the olefinic region: (A) 8, (B) 23; (C) 23 + DCDMH.
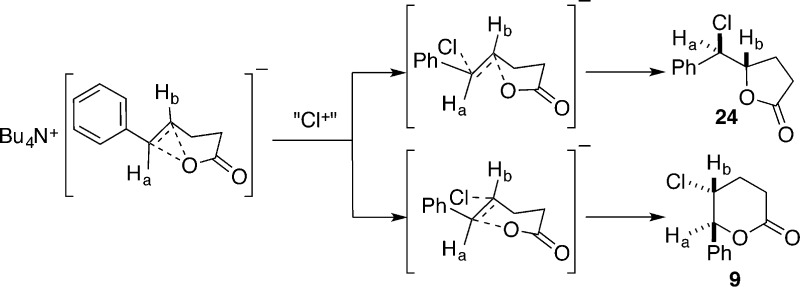
The dramatic changes in the chemical shifts for 23 cannot be explained by the inductive effect of the carboxylate functional group, which is too remote to have such a pronounced effect. Alternatively, the change in chemical shifts could arise from an interaction of carboxylate ion with the alkene such that it causes a redistribution of the electron density in the π-system (Scheme 12). The electronically activated alkene thus reacts readily with a weak chlorinating agent, such as DCDMH, to afford chloro lactone 9, the product of anti addition, potentially bypassing the intermediacy of a chloriranium ion.
Scheme 12.
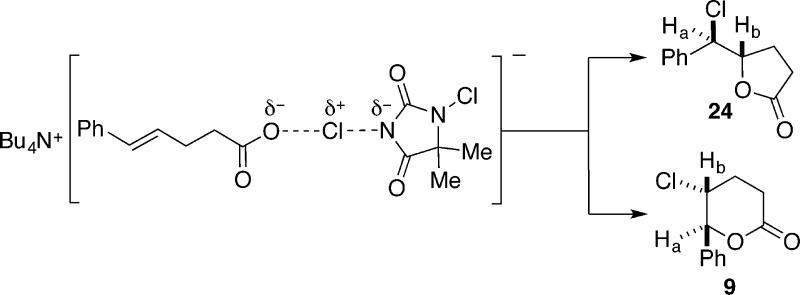
Although the influence of the carboxylate on the electronic properties of the alkene present a compelling explanation of the dramatic rate of chlorolactonization of 23, this influence may not be relevant in the presence of a chlorinating agent. Thus, a spectroscopic analysis of a 1:1 mixture of 23 and DCDMH was undertaken. Inspection of the 1H NMR spectrum of the reaction mixture corresponding to entry 1, Table 2, revealed a number of important features. First, after only 10 min, ca. 20% of 23 was converted to a mixture of 9 and 24. Second, the olefinic protons of 23 returned to a normal splitting pattern with signals centered at 6.277 ppm for Ha and 6.204 ppm for Hb, indicating that the interaction of the alkene with the carboxylate had diminished though still with a significant upfield shift of Ha (Figure 2C). Third, the methylene protons next to the carboxylate group moved downfield by ca. 0.07 ppm, while those next to the olefin remained unchanged. Taken together, these spectral clues suggest that the carboxylate is engaged in an equilibrium interaction with the chlorinating agent. However, on the basis of the change in chemical shift for the methylene protons next to the carboxylate, it does not appear that an acyl hypochlorite has been formed in spectroscopically detectable amounts.37 Rather, the observed changes are more consistent with a halogen-bonding interaction38 with the carboxylate which results in enhanced electrophilic character of the chlorine (i.e., Lewis base activation of Lewis acidity, Scheme 13).10,39 At this point, is not possible to deconstruct the overall rate enhancement to identify the relative contributions of these two factors, olefin activation vs electrophile activation.
Scheme 13.
Finally, and perhaps most importantly, it was essential to establish if these interactions are spectroscopically detectable under more relevant chlorocyclization reaction conditions. Thus, 1H NMR spectra of the combination of 8 with 1.0 equiv of quinuclidine and with 1.0 equiv of DCDMH were collected, Figure 3. On the basis of pKa, it is expected that the carboxylate would be stoichiometrically deprotonated (quinuclidine pKbH 12.1), and accordingly, the olefinic region shows a modest contraction of the chemical shifts compared to 8 with signals centered at 6.400 ppm for Ha and 6.218 ppm for Hb (Figure 3B). Clearly, though detectable, the polarization of the alkene electron density is significantly attenuated compared to 23. Interestingly, upon addition of DCDMH, no change in the olefinic region of the salt is detectable (Figure 3C).
Figure 3.
1H NMR spectra of the olefinic region: (A) 8; (B) 8 + quinuclidine; (C) 8 + quinuclidine + DCDMH.
Catalysis of Chlorolactonization of 23
Two interesting differences in the behavior of 8 and 23 in the presence of DCDMH with and without quinuclidine merit comment. The first concerns the rate of chlorolactonization. Under identical conditions in the absence of quinuclidine, the rates are dramatically different; acid 8 undergoes 12% conversion in 20 h whereas 23 undergoes 64% conversion in only 2.5 h. As detailed above, the carboxylate provides activation of either or both components in the reaction. This difference in reactivity of the alkene is also manifest in the relative rates of chlorolactonization of 8 and 23 in the presence of quinuclidine. With 10 mol % of the catalyst, 8 is completely consumed in 20 h compared to full conversion of 23 in 20 min. The reasons for the different rates in the presence of quinuclidine are more subtle. Considering the Brønsted basicity of quinuclidines (and the other tertiary amines tested), we suspect that these amines are fully protonated by the carboxylic acid under catalytic conditions. Thus, whereas the Lewis basicity of the amines is now quenched and unable to generate an activated chlorinating agent, the carboxylate (ion paired with the quinuclidinium ion) can provide electron density to activate the alkene as described above. Moreover, it is also possible that the quinuclidinium ion can provide electrophilic activation of DCDMH (Scheme 14). Thus, what was initially interpreted as Lewis base catalysis is, in fact, Brønsted base/Brønsted acid catalysis. The final aspect of the rate differences involves the effect of quinuclidine on the rate of chlorolactonization of 23 (cf. Table 2, entries 1 and 3). The significant increase in rate induced by 10 mol % of quinuclidine cannot be ascribed to its function as a Brønsted base as the carboxylate is already fully deprotonated. Thus, the Lewis basic character of the quinuclidine can now be expressed in the formation of an active chlorinating agent from DCDMH and thereby further accelerate the functionalization of the double bond (Scheme 14).
Scheme 14.
The second difference concerns the constitutional site selectivity of the chlorolactonization of the two substrates under catalysis by quinuclidine. Reaction of free acid 8 affords exclusively trans chloro lactone 9, whereas ammonium carboxylate 23 affords a 4:1 mixture of δ- and γ-lactones, 9 and 24, respectively. The clean anti addition in a Markovnikov sense is the generally expected outcome of these kinds of Ad reactions characterized by an unsymmetrical buildup of positive charge next to the phenyl group in the transition state.33 However, the formation of both constitutional isomers from Markovnikov and anti-Markovnikov addition with 23 is unusual. The rate enhancement observed by forming the tetrabutylammonium salt suggests that the electronic activation of either the alkene or the chlorinating agent shifts the transition state to an earlier position on the reaction coordinate, thus requiring less interaction with the chlorenium ion source and a correspondingly lower difference in the accumulation of positive charge.
