Abstract
To act on the environment, organisms must perceive object locations in relation to their body. Several neuroscientific studies provide evidence of neural circuits that selectively represent space within reach (i.e., peripersonal) and space outside of reach (i.e., extrapersonal). However, the developmental emergence of these space representations remains largely unexplored. We investigated the development of space coding in infant macaques and found that they exhibit different motor strategies and hand configurations depending on the objects’ size and location. Reaching-grasping improved from 2 to 4 weeks of age, suggesting a broadly defined perceptual body schema at birth, modified by the acquisition and refinement of motor skills through early sensorimotor experience, enabling the development of a mature capacity for coding space.
Keywords: premotor cortex, space representation, peripersonal space, body schema, motor development
1. Introduction
A central issue in cognitive development is how the brain constructs a map of the surrounding world and how this map interacts with the internal representation of one’s body. To interact with an object, it is necessary to determine whether an object is in the near-reachable space or in the far-unreachable space. The capacity to discriminate an object as reachable involves not only information about properties of objects and affordances for action and interaction, but also spatial information of objects and their relation to the body and the possibilities of acting (Witt, Profitt, & Epstein, 2004).
Objects in peripersonal space, that is, the space immediately surrounding the body, can be easily grasped and manipulated, whereas objects located beyond this space (i.e., extrapersonal space) cannot be reached without moving the torso towards the object. To plan appropriate behavioral patterns, the brain needs to differentiate objects situated in peripersonal space from those in extrapersonal space (Previc, 1990, 1998; Rizzolatti et al., 1988; Rizzolatti, Fadiga, Fogassi, & Gallese, 1997). In support of this, neurophysiological experiments in adult nonhuman primates and neurologically impaired human patients reveal that the space immediately surrounding the body is represented differently than space farther away (Brain, 1941; Sommer, 1969). Furthermore, the brain has different ways of coding the position of objects placed at different locations with respect to the body (Iriki, Tanaka, & Iwamura, 1996; Làdavas, 2002; Shelton, Bowers, & Heilman, 1990).
Other neurophysiological studies in monkeys reveal additional details of how different brain areas are involved in space coding (Rizzolatti & Luppino, 2001). For example, bimodal neurons, which code for peripersonal and extrapersonal space, have been described in inferior parietal areas and the ventral premotor cortex (Duhamel, Colby, & Goldberg, 1998; Fogassi et al., 1992, 1996; Graziano & Cooke, 2006; Graziano, Yap, & Gross 1994; Graziano & Gross, 1998). These neurons are activated by visual as well as somatosensory stimulation applied to specific body parts, and exhibit higher activity when visual stimuli are within peripersonal space, compared to extrapersonal space. Recent behavioral and neuroimaging studies with healthy individuals suggest the presence of a functionally homologous space coding system in humans (Bremner, Schlack, Duhamel, Graf, & Fink, 2001; Macaluso & Maravita, 2010; Pavani & Castiello, 2004; Spence, Pavani, & Driver, 2000; Spence, Pavani, Maravita, & Holmes, 2004). Moreover, there are reports of brain-damaged patients with specific impairments in detecting information within peripersonal space, but not extrapersonal space, or vice versa in extrapersonal space (Brozzoli, Demattè, Pavani, Frassinetti, & Farnè, 2006; Cowey, Small, & Ellis, 1994; Farnè, Demattè, & Làdavas, 2003; Làdavas & Farnè, 2004; Vuilleumier, Valenza, Mayer, Reverdin, & Landis, 1998).
Studies in monkeys have shown that several parietal-premotor circuits work in parallel supporting sensorimotor transformation to control arm-hand movements in space. In fact, in the posterior parietal area PFG there are neurons that code not only for space, but are also active while the monkey executes a grasping action. In fact, about half of PFG neurons showing responses to visual stimuli moving in peripersonal space fired also during the active arm movement (Rozzi, Ferrari, Bonini, Rizzolatti, & Fogassi, 2008). Similar properties have been found in anatomically connected ventral premotor areas F4 and F5 (Maranesi et al., 2012). Other parietal cortical areas, located within the intraparietal sulcus (area AIP), possess neurons that are critically involved in grasping. For example, in both areas AIP and F5 there are neurons that have visuo-motor properties, firing both during the observation of objects and during grasping (Murata, Gallese, Luppino, Kaseda, & Sakata, 2000; Raos, Umiltá, Murata, Fogassi, & Gallese, 2006). Together, these and other investigations demonstrate the existence of several cortical parietal and premotor areas that code for several aspects of actions, and suggest that neuronal visual responses related to objects and space are tightly linked with the possibility of the body to move in space and to reach targets. These circuits have been proposed to play an important role in the organization of goal-directed movements in space, and lesions to these circuits can produce several impairments in visually-guided reaching and grasping (Fogassi, Gallese, Buccino, Craighero, Fadiga, & Rizzolatti, 2001).
The link between the control of movement and space perception has been emphasized by several neurophysiological and neuropsychological studies (Matelli & Luppino, 2001; Rizzolatti, Fogassi, & Gallese, 2002). Interestingly, space coding for peripersonal and extrapersonal space appears to be dynamic in that it can be modified by active movement and sensorimotor experience. For example, tool use can change the perception of the spatial relation between the body and the object upon which the tool is acting (Berti & Frassinetti, 2000; Berti & Rizzolatti, 2002; Halligan, Fink, Marshall, & Vallar, 2003; Iriki et al., 1996; Longo & Lourenco, 2007; Maravita & Iriki, 2004; Rizzolatti, Matelli, & Pavesi, 1983; Weiss, Marshall, Zilles, & Fink, 2003). Another example is that of a patient affected by selective neglect for the space close to the body, whose symptoms were transferred into the far space after using an extension tool, as if the use of the tool remapped the far space as near-reachable space (Berti & Frassinetti, 2000).
Although developmental research on space coding is sparse, behavioral studies provide initial indications that young human infants have some spatial representation of peripersonal space when planning and executing reach movements (Clifton, Rochat, Litovsky, & Perris, 1991; Field, 1977). For example, 3-month-old human infants appear to encode locations egocentrically, showing initial evidence of a gradual shift from egocentric to object-centered references by 6 months (Gilmore & Johnson, 1997; Newcombe & Huttenlocher, 2003). It has been also reported that, between 4 and 5 months of age, human infants develop the ability to coordinate and update their spatial reference frames in relation to their capacity to lean the body forward towards objects (Yonas & Hatman, 1993). Moreover, 12-month-old human infants can use a tool to extend their spatial interaction range (McKenzie, Skouteris, Day, Hartman, & Yonas, 1993). Despite these studies highlighting important aspects of the development of space processing, the development of spatial discrimination—distinguishing peripersonal and extrapersonal space—remains largely unexplored.
Some studies have shown that infant macaques develop the capacity to reach and grasp starting from the third week of life (Lawrence & Hopkins, 1976; Nelson et al., 2011). In addition, other studies report that infant macaques’ capacity to move autonomously in their surrounding environment is precocious, beginning in the first week of life (Hinde, Rowell, & Spencer-Booth, 1964; Maestripieri, 1996). This suggests that the first weeks of life are critical for the development of body maps, which support infants’ capacity to move in the environment and interact with objects. Although there is a strict dependence between the development of motor skills and space representation, the relationship between these two aspects of motor development has, to our knowledge, not been thoroughly explored. In the present study, we investigated the development of space processing in rhesus macaques between the second and the fourth weeks of life. Specifically, we presented infants with objects of different sizes at different distances from the body (i.e., peripersonal and extrapersonal space) and examined both the behavioral patterns used to reach objects and grip configurations for grasping objects.
A nonhuman primate model, and a macaque monkey model specifically, is advantageous for investigating the development of space perception. First, the neural mechanisms underpinning the control of reaching-grasping movement and space coding in humans and macaques are similar and have been described in great detail. In fact, homologue cortical areas control reaching-grasping and process peripersonal space in both species (Bremner, Schlack, Duhamel, Graf, & Fink, 2001; Macaluso & Maravita, 2010; Pavani & Castiello, 2004; Spence, Pavani, & Driver, 2000; Spence, Pavani, Maravita, & Holmes, 2004). Second, given human infants’ limited response repertoire in the first few months of life, it would be difficult to investigate the development of grasping in different space sectors. In contrast, macaques have a precocious motor development, making it possible to follow each infant’s motor skill development, including their grasping and locomotion in relation to their perception of space.
2. Materials and Methods
2.1. Pilot Testing
2.1.1. Subjects and Procedures
To develop a reaching-grasping task, we carried out a pilot study in the first and second weeks of infants’ life, in which we tested 4 infants in their incubators, while still clinging to their surrogate, by presenting them with a large colored ball (21 mm diameter). We initially moved the ball both horizontally and vertically in front of them in order to evaluate the timing of the emergence of their ability to visually track the object and to adjust their posture. We then presented the ball in front of the infants and recorded any attempts to reach-grasp the target.
2.1.2 Preliminary Behavioral Observations
Preliminary observations revealed that from the fourth day of life all infants were able to visually track the moving object. Moreover, their capacity to cling firmly to the surrogate and move one arm in space to grasp objects improved in the first days of life, suggesting that in the first week of life important postural adjustment and coordinated visuomotor movements are emerging. However, their first attempts to reach and grasp the ball appeared only at day 7. In fact, starting from this age and across the second week of life, we scored a total of 81 reaching attempts, 51 (63%) of which ended with successful grips.
Based on these preliminary observations, we designed a task aimed at investigating the development of infants’ capacity to perceive objects’ size and space location.
