Abstract
DNM1L encodes dynamin-related protein (DRP1/ DLP1), a key component of the mitochondrial fission machinery that is essential to proper functioning of the mammalian brain. Two previously reported probands with de novo missense mutations in DNM1L presented in the first year of life with severe encephalopathy and refractory epilepsy, one with death at 37 days after birth. In contrast, we report identical novel missense mutations in DNM1L in two unrelated probands who experienced normal development for several years before presenting with refractory focal status epilepticus and subsequent rapid neurological decline. We expand the phenotype of DNM1L-related mitochondrial fission defects, reveal common unique clinical characteristics and imaging findings, and compare the cellular impact of this novel mutation to the previously reported A395D lethal variant. We demonstrate that our R403C mutation acts by a dominant-negative mechanism and reduces oligomerization, mitochondrial fission activity, and mitochondrial recruitment of DRP1, but to a lesser extent compared to the A395D mutation. In contrast to the initial report of neonatal lethality resulting from DNM1L mutation and DRP1 dysfunction, our results show that milder DRP1 impairment is compatible with normal early development and subsequently results in a distinct set of neurological findings. In addition, we identify a common pathogenic mechanism whereby DNM1L mutations impair mitochondrial fission.
Keywords: DNM1L, DRP1, DLP1, mitochondria, fission, epileptic encephalopathy, seizures, developmental regression
INTRODUCTION
Mitochondria are essential for proper cellular function. Classically, “mitochondrial disease” implies a primary defect in a nuclear- or mitochondrial-encoded gene, whose disrupted protein product precludes adequate oxidative phosphorylation, and thus energy production in cells [Lightowlers et al., 2015]. Mitochondrial disease can present with a variety of disparate phenotypic features affecting multiple organ systems and may include developmental delay and regression, which can worsen with intercurrent illness, as well as myopathy, seizures, and other findings [Lightowlers et al., 2015]. Recently, with the advent of clinical whole exome sequencing, the list of conditions associated with mitochondrial dysfunction has greatly expanded and the term mitochondrial disease has been used more broadly. One group of conditions that has emerged involves disrupted mitochondrial dynamics [Chan 2007].
Mitochondrial dynamics, consisting of fusion and fission, is an important regulator of mitochondrial function. Dynamin-related protein 1 (DRP1), or dynamin-like protein 1 (DLP1), encoded by DNML1 [Smirnova et al., 2001], is the central molecular player that mediates mitochondrial fission. It is produced in the cytosol but can be recruited to the mitochondrial surface by receptors located on the outer membrane. There are currently four known DRP1 receptors: FIS1, MFF, MID49, and MID51 [Chan 2012]. MFF appears to be the major DRP1 receptor, because removal of MFF causes the greatest defect in mitochondrial fission [Loson et al., 2013; Otera et al., 2010]. Once recruited to mitochondria, DRP1 assembles into an oligomeric ring that drives constriction and scission of the mitochondrial tubule [Ingerman et al., 2005; Mears et al., 2011]. DRP1 has also been shown to be important for peroxisomal division [Koch et al., 2003].
DRP1 belongs to the GTP-hydrolyzing dynamin superfamily of mechanoenzymes whose activity is regulated by self-assembly via a stalk domain with multiple interacting regions (classically referred to as the middle domain) [Ferguson and De Camilli 2012; Schmid and Frolov 2011]. DRP1 initially forms dimers that are stabilized by stalk-stalk interactions. Via a separate set of stalk-stalk interactions, these dimers then build higher order assemblies that form rings wrapping around the mitochondrial tubule. These rings are thought to constrict the diameter of the mitochondrial tubule and facilitate close lipid membrane interactions that are needed for scission [Ingerman et al., 2005; Mears et al., 2011]. DRP1 self-assembly is also important because it stimulates GTP hydrolysis, which is necessary for scission. Finally, self-assembly facilitates DRP1 recruitment to the mitochondrial surface, because the major DRP1 receptor, MFF, only binds stably to oligomerized DRP1 [Liu and Chan 2015].
As a key component of mitochondrial fission, DRP1 is essential for proper mitochondrial function and furthermore, is critically important in the proper functioning of the mammalian brain and for survival in general. Constitutive homozygous Dnm1l knockout mice die during embryogenesis, and conditional Dnm1l ablation in mouse brain leads to developmental defects, both of which are associated with impaired mitochondrial fission [Ishihara et al., 2009; Wakabayashi et al., 2009]. In humans, two probands with distinct de novo missense mutations in DNM1L have been reported in the literature. One presented in the first days of life with severe neonatal encephalopathy, microcephaly, abnormal brain development in the form of dysmyelination and altered gyral pattern, and optic atrophy. She died at 37 days of age after attaining no developmental milestones [Waterham et al., 2007]. She was found to have a de novo missense mutation in the middle domain of DRP1 (p.A395D), resulting in abnormal mitochondrial and peroxisomal fission. The second proband presented at 6 months of age with global developmental delay, developed refractory epilepsy at one year of age with multiple subsequent episodes of status epilepticus, and remains profoundly globally developmentally delayed. He harbored a de novo missense change in the middle domain of DRP1 (p.G362D), again causing abnormal mitochondrial fission [Vanstone et al., 2015]. In addition, two abstracts have reported additional individuals with DNM1L variants or mutations. The first reported two siblings with an autosomal recessive disorder due to compound heterozygous truncating mutations, both of whom died within the first month of life (Yoon et al., 2014, Neuromuscular disorders, abstract), and the second reported two unrelated individuals with distinct de novo missense changes in DNM1L and global developmental delay (L. Roback et al., 2015, American Society of Human Genetics, abstract).