Ground-State Conformation of Chlorolactone 9
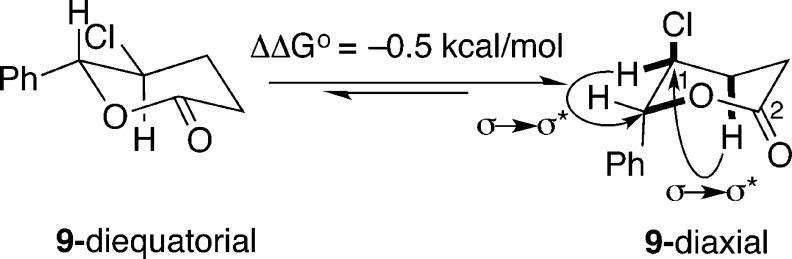
Although it is mentioned in the literature, chloro lactone 9 has never been fully described.21 As part of the spectroscopic characterization, we noted that the vicinal coupling constant between the hydrogens on the two stereogenic centers was unexpectedly small (3J = 5.9 Hz), suggesting that the hydrogens are both equatorial and that the chlorine atom and phenyl group are in axial positions. This surprising observation was confirmed by straightforward calculations of the ground-state energies of the two limiting chair conformations using semiempirical methods to obtain geometries and DFT calculations (B3LYP//6-31G*) for their energies (Scheme 15). The modest preference for the diaxial conformation likely results from energetic gains arising from favorable σ → σ* overlap (gauche effect)40 which are not offset by unfavorable steric interactions because of the lack of axial substituents at O(1) and C(2).
Scheme 15.
Solvolysis Experiments
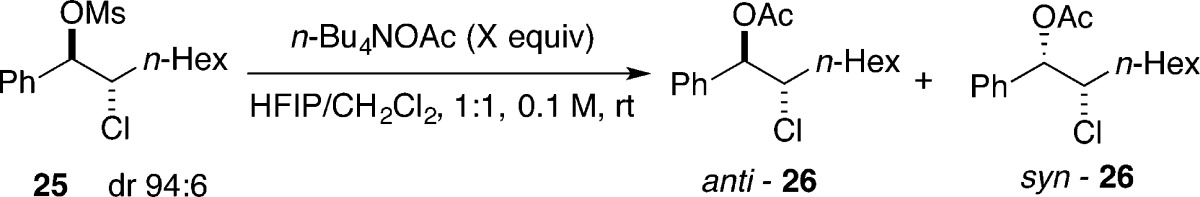
The results in Table 1 show that the more Lewis basic but less Brønsted basic sulfur- and selenium-containing catalysts were not efficient for chlorolactonization of 8. One possible explanation is that various side products arose because of the instability of intermediate chloriranium ion. To test this hypothesis, the solvolytic substitution of a disubstituted, benzylic chloriranium ion was examined. The ion was generated by solvolytic substitution of configurationally defined β-chloro mesylate 25 with tetrabutylammonium acetate in strongly ionizing media (Table 3). In a previous study on stability of haliranium ions, it was found that acetolysis of dialkyl-substituted chloriranium ions proceeds with high diastereospecificity, giving exclusively the anti-chloro acetate.8
Table 3. Solvolytic Substitution with 25.
| entry | X | conva (%) | time | anti/syna |
|---|---|---|---|---|
| 1 | 1 | 100 | 15 min | 62:38 |
| 2 | 2 | 100 | 17 min | 60:40 |
| 3 | 5 | 100 | 70 min | 62:38 |
| 4 | 10 | 76 | 48 h | 62:38 |
| 5 | 20 | 0 | 48 h | NA |
Determined by integration of 1H NMR signals against tetramethylsilane internal standard.
Acetolysis of 25 in a 1:1 mixture of HFIP/DCM with 1 equiv of tetrabutylammonium acetate afforded an anti/syn mixture of chloro acetates in a 62:38 ratio along with various products of elimination (Table 3, entry 1). Acetate product anti-26 can be formed by the opening of a chloriranium ion or by direct addition of acetate to a benzylic carbocation. Moreover, syn-26 may also arise from an open carbocation or by direct SN2-type displacement of mesylate 24. The formation of syn-26 by direct displacement was excluded by demonstrating that the diastereomeric ratio of the products remained unchanged as the amount of the nucleophile was increased (Table 3, entries 2–4). Interestingly, the rate of the reaction decreased with larger quantities of n-Bu4NOAc, ultimately leading to complete loss of reactivity with 20 equiv of n-Bu4NOAc (Table 3, entry 5).41 On the basis of these experiments, it can be concluded that the benzylic chloriranium ion, if formed, is in equilibrium with an open benzylic carbocation, giving rise to poorly selective nucleophilic capture and formation of unwanted elimination products.
Conclusions
In summary, depending on the catalyst, two modes of operation for catalytic chlorolactonization of 1,2-disubstituted styrenyl carboxylic acids have been identified. In the first scenario, catalysts with Brønsted basic character (i.e., quinuclidines) promote chlorolactonization by deprotonation of the carboxylic acid. The resulting carboxylate activates either or both the alkene and the chlorinating agent by donation of electron density into the olefinic π-system and potentially by hydrogen bonding to the hydantoin. In the second scenario, catalysts act as Lewis bases which transfer the chlorenium ion from the source to the substrate. However, a chloriranium ion, if formed, is unstable and leads to various side products. The greatest challenge in developing an enantioselective chlorolactonization is capturing the chloriranium ion faster than it can decompose or open to a free carbocation. Other challenges include designing catalysts that are stable under reaction conditions and avoiding unproductive side reactions between the chlorine electrophile and other functionality in the substrate.
Despite the recent progress the area of enantioselective halolactonization reactions, enantioselective chlorolactonization is still limited to 1,1-substituted aryl alkenoic acids. The study reported herein highlights main challenges associated with the development of catalytic asymmetric chlorolactonization and provides insights for further development of this important class of reactions.
