Abstract
Background
Jumping to conclusions due to impulsivity has been shown to be a sensitive marker for dopamine dysregulation and addictive behaviour patterns in treated patients with Parkinson's disease (PD). It is unknown whether drug naïve PD patients, who have never received dopaminergic therapy also have deficits in information sampling.
Methods
Twenty five de novo PD patients and twenty matched healthy controls were recruited and tested on the beads task, which is a validated information sampling task to assess reflection impulsivity and a temporal discounting questionnaire.
Results
Patients gathered significantly less information and made more irrational choices than matched controls. There was, however, no group difference on the temporal discounting questionnaire.
Conclusions
Poor information sampling and irrational decision making may be an inherent component of the neuropsychological deficit in Parkinson's disease. These findings suggest that underlying impulsivity detected by a metric task is common in de novo PD.
Introduction
Impulsive compulsive behaviours (ICBs) are increasingly recognized as a devastating complication of Parkinson's disease (PD) treatment, and include pathological gambling, compulsive sexual disorder, excessive inappropriate shopping, binge eating, compulsive shopping, and the dopamine dysregulation syndrome (Lees syndrome) [1, 2]. ICBs have been reported to be no more frequent in untreated PD than in the general population, which would suggest that PD pathology alone is unlikely to increase the risk of addictive behaviours [3, 4]. However, the relevance of pre-morbid neuropsychological characteristics in the subsequent development of ICBs is suggested by studies describing high novelty seeking personality traits and a history of alcohol or substance dependence and depression as ICB risk factors [5, 6].
The nature of the neuropsychological deficits in early de novo PD is still under investigation despite the first studies being carried out more than thirty years ago [7, 8]. Difficulties in set switching as well as perseverative errors on the Wisconsin Card Sorting Test and on verbal fluency task [7], impairments in reward learning, novelty processing, visuospatial functions and verbal memory have been described in untreated PD patients [9-11] but see [12].
In this study we use the “beads task” to examine “reflection impulsivity” in untreated PD, which is the ability to gather and evaluate information before making a definitive decision. Reflection impulsivity correlates with the presence of impulsive behavior in PD patients treated with dopamine replacement therapies, and is also abnormal in other populations with addictive behaviours including substance abusers and non-PD pathological gamblers [6]. Considering that dopaminergic drugs are regarded as the main cause of ICBs in PD [13] we hypothesized that untreated PD patients would not differ from controls in an information sampling task.
Patients and Methods
All participants provided written informed consent according to the declaration of Helsinki and were recruited consecutively in outpatient clinics to avoid a selection bias. The study was approved by the Ethics Committee of Clementino Fraga Filho University Hospital.
Twenty five PD patients that have never been treated with dopaminergic drugs and twenty healthy controls were recruited from the Federal University of Rio de Janeiro-Brazil. All patients fulfilled the Queen Square Brain Bank criteria for the diagnosis of PD [14] and were screened for subclasses of ICBs in a semi structured interview using accepted diagnostic criteria for pathological gambling [15], compulsive sexual behavior [16] and punding [17]. We also used a self-rated validated questionnaire for impulsive compulsive disorders in Parkinson's disease (QUIP)[18]. Subjects screening positive for ICBs were excluded. Major depression as well as a history of anxiety disorder or apathy was an exclusion criteria.
The Frontal Access Battery (FAB), the Mini-Mental State Examination (MMSE) and UPDRS part III were performed in all participants. Participants who scored less than 26 points on the MMSE were excluded.
