Sec31B protein is crucial for pollen wall formation by participating in COPII-coated vesicle trafficking in the tapetum in Arabidopsis.
Abstract
The pollen wall protects pollen grains from abiotic and biotic stresses. During pollen wall development, tapetal cells play a vital role by secreting proteins, signals, and pollen wall material to ensure microspore development. But the regulatory mechanism underlying the secretory pathway of the tapetum is largely unknown. Here, we characterize the essential role of the Arabidopsis (Arabidopsis thaliana) COPII protein SECRETORY31B (SEC31B) in pollen wall development and the secretory activity of tapetal cells. The sporophyte-controlled atsec31b mutant exhibits severe pollen and seed abortion. Transmission electron microscopy observation indicates that pollen exine formation in the atsec31b mutant is disrupted significantly. AtSEC31B is a functional COPII protein revealed by endoplasmic reticulum (ER) exit site localization, interaction with AtSEC13A, and retarded ER-Golgi protein trafficking in the atsec31b mutant. A genetic tapetum-specific rescue assay indicates that AtSEC31B functions primarily in the tapetum. Moreover, deletion of AtSEC31B interrupted the formation of the ER-derived tapetosome and altered the location of the ATP-BINDING CASSETTE TRANSPORTER9 protein in the tapetum. Therefore, this work demonstrates that AtSEC31B plays a vital role in pollen wall development by regulating the secretory pathway of the tapetal cells.
The pollen cell wall is a complex and robust structure surrounding the microspore cytoplasm that helps to resist various severe environments and also is involved in pollination and pollen-stigma recognition. The pollen wall of mature pollen grains comprises intine and exine, plus tryphine deposited on the surface of the exine (Scott et al., 2004). Intine enriched in cellulose and hemicelluloses is comparable to the primary cell wall of somatic cells. Exine consists of inner nexine and outer sexine, which contains tectum and bacula. The main composition of exine is sporopollenin derived from the tapetum, the innermost layer of the anther locule wall. More importantly, normal pollen wall development is crucial for microspore development (Scott et al., 2004; Ariizumi and Toriyama, 2011). Aberrant pollen wall formation frequently leads to severe pollen abortion.
In higher plants, during microspore development, tapetum secretes signals, enzymes, and pollen wall materials to ensure successful microspore development. At the anther stage, while going through the callose wall of degenerating tetrads, tapetal cells continuously secrete enzymes (i.e. callose-degrading glucanases; Stieglitz, 1977) into the anther locule to release the microspores. Simultaneously, primexine forms outside the surface of the microspores and serves as a mold for the subsequent sporopollenin deposition. Impaired primexine formation often results in abnormal exine and abortive pollen. Many reports describe members involved in the formation of primexine, such as DEFECTIVE IN EXINE FORMATION1 (DEX1), MALE STERILE1 (MS1)/HACKLY MICROSPORE (HKM), NO PRIMEXINE AND PLASMA MEMBRANE UNDULATION1 (NPU1), RUPTURED POLLEN GRAIN1 (RPG1), NO EXINE FORMATION1 (NEF1), the recently reported SPONGY2 (SPG2), and UNEVEN PATTERN OF EXINE1 (UPEX1), which are all highly expressed in the tapetum and sporophyte-controlled tissues (Paxson-Sowders et al., 2001; Ariizumi et al., 2004, 2008; Guan et al., 2008; Chang et al., 2012; Li et al., 2016; Xu et al., 2016). After the microspores are released, tapetal cells constantly synthesize precursors of sporopollenin and release them into the locule to deposit on the surface of the pollen exine. MS2, ACOS5, CYP703A2, and CYP704B2 are reportedly involved in the synthesis of sporopollenin precursors in Arabidopsis (Arabidopsis thaliana; Morant et al., 2007; de Azevedo Souza et al., 2009; Dobritsa et al., 2009; Chen et al., 2011). Additionally, DEFECTIVE POLLEN WALL2, encoding an acyltransferase, is required for rice (Oryza sativa) pollen development (Xu et al., 2016). Then, these precursors and other structural materials are transported outside tapetal cells via lipid transfer proteins or ATP-BINDING CASSETTE TRANSPORTER (ABCG) proteins (McFarlane et al., 2010; Quilichini et al., 2010; Choi et al., 2011, 2014; Huang et al., 2013).
The tapetum is a temporary cell layer. As the tapetal programmed cell death proceeds, the cell contents are released into the locule; thus, the lipids and proteins accumulated in tapetosomes and elaioplasts are deposited on the exine of the microspores, forming the tryphine. Some ABCG transporters in the tapetum also are involved in pollen coat deposition. ABCG9 and ABCG31 show high expression in the tapetum, and abcg9 abcg31 mutants mirror the immature pollen coat of wild-type pollen (Choi et al., 2014). ABCG26 is required for the trafficking of polyketide and hydroxycinnamoyl spermidines, which facilitate the formation of pollen exine in Arabidopsis (Quilichini et al., 2010, 2014). In addition, rice ABCG26 and ABCG15 play important roles in male reproduction (Zhao et al., 2015). Therefore, successful pollen wall development relies largely on normal secretory activity in the tapetum. However, very little information is available about the regulatory mechanism underlying the secretory activity of tapetal cells.