Experimental Section
Chlorolactonization of 8 with DCDMH in the Presence of 1 equiv of Quinuclidine. Preparation of rel-(5R,6S)-5-Chlorotetrahydro-6-phenyl-2H-pyran-2-one (9)
A flame-dried 50 mL Schlenk flask was charged with quinuclidine (333 mg, 3.00 mmol, 1.00 equiv) and chloroform (15.0 mL, 0.200 M) at room temperature. (E)-5-Phenyl-4-pentenoic acid 8 (529 mg, 3.0 mmol) was added to the solution, followed by DCDMH (709 mg, 3.6 mmol, 1.20 equiv), and the resulting mixture was stirred at room temperature for 3 h. The reaction was quenched by the addition of a solution of butyl vinyl ether (388 μL, 300 mg, 3.0 mmol, 1.0 equiv) in ethanol (4.0 mL, approximately 10% v/v solution). After being stirred for 10 min, the solution was poured into a separatory funnel, washed with water (2 × 10 mL) and brine (1 × 10 mL), dried over sodium sulfate (4 g), filtered, and concentrated in vacuo (30 °C, 10 mmHg) to form a thick, yellow oil. Integration of the crude 1H NMR indicated a mixture of 9 and 24 in a ca. 100:17 ratio. Filtration through a silica plug (4 cm ⌀ × 10 cm) eluting with dichloromethane provided a white solid that was subsequently recrystallized from tert-butyl methyl ether to provide 9 as white needles (398 mg, 63%). Residual TBME incorporated into the crystalline product can be removed by sublimation of the needles at 60 °C/0.1 mmHg. Purification of the mother liquor by radial silica gel chromatography (4 mm, 9:1 hexanes/ethyl acetate) provided the minor isomer 24 as a clear, colorless oil (95.0 mg, 15%). Data for 9: mp (sublim) 99–100 °C; 1H NMR (500 MHz, CDCl3) δ 7.48–7.34 (m, 3 H, HC(9 and 10)), 7.35–7.29 (m, 2 H, HC(8)), 5.49 (d, J = 5.9 Hz, 1 H, HC(6)), 4.32 (ddd, J = 6.2, 5.9, 4.2 Hz, 1 H, HC(5)), 2.96 (ddd, J = 18.2, 9.0, 7.1 Hz, 1 H, HaC(3)), 2.71 (ddd, J = 18.2, 6.5, 5.5 Hz, 1 H, HbC(3)), 2.35 (dddd, J = 14.1, 8.9, 6.5, 4.2 Hz, 1 H, HaC(4)), 2.20 (dddd, J = 14.1, 7.2, 6.3, 5.5, 0.89 Hz, 1 H, HbC(4)); 13C{1H} NMR (126 MHz, CDCl3) δ 169.2 (C(2)), 137.1 (C(7)), 129.2 (C(10)), 129.0 (C(9)), 126.4 (C(8)), 85.4 (C(6)), 56.4 (C(5)), 27.3 (C(3)), 26.8 (C(4)); IR (neat, cm–1): 3445 (w), 2963 (w), 1724 (s, C=O), 1495 (w), 1458 (w), 1382 (w), 1221 (s), 1066 (m), 1031 (s), 949 (m), 920 (m), 756 (m), 697 (s), 648 (m), 515 (m); MS (EI+, TOF, 70 eV) 212.1 (22, M+, 37Cl), 210.1 (66, M+, 35Cl), 148.1 (18), 138.0 (10), 120.1 (10), 105.1 (74), 104.0 (100), 91.1 (9), 76.0 (41); HRMS (EI+, TOF) m/z calcd for C11H11O2Cl 210.0448, found 210.0446; TLC Rf 0.24 (hexanes/EtOAc 3:1) [UV, KMnO4]. Anal. Calcd for C11H11O2Cl (210.66): C, 62.72; H, 5.26; Cl, 16.83. Found: C, 62.37; H, 5.28; Cl, 16.91.
Data for 24: 1H NMR (500 MHz, CDCl3) δ 7.52–7.30 (m, 5
H, HC(aryl)), 5.05 (d, J = 5.7 Hz, 1 H, HC(6)), 4.85
(td, J = 7.1, 5.8 Hz, 1 H, HC(5)), 2.60–2.44
(m, 2 H, H2C(3)), 2.42–2.21 (m, 2 H, H2C(4)); 13C{1H} NMR (126 MHz, CDCl3) δ 176.3 (C(2)), 136.5 (C(7)), 129.2 (C(10)), 128.9 (C(9)),
128.0 (C(8)), 82.1 (C(5)), 64.2 (C(6)), 28.4 (C(3)), 24.3 (C(4));
IR (neat, cm–1) 3546 (w), 2928 (w), 1776 (s, γ-lactone
C=O stretch), 1495 (w), 1454 (m), 1419 (w), 1331 (w), 1173
(s), 1027 (m), 910 (m), 838 (w), 750 (w), 700 (m), 533 (w); MS (EI+, Quad, 70 eV) 212.1 (4, M+, 37Cl),
210.1 (10, M+, 35Cl), 125.0 (17), 114.9 (9),
85.1 (100); HRMS (ES+, TOF) m/z calcd for C11H12O2Cl,
211.0526, found 211.0532; TLC Rf 0.22 (hexanes/EtOAc 3:1) [UV, KMnO4].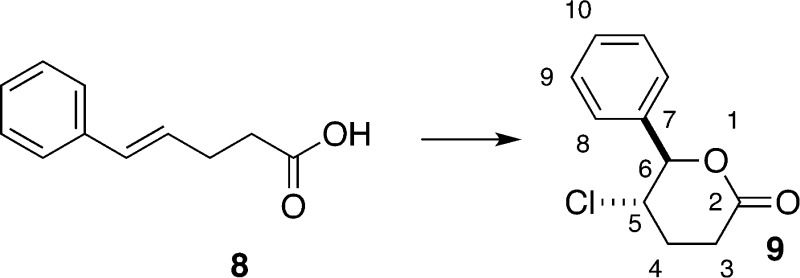
General Procedure: Chlorolactonization of 8 with 1-CBT in the Presence of (DHQD)2PHAL. Preparation of rel-(5R,6S)-5-Chlorotetrahydro-6-phenyl-2H-pyran-2-one (9)
A 5 mm, oven-dried
NMR tube equipped with a septum was charged with 1-CBT (10 mg, 0.078
mmol, 1.2 equiv) and 1,2,4,5-C6H2Cl4 (10.2 mg). The tube was then purged with Ar through a needle. Deuterochloroform
(600 μL) was added via syringe, and then the tube was agitated
with a vortex mixer. Acid 8 (12.1 mg, 0.066 mmol) was
added as a solid, and then the tube was agitated with a vortex mixer.
