Transcriptomic and metabolomic profiling of grapevine berries after harvest in different Vitis vinifera genotypes reveals the molecular basis of cluster detachment, senescence and dehydration stress.
Abstract
The molecular events that characterize postripening grapevine berries have rarely been investigated and are poorly defined. In particular, a detailed definition of changes occurring during the postharvest dehydration, a process undertaken to make some particularly special wine styles, would be of great interest for both winemakers and plant biologists. We report an exhaustive survey of transcriptomic and metabolomic responses in berries representing six grapevine genotypes subjected to postharvest dehydration under identical controlled conditions. The modulation of phenylpropanoid metabolism clearly distinguished the behavior of genotypes, with stilbene accumulation as the major metabolic event, although the transient accumulation/depletion of anthocyanins and flavonols was the prevalent variation in genotypes that do not accumulate stilbenes. The modulation of genes related to phenylpropanoid/stilbene metabolism highlighted the distinct metabolomic plasticity of genotypes, allowing for the identification of candidate structural and regulatory genes. In addition to genotype-specific responses, a core set of genes was consistently modulated in all genotypes, representing the common features of berries undergoing dehydration and/or commencing senescence. This included genes controlling ethylene and auxin metabolism as well as genes involved in oxidative and osmotic stress, defense responses, anaerobic respiration, and cell wall and carbohydrate metabolism. Several transcription factors were identified that may control these shared processes in the postharvest berry. Changes representing both common and genotype-specific responses to postharvest conditions shed light on the cellular processes taking place in harvested berries stored under dehydrating conditions for several months.
Grapevine berries are nonclimacteric fruits characterized by relatively slow development and ripening that occurs without any clear involvement of the plant hormone ethylene. Berry development is marked by dramatic physiological, metabolic, and textural changes that coincide with the onset of ripening (veraison) followed by the gradual emergence of sweet, low-acid, ripe fruit with desirable quality traits. Transcriptome analysis has shown that profound transcriptomic remodeling drives the transition from the vegetative to the ripening phase and that ripening is highly coordinated at the transcriptional level (Terrier et al., 2005; Fasoli et al., 2012). Wine grapes are harvested at the commercial ripening stage, when the major ripening parameters achieve the enological requirements for different types of wine. To make some particular wine styles (e.g. Amarone), berries may be left on the plant beyond ripening, or they may be harvested and stored in dehydrating rooms before vinification. These commercial practices significantly impact some organoleptic traits of wines, including the enhancement of sugar/alcohol content, robustness, and development of particular overripening- and/or withering-related aromas. The few studies focusing on postripening events have shown that active biochemical and molecular changes continue in these berries, probably reflecting processes involved in overripening and/or senescence (Zamboni et al., 2008; Guillaumie et al., 2011; Gapper et al., 2013; Cramer et al., 2014). Postharvest dehydration has a significant impact on general berry metabolism and physiology, which is difficult to distinguish from overripening or senescence. Water loss increases the solute concentration and progressively induces stress in the pericarp cells. Current dehydration techniques can last from 3 weeks to 4 months, causing a weight loss of 30% to 40% and yielding a final product that is richer in sugars, solutes, and aroma compounds. Physical, physiological, and biochemical changes have been described in postharvest berries dehydrated in ventilated rooms, suggesting a dependence on both environmental parameters and endogenous factors, such as berry morphology and genotype (Barbanti et al., 2008; Tonutti and Bonghi, 2013). During postharvest withering, ongoing or specifically activated metabolic processes may affect berry compositional parameters, such as the polyphenol and volatile metabolite profiles, the structure of cell wall polysaccharides, and the activity of enzymes involved in anaerobic respiration (Costantini et al., 2006; Toffali et al., 2011; Zoccatelli et al., 2013). At the molecular level, the induction of stilbene synthase (STS) gene expression (Versari et al., 2001) was the first indication of large-scale changes in the berry transcriptome described in subsequent reports. Indeed, cDNA-AFLP analysis by Zamboni et al. (2008), microarray analysis by Rizzini et al. (2009) based on an incomplete genome representation, and the exhaustive transcriptomic atlas, including postharvest samples generated by Fasoli et al. (2012), revealed the extensive modulation of gene expression in berry tissues during prolonged postharvest dehydration. The induction of genes involved in secondary metabolism, cell wall metabolism, stress responses, and carbohydrate metabolism and transport, indicates that the active transcriptional control of these processes facilitates structural and compositional changes, including the de novo biosynthesis of certain classes of compounds in postharvest dehydrating berries.
The study of grape postripening phase is of great interest to plant biologists and winemakers, providing particular insights into the genetic and environmental factors affecting the postharvest berry physiology and metabolism, in turn directly influencing the organoleptic properties of wines. Although some of the major metabolic processes affected during postharvest dehydration have been identified in certain genotypes (grape varieties) and under specific conditions, there has been no comprehensive analysis that reveals both the general events shared among different varieties and the genotype-specific responses. Here, we report an exhaustive analysis of metabolomic and transcriptomic responses to postharvest dehydration in six red berry genotypes (Corvina, Sangiovese, Merlot, Syrah, Oseleta, and Cabernet Sauvignon) stored after detachment under identical controlled environmental conditions. Multivariate data analysis allowed us to define the common molecular events that characterize postharvest grapevine berries and how these events correlate with major changes in the berry metabolic profile.
RESULTS
Characterization of Postharvest Dehydration in Six Red Berry Varieties
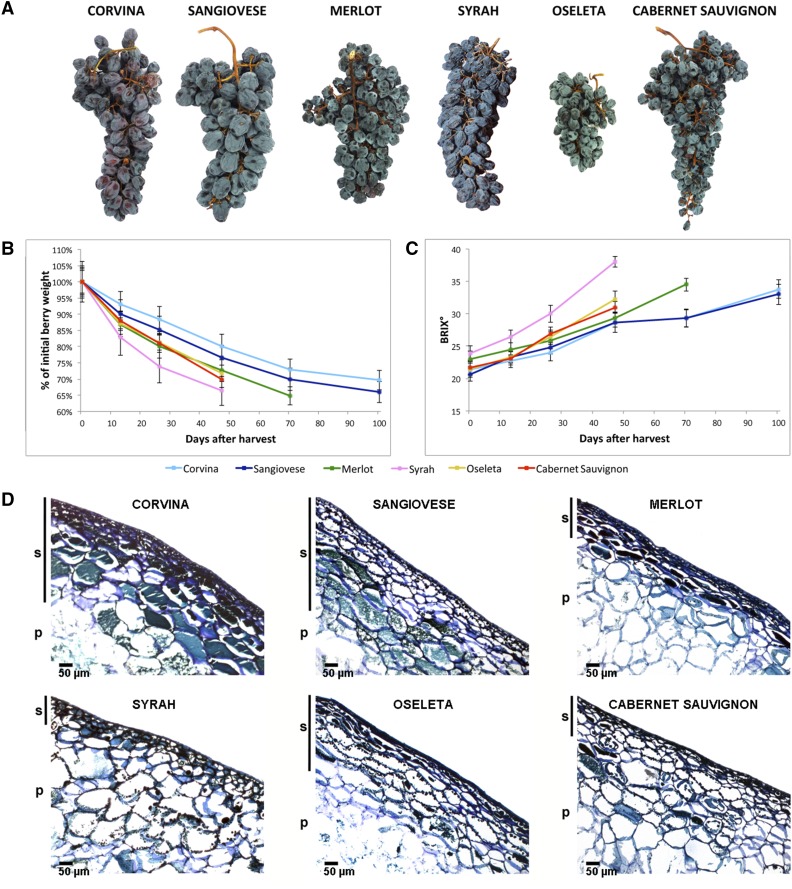
Corvina, Sangiovese, Merlot, Oseleta, Syrah, and Cabernet Sauvignon berries were collected at commercial maturity, and the detached bunches were stored under identical controlled environmental conditions. The six cultivars differed greatly in terms of dehydration kinetics. Corvina berries showed the slowest weight loss, followed by Sangiovese and Merlot, whereas Cabernet Sauvignon, Oseleta, and Syrah lost weight more rapidly. The drying process was stopped until all genotypes reached ∼30% weight loss (Fig. 1A), i.e. after 47 d for Oseleta, Syrah, and Cabernet Sauvignon, after 70 d for Merlot, and after 100 d for Sangiovese and Corvina (Fig. 1B). Berry samples were collected at the same times after harvest, so four time points encompassed the entire process for Oseleta, Syrah, and Cabernet Sauvignon, but five sampling points were needed for Merlot and six time points were needed for Corvina and Sangiovese. We also investigated dehydration kinetics by measuring the total soluble solids content. As expected, the soluble solids content increased more rapidly for the fastest drying varieties (e.g. Syrah; Fig. 1C). We also monitored the berry juice acidity, revealing that Corvina berries were characterized by slower organic acid catabolism (Supplemental Fig. S1, A and B).
Figure 1.
Overview of the postharvest dehydration process and sampling strategy for Corvina, Sangiovese, Merlot, Syrah, Oseleta, and Cabernet Sauvignon grapevine varieties. A, Berry clusters representing each variety were collected at the final postharvest withering stage (corresponding to ∼30% weight loss). B and C, Berry weight loss and °Brix trends. D, Transverse berry section at harvest for each variety (100× magnification) stained with toluidine blue, showing the epidermal and hypodermal skin layers (s) plus mesocarp (pulp, p). Scale bar = 50 µm.
The physical proprieties of berries that are likely to be responsible for the variety-specific dehydration kinetics were investigated by weighing the berries at harvest and by the analysis of the berry skin (exocarp) by optical microscopy (Fig. 1D; Supplemental Fig. S1C). The initial berry weight provides an indirect measure of the size and allows us to make a reasonable estimation of the surface area/volume ratio (S/V), which is inversely related to the dehydration rate (Barbanti et al., 2008). A rough calculation of S/V revealed that Sangiovese (3.5 cm−1) showed the lowest ratio, followed by Corvina (3.7 cm−1), whereas Oseleta (4.6 cm−1) showed the highest ratio, followed by Syrah and Cabernet Sauvignon (Supplemental Fig. S1C). The exocarp was shown to comprise one or two layers of compact epidermis and an underlying hypodermis with genotype-dependent differences in the number and structure of layers. Corvina, Sangiovese, and Oseleta berries contained more layers than the other varieties. The Corvina exocarp cells were particularly compact, whereas the Oseleta berries were covered by a thick cuticle that prevents the evaporation of water (Fig. 1D). Merlot berry skins were thinner, although with several cell layers, whereas Cabernet Sauvignon and Syrah berries had much thinner skins with fewer layers of generally loosely packed cells. This phenomenon was particularly evident in Syrah berries, which experienced the fastest loss of water (Fig. 1D).
Together, these data suggest that the combination of high berry weight, thick skin, and multiple layers of compact hypodermis may account for the slower postharvest dehydration of Corvina and Sangiovese berries.
The accumulation of d-gluconic acid, one of the most important markers associated with grape infection by Botrytis cinerea, was analyzed at the initial and final sampling point in all varieties to evaluate the healthiness of grapes. In all samples, d-gluconic acid levels were lower than 0.5 g/L, considered the threshold value for the presence of B. cinerea (Supplemental Fig. S1D). A slight increase in Tend compared to T0 was observed in some genotypes, probably reflecting the concentration effect of the dehydrated samples.
Characterization of the Metabolome in Six Berry Varieties at Harvest
High-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) analysis was used to measure the nonvolatile metabolites in the six berry varieties during postharvest dehydration. Among the 1884 recorded m/z features, 194 were putatively assigned to molecules, 260 to aglycones, fragments, and molecular adducts, and the remaining 1431 remained unidentified. The identified metabolites included 25 anthocyanins, 38 flavonoids, 14 hydroxycinnamic acids, seven hydroxybenzoic acids, three hydroxytorosol/hydroxytyrosol derivatives, 35 proanthocyanins and flavanols, 56 stilbenes, and a small number of sugars and nonaromatic organic acids (Supplemental Dataset S1).