2.2. Reaching-Grasping Task
2.2.1 Subjects and Housing
Subjects were 16 infant macaques (Macaca mulatta), 10 males and 6 females, followed longitudinally from age 7 to 30 days (mean infant age: week 2 M = 10.19 days, SD = 1.03, week 3 M = 17.33 days, SD = .90, week 4 M = 24.31 days, SD = 1.11). Infants were separated from their mother on day 1 post-partum and reared in a nursery facility according to procedures described by Shannon, Champoux, and Suomi (1998). Infants were individually housed in plastic incubators (51 × 38 × 43 cm) during the first two weeks of life, and housed in metal cages (65 × 73 × 83 cm) from the third week onward. Both housing arrangements contained an inanimate surrogate mother as well as loose pieces of fleece fabric, one plush toy, and various plastic toys. During the first week of life, the surrogate mother was composed of 16.5 cm circumference polypropylene cylinder, wrapped in fleece fabric and attached by a flexible metal component to an 11.5 cm wide circular metal base. In the second week, infants were provided with a hanging surrogate mother (see also Dettmer, Novak, Meyer, Ruggiero, & Suomi, 2008), consisting of a plastic cylinder core (20 cm high and 19 cm circumference) with a wide soft cloth cover (20 × 25 cm). For the first month of life, infants could see and hear, but not physically touch, other infants. For further details about rearing procedures, see also Ferrari, Visalberghi, Paukner, Fogassi, Ruggiero, and Suomi (2006).
Testing was conducted in accordance with regulations governing the care and use of laboratory animals. The Animal Care and Use Committee of the National Institutes of Health approved this study.
2.2.2. Experimental Setting
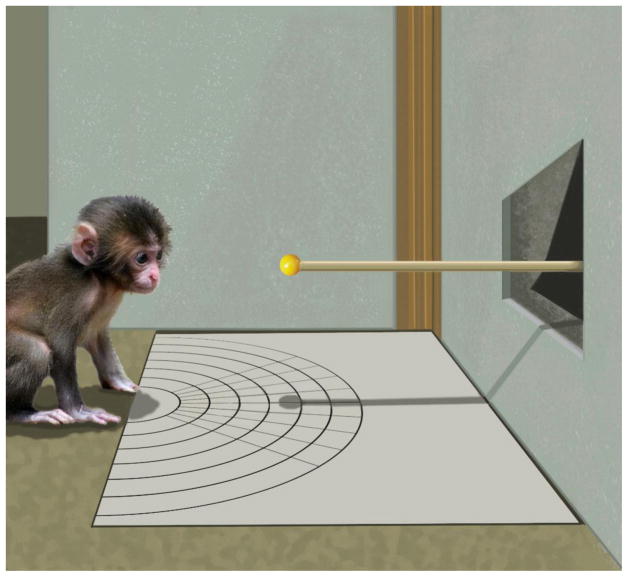
For the experimental setting, we positioned a rectangular board (31.6 cm × 36.5 cm) on a table. On the board there were 7 semicircles drawn as markers to allow us to calculate the exact distance between the stimulus and the infant. Opaque vertical panels were positioned along two sides of the table to prevent the infant from seeing the person presenting the stimuli and other distractions. The panel immediately in front of the infant contained a small window, through which one experimenter presented ball stimuli to the infant (Figure 1). We recorded all sessions with two Sony Digital Video camcorders (ZR600 and HDR-CX560V), one with a side view of the infant, and one with an aerial view of the infant.
Figure 1.
Schematic illustration of the experimental setting from a side view.
2.2.3. Procedure
At the start of the test session, infants were placed on the board and positioned within the area defined by the first semicircle. One experimenter held the infant in a stable position, supporting the infant’s chest with her hands and ensuring the infant’s arms and shoulders remained free to move. Once the infant was positioned, a second experimenter presented the infant with one ball at a time. Balls were of two sizes—large (21 mm diameter) and small (7 mm diameter)—, and were presented at two distances: (i) at a reachable distance (i.e., near or peripersonal space, within 5 cm from the infant), which was calculated using the average length of infants’ arms (M = 10.71 cm, SD = .95 cm), and (ii) at an unreachable distance (i.e., far or extrapersonal space, 14 cm from the infant), calculated as 20% longer than the average length of the infants’ arms (M = 12. 85 cm, SD = 1.14 cm). The ball was attached to a stick and presented through the window of the vertical panel in front of the infant. Infants participated in one session per day of up to 8 trials.
There were four types of trials (small and large balls presented in near and far space), each presented twice per session, in a predetermined counter-balanced order. Each trial lasted 20 seconds. If the infant interacted with the ball, the experimenter let the infant manipulate the ball for few seconds, after which the ball was removed. If the infant made no interaction attempt within 20 seconds, the trial was terminated and the ball removed. Infants were tested twice per week, for 4 weeks, between days 7 and 30.
2.2.4. Behavioral analysis
We analyzed videos frame-by-frame (33 frames per sec) using VirtualDub software (virtualdub.org). We scored infants’ motor strategies to approach and reach the ball: (i) arm extension, if infants attempted to contact the ball by extending their arms and fingers toward the target, without stepping forward; (ii) mouth grasping, if infants leaned their torsos and heads forward, without stepping toward the target. During the movement and the approach to the target, behaviors were classified as (iii) locomotion-arm, if infants approached the target with one or more steps, followed by arm extension and ball grasping with their hands; (iv) locomotion-mouth, if infants approached the target through one or more steps, followed by grasping the ball with their mouths (see Supplementary Material, Videos S1–S4). Only the first effector used by infants to reach and grasp the ball, either the hand or the mouth, was included in the analysis. However, we assessed, for each infant, the percentage of attempts in which there was hand grasping followed by bringing the object to the mouth, or, vice versa, of mouth grasping followed by grasping and holding the object with the hand. This analysis included all successful grasping attempts made by infants. A grasp was considered successful when infants contacted the ball either with the mouth or the fingers flexed around the target.
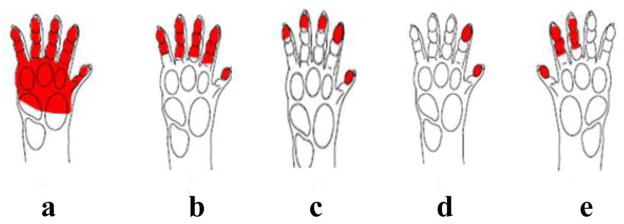
We also scored infants’ hand grip configurations during ball grasping and classified them as (a) hand wrap, (b) thumb-and-four-finger grip, (c) all-tips grip, (d) pad-to-pad grip, or (e) thumb-to-second-third grip (Figure 2; see also McFarlane & Graziano, 2009 for grip definitions, and Supplementary Material, Videos S4–S7). We then categorized these grip configurations depending on the parts of the hand that touched the ball. Configurations were classified as power grips (Figure 2 a–c), if the ball was held between the palm and the fingers, and with the fingers flexed around the ball, or were classified as precision grips (Figure 2 d–e), if the ball was pinched between the second or third digit and the opposing thumb (Napier, 1956). This analysis included only successful grasps.
Figure 2.
Hand grip configurations. (a) hand wrap, (b) thumb-and-four-finger grip, (c) all-tips grip, (d) pad-to-pad grip, (e) thumb-to-second-third grip. Configurations a, b, and c were classified as power grips; configurations d and e were classified as precision grips.
2.2.5. Statistical Analysis
To determine whether infants’ motor strategies varied across weeks and as a function of presentation distance, we applied a 4 (Motor Strategy: arm extension, mouth, locomotion-arm, locomotion-mouth) × 2 (Space: extrapersonal, peripersonal) × 3 (Age: week 2, week 3, week 4) two-way repeated measures analysis of variance (ANOVA) on the mean frequency of successful grips.
To investigate whether infants’ reaction times from the presentation of the target to the onset of reaching movements varied across weeks as a function of the effector used to grasp the ball and the presentation distance, we applied a 2 (Effector: hand, mouth) × 2 (Space: extrapersonal, peripersonal) × 3 (Age: week 2, week 3, week 4) two-way repeated measures ANOVA on the mean latency of successful grips.
To investigate whether infants’ grip configurations changed across weeks and according to ball size, we carried out a 2 (Grip Type: power, precision) × 2 (Ball Size: small, large) × 3 (Age: week 2, week 3, week 4) two-way repeated measures ANOVA on the mean frequency of successful grips.
To adjust for multiple comparisons, we performed post-hoc comparisons with Bonferroni corrections. Since the data were non-normally distributed, they were square root transformed prior to analysis.
3. Results
3.1. Motor Strategy
Overall, the present results revealed that infant monkeys perceived objects at varying distances in space, and successfully reached and grasped objects by using either their hands or mouths as effectors. Moreover, it is interesting to note that infants often performed hand-mouth coordinated actions to explore the objects. In fact, we observed several occurrences in which infants reached for objects using their hands first, followed by bringing the object to their mouth, or they approached the object with their mouth first, followed by their hand grasping and holding the object. In peripersonal space, infants’ mean percentage of hand-mouth actions was 21.4 (SD = 5.9) in the second week of life, 29.7 (SD = 8.7) in the third week, and 16.6 (SD = 7.0) in the fourth week, whereas in the extrapersonal space, it was 8.0 (SD = 3.2) in the second week, 15.4 (SD = 3.1) in the third week, and 12.5 (SD = 4.1) in the fourth week.
Over the course of development, infants’ use of behavioral strategies to obtain objects changed, suggesting that critical improvements in sensorimotor integration were occurring. Analysis of motor strategies revealed main effects for strategy, F (3, 15) = 17.85, p < .001, η2p = .543, space, F (1,15) = 15.76, p = .001, η2p = .512, and week, F (2, 15) = 4.76, p = .016, η2p = .241, showing that infants performed more successful grips in the peripersonal space (M = .657, SD = .031) than in extrapersonal space (M = .559, SD = .041, p = .001), and that the frequency of grips increased significantly from week 2 (M = .434, SD = .073) to week 3 (M = .700, SD = .060, p = .001), and from week 3 to week 4 (M = .691, SD = .066, p = .049).