A solution of (DHQD)2PHAL (5.0 mg in 50 μL of CDCl3, 0.0065 mmol, 0.1 equiv) was added via syringe, and then
the tube was agitated with a vortex mixer. The reaction mixture was
analyzed by 1H NMR spectroscopy after 1 and 24 h. After
24 h, a solution of butyl vinyl ether in ethanol (15 vol %, 100 μL)
was added to quench the reaction. The resulting solution was concentrated
in vacuo (23 °C, 6 mmHg). The residue was purified by column
chromatography (silica gel, 1 g, 1 cm diam, CH2Cl2/hexane, 4:1) to afford 8.8 mg (66%) of 9 as a colorless
oil. CSP-SFC: (5R,6S)/(5S,6R)-9, tR 7.0 min (45.0%); (5S,6R)/(5R,6S)-9, tR 11.5 min (55.0%) (Chirapak AD, 125 bar, 3
mL/min, 5% MeOH in CO2).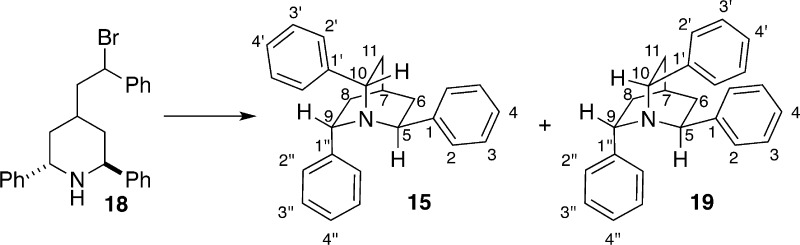
Preparation of 2,6,7-Triphenylquinuclidines (15 and 19)
A flame-dried, 100 mL, single-neck, round-bottomed flask fitted with a magnetic stir bar, reflux condenser, and Ar inlet was charged with bromoamine 18 (879 mg, 2.09 mmol, 1.0 equiv) and dissolved in toluene (30 mL). To the obtained solution was added sodium bicarbonate (176 mg, 4.18 mmol, 2.0 equiv), and the reaction mixture was heated at 115 °C for 12 h. Then the reaction mixture was cooled to room temperature and quenched with 30 mL of distilled water. The mixture was transferred to a 125 mL separatory flask, and the aqueous layer was extracted with dichloromethane (3 × 60 mL). The combined organic layer was washed with saturated brine solution (1 × 50 mL), dried over MgSO4, and concentrated by rotary evaporation to give the crude mixture of quinuclidines as yellow oil. Purification by flash chromatography (SiO2, 200 mm × 25 mm, 10 mL fractions, hexanes/CH2Cl2, 4:1 then hexane/EtOAc, 19:1) yielded C3-symmetric quinuclidine 15 as a white solid (349 mg, 49%) and C1-symmetric quinuclidine 19 as a colorless oil (183 mg, 26%, Rf 0.39 (hexane/EtOAc, 19:1) [UV, I2]). The 1H NMR spectroscopic data matched those from an alternative preparation.31 Data for 15: 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 8.2 Hz, 6 H, HC(2)), 7.37 (t, J = 7.7 Hz, 6 H, HC(3)), 7.25 (t, J = 7.7 Hz, 3 H, HC(4)), 4.18 (t, J = 8.8 Hz, 3 H, HC(5, 9, 10)), 2.35–2.25 (m, 4 H, HC(6, 7, 8, 11)), 1.76 (t, J = 10.8 Hz, 3 H, HC(6′, 8′, 11′)); TLC Rf 0.48 (hexane/EtOAc, 19:1) [UV, I2].
Data for (2R,6R,7S)-2,6,7-triphenylquinuclidine (19): 1H NMR (400 MHz, CDCl3) δ
7.76 (d, J = 7.5 Hz, 2 H, HC(aryl)), 7.39 (t, J = 7.5 Hz, 2 H), 7.25 (t, J = 8.5 Hz,
3 H, HC(aryl)), 7.01 (d, J = 7.1 Hz, 2 H, HC(aryl)),
6.96–6.74 (m, 6 H, HC(aryl)), 4.44 (dd, J =
10.8, 4.4 Hz, 1 H, HC(9/10)), 4.37 (t, J = 8.7 Hz,
1 H, HC(9/10)), 3.88 (t, J = 9.6 Hz, 1 H, HC(5)),
2.38 (s, 1 H, HC(7)), 2.30 (m, 2 H, HC(8, 11)), 1.98 (m, 9.6 Hz, 4
H, HC(6, 8′, 11′)); 13C{1H} NMR
(126 MHz, CDCl3) δ 144.3 (C(1)), 143.7 (C(1′)),
142.1 C(1″), 129.6, 128.2, 127.3, 127.2, 126.8, 126.7, 126.6
(C(2/3/4)), 126.3 (C(4′)), 124.8 (C(4″)), 63.1 (C(9/10)),
62.4 (C(9/10)), 51.7 (C(5)), 32.6 (C(6)), 31.4 (C(8/11)), 29.5 (C(8/11)),
24.4 (C(7)); IR (neat) 3058 (w), 3026 (w), 2929 (w), 2865 (w), 1684
(w), 1601 (w), 1493 (w), 1466 (w), 1447 (w), 1337 (m), 1299 (w), 1202
(w), 1179 (w), 1074 (w), 1043 (w), 984 (m), 908 (w), 841 (w), 825
(w), 805 (w), 788 (w), 741 (s), 692 (s), 649 (w), 612 (w), 592 (w),
550 (w); LR MS (EI+, 70 eV) 340.2.; HR MS (EI+, [M + H]+) m/z calcd
for C25H26N 340.2065, found 340.2065. CSP-HPLC:
(R,R,S)-19, tR 9.71 min (99%); (S,S,R)-19, tR 12.24 min (1%) (NP-HPLC, CHIRALPAK OJH, 98.0:2.0
hexane/i-PrOH, 0.5 mL/min, 210 nm, 22 °C); [α]D −90.25 (22 °C, c = 1.3, THF).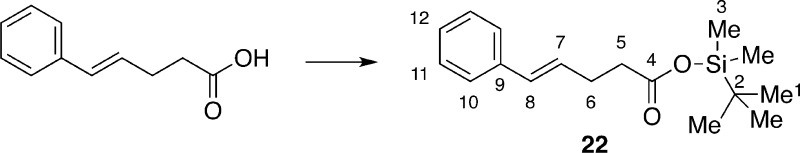
Preparation of tert-Butyldimethylsilyl (E)-5-Phenylpent-4-enoate (22)
A flame-dried,
25 mL, single-neck, round-bottomed flask fitted with a magnetic stir
bar and Ar inlet was charged with (E)-5-phenylpent-4-enoic
acid (176 mg, 1.0 mmol, 1.0 equiv) and dissolved in DMF (3 mL). tert-Butyldimethylsilyl chloride (226 mg, 1.5 mmol, 1.5
equiv) and 1H-imidazole (136 mg, 2.0 mmol, 2.0 equiv)
were added successively. The solution was heated to 40 °C in
an oil bath for 7 h and then cooled to room temperature and stirred
overnight. The mixture was transferred to a 125 mL separatory flask,
diluted with 50 mL of ethyl acetate, and washed with saturated brine
solution (5 × 50 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation to give the product
as a clear, colorless oil (253 mg, 87%). The silyl ester was used
immediately for further reactions without purification. Data for 22: 1H NMR (400 MHz, CDCl3) δ
7.31 (dt, J = 15.2, 7.6 Hz, 4 H, HC(10 and 11)),
7.21 (t, J = 7.2 Hz, 1 H, HC(12)), 6.44 (d, J = 15.8 Hz, 1 H, HC(8)), 6.30–6.16 (m, 1 H, HC(7)),
2.52 (s, 4 H, HC(5 and 6)), 0.95 (s, 9H, HC(1)), 0.28 (s, 6 H, HC(3)); 13C{1H} NMR (126 MHz, CDCl3) δ
173.5 (C(4)), 137.5 (C(9)), 130.9 (C(8)), 128.7 (C(12)), 128.6 (C(11)),
127.2 (C(7)), 126.1 (C(12)), 35.8 (C(6)), 28.6 (C(5)), 25.7 (C(1)),
17.73 (C(2), −4.65 (C(3)); IR (neat) 3027 (w), 2955 (w), 2930
(w), 2858 (w), 1717 (s), 1599 (w), 1495 (w), 1472 (w), 1463 (w), 1447