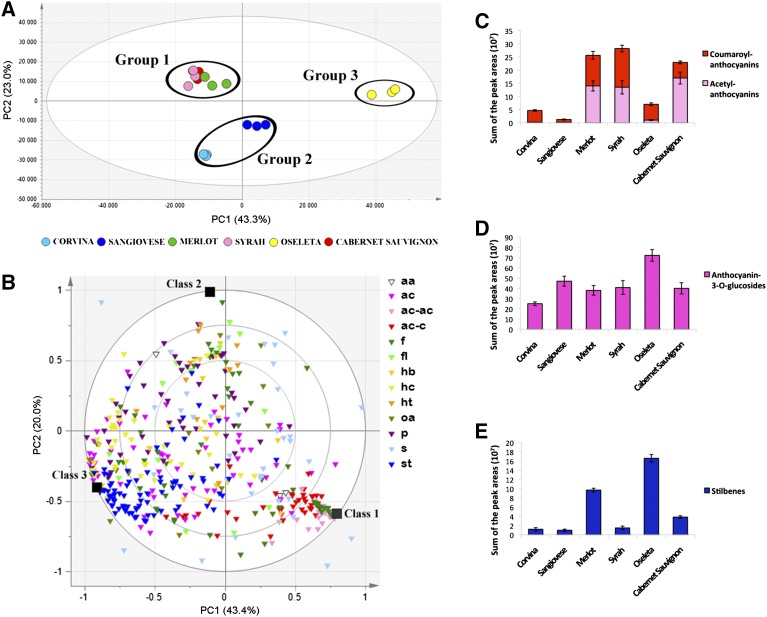
We analyzed the berry metabolomes at T0 to find metabolic differences among the six varieties at harvest. The data matrix was explored by principal component analysis (PCA), partitioning the six varieties into three groups based on their metabolic content (Fig. 2A). The first group is represented by Merlot, Syrah, and Cabernet Sauvignon, the second group by Corvina and Sangiovese, and Oseleta was clearly separated from the other varieties (Fig. 2A). On this basis, a three-class orthogonal bidirectional partial least squares discriminant analysis (O2PLS-DA) model was constructed to characterize each group of varieties by berry metabolic composition. The correlation loading plot of the model (R2X(cum):0.74 and Q2(cum):0.968) clearly showed that class 1 (Merlot, Syrah, and Cabernet Sauvignon) and class 3 (Oseleta) were positively characterized by many distinct metabolites, whereas Corvina and Sangiovese (class 2) were positively characterized by only a few metabolites, and showed an intermediate composition between the other two classes (Fig. 2B; Supplemental Dataset S2). In particular, Merlot, Syrah, and Cabernet Sauvignon were characterized by higher amounts of anthocyanin (acetyl) and (coumaroyl)-3-O-glucoside (Fig. 2, B and C), whereas Oseleta showed high levels of anthocyanin-3-O-glucoside and stilbenes (Fig. 2, B, D, and E). The three groups of varieties were analyzed in more detail based on specific classes of metabolites and the metabolic differences between individual genotypes by building new PCA models and by further O2PLS-DA correlation plots as discussed in the Supplemental Data.
Figure 2.
Characterization of the berry metabolome in the six varieties at harvest. A, PCA score scatter plot of the model obtained for the metabolites detected by HPLC-ESI-MS at harvest. Samples, corresponding to the three biological replicates of each variety at harvest, are separated into three groups (explained variance = 66.3%). The PCA score scatter plot is colored according to the variety. B, O2PLS-DA correlation loading plot of the three-class model for the metabolites detected by HPLC-ESI-MS according to the unsupervised PCA in A. Groups of metabolites are shown in different colors. Abbreviations: aa, amino acids; ac, anthocyanins; ac-ac, acylated anthocyanins; ac-c, coumaroyl anthocyanins; f, other flavonoids; fl, flavonols; hb, hydroxybenzoic acids; hc, hydroxycinnamic acids; ht, hydroxytyrosol; oa, organic acids; p, procyanidins; s, sugars; st, stilbenes. C, D, and E, Distribution of coumaroyl and acetyl anthocyanins, anthocyanin-3-O-glucosides, and stilbenes, respectively, indicated as the sum of peak areas, among the varieties at harvest.
Metabolomic Changes in Berries during Postharvest Dehydration
The active metabolic changes during postharvest dehydration were investigated by normalizing the data matrix to the level of weight loss in each variety, in order to exclude or minimize the concentration effect due to water loss. PCA revealed the same clustering into three groups observed at T0 (Fig. 2A), suggesting that the influence of genotype has a greater effect on the variability described by the two principal components than the metabolomic changes during dehydration (Supplemental Fig. S2). We therefore investigated the metabolomic changes characterizing the six varieties by PCA applied to the three groups of varieties separately. Merlot, Syrah, and Cabernet Sauvignon showed similar dynamic metabolic profiles characterized by a steady increase in the PC1 value until the penultimate stage, with a sharp reversal at the final stage to a PC1 value lower than that at T0 (Fig. 3A). The PCA loadings plot indicated that this pattern reflects the initial accumulation of some molecules during dehydration followed by a sudden decrease. The PC1 variability was primarily due to the levels of anthocyanin-3-O-glucosides, mainly malvidin (Fig. 3B). Other molecules followed the same PC1 dynamic profile, such as quercetin-3-O-glucoside, miricetin-O-hexose, delfinidin-3-O-glucoside, and malvidin-(acetyl)-3-O-glucoside, but these also separated Cabernet Sauvignon from Merlot and Syrah, as described by PC2 (Fig. 3B).
Figure 3.
Global metabolomic trends during the postharvest dehydration process. A, C, and E, PCA score scatter plots of the three models obtained for the metabolites detected by HPLC-ESI-MS during postharvest dehydration for varieties belonging to class 1 (Merlot, Syrah, and Cabernet Sauvignon; explained variance = 48.8%), class 2 (Corvina and Sangiovese; explained variance = 61.3%), and class 3 (Oseleta; explained variance = 60.0%). Samples comprised three biological replicates at each stage. The PCA score scatter plot is colored according to the variety. CO, Corvina; SA, Sangiovese; ME, Merlot; SY, Syrah; OS, Oseleta; CA, Cabernet Sauvignon. B, D, and F, PCA correlation loading plots of the PCA models for classes 1, 2, and 3. Groups of metabolites are shown in different colors. Abbreviations: aa, amino acids; ac, anthocyanins; ac-ac, acylated anthocyanins; ac-c, coumaroyl anthocyanins; f, other flavonoids; fl, flavonols; hb, hydroxybenzoic acids; hc, hydroxycinnamic acids; ht, hydroxytyrosol; oa, organic acids; p, procyanidins; s, sugars; st, stilbenes.
The Corvina and Sangiovese berry metabolomes changed predominantly along PC2 (Fig. 3C). The PC2 value of the Corvina berries declined progressively, whereas the PC2 value of the Sangiovese berries fluctuated from T0 to T5. The decline in PC2 was predominantly caused by the accumulation of stilbenes in both varieties. In contrast, the levels of quercetin-3-O-desoxyhexoside, catechin, peonidin-3-O-glucoside, and hydroxytyrosol declined during dehydration, the latter in Corvina berries only (Fig. 3D). PCA applied to the Oseleta metabolome showed a steady increase from T1 to T3 along PC1 and an increase at T2 along PC2 (Fig. 3E). The PCA loadings plot revealed that the increase along PC1 reflected the accumulation of resveratrols and viniferins, as observed for Corvina and Sangiovese, whereas the transient increase along PC2 reflected the accumulation of some anthocyanin-3-O-glucosides and caffeoyl tartaric acid (Fig. 3F).
Changes in the berry metabolome during postharvest dehydration were highlighted by grouping the annotated metabolites into five classes: (1) acylated (acetyl and coumaroyl) and nonacylated anthocyanidin-3-O-glucosides, (2) flavonols and other flavonoids, (3) hydroxycinnamic and hydroxybenzoic acids and hydroxytyrosol, (4) flavan-3-ols and proanthocyanidins, and (5) stilbenes. The quantity of each class was calculated by summing the total intensities of metabolites in each class for each variety. The ratio of values at the final stage (Tend) and harvest stage (T0) summarized the global effect of dehydration on each class of compounds (Fig. 4A). This revealed that anthocyanins, flavonols, and other flavonoids were slightly negatively affected by dehydration in all varieties, whereas the positive or negative final balance of hydroxycinnamic/hydroxybenzoic acids, flavan-3-ols, and proanthocyanidins depended on genotype and was clearly negative in Corvina.
Figure 4.
The dynamic phenylpropanoid pathway during postharvest dehydration. A, The distribution of phenylpropanoid-related metabolites is represented as the fold change between the quantity determined by HPLC-MS analysis at the last (Tend) and the harvest (T0) stages for each variety. Groups of metabolites are shown in different colors. The striped bar indicates nonsignificant fold change values. CO, Corvina; SA, Sangiovese; ME, Merlot; SY, Syrah; OS, Oseleta; CA, Cabernet Sauvignon. B, Distribution of stilbenes, determined by HPLC-MS analysis and indicated as the sum of the peak areas (arbitrary units), among the six varieties at all stages of the postharvest drying process. The details of resveratrol monomers, dimers, trimers, and tetramers are indicated for each time point in different shades.
A closer inspection of the trend for each class of metabolites revealed characteristic genotype-dependent fluctuations in some classes that were not indicated by the Tend/T0 ratio (Supplemental Fig. S3). This was true for anthocyanins, flavonols, and other flavonoids in Syrah, Cabernet Sauvignon, and Merlot (Supplemental Fig. S3, A and B) as stated above (Fig. 3B).
The stilbene class was the most strongly genotype dependent, showing particularly high levels of accumulation in withered Sangiovese (Tend/T0 = 14.8), Corvina (Tend/T0 = 7.9), Syrah (Tend/T0 = 5.3), and, to a lesser extent, Oseleta (Tend/T0 = 1.9) berries, whereas the same metabolites showed little or no change in Merlot and Cabernet Sauvignon berries (Fig. 4A). The profile of monomeric and oligomeric stilbenes during dehydration differed among varieties in terms of both accumulation levels and degrees of polymerization, further highlighting the genotype-dependent effect of postharvest dehydration on stilbene metabolism (Fig. 4B). Corvina and Sangiovese berries initially contained minimal amounts of stilbenoids and a steady increase was observed during dehydration. In Syrah berries, the low initial level of these compounds increased more rapidly than Corvina and Sangiovese, peaking at T2, whereas in Oseleta the initial level was much higher and doubled between T1 and T2. Conversely, there was no clear accumulation trend during the dehydration of Merlot and Cabernet Sauvignon berries (Fig. 4B).
Together, these data showed that the postharvest period involves genotype-dependent changes in the berry metabolome. In some genotypes, the major contribution to the dynamic metabolic profile reflected stilbene accumulation, whereas in varieties that do not accumulate stilbenoids the greatest source of variation was the transient accumulation/depletion of other classes of metabolites, such as anthocyanins and flavonols.
The accumulation of volatile organic compounds was analyzed in Corvina berries by gas chromatography-mass spectrometry (GC-MS) revealing 72 compounds: four alcohols, seven aldehydes, three carboxylic acids, one ester, 14 benzene derivatives, four C13-norisoprenoids, seven monoterpenes, and 32 sesquiterpenes (Supplemental Dataset S3). PCA of the dataset normalized for berry weight loss explained 79.35% of the total variance (PC1 69.4% and PC2 9.95%) and clearly showed the postharvest progression from ripe berries (left side) to later stages (right side; Supplemental Fig. S4). The modulation of volatiles was described by a heat map, grouping compounds on the basis of their dynamic accumulation/depletion (Supplemental Fig. S5). Only a few metabolites were depleted (in particular, n-hexanal, 2-hexenal, and eugenol), whereas many compounds accumulated after harvest, following diverse trends. These included a large number of sesquiterpenes that increased gradually, peaking at T4 (Supplemental Fig. S5). Intriguingly, three monoterpenes (γ-terpinene, paracymene, and 1-terpinen-4-ol) increased suddenly and only at the final postharvest stage.
Postharvest Changes in the Berry Transcriptome
Next we carried out a transcriptomic analysis of the same samples used for the metabolomic study to provide a complete overview of molecular changes taking place during berry postharvest. We removed from the dataset those genes whose expression was always lower than a threshold value calculated for each subarray, reducing the number of genes to 25,402 (Supplemental Dataset S4).
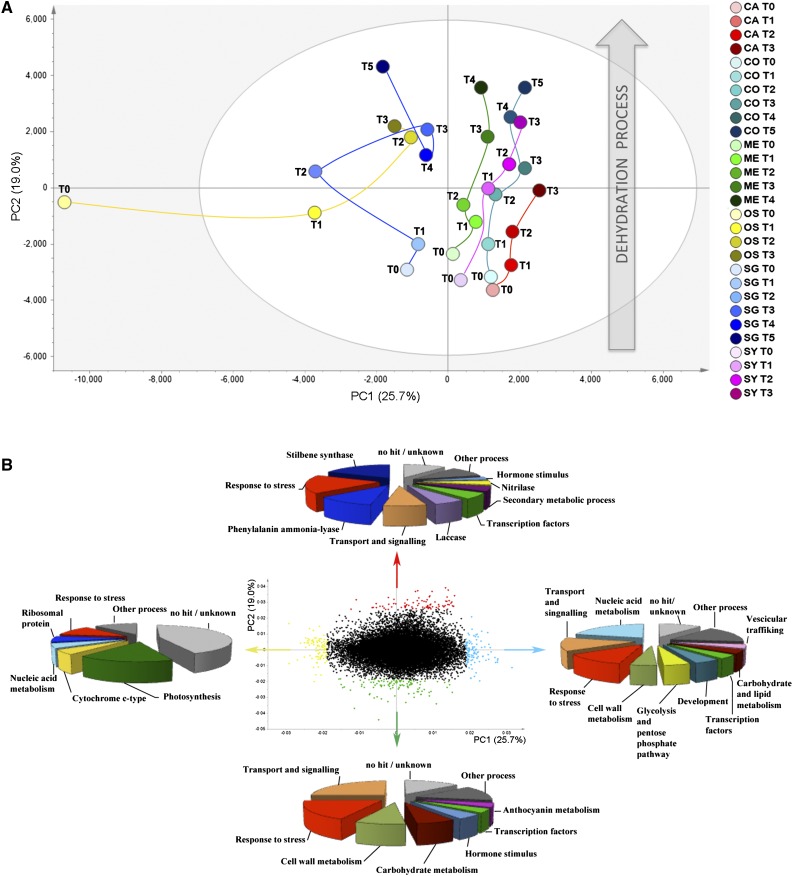
PCA applied to the six varieties at harvest revealed that berries could clearly be separated into three groups. The Oseleta berries formed a unique group, as observed during the metabolomic analysis, whereas Sangiovese grouped with Merlot, and Corvina grouped with Cabernet Sauvignon and Syrah (Supplemental Fig. S6). However, by applying PCA to the entire collection of samples representing the six varieties, the grouping was no longer evident (Fig. 5A). Postharvest transcriptomic changes were primarily described by PC2 in all varieties. The dynamic changes in the berry transcriptome along this principal component were particularly pronounced for Corvina, Sangiovese, Merlot, and (to a lesser extent) Syrah, with high component values reached at the final time point. Cabernet Sauvignon had a similar PC2 value to the other varieties at T0, but showed relatively small postharvest transcriptomic changes, the final sample reaching the same PC2 value as the Syrah T1 sample. In Oseleta, the PC2 values increased only after T1, the transition between T0 and T1 being mostly attributable to PC1. With the exception of this particular case, PC1 mainly described the genotypic differences among samples (Fig. 5A).