We found an interaction between strategy and space, F (3, 15) = 27.07, p < .001, η2p = .643, which indicates that infants reached and grasped the ball by simply extending their own hand or mouth more in the peripersonal space (hand: M = 1.10, SD = .091; mouth: M = .499, SD = .077) than in the extrapersonal space (hand: M = .286, SD = .062, p < .001; mouth: M = .042, SD = .028, p < .001), whereas locomotion followed by hand or mouth grasp was more frequent in the extrapersonal space (locomotion-arm: M = .788, SD = .116; locomotion-mouth: M = 1.121, SD = .147) than in the peripersonal space (locomotion-arm: M = .142, SD = .068, p = .001; locomotion-mouth: M = .884, SD = .099, p = .026).
Moreover, there was an interaction between strategy and week, F (6, 15) = 3.20, p = .007, η2p = .176, in which the use of the arm increased from week 2 (M = .232, SD = .069) to week 3 (M = .724, SD = .142, p = .014), and from week 2 to week 4 (M = 1.127, SD = .175, p = .001), but there was no difference between week 3 (M = .724, SD = .142) and week 4 (p = .127). Similarly, the use of the hand in association with locomotion increased from week 2 (M = .289, SD = .094) to week 3 (M = .609, SD = .117, p = .047).
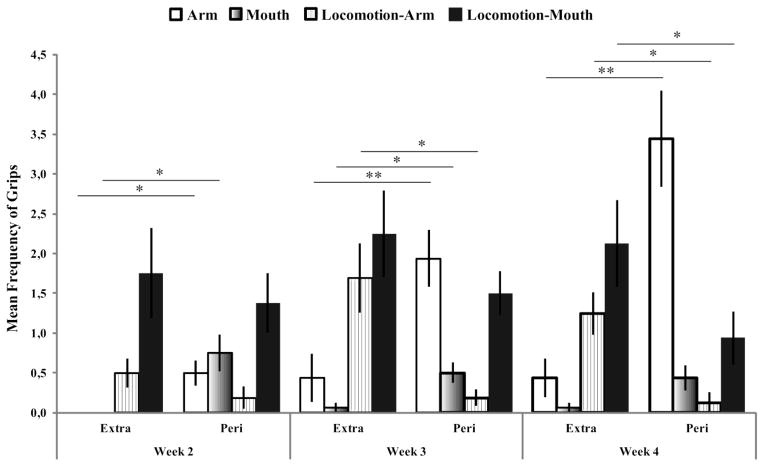
Finally, we found a three-way interaction among strategy, space, and week, F (6, 15) = 2.65, p = .020, η2p = .150 (Figure 3). Post-hoc comparisons revealed that in peripersonal space there was a progressive increase of the use of the arm from week 2 (M = .463, SD = .138) to week 3 (M = 1.215, SD = .175, p = .009), and from week 2 to week 4 (M = 1.631, SD = .227, p = .001), with a parallel decrease of the use of locomotion in association with the mouth from week 3 (M = 1.140, SD = .115) to week 4 (M = .655, SD = .184, p = .049). Similarly, in extrapersonal space, infants exhibited an increase in successful grips performed with the hand, although still in combination with locomotion, from week 2 (M = .427, SD = .146) to week 3 (M = 1.030, SD = .205, p = .029), and from week 2 to week 4 (M = .908, SD = .168, p = .034), but there was no difference between week 3 (M = 1.030, SD = .205) and week 4 (M = .908, SD = .168, p = .586). Across all the three weeks, infants used their arm more in peripersonal space (week 2: M = .463, SD = .138; week 3: M = 1.215, SD = .175; week 4: M = 1.631, SD = .227) than in extrapersonal space (week 2: M = .00, SD = .00, p = .004; week 3: M = .233, SD = .160, p < .001; week 4: M = .623, SD = .168, p < .001). Similarly, across all weeks, infants’ mouths were used more in peripersonal space (week 2: M = .598, SD = 162; week 3: M = .500, SD = .129; week 4: M = .401, SD = .136) than in extrapersonal space (week 2: M = .00, SD = .00, p = .002; week 3: M = .063, SD = .063, p = .004; week 4: M = .063, SD = .063, p = .021). In contrast, infants’ frequency of locomotion-arm, both in the third and fourth week of life, was higher in far space (week 3: M = 1.030, SD = .205; week 4: M = .908, SD = .168) compared to near space (week 3: M = .188, SD = .101, p = .002; week 4: M = .088, SD = .088, p = .002). Finally, in the fourth week of life, infants’ frequency of locomotion followed by mouth contact was higher in far space (M = 1.157, SD = .229) compared to near space (M = .655, SD = 184, p = .004).
Figure 3.
Motor strategies adopted by infant macaques to successfully reach the ball in the peripersonal and extrapersonal space across age. In peripersonal space, with age, infants increased use of their arm and decreased use of locomotion-mouth. Similarly, in the extrapersonal space infants, with age, showed an increase in the frequency of successful grips performed with their hand. Across all weeks, infants used their arm more in peripersonal space than in the extrapersonal space. Similarly, infants used their mouth more in peripersonal space than in extrapersonal space. Locomotion followed by both arm and mouth grasping occurred more often in far space compared to near space. Only significant differences between extrapersonal and peripersonal space within each week are reported in the graph. Error bars show the S.E.M. *p < .01, **p < .001.
There were no other statistically significant effects, ps > .05.
3.2 Reaction Times from Stimulus Presentation to the Onset of Reaching Movement
Analysis of reaction times revealed main effects for space, F (1, 15) = 8.06, p = .012, η2p = .35, and week, F (2, 15) = 5.93, p = .007, η2p = .283, showing that infants were faster in the peripersonal space (M = 7.44, SD = .076) than in extrapersonal space (M = 9.76, SD = 1.01, p = .012), and that the reaction times to grasp the ball decreased significantly from week 2 (M = 11.86, SD = 1.21) to week 4 (M = 6.45, SD = 1.19, p = .008), while the difference between week 2 and week 3 only approached significance (M = .7.49, SD = 1.51, p = .068).
We found an interaction between effector and week, F (2,15) = 3.83, p = .33, η2p = .203, which indicates that the reaction time to grasp the ball with the hand decreased significantly from week 2 (M = 15.04, SD = 1.53) to week 3 (M = 8.15, SD = 1.99, p = .036) and from week 2 to week 4 (M = 3.91, SD = 1.45, p < .001), whereas there were no differences in mouth reaction times across weeks (week 2 M = 8.69, SD = 1.68; week 3 M = 6.82, SD = 1.69; week 4 M = 8.99, SD = 1.85, ps > .05). Moreover, infants were faster when grasping the ball with their hand than with their mouth in week 2 (hand M = 15.04, SD = 1.53; mouth M = 8.69, SD = 1.68, p = .013) and week 4 (hand M = 3.91, SD = 1.45; mouth M = 8.99, SD = 1.85, p = .038), whereas no difference between the two effectors was found in the third week (hand M = 8.15, SD = 1.99; mouth M = 6.82, SD = 1.69, p > .05).
3.3. Hand Grip Repertoire
Infant macaques showed five different hand configurations during ball grasping. As we mentioned (see paragraph 2.2.4.), these hand configurations were classified as (a) hand wrap, (b) thumb-and-four-finger grip, (c) all-tips grip, (d) pad-to-pad grip, or (e) thumb-to-second-third grip (Figure 2). However, we observed that in the second week of life infants relied on a small grip repertoire exhibiting only three configurations (i.e. hand wrap, thumb-to-four-finger, thumb-to-second-third). More specifically, starting from day 7, 11 infants out of 16 infants grasped the target using a whole hand configuration, whereas, starting from day 11, only 3 out of 16 infants used either thumb-to-four finger grip or thumb-to-second-third grip. In the third week, a greater number of infants continued to use these grip configurations: all infants grasped the object by using a hand wrap configuration, 14 infants showed the thumb-to-second-third grip, and 8 infants performed the thumb-to-four finger grip. Concurrently, 4 infants enlarged their grip repertoires, starting to show other configurations such as all-tips grip and pad-to-pad grip. Finally, in the fourth week, the full range of grip types was evident, and all infants performed hand wrap and thumb-to-second-third grips, whereas configurations, such as thumb-to-four finger, all-tips, and pad-to-pad were used by 10, 7, and 7 infants, respectively.
3.4. Hand Grip Configurations According to the Size of the Object
3.4.1. Grips in Peripersonal Space
Analysis of infants’ grip configurations when the ball was in near space revealed main effects for grip type, F (1, 15) = 12.18, p = .003, η2p = .448, ball size, F (1,15) = 10.41, p = .006, η2p = .410, and week, F (2, 15) = 13.12, p = .000, η2p = .467. Infants used more power grips (M = .692, SD = .078) than precision grips (M = .300, SD = .057), reached and grasped the ball more when it was large (M = .595, SD = .042) than when it was small (M = .397, SD = .057), and increased the number of successful grips from week 2 (M = .207, SD = .069) to week 3 (M = .572, SD = .055, p = .004), and from week 2 to week 4 (M = .710, SD = .085, p = .001).
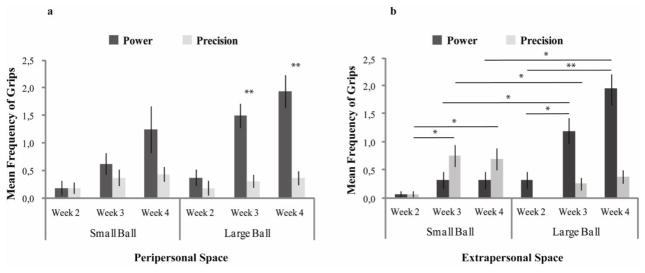
We found an interaction between grip type and ball size, F (1, 15) = 10.61, p = .005, η2p = .414, which indicates that infants used the power configuration more for the large ball (M = .911, SD = .088) than for the small ball (M = .473, SD = .098, p = .001), whereas precision grip was used equally for the large (M = .279, SD = .064) and the small balls (M = .321, SD = .080, p = .645) (Figure 4a). Moreover, the power configuration (M = .911, SD = .088) was used more than precision grip for grasping the large ball (M = .279, SD = .064, p < .001), whereas the two configurations were equally used for grasping the small ball (power: M = .473, SD = .098; precision: M = .321, SD = .080, p = .290).