(w), 1413 (w), 1363 (w), 1252 (s), 1177 (m), 1077 (w), 1029 (w), 1006
(w), 962 (m), 938 (m), 892 (w), 840 (s), 813 (s), 788 (s), 741 (s),
691 (s), 609 (w); LR MS (EI+, 70 eV) 291.2; HR MS (EI+,
[M + H]+) m/z calcd for
C17H27O2Si 291.1775, found 291.1780.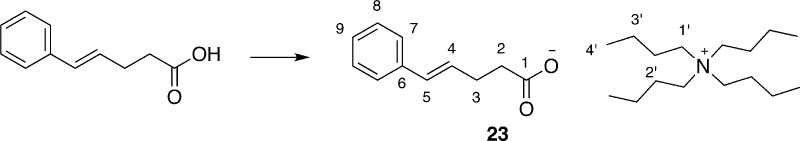
Preparation of Tetrabutylammonium (E)-5-Phenylpent-4-enoate (23)
To a 20 mL scintillation vial equipped
with a plastic screw cap and a magnetic stir bar was added (E)-5-phenylpent-4-enoic acid (176 mg, 1.0 mmol) followed
by the addition of 40% aqueous solution of tetrabutylammonium hydroxide
solution (8.0 mL, 12.0 mmol, 12 equiv). The resulting homogeneous
reaction mixture was stirred for 30 min and then was transferred to
a 50 mL separatory funnel and extracted with chloroform (3 ×
50 mL). The combined organic layers were washed with water (3 ×
20 mL) and brine (1 × 50 mL), dried over MgSO4, and
concentrated by rotary evaporation to give the product as a pale yellow
oil (374 mg, 90%). Data for 23: 1H NMR (400
MHz, CDCl3) δ 7.31 (d, J = 8.0 Hz,
2 H, HC(7)), 7.24 (t, J = 7.6 Hz, 2 H, HC(8)), 7.14
(t, J = 7.1 Hz, 1 H, HC(9)), 6.37 (d, J = 3.6 Hz, 2 H, HC(4 and 5)), 3.41–3.24 (m, 8H, HC(1′)),
2.70–2.47 (m, 2 H, HC(3)), 2.41–2.28 (m, 2 H, HC(2)),
1.61 (p, J = 7.6 Hz, 8H, HC(2′)), 1.41 (h, J = 7.3 Hz, 8H, HC(3′)), 0.98 (t, J = 7.3 Hz, 12 H, HC(4′)); 13C{1H} NMR
(126 MHz, CDCl3) δ 177.8 (C(1)), 138.5 (C(6)), 132.7
(C(5)), 128.7 (C(9)), 128.6 (C(8)), 126.4 (C(4)), 125.9 (C(7)), 58.8
(C(1′)), 38.6 (C(3)), 30.9 (C(2)), 24.2 (C(2′)), 19.9
(C(3′)), 13.8 (C(4′)); IR (neat) 2960 (m), 2874 (m),
1717 (w), 1649 (w), 1579 (s), 1489 (m), 1462 (m), 1378 (s), 1275 (m),
1151 (w), 1106 (w), 1069 (w), 1027 (w), 963 (m), 882 (m), 802 (m),
742 (s), 694 (s), 491 (m); HR MS (EI+, [M]−) m/z calcd for C11H11O2 175.0759, found 175.0752.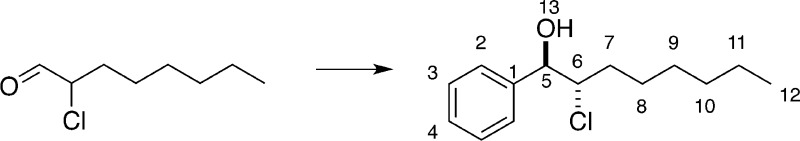
Preparation of rel-(1R,2S)-2-Chloro-1-phenyloctan-1-ol
A flame-dried, 100
mL Schlenk flask fitted with a magnetic stir bar and rubber septum
under argon was charged with 2-chlorooctanal42 (486 mg, 3.0 mmol) and dissolved in THF (60 mL). The reaction mixture
was then cooled in a dry ice/2-propanol bath (internal temperature
−73 °C), and 1.9 M phenyllithium in dibutyl ether (1.58
mL, 3.0 mmol, 1.0 equiv) was added dropwise by syringe at a rate in
which the internal temperature was maintained below −70 °C
to give a light yellow homogeneous solution. The reaction mixture
was stirred for 20 min at −70 °C and quenched with 40
mL of satd aq NH4Cl solution. The mixture was allowed to
warm to room temperature and then was transferred to 250 mL separatory
funnel and was diluted with diethyl ether (100 mL). The aqueous layer
was extracted with diethyl ether (3 × 50 mL); the combined organic
layers were washed with brine (1 × 100 mL), dried over MgSO4, and concentrated by rotary evaporation to give the crude
product as yellow oil. Purification by flash chromatography (SiO2, 200 mm × 25 mm, 10 mL fractions, hexane/EtOAc, 19:1
increased to hexane/EtOAc, 9:1) yielded anti-2-chloro-1-phenyloctan-1-ol
(94:6 mixture of anti/syn diastereomers
as determined my 1H NMR spectroscopy) as a colorless oil
(1.35 g, 44%). An analytical sample was obtained by Kugelrohr distillation
(air bath = 170 °C, 1.0 mmHg). Data for anti-2-chloro-1-phenyloctan-1-ol: 1H NMR (500 MHz, CDCl3) δ 7.41–7.29 (m, 5 H, HC(2, 3, 4)), 4.94 (d, J = 4.1 Hz, 1 H, HC(5)), 4.18 (dt, J =
8.6, 4.3 Hz, 1 H, HC(6)), 2.57 (s, 1 H, HO(13)), 1.76–1.62
(m, 2 H, HC(7)), 1.62–1.43 (m, 2 H, HC(8)), 1.38–1.14
(m, 6 H, HC(9, 10, 11)), 0.87 (t, J = 7.0 Hz, 3 H,
HC(12)); 13C{1H} NMR (126 MHz, CDCl3) δ 140.0 (C(4)), 128.5 (C(2)), 128.1 (C(4)), 126.7 (C(3)),
77.16 (C(5)), 68.6 (C(6)), 31.7 (C(7)), 31.5 (C(8)), 28.8 (C(9)),
26.7 (C(10)), 22.7 (C(11)), 14.2 (C(12)); IR (neat) 3420 (w), 3031
(w), 2954 (w), 2857 (w), 2926 (w), 2857 (w), 1495 (w), 1453 (w), 1378
(w), 1189 (w), 1123 (w), 1028 (w), 915 (w), 800 (w), 727 (w), 761
(m), 699 (s), 602 (w), 518 (w); TLC Rf 0.30 (hexane/EtOAc, 9:1) [KMnO4]; LR
MS (EI+, 70 eV) 240.1; HR MS (EI+, [M]+) m/z calcd for C14H21ClO 240.1281, found 240.1283. Anal. Calcd for C14H21ClO (240.77): C, 69.84; H, 8.79. Found: C, 69.75; H,
8.65.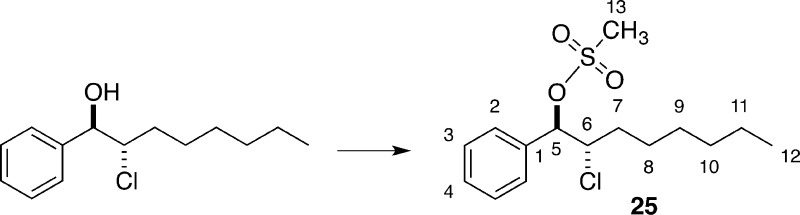
Preparation of rel-(1R,2S)-2-Chloro-1-phenyloctyl Methanesulfonate (25)
A flame-dried, 25 mL, two-neck, round-bottomed
flask fitted
with a magnetic stir bar, and Ar inlet, and a rubber septum was charged
with anti-2-chloro-1-phenyloctan-1-ol (197 mg, 0.82
mmol) and dissolved in dichloromethane (5 mL). Triethylamine (228
μL, 1.64 mmol, 2.0 equiv) was added, and the reaction mixture
was cooled in an ice bath (internal temperature 3 °C). Methanesulfonyl
chloride (95 μL, 1.23 mmol, 1.5 equiv) was added in one portion
by syringe, and the reaction mixture was allowed to warm to room temperature
and was stirred overnight. The reaction was quenched by the addition
of satd aq NH4Cl solution (3 mL) with vigorous stirring.