Figure 5.
The dynamic berry transcriptome during postharvest dehydration. A, PCA score scatter plot of the model obtained for transcripts detected by microarray analysis during postharvest dehydration in each variety (explained variance = 44.7%). Samples, corresponding to the averaged value of the three biological replicates at each stage, are colored according to variety. CO, Corvina; SA, Sangiovese; ME, Merlot; SY, Syrah; OS, Oseleta; CA, Cabernet Sauvignon. B, PCA correlation loading plot of the PCA model. Groups of genes more correlated to the first and second components are shown in different colors: first principal component negatively correlated, yellow; first principal component positively correlated, light blue; second principal component negatively correlated, green; second principal component positively correlated, red. The most represented functional categories for each group of genes are indicated by the arrows.
We sought the most important genes associated with sample distribution along the two principal components by selecting 100 genes with the highest and the lowest score values (Fig. 5B; Supplemental Dataset S5). Genes with the lowest PC1 score values, down-regulated during the transition from T0 to T1 in Oseleta, predominantly represented photosynthesis, response to stress, cytochrome, and ribosomal proteins. This suggests that Oseleta berries are still characterized by high photosynthetic activity at the harvest stage (T0) and may represent a less-ripe physiological state than other cultivars. In contrast, genes positively associated with PC1, particularly in the Cabernet Sauvignon, Corvina, Syrah, and Merlot cultivars, represented many functional categories and thus covered diverse biological functions. The most represented categories were related to stress responses, including heat shock proteins and genes involved in the scavenging of reactive oxygen species, nucleic acid metabolism, transport and signaling.
The functional annotation of 100 genes with the highest PC2 score values, i.e. those strongly associated with and up-regulated during the final postharvest stages (Fig. 5B, red triangles), revealed the common induction of many genes encoding STS, Phe ammonia-lyase (PAL), stress response proteins (including osmotins and pathogenesis-related proteins), proteins involved in transport and signaling, as well as laccases and dirigent proteins involved in the oxidation of phenolic substances (Morreel et al., 2004; Pourcel et al., 2005; Schuetz et al., 2014). We also identified two genes encoding cinnamate 4-hydroxylase (C4H) and 4-coumarate:CoA ligase (4CL), which, together with PAL, catalyze the earliest steps of phenylpropanoid biosynthesis. We found also a cinnamoyl-CoA reductase, the first committed enzyme of the lignin biosynthesis pathway, and a caffeic acid 3-O-methyltransferase, which catalyzes the multistep methylation of hydroxylated monomeric lignin precursors. We identified several genes related to hormone synthesis and activity, including two encoding ethylene-related proteins—1-amino-cyclopropane-1-carboxylate oxidase1 (ACO1) and an ethylene-responsive transcription factor—and an auxin efflux carrier, supporting a role for these hormones in the berry dehydration response. Interestingly, this list included four nitrilase genes that may be involved in hormone metabolism (Howden and Preston, 2009). We also identified genes encoding transcription factors of the NAC family (VvNAC60 and VvNAC61), two WRKY factors, and VvJAZ9, the latter associated with biotic and abiotic stress responses (Zhang et al., 2012; Supplemental Dataset S5).
The functional annotation of 100 genes with the lowest PC2 score values, i.e. those strongly associated with the initial postharvest stages and down-regulated thereafter (Fig. 5B, green triangles), revealed genes encoding proteins involved in cell wall metabolism (four expansins, two pectinesterases, and a xyloglucan endotransglucosylase/hydrolase), sugar metabolism (two Suc-phosphate synthases), and hormone signaling (IAA19 and an auxin-independent growth promoter, and the VvERF045 ethylene response factor). We also found genes directly involved in anthocyanin synthesis and transport, such as VvMYBA2, VvLDOX, VvGST4, and VvanthoMATE1, strongly indicating that anthocyanin synthesis is switched off during postharvest dehydration in all six varieties (Supplemental Dataset S5).
Altogether, PCA revealed that all six varieties share common transcriptomic changes during the dehydration process, albeit with different and genotype-dependent dynamic profiles.
General Molecular Events Characterizing the Postharvest Life of Grapevine Berries
The molecular changes shared by all six varieties during postharvest dehydration were built into a comprehensive model by focusing on the common differentially expressed genes. A significance analysis of microarrays multiclass comparison using a false discovery rate (FDR) of 0.1% and a fold change threshold (|FC| > 2 in at least one comparison) was performed separately in Corvina, Sangiovese, Merlot, Syrah, Oseleta, and Cabernet Sauvignon berries (Supplemental Dataset S6). This identified 49 and 45 genes that were up-regulated and down-regulated, respectively, in all varieties (Supplemental Fig. S7).
The 49 commonly up-regulated genes included some encoding proteins related to secondary metabolism, i.e. three STSs, two laccases, and a dirigent protein probably involved in the oxidative polymerization of phenolic compounds. Moreover, the VvROMT gene, a close homolog of VvROMT1 that is involved in the methylation of stilbenes (Schmidlin et al., 2008), was also up-regulated in all six varieties. Many of the up-regulated genes were found to encode transport and signaling components, e.g. a MATE efflux protein, a magnesium transporter, the sugar transporter ERD6-like 16, an ammonium transporter, a potassium channel, a mitochondrial phosphate transporter, an intramitochondrial sorting protein, a calmodulin-binding protein, and two receptor-like kinases. We also identified a zinc finger C3HC4-type regulator, an R2R3 MYB homolog of Arabidopsis (Arabidopsis thaliana) AtMYB62, and two WD40-repeat proteins. We also observed the common up-regulation of a pyruvate kinase and a lactate dehydrogenase involved in carbohydrate metabolism.
The 45 commonly down-regulated genes encoded some proteins involved in cell wall metabolism (including a pectate lyase, a 1,4-β-mannan endohydrolase, VvEXPA1, a cellulase, an endo-1,4-β-glucanase, and a polygalacturonase) and in lipid metabolism, e.g. three ketoacyl-CoA synthases and transcription factor SHINE1, which regulates wax biosynthesis (Aharoni et al., 2004; Supplemental Fig. S7). Another important group of commonly down-regulated genes encoded transcription factors, including two anthocyanin MYB regulators (Walker et al., 2007), the ERF/AP2 factor VvERF045, a basic helix-loop-helix factor, and the transcription factor VvbZIP07. The down-regulation of the aforementioned anthocyanin MYBs as well as cytochrome b5 DIF-F, which is required for the full activity of flavonoid 3′5'-hydroxylase (de Vetten et al., 1999; Bogs et al., 2006), provides further evidence for the postharvest cessation of anthocyanin biosynthesis.
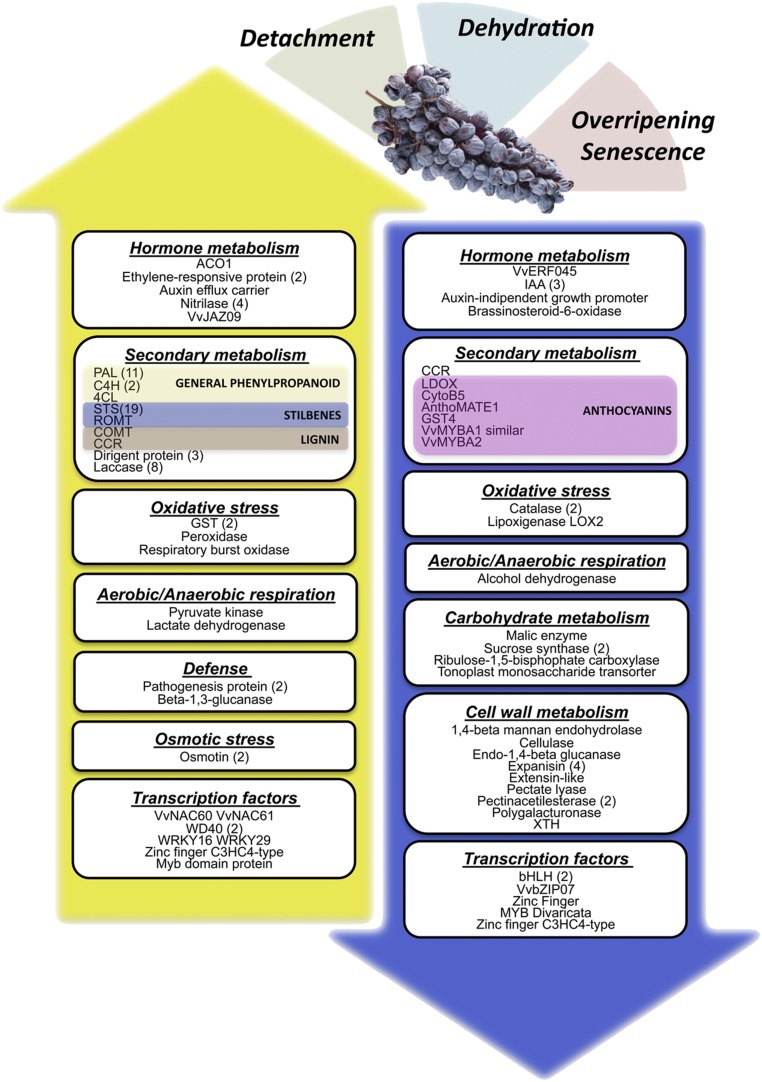
A comprehensive picture of the principal molecular events activated or repressed during postharvest dehydration was presented by integrating data representing (1) the commonly-modulated genes, and (2) genes associated with the initial and final postharvest stages as identified by PCA. Both approaches highlighted either the same 20 genes as indicated in Supplemental Fig. S7, or genes often related to the same specific metabolic or cellular functions. The common responses to postharvest dehydration in grapevine berries were therefore assembled into a comprehensive model that also shows putative major triggers, i.e. fruit detachment, dehydration and over-ripening/senescence (Fig. 6; Supplemental Dataset S7).
Figure 6.
Blueprint describing metabolic processes and functional categories of the genes primarily involved in detachment, dehydration, and senescence stress that grape berries experience during the postharvest dehydration process regardless of the variety. In particular, the yellow arrow describes gene categories that are up-regulated during the postharvest drying process, whereas the blue arrow describes gene categories that are down-regulated.
Genotype-Dependent Responses to Postharvest Dehydration
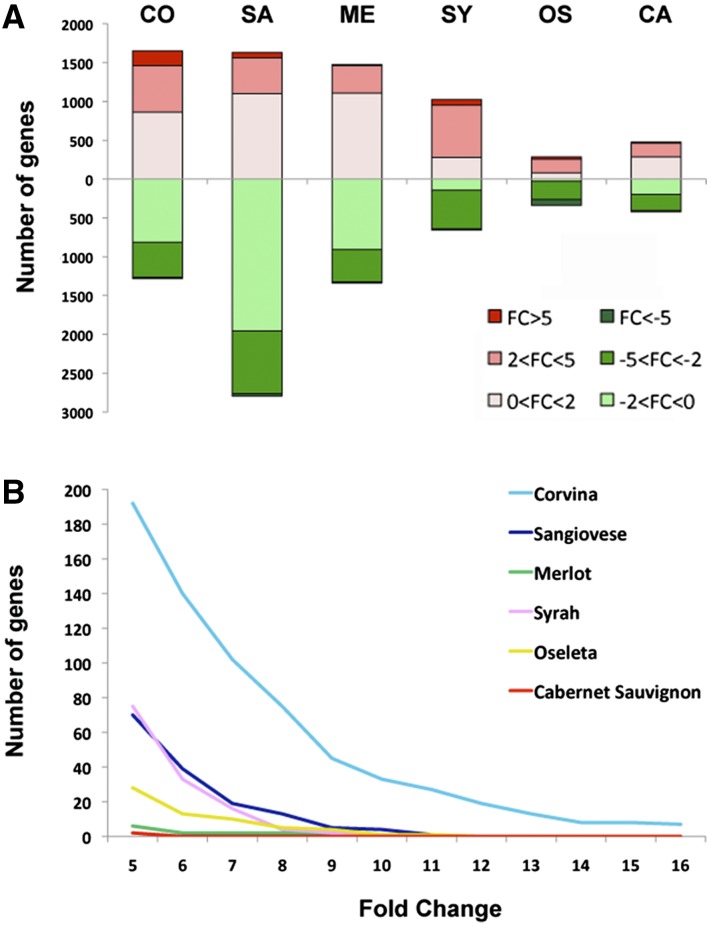
Although many common features are shared by the six genotypes, the number of genes modulated after harvest differs greatly among them. The highest number was observed in Sangiovese berries (4427), followed by Corvina (2933), Merlot (2803), Syrah (1681), Cabernet Sauvignon (886), and Oseleta (622) (Supplemental Dataset S6). Although the modulation of many genes was shared among two or more genotypes, each variety was characterized by large numbers of uniquely modulated genes as listed in Supplemental Dataset S6. The ratio between up-regulated and down-regulated genes also differed among the six varieties. For example, Sangiovese was characterized by a higher proportion of down-regulated genes (Fig. 7A). By comparing the average FC value of the differentially expressed genes in each variety after harvest, the distribution of gene modulation intensity was found to be genotype dependent. In particular, the highest number of strongly up-regulated genes (FC > 5) was found in Corvina, Sangiovese, and Syrah berries (Fig. 7A). A closer inspection of these genes revealed that Corvina was characterized by the strongest up-regulation intensity, with many genes showing an FC > 10 (Fig. 7B). In Sangiovese, Syrah, and (to a lesser extent) Oseleta, many genes were characterized by FC values of 5 to 10, whereas the other genotypes revealed few genes with an average FC > 5. These observations clearly show that the transcriptomic responses to postharvest dehydration are strongly genotype dependent.