Figure 4.
Mean frequency of successful power and precision grips in (a) peripersonal and (b) extrapersonal space across weeks. In peripersonal space, infants used the power configuration more than the precision grip for grasping the large ball, whereas they equally used the two grip configurations for grasping the small ball. When balls were presented in extrapersonal space, starting from the third week, infants used the power grip more often for the large ball than for the small ball, and conversely used the precision grip more for the small ball than for the large ball. Error bars show the S.E.M. *p < .01, **p < .001.
There were no interactions between grip type and week, F (2, 15) = 3.11, p = .059, ball size and week, F (2, 15) = 1.45, p = .256, or among grip type, ball size, and week, F (2, 15) = 0.90, p = .414.
3.4.2. Grips in Extrapersonal Space
Analysis of infants’ grip configurations when the ball was in far space revealed main effects for ball size, F (1,15) = 14.75, p = .002, η2p = .496, and week, F (2, 15) = 19.24, p < .001, η2p = .562, indicating that infants reached and grasped the ball more when it was large (M = .516, SD = .040) than when it was small (M = .310, SD = .061, p = .002) and increased the number of successful grips from week 2 (M = .100, SD = .034) to week 3 (M = .523, SD = .079, p < .001), and from weeks 2 to 4 (M = .625, SD = .081, p < .001). There was no main effect of grip type, F (1, 15) = 4.19, p = .059.
We found an interaction between grip type and ball size, F (1, 15) = 35.38, p < .001, η2p = .702, in which infants used the power configuration more for the large ball (M = .825, SD = .078) than for the small ball (M = .205, SD = .080, p < .001), and used the precision grip more for the small ball (M = .427, SD = .089) than for the large ball (M = .208, SD = .067, p = .030).
We also found an interaction between ball size and week, F (2, 15) = 3.91, p = .031, η2p = .207, in which the frequency of grasp attempts with the small ball increased from week 2 (M = .063, SD = .043) to week 3 (M = .458 SD = .104, p = .001), and from weeks 2 to 4 (M = .427, SD = .098, p = .002). Similarly, grasp attempts with the large ball increased from week 2 (M = .138, SD = .063) to week 3 (M = .588 SD = .081, p < .001), and from weeks 2 to 4 (M = .824, SD = .083, p < .001). Frequencies of grasp attempts with both the small and the large ball did not differ between weeks 3 and 4 (ps > .05).
Furthermore, we found a three-way interaction between grip type, ball size, and week, F (2, 15) = 4.97, p = .016, η2p = .242. Post-hoc comparisons showed that infants started using the power grip more often for the large ball (M = .925, SD = .149) than for the small ball (M = .276, SD = .126, p = .001) in the third week of life. Similarly, starting from the third week, they used the precision grip more for the small ball (M = .640, SD = .151) than for the large ball (M = .250, SD = 112, p = .025). Moreover, the use of power grip for grasping the large ball increased progressively from week 2 (M = .276, SD = .126) to week 3 (M = .925, SD = .149, p = .003) and from week 3 to week 4 (M = 1.273, SD = .146, p < .001). Likewise, there was an increase in the use of precision grip for the small ball from week 2 (M = .063, SD = .063) to week 3 (M = .640, SD = .151, p = .002) and from week 3 to week 4 (M = .578, SD = .154, p = .003) (Figure 4b).
There were no other statically significant differences, ps > .05.
4. Discussion
To successfully reach for an object, infants need information about: the object’s position and orientation in space, and its properties (e.g., size); the position and movements of their arms and hands relative to their bodies (i.e., proprioception); and the position of their arms and hands relative to the object. The results of the present study reveal that infant monkeys perceive objects at varying distances in space and use this information to plan their motor strategies and adjust their hand configurations. Over the course of the first weeks of life there are changes in infants’ use of behavioral strategies to obtain objects, which may reflect critical improvements in their motor development and sensorimotor integration. This developmental trajectory seems to occur in parallel with body schema modifications and, consequently, with space perception.
Thus, data reported in the current study support the idea that infants’ reaching-grasping behaviours are voluntary and goal-directed, in contrast with the traditional description of newborn infants’ behaviors as reflexes. By definition, reflexes are involuntary, automatic and fixed responses, and therefore stereotyped, not altered by learning, and not adjusted to meet a goal. Our preliminary behavioural observations (see paragraph 2.1.2) are in agreement with previous experiments in infant rhesus monkeys (Castell & Sackett, 1972; Milbrath 1968; Mowbray & Cadell, 1962), demonstrating that a stage of involuntary grasp response is present on the first 10 days of life, and that after this time grasping becomes largely voluntary. Moreover, these studies reported that infants’ abilities to respond to visual stimuli, and to visually follow objects, rapidly develop in the first postpartum days (days 4–7; Harlow et al., 1956). In addition, we showed evidences that the reaching-grasping behaviours recorded in this study are voluntary, goal-directed, and flexible. Indeed, we report that with age infant demonstrate an improved capacity to successfully grasp objects and a decrease in errors (see Supplementary Results), suggesting that learning is occurring. This result is incompatible with the interpretation that these behaviors are reflexes. Furthermore, infants often made postural adjustments before starting a reaching movement, and before initiating an arm movement, they looked at the target toward which the action was directed (see Supplementary Material, Videos S1–S7).
4.1. Perception of Object Distance and Infants’ Motor Strategies
Whether an object is reachable or not is determined by both the object’s characteristics and by the actor’s capacity to act (i.e., motor ability) in the environment (Rochat & Goubet, 1995; Rochat, Goubet, & Senders, 1999; Turvey, 1992). In the present study, infants made more reach-grasp attempts when objects were presented in peripersonal space, compared to extrapersonal space, suggesting that, from early in development, monkey infants may have perceived whether objects were reachable, and discriminated objects in extrapersonal and peripersonal space. This interpretation is in agreement with studies in human infants, which suggested that, from the moment infants begin to reach, around 4–5 months, they also detect the affordable distance at which an object is reachable (Cruikshank, 1941; Field, 1976; Rochat & Goubet, 1995; Rochat et al., 1999).
Anecdotally, we observed that in the first week of life, infants made some attempts to grasp objects while clinging to the surrogate. This observation extends those previously reported (Hinde et al., 1964; Nelson et al., 2011), but also shows that macaque infants’ capacity to grasp objects is precocious and develops earlier than previously thought. In particular, early attempts to grasp are displayed by infants already in the first week of life and appear to become more frequent starting from the second week. These findings indicate that significant modifications in motor development and visuo-motor coordination are occurring between the first and the second week of life. These changes might be critical for the development of body perception in relation to the capacity of the individual to act on objects and to navigate through space.
Although the timing of development in the use of the hand and the mouth is different between human and monkey infants (Antinucci, 1990; Spinozzi & Natale, 1986; Vauclair, 1984, Bard & Vauclair, 1984), we observed interesting similarities in their pattern of emergence. Infant monkeys approached and grasped objects with their mouths earlier in development than they grasped with their hands. This mouth grasping was common in the second week of life, during which time they explored objects with their mouths, both in peripersonal and extrapersonal space. Clearly, the mouth predominated as the main effector to reach and grasp the target. From the third week of life, hand grasping became more prominent and skilled, even if the pattern of object exploration with the mouth continued to be used concurrently with hand prehension. Moreover, with age, in peripersonal space, as hand prehension became more frequent and precise, mouth grasping decreased. It is interesting to note that previous pioneering anecdotal observations by Hinde and colleagues (1964) reported that infant macaques started to make grasp attempts in the first week of life and that the mouth is often used to contact objects during the second week of life. Although this study is mainly observational, and lacks quantitative analysis, it is, however, consistent with our findings.
Similarly, in humans, the mouth is used as the first haptic exploration tool, and with age this function is lost (McCall, 1974; Ruff, 1984; Rochat & Goubet, 1995; Rochat et al., 1999). During the first six months of life, human infants progress from oral (proximal) preference to arm movements (distal) to reach objects. By the end of the first year, instances of oral exploration decrease, as fine object manipulation and visuomotor coordination increase (McCall, 1974; Ruff, 1984; Rochat & Goubet, 1995; Rochat et al., 1999).
The present findings thus indicate that in monkeys, similarly to humans, there is a tight link between hand and mouth actions, which are often coordinated in order to explore objects. In fact, we observed that infants often reached for objects using two types of coordinated actions. In the first type, infants used their hands first, followed by bringing the object to the mouth; in the second type, infants approached the object with the mouth first, followed by the hand grasping and holding the object. Interestingly, these motor patterns involving the coordination between the hand and the mouth occurred prior to the development of independent locomotion, probably reflecting motor synergies present even before birth, as demonstrated by ultrasonographic research in human fetuses (Butterworth & Hopkins, 1988; De Vries, Visser, & Prechtl, 1984; Lew & Butterworth, 1997; Reissland, Francis, Aydin, Mason, & Schaal, 2013).
From a neurophysiological perspective, parietal-premotor cortical circuits support hand-mouth coordination (Graziano, Taylor, & Moore, 2002; Rizzolatti et al., 1988). In particular, in both the ventral premotor cortex and the posterior parietal lobule there are neurons that code actions performed with either the hand, or the mouth, or both, thus suggesting that often such coding is not limited to a movement performed with a specific effector. Interestingly, the motor representations of hand and mouth, both in the ventral premotor cortex and the posterior parietal lobule, are adjacent and partially overlapping (Buccino et al. 2001; Ferrari, Gallese, Rizzolatti, & Fogassi 2003; Rozzi et al., 2008), thus further supporting the hypothesis that the coordination of hand and mouth actions relies on neurophysiological mechanisms that, at the cortical level, are functionally and anatomically connected.