Here, we report identical, novel, de novo, heterozygous missense mutations in DNM1L [c.1207C>T (p.R403C)] in two unrelated individuals who share a remarkably similar phenotype that is delayed in onset compared to the previously reported cases. Despite the later onset, the course remains quite devastating. Both young boys had undergone a period of essentially normal development but then presented acutely in childhood with status epilepticus after minor metabolic insults and had variably progressive, yet remarkably similar courses involving refractory epilepsy, encephalopathy, developmental regression, myoclonus, and characteristic MRI findings. Through functional studies, we demonstrate that this mutation impacts a critical amino acid residue within the middle domain of DRP1 and exhibits a dominant negative effect involving decreased recruitment of DRP1 to mitochondria, decreased DRP1 oligomerization, and impaired mitochondrial fission, though not as marked as the previously reported A395D lethal variant. Based on these investigations, the R403C mutation appears to be less severe and better tolerated at the phenotypic and cellular level, expanding the clinical presentation of disease associated with DNM1L-related mitochondrial fission defects.
MATERIALS AND METHODS
Appropriate informed consent was obtained from human subjects.
Exome Sequencing and Variant Filtering
Clinical exome sequencing, including variant filtering, was performed by Baylor Miraca Genetics laboratories.
Cloning
Isoform b of mouse dynamin-like protein 1 isoform b (699 amino acids, NCBI NP_001021118.1) was used. DRP1 mutants A395D and R403C were generated by overlapping PCR mutagenesis and verified by sequencing.
Cell lines and cell culture
Cell lines were cultured in DMEM containing 10% fetal bovine serum, 100 I.U./ml streptomycin and 100 μg/ml penicillin. Drp1-null mouse cell lines were a generous gift from Katsuyoshi Mihara (Kyushu University, Fukuoka, Japan). Mff-null mouse cell lines were as previously described [Loson et al., 2013]. Wild-type and Drp1-null MEF cell lines stably expressing mouse DRP1 and DRP1 mutants were generated by retroviral transduction of pQCXIP-based (Clontech, Mountain View, CA) vectors with DRP1 cloned into the BamHI/EcoRI sites with a Kozak sequence. Transduced cells were selected in 0.5 μg/mL puromycin.
Immunostaining and imaging
Antibodies to DRP1 (mouse anti-DLP1, BD Biosciences, San Diego, CA) and TOM20 (rabbit anti-TOM20, Santa Cruz Biotechnology, Santa Cruz, CA) were used for immunostaining. Cells were grown in LabTek chambered glass slides (Nunc, Rochester, NY), then fixed in prewarmed 4% formaldehyde for 10 minutes at 37°C, permeabilized in 0.1% Triton X-100, and incubated with antibodies in 5% fetal calf serum. For cytosol clearing of soluble DRP1, cells were permeabilized with 0.005% digitonin in buffer containing 20 mM HEPES, pH 7.3, 110 mM potassium acetate, 2 mM magnesium acetate, 0.5 mM ethylene glycol tetraacetic acid (EGTA), 220 mM mannitol, 70 mM sucrose, and 2 mM fresh dithiothreitol for 90 seconds at room temperature, then fixed and immunostained as above. Scoring of mitochondrial morphology was performed blind to genotype in triplicates of 100 cells. Imaging was performed with a Plan-Apochromat 63x/1.4 oil objective on a Zeiss LSM 710 confocal microscope driven by Zen 2009 software (Carl Zeiss, Jena, Germany). Images were cropped, globally adjusted for contrast and brightness, and median filtered using ImageJ (National Institutes of Health, Bethesda, MD).
Yeast Two-Hybrid Assay
DRP1 and DRP1 mutants were cloned into either the pGAD-C1 or pGBDU-C1 vectors and transformed into PJ69-4α and PJ69-4a yeast strains, respectively. Transformants were selected with leucine- and uracil-deficient plates, respectively, and haploid combinations for interaction testing were mated by spotting on YPD plus adenine plates, at 30°C. Diploids were selected by replica-plating onto leucine- and uracil-deficient plates (labeled as +Adenine) at room temperature, and interactions were assayed following replica-plating on leucine-, uracil- and adenine-deficient plates (labeled as −Adenine), at room temperature. Growth on adenine-deficient plates indicates a positive interaction, and interaction tests were performed at least three times.