Beads Task
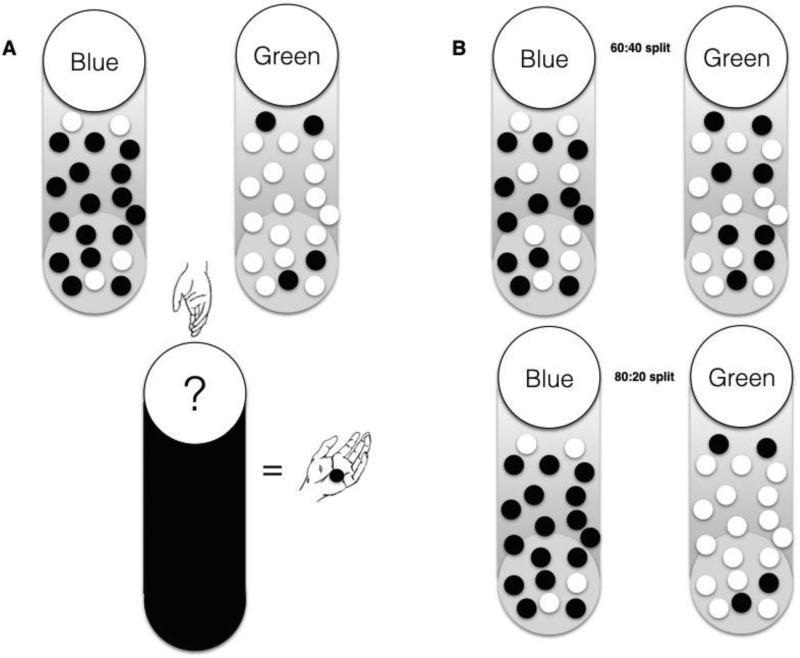
The “beads task” [6, 19] was explained to the participants by the first author (FC) who made sure that all participants fully understood the task before conducting the trial in a quiet room. Participants were told that there were two cups with 200 beads each, one cup containing mostly green beads and fewer blue beads, the other with mostly blue beads and fewer green beads. The computer would pick one of the two cups and begin drawing beads from the cup one at a time. After each bead the participant had to decide whether they knew which cup the bead was being drawn from (i.e. guess either blue or green cup) or ask to see another bead (see figure 1). At the start of the trial they had been told they could draw up to 10 beads before deciding. In general, the more beads drawn by the participant, the better their expected outcome will be, but participants were not told this. Participants had to complete four blocks with two blocks containing an 80:20 ratio of beads and the other two a 60:40 ratio. Incorrect choices resulted in either a loss of nothing (two blocks) or a loss of 10 units (two blocks). All participants had also been informed of the loss condition and the beads ratio (e.g. 80:20 versus 60:40) before each trial and knew each draw would be “charged” with 0.2 units. Each of the four blocks (80:20 loss condition, 80:20 no loss, 60:40 loss condition and 60:40 no loss) was repeated 3 times making a total of twelve trials and was randomly started. We recorded the number of beads drawn before the participant guessed a cup and whether the choice represented a rational (e.g. if more blue beads were drawn the participant guessed blue) or irrational (“opposite”- i.e. guessing the less likely cup colour) choice. Participants were not paid at the end of the study.
Fig 1.
A: Subjects were told that beads could be drawn from one of two cups. One of the cups contained more blue beads than green beads and one contained more green beads than blue beads. One cup was drawn from in each trial. A bead was drawn from the cup and shown to the participant. After seeing the bead the participant could either guess which urn was being drawn from, or choose to see another bead. B: Two different ratios were used. One 60/40 ratio (above) and one 80/20 split (below).
Kirby temporal discounting questionnaire
The Kirby delayed discounting questionnaire was obtained from all participants to assess temporal discounting[20]. A series of 27 choices between smaller, immediate rewards, and larger, delayed rewards was presented e.g. “would you prefer 300 Reais today, or 450 Reais in 30 days?”. The delayed larger rewards were subdivided into three small (150 –200 Reais), medium (300–350 Reais), and large (400–450 Reais) rewards. The hyperbolic discount parameter (k) was calculated for each participant for high, low, and medium monetary rewards. Higher k values correlate with steeper temporal discounting, (taking the smaller immediate reward preferentially).
Data Analysis
Statistical analysis was performed using SPSS, version 21. For the demographic variables, age, gender, years of education, age of disease onset, UPDRS scores, were separately used as dependent variables and group (PD versus controls), where appropriate, was modelled as a between subject factor. We used ANOVA, t-test or χ 2 test where appropriate.