Angiosperms exhibit two primary types of tapetum. One is the secretory tapetum, which releases the materials required for pollen development via locular fluid. The other is the amoeboid tapetum, which can move to and contact microspores directly (Pacini et al., 1985). Compared with the amoeboid tapetum, the secretory tapetum may produce more pollen grains in larger anthers. Arabidopsis possesses a secretory tapetum. However, few mutants have been reported to affect the secretory pathway in Arabidopsis tapetal cells. Disruption of Arabidopsis GLYCEROL-3-PHOSPHATE ACYLTRANSFERASE1 (AtGPAT1) and AtGPAT6, which is involved in the initial step of glycerolipid biosynthesis, leads to abnormal endoplasmic reticulum (ER) structure and decreased secretion in tapetal cells (Zheng et al., 2003; Li et al., 2012). Male gametogenesis impaired anthers, a P-type ATPase cation pump, may regulate the cation homeostasis of the secretory pathway compartments in the tapetum (Jakobsen et al., 2005). Moreover, deletion of MS1, a PHD-type transcriptional factor, also causes the abnormal secretion of pollen wall material in the tapetum (Ito et al., 2007). Recently, ECHIDNA was reported to be involved in trans-Golgi network transport, and echidna showed multiple male fertility defects (Fan et al., 2014). However, the key components or factors that participate in the secretory pathway of tapetum cells are less understood.
COPII-coated vesicles specifically mediate anterograde transport from the ER to the Golgi apparatus and play a vital role in the early secretory pathway (Wuestehube et al., 1996). Through COPII vesicle trafficking, proteins and lipids can be transported from the ER to the Golgi and then sorted to the plasma membrane, apoplastic space, and other organelles. In yeast (Saccharomyces cerevisiae), the initiation of COPII complex assembly starts with the recruitment of Sar-1-GDP by Sec12p. Sar-1p initiates the recruitment of Sec23/24p and Sec13/31p from cytosol. Sec24p is responsible for cargo selecting, and Sec13/31p forms the vesicle coat (Wuestehube et al., 1996; Salama et al., 1997; Shugrue et al., 1999; Robinson et al., 2007). However, little is known regarding COPII vesicle transport in plants. Arabidopsis SEC24A is required for the maintenance of ER morphology, and deletion of AtSEC24A affects the size of sepal cells and pollen germination (Faso et al., 2009; Nakano et al., 2009; Qu et al., 2014). Knockout of both AtSEC24B and AtSEC24C leads to defective gametophyte development (Tanaka et al., 2013). More recently, AtSAR1A and AtSEC23A were shown to interact as a pair, which is essential for protein ER export in Arabidopsis (Zeng et al., 2015). However, the function of other COPII proteins in Arabidopsis has yet to be determined.
In this study, we found that disruption of AtSEC31B, a putative homolog of yeast Sec31p, caused dramatically reduced viable pollen grain and seed abortion. Transmission electron microscopy (TEM) analysis indicates that pollen wall development is impaired significantly. Although AtSEC31B is expressed ubiquitously, particularly strong transcriptional and protein expression are detected in the tapetum. SEC31B driven by the tapetum-specific A9 promoter could fully recover the atsec31b mutant phenotype. AtSEC31B served as a functional COPII protein revealed by subcellular location, coimmunoprecipitation (Co-IP), and fluorescence recovery after photobleaching (FRAP) assays. The alterations in the size and structure of the lipid storage organelles in the tapetum also were observed in the atsec31b mutant. Finally, we found that the distribution of ABCG9 in the tapetum is altered in the atsec31b mutant. Therefore, our work suggests that SEC31B is crucial for pollen wall development and may be involved in the early secretory pathway of tapetal cells.
RESULTS
The Homozygous atsec31b Mutant Shows Significantly Reduced Male Fertility
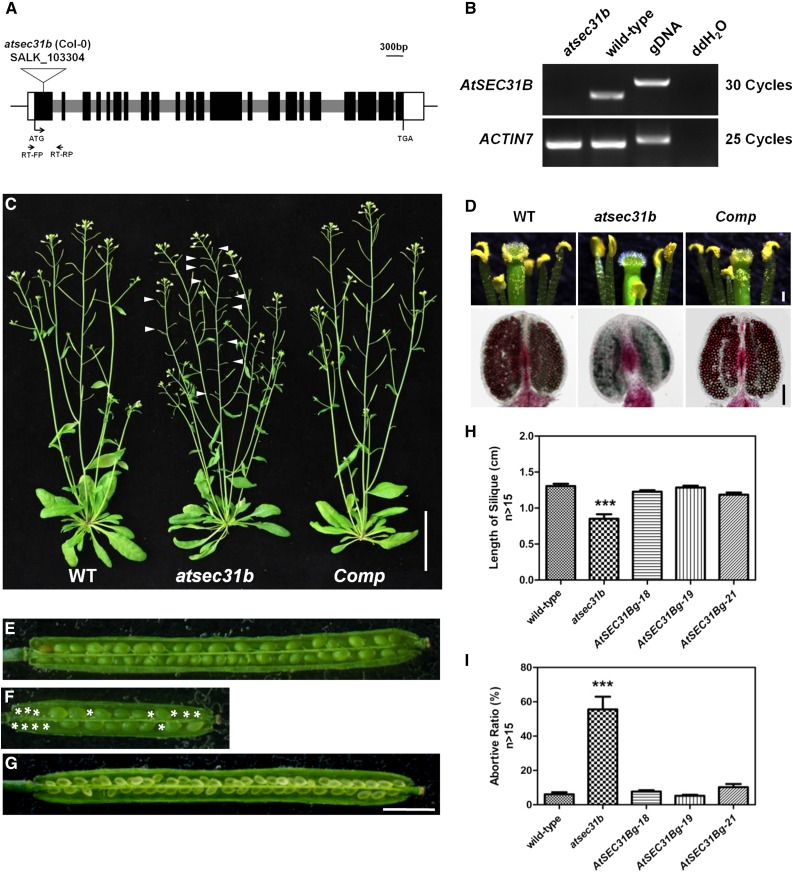
AtSEC31B (AT3G63460) was selected from our previous proteomic analyses in germinated Arabidopsis pollen grains (Ge et al., 2011). AtSEC31B shows 24% identity with Saccharomyces cerevisiae SEC31p (ScSEC31p; Supplemental Fig. S1; Robinson et al., 2007). To explore the detailed functions of AtSEC31B, we obtained a knockout T-DNA insertion mutant, atsec31b (SALK_103304), from the Arabidopsis Biological Resource Center (ABRC; Fig. 1, A and B). During vegetative growth, atsec31b mutant plants exhibit normal development (Fig. 1C). However, in the reproductive stage, the anthers of the atsec31b mutant become shriveled and dark yellow, and little pollen appears in the pistil, whereas wild-type plants exhibit bright yellow anthers and abundant pollen (Fig. 1D, top row). Most of the pollen grains in atsec31b anthers were not stained purple by Alexander’s staining and were likely aborted (Fig. 1D, bottom row). Moreover, atsec31b mutant plants produced short, small siliques and showed an abortion ratio of approximately 55% (Fig. 1, E–I). This mutation is recessive, because heterozygous atsec31b/+ plants show normal growth. Artificial pollination with excess mutant pollen on the stigma of atsec31b could recover the abortion phenotype, indicating that decreased viable pollen number is responsible for severe seed abortion (Supplemental Fig. S2).