Figure 7.
Genotype-dependent responses to the postharvest dehydration process. A, Genes significantly up-regulated and down-regulated in each variety. The modulation intensity is indicated by different shades of red (up-regulation) and green (down-regulation). CO, Corvina; SA, Sangiovese; ME, Merlot; SY, Syrah; OS, Oseleta; CA, Cabernet Sauvignon. B, Number of up-regulated genes with a fold change > 5 in each variety.
To evaluate differences in the responsiveness of the six varieties to postharvest dehydration, the 50 genes with the highest induction ratio (top50) were identified in each cultivar, and their expression profiles were compared (Fig. 8; Supplemental Dataset S8). Heat maps representing FC values at each time point compared to T0 showed that the six varieties are characterized by different trends in gene activation. Corvina showed a strong and progressive induction of gene expression during postharvest dehydration starting at T1. A similar trend, with a lower induction ratio, was observed in Syrah. In the other genotypes, the highest induction ratio was delayed (to T2 in Oseleta and T3 in the remaining varieties). In Sangiovese berries, a temporary decrease in gene induction at T4 was observed, confirming the behavior shown in Figure 5A.
Figure 8.
Differences in the responsiveness of the six varieties to the postharvest dehydration process. Heat maps of the top50 activated transcripts during postharvest dehydration in each variety. Values represent the ratios between the fold change at each time point and the T0 sample. A schematic representation of the general up-regulation trend is provided above each heat map, and the functional annotations of each gene are reported in different colors.
The six varieties were also distinguishable by the function of the top50 genes (Fig. 8; Supplemental Dataset S8). Corvina was characterized by a high number (19) of STSs. Many genes involved in phenylpropanoid and terpene metabolism were also present. The top50 Corvina genes also included genes involved in biotic and abiotic stress responses, two pectinesterases, and only one WRKY transcription factor (VvWRKY24). The top50 Sangiovese genes included many laccases (20), two STSs, and many genes (14) involved in biotic and abiotic stress responses. The top50 Merlot genes were mainly related to abiotic stress (15) and transport and signaling (7). The top50 Syrah genes included 20 laccases, 12 of which were common to Sangiovese berries, and VvROMT. We found also many genes (10) involved in biotic and abiotic stress responses. We also found a terpene synthase and a pectinesterase, similar to Corvina berries. Oseleta berries expressed a unique group of highly up-regulated genes. In particular, we found seven genes previously shown by significance analysis of microarray to be specifically modulated in Oseleta berries (Supplemental Datasets S6 and S8), including three involved in nucleic acid metabolism. The strong induction of stilbene metabolism was inferred by the presence of six STSs and VvROMT among the Oseleta top50 genes. Interestingly, many genes were found in the transport, signaling, and transcription factors category. The top50 Cabernet Sauvignon genes included many laccases (15) and several genes involved in stress responses. Interestingly, we observed the induction of four genes related to hormone metabolism, i.e. an ethylene responsive protein, an auxin efflux carrier, VvJAZ6 and a methyl jasmonate esterase, that were modulated solely in this variety (Supplemental Dataset S8).
The top50 genes of each genotype were analyzed in more detail to determine whether the up-regulation of a single gene in a given genotype is shared with any of the remaining five genotypes. We found that a relatively large number of genes (indicated with an asterisk in Supplemental Dataset S8) in the top50 list of one genotype was present in the top50 list of one or more other genotypes. This was particularly evident for Sangiovese, Syrah, and Cabernet Sauvignon, which shared several common laccases and germin-like proteins. Moreover, we calculated the ratio of the average fold change of each gene in one genotype and the higher average fold change of the same gene in the other five genotypes. This relative up-regulation index (RUI) indicates whether the up-regulation of a given gene is peculiar to a specific genotype, revealing that the majority of the top50 genes in Cabernet Sauvignon and (to a lesser extent) Merlot, Sangiovese, and Syrah, are up-regulated at higher levels in at least one other of the five remaining genotypes. In contrast, almost all top50 Corvina genes showed very high RUI values, indicating a high level of relative up-regulation compared to the other varieties. Interestingly, Oseleta berries were characterized by relatively weak postharvest gene induction (Fig. 7), but the up-regulation of many top50 genes was restricted to this genotype, with RUI values > 1.
A Closer Investigation of the Differential Expression of Phenylpropanoid/Stilbene Genes
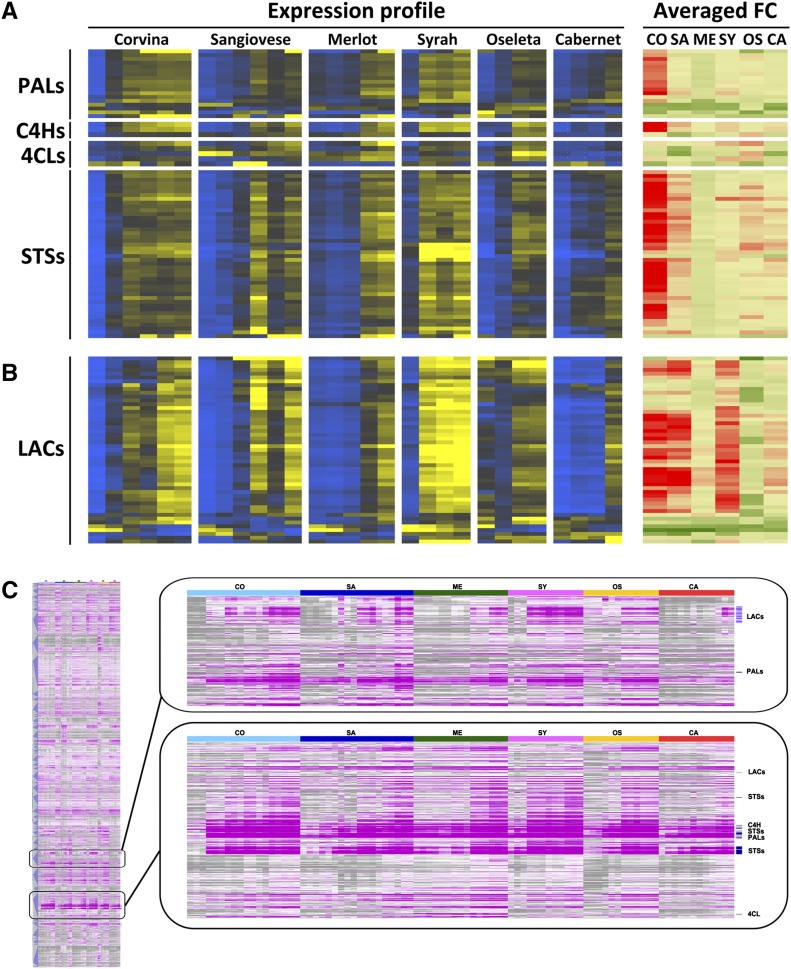
The transcriptomic and metabolomic data described above indicate that stilbene compounds play a major role in the postharvest dehydration response, so we focused on the expression of genes related to stilbene metabolism. We selected PAL, C4H, 4CL, and STS genes from the list of 6904 genes differentially expressed after harvest in at least one variety (FDR = 0.1% and |FC| ≥ 2 in at least one comparison; Supplemental Dataset S6). The expression profiles of the resulting 18 PAL, 3 C4H, 5 4CL, and 44 STS genes together with their averaged FC values are shown in Fig. 9A. We observed the generally coordinated up-regulation of genes representing the phenylpropanoid/stilbene biosynthesis pathway, albeit with genotype-dependent expression profiles. Syrah displayed the highest expression levels, followed by Sangiovese and Corvina. In contrast, the lowest expression of phenylpropanoid/stilbene biosynthesis genes, in particular C4H and 4CL genes, was observed in Cabernet Sauvignon berries. Based on the averaged FC value, Corvina showed the highest modulation for all genes except those encoding 4CLs. A general high level of induction for STS genes was also observed in Sangiovese berries. Merlot berries showed the least significant modulation of almost all the genes we tested.
Figure 9.
The phenylpropanoid pathway during grapevine postharvest dehydration in six different varieties. A and B, Heat maps of the expression profile (left: blue indicates low expression while yellow indicates high expression) and the average fold change (right: green indicates low FC while red indicates high FC) for a selection of phenylpropanoid-related genes (A) and laccase genes (B) during the postharvest dehydration process in each variety. C, Hierarchical cluster analysis of differentially expressed genes during dehydration in at least one variety (FDR = 0.1% and |FC| > 2; gray indicates low expression while pink indicates high expression). Enlargements show examples of 2 out of 91 hierarchical clusters. CO, Corvina; SA, Sangiovese; ME, Merlot; SY, Syrah; OS, Oseleta; CA, Cabernet Sauvignon. LAC, Laccase.
Several laccase genes were found among the common and genotype-specific molecular responses to dehydration, and the corresponding enzymes may control the oxidative polymerization of phenols, thus accounting for the accumulation of stilbene oligomers after harvest (Fig. 4B). We therefore inspected the expression profiles and the averaged FC values of laccases selected using the approach described above, and these are listed in Supplemental Dataset S6. The heat map indicates that the expression of most of the 49 laccases mirrors the expression of stilbene biosynthesis genes, strongly suggesting their involvement in the same metabolic pathway (Fig. 9B). The averaged FC values reveal by far the highest level of induction in Corvina, Sangiovese, and Syrah berries.
The relationship among genes related to stilbene metabolism and other pathway components was investigated by hierarchical clustering of the 6904 differentially expressed genes, resulting in 91 clusters (Fig. 9C). We found that 31 of the 44 modulated STS genes described above belonged to the same cluster together with 12 PALs, a 4CL, and 3 C4H genes, strongly supporting the common regulation of STSs and early steps in the phenylpropanoid pathway during postharvest dehydration (Fig. 9C; Supplemental Dataset S9). In addition, VvROMT and three dirigent proteins were found in the same cluster, supporting their involvement in stilbene metabolism. Interestingly, the expression profile of the ABC transporter VvPDR12 was similar to the STSs. Together with the presence of other three members of the ABC transporter family, this may indicate the involvement of these transporters in stilbene compartmentalization. The cluster included eight MYB transcription factors, including the known stilbene regulator VvMYB15 (Höll et al., 2013) and the phenylpropanoid/flavonoid regulator VvMYB5a (Cavallini et al., 2014). The other putative regulators in the cluster were three bHLHn proteins, VvbZIP20, two JAZ proteins, three NAC proteins, four WRKY proteins, and seven C3HC4-type zinc fingers. Notably, the same cluster contained the ethylene biosynthesis genes, ACO1 and SAM (S-adenosyl-Met) synthase.
A separate cluster contained 38 of the 49 laccases together with two PALs, four cinnamyl alcohol dehydrogenases, and a caffeic acid 3-O-methyltransferase (Fig. 9C; Supplemental Dataset S10). Interestingly, this cluster included several genes involved in abiotic stress responses and/or in senescence, such as 11 germin-like proteins, 11 nitrilases, six glutathione S-transferases, three heat-shock proteins, a peroxidase, and a thioredoxin. The cluster also contained several transcription factors, i.e. four WRKY proteins, six zinc fingers, three NAC proteins, two MYB factors, and VvbZIP38.
Taken together, these data suggested a clear genotype-dependent effect on the activation of stilbenoid metabolism, apparently associated with the expression of several laccase genes. However, a closer inspection of expression profiles revealed that differential transcriptional fine tuning is probably responsible for the distinct expression pattern of STS and laccase genes during the postharvest dehydration process.