In addition to these studies, others indicate that, within these cortical sectors, visuomotor neurons are also present and they activate both when a stimulus is applied to the body surface (around the face or the arm) and when a visual stimulus is approaching or moving away from that body part (Fogassi et al., 1996; Gentilucci et al., 1988; Graziano, 2006; Maranesi et al., 2012; Rozzi et al., 2008). The co-occurrence of these bimodal neurons with motor neurons within the same cortical sector, and the anatomical connection and proximity of the cortical areas containing such neurons, supports the idea of a tight link between space coding and goal-directed movements.
Another factor to consider is the challenge posed by postural adjustments and improved locomotion coordination during development. As already noted by Piaget (1954) and other psychologists (Bremner, Holmes, & Spence, 2008), infants’ capacity to autonomously move in three-dimensional space expands not only their motor possibilities but also their capacity to plan movements in time and space. These landmark developmental changes are accompanied by the maturation of the skeleton-muscle structure and body biomechanics. For example, changes in the body (e.g., skeleton growth) alter the perception of external space (Maravita, Spence, & Driver, 2003), therefore requiring a parallel update of the body schema (Bremner, Mareschal, Lloyd-Fox, & Spence, 2008; Bremmer et al., 2008; Spence, Pavani, & Driver, 1998; Pavani, Spence, & Driver, 2000; Shore, Spry, & Spence, 2002). Thus, infant monkeys’ improved reach-grasp performance (i.e., increase with age in number of reach-grasp attempts with the hand), starting from the second week of life, should be considered in terms of their capacity to overcome the constraints posed by their posture and increased sensorimotor coordination of their bodies, and especially their arms. Our results in fact showed that, between the second and fourth week, there was a change in infants’ strategies to reach objects in peripersonal space, with increases in the use of the arm and decreases in movements of the body and mouth toward the target. Parallel to this, there was a change in infants’ motor strategy in extrapersonal space, with increases in arm actions associated with locomotion.
These data seem to suggest that infants encode spatial locations in relation to their opportunities to generate active movements towards objects located at varying distances. It is possible that the sensorimotor experience of reaching and grasping objects, displayed in the second week of life, support infants’ construction of their body representation, centered mainly on the mouth, but also more generally to the whole body. At this age, infants reach objects primarily through body movement and extension toward objects, which are then grasped by the mouth. Subsequently in development, by the third and fourth weeks of life, infants’ increased use of their arms and hands to reach objects may modify their space representation in relation to the enlarged possibilities of the motor system.
This sequela of events in the use of different effectors likely facilitates the process of discriminating near and far space in relation to the different body effectors. During the first weeks of life, the improved body motor maturation of the skeleton and of general postural adjustments, allowed infant macaques in the present study to improve their arm motor skills, thus expanding their motor possibilities to reach objects outside the near space by better coordinating their use of their arms with their body (Rochat et al., 1999).
Our data clearly demonstrate that, from the third week of life, but likely not earlier, infants are accurately discriminating distances and choosing appropriate motor strategies for varying distances, reaching with only their arm or moving their entire body, thus suggesting that only at a later stages of early development infants appear to code space in coordinates that can also include their arms. With age, the gradual increase in the use of the arm to actively reach for objects seems to be crucial for inducing changes in the body schema and for remapping near-space representations based on an arm-centered coordinate system.
Neurophysiological work in monkeys reveals specific brain areas in the parietal and premotor cortices that code for peripersonal space and containing neurons showing both motor and visuotactile properties (Fogassi et al., 1996; Graziano & Cooke, 2006; Graziano, Gross, Taylor, & Moore, 2004). Such representations are adjacent to the cortical motor representation of the hand and the arm, thus suggesting a neuro-anatomical connection and a possible functional link in the way space representations for the mouth and arm are constructed during development. Thus, it is likely that arm, body, and face cortical motor maps are integrated, even though their representations might still have independent (although partially overlapping) anatomical distributions and different functional roles in sensorimotor integration (Rizzolatti & Luppino, 2001). Our data seems to suggest such independence in the timing of their developmental emergence, with body and face representations developing first, followed by arm representations.
4.2. Grip Types for Targets of Varying Size and Distance
To grasp objects, infants need to shape their hands to match the three-dimensional structure of the object. This behavior requires a transformation from the visual representation of the object’s geometrical properties to the motor commands acting on the muscles of the hand. Our results revealed that, over the course of the first weeks of life, infants performed adjustments in their reaching and hand grasping configurations, depending on the objects’ size and distance. As early as the second week of life, infants showed the ability to used power and precision grips, even though their capacity to display these hand motor patterns did not reach the level of a fully matured adult configuration.
Previous studies in infant monkeys failed to describe fine and skilled hand and digit movements in the first month of life. The absence of such descriptions might be related to the hypotheses concerning the maturation and function of the pyramidal tract and its direct connections to the lateral motor-nuclei of the spinal cord (Evarts, 1981; Porter & Lemon, 1993). Both in humans and monkeys, the result of anatomical and electrophysiological studies support the idea that cortico-motoneuronal (CM) connections, which arise from the primary motor area, are important in the development of dexterity and in the central control of relatively independent finger movements (RIFM) (Kuypers, 1981; Dum & Strick 1991; Porter & Lemon, 1993). Kuypers (1962) suggested that the inability of infant monkeys to perform RIFM at birth was in part attributable to the immaturity of the CM projections. Lawrence and Hopkins (1976) reported that infant macaques started reaching at 3–4 weeks, but the earliest signs of RIFM occurred at 2–3 months, with a mature pattern at 7–8 months. Clearly, the study by Lawrence and Hopkins, although conducted on a small number of infants, contrasts the early hypothesis that a fully matured corticospinal tract is needed to display RIFM. Moreover, when in the adult monkey the monosynaptic connections are experimentally interrupted, dexterous movements can still be present as well as corticomotoneuronal excitation mediated through disynaptic pathways (Sasaki et al., 2004).
Galea and Darian-Smith (1995) reported that performance on a reach-and-grasp test by a group of young macaques approached adult levels by 6 months and correlated with the emergence of an adult-like distribution of cortical motor areas contributing to the corticospinal tract. Armand and colleagues (1997) showed that the CM projection was weak at birth and, although it developed rapidly in the first few postnatal months, it was not fully mature until the second year of life. This study demonstrates that the process of maturation of CM pathway is complex and follows a slow developmental trajectory. Furthermore, transcranial magnetic stimulation (TMS) studies exploring the maturation of the corticospinal system in the infant macaque (Eyre, Miller, & Ramesh, 1991; Flament, Hall, & Lemon, 1992; Müller, Hömberg, & Lenard, 1991: Müller, Ebner, & Hömberg, 1994; Olivier, Edgley, Armand, & Lemon, 1997) reported that the earliest component of electromyogram (EMG) responses to TMS, which in the adult is mediated by the CM system (Edgley, Eyre, Lemon, & Miller, 1990; Baker, Olivier, & Lemon, 1994), could not be obtained before 2–3 months of age. According to some authors, this finding supports the idea that functional CM connections must be established for the capacity to perform fine finger movements. Together these findings do not provide a univocal picture of how the maturation of the corticospinal tract in the first months of development supports the emergence of more complex skilled hand movements. Although it seems that there is a relation between these two aspects, the course of the changes occurring at behavioral, neuroanatomical, and physiological levels, especially in the first months of life, remain poorly understood.
Thus, in the absence of corticospical connections, it might be expected that there would be no hand and digit movements of interest prior to the emergence of a fully mature precision grip. Conversely, the present study provides a novel description of the development of different grasping configurations in early infancy, suggesting a gradual development of the motor system’s structure and function. It should be noted that the early behaviors described in the current study are certainly not the equivalent of the well-developed reaching-grasping behaviors seen later in infancy or in mature individuals. As described in the Behavioral Analysis paragraph (2.2.4), we classified hand configurations as power and precision according to Napier’s definition (1960), which differs from the definition used by Lawrence and Hopkins (1976) for RIFM. In fact, the precision grip described by Napier (1960) is not considered a complex movement dependent upon fibres passing in the pyramidal tracts (Hepp-Reymond & Wiesendanger, 1972).
Nevertheless, it is possible that the occurrence of the developmental changes in the grasping patterns displayed by infants in this study may be mediated by the developing pyramidal tract and the maturation of functional CM connections, which although they are weak in the first month of life, are nevertheless present with a small proportion depending on the selected area of the lamina IX (Armand et al., 1997). Interestingly, more recent electrophysiological and anatomical studies in humans suggest that direct connections of the pyramidal tract to motor neurons are established before birth (Eyre, Miller, Clowry, Conway, & Watts, 2000). Our observations of the early development of different grasping patterns might suggest that also in monkeys the refinement of CM connections occurred prenatally, which would also explain the precocious motor development displayed by newborn macaques compared to other animals (Martin, 2005), and might account for why infant macaques begin reaching in their first weeks of life (Hinde et al., 1964; Lawrence & Hopkins, 1976). However, several other lines of evidence suggest that the development of segmental corticospinal terminations in monkeys follow a different pattern (Kuypers, 1962; Armand et al., 1997). Between birth and about 8 months, as the corticospinal tract increased in its overall size (Heffner & Masterton 1983; Nudo, Sutherland, & Masterton, 1995), along with the emergence of corticospinal terminations in the ventral horn, where the lateral motor-nuclei are located (Kuypers, 1962; Armand et al., 1997), monkeys begin to move their fingers more independently and develop precision grip (Lawrence & Hopkins 1976; Flament et al., 1992; Galea & Darian-Smith, 1995).