Figure 1.
Disruption of AtSEC31B led to a male-sterile phenotype. A, Schematic representation of the T-DNA insertion in AtSEC31B. Black, gray, and white boxes indicate the exons, introns, and untranslated regions, respectively. B, Reverse transcription (RT)-PCR analysis of the transcripts across the insertion sites of AtSEC31B expressed in mutant inflorescences. Primers for transcript amplification are indicated by arrows in A, with RT-FP/RP for AtSEC31B. ACTIN7 was used as an internal control. Three biological and technical replicates were conducted. Genomic DNA (gDNA) as a positive control and distilled, deionized water (ddH2O) as a negative control also are presented. C, Comparison of vegetative and reproductive development between 40-d-old wild-type (WT), atsec31b mutant, and complementary (Comp) plants. The white arrowheads indicate short siliques. Bar = 5 cm. D, Details of wild-type, atsec31b mutant, and complementary buds. Note that there are fewer mature pollen grains on the mutant anthers and stigma (top row; bar = 200 μm). By Alexander’s staining, the anther of the wild-type plants is filled with viable, purple-stained pollen grains (purple), while that of the atsec31b mutants contains only a few viable pollen grains (bottom row, corresponding to each image above; bar = 100 μm). E to G, Siliques were harvested after natural self-pollination (wild-type [E], atsec31b [F] and Complement [G]), then were dissected for statistical analysis. The white asterisks indicate the abortive seeds. Bar = 5 mm. H and I, Statistical analysis for the seed sets in E to G, including length of siliques (H) and abortive ratio of seed sets (I). n > 15. AtSEC31Bg-8, -19, -21 are three independent complementation lines. Each data point represents mean ± SE (n > 15). Asterisk indicates significant difference relative to wild type (Student's t test, ***P < 0.001).
Next, we performed a genetic analysis of atsec31b mutants. The progeny of the self-pollinated heterozygous plants deviated significantly from classic Mendelian segregation (Supplemental Table S1). Male gametophyte transmission efficiency is reduced significantly to 17% in the atsec31b mutant, whereas the female function is unaffected (Supplemental Table S1). Therefore, the disruption of AtSEC31B dramatically impairs male fertility.
Moreover, we obtained another knockout T-DNA insertion line, designated atsec31b-n (CS918444), from the ABRC. atsec31b-n contains a T-DNA insertion fragment in the 12th exon of AtSEC31B (Supplemental Fig. S3A). The phenotype of homozygous atsec31b-n is similar to that of the atsec31b mutant (Supplemental Fig. S3, B and C). Subsequently, genetic complementation was performed. An AtSEC31B genome fragment was transformed to the atsec31b mutant. Approximately 20 T1 transgenic lines are fully recovered to wild-type plants. The phenotypes of three independent single insertion lines were analyzed in detail, including seedling height, seed abortion rate, pollen fertility, and silique length (Fig. 1, C, D, and G–I). We selected the atsec31b mutant for subsequent experiments.
The atsec31b Mutant Exhibits an Abnormal Pollen Wall and Compromised Pollen Germination and Pollen Tube Growth
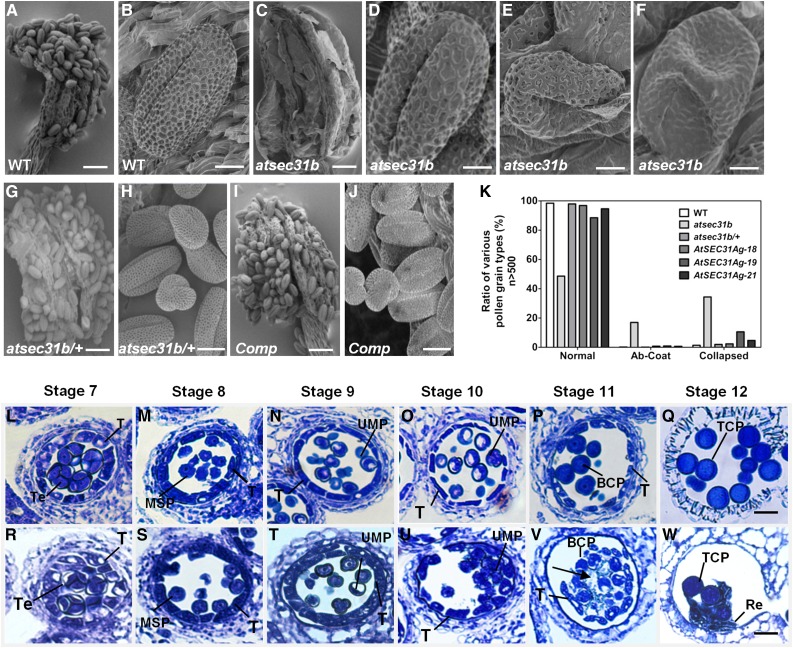
Scanning electron microscopy (SEM) was performed to examine the detailed morphology of mature pollen. In wild-type and rescued lines, mature pollen grains were abundant and plump and showed a typical reticular exine pattern (Fig. 2, A, B, I, and J). By contrast, few mature pollen grains were observed in the atsec31b (Fig. 2, C–F) and atsec31b-n (Supplemental Fig. S3D) anthers. In the atsec31b/+ anthers, mature pollen grains were comparable with the wild type (Fig. 2, G and H), which implies that AtSEC31B functions sporophytically to control pollen development. In addition, some remnants were attached to the mutant anthers and were assumed to be degradation products of pollen (Fig. 2C). Thus, we carefully examined these pollen grains recognized in atsec31b plants. They could be classified into three types (n > 500): (1) grains with a normal exine, as observed in wild-type plants, accounting for 40% of the grains (Fig. 2, D and K); (2) grains with an abnormal pollen