RESULTS
Exome sequencing reveals identical de novo missense DNM1L mutations in two unrelated, previously healthy children with sudden-onset epileptic encephalopathy
Proband 1 was a previously healthy 4 year old male who presented with partial status epilepticus characterized by right hemibody clonus and impaired consciousness two weeks following his Diptheria, Tetanus, and Pertussis (DTaP) booster. The family denied any other illness or trauma in the weeks prior to presentation. Video electroencephalogram on admission revealed diffuse slowing with left central spikes time locked to right hand clonus, and magnetic resonance imaging (MRI) demonstrated curvilinear diffusion changes in the left thalamus (Figure 1A, arrow). MR angiogram was normal. He failed aggressive treatment with multiple antiepileptic drugs, ultimately being placed in pharmacologic coma with pentobarbital titrated to burst suppression for 5 days. Following resolution of his status epilepticus he had expressive and receptive dysphasia, difficulty ambulating without maximum assistance, and decline in cognitive level to that of a toddler. Although his seizures improved on a regimen of phenytoin, levetiracetam, lacosamide, and clonazepam, he was readmitted 6 months after initial discharge with epilepsia partialis continua, which resolved over several days.
Figure 1. Magnetic resonance imaging of brains from probands 1 and 2.
(A) Axial diffusion-weighted magnetic resonance image with white arrow showing curvilinear intensity in the left thalamus in proband 1. (B,C) Coronal magnetic resonance images obtained from proband 1 at (B) initial presentation and (C) one month following resolution of status epilepticus show progressive atrophy of the brain, most marked in the hippocampi. (D) Axial T2 FLAIR magnetic resonance image with white arrow showing hyperintensity in the right central thalamus in proband 2, which was faintly hyperintense on diffusion-weighted imaging as well. (E,F) Coronal magnetic resonance images obtained from proband 2 at (E) initial presentation and (F) one year following resolution of status epilepticus show progressive atrophy of the brain, particularly involving the right posterior putamen and bilateral hippocampi (left greater than right).
His evaluation was extensive and included normal electrolytes, lactate, pyruvate, plasma amino acids, urine organic acids, acylcarnitine profile, very long chain fatty acids and ammonia. Cerebrospinal fluid cell count, protein, glucose, culture, HSV PCR and encephalitis panels were normal. Autoimmune testing for anti-NMDA, anti-GAD, anti-thyroglobulin and ANA were negative. Enzyme testing of palmitoyl-protein thioesterase 1 and tripeptidyl peptidase 1 were normal. Mutation analysis for MELAS (MIM# 540000) and DNA sequencing of POLG (MIM#174763) were negative. Muscle biopsy was normal. Whole exome sequencing revealed a heterozygous de novo variant of unknown significance in DNM1L [c.1207C>T (p.R403C)].
At 8 years of age, seizures continue to occur on average 1-3 times per month without subsequent hospitalizations. He can ambulate short distances without assistance, but requires a wheelchair for longer distance. He has slow processing, inattention, cognitive delay, and intermittent aggressive behavior. He is able to speak, but has dysphasia. Follow-up MRI demonstrates mild diffuse cerebral atrophy, which is marked in the bilateral hippocampi (Figure 1B, C).
Proband 2 was a previously healthy typically developing 5 year old male, except for mild expressive speech delay in the form of dysarthria, who presented suddenly with focal status epilepticus and encephalopathy after minor head trauma involving a collision with another child without loss of consciousness and in the setting of a normal head CT. He had had a viral illness the week prior to presentation, during which he reportedly experienced increased clumsiness and subtle changes in behavior. Prior to that, he had tolerated routine childhood illnesses well. Initial electroencephalogram showed diffuse cerebral disturbance with widespread epileptiform activity maximal over the right fronto-temporal region, correlating with his clinical presentation of status epilepticus involving the left side of his body. His status epilepticus was refractory to multiple therapies, requiring several anti-epileptic medications, steroids, pharmacologic coma with pentobarbital, intravenous immune globulin (IVIg), ketogenic diet, and plasmapheresis prior to resolution. He experienced significant developmental regression and developed sustained myoclonus. He briefly recovered some function, including walking and speaking a few words initially. However, a few months later he presented again in refractory status epilepticus and has not recovered as well. Currently, at age 7, he has profound global developmental delay and remains hypertonic, hyperreflexic with myoclonus, tracheostomy and G-tube dependent, and wheelchair bound.