However, the maturation of the CM system is only one factor in the development of hand motor skill. Although the establishment of functional CM connections may be an important milestone in the development of motor skills, this aspect should be considered as one of many markers of the developmental process and may not parallel other measures of functional maturity of the motor system. It is possible that, at this early developmental stage, other elements of the infant’s motor system are maturing and that the emergence of infant’s correct hand shaping (to match three-dimensional objects) also involves parieto-frontal circuits and connections between the anterior intraparietal (AIP) area and ventral premotor cortex (Murata, Fadiga, Fogassi, Gallese, Raos, & Rizzolatti, 1997; Rizzolatti et al., 1988). Parieto-frontal circuits provide an anatomical basis for the transformation of sensory information into actions (Matelli & Luppino, 2001; Rizzolatti et al., 1998). Both the ventral premotor cortex and AIP contain neurons that code for selective hand manipulations, grasping movements, and various visual characteristics of three-dimensional objects (Rizzolatti et al., 1988; Murata et al., 1997). Jeannerod and colleagues (1995) suggested that this circuit may transform the three-dimensional characteristics of objects from sensory information into the appropriate hand movements for grasping. Interestingly, although these areas have a weak direct influence on the spinal motor neurons, with the majority of their corticospinal neurons terminating in the intermediate zone (Dum & Strick, 1996; 2002), they nevertheless can influence motor output at the level of both the primary motor area and the spinal cord (Dum & Strick, 1991). Indeed, several reports demonstrate that the ventral premotor area can influence hand motor function through both corticospinal projections and corticocortical projections to the primary motor cortex. Stimulation of the macaque ventral premotor cortex, which by itself evoked little or no detectable corticospinal output (Maranesi et al. 2012), can produce a robust modulation of motor outputs from the primary motor cortex (Shimazu Maier, Cerri, Kirkwood, & Lemon, 2004) in a muscle- and grasp-specific manner (Prabhu et al., 2009).
Together, these findings suggest that parieto-premotor circuits and the corticospinal system might work in parallel in the control of motor functions. However, these two systems might follow two different developmental patterns, with the former emerging earlier than the latter. Probably, the early motor behaviors exhibited by our infant monkeys and the sensory consequences of their actions are crucial in shaping the developing patterns of connection between motor cortex neurons and spinal motor circuits and the functional organization of the motor system. This mechanism would help to refine and stabilize mature connections and therefore to expand infant’s behavioural repertoire and motor planning capabilities. Accordingly, the longer corticospinal development could reflect the need to adapt to changing motor control demands as the infants grow throughout life (Martin, 2005), thus supporting more independent finger movements and fine hand skills.
Moreover, it is interesting to note that two different developmental patterns of grasping emerged when targets were located in near and far space. When tested in peripersonal space, infants used the power grip more often when the ball was large, but they did not preferentially use a specific configuration when the ball was small. In fact, when a small ball was presented in the peripersonal space, infants kept using a whole hand configuration to grasp the object. One interpretation is that, in near space, the whole hand configuration can be used to successfully take possession of the object and postural stability may facilitate this motor pattern, which, in terms of sensorimotor efforts, is more efficient and less demanding. In fact, the advantage of opening the hand more fully is that it requires less endpoint accuracy relative to precision grips (von Hofsten & Rönnqvist, 1988). This behavior could be adaptive, since a fully opened hand will optimize the possibility of capturing the object efficiently (Wing, Turton, & Fraser, 1986). Thus, preparatory adjustments to object size are less crucial than are preparatory adjustments to object distance.
Conversely, our data showed that infants’ correct configuration of their hands and performance of precision grips when the ball was small appeared only when the target was presented in extrapersonal space. This pattern emerged starting from the third week of life, during which we recorded an increased use of the arm, likely reflecting a maturation of infants’ motor skills, as previously discussed. Possibly, coding the spatial relationship between more distant objects and one’s body parts requires more complex processing than when objects are presented in peripersonal space (Rizzolatti & Sinigaglia, 2008). The extrapersonal task, being more demanding in terms of integration of sensory information, may have required infants to plan more effective motor strategies for reaching and grasping small objects, thus promoting the emergence of an advanced differentiation of grip configurations. In support of this idea, Bushnell and Boudreau (1993) suggest a high correspondence between unfolding perceptual abilities and the acquisition of particular motor patterns, consistent with our notion that motor limitations are of critical importance for the development and the dynamic modulation of different motor patterns (e.g., motor strategies and grip types). According to McEwan, Dihoff and Brosvic (1991), when an object is in far space and an infant has the opportunity to move toward the object, the infant will plan reaching and grasping more effectively. The dynamic interactions between objects’ properties and infants’ abilities to act on their environment shape and refine infants’ sensori-motor systems, thus producing more effective strategies for grasping objects. Thus, through locomotion infants may acquire knowledge about the space and distance between themselves and objects, and through body movements they more accurately perceive distances and object sizes (Bertenthal, 1996).
In this regard, we hypothesize that our experimental setting and the postural support we provided to infants was more appropriate to examine the developmental aspects of infant prehension compared to previous behavioral studies on infant monkeys (e.g. Lawrence & Hopkins, 1976). Differences in the task constraints might explain why infants in the present study exhibited precocious reaching ability and the emergence of different grasping configurations.
5. General Conclusions
Our results support the view that perception and action form a closely, interconnected loop (Jeannerod, 1994), since improvements in infants’ perceptual capabilities are paced by the emergence of different motor patterns and vice versa, motor skills are facilitated by concurrent improvement in perception (Bertenthal, Rose, & Bai, 1997; Thelen, Smith, Karmiloff-Smith, & Johnson, 1994). The behavioral results of the present study collectively suggest that in macaques’ early development there are, very likely, brain mechanisms of intermodal integration of visual, proprioceptive, and motor movements similar to those described in adults (Fogassi et al., 1996; Graziano & Cooke, 2006; Graziano et al., 2004). Moreover, these findings point to the presence of an early perceptual body schema, already formed soon after birth (if not before), though only broadly defined, and subsequently modified by the acquisition and refinement of motor skills. Such modifications demonstrate the importance of early brain plasticity and of the role of early sensorimotor experience in establishing a mature capacity for coding space.
Supplementary Material
Highlights.
We traced the development of space coding in 2 to 4 week-old infant macaques
Infants’ reaching-grasping skills improved from week 2 to week 4 of age
Infants showed different motor strategies depending on the objects’ location
Infants adjusted their grip configurations according to the objects’ size
Visuo-motor transformation might start developing early in development
Acknowledgments
This research was supported by the Division of Intramural Research, NICHD, and by NIH PO1HD064653 grant. We thank nursery staff in the Laboratory of Comparative Ethology for assisting in data collection. We thank three anonymous reviewers for feedback on earlier versions of this manuscript
Footnotes
Author contributions
Principal Investigator who obtained funding and supervised data collection: PFF. Senior Research Associate who supervised study: SJS. Conceived and designed the experiments: VS PFF. Performed experiments: VS EAS. Coded data: VS EAS. Analyzed data and prepared figures: VS. Interpreted data and wrote paper: VS PFF. All authors reviewed the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antinucci F. The comparative study of cognitive ontogeny in four primate species. Language and intelligence in monkeys and apes: Comparative developmental perspectives. 1990:157–171. [Google Scholar]
- Armand J, Olivier E, Edgley SA, Lemon RN. Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. The Journal of neuroscience. 1997;17(1):251–266. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Recording an identified pyramidal volley evoked by transcranial magnetic stimulation in a conscious macaque monkey. Experimental Brain Research. 1994;99:529–532. doi: 10.1007/BF00228989. [DOI] [PubMed] [Google Scholar]
- Bard KA, Vauclair J. The communicative context of object manipulation in ape and human adult-infant pairs. Journal of Human Evolution. 1984;13(2):181–190. doi: 10.1016/S0047-2484(84)80062-7. [DOI] [Google Scholar]
- Bertenthal BI. Origins and early development of perception, action, and representation. Annual Review of Psychology. 1996;47(1):431–459. doi: 10.1146/annurev.psych.47.1.431. [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Rose JL, Bai DL. Perception–action coupling in the development of visual control of posture. Journal of Experimental Psychology: Human Perception and Performance. 1997;23(6):1631. doi: 10.1037/0096-1523.23.6.1631. [DOI] [PubMed] [Google Scholar]
- Berti A, Frassinetti F. When far becomes near: Remapping of space by tool use. Journal of Cognitive Neuroscience. 2000;12(3):415–420. doi: 10.1162/089892900562237. [DOI] [PubMed] [Google Scholar]
- Berti A, Rizzolatti G. Visual processing without awareness: Evidence from unilateral neglect. Journal of Cognitive Neuroscience. 2002;4(4):345–351. doi: 10.1162/jocn.1992.4.4.345. [DOI] [PubMed] [Google Scholar]