coat, accounting for 25% (Fig. 2, E and K); and (3) collapsed grains with a concave and shrunken morphology without a typical exine, accounting for 35% (Fig. 2, F and K). These observations revealed that more than half of the pollen in the atsec31b mutant displayed an abnormal exine pattern (Fig. 2K). The pollen grains stuck to the remnants of atsec31b anthers, and only a portion of the grains could be obtained and examined. We next performed pollen germination assays in vitro and in vivo to observe germination rates and tube elongation. In vitro, only 25% (n > 300) of the available atsec31b mutant pollen grains could germinate, compared with 82% (n > 300) of wild-type pollen grains (Supplemental Fig. S4A). The ratio of pollen germination in rescued lines has no significant difference from that of the wild type (Supplemental Fig. S4A). The length of the atsec31b mutant pollen tubes is significantly shorter than that of wild-type plants (Supplemental Fig. S4B). In vivo, the atsec31b pollen tubes also grew slower than those of wild-type plants, but they could reach the bottom at 24 h after pollination (hap; Supplemental Fig. S4C). These results indicate that the pollen germination rate and pollen tube elongation are seriously compromised in the atsec31b plants.
Figure 2.
The atsec31b mutant exhibits defective microspore development. A to J, SEM observation of mature pollen grains and dehiscent anthers. A and C, Wild-type (WT) anther (A) contained numerous pollen grains, whereas most atsec31b pollen grains degenerated (C). B, Wild-type pollen grains with a regular reticulate exine pattern. D to F, Three types of pollen grains from the anthers of atsec31b plants: normal pollen grains (D), plump pollen grains with defective pollen wall (E), and collapsed pollen grains (F). G and H, The atsec31b/+ plants showed abundant and normal pollen grains, respectively. I and J, ProAtSEC31B:AtSEC31Bg/atsec31b could complement the defective pollen development in atsec31b mutant plants. Comp, Complementary plants. For A, C, G, and I, bars = 50 μm; for B, D, E, and F, bars = 5 μm; and for H and J, bars = 10 μm. K, Statistical analysis of the three types of pollen grains shown in D to F. Ab-Coat, Pollen grains with an abnormal coat; Collapsed, collapsed pollen grains without a typical exine. L to W, Locules from anther sections of wild-type (L–Q) and atsec31b (R–W) plants are shown from anther stages 7 to 12. The black arrow in V indicates abnormal structures surrounding the microspores. BCP, Bicellular pollen; MSP, microspores; Re, remnants; T, tapetum; TCP, tricellular pollen; Te, tetrad; UMP, uninuclear microspore. Bars = 20 μm.
Microspores of atsec31b Plants Exhibit Defective Primexine Development
Next, we further explored the progress of pollen development in atsec31b and wild-type plants by examining transverse sections of the anthers. According to Sanders’ morphological characteristics, anther development can be divided into 14 well-ordered stages in Arabidopsis (Sanders et al., 1999). At anther stage 7, the anther locules were filled with tetrads wrapped in callose walls in wild-type plants (Fig. 2L), and no morphological defects were observed in atsec31b plants (Fig. 2R). At stages 8 and 9, the atsec31b plants (Fig. 2, S and T) appeared to be similar to wild-type plants (Fig. 2, M and N). The first significant developmental defects were observed at anther stage 10, in which the microspores of wild-type plants generated a significant pollen wall (Fig. 2O), whereas the pollen wall of atsec31b microspores was ambiguous and incomplete (Fig. 2U). At stage 11, the microspores of wild-type plants exhibited a typically regular exine wall (Fig. 2P). In atsec31b plants, only a small amount of pollen survived (Fig. 2V). At stage 12, pollen development was completed in wild-type plants (Fig. 2Q), but in the locules of atsec31b plants, debris and a few normal pollen grains could be observed (Fig. 2W). Additionally, the tapetum in the atsec31b mutant exhibits a normal structure and undergoes similar programmed cell death to that of the wild type (Fig. 2, R–W). Thus, these observations suggest that the exine formation of microspores was remarkably impaired in the atsec31b mutant.