Rapid and Slow Transcriptomic Responses to Postharvest Dehydration in Corvina Berries Take Place Predominantly in the Skin
Our data revealed that the induction of gene expression during postharvest dehydration is strongest in the Corvina variety. For this reason and because the postharvest dehydration of Corvina berries has already been profiled at the level of the transcriptome (Zamboni et al., 2008; Zamboni et al., 2010; Fasoli et al., 2012), we investigated the dynamic molecular changes in Corvina berries in more detail. The first percentile (i.e. the most strongly up-regulated genes) and the last percentile (i.e. the most strongly down-regulated genes) of the averaged FC ranking of the 2933 genes differentially expressed in Corvina with a |FC| ≥ 2 (Supplemental Dataset S6) were selected and analyzed by k-means clustering. Only two clusters of genes were observed based on coexpression profiles, for both the up-regulated and down-regulated genes (Fig. 10, A and B; Supplemental Dataset S11). We found that 142 up-regulated and 172 down-regulated genes showed rapid induction and repression, respectively (Fig. 10A), whereas 151 up-regulated and 121 down-regulated genes were characterized by slower induction and repression, respectively (Fig. 10B).
Figure 10.
Rapid and slow transcriptomic responses during postharvest dehydration in Corvina berries. Line plots showing the rapid (A) and slow (B) up-regulated (yellow) and down-regulated (blue) transcripts in Corvina berries undergoing postharvest dehydration. Functional categories are shown as pie charts beside each combination. C, Gene expression of the most strongly up-regulated (first percentile) and down-regulated (99th percentile) transcripts we identified during the ripening and postharvest process in Corvina berry pericarp, as reported in the expression atlas (Fasoli et al., 2012). D, Ratio of the average expression level in the three postharvest stages analyzed by Fasoli et al. (2012) in pulp and skin of most up-regulated genes. Positive fold changes are shown in pink, and negative fold changes are shown in green.
The rapid responses mainly involved the up-regulation of STS, PAL, and terpene synthase genes and the simultaneous down-regulation of genes related to anthocyanin metabolism, including many genes encoding flavonoid 3′,5′-hydroxylase, suggesting that the rapid reaction to postharvest dehydration is mainly represented by the rearrangement of secondary metabolism (Fig. 10A). Genes involved in defense and stress responses, such as those encoding pathogen-related proteins and germin-like proteins, were also rapidly induced, whereas many genes involved in transport, in particular several ABC transporters, and genes involved in abiotic stress responses, were rapidly repressed (Fig. 10A).
Among the slowly up-regulated transcripts were many encoding laccases and proteins involved in transport and signaling (Fig. 10B). We also found several genes involved in abiotic stress responses, hormone metabolism, (mainly auxin and ethylene), and the three pectin-methylesterases described by Zoccatelli et al. (2013; Fig. 10B). Genes that were slowly down-regulated were mainly involved in transport, signaling, photosynthesis, and cell wall metabolism. The latter included five expansins, an endo1-4-β-glucanase, three 1,4-β-mannanases, a cellulase, and a cellulose synthase (Fig. 10B).
These data allowed us to identify the most important transcriptional events taking place a few days after harvest, potentially representing the berry’s response to removal from the plant. We also identified a group of transcripts that were modulated more slowly and gradually during the postharvest period, probably representing the final developmental program (senescence) and/or a response to the increasing stress caused by water loss.
The same selection of strongly modulated genes was further investigated by taking advantage of the Corvina expression atlas (Fasoli et al., 2012). This allowed us to profile gene expression over a period extended to preharvest ripening stages (starting from veraison) and to look at separate profiles in the skin and pulp (Supplemental Fig. S8; Supplemental Dataset S12). The averaged expression levels of the most strongly up-regulated genes clearly revealed their steady, low-level expression during ripening followed by a marked induction at the first postharvest stage, whereas the expression of the most strongly down-regulated genes declined progressively from veraison to the last postharvest stage, with a less dramatic change after harvest (Fig. 10C). We then focused on the expression profiles of the same set of genes separately in the pulp and skin, in order to investigate the relative contribution of each tissue to the general expression level in the pericarp. The average expression level in the pulp and skin at the three postharvest stages analyzed by Fasoli et al. (2012) was used to determine the skin/pulp expression ratio. This revealed that most (90%) of the 293 most strongly up-regulated genes were expressed at higher levels in the skin than the pulp, with generally high FC values, whereas the small group of genes expressed more strongly in the pulp showed less pronounced FC values compared to the skin (Fig. 10D; Supplemental Dataset S12). A similar result was obtained for the most down-regulated genes, with about 70% of the genes showing higher expression levels in the skin (Supplemental Fig. S9; Supplemental Dataset S12). Interestingly, among the up-regulated genes expressed at higher levels in skin, we found genes involved in biotic stress resistance, many encoding germin-like proteins and some stilbenoid-related genes. Among the genes expressed at higher levels in the pulp, we found one 1-aminocyclopropane-1-carboxylate synthase (ACS), the abscisic acid receptor PYL4 RCAR10, and two genes involved in water transport (a nodulin and an aquaporin). Taken together, these data indicate that a new postripening transcriptional program is launched in the berry pericarp after harvest and that the berry skin contributes more to this program than the pulp.
DISCUSSION
In addition to the obvious changes in solute concentration, detached grapevine berries actively modulate their metabolism during postharvest dehydration as revealed by the transcriptional and metabolic changes reported in early studies (Versari et al., 2001; Costantini et al., 2006) and in more recent large-scale investigations (Zamboni et al., 2008, 2010; Rizzini et al., 2009; Fasoli et al., 2012). Our comprehensive metabolomic and transcriptomic study of six grapevine genotypes allowed us to identify common and genotype-specific behaviors, supporting earlier reports and providing a general map of the biochemical and molecular processes in the berry after harvest.
Differences in Water Loss Kinetics May Reflect the Distinct Skin Morphology of the Six Varieties
The six red grapevine varieties were characterized by distinct water loss kinetics and lost ∼30% of their total weight after 47 to 100 d, representing a wide experimental comparative platform that has never been exploited before. The differences in water loss kinetics we observed may in part reflect differences in berry skin morphology and initial berry weight among the six varieties (Fig. 1; Supplemental Fig. S1C). Corvina berries, which are large and have thick skins with compact layers, lost the least weight after harvest, whereas the smaller Syrah berries with their thin skins lost weight the most rapidly. Few studies have linked berry morphological features, such as size and skin texture parameters, with the dehydration rate (Barbanti et al., 2008; Rolle et al., 2011; Giacosa et al., 2012; Rolle and Gerbi, 2013). In this context, our data indicate that berry morphological features that potentially influence water loss may have a major impact on the extent of dehydration stress.
Postharvest Metabolomic Changes Are in Part Dependent on the Initial Metabolic Profile
The accumulation or depletion of phenolic compounds varies extensively during postharvest dehydration, indicating that factors such as genotype, dehydration kinetics, and environmental conditions have a major impact (Bellincontro et al., 2009; Mencarelli et al., 2010).
The dynamic profile of nonvolatile metabolites was based on a metabolomic dataset normalized for the weight loss of each variety after harvest. The differences among the varieties confirmed that some of the observed metabolic responses to postharvest dehydration were not passive effects caused by concentration changes but were evidence of an active process. We found that the initial grouping of the six varieties based on the qualitative content of some classes of molecules, such as anthocyanins and other flavonoids, was related to the dynamic metabolic changes after harvest (Fig. 3). In particular, the first group (Merlot, Syrah, and Cabernet Sauvignon) showed a temporary increase followed by a dramatic fall in the quantity of anthocyanins (mainly 3-O-glucosides) together with some other flavonoid compounds, whereas in the other two groups these compounds declined gradually during postharvest dehydration. The gradual loss of delphinidin-3-O-glucoside during the withering process in Corvina berries has already been described (Toffali et al., 2011).
The stilbenoids showed the highest dynamic range of concentrations and accumulated to high levels at the end of postharvest dehydration in four of the six genotypes (Fig. 4). The accumulation of stilbenoids was unrelated to the initial grouping of varieties defined on the basis of their T0 metabolome. Moreover, the presence of several oligomeric forms (viniferins) in dehydrating berries of the stilbenoid-accumulating varieties indicated that the activation of stilbene metabolism after harvest facilitates the synthesis of a plethora of related compounds.
The accumulation of monomeric stilbenes after harvest was previously reported in Corvina and Raboso Piave berries (Versari et al., 2001; Bonghi et al., 2012). These compounds (phytoalexins) have antifungal activity and are rapidly synthesized in response to fungal colonization (Pezet et al., 2003; Jean-Denis et al., 2006; Püssa et al., 2006; Jerkovic et al., 2007) and abiotic stress (Fritzemeier and Kindl, 1981; Versari et al., 2001). However, the accumulation of stilbenes was also reported in ripening berries in the absence of biotic or abiotic elicitors (Gatto et al., 2008). Under our experimental conditions, B. cinerea infection was excluded, and the accumulation of stilbenes may therefore reflect osmotic stress due to water loss and/or a feature of berry development (senescence). Nevertheless, stilbene production after ripening is not a common feature and is genotype dependent, with Cabernet Sauvignon and Merlot berries showing minimal responses, as previously observed by Panceri et al. (2013).
The untargeted analysis of volatile metabolites in Corvina berries revealed profound changes in the abundance of sesquiterpenes (Supplemental Fig. S5), which are usually present in a free form and are rapidly produced in response to stress, external stimuli, and developmental cues (Holopainen, 2004; Gordon-Weeks and Pickett, 2009; Schwab and Wüst, 2015; Zhang et al., 2016). The sesquiterpenes we detected showed diverse profiles in Corvina berries, although most accumulated toward the end of the dehydration process. The sesquiterpene profiles were clearly distinct from those of three monoterpenes (1-terpinen-4-ol, paracymene, and γ-terpinene), which accumulated dramatically only at the final stage.
The Metabolomic Changes Correlated with Transcriptional Reprogramming
The dynamic metabolic profiles discussed above were often supported by the results of our transcriptome analysis. The expression of stilbene biosynthesis genes (encoding PAL, C4H, 4CL, and STS) increased after harvest in a genotype-dependent manner that matched the varietal stilbene accumulation profiles (Fig. 9). The strong induction of stilbene-related genes after harvest has already been described in Corvina berries (Versari et al., 2001; Zamboni et al., 2008, 2010; Fasoli et al., 2012) and in other varieties (Rizzini et al., 2009). In our study, the massive induction of STSs in Corvina was observed to a lesser extent also in Sangiovese, Syrah, and Oseleta, whereas the same genes were induced to a minimal extent and their induction was delayed in Merlot and Cabernet Sauvignon. This confirms the genotype-dependent regulation of stilbene-related genes, which appears unrelated to dehydration stress kinetics. Variations in stilbene accumulation in response to abiotic stress were recently reported in cultivated and wild grape accessions, allowing the separation of genotypes into two subpopulations characterized by high and low levels of stilbene induction, respectively (Duan et al., 2015). This behavior may depend on differences in the promoter region of the stilbene pathway regulators, as reported for VvMYB14 (Duan et al., 2016).
We found that STS genes clustered with certain transcription factors of the MYB, WRKY, NAC, zinc finger, bZIP, and JAZ families (Supplemental Dataset S9). The presence of the known stilbene regulator VvMYB15 (Höll et al., 2013) in the same cluster suggests these candidates may also be transacting regulatory factors in the stilbene biosynthesis pathway. Sequence alignment and the functional analysis of those candidates could help to identify the molecular basis of genotype-dependent stilbene synthesis responsiveness. The same cluster contained VvROMT, an inducible gene involved in the methylation of resveratrol (Schmidlin et al., 2008), suggesting that the postharvest induction of stilbene biosynthesis also includes genes involved in stilbene modification. Notably, a small set of ABC transporters, including VvPDR12, showed a similar expression profile to most of the STSs, strongly suggesting a role in the transport of stilbenes and/or their precursors into the apoplast.
Interestingly, a strict relationship was observed between the induction of STS and laccase genes, which mirrored the accumulation trends of the different resveratrol polymers in the six varieties. Laccases are multicopper oxidases that catalyze the oxidation of a range of aromatic compounds, including the oxidative polymerization of monolignols and flavonoids in plants (Pourcel et al., 2005; Schuetz et al., 2014). Recently, an intracellular laccase in lychee pericarp was proposed to facilitate anthocyanin degradation (Fang et al., 2015). Laccases may therefore participate in the oxidative polymerization of monomeric stilbenes to produce viniferins during the postharvest dehydration of grapevine berries.
Despite the data discussed above, the grouping of STSs and laccases in two different expression clusters, as confirmed by the deeper analysis of the dataset in all six varieties and also by the separation of rapidly and slowly induced genes in Corvina berries, suggests that the expression of laccases is delayed compared to genes involved in stilbene synthesis. Even so, we cannot rule out the possibility that the massive induction of laccases could be related to lignin biosynthesis, as previously proposed (Wang et al., 2015). This is also supported by the clustering of laccases with several cinnamyl alcohol dehydrogenases (Supplemental Dataset S10).
The depletion of anthocyanins during postharvest dehydration offers another clear relationship between metabolite and transcript modulation, as reflected by the common down-regulation of anthocyanin-related genes such as VvMYBAs, VvGST4, VvANTHOMATE1, VvLODX, and VvCYTOB5 (Supplemental Dataset S7). This behavior was highlighted by the deeper analysis of Corvina berries, which identified additional anthocyanin genes clustered with the rapidly down-regulated transcripts. The cessation of anthocyanin biosynthesis would lead to a declining profile due to natural anthocyanin turnover or the active degradation of anthocyanins after harvest by specific oxidizes, such as the laccases described above.