- Brain WR. Visual disorientation with special reference to lesions of the right cerebral hemisphere. Brain. 1941;64:224–272. doi: 10.1093/brain/64.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Duhamel JR, Graf W, Fink GR. Space coding in primate posterior parietal cortex. Neuroimage. 2001;14(1):46–51. doi: 10.1006/nimg.2001.0817. [DOI] [PubMed] [Google Scholar]
- Bremner AJ, Holmes NP, Spence C. Infants lost in (peripersonal) space? Trends in Cognitive Sciences. 2008;12(8):298–305. doi: 10.1016/j.tics.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Bremner AJ, Mareschal D, Lloyd-Fox S, Spence C. Spatial localization of touch in the first year of life: Early influence of a visual spatial code and the development of remapping across changes in limb position. Journal of Experimental Psychology: General. 2008;137(1):149–162. doi: 10.1037/0096-3445.137.1.149. [DOI] [PubMed] [Google Scholar]
- Brozzoli C, Demattè ML, Pavani F, Frassinetti F, Farnè A. Neglect and extinction: Within and between sensory modalities. Restorative Neurology and Neuroscience. 2006;24(4–6):217–232. [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13(2):400–404. doi: 10.1111/j.1460-9568.2001.01385.x. [DOI] [PubMed] [Google Scholar]
- Bushnell EW, Boudreau JP. Motor development and the mind: The potential role of motor abilities as a determinant of aspects of perceptual development. Child Development. 1993;64(4):1005–1021. doi: 10.1111/j.1467-8624.1993.tb04184.x. [DOI] [PubMed] [Google Scholar]
- Butterworth G, Hopkins B. Hand-mouth coordination in the new-born baby. British Journal of Developmental Psychology. 1988;6(4):303–314. doi: 10.1111/j.2044-835X.1988.tb01103.x. [DOI] [Google Scholar]
- Butterworth G, Verweij E, Hopkins B. The development of prehension in infants: Halverson revisited. British Journal of Developmental Psychology. 1997;15(2):223–236. doi: 10.1111/j.2044-835X.1997.tb00736.x. [DOI] [Google Scholar]
- Clifton RK, Rochat P, Litovsky RY, Perris EE. Object representation guides infants’ reaching in the dark. Journal of Experimental Psychology: Human Perception and Performance. 1991;17(2):323–329. doi: 10.1037/0096-1523.17.2.323. [DOI] [PubMed] [Google Scholar]
- Cowey A, Small M, Ellis S. Left visuo-spatial neglect can be worse in far than in near space. Neuropsychologia. 1994;32(9):1059–1066. doi: 10.1016/0028-3932(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Cruikshank RM. The development of visual size constancy in early infancy. The Pedagogical Seminary and Journal of Genetic Psychology. 1941;58(2):327–351. doi: 10.1080/08856559.1941.10534574. [DOI] [Google Scholar]
- Dettmer AM, Ruggiero AM, Novak MA, Meyer JS, Suomi SJ. Surrogate mobility and orientation affect the early neurobehavioral development of infant rhesus macaques (Macaca mulatta) Developmental Psychobiology. 2008;50(4):418–422. doi: 10.1002/dev.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries JIP, Visser GHA, Prechtl HFR. Fetal motility in the first half of pregnancy. Clinics in Developmental Medicine. 1984;94:46–64. [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. Journal of Neuropsychology. 1998;79(1):126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. The Journal of neuroscience. 1991;11(3):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. The Journal of neuroscience. 1996;16(20):6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiology & behavior. 2002;77(4):677–682. doi: 10.1016/S0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. The Journal of Physiology. 1990;425(1):301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Role of motor cortex in voluntary movements in primates. Comprehensive Physiology 1981 [Google Scholar]
- Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. The Journal of physiology. 1991;434(1):441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123(1):51–64. doi: 10.1093/brain/123.1.51. http://dx.doi.org/10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- Farnè A, Demattè ML, Làdavas E. Beyond the window: Multisensory representation of peripersonal space across a transparent barrier. International Journal of Psychophysiology. 2003;50(1–2):51–61. doi: 10.1016/S0167-8760(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Felix D, Wiesendanger M. Pyramidal and non-pyramidal motor cortical effects on distal forelimb muscles of monkeys. Experimental brain research. 1971;12(1):81–91. doi: 10.1007/BF00234417. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. European Journal of Neuroscience. 2003;17(8):1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biology. 2006;4(9):e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field TM. Effects of early separation, interactive deficits, and experimental manipulations on infant-mother face-to-face interaction. Child Development. 1977;48(3):763–771. [Google Scholar]
- Flament D, Hall EJ, Lemon RN. The development of cortico-motoneuronal projections investigated using magnetic brain stimulation in the infant macaque. The Journal of Physiology. 1992;447:755–768. doi: 10.1113/jphysiol.1992.sp019027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey. A reversible inactivation study. Brain. 2001;124(3):571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Di Pellegrino G, Fadiga L, Gentilucci M, Luppino G, Rizzolatti G. Space coding by premotor cortex. Experimental Brain Research. 1992;89(3):686–690. doi: 10.1007/BF00229894. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (Area F4) Journal of Neurophysiology. 1996;76(1):141–57. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cerebral Cortex. 1995;5(6):518–540. doi: 10.1093/cercor/5.6.518. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. Experimental Brain Research. 1988;71(3):475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The Ecological Approach to Visual Perception. Boston: Houghton Mifflin; 1979. [Google Scholar]
- Gilmore RO, Johnson MH. Body-centered representations for visually-guided action emerge during early infancy. Cognition. 1997;65(1):B1–B9. doi: 10.1016/S0010-0277(97)00038-3. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44(6):845–859. doi: 10.1016/j.neuropsychologia.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Gross CG. Spatial map for the control of movement. Current Opinion in Neurobiology. 1998;8(2):195–201. doi: 10.1016/S0959-4388(98)80140-2. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gross CG, Taylor CS, Moore T. A system of multimodal areas in the primate brain. In: Spence C, Driver J, editors. Crossmodal Space and Crossmodal Attention. Oxford: Oxford University Press; 2004. pp. 51–67. [Google Scholar]
- Graziano MS, Yap GS, Gross CG. Coding of visual space by premotor neurons. Science. 1994;266(5187):1054–1054. doi: 10.1126/science.7973661. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34(5):841–851. doi: 10.1016/S0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Masterton RB. The role of the corticospinal tract in the evolution of human digital dexterity. Brain, behavior and evolution. 1983;23(3–4):165–183. doi: 10.1159/000121494. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Wiesendanger M. Unilateral pyramidotomy in monkeys: effect on force and speed of a conditioned precision grip. Brain research. 1972;36(1):117–131. doi: 10.1016/0006-8993(72)90770-6. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Rowell T, Spencer-Booth Y. Behaviour of socially living rhesus monkeys in their first six months. Proceedings of the Zoological Society of London. 1964;143(4):609–649. doi: 10.1111/j.1469-7998.1964.tb03884.x. [DOI] [Google Scholar]
- Häger-Ross C, Schieber MH. Quantifying the independence of human finger movements: comparisons of digits, hands, and movement frequencies. The Journal of Neuroscience. 2000;20(22):8542–8550. doi: 10.1523/JNEUROSCI.20-22-08542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan PW, Fink GR, Marshall JC, Vallar G. Spatial cognition: Evidence from visual neglect. Trends in Cognitive Sciences. 2003;7(3):125–133. doi: 10.1016/S1364-6613(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Halverson HM. An experimental study of prehension in infant by means of systematic cinema records. Genetic Psychology Monographs. 1931;10:107–285. [Google Scholar]
- Holmes NP, Spence C. The body schema and multisensory representation(s) of peripersonal space. Cognitive Processing. 2004;5(2):94–105. doi: 10.1007/s10339-004-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. Object oriented action. Insights into the reach to grasp movement. In: Bennet KMB, Castiello U, editors. Advances in psychology. Amsterdam, Netherlands: North-Holland/Elsevier Science Publishers; 1994. pp. 3–15. [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends in neurosciences. 1995;18(7):314–320. [PubMed] [Google Scholar]
- Kuypers HG. Corticospinal connections: postnatal development in the rhesus monkey. Science. 1962;138(3541):678–680. doi: 10.1016/0166-2236(95)93921-j. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Anatomy of the descending pathways. Comprehensive Physiology. 1981 doi: 10.1002/cphy.cp010213. [DOI] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7(14):2325–30. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Làdavas E. Functional and dynamic properties of visual peripersonal space in humans. Trends in Cognitive Sciences. 2002;6(1):17–22. doi: 10.1016/S1364-6613(00)01814-3. [DOI] [PubMed] [Google Scholar]
- Làdavas E, Farnè A. Visuo-tactile representation of near-the-body space. Journal of Physiology-Paris. 2004;98(1–3):161–170. doi: 10.1016/j.jphysparis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Làdavas E, Farnè A. Multisensory representation of peripersonal space. In: Knoblich G, Thornton IM, Grosjen M, Shiffrar M, editors. Human body perception from the inside out. Oxford: Oxford University Press; 2006. pp. 89–104. [Google Scholar]
- Lawrence DG, Hopkins DA. The development of motor control in the rhesus monkey: evidence concerning the role of corticomotoneuronal connections. Brain: a journal of neurology. 1976;99(2):235–254. doi: 10.1093/brain/99.2.235. [DOI] [PubMed] [Google Scholar]
- Lew AR, Butterworth G. The development of hand-mouth coordination in 2-to 5-month-old infants: Similarities with reaching and grasping. Infant Behavior and Development. 1997;20(1):59–69. doi: 10.1016/S0163-6383(97)90061-8. [DOI] [Google Scholar]
- Longo MR, Lourenco SF. On the nature of near space: Effects of tool use and the transition to far space. Neuropsychologia. 2006;44(6):977–981. doi: 10.1016/j.neuropsychologia.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Maternal encouragement of infant locomotion in pigtail macaques, Macaca nemestrina. Animal Behaviour. 1996;51(3):603–610. doi: 10.1006/anbe.1996.0064. [DOI] [Google Scholar]