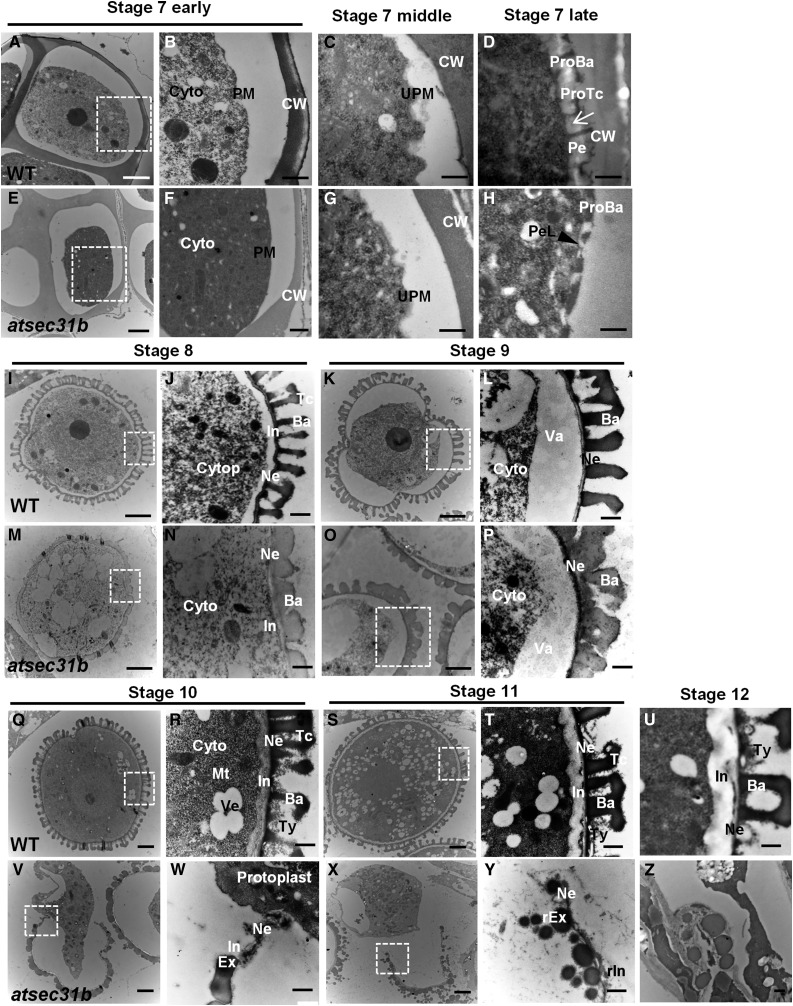
Next, TEM was performed. At anther stage 7, a primexine gradually formed between the callose wall and the microspore plasma membrane in wild-type plants, which was initiated simultaneously with plasma membrane undulation (Fig. 3, A–C). In the atsec31b mutant plants, the plasma membrane of the microspores undulated more lightly than in wild-type plants (Fig. 3, E–G). The formation of primexine was completed in wild-type plants, and a regular structure of the probaculae and protectum was gradually built and accomplished in the primexine matrix (Fig. 3D). However, in the atsec31b mutant, primexine-like structures that were significantly thinner than those of wild-type plants were deposited, and the probaculae showed a podgy morphology without any observable protectum (Fig. 3H). At anther stages 8 and 9, the wild-type microspores developed a regular lattice-patterned exine containing the tectum, the bacula, and the foot layer (Fig. 3, I–L). By contrast, the tectum was absent in atsec31b microspores, and the baculas appeared to be stubbier than those of wild-type microspores (Fig. 3, M–P). At stages 10 and 11, wild-type microspores showed further development (Fig. 3, Q–T), whereas in atsec31b microspores, the exine degenerated and was ultimately fragmented (Fig. 3, V and W), and abnormal spherical sporopollenin aggregated around the microspores (Fig. 3, X and Y). At anther stage 12, the formation of a typical pollen wall and pollen coat was completed in wild-type microspores (Fig. 3U). In atsec31b anthers, only remnants could be observed (Fig. 3Z). These TEM observations imply that knocking out AtSEC31B severely disrupted exine development. Moreover, random deposits of sporopollenin on the locule wall (the inner surface of the locule) appeared at stage 8 in atsec31b mutant plants (Supplemental Fig. S5).
Figure 3.
Ultrastructure of pollen wall development in wild-type (WT) and atsec31b plants. The tetrads at anther stage 7 (B and F) are high magnifications of the insets in A and E. The microspores of stage 8 (J and N), stage 9 (L and P), stage 10 (R and W), and stage 11 (T and Y) are high magnifications of the insets in I and M, K and O, Q and V, and S and X, respectively. The white arrow and black arrowhead in D and H indicate the primexine (Pe) and distorted primexine-like structure (PeL), respectively. Ba, Baculae; Cytop, cytoplasm; CW, callose wall; Ex, exine; G, Golgi body; In, intine; Mt, mitochondria; Ne, nexine; P, plastid; PM, plasma membrane; ProBa, probaculae; ProTc, protectum; rEx, remnants of exine; rIn, remnants of intine; Tc, tectum; Ty, tryphine; UPM, undulated plasma membrane; Va, vacuole; Ve, vesicle. Bars = 2 μm for A, E, I, M, Q, V, S, and X and 500 nm for the other images.