The biphasic layer was poured into a 25 mL separatory funnel containing
10 mL of dichloromethane, and the organic layer was washed with distilled
water (1 × 10 mL), satd aq sodium bicarbonate solution (1 ×
10 mL), and brine (1 × 10 mL). The organic layer was dried over
MgSO4 and concentrated by rotary evaporation to give the
crude product as colorless oil. The crude product was passed through
a short silica plug (1 cm) in a Pasteur pipet with a 1:1 mixture of
EtOAc/hexane (10 mL), and concentrated by rotary evaporation to give
the 25 (94:6 mixture of anti/syn diastereomers as determined by 1H NMR spectroscopy)
as a colorless oil (226 mg, 87%). Data for 25: 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 5 H, HC(2, 3,
4)), 5.61 (d, J = 6.1 Hz, 1 H, HC(5)), 4.20 (ddd, J = 9.7, 6.1, 2.8
Hz, 1 H, HC(6)), 2.77 (s, 3 H, HC(13)), 2.03–1.86 (m, 1 H,
HC(7)), 1.78–1.49 (m, 2 H, HC(7′, 8)), 1.45–1.16
(m, 7 H, HC(8′, 9, 10, 11)), 0.87 (t, J = 6.9 Hz, 3 H, HC(12)); 13C{1H} NMR (126 MHz, CDCl3) δ
135.5 (C(1)), 129.6 (C(4)), 128.9 (C(2/3)), 127.6 (C(2/3)), 85.7 (C(5)),
63.9 (C(6)), 39.3 (C(13)), 33.0 (C(7)), 31.7 (C(9/10/11)), 28.7 (C(9/10/11)),
26.1 (C(8)), 22.6 (C(9/10/11)), 14.2 (C(12)); IR (neat) 2927 (m),
2857 (w), 1746 (s), 1455 (s), 1371 (w), 1227 (s), 1029 (w), 761 (w),
701 (m); TLC Rf 0.19
(hexane/EtOAc, 9:1) [UV]; LR MS (EI+, 70 eV) 318.1; HR MS (EI+, [M]+) m/z calcd
for C15H23O3ClS 318.1056, found 318.1048.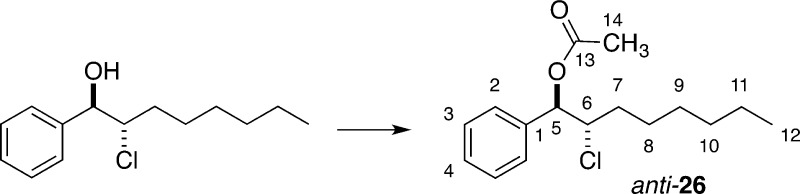
Preparation of rel-(1R,2S)-2-Chloro-1-phenyloctyl Acetate (anti-26)
To a 1 dram glass vial equipped with a plastic screw cap and a Teflon coated stir bar were added anti-2-chloro-1-phenyloctan-1-ol (12 mg, 0.05 mmol), pyridine (12 μL, 0.15 mmol, 3.0 equiv), and dichloromethane (1 mL). The reaction mixture was stirred at room temperature for 5 min, and acetic anhydride (9.5 μL, 0.10 mmol, 2.0 equiv) was added in one portion via syringe. After being stirred at room temperature for 12 h, the reaction mixture was transferred to a 25 mL separatory funnel and was diluted with dichloromethane 10 mL. The organic layer was washed with 2 M HCl solution (2 × 10 mL), distilled water (1 × 10 mL), satd aq sodium bicarbonate solution (1 × 10 mL), and brine (1 × 10 mL). The organic layers were dried over MgSO4 and concentrated by rotary evaporation to give the crude product as colorless oil. The crude product was passed through a short silica plug (1 cm) in a Pasteur pipet with hexane/EtOAc, 9:1 (10 mL) and was concentrated by rotary evaporation to give anti-26 (94:6 mixture of anti/syn diastereomers as determined by 1H NMR spectroscopy) as a colorless oil (8 mg, 56%). Data for anti-26: 1H NMR (500 MHz, CDCl3) δ 7.43–7.31 (m, 5 H, HC(2, 3, 4)), 5.93 (d, J = 5.4 Hz, 1 H, HC(5)), 4.18 (dq, J = 9.5, 3.1 Hz, 1 H, HC(6)), 2.14 (s, 3 H, HC(14)), 1.87–1.71 (m, 1 H, HC(7)), 1.67–1.51 (m, 2 H, HC(7′, 8)), 1.41–1.09 (m, 7 H, HC(8′, 9, 10, 11)), 0.87 (t, J = 6.9 Hz, 3 H, HC(12)); 13C{1H} NMR (126 MHz, CDCl3) δ 136.9 (C(1)), 128.6 (C(4)), 128.4 (C(2/3)), 127.6 (C(2/3)), 77.8 (C(5)), 64.36 (C(6)), 33.2 (C(7)) 31.8 (C(9/10/11)), 28.8 (C(9/10/11)), 26.4 (C(8)), 22.7 (C(9/10/11)), 21.2 (C(14)), 14.2 (C(12)); IR (neat) 2927 (m), 2857 (w), 1746 (s), 1455 (s), 1371 (w), 1227 (s), 1029 (w), 761 (w), 701 (m); TLC Rf 0.48 (hexane/EtOAc, 9:1) [UV]; LR MS (EI+, 70 eV) 246.1; HR MS (EI+, [M – HCl]+) m/z calcd for C16H22O2 246.1620, found 246.1621.
Acknowledgments
We are grateful to the National Institutes of Health (GM R01-085235) for generous financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.6b01455.
Optimization studies, and 1H and 13C spectra of all described compounds (PDF)
The authors declare no competing financial interest.
Author Status
† ISHC member.