Initial magnetic resonance imaging revealed a non-specific T2 hyperintensity in the right thalamus (Figure 2D, arrow) with follow up imaging showing similar T2 hyperintensities in the right putamen and right frontal lobe (data not shown). Serial magnetic resonance imaging also revealed progressive global cerebral volume loss, particularly involving the right posterior putamen and bilateral hippocampi, left greater than right (Figures 2E,F). Magnetic resonance angiogram was normal. Magnetic resonance spectroscopy revealed decreased N-acetyl aspartate consistent with neuroaxonal loss or dysfunction, as well as elevated lactate, which can be seen in status epilepticus and in disorders involving mitochondrial dysfunction. An elevated glutamate/glutamine (Glx) peak was also observed, the significance of which is unclear, though it may be related to seizure activity. EEGs during his two hospital admissions continued to show intermittent focal status epilepticus mainly over the right hemisphere, sometimes associated with clinical seizure activity and sometimes subclinically. His EEG progressed to showing bilateral background slowing with rhythmic slowing over the right hemisphere, consistent with a diffuse encephalopathy and focal seizure disorder.
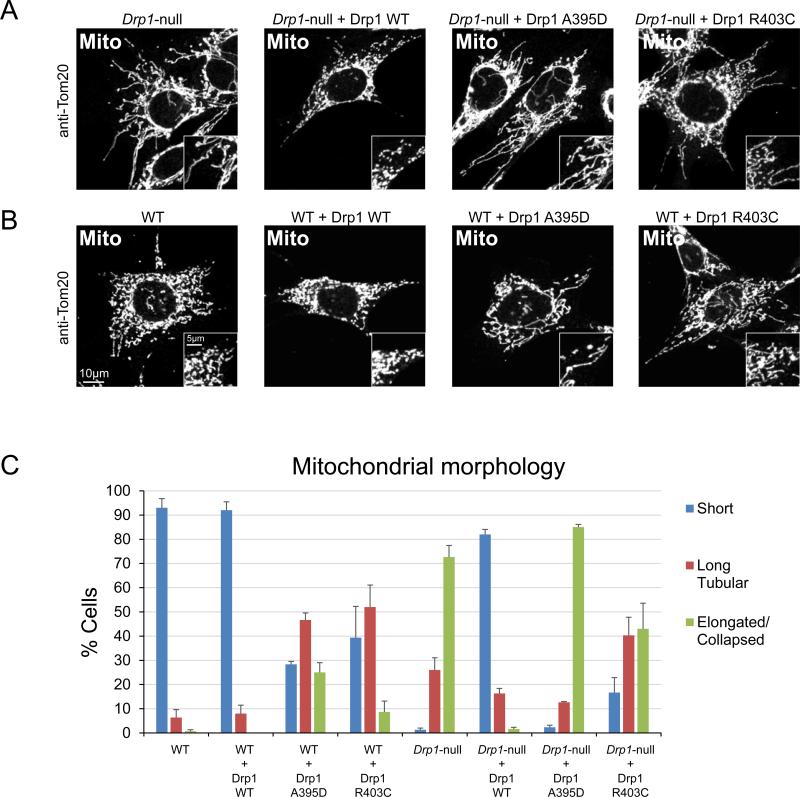
Figure 2. Mutants R403C and A395D have dominant-negative effects on mitochondrial fission.
(A) Fission activity upon expression in Drp1-null MEFs. Drp1-null MEFs expressing wild-type Drp1, Drp1A395D, and Drp1R403C were fixed and immunostained against the outer membrane protein TOM20 to visualize mitochondrial morphology. (B) Same as (A) except that wildtype MEFs were used. (E) Quantitation of mitochondrial morphology. Cells were scored into three categories of mitochondrial profiles: short, long tubular, or elongated and/or collapsed mitochondrial tubules. Quantitation was done in triplicate, with 100 cells scored per experiment. Error bars, SEM.
Extensive neurologic, infectious, rheumatologic, and biochemical genetics evaluations were mostly negative. Notably, lactate and amino acids in plasma and in cerebrospinal fluid were normal, as were analysis of very long chain fatty acids and two separate analyses of urine organic acids. He was found on SNP array to harbor an interstitial duplication on Xp21.3p21.2 involving the IL1RAPL1 gene (MIM#300206), which was inherited from his unaffected mother and is thought to be non-contributory. A forty-gene childhood-onset epilepsy panel, which includes among others the neuronal ceroid lipofuscinosis genes, MECP2 (MIM#300005), and POLG (MIM#174763), was negative.
Whole exome sequencing, including mitochondrial sequencing, was performed. As an aside, he carries two likely tolerated/benign missense variants in ALDH5A1 (MIM#610045), which is the causative gene for the autosomal recessive condition succinic semialdehyde dehydrogenase (SSADH) deficiency (MIM#271980). However, the diagnosis of SSADH deficiency can be ruled out because he lacks the ubiquitous gamma hydroxybutyric aciduria on repeated urine organic acid analyses and has normal levels of free and total gamma-aminobutyric acid (GABA) in cerebrospinal fluid. Ultimately, the proband was found to have a likely causative de novo heterozygous variant of unknown clinical significance in the DNM1L gene (MIM#603850), which encodes DRP1. The novel heterozygous missense change [c.1207C>T (p.R403C)] has not been reported previously in diseased or healthy individuals and is predicted to be not tolerated and damaging by SIFT and Polyphen-2, respectively.