AtSEC31B Is Widely Expressed and Functions Mainly in the Tapetum
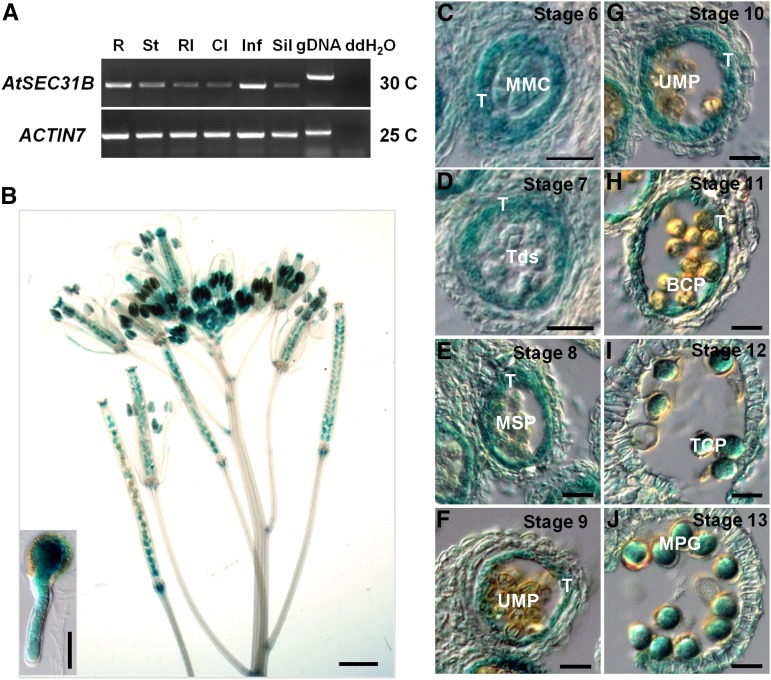
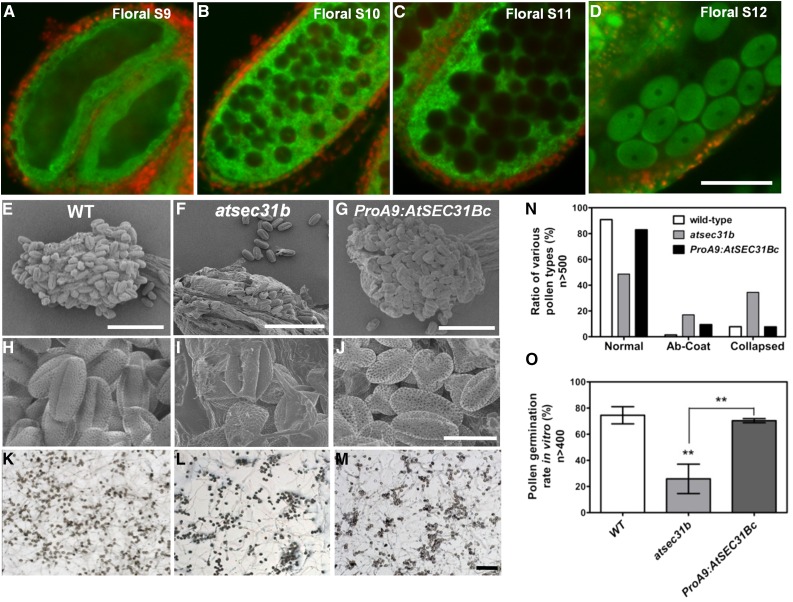
We next investigated the expression pattern of AtSEC31B. RT-PCR analysis showed that AtSEC31B is universally expressed, predominantly in the inflorescences and root (Fig. 4A). In ProAtSEC31B:GUS transgenic plants, GUS activity was detected in the stamens, pistils, young siliques, leaves, roots, and cotyledons (Fig. 4B; Supplemental Fig. S6). In cross sections, AtSEC31B was expressed at a high level in all cell types of the anther before anther stage 9 (Fig. 4, C–E). However, at anther stages 9 to 11, GUS expression was detected prominently in the tapetum (Fig. 4, F–H), in accordance with the stage at which the tapetum continuously secreted a large amount of exine materials into the anther locule. In addition, AtSEC31B transcripts were expressed highly in mature pollen grains and pollen tubes (Fig. 4, B, I, and J). We also generated ProAtSEC31B:AtSEC31Bc:EGFP (for enhanced GFP), which fully complements the atsec31b mutant phenotype (Supplemental Fig. S7). Consistent with GUS staining, GFP signals were detected in tapetal cells at floral stage 9 (corresponding to anther stages 5–7; Fig. 5A), in both the tapetum and locular fluid at floral stages 10 and 11 (Fig. 5, B and C), and in mature pollen at floral stage 12 (Fig. 5D).
Figure 4.
Expression analysis of Arabidopsis SEC31B. A, Expression pattern of AtSEC31B in wild-type plants by RT-PCR. Total RNA was isolated from 6-week-old wild-type plants. ACTIN7 was used as an internal control. R, Root; St, stem; Rl, rosette leaf; Cl, cauline leaf; Inf, inflorescence; Sil, silique. Genomic DNA (gDNA) as a positive control and distilled, deionized water (ddH2O) as a negative control also are presented. B, GUS expression profile in the inflorescence transformed with ProAtSEC31B:GUS. The internal view in the bottom left corner shows GUS signal in a germinating pollen tube. Bar = 1 mm; for the internal view, bar = 20 μm. C to J, Paraffin sections of anthers from plants transformed with ProAtSEC31B:GUS. GUS expression was detected continuously in tapetal cells (T) before tapetum degenerated and was expressed highly in microspore mother cells (MMC), then decreased gradually in tetrads (Tds), uninuclear microspores (UMP), and bicellular pollen grains (BCP) but increased in tricellular pollen grains (TCP) and mature pollen grains (MPG). Bars = 50 μm.
Figure 5.
AtSEC31B-GFP is expressed in tapetal cells, and ProA9:AtSEC31Bc/atsec31b could rescue the mutant phenotype. A to D, A transgenic plant line expressing full-length AtSEC31B cDNA fused with GFP under the control of the AtSEC31B promoter (ProAtSEC31B:AtSEC31Bc:GFP) in the atsec31b mutant plant shows green fluorescent signals in the tapetal layer and pollen grains (green) and autofluorescence signals of chlorophyll (red). Bar = 50 μm. E and H, SEM observation for the wild type (WT). F and I, SEM observation for atsec31b. G and I, SEM observation for ProA9:AtSEC31Bc/atsec31b. K to M, Pollen germination in vitro for the wild type, atsec31b, and ProA9:AtSEC31Bc/atsec31b, respectively. N, Statistical analysis for H to J. Ab-Coat, Pollen grains with an abnormal coat; Collapsed, collapsed pollen grains without a typical exine. O, Statistical analysis for K to M. Each data point represents a mean value ± se (n > 400). Asterisks in U indicate significant differences relative to the wild type (Student’s t test, **, P < 0.01). For E to G, bars = 100 μm; and for H to J, bars = 20 μm.