Supplementary Material
References
- a Collins I. J. Chem. Soc., Perkin Trans. 1 1998, 1869–1888. 10.1039/a800740c. [DOI] [Google Scholar]; b Donnelly D. M. X.; Meegan M. J.. Comprehensive Heterocyclic Chemistry; Katritzky A. R., Ed.; Pergamon: New York, 1984; Vol. 4, pp 657–712. [Google Scholar]; c Devon T. K.; Scott A. I. In Handbook of Naturally Occurring Compounds; Academic Press: New York, 1975; Vol. 1, p 249. [Google Scholar]; d Martin S. F.; Guinn D. E. Synthesis 1991, 245–262. 10.1055/s-1991-26438. [DOI] [Google Scholar]
- a Rashid S.; Bhat B. A.; Mehta G. Org. Lett. 2015, 17, 3604–3607. 10.1021/acs.orglett.5b01707. [DOI] [PubMed] [Google Scholar]; b Cao J.; Perlmutter P. Org. Lett. 2013, 15, 4327–4329. 10.1021/ol401801g. [DOI] [PubMed] [Google Scholar]
- Maier M. In Science of Synthesis; Carreira E. M., Panek J. S., Eds.; Thieme: Stuttgart, 2010; Vol. 20; pp 1421–1551. [Google Scholar]
- Ranganathan S.; Muraleedharan K. M.; Vaish N. K.; Jayaraman N. Tetrahedron 2004, 60, 5273–5308. 10.1016/j.tet.2004.04.014. [DOI] [Google Scholar]
- a Zheng S.; Schienebeck C. M.; Zhang W.; Wang H.-Y.; Tang W. Asian J. Org. Chem. 2014, 3, 366–376. 10.1002/ajoc.201400030. [DOI] [Google Scholar]; b Tan C. K.; Yu W. Z.; Yeung Y.-Y. Chirality 2014, 26, 328–343. 10.1002/chir.22272. [DOI] [PubMed] [Google Scholar]; c Fujioka H.; Murai K. Heterocycles 2013, 87, 763–805. 10.3987/REV-12-762. [DOI] [Google Scholar]; d Mendoza A.; Fananas F. J.; Rodriguez F. Curr. Org. Synth. 2013, 10, 384–393. 10.2174/1570179411310030004. [DOI] [Google Scholar]; e Tan C. K.; Yeung Y.-Y. Chem. Commun. 2013, 49, 7985–7996. 10.1039/c3cc43950j. [DOI] [PubMed] [Google Scholar]; f Denmark S. E.; Kuester W. E.; Burk M. T. Angew. Chem., Int. Ed. 2012, 51, 10938–10953. 10.1002/anie.201204347. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Hennecke U. Chem. - Asian J. 2012, 7, 456–465. 10.1002/asia.201100856. [DOI] [PubMed] [Google Scholar]; h Castellanos A.; Fletcher S. P. Chem. - Eur. J. 2011, 17, 5766–5776. 10.1002/chem.201100105. [DOI] [PubMed] [Google Scholar]; i Tan C. K.; Zhou L.; Yeung Y.-Y. Synlett 2011, 1335–1339. 10.1055/s-0030-1260578. [DOI] [Google Scholar]
- Suess H. E.; Urey H. C. Rev. Mod. Phys. 1956, 28, 53–74. 10.1103/RevModPhys.28.53. [DOI] [Google Scholar]
- a Gribble G. W. Environ. Chem. 2015, 12, 396–405. 10.1071/EN15002. [DOI] [Google Scholar]; b Gribble G. W. Progress in the Chemistry of Organic Natural Products 2009, 91, 1–613. 10.1007/978-3-211-99323-1_1. [DOI] [PubMed] [Google Scholar]; c Gribble G. W. Chemosphere 2003, 52, 289–297. 10.1016/S0045-6535(03)00207-8. [DOI] [PubMed] [Google Scholar]; d Gribble G. W. Acc. Chem. Res. 1998, 31, 141–152. 10.1021/ar9701777. [DOI] [Google Scholar]; e Gribble G. W. Progress in the Chemistry of Organic Natural Products 1996, 68, 1–498. 10.1007/978-3-7091-6887-5_1. [DOI] [PubMed] [Google Scholar]
- a Olah G. A.; Bollinger J. M. J. Am. Chem. Soc. 1967, 89, 4744–4752. 10.1021/ja00994a031. [DOI] [PubMed] [Google Scholar]; b Olah G. A.; Bollinger J. M. J. Am. Chem. Soc. 1968, 90, 947–953. 10.1021/ja01006a019. [DOI] [Google Scholar]; c Olah G. A.; Bollinger J. M.; Brinich J. J. Am. Chem. Soc. 1968, 90, 2587–2594. 10.1021/ja01012a025. [DOI] [Google Scholar]
- a Chung W.-j.; Vanderwal C. D. Angew. Chem., Int. Ed. 2016, 55, 4396–4434. 10.1002/anie.201506388. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chung W.-j.; Vanderwal C. D. Acc. Chem. Res. 2014, 47, 718–728. 10.1021/ar400246w. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nilewski C.; Carreira E. M. Eur. J. Org. Chem. 2012, 1685–1698. 10.1002/ejoc.201101525. [DOI] [Google Scholar]
- Denmark S. E.; Beutner G. L. Angew. Chem., Int. Ed. 2008, 47, 1560–1638. 10.1002/anie.200604943. [DOI] [PubMed] [Google Scholar]
- Frost B. Liebigs Ann. Chem. 1884, 226, 363–376. 10.1002/jlac.18842260312. [DOI] [Google Scholar]
- a Stobbe H.; Strigel A.; Meyer C. Justus Liebigs Ann. Chem. 1902, 321, 105–126. 10.1002/jlac.19023210109. [DOI] [Google Scholar]; b Stobbe H. Liebigs Ann. Chem. 1894, 282, 280–319. 10.1002/jlac.18942820304. [DOI] [Google Scholar]
- Bartlett P. A.; Myerson J. J. Am. Chem. Soc. 1978, 100, 3950–3952. 10.1021/ja00480a061. [DOI] [Google Scholar]
- Kitagawa O.; Hanano T.; Tanabe K.; Shiro M.; Taguchi T. J. Chem. Soc., Chem. Commun. 1992, 1005–1007. 10.1039/c39920001005. [DOI] [Google Scholar]
- Haas J.; Piguel S.; Wirth T. Org. Lett. 2002, 4, 297–300. 10.1021/ol0171113. [DOI] [PubMed] [Google Scholar]
- Cui X. L.; Brown R. S. J. Org. Chem. 2000, 65, 5653–5658. 10.1021/jo000449a. [DOI] [PubMed] [Google Scholar]
- Garnier J. M.; Robin S.; Rousseau G. Eur. J. Org. Chem. 2007, 2007, 3281–3291. 10.1002/ejoc.200700041. [DOI] [Google Scholar]
- Mellegaard S. R.; Tunge J. A. J. Org. Chem. 2004, 69, 8979–8981. 10.1021/jo048460o. [DOI] [PubMed] [Google Scholar]
- Whitehead D. C.; Yousefi R.; Jaganathan A.; Borhan B. J. Am. Chem. Soc. 2010, 132, 3298–3300. 10.1021/ja100502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi R.; Whitehead D. C.; Mueller J. M.; Staples R. J.; Borhan B. Org. Lett. 2011, 13, 608–611. 10.1021/ol102850m. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Liu N.; Schienebeck C. M.; Decloux K.; Zheng S.; Werness J. B.; Tang W. Chem. - Eur. J. 2012, 18, 7296–7305. 10.1002/chem.201103809. [DOI] [PubMed] [Google Scholar]
- Han X.; Dong C.; Zhou H.-B. Adv. Synth. Catal. 2014, 356, 1275–1280. 10.1002/adsc.201300915. [DOI] [Google Scholar]
- Relative and absolute configurations of the products were not assigned.