For the behavioral variables we performed analyses using a generalized linear model (SPSS) as described elsewhere [6]. As a dependent variable we used either the number of draws before making a decision or the number of decisions that were contrary to the evidence participants had at the time (opposite colour choice, eg. green bead shown, blue cup selected). A Poisson model which had a log-linear link function was used. For the analyses, beads ratio (80/20 or 60/40) and loss condition (loss, no loss), were modeled as fixed factors. Group (PD versus controls) was modeled as a between factor and subject was a random factor nested under group.
Results
Demographic variables (Table 1) were analyzed using analysis of variance, t-test, or chi-square tests. There were no differences between the control and the patient group on any of the demographic variables. Two patients were taking serotonin reuptake inhibitors but were on stable doses for more than 6 months.
Table 1.
Demographic characteristics.
| PD | Controls | t-value, χ2 | p | |
|---|---|---|---|---|
| Participants (n) | 25 | 20 | ||
| Age (years) | 54.2 ± 10.5 | 52.5 ± 10.3 | 36.0 | 0.57 |
| Gender n (%) (male) | 17 (68) | 12 (60) | χ2 =10.0 | 0.58 |
| Disease Duration(yrs) | 1.4 ± 0,9 | |||
| Education(yrs) | 14.0 ± 3.7 | 14.3 ± 3.22 | 27.0 | 0.83 |
| Hoehn Yahr | 1.9 ± 0.9 | |||
| UPDRS III | 20.6 ± 9.9 | |||
| MMSE | 29.1 ± 0.8 | 29.3 ± 1.1 | 197.6 | 0.55 |
| FAB | 15.5 ± 2.8 | 16.5 ± 1.3 | 45.7 | 0.16 |
| FAB Go-NoGo | 2.53 ± 0.92 | 2.7 ± 0.46 | 1.72 | 0.08 |
| RT (ms) | 5535 ± 2898 | 5066 ± 3692 | 1.60 | 0.11 |
All values are mean ± standard deviation. FAB = Frontal Access Battery, MMSE = Mini-Mental State Examination, UPRS = Unified Parkinson Disease Rating Scale, yrs = years. RT (ms)= Response Time.
Beads Task
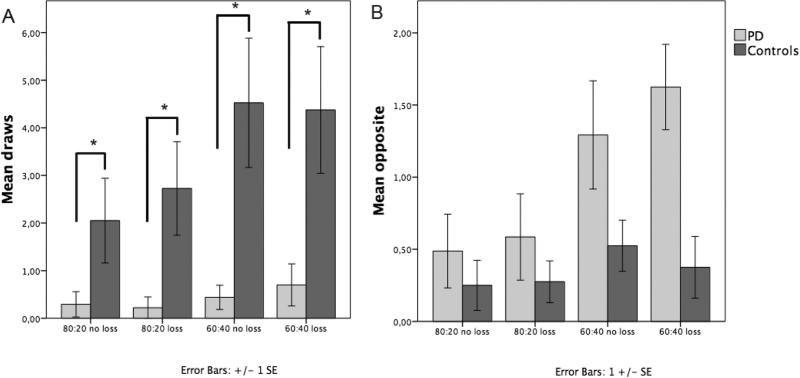
We examined the number of draws each participant made in the different conditions: 80/20 split (loss or no loss) and 60/40 split (loss and no loss) (Fig 2A). Groups (PD and controls), beads ratio (80:20 or 60:40), condition (loss, no loss) were modelled as fixed factors. We found significant effects of group (Wald χ2 = 36,2, p<0.001) and ratio (80/20 versus 60/40) (Wald χ2 = 35,2, p<0.001), but no effect of loss condition (loss or no loss) (Wald χ2 = 0,7, p>0.5). Pairwise comparison showed that all patients gathered less evidence than controls (p<0.001).
Fig 2.
One bead is always shown before the participant must make a decision, so total beads seen are mean draws plus one. A: Average number of draws per condition by group. B: Number of times participants chose the opposite colour. Error bars are +/− 1 SE. Significant differences have been labelled with “*”.
We then examined the number of times participants guessed the less probable cup given the evidence they had (Fig. 2B). We found a main effect of group (Wald χ2= 12.1, p<0.001) with patients making more irrational choices. Furthermore, there was an effect of ratio (Wald χ2 =20.1, p<0.001) but no effect of condition (Wald χ2=0.02, p=0.8) and no interaction of group and ratio (Wald χ2 =0.6, p=0.4).