Combined with the sporophyte-controlled mutant phenotype, defective exine formation in the atsec31b mutant, and high expression of AtSEC31B in the tapetum (Figs. 3 and 4, C–F), we speculate that AtSEC31B functions primarily in the tapetum. Then, we fused AtSEC31B cDNA with the Arabidopsis A9 promoter (Paul et al., 1992), which is expressed exclusively in tapetum cells, and transformed the resulting plasmid into atsec31b plants. In approximately 20 T1 transgenic lines, A9 promoter-driven AtSEC31B nearly completely rescued the male-sterile phenotype of atsec31b mutants, including the defective pollen development and pollen germination rate in vitro (Fig. 5, E–U). All of these observations suggest the crucial role of AtSEC31B in tapetal cells.
AtSEC31B-GFP Is a Functional COPII Component
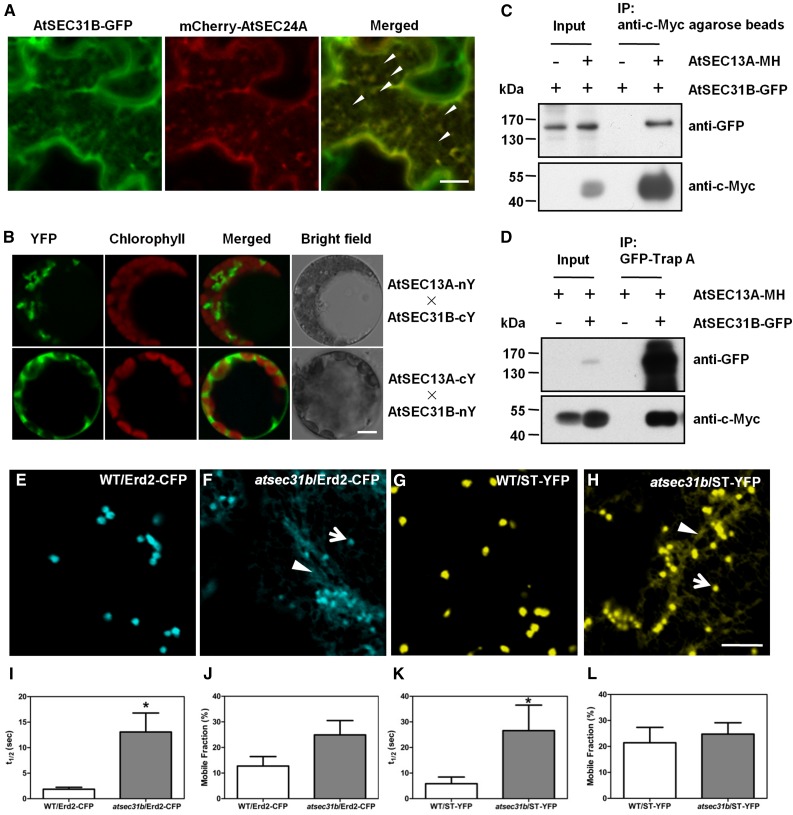
To demonstrate that AtSEC31B is a bona fide COPII protein, we further investigated the subcellular location of AtSEC31B. We crossed AtSEC31B-EGFP with stable transgenic plants expressing the ER marker HDEL-mCherry, the cis-Golgi marker Sed5/SYP31-yellow fluorescent protein (YFP), and the trans-Golgi marker sialyltransferase (ST)-YFP (Chatre et al., 2005; Nelson et al., 2007; Li et al., 2009). AtSEC31B-EGFP was distributed in punctate regions and spread along the ER network (Supplemental Fig. S8A), whereas AtSEC31B-GFP foci were embedded in the mouth of the U-shaped Sed5-YFP signal (Supplemental Fig. S8B). We also found that the smaller AtSEC31B-GFP foci were accumulated around the larger punctate-like ST-YFP (Supplemental Fig. S8C). These results indicate that AtSEC31B-GFP is closely associated with ER and cis-Golgi markers and most likely localizes to endoplasmic reticulum exit sites (ERESs). Next, we coexpressed AtSEC31B-GFP and the ERES marker mCherry-SEC24A in tobacco (Nicotiana tabacum) leaves (Hanton et al., 2007; Wei and Wang, 2008). The AtSEC31B-GFP signals strongly overlapped with the mCherry-SEC24A signals (Fig. 6A).
Figure 6.
ERES-localized AtSEC31B is colocalized and interacts with AtSEC13A, and its disruption causes traffic defects of two Golgi markers. A, AtSEC31B-GFP well overlapped with the ER exit site marker, mCherry-AtSEC24A, in tobacco leaf cells. Bar = 10 μm. B, BiFC assay showing the interaction between AtSEC31B and AtSEC13A directly in Arabidopsis living protoplasts. cY, C-terminal fragment of YFP; nY, N-terminal fragment of YFP. Bar = 10 μm. C and D, Co-IP between AtSEC31B and AtSEC13A in vivo by stably transgenic plants driven by native promoters. IP, Immunoprecipitate. E to L, Confocal images and FRAP analysis of the subcellular pattern for Erd2-CFP (E and F) and ST-YFP (G and H) in epidermal cells of 3-week-old rosette leaves. The CFP and YFP signals were partially retained in the ER, as observed in the confocal laser scanning microscopy images, which were reflected by the larger fluorescence recovery half-time (t1/2) in atsec31b plants than in wild-type (WT) plants (I and K), despite the mobile fraction being similar (J and L). The white arrows and arrowheads indicate Golgi stacks and the ER, respectively. Sample size in each test was more than 10 Golgi spots. Each data point represents a mean value ± se (n > 10). Asterisks in I to L indicate significant differences relative to the wild type (Student’s t test, *, P < 0.05). Bars = 5 μm.