- Yousefi R.; Ashtekar K. D.; Whitehead D. C.; Jackson J. E.; Borhan B. J. Am. Chem. Soc. 2013, 135, 14524–14527. 10.1021/ja4072145. [DOI] [PubMed] [Google Scholar]
- For an example of enantioselective bromolactonization of 1,2-disubstituted alkenes, see:Tan C. K.; Zhou L.; Yeung Y.-Y. Org. Lett. 2011, 13, 2738–2741. 10.1021/ol200840e. [DOI] [PubMed] [Google Scholar]
- Bromination:; a Denmark S. E.; Burk M. T. Chirality 2014, 26, 344–355. 10.1002/chir.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]; Selenylation:; b Denmark S. E.; Kalyani D.; Collins W. R. J. Am. Chem. Soc. 2010, 132, 15752–15765. 10.1021/ja106837b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sulfenylation:; c Denmark S. E.; Kornfilt D. J. P.; Vogler T. J. Am. Chem. Soc. 2011, 133, 15308–15311. 10.1021/ja2064395. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Denmark S. E.; Jaunet A. J. Org. Chem. 2014, 79, 140–171. 10.1021/jo4023765. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Denmark S. E.; Chi H.-M. J. Am. Chem. Soc. 2014, 136, 8915–8918. 10.1021/ja5046296. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Denmark S. E.; Rossi S.; Webster M. P.; Wang H. J. Am. Chem. Soc. 2014, 136, 13016–13028. 10.1021/ja506133z. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Denmark S. E.; Hartmann E.; Kornfilt D. J. P.; Wang H. Nat. Chem. 2014, 6, 1056–1064. 10.1038/nchem.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmark S. E.; Burk M. T.; Hoover A. J. J. Am. Chem. Soc. 2010, 132, 1232–1233. 10.1021/ja909965h. [DOI] [PubMed] [Google Scholar]
- Maddox S. M.; Nalbandian C. J.; Smith D. E.; Gustafson J. L. Org. Lett. 2015, 17, 1042–1045. 10.1021/acs.orglett.5b00186. [DOI] [PubMed] [Google Scholar]
- Samanta R. C.; Yamamoto H. Chem. - Eur. J. 2015, 21, 11976–11979. 10.1002/chem.201502234. [DOI] [PubMed] [Google Scholar]
- a Smith J. R. L.; McKeer L. C.; Taylor J. M. J. Chem. Soc., Perkin Trans. 2 1987, 1533–1537. 10.1039/p29870001533. [DOI] [Google Scholar]; b Smith J. R. L.; McKeer L. C.; Taylor J. M. J. Chem. Soc., Perkin Trans. 2 1988, 385–391. 10.1039/p29880000385. [DOI] [Google Scholar]; c Smith J. R. L.; McKeer L. C.; Taylor J. M. J. Chem. Soc., Perkin Trans. 2 1989, 1529–1536. 10.1039/p29890001529. [DOI] [Google Scholar]
- Gao X.; Singh R. P.; Corey E. J. Org. Lett. 2010, 12, 1812–1814. 10.1021/ol100426m. [DOI] [PubMed] [Google Scholar]
- Corey E. J.; Yuen P.-W. Tetrahedron Lett. 1989, 30, 5825–5828. 10.1016/S0040-4039(01)93481-1. [DOI] [Google Scholar]
- For a thorough treatment of the stereochemistry and mechanism of AdE reactions, see:; a Kočovskí P.Addition Reactions: Polar Addition. In Organic Reaction Mechanisms; Knipe A. C., Ed.; Wiley: Chichester, 2007. [Google Scholar]; b De la Mare P. B. D.; Bolton R.. Electrophilic Additions to Unsaturated Systems, 2nd ed.; Elsevier: New York, 1982; pp 136–197. [Google Scholar]; c Vʼyunov K. A.; Ginak A. I. Russ. Chem. Rev. 1981, 50, 151–163. 10.1070/RC1981v050n02ABEH002552. [DOI] [Google Scholar]; d Schmid G. H.; Garratt D. G In The Chemistry of Doubly Bonded Functional Groups; Patai S., Ed.; Wiley-Interscience: New York, 1977; Supplement A, Part 2, Chapter 9. [Google Scholar]; e Freeman F. Chem. Rev. 1975, 75, 439–490. 10.1021/cr60296a003. [DOI] [Google Scholar]; f Fahey R. C. In Topics in Stereochemistry; Eliel E. L., Allinger N. L., Eds.; Wiley: New York, 1968; Vol. 3, pp 237–342. [Google Scholar]
- a Staninets V. I.; Shilov E. A. Ukrain. Khim. Zh. 1968, 34, 372. [Google Scholar]; b Staninets V. I.; Shilov E. A. Russ. Chem. Rev. 1971, 40, 272–283. 10.1070/RC1971v040n03ABEH001918. [DOI] [Google Scholar]
- a Isaacs N. S.Physical Organic Chemistry; Longman Scientific & Technical: London, 1987; Chapter 12. [Google Scholar]; b Lowry T. H.; Richardson K. S.. Mechanism and Theory in Organic Chemistry, 2nd ed.; Harper and Row: New York, 1981; pp 506–526. [Google Scholar]
- During the course of our studies, we became aware of a comprehensive investigation of the role of carboxylate ions in the activation of chlorolactonization reactions by Borhan, Jackson, and co-workers. We are grateful to Prof. Borhan for sharing the results of those studies.Ashtekar A. D.; Vetticatt M.; Yousefi R.; Jackson J. E.; Borhan B. J. Am. Chem. Soc. 2016, 138, 8114–8119. 10.1021/jacs.6b02877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The chemical shifts for acetic acid and acetyl hypochlorite (in CCl4) are 2.10 and 2.24 ppm, respectively.Evans J. C.; Lo G. Y-S.; Chang Y.-L. Spectrochim. Acta 1965, 21, 973–979. 10.1016/0371-1951(65)80165-5. [DOI] [Google Scholar]
- a Metrangolo P., Resnati G., Eds. Halogen Bonding I. In Topics in Current Chemistry; Springer: Heidelberg, 2015; Vol. 358. [Google Scholar]; b Metrangolo P.; Resnati G., Eds. Halogen Bonding. In Topics in Current Chemistry; Springer: Heidelberg, 2008; Vol. 126. [Google Scholar]
- Weinhold F.; Landis C.. Valency and Bonding: A Natural Bond Orbital Donor–Acceptor Perspective; Cambridge University Press: Cambridge, 2005; pp 275–306. [Google Scholar]
- Wolfe S. Acc. Chem. Res. 1972, 5, 102–111. 10.1021/ar50051a003. [DOI] [Google Scholar]
- Because HFIP possesses extremely high ionizing power and low nucleophilicity, it primarily functions by stabilizing the leaving group (mesylate). The attenuation of solvolysis in the presence of a large excess of tetrabutylammonium acetate might arise from a buffering effect on the hydrogen bonding ability of the solvent.Schadt F. L.; Schleyer P. v. R.; Bentley T. W. Tetrahedron Lett. 1974, 15, 2335–2338. 10.1016/S0040-4039(01)92248-8. [DOI] [Google Scholar]
- Amatore M.; Beeson T. D.; Brown S. P.; MacMillan D. W. C. Angew. Chem., Int. Ed. 2009, 48, 5121–5124. 10.1002/anie.200901855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.