Functional characterization of R403C in mouse embryonic fibroblasts reveals a dominant negative mechanism and impaired mitochondrial fission
To determine whether the R403C mutation affects DRP1 function, we examined the effect of Drp1R403C in mouse embryonic fibroblasts (MEFs), where both wild-type and Drp1 mutant cells are available. In wild-type MEFs under basal conditions, mitochondria exist as a population of short and/or fragmented tubules (Figure 2B, C). In contrast, Drp1-null cells, due to unopposed fusion, have highly elongated and interconnected mitochondria (Figure 2A, C). Expression of wild-type Drp1 in these mutant cells restores fission, resulting in shorter mitochondrial tubules, whereas expression of Drp1A395D fails to rescue fission (Figure 2A, C). Expression of Drp1R403C only partially rescues fission in Drp1-null cells, resulting in an intermediate phenotype. The R403C mutation therefore impairs DRP1 function in MEFs, but not as severely as the A395D mutation.
Because the R403C mutation was found to be heterozygous in the patients, we sought to determine whether Drp1R403C has a dominant-negative effect that can interfere with the function of wild-type DRP1 within the same cell. We expressed the mutant in wild-type MEFs containing endogenous DRP1. Indeed, we find that expression of Drp1R403C interferes with fission activity, resulting in mitochondrial elongation compared to control cells or cells expressing wild-type Drp1 (Figure 2B, C). Consistent with the above results, we also find that expression of Drp1A395D has an even more severe dominant-negative effect, resulting in a higher proportion of cells with elongated mitochondria.
R403C reduces DRP1 recruitment to mitochondria and self-assembly
We wondered whether recruitment of DRP1 to mitochondria might be affected by the R403C mutation. To test this idea, we expressed wild-type Drp1, Drp1A395D, and Drp1R403C in Drp1-null cells and examined their co-localization with mitochondria (Figure 3A). For immunostaining of DRP1, we treated the cells with digitonin to remove cytosolic DRP1 before immunostaining. This treatment fragments mitochondria, but allows clear visualization of DRP1 puncta on mitochondria without interference from cytosolic DRP1 signals. For reference, we examined DRP1 localization in a cell line devoid of a major receptor, MFF, and found that DRP1 co-localization with mitochondria is much reduced compared to wild-type MEFs. In Drp1-null cells, we find that expressed wild-type DRP1 co-localizes with mitochondria, whereas expressed DRP1R403C and DRP1A395D show reduced signals, indicating loss of recruitment (Figure 3A).
Figure 3. Recruitment to mitochondria is impaired in the A395D and R403C mutants.
(A) Analysis of Drp1 alleles in Drp1-null MEFs. Cells expressing the indicated alleles were briefly permeabilized with digitonin to clear cytosolic DRP1 before fixation. This resulted in mitochondrial fragmentation, but DRP1 puncta remained on mitochondria for visualization by immunostaining with an antibody against DRP1 (DRP1, green). Mitochondria were visualized by immunostaining against TOM20 (Mito, red). The DRP1 signal, mitochondrial signal, and merged signals are shown. Inset squares are magnifications of the boxed region in the main image. In the first column, Mff-null MEFs are shown as a reference for defective DRP1 recruitment to mitochondria. (B) Same as (A), except that wildtype MEFs are used.
To examine whether expression of the mutants disrupts localization of endogenous Drp1, we expressed Drp1A395D and Drp1R403C in wild-type cells (Figure 3B). We find that both mutants reduce the amount of DRP1 co-localizing with mitochondria, suggesting that the mutants dominantly interfere with recruitment of endogenous DRP1 to mitochondria.
In previous work, we showed that DRP1 recruitment by the major receptor MFF is dependent on the ability of DRP1 to assemble [Liu and Chan 2015]. We therefore tested whether DRP1 assembly is affected by the R403C mutation in a yeast two-hybrid interaction assay (Figure 4A). Wild-type DRP1 interacts with wild-type DRP1, as indicated by growth of AD--DRP1 against BD-DRP1, whereas DRP1A395D fails to interact with wild-type DRP1 (Figure 4A), indicating loss of higher order oligomer formation between the two. DRP1R403C weakly interacts with wild-type DRP1, suggesting that the R403C mutation affects DRP1 oligomerization but not to the extent of the A395D mutation (Figure 4A). Both A395 and R403 reside in a region of DRP1 thought to be important for mediating the dimer-to-dimer interactions required for assembly into oligomeric rings[Frohlich et al., 2013] (Figure 4B). In dynamin, the homologous residue of DRP1 R403 is R399, which has been demonstrated to be critical for higher order oligomerization [Ramachandran et al., 2007]. The crystal structure of the dynamin tetramer shows that the R399 residue from one dimer forms polar interactions with glutamate residues on adjacent dimers, helping to link one dimer to the next to form tetramers [Reubold et al., 2015] (Figure 4C).