In yeast, the coat components of the COPII complex, Sec13p and Sec31p, interact directly with each other, forming a tetramer complex. AtSEC13A, a homolog of ScSEC13p in Arabidopsis, is supposed to be a subunit of the COPII complex in Arabidopsis and was reported to interact with AtSEC31B in leek (Allium porrum) inner epidermal cells (Hino et al., 2011). Thus, we next performed a bimolecular fluorescence complementation (BiFC) assay to test the interaction of AtSEC31B and AtSEC13A in Arabidopsis. YFP signals were detected only in the cytosol of protoplasts cotransformed with AtSEC13A-nY plus AtSEC31B-cY or AtSEC13A-cY plus AtSEC31B-nY (Fig. 6B) but not in other combinations (Supplemental Fig. S9). Co-IP also was performed using stable transgenic lines of native promoter-driven AtSEC31B-EGFP and 7Myc-6His-AtSEC13A. We found that AtSEC31B-EGFP and 7Myc-6His-AtSEC13A could coimmunoprecipitate with each other (Fig. 6, C and D). These data suggest that AtSEC31B can interact directly with AtSEC13A in vivo.
To further explore protein trafficking processes in the atsec31b mutant, we observed the subcellular localization of Golgi marker proteins in wild-type and atsec31b mutant plants. ST-YFP and Erd2-cyan fluorescent protein (CFP) are reported to be transported from the ER to the Golgi via anterograde trafficking (Brandizzi et al., 2002). ST-YFP and Erd2-CFP signals were clearly retained in the ER in atsec31b plants (Fig. 6, E–H). Subsequently, FRAP was conducted to determine whether the retained signals resulted from defective ER export. The half-time values for both markers in atsec31b mutants were significantly longer than those in wild-type plants (Fig. 6, I and K). However, the mobile fraction in each individual test was not affected, indicating that the available mobile fraction of the marker proteins was not altered in atsec31b plants (Fig. 6, J and L). These data suggest that disruption of AtSEC31B dramatically affects ER-Golgi vesicle trafficking. Therefore, all the above date indicate that AtSEC31B-GFP is a functional COPII protein.
The Development of Elaioplasts and Tapetosomes in the Tapetum Was Impaired in the atsec31b Mutants
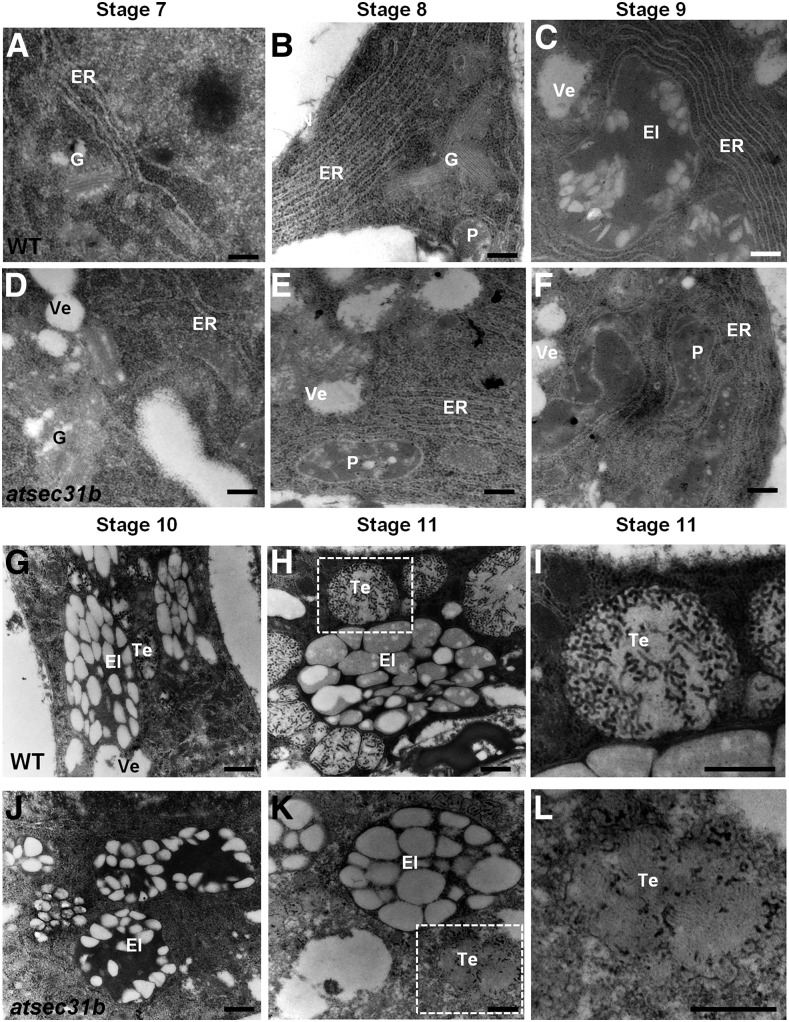
We next examined whether the disruption of AtSEC31B would result in any defects in the morphology (especially ER, Golgi, and other organelles) of the tapetum by TEM, although no obvious defect in tapetal cells could be detected in the atsec31b mutant by light microscopy (Fig. 2). At anther stages 7 to 9, both in wild-type and mutant plants, the tapetal cells exhibited large stacks of ER, Golgi, and many plastids and in the peripheral region (Fig. 7, A, B, D, and E). At stages 8 and 9, the tapetal cells developed two main organelles, the plastid-derived elaioplasts and the ER-derived tapetosomes, which could release lipids and other materials on the surface of the pollen-forming pollen coat (Suzuki et al., 2013). The elaioplasts of wild-type tapetal cells contained many electron-translucent lipid plastoglobules at stage 9 (Fig. 7C). However, in atsec31b plants, the differentiated elaioplasts were first observed in atsec31b plants at anther stage 10, which contained fewer, smaller plastoglobules than wild-type plants (Fig. 7, F and J). Additionally, tapetosomes were observed in wild-type tapetal cells (Fig. 7G) but were not found in contemporary atsec31b plants (Fig. 7J). At anther stage 11, the elaioplasts and tapetosomes of wild-type tapetal cells enlarged and underwent further development (Fig. 7, H and I). By contrast, except for a few, smaller elaioplasts, the atsec31b tapetum contained obscure, malformed tapetosomes, with a fragmented interior structure and no obvious