Finally, by profiling the volatile metabolites in Corvina berries over the course of postharvest dehydration, we observed the accumulation of sesquiterpenes and the induction of several terpene synthase genes (Fig. 8). This suggests that the sesquiterpene biosynthesis pathway is commonly regulated in Corvina berries and other genotypes (such as Syrah and Cabernet Sauvignon) that similarly activate terpene synthases after harvest, even though we did not analyze the volatile compound profile in these other varieties. The induction of several members of the terpene synthase family has previously been observed in berry skins during the late stages of ripening (Cramer et al., 2014).
Common Molecular Responses to Postharvest Dehydration in Berries
Our transcriptomic survey identified common molecular events that characterize the postharvest dehydration process in grapevine berries. The integration of two complementary statistical approaches provided a comprehensive overview of changes in cellular functions, highlighted by the shared transcriptional modulation in harvested berries (Fig. 6). This representation is dominated by the massive activation of genes related to phenylpropanoid, stilbene, and lignin metabolism and the general repression of anthocyanin-related genes as discussed above.
The modulation of several genes related to ethylene and auxin metabolism confirmed previous reports that these hormones are involved in the perception of stress caused by postharvest dehydration, and there may be crosstalk between them (Tonutti and Bonghi, 2013). Grapevine produces nonclimacteric fruits and therefore does not show a clear correlation between ripening induction and ethylene production. However, the up-regulation of ethylene biosynthesis genes such as SAM synthase and ACO was observed in earlier transcriptome surveys on postharvest berries (Zamboni et al., 2008; Rizzini et al., 2009). Here we observed the up-regulation of ACO1 and two ethylene-responsive genes as a common feature of all six genotypes after harvest. The induction of further ethylene-related genes was revealed by the deeper investigation of our dataset, e.g. cluster analysis revealed that a SAM synthase, Ethylene Overproducer1, VvERF050, and VvERF086 grouped in the same expression cluster as ACO1 (Supplemental Dataset S9), whereas an ACS gene was identified by separating the rapid and slow transcriptional responses in Corvina berries. Interestingly, the inspection of our Corvina transcriptome atlas (Fasoli et al., 2012) revealed that the main tissue contributing to the postharvest induction of ACS was the berry pulp. These observations, together with the reported effect of postharvest ethylene treatment on berry metabolism (Botondi et al., 2011; Becatti et al., 2014), strongly suggest that the complex reprogramming of the metabolome and transcriptome during postharvest dehydration relies in part on the regulation of ethylene production. This contrasts with recent physiological data pertaining to the postharvest ripening of citrus fruits, in which the down-regulation of grapevine ACO and ACS genes was part of a comparative survey of fruit species after harvest (Ding et al., 2015). By inspecting a previous transcriptomic dataset, the authors compared grape berry samples that are not representative of postharvest versus ripe fruits, so the reported down-regulation of ethylene biosynthesis genes cannot be attributed to specific molecular events related to postripening development and senescence. Furthermore, the common up-regulation of several nitrilase genes, possibly acting to detoxify ethylene coproducts such as cyanide (Howden and Preston, 2009), also supports the role of ethylene in the postharvest dehydration of grapevine berries.
Other hormone-related molecular events shared by all genotypes include the modulation of some auxin-related genes, the down-regulation of brassinosteroid-6-oxidase, and the up-regulation of VvJAZ09. There are 11 JAZ transcription factors in grapevine, and they are thought to mediate stress responses (Zhang et al., 2012) by modulating jasmonic acid signaling and participating in cross talk with other hormone signaling pathways. VvJAZ09 is the most strongly induced by abiotic stress factors such as drought, salinity, and low temperature. Although previous studies have reported an increase in abscisic acid (ABA; Costantini et al., 2006) together with the up-regulation of genes related to ABA metabolism as part of a general berry response to harvesting and/or water loss, ABA-related genes were not among the commonly modulated genes identified in our study, although some candidates were modulated in specific genotypes.
Shared transcriptional modulation was also observed for other processes including oxidative and osmotic stress responses, anaerobic respiration, defense responses, cell wall metabolism, and carbohydrate metabolism. The regulation of most of these processes was consistent with previous transcriptomic data (Zamboni et al., 2008; Rizzini et al., 2009), including the activation of a lactate dehydrogenase and the down-regulation of an alcohol dehydrogenase. The latter result appears to conflict with the increase in alcohol dehydrogenase expression and activity observed during several other postharvest studies in grapevine (Costantini et al., 2006; Cirilli et al., 2012). However, our data were based on berries subjected to slower and more prolonged dehydration compared to these other studies, and inconsistencies in the expression of genes related to anaerobic respiration may therefore reflect such differences.
Finally, in addition to transcription factors controlling metabolism as described above, we also identified many other modulated transcription factors whose function is currently unknown. We observed the up-regulation of two WRKY proteins and two NAC proteins, one of which (VvNAC60) was recently proposed to control vegetative-to-mature phase transition in grapevine berries (Palumbo et al., 2014). WRKY and NAC genes are often involved in plant responses to drought and salinity, and their overexpression in transgenic plants confers tolerance to abiotic and biotic stress (Puranik et al., 2012; Bakshi and Oelmüller, 2014). Furthermore, members of both families are associated with senescence in different plant organs, including fruits. The WRKY and NAC genes induced in all six genotypes we investigated may represent important signaling components in the dehydration stress response or in the control of late berry developmental processes such as senescence (Gómez et al., 2014).
The physiological basis of the mass gene modulation in all six genotypes grapes may reflect common events in berries on and off the vine, because most of the strongly up-regulated genes are shared with berries left on the vine after commercial ripening (data not shown), and such berries undergo only limited dehydration (Zamboni et al., 2008; Guillaumie et al., 2011; Cramer et al., 2014). The transcriptional reprogramming may therefore represent a more general postripening developmental phase in the berry, i.e. the onset of overripening and/or senescence. Nevertheless, by looking at the expression profiles in the grapevine transcriptomic atlas (Fasoli et al., 2012), we observed that genes up-regulated in our study tended to show steady expression during preripening stages of berry development and were rapidly induced just after harvesting (Fig. 10C). This behavior may represent the berry response to removal from the plant and/or sudden changes in the environmental conditions (from open field to the storage room). Harvesting may therefore accelerate the expression of genes related to overripening and senescence as well as activating new processes unique to postharvest development.
Genotype-Related Molecular Responses to Postharvest Dehydration
The unique aspects of dehydration kinetics in the six varieties seem to have a significant impact on the transcriptome in terms of the number of differentially expressed genes observed after harvest (Fig. 7A). The positive correlation between the slow rate of water loss and the total number of modulated genes has already been reported for berries of the same genotype under different environmental conditions affecting the total length of the postharvest process (Rizzini et al., 2009). These data strongly suggest that long periods of berry dehydration result in more significant transcriptional modulation, which in turn influences the metabolic changes during the postharvest process.
The six varieties were also characterized by distinct gene expression profiles and modulation intensities. Under our experimental conditions, Corvina berries were particularly responsive to the postharvest process, with the highest number of strongly induced genes (FC > 5), whereas Cabernet Sauvignon berries showed the weakest response (Fig. 7B). We investigated these different responses by focusing on the top50 list of genes characterized by the highest FC for each genotype (Fig. 8). Interestingly, by following the expression trends of these genes, we found that each genotype is characterized by a unique gene induction profile that may reflect differences in the perception of postharvest stress. For example, Corvina was characterized by rapid gene induction, whereas the process was much slower in Merlot and Cabernet Sauvignon berries, reflecting a weaker stress perception or response.
Further investigation of the top50 induced genes in Merlot, Sangiovese, and Syrah berries using the RUI approach revealed that most of these genes showed a similar induction ratio in at least one other variety (Supplemental Dataset S8). The low RUI (often < 1) of the top50 up-regulated genes in Cabernet Sauvignon berries indicated that the same genes are usually induced more strongly in one of the remaining five genotypes. In contrast, the top50 induced genes in Corvina and Oseleta were characterized by very high RUI values, suggesting these genes were not strongly induced in other varieties. Furthermore, the functional annotation of the top50 genes highlighted differences among the genotypes in terms of the response to postharvest conditions. Although the induction of laccases and stress-related genes was shared generally by Sangiovese, Syrah, and Cabernet Sauvignon berries, many genes featured in the top50 list of only a single genotype. This was the case, e.g. for terpene and STSs in Corvina berries and genes involved in transcription and translation in Oseleta berries, highlighting the unique postharvest responses in these varieties.
Overall, these data highlight the different extent of transcriptome rearrangements upon harvesting in the six genotypes, suggesting a major dependence on genotype responsiveness. However, the influence of cell death in pericarp tissues cannot be ruled out as a cause of changes in mRNA and metabolites. The differential occurrence of a continuous decline of vitality affecting mainly the flesh cells during berry ripening has been demonstrated in a number of grape varieties (Tilbrook and Tyerman, 2008). Although we did not set up a specific analysis to assess the vitality of berry cells, it could be hypothesized that postharvest stress conditions may have induced this phenomenon to a different extent in the six grape genotypes. In particular, in the case of thicker-skinned varieties, the proportion of live skin cells may be higher and therefore the transcript and metabolite changes in these berries may be dominated by these cells. Further analysis specifically addressed to ascertain the occurrence of cell death during grape berry postharvest life will be fundamental for an accurate interpretation of molecular and metabolite changes observed.
CONCLUSION
We have carried out an exhaustive survey of metabolomic and transcriptomic changes in the grapevine berry during prolonged postharvest dehydration. We analyzed six red berry genotypes, with different dehydration rates and thus differences in the timing of dehydration stress, when stored under identical controlled environmental conditions. By exploiting these experimental features, we are likely to have identified most of the general molecular events that occur in dehydrating berries after harvest. Our analysis showed that basic cellular mechanisms such as transcription, and thus possibly the transcriptional control of cell metabolism, are maintained in berries that have been harvested and stored in dehydrating conditions for several months. We identified genotype-dependent changes that reflect characteristic responses to postharvest conditions, including differences in the modulation of large number of genes/metabolites and the intensity of modulation, indicating that dehydrating berries are subject to massive transcriptional and metabolic reprogramming.
MATERIALS AND METHODS
Experimental Setup
Berries from the grapevine (Vitis vinifera) varieties Corvina, Sangiovese, Merlot, Syrah, Oseleta, and Cabernet Sauvignon grown in neighboring vineyards in the Verona-Valpolicella region of northeast Italy were harvested at commercial maturity (when the soluble solid content reached the winemaker requirements) in 2010. Approximately 100 kg of grape bunches from each cultivar were placed on wooden trays and stored in the same dehydrating room, which had an area of ∼750 m3 and contained dehumidifying/refrigeration air-conditioning equipment (Zanoni Impianti). The environmental conditions represented the average values registered in nonconditioned dehydrating rooms over the same period for the previous 10 years, i.e. a gradually decreasing temperature (from 16°C to 7°C) and a gradually increasing relative humidity (from 55% to 80%) starting on day 0 and ending on day 100 after harvest.
Berry Weight Loss and Quality
Samples from the six varieties were taken on harvest day (T0) and days 13 (T1), 26 (T2), 47 (T3), 69 (T4), and 100 (T5) to determine weight loss and provide material for molecular analysis. The drying process was stopped when the weight loss reached ∼30%. Therefore, the drying of Syrah, Oseleta, and Cabernet Sauvignon berries (the three varieties that lost weight most rapidly) was stopped at T3, Merlot berries were collected until T4, and Corvina and Sangiovese took the longest time to wither and were collected at T5. Three groups of 100 berries were randomly collected from bunches at each time point to create three biological replicates. The same biological material was used for quality assessment (total soluble solids, titratable acidity, and pH), and molecular analysis (metabolome and transcriptome). For molecular analysis, the samples were immediately frozen in liquid nitrogen without the pedicel. Soluble solids (°Brix) were measured using a DBR35 bench-top digital refractometer (Tsingtao Unicom-Optics Instruments). Titratable acidity was determined by titrating 7.5 mL of berry juice to pH 8.1 with 0.1 m NaOH, using bromothymol blue as a colorimetric indicator. Berry weight was measured at harvest.
To ensure the absence of fungal infection, a d-gluconic acid/d-glucono-δ-lactone assay kit was used to analyze powdered berry pericarp according to the manufacturer’s instructions (Megazyme International). The powdered material (1 g) was diluted in 10 mL of buffer containing 500 µL Carrez 1 solution, 500 µL Carrez 2 solution, and 1 mL 0.1 m NaOH topped up to 10 mL with water, and was filtered twice with standard filter paper cones. Before the second filtration step, the samples were treated with polyvinylpolypyrrolidone to remove colored solutes.
Optical Microscopy
Fresh Corvina berries were cut into small pieces and fixed in 2% formaldehyde and 0.25% glutaraldehyde in phosphate-buffered saline (pH 7.5) under vacuum overnight. The fixed berries were rinsed five times in phosphate-buffered saline and then dehydrated with increasing concentrations of ethanol (25%, 50%, 75%, and 100%) before postfixing overnight in ethanol containing increasing concentrations of xylene (25%, 50%, 75%, and 100%). The samples were embedded by progressively substituting the xylene with Paraplast Plus (Thermo Fisher Scientific). Tissue sections (7 μm) were prepared using a 2035 Leica microtome (Leica Microsystems), floated on warm water, and immobilized on slides coated with poly-l-Lys to facilitate handling. The slides were then air-dried at 37°C. After removing the paraffin by incubating in 100% xylene for 2× 15 min and passing quickly through decreasing concentrations of ethanol (100%, 75%, 50%, and 25%), the sections were stained with toluidine blue and viewed under a Leica DMRB optical microscope (Leica Microsystems).