- Macaluso E, Maravita A. The representation of space near the body through touch and vision. Neuropsychologia. 2010;48(3):782–795. doi: 10.1016/j.neuropsychologia.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Maranesi M, Rodà F, Bonini L, Rozzi S, Ferrari PF, Fogassi L, Coudé G. Anatomo-functional organization of the ventral primary motor and premotor cortex in the macaque monkey. European Journal of Neuroscience. 2012;36(10):3376–3387. doi: 10.1111/j.1460-9568.2012.08252.x. [DOI] [PubMed] [Google Scholar]
- Maravita A, Spence C, Driver J. Multisensory integration and the body schema: close to hand and within reach. Current Biology. 2003;13(13):R531–R539. doi: 10.1016/S0960-9822(03)00449-4. [DOI] [PubMed] [Google Scholar]
- Maravita A, Spence C, Kennett S, Driver J. Tool-use changes multimodal spatial interactions between vision and touch in normal humans. Cognition. 2002;83(2):B25–B34. doi: 10.1016/S0010-0277(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema) Trends in Cognitive Sciences. 2004;8(2):79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. The Neuroscientist. 2005;11(2):161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage. 2001;14(1):S27–S32. doi: 10.1006/nimg.2001.0835. [DOI] [PubMed] [Google Scholar]
- McCall RB. Exploratory manipulation and play in the human infant. Monographs of the Society for Research in Child Development. 1974;39(2):88. doi: 10.2307/1166007. [DOI] [PubMed] [Google Scholar]
- McEwan MH, Dihoff RE, Brosvic GM. Early infant crawling experience is reflected in later motor skill development. Perceptual and Motor Skills. 1991;72:75–79. doi: 10.2466/pms.1991.72.1.75. [DOI] [PubMed] [Google Scholar]
- MacFarlane NB, Graziano MS. Diversity of grip in Macaca mulatta. Experimental Brain Research. 2009;197(3):255–268. doi: 10.1007/s00221-009-1909-z. [DOI] [PubMed] [Google Scholar]
- McKenzie BE, Skouteris H, Day RH, Hartman B, Yonas A. Effective action by infants to contact objects by reaching and leaning. Child Development. 1993;64(2):415–429. doi: 10.1111/j.1467-8624.1993.tb02918.x. [DOI] [PubMed] [Google Scholar]
- Müller K, Ebner B, Hömberg V. Maturation of fastest afferent and efferent central and peripheral pathways: no evidence for a constancy of central conduction delays. Neuroscience letters. 1994;166(1):9–12. doi: 10.1016/0304-3940(94)90828-1. [DOI] [PubMed] [Google Scholar]
- Müller K, Hömberg V, Lenard HG. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1991;81(1):63–70. doi: 10.1016/0168-5597(91)90105-7. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. Journal of neurophysiology. 1997;78(4):2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. Journal of Neurophysiology. 2000;83(5):2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Napier JR. Proceedings of the Zoological Society of London. 4. Vol. 134. Blackwell Publishing Ltd; 1960. Studies of the hands of living primates; pp. 647–657. [Google Scholar]
- Nelson EL, Emery MS, Babcock SM, Novak MF, Suomi SJ, Novak MA. Head orientation and handedness trajectory in rhesus monkey infants (Macaca mulatta) Developmental Psychobiology. 2011;53(3):246–255. doi: 10.1002/dev.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe NS, Huttenlocher J. Making space: The development of spatial representation and reasoning. MIT Press; 2003. [Google Scholar]
- Nudo RJ, Sutherland DP, Masterton RB. Variation and evolution of mammalian corticospinal somata with special reference to primates. Journal of Comparative Neurology. 1995;358(2):181–205. doi: 10.1002/cne.903580203. [DOI] [PubMed] [Google Scholar]
- Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. The Journal of Neuroscience. 1997;17(1):267–276. doi: 10.1523/JNEUROSCI.17-01-00267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavani F, Castiello U. Binding personal and extrapersonal space through body shadows. Nature Neuroscience. 2004;7(1):14–16. doi: 10.1038/nn1167. [DOI] [PubMed] [Google Scholar]
- Pavani F, Spence C, Driver J. Visual capture of touch: Out-of-the-body experiences with rubber gloves. Psychological Science. 2000;11(5):353–359. doi: 10.1111/1467-9280.00270. [DOI] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. New York: Basic Books; 1954. [Google Scholar]
- Porter R, Lemon R. Corticospinal function and voluntary movement. Oxford: Clarendon Press; 1993. pp. 122–209. [Google Scholar]
- Prabhu G, Shimazu H, Cerri G, Brochier T, Spinks RL, Maier MA, Lemon RN. Modulation of primary motor cortex outputs from ventral premotor cortex during visually guided grasp in the macaque monkey. The Journal of physiology. 2009;587(5):1057–1069. doi: 10.1113/jphysiol.2008.165571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previc FH. Functional specialization in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behavioral and Brain Sciences. 1990;13(3):519–542. doi: 10.1017/S0140525X00080018. [DOI] [Google Scholar]
- Previc FH. The neuropsychology of 3-D space. Psychological bulletin. 1998;124(2):123. doi: 10.1037/0033-2909.124.2.123. [DOI] [PubMed] [Google Scholar]
- Raos V, Umiltá MA, Murata A, Fogassi L, Gallese V. Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. Journal of neurophysiology. 2006;95(2):709–729. doi: 10.1152/jn.00463.2005. [DOI] [PubMed] [Google Scholar]
- Reissland N, Francis B, Aydin E, Mason J, Schaal B. The development of anticipation in the fetus: A longitudinal account of human fetal mouth movements in reaction to and anticipation of touch. Developmental Psychobiology. 2013 doi: 10.1002/dev.21172. Advance Online Publication. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. Experimental Brain Research. 1988;71(3):491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. The space around us. Science. 1997;277(5323):190–191. doi: 10.1126/science.277.5323.190. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Current Opinion in Neurobiology. 2002;12(2):149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31(6):889–901. doi: 10.1016/S0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M, Pavesi G. Deficits in attention and movement following the removal of postarcuate (area 6) and prearcuate (area 8) cortex in macaque monkeys. Brain. 1983;106(3):655–73. doi: 10.1093/brain/106.3.655. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. Mirrors in the brain. How our minds share actions and emotions. Oxford: Oxford University Press; 2008. [Google Scholar]
- Rochat P, Goubet N. Development of sitting and reaching in 5-to 6-month-old infants. Infant Behavior and Development. 1995;18(1):53–68. doi: 10.1016/0163-6383(95)90007-1. [DOI] [Google Scholar]
- Rochat P, Goubet N, Senders SJ. To reach or not to reach? Perception of body effectivities by young infants. Infant and Child Development. 1999;8(3):129–148. doi: 10.1002/(SICI)1522-7219(199909)8:3<129::AID-ICD193>3.0.CO;2-G. [DOI] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: Electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. European Journal of Neuroscience. 2008;28(8):1569–1588. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- Ruff HA. Infants’ manipulative exploration of objects: Effects of age and object characteristics. Developmental Psychology. 1984;20(1):9–20. doi: 10.1037/0012-1649.20.1.9. [DOI] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. Journal of neurophysiology. 2004;92(5):3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behavior and Development. 1992;15(2):155–177. doi: 10.1016/0163-6383(92)80021-L. [DOI] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology. 1998;46(4):311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shelton PA, Bowers D, Heilman KM. Peripersonal and vertical neglect. Brain. 1990;113(1):191–205. doi: 10.1093/brain/113.1.191. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. The Journal of Neuroscience. 2004;24(5):1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore DI, Spry E, Spence C. Confusing the mind by crossing the hands. Cognitive Brain Research. 2002;14(1):153–163. doi: 10.1016/S0926-6410(02)00070-8. [DOI] [PubMed] [Google Scholar]
- Sommer R. Personal space: The behavioral basis of design. Englewood Cliffs, NJ: Prentice-Hall; 1969. [Google Scholar]
- Spence C, Pavani F, Driver J. What crossing the hands can reveal about crossmodal links in spatial attention. Psychonomic Society. 1998;3:13. [Google Scholar]
- Spence C, Pavani F, Driver J. Crossmodal links between vision and touch in covert endogenous spatial attention. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(4):1298–1319. doi: 10.1037/0096-1523.26.4.1298. [DOI] [PubMed] [Google Scholar]
- Spence C, Pavani F, Maravita A, Holmes N. Multisensory contributions to the 3-D representation of visuotactile peripersonal space in humans: Evidence from the crossmodal congruency task. Journal of Physiology. 2004;98(1–3):171–189. doi: 10.1016/j.jphysparis.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Natale F. The interaction between prehension and locomotion in macaque’s, gorilla’s and child’s cognitive development. In: Else JG, Lee PC, editors. Primate Ontogeny, Cognition, and Social Behaviour. Cambridge: Cambridge University Press; 1986. pp. 155–159. [Google Scholar]
- Thelen E, Smith LB, Karmiloff-Smith A, Johnson MH. A dynamic systems approach to the development of cognition and action. Vol. 10. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Turvey MT. Affordances and prospective control: An outline of the ontology. Ecological Psychology. 1992;4(3):173–187. doi: 10.1207/s15326969eco0403_3. [DOI] [Google Scholar]
- Vauclair J. Phylogenetic approach to object manipulation in human and ape infants. Human Development. 1984;27(5–6):321–328. doi: 10.1159/000272925. [DOI] [Google Scholar]
- von Hofsten C, Rönnqvist L. Preparation for grasping an object: a developmental study. Journal of Experimental Psychology: Human Perception and Performance. 1988;14(4):610–621. doi: 10.1037/0096-1523.14.4.610. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Valenza N, Mayer E, Reverdin A, Landis T. Near and far visual space in unilateral neglect. Annals of Neurology. 1998;43(3):406–410. doi: 10.1002/ana.410430324. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Marshall JC, Zilles K, Fink GR. Are action and perception in near and far space additive or interactive factors? Neuroimage. 2003;18(4):837–846. doi: 10.1016/S1053-8119(03)00018-1. [DOI] [PubMed] [Google Scholar]
- Wing AM, Turton A, Fraser C. Grasp size and accuracy of approach in reaching. Journal of Motor Behavior. 1986;18(3):245–260. doi: 10.1080/00222895.1986.10735380. [DOI] [PubMed] [Google Scholar]
- Witt JK, Proffitt DR, Epstein W. Perceiving distance: A role of effort and intent. Perception. 2004;33(5):577–590. doi: 10.1068/p5090. [DOI] [PubMed] [Google Scholar]
- Yonas A, Hartman B. Perceiving the affordance of contact in four and five-month-old infants. Child Development. 1993;64(1):298–308. doi: 10.1111/j.1467-8624.1993.tb02911.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.