Finally, we have also assessed response time, which did not show a significant group difference (t=1.07, p=0.11).
Kirby temporal discounting questionnaire
There was no group difference in the K scores (large K, p=0.25, medium K, p=0.55 and small K p=0.83).
There were also no significant relationships between the beads draws with Large K (−0.100, p= 0.64); Medium K (0.113, p=0.59) or Small K (− 0.150, p= 0.48).
FAB Go-NoGo score and beads task
Furthermore, there was no significant correlation between the FAB Go-NoGo score and the beads task (Pearson's correlation coefficient =0.198, p=0.79) and between drug naïve PD versus healthy controls (t=1.72, p=0.08).
Discussion
This is the first study to assess reflection impulsivity in untreated PD, demonstrating that patients gathered significantly less information and made more irrational decisions than healthy controls. These results are surprising as previous studies using the same task showed that PD patients on levodopa monotherapy performed as well as healthy controls, while those who took dopamine agonists jumped to conclusions and made more irrational choices [6, 13]. Furthermore, none of our patients had any ICB symptoms as assessed by the semi structured interview or the QUIP. While premature choices and jumping to conclusions are found in about 20% of the general population [21], our results suggest that subtle underlying impulsivity, only detectable by metric tasks may be even more common in the early stages of PD. Computerized tasks may be more valid than using self-filled questionnaires as people may either lack insight into their impulsivity or deliberately deny such impulsive tendencies [22].
In line with a previous study some patients justified their irrational behaviour by saying that they anticipated the drawn bead belonged to the opposite cup, or that they had “enough evidence” after a single bead was shown [6].
An uneven pattern of dopamine loss, particularly affecting the dorsal striatum, has been described in early PD patients [23]. Recent studies have shown that the dorsal striatum is important for several cognitive tasks including task shifting [24]. Difficulties with decision making, such as reward learning [9] and task switching behavior [25, 26], can be improved by dopamine replacement therapy.
Dorsal striatal dysfunction which has been also linked with impulsivity [27, 28] may thus be responsible for reflection impulsivity in our cohort.
Given the poor task performance in both PD patients on dopamine agonists [13, 29] and de novo patients, it is also possible that an inverted U-shaped curve, where too little or too much dopamine D2/D3 receptor stimulation causes poor task performance, may cause reflection impulsivity. Further studies in a larger cohort of PD patients treated only with dopamine agonists in their on and off state are necessary to confirm this hypothesis.
Finally, it is also possible that cultural differences, which are known in PD [30] are responsible for the results presented here.
It is unlikely that early decisions in our patient cohort are driven by immediate reward seeking behavior, given the lack of effect of punishment condition for incorrect choices in this task and results of a recent functional magnetic imaging study in drug naïve PD patients, showing attenuated mesolimbic and mesocortical regions during gambling [31].
As the beads task is also related to temporal discounting [32] we obtained a temporal discounting questionnaire to assess whether jumping to conclusion is driven by “waiting impulsivity”, which is the inability to wait for a larger but delayed reward over a small immediate gratification. In line with a previous study in the non-PD population [33] there was no correlation between the beads task and temporal discounting and no difference between the PD group and controls. While in standard temporal discounting tasks rewards are delayed by weeks, in the beads task, drawing more of the beads delays a possible reward only by a few seconds and correct responses resulted in small fixed rewards, compared to anticipated large rewards in temporal discounting tasks. Furthermore, the neuronal correlate of waiting impulsivity has been associated with slightly different brain areas including the hippocampus, the amygdala [34]. Furthermore, a lower connectivity of the subthalamic nucleus with ventral striatum has been recently also linked with waiting impulsivity, albeit in the non PD population [35].
A potential limitation of this study is that while we investigate reflection impulsivity and temporal discounting, we cannot assess all other postulated aspects of impulsivity, such as motor impulsivity and risk taking [36-38]. We have, however, found no correlation between the NoGo scores of the FAB and the beads task, making motor impulsivity as the main trigger for poor performance on the beads task unlikely.