Extraction, Analysis, and Identification of Metabolites
The metabolites were extracted, analyzed by HPLC-ESI-MS, and GC-MS and tentatively identified following the protocols described by Anesi et al. (2015).
HPLC with diode array detection analysis was carried out using a Beckman Coulter Gold 126 Solvent Module coupled with a Gold 168 Diode Array Detector (Beckman Coulter). The chromatography conditions were the same as described above for LC-MS, except the injection volume was set to 100 μL. Signals were recorded over the range 190 to 600 nm, and data were collected and analyzed using 32 Karat Software v7.0 (Beckman Coulter). Resveratrol and stilbenes were quantified by comparing with a calibration curve based on a trans-resveratrol commercial standard (Sigma-Aldrich). Resveratrol peak areas were assessed at 310 nm to determine the final equation (y = 4 × 107 + 442490, R2 = 0.99868). The stilbene peak area was quantified at 310 nm, within the linear dynamic range of the calibration curve, and converted to mg resveratrol equivalents in 100 g fresh weight.
LC-MS Data Processing and Modeling
The HPLC-MS data files were processed using MzMine v2.10 and the values included in the final data matrix were normalized against berry weights. The data matrix was analyzed using SIMCA-P v13.0 (Umetrix AB). Pareto scaling was applied to all analytical methods. Unsupervised PCA was applied to 18 samples, corresponding to harvest time points for the six varieties, to identify clusters of samples. The clusters were used as classes for O2PLS-DA to characterize class-specific metabolites (loadings plot) considering the pq(corr) parameter that defines the correlation between p (the metabolites) and q (the classes). Only metabolites with pq(corr) > 0.7 were considered. O2PLS-DA models were statistically validated by ANOVA of the cross-validated residuals (CV-ANOVA, P < 0.01) and the permutation test (200 permutations).
Extraction, Analysis, and Identification of Volatile Metabolites in Corvina Berries
Twenty grams of frozen homogenized berry tissue were placed in 100-mL cylinders with stoppers and thawed at room temperature for 1.5 h prior to extraction. The grape homogenate was mixed with 10 mL of deionized water and the deuterated internal standards d13-hexanol (1000 µg/kg), α-copaene (200 µg/kg), and d3-β-ionone (50 µg/kg) dissolved in ethanol (Australian Wine Research Institute) were added. Finally, 5 mL of 1:1 (v/v) n-pentane:ethyl acetate were added (unisolve-grade n-pentane and suprasolv-grade ethyl acetate; Merck) and the mixture was sonicated for 15 min at room temperature in an ultrasonic bath (Branson 3510 Ultrasonic). Metabolites were extracted for 2 h at room temperature on a Rocking Platform Mixer (Ratex Instruments Pty) at 25 rpm. Organic phases were collected and stored in sealed glass vials at −20°C.
An Agilent Technologies 6890 GC (Agilent Technologies), equipped with a Gerstel MPS2 multipurpose sampler (Gerstel), was coupled to an Agilent 5973N mass selective detector. The instrument was also equipped with a Gerstel CIS-4 cool inlet fitted with a resilanized borosilicate glass liner with glass wool insert. The instrument was controlled by Agilent G1701CA ChemStation and Gerstel Master v1.81.
Each sample (2.5 µl) was cryofocused in the Gerstel CIS-4 held at −10°C, and injected in solvent vent mode. The injector temperature was set to −10°C and ramped to 240°C at 10°C/min and then held at the final temperature for 3 min to ensure no metabolite carry over, as confirmed by the analysis of blanks.
The gas chromatograph was fitted with a 60 m × 0.25 mm, 0.25 µm DB-5 MS+ Agilent column. The carrier gas was helium flowing at 26 cm/s. The oven temperature was initially 40°C and was held for 7 min, then increased to 150°C at 7°C/min, to 170°C at 2°C/min and then to 240°C at 20°C/min and held for 15 min.
The MS transfer line was set at 250°C, the MS quadrupole at 150°C, and the source at 230°C. Positive ion electron impact spectra at 70 eV were recorded in the range 35 to 350 m/z for scan runs. The C8-C20 n-alkane standard solution (Fluka, Sigma-Aldrich) was used to benchmark retention indices. The identity of compounds was verified by comparison with (1) Kovats retention indices, (2) mass spectra available on the National Institute of Standards and Technology database, and (3) the terpenoids library in MassFinder v4.1 (Dr. Hochmuth Scientific Consulting).
Data were analyzed using Agilent C1701 ChemStation software and expressed on a “per-berry basis,” taking weight loss into account. To avoid differences based on detection efficiencies, the monoterpene and sesquiterpene peak areas were normalized to α-copaene, norisoprenoids to d3-β-ionone, and the remaining compounds to d13-hexanol. The data matrix was imported into SIMCA-P v13.0 before the data were log (x+1) transformed and Pareto scaled.
RNA Extraction and Microarray Analysis
Total RNA was extracted from ∼200 mg of berry tissue (pericarp without seeds) using the Spectrum Plant Total RNA kit (Sigma-Aldrich) as previously described (Fasoli et al., 2012). According to the manufacturer’s instructions, we hybridized 10 μg of total RNA per sample to a NimbleGen microarray 090818_Vitus_exp_HX12 chip (Roche, NimbleGen), which contains probes targeting 29,549 predicted grapevine genes (http://ddlab.sci.univr.it/FunctionalGenomics/) representing ∼98.6% of the genes predicted from the V1 annotation of the 12 grapevine genome (http://srs.ebi.ac.uk/) and 19,091 random probes as negative controls.
Evaluation of Gene Expression and Statistical Analysis
The threshold expression level, which defines a gene as “expressed” or “nonexpressed,” was found by calculating the control group average value for the 19,091 random probes in each experiment, thus estimating a background threshold for each subarray. For each group of samples (three biological replicates per time point), we defined a transcript as expressed for the specific time point/condition only if at least two (out of three) expression values exceeded the estimated threshold. This approach revealed that 25,402 transcripts were expressed under at least one condition. To compare the expression fold changes among the six varieties during the dehydration process, the background intensities were averaged within each variety to yield a single estimated value for each, and then substituted for the actual expression of a gene when below the threshold.
PCA was performed using SIMCA-P v13.0 on the dataset of expressed genes (25,402 genes) for both the T0-reduced data matrix (18 samples) and the complete (87 samples) data matrix, separately. In the latter case, the loadings of the first and second principal components were ordered, and the first and last 100 genes were extracted to investigate their functional categories.
Multiclass significance analysis of microarray was carried out using TMeV v4.8 (http://mev.tm4.org) with a FDR of 0.01%, to extract genes that were significantly modulated during the dehydration process in the six varieties. The modulated genes were then normalized against a common negative value and filtered on the basis of an absolute FC > 2 in at least one comparison. The FC values were calculated for each time point (from T1 to T5 depending on the varietal-specific dehydration rate) compared to harvest (T0). Differentially expressed Corvina genes were filtered by absolute FC ≥ 2 (2933 genes), the genes were ordered by average FC and those within the first and last percentiles (representing up-regulated and down-regulated genes, respectively) were extracted to investigate their expression profiles during postharvest dehydration. The 586 genes were clustered to distinguish rapid and slow responses to the postharvest conditions. Expression profiles (values computed as log2 of the fluorescence intensity) were plotted in two different graphs (rapid and slow) describing the peculiar trends of up-regulated and down-regulated genes using R software (R Foundation for Statistical Computing). Expression profiles (values computed as log2 of the fluorescence intensity) of the same 586 genes in Corvina berry flesh and skin during maturation (veraison, midripening and ripening) and postharvest dehydration (postharvest withering first, second and third months) were obtained from Fasoli et al. (2012) via the Gene Expression Omnibus series entry GSE36128, and were plotted separately using R software.
Accession Numbers
Grape berry microarray expression data are available in the Gene Expression Omnibus under the series entry GSE75498.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Overview of the postharvest dehydration process.
Supplemental Figure S2. PCA score scatter plot of the model obtained for the metabolites detected by HPLC-ESI-MS in the six genotypes in all stages of postharvest.
Supplemental Figure S3. Accumulation trends of anthocyanins (A), flavonols and other flavonoids (B), hydroxycinnamic/hydroxybenzoic acids (C), and flavan-3-ols and proanthocyanidins (D) during the postharvest process in the six varieties.
Supplemental Figure S4. PCA score scatter plot of the model obtained for the volatile metabolites detected by GC-MS in all stages of Corvina berries.
Supplemental Figure S5. Hierarchical clustering of volatile organic compounds modulation during postharvest in Corvina berries.
Supplemental Figure S6. PCA score scatter plot of the model obtained for the transcripts detected by microarray analysis at harvest in each variety (explained variance = 69.2%).
Supplemental Figure S7. Heat map of commonly differentially modulated genes in the six genotypes during the postharvest process.
Supplemental Figure S8. Gene expression of the most up-regulated (first percentile) and down-regulated (99th percentile) Corvina transcripts we identified during the ripening and postharvest process in Corvina skin and pulp, as reported in the expression atlas (Fasoli et al., 2012).
Supplemental Figure S9. Ratio of the average expression level in the three postharvest stages analyzed by Fasoli et al. (2012) in pulp and skin of most down-regulated genes.
Supplemental Figure S10. PCA score scatter plots of the models obtained for anthocyanins flavonols and other flavonoids.
Supplemental Figure S11. PCA score scatter plot of the model obtained for the metabolites detected by HPLC-ESI-MS at harvest for Merlot, Cabernet Sauvignon and Syrah.
Supplemental Dataset S1. Nonvolatile metabolites data matrix of the six berry genotypes during postharvest dehydration.
Supplemental Dataset S2. Classification of nonvolatile metabolites on the basis of the three-class O2PLS-DA model constructed for the six genotypes at harvest.
Supplemental Dataset S3. Volatile metabolites data matrix of Corvina berries during postharvest dehydration.
Supplemental Dataset S4. Transcriptomic dataset of the 25,402 genes filtered by each subarray threshold.
Supplemental Dataset S5. Loadings of two principal components (PC1 and PC2) of the PCA model performed on the entire transcriptomic dataset of the six genotypes during postharvest dehydration.
Supplemental Dataset S6. Differentially expressed genes during postharvest dehydration.
Supplemental Dataset S7. List of commonly up-regulated and down-regulated genes, as described in Figure 6.
Supplemental Dataset S8. List of the top50 genes.
Supplemental Dataset S9. List of genes belonging to cluster 5 of the heat map shown in Figure 9C.
Supplemental Dataset S10. List of genes belonging to cluster 4 of the heat map shown in Figure 9C.
Supplemental Dataset S11. List of genes rapidly or slowly up-regulated or down-regulated in postharvest Corvina berries.
Supplemental Dataset S12. Most strongly up-regulated (first percentile) and down-regulated (99th percentile) Corvina transcripts identified during the ripening and postharvest process in Corvina berry pericarp, flesh, and skin, as reported in the expression atlas (Fasoli et al., 2012).
Supplemental Dataset S13. Classification of anthocyanins, flavonols, and other flavonoids, and hydroxycinnamic and hydroxybenzoic acids and hydroxytyrosol molecules (listed in three separate sheets) on the basis of the three-class O2PLS-DA models constructed for the six varieties at harvest.
Supplemental Dataset S14. Classification of nonvolatile metabolites on the basis of the three-class O2PLS-DA model constructed for Merlot, Cabernet Sauvignon and Syrah at harvest.
Supplemental Dataset S15. Classification of nonvolatile metabolites on the basis of the two-class O2PLS-DA model constructed for Corvina and Sangiovese at harvest.
Supplementary Material
Acknowledgments
We thank Masi Agricola SpA for kindly providing grapes, structures, and dehydration technologies. Andrea Dal Cin, Anita Boscaini, and Vittorio Zandonà are acknowledged for valuable support during berry sampling and technological analysis. We thank Tracey Siebert for providing training in GC-MS and supporting the analysis of volatile metabolites.
Glossary
- HPLC-ESI-MS
high-performance liquid chromatography-electrospray ionization-mass spectrometry
- PCA
principal component analysis
- O2PLS-DA
orthogonal bidirectional partial least squares discriminant analysis
- GC-MS
gas chromatography-mass spectrometry
Footnotes
This work was supported by the Joint Project 2010 and 2014 between Masi Agricola SpA and the Biotechnology Department of the University of Verona, by the INNOVINE European Project FP7-311775 “Combining innovation in vineyard management and genetic diversity for a sustainable European viticulture,” and benefited from the networking activities coordinated under the EU-funded Cost Action FA1106 “An integrated systems approach to determine the developmental mechanisms controlling fleshy fruit quality in tomato and grapevine.” S.D.S. was financed by the Italian Ministry of University and Research FIRB RBFR13GHC5 project “The Epigenomic Plasticity of Grapevine in Genotype per Environment Interactions.”