Figure 4. Assembly defect of DRP1 mutants.
(A) DRP1 oligomerization assessed by the yeast two-hybrid assay. DRP1 and DRP1 mutants expressed from the pGAD vector as GAL4 activation domain (AD) fusion proteins were tested against DRP1 expressed from the pGBDU vector as a GAL4 DNA-binding domain (BD) fusion proteins. Growth on adenine-deficient plates indicates an interaction. (B) Ribbon diagram of DRP1 (PDB file: 4BEJ) depicting the location of the R403 and A395 residues. Circled is the region (Interface 3) predicted to mediate dimer-to-dimer interactions during DRP1 oligomerization. (C) Ribbon diagram of the dynamin tetramer (PBD file: 5A3F). The violet and green dynamin monomers comprise the dimer on the left, and the gray and blue monomers comprise the dimer on the right. The inset depicts interface 3 of the dimer:dimer interaction, with the green monomer of the left dimer removed for clarity. The dynamin residues depicted are labeled on the left, next to the corresponding residues on DRP1.
DISCUSSION
Our results indicate that the R403C mutation is a dominant-negative allele that is defective in oligomerization, recruitment to mitochondria, and mitochondrial fission activity. Each of these deficits is also present, and more severe, in the lethal A395D mutation, discovered in an individual who presented with abnormal brain development, encephalopathy, and lactic acidosis [Waterham et al., 2007]. The milder clinical phenotype of the R403C mutation is consistent with its milder defects in DRP1 function, compared to the original A395D mutation.
Interestingly, the higher order oligomerization of DRP1 dimers appears to be critical for recruitment by MFF, the major DRP1 receptor on the mitochondrial surface [Liu and Chan 2015]. In a screen for DRP1 mutants that fail to bind MFF, it was found that mutations impairing DRP1 self-assembly secondarily reduce binding to MFF, whereas binding to the alternative receptors MID49 and MID51 is less affected. Consistent with these findings, the R403C mutant shows reduced self-assembly and impaired recruitment to the mitochondrial surface. Because all the reported heterozygous DNM1L mutations [Vanstone et al., 2015; Waterham et al., 2007] localize to the stalk domain and appear to act as dominant-negative alleles, the impaired DRP1 self-assembly mechanism shown here may be a major pathogenic mode affecting DRP1 function. Self-assembly is critical for DRP1 function, because it enhances GTP hydrolysis activity and facilitates DRP1 recruitment via MFF. Importantly, the A395D and R403C mutations impair higher order assembly but allow dimer formation. Therefore, they act as dominant-negative alleles, allowing heterozygous mutations to greatly impact neuronal function.
We found the R403C mutation in two unrelated individuals with strikingly similar clinical features. Both had been developing essentially normally for 4-5 years before presenting with sudden-onset refractory status epilepticus after distinct minor metabolic insults. Both subsequently had devastating courses with refractory epilepsy, myoclonus, brain atrophy on MRI, and regression, resulting in profound global developmental delay. The predicted damaging nature of the change, which is corroborated by the functional studies herein, as well as the strikingly similar phenotypes observed, make this the likely cause of the probands’ clinical findings. Of the individuals reported to have DNM1L-related mitochondrial fission defects, all except for one sibship have had heterozygous de novo missense mutations impacting the middle domain of DRP1, and both the R403C (reported here) and A395D [Waterham et al., 2007] mutations exhibit a common dominant-negative mutation mechanism, with the A395D mutation exhibiting a more severe cellular and clinical phenotype compared to R403C.
The previously reported individuals with DNM1L-associated mitochondrial fission defects had more severe phenotypes than the two probands reported here with regard to age of onset and severity. Our report therefore expands the phenotypic spectrum of this group of disorders. The patient with the A395D mutation [Waterham et al., 2007] presented in the first days of life with neonatal encephalopathy and died at 37 days of age after attaining no developmental milestones [Waterham et al., 2007]. The patient with the G362D mutation [Vanstone et al., 2015] presented at 6 months of age with global developmental delay and went on to develop refractory epilepsy with repeated bouts of status epilepticus. Finally, the sibship containing compound heterozygous truncating mutations, which would predict little to no DRP1 function, died at 5 days and 3 weeks of life (Yoon et al., 2014, Neuromuscular disorders, abstract). In contrast, the two unrelated probands reported here had periods of essentially normal development until 4-5 years of age when they presented with sudden-onset status epilepticus and subsequent developmental regression and encephalopathy. In all cases, the developmental delay was ultimately profound. The previously reported individuals were not reported to exhibit developmental regression, unlike the probands reported here.