Both reflection impulsivity and risk taking fall into the broad category of “cognitive impulsivity” [36, 39]. While altered reward learning and risk taking as well as increased reflection impulsivity can be found in PD [6, 40, 41], the relationship between feedback learning, risk taking and reflection impulsivity is not clear. A functional magnetic resonance imaging (fMRI) study in healthy controls showed activation of a wide brain network including the parietal and prefrontal cortex, the anterior insula, and also the striatum when participants decided to stop sampling and made a decision making on the beads task [19] whereas activation of networks including the orbitofrontal cortex are more associated with a feedback learning task [41]. Behaviourally these tasks are also distinct. For example, previous studies on substance abusers showed this cohort gather significantly less evidence on the beads task but did not perform worse on feedback learning and risk taking tasks [42]. Furthermore, a recent study has suggested that the beads task may be particularly sensitive to assess impulsivity as it is visually less explicit and requires individuals to rely on their internal task representation [33], which is not the case in classical risk taking [43] and feedback learning tests.
Although this is one of the largest behavioural studies in de novo PD patients, the sample size is still small, reflecting the world wide difficulty in obtaining patients for research prior to the initiation of treatment.
Conclusions
We found that irrational decision making and poor ability to gather information is seen in de novo PD patients, who have never been exposed to dopamine replacement therapy. Dysfunction of a brain network including the prefrontal cortex, the striatum and the insula, which is necessary for decision making and inhibitory control may underlie this phenomenon. A follow up study on this patient cohort is currently underway to assess whether levodopa or dopamine agonists can influence this impairment, and whether reflection impulsivity in an untreated state correlates with a subsequent increased risk of ICBs when on dopamine replacement therapies.
References
- 1.Averbeck BB, O'Sullivan SS, Djamshidian A. Impulsive and compulsive behaviors in Parkinson's disease. Annu Rev Clin Psychol. 2014;10:553–580. doi: 10.1146/annurev-clinpsy-032813-153705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker N, Munhoz RP, Teive HA. Lees' syndrome: a case series. Arq Neuropsiquiatr. 2011;69:756–759. doi: 10.1590/s0004-282x2011000600006. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub D, Papay K, Siderowf A, Parkinson's Progression Markers I. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology. 2013;80:176–180. doi: 10.1212/WNL.0b013e31827b915c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonini A, Siri C, Santangelo G, Cilia R, Poletti M, Canesi M, Caporali A, Mancini F, Pezzoli G, Ceravolo R, Bonuccelli U, Barone P. Impulsivity and compulsivity in drug-naive patients with Parkinson's disease. Mov Disord. 2011;26:464–468. doi: 10.1002/mds.23501. [DOI] [PubMed] [Google Scholar]
- 5.Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, Weintraub D, Wunderlich GR, Stacy M. Impulse control disorders in Parkinson disease: a multicenter case--control study. Ann Neurol. 2011;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 6.Djamshidian A, O'Sullivan SS, Sanotsky Y, Sharman S, Matviyenko Y, Foltynie T, Michalczuk R, Aviles-Olmos I, Fedoryshyn L, Doherty KM, Filts Y, Selikhova M, Bowden-Jones H, Joyce E, Lees AJ, Averbeck BB. Decision making, impulsivity, and addictions: do Parkinson's disease patients jump to conclusions? Mov Disord. 2012;27:1137–1145. doi: 10.1002/mds.25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson's disease. Brain. 1983;106(Pt 2):257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- 8.Levin BE, Llabre MM, Weiner WJ. Cognitive impairments associated with early Parkinson's disease. Neurology. 1989;39:557–561. doi: 10.1212/wnl.39.4.557. [DOI] [PubMed] [Google Scholar]
- 9.Bodi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N, Dibo G, Takats A, Bereczki D, Gluck MA. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 11.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 12.Poletti M, Frosini D, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Decision making in de novo Parkinson's disease. Mov Disord. 2010;25:1432–1436. doi: 10.1002/mds.23098. [DOI] [PubMed] [Google Scholar]