References
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A (2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16: 2463–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anesi A, Stocchero M, Dal Santo S, Commisso M, Zenoni S, Ceoldo S, Tornielli GB, Siebert TE, Herderich M, Pezzotti M, et al. (2015) Towards a scientific interpretation of the terroir concept: plasticity of the grape berry metabolome. BMC Plant Biol 15: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi M, Oelmüller R (2014) WRKY transcription factors: jack of many trades in plants. Plant Signal Behav 9: e27700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanti D, Mora B, Ferrarini R, Tornielli GB, Cipriani M (2008) Effect of various thermo-hygrometric conditions on the withering kinetics of grapes used for the production of “Amarone” and “Recioto” wines. J Food Eng 85: 350–358 [Google Scholar]
- Becatti E, Genova G, Ranieri A, Tonutti P (2014) Postharvest treatments with ethylene on Vitis vinifera (cv Sangiovese) grapes affect berry metabolism and wine composition. Food Chem 159: 257–266 [DOI] [PubMed] [Google Scholar]
- Bellincontro A, Nicoletti I, Valentini M, Tomas A, De Santis D, Corradini D, Mencarelli F (2009) Integration of nondestructive techniques with destructive analyses to study postharvest water stress of winegrapes. Am J Enol Vitic 60: 57–65 [Google Scholar]
- Bogs J, Ebadi A, McDavid D, Robinson SP (2006) Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development. Plant Physiol 140: 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonghi C, Rizzini FM, Gambuti A, Moio L, Chkaiban L, Tonutti P (2012) Phenol compound metabolism and gene expression in the skin of wine grape (Vitis vinifera L.) berries subjected to partial postharvest dehydration. Postharvest Biol Technol 67: 102–109 [Google Scholar]
- Botondi R, Lodola L, Mencarelli F (2011) Postharvest ethylene treatment affects berry dehydration, polyphenol and anthocyanin content by increasing the activity of cell wall enzymes in Aleatico wine grape. Eur Food Res Technol 232: 679–685 [Google Scholar]
- Cavallini E, Zenoni S, Finezzo L, Guzzo F, Zamboni A, Avesani L, Tornielli GB (2014) Functional diversification of grapevine MYB5a and MYB5b in the control of flavonoid biosynthesis in a petunia anthocyanin regulatory mutant. Plant Cell Physiol 55: 517–534 [DOI] [PubMed] [Google Scholar]
- Cirilli M, Bellincontro A, De Santis D, Botondi R, Colao MC, Muleo R, Mencarelli F (2012) Temperature and water loss affect ADH activity and gene expression in grape berry during postharvest dehydration. Food Chem 132: 447–454 [DOI] [PubMed] [Google Scholar]
- Costantini V, Bellincontro A, De Santis D, Botondi R, Mencarelli F (2006) Metabolic changes of Malvasia grapes for wine production during postharvest drying. J Agric Food Chem 54: 3334–3340 [DOI] [PubMed] [Google Scholar]
- Cramer GR, Ghan R, Schlauch KA, Tillett RL, Heymann H, Ferrarini A, Delledonne M, Zenoni S, Fasoli M, Pezzotti M (2014) Transcriptomic analysis of the late stages of grapevine (Vitis vinifera cv. Cabernet Sauvignon) berry ripening reveals significant induction of ethylene signaling and flavor pathways in the skin. BMC Plant Biol 14: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vetten N, ter Horst J, van Schaik HP, de Boer A, Mol J, Koes R (1999) A cytochrome b5 is required for full activity of flavonoid 3′, 5′-hydroxylase, a cytochrome P450 involved in the formation of blue flower colors. Proc Natl Acad Sci USA 96: 778–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Chang J, Ma Q, Chen L, Liu S, Jin S, Han J, Xu R, Zhu A, Guo J, et al. (2015) Network analysis of postharvest senescence process in citrus fruits revealed by transcriptomic and metabolomic profiling. Plant Physiol 168: 357–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Fischer S, Merz P, Bogs J, Riemann M, Nick P (2016) An ancestral allele of grapevine transcription factor MYB14 promotes plant defence. J Exp Bot 67: 1795–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Halter D, Baltenweck R, Tisch C, Tröster V, Kortekamp A, Hugueney P, Nick P (2015) Genetic diversity of stilbene metabolism in Vitis sylvestris. J Exp Bot 66: 3243–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Zhang XL, Luo HH, Zhou JJ, Gong YH, Li WJ, Shi ZW, He Q, Wu Q, Li L, et al. (2015) An intracellular laccase is responsible for epicatechin-mediated anthocyanin degradation in litchi fruit pericarp. Plant Physiol 169: 2391–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasoli M, Dal Santo S, Zenoni S, Tornielli GB, Farina L, Zamboni A, Porceddu A, Venturini L, Bicego M, Murino V, et al. (2012) The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 24: 3489–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzemeier KH, Kindl H (1981) Coordinate induction by UV light of stilbene synthase, phenylalanine ammonia-lyase and cinnamate 4-hydroxylase in leaves of vitaceae. Planta 151: 48–52 [DOI] [PubMed] [Google Scholar]
- Gapper NE, McQuinn RP, Giovannoni JJ (2013) Molecular and genetic regulation of fruit ripening. Plant Mol Biol 82: 575–591 [DOI] [PubMed] [Google Scholar]
- Gatto P, Vrhovsek U, Muth J, Segala C, Romualdi C, Fontana P, Pruefer D, Stefanini M, Moser C, Mattivi F, et al. (2008) Ripening and genotype control stilbene accumulation in healthy grapes. J Agric Food Chem 56: 11773–11785 [DOI] [PubMed] [Google Scholar]
- Giacosa S, Torchio F, Segade SR, Caudana A, Gerbi V, Rolle L (2012) Varietal relationship between skin break force and off-vine withering process for winegrapes. Dry Technol 30: 726–732 [Google Scholar]
- Gómez MD, Vera-Sirera F, Pérez-Amador MA (2014) Molecular programme of senescence in dry and fleshy fruits. J Exp Bot 65: 4515–4526 [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks R, Pickett JA (2009) Role of natural products in nature: plant—insect interactions. In Osborn AE, Lanzotti V, eds, Plant-Derived Natural Products. Springer, Berlin, pp 321–347 [Google Scholar]
- Guillaumie S, Fouquet R, Kappel C, Camps C, Terrier N, Moncomble D, Dunlevy JD, Davies C, Boss PK, Delrot S (2011) Transcriptional analysis of late ripening stages of grapevine berry. BMC Plant Biol 11: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höll J, Vannozzi A, Czemmel S, D’Onofrio C, Walker AR, Rausch T, Lucchin M, Boss PK, Dry IB, Bogs J (2013) The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 25: 4135–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JK. (2004) Multiple functions of inducible plant volatiles. Trends Plant Sci 9: 529–533 [DOI] [PubMed] [Google Scholar]
- Howden AJM, Preston GM (2009) Nitrilase enzymes and their role in plant-microbe interactions. Microb Biotechnol 2: 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Denis JB, Pezet R, Tabacchi R (2006) Rapid analysis of stilbenes and derivatives from downy mildew-infected grapevine leaves by liquid chromatography-atmospheric pressure photoionisation mass spectrometry. J Chromatogr A 1112: 263–268 [DOI] [PubMed] [Google Scholar]
- Jerkovic V, Nguyen F, Nizet S, Collin S (2007) Combinatorial synthesis, reversed-phase and normal-phase high-performance liquid chromatography elution data and liquid chromatography/positive atmospheric pressure chemical ionization tandem mass spectra of methoxylated and glycosylated resveratrol analogues. Rapid Commun Mass Spectrom 21: 2456–2466 [DOI] [PubMed] [Google Scholar]
- Mencarelli F, Bellincontro A, Nicoletti I, Cirilli M, Muleo R, Corradini D (2010) Chemical and biochemical change of healthy phenolic fractions in winegrape by means of postharvest dehydration. J Agric Food Chem 58: 7557–7564 [DOI] [PubMed] [Google Scholar]
- Morreel K, Ralph J, Kim H, Lu F, Goeminne G, Ralph S, Messens E, Boerjan W (2004) Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol 136: 3537–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo MC, Zenoni S, Fasoli M, Massonnet M, Farina L, Castiglione F, Pezzotti M, Paci P (2014) Integrated network analysis identifies fight-club nodes as a class of hubs encompassing key putative switch genes that induce major transcriptome reprogramming during grapevine development. Plant Cell 26: 4617–4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panceri CP, Gomes TM, De Gois JS, Borges DLG, Bordignon-Luiz MT (2013) Effect of dehydration process on mineral content, phenolic compounds and antioxidant activity of Cabernet Sauvignon and Merlot grapes. Food Res Int 54: 1343–1350 [Google Scholar]
- Pezet R, Viret O, Perret C, Tabacchi R (2003) Latency of Botrytis cinerea Pers.: Fr. and biochemical studies during growth and ripening of two grape berry cultivars, respectively susceptible and resistant to grey mould. J Phytopathol 151: 208–214 [Google Scholar]
- Pourcel L, Routaboul JM, Kerhoas L, Caboche M, Lepiniec L, Debeaujon I (2005) TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 17: 2966–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17: 369–381 [DOI] [PubMed] [Google Scholar]
- Püssa T, Floren J, Kuldkepp P, Raal A (2006) Survey of grapevine Vitis vinifera stem polyphenols by liquid chromatography-diode array detection-tandem mass spectrometry. J Agric Food Chem 54: 7488–7494 [DOI] [PubMed] [Google Scholar]
- Rizzini FM, Bonghi C, Tonutti P (2009) Postharvest water loss induces marked changes in transcript profiling in skins of wine grape berries. Postharvest Biol Technol 52: 247–253 [Google Scholar]
- Rolle L, Caudana A, Giacosa S, Gerbi V, Río Segade S (2011) Influence of skin hardness on dehydration kinetics of wine grapes. J Sci Food Agric 91: 505–511 [DOI] [PubMed] [Google Scholar]
- Rolle L, Gerbi V (2013) Changes in physical and mechanical properties of dehydrating berries. In Mencarelli F, Tonutti E, eds, Sweet, Reinforced and Fortified Wines. Wiley-Blackwell, Hoboken, NJ, pp 119–130 [Google Scholar]
- Schmidlin L, Poutaraud A, Claudel P, Mestre P, Prado E, Santos-Rosa M, Wiedemann-Merdinoglu S, Karst F, Merdinoglu D, Hugueney P (2008) A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol 148: 1630–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz M, Benske A, Smith RA, Watanabe Y, Tobimatsu Y, Ralph J, Demura T, Ellis B, Samuels AL (2014) Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiol 166: 798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab W, Wüst M (2015) Understanding the constitutive and induced biosynthesis of mono- and sesquiterpenes in grapes (Vitis vinifera): a key to unlocking the biochemical secrets of unique grape aroma profiles. J Agric Food Chem 63: 10591–10603 [DOI] [PubMed] [Google Scholar]
- Terrier N, Glissant D, Grimplet J, Barrieu F, Abbal P, Couture C, Ageorges A, Atanassova R, Léon C, Renaudin JP, et al. (2005) Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222: 832–847 [DOI] [PubMed] [Google Scholar]
- Tilbrook J, Tyerman SD (2008) Cell death in grape berries: varietal differences linked to xylem pressure and berry weight loss. Funct Plant Biol 35: 173–184 [DOI] [PubMed] [Google Scholar]
- Toffali K, Zamboni A, Anesi A, Stocchero M, Pezzotti M, Levi M, Guzzo F (2011) Novel aspects of grape berry ripening and post-harvest withering revealed by untargeted LC-ESI-MS metabolomics analysis. Metabolomics 7: 424–436 [Google Scholar]
- Tonutti P, Bonghi C (2013) Biochemistry and physiology of dehydrating berries. In Mencarelli F, Tonutti E, eds, Sweet, Reinforced and Fortified Wines: Grape Biochemistry, Technology and Vinification, Wiley-Blackwell, Hoboken, NJ, pp 77–90 [Google Scholar]
- Versari A, Parpinello GP, Tornielli GB, Ferrarini R, Giulivo C (2001) Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J Agric Food Chem 49: 5531–5536 [DOI] [PubMed] [Google Scholar]
- Walker AR, Lee E, Bogs J, McDavid DAJ, Thomas MR, Robinson SP (2007) White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J 49: 772–785 [DOI] [PubMed] [Google Scholar]
- Wang J, Feng J, Jia W, Chang S, Li S, Li Y (2015) Lignin engineering through laccase modification: a promising field for energy plant improvement. Biotechnol Biofuels 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni A, Di Carli M, Guzzo F, Stocchero M, Zenoni S, Ferrarini A, Tononi P, Toffali K, Desiderio A, Lilley KS, et al. (2010) Identification of putative stage-specific grapevine berry biomarkers and omics data integration into networks. Plant Physiol 154: 1439–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni A, Minoia L, Ferrarini A, Tornielli GB, Zago E, Delledonne M, Pezzotti M (2008) Molecular analysis of post-harvest withering in grape by AFLP transcriptional profiling. J Exp Bot 59: 4145–4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Fuentes S, Siebert T, Krstic M, Herderich M, Barlow EWR, Howell K (2016) Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes. Food Chem 204: 463–474 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao M, Singer SD, Fei Z, Wang H, Wang X (2012) Genome-wide identification and analysis of the TIFY gene family in grape. PLoS One 7: e44465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccatelli G, Zenoni S, Savoi S, Dal Santo S, Tononi P, Zandona V, Dal Cin A, Guantieri V, Pezzotti M, Tornielli GB (2013) Skin pectin metabolism during the postharvest dehydration of berries from three distinct grapevine cultivars. Aust J Grape Wine Res 19: 171–179 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.