Furthermore, all three living probands have experienced one or more bouts of refractory status epilepticus; however, only two of the three individuals (those reported here) were thought to exhibit similar findings on brain MRI, including diffuse cerebral atrophy involving the hippocampi and non-specific thalamic hyperintensities. Notably, proband 2 was on steroids during his course, which could have contributed to the cerebral atrophy, but the same was not the case for proband 1, supporting the idea that atrophy is part of the disease process rather than being iatrogenic.
Importantly, with respect to laboratory work up, the initially reported A395D mutation exhibited persistently elevated lactate and alanine levels in blood and CSF, and elevated plasma very long chain fatty acids due to the defects in mitochondrial and peroxisomal fission [Waterham et al., 2007]. However, none of the three living probands exhibited these laboratory findings on routine biochemical screening tests, suggesting a less severe defect. Therefore, routine biochemical screening tests will not necessarily identify individuals with these disorders. Because of this and the extremely rare nature of these conditions, whole exome sequencing is currently the most efficient and effective way to diagnose these conditions.
Another provocative similarity between the two cases reported here, particularly with respect to presentation of more classic mitochondrial disorders, is that there is often a preceding illness or other metabolic insult prior to initial presentation and/or prior to episodes of developmental regression or worsening of seizures. Interestingly, each individual reported here experienced a minor metabolic insult prior to initial presentation. Proband one received a DTaP booster a few weeks prior to his presentation, and proband two had a viral illness the week prior to his presentation. These remain interesting correlations at this point, and there is no way to prove causality. Intriguingly however, a similar correlation exists in three individuals with mitochondrial fission defects resulting from homozygous truncating mutations in STAT2 (MIM#600556), a novel regulator of DRP1 [Shahni et al., 2015]. All three presented shortly after receiving the measles, mumps, rubella (MMR) vaccine with febrile illness, and one of them progressed to having opsoclonus-myoclonus, refractory epilepsy, spasticity, and cortical vision impairment [Shahni et al., 2015].
Finally, it is worth noting that several members of the dynamin family of large GTPases [Ferguson and De Camilli 2012; Schmid and Frolov 2011], of which Drp1 is a member, have now been implicated in a range of neurologic disorders. Recently, mutations in dynamin 1 (DNM1;MIM# 602377), a GTPase involved in synaptic vesicle recycling and endocytosis, have been found in seven individuals with epileptic encephalopathy [Euro et al., 2014; Nakashima et al., 2015]. Mutations in dynamin2 (DNM2; MIM#602378), also involved in endocytosis, are responsible for some forms of Charcot-Marie-Tooth [Zuchner et al., 2005], centronuclear myopathy [Bitoun et al., 2007], and a lethal congenital syndrome [Koutsopoulos et al., 2013]. Mutations in atlastin-1 (ATL1; MIM#606439), involved in homotypic fusion of the endoplasmic reticulum, have been implicated in some forms of spastic paraplegia [Zhao et al., 2001] and hereditary sensory neuropathy [Guelly et al., 2011]. Furthermore, mutations in the dynamin family genes MFN2 (MIM#608507) and OPA1 (MIM#605290), involved in mitochondrial fusion, also cause neurological defects in humans [Carelli and Chan 2014; Reddy et al., 2011]. The latter observation, along with the DNM1L mutation reported here, suggests that defects in either mitochondrial fusion or fission can cause neurological disease. Indeed, more common late-onset neurodegenerative conditions, like Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis (ALS), have been shown to exhibit abnormal mitochondrial dynamics [Reddy et al., 2011]. These observations further support mitochondrial fission and fusion defects as a pathological mechanism causing significant neurocognitive compromise, and thus morbidity and mortality, for individuals of all ages with both rare and common diseases, and sets the stage for the design of novel therapeutic strategies aimed at restoring the disrupted balance of mitochondrial dynamics.
ACKNOWLEDGEMENTS
We first and foremost thank the patients and their families for permission to publish this work. We thank Zhiyv Niu, PhD, FACMG, for connecting the authors. We thank Vera Joanna Burton, MD, PhD, and Thangamadhan Bosemani, MBBS, for assistance with obtaining MR images. This work was supported by NIH grant GM110039.
Footnotes
WEB RESOURCES
The URLs for data presented herein are as follows:
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
SIFT, http://sift.jcvi.org/
The authors have no conflicts of interest to declare.