- 13.Djamshidian A, O'Sullivan SS, Foltynie T, Aviles-Olmos I, Limousin P, Noyce A, Zrinzo L, Lees AJ, Averbeck BB. Dopamine agonists rather than deep brain stimulation cause reflection impulsivity in Parkinson's disease. J Parkinsons Dis. 2013;3:139–144. doi: 10.3233/JPD-130178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC.: 2000. [Google Scholar]
- 16.Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- 17.Evans AH, Katzenschlager R, Paviour D, O'Sullivan JD, Appel S, Lawrence AD, Lees AJ. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, Adler CH, Potenza MN, Miyasaki J, Siderowf AD, Duda JE, Hurtig HI, Colcher A, Horn SS, Stern MB, Voon V. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson's disease. Mov Disord. 2009;24:1461–1467. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J Neurosci. 2011;31:17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 21.Freeman D, Pugh K, Garety P. Jumping to conclusions and paranoid ideation in the general population. Schizophr Res. 2008;102:254–260. doi: 10.1016/j.schres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Avila A, Cardona X, Bello J, Maho P, Sastre F, Martin-Baranera M. Impulse control disorders and punding in Parkinson's disease: the need for a structured interview. Neurologia. 2011;26:166–172. doi: 10.1016/j.nrl.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald PA, Monchi O. Differential effects of dopaminergic therapies on dorsal and ventral striatum in Parkinson's disease: implications for cognitive function. Parkinsons Dis. 2011;2011:572743. doi: 10.4061/2011/572743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cools R. Dopaminergic modulation of cognitive function-implications for LDOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Djamshidian A, O'Sullivan SS, Lees A, Averbeck BB. Stroop test performance in impulsive and non impulsive patients with Parkinson's disease. Parkinsonism Relat Disord. 2011;17:212–214. doi: 10.1016/j.parkreldis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djamshidian A, O'Sullivan SS, Tomassini A, Foltynie T, Limousin P, Aviles-Olmos I, Warner TT, Lees AJ, Averbeck BB. In a rush to decide: deep brain stimulation and dopamine agonist therapy in Parkinson's disease. J Parkinsons Dis. 2014;4:579–583. doi: 10.3233/JPD-140388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 31.van der Vegt JP, Hulme OJ, Zittel S, Madsen KH, Weiss MM, Buhmann C, Bloem BR, Munchau A, Siebner HR. Attenuated neural response to gamble outcomes in drug-naive patients with Parkinson's disease. Brain. 2013;136:1192–1203. doi: 10.1093/brain/awt027. [DOI] [PubMed] [Google Scholar]
- 32.Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, Fernandez H, Potenza MN, Dolan RJ, Hallett M. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banca P, Lange I, Worbe Y, Howell NA, Irvine M, Harrison NA, Moutoussis M, Voon V. Reflection impulsivity in binge drinking: behavioural and volumetric correlates. Addict Biol. 2015 doi: 10.1111/adb.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Morris LS, Kundu P, Baek K, Irvine MA, Mechelmans DJ, Wood J, Harrison NA, Robbins TW, Bullmore ET, Voon V. Jumping the Gun: Mapping Neural Correlates of Waiting Impulsivity and Relevance Across Alcohol Misuse. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 37.Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- 38.Voon V. Models of Impulsivity with a Focus on Waiting Impulsivity: Translational Potential for Neuropsychiatric Disorders. Curr Addict Rep. 2014;1:281–288. doi: 10.1007/s40429-014-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Djamshidian A, Jha A, O'Sullivan SS, Silveira-Moriyama L, Jacobson C, Brown P, Lees A, Averbeck BB. Risk and learning in impulsive and nonimpulsive patients with Parkinson's disease. Mov Disord. 2010;25:2203–2210. doi: 10.1002/mds.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, Hallett M. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djamshidian A, Sanotsky Y, Matviyenko Y, O'Sullivan SS, Sharman S, Selikhova M, Fedoryshyn L, Filts Y, Bearn J, Lees AJ, Averbeck BB. Increased reflection impulsivity in patients with ephedrone-induced Parkinsonism. Addiction. 2013;108:771–779. doi: 10.1111/add.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]