REFERENCES
- Bitoun M, Bevilacqua JA, Prudhon B, Maugenre S, Taratuto AL, Monges S, Lubieniecki F, Cances C, Uro-Coste E, Mayer M, Fardeau M, Romero NB, Guicheney P. Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol. 2007;62(6):666–670. doi: 10.1002/ana.21235. [DOI] [PubMed] [Google Scholar]
- Carelli V, Chan DC. Mitochondrial DNA: impacting central and peripheral nervous systems. Neuron. 2014;84(6):1126–1142. doi: 10.1016/j.neuron.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial dynamics in disease. N Engl J Med. 2007;356(17):1707–1709. doi: 10.1056/NEJMp078040. [DOI] [PubMed] [Google Scholar]
- Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- Euro E-RESC, Epilepsy Phenome/Genome P, Epi KC De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet. 2014;95(4):360–370. doi: 10.1016/j.ajhg.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13(2):75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich C, Grabiger S, Schwefel D, Faelber K, Rosenbaum E, Mears J, Rocks O, Daumke O. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein. EMBO J. 2013;32(9):1280–1292. doi: 10.1038/emboj.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelly C, Zhu PP, Leonardis L, Papic L, Zidar J, Schabhuttl M, Strohmaier H, Weis J, Strom TM, Baets J, Willems J, De Jonghe P, Reilly MM, Frohlich E, Hatz M, Trajanoski S, Pieber TR, Janecke AR, Blackstone C, Auer-Grumbach M. Targeted high-throughput sequencing identifies mutations in atlastin-1 as a cause of hereditary sensory neuropathy type I. Am J Hum Genet. 2011;88(1):99–105. doi: 10.1016/j.ajhg.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170(7):1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11(8):958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Koch A, Thiemann M, Grabenbauer M, Yoon Y, McNiven MA, Schrader M. Dynamin-like protein 1 is involved in peroxisomal fission. J Biol Chem. 2003;278(10):8597–8605. doi: 10.1074/jbc.M211761200. [DOI] [PubMed] [Google Scholar]
- Koutsopoulos OS, Kretz C, Weller CM, Roux A, Mojzisova H, Bohm J, Koch C, Toussaint A, Heckel E, Stemkens D, Ter Horst SA, Thibault C, Koch M, Mehdi SQ, Bijlsma EK, Mandel JL, Vermot J, Laporte J. Dynamin 2 homozygous mutation in humans with a lethal congenital syndrome. Eur J Hum Genet. 2013;21(6):637–642. doi: 10.1038/ejhg.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowlers RN, Taylor RW, Turnbull DM. Mutations causing mitochondrial disease: What is new and what challenges remain? Science. 2015;349(6255):1494–1499. doi: 10.1126/science.aac7516. [DOI] [PubMed] [Google Scholar]
- Liu R, Chan DC. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol Biol Cell. 2015 doi: 10.1091/mbc.E15-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24(5):659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18(1):20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Kouga T, Lourenco CM, Shiina M, Goto T, Tsurusaki Y, Miyatake S, Miyake N, Saitsu H, Ogata K, Osaka H, Matsumoto N. De novo DNM1 mutations in two cases of epileptic encephalopathy. Epilepsia. 2015 doi: 10.1111/epi.13257. [DOI] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191(6):1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Surka M, Chappie JS, Fowler DM, Foss TR, Song BD, Schmid SL. The dynamin middle domain is critical for tetramerization and higher-order self-assembly. EMBO J. 2007;26(2):559–566. doi: 10.1038/sj.emboj.7601491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67(1-2):103–118. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubold TF, Faelber K, Plattner N, Posor Y, Ketel K, Curth U, Schlegel J, Anand R, Manstein DJ, Noe F, Haucke V, Daumke O, Eschenburg S. Crystal structure of the dynamin tetramer. Nature. 2015;525(7569):404–408. doi: 10.1038/nature14880. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Frolov VA. Dynamin: functional design of a membrane fission catalyst. Annu Rev Cell Dev Biol. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- Shahni R, Cale CM, Anderson G, Osellame LD, Hambleton S, Jacques TS, Wedatilake Y, Taanman JW, Chan E, Qasim W, Plagnol V, Chalasani A, Duchen MR, Gilmour KC, Rahman S. Signal transducer and activator of transcription 2 deficiency is a novel disorder of mitochondrial fission. Brain. 2015;138(Pt 10):2834–2846. doi: 10.1093/brain/awv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstone JR, Smith AM, McBride S, Naas T, Holcik M, Antoun G, Harper ME, Michaud J, Sell E, Chakraborty P, Tetreault M, Care4Rare C, Majewski J, Baird S, Boycott KM, Dyment DA, MacKenzie A, Lines MA. DNM1L-related mitochondrial fission defect presenting as refractory epilepsy. Eur J Hum Genet. 2015 doi: 10.1038/ejhg.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186(6):805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356(17):1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- Zhao X, Alvarado D, Rainier S, Lemons R, Hedera P, Weber CH, Tukel T, Apak M, Heiman-Patterson T, Ming L, Bui M, Fink JK. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat Genet. 2001;29(3):326–331. doi: 10.1038/ng758. [DOI] [PubMed] [Google Scholar]
- Zuchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De Jonghe P, Merory J, Oliveira SA, Speer MC, Stenger JE, Walizada G, Zhu D, Pericak-Vance MA, Nicholson G, Timmerman V, Vance JM. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet. 2005;37(3):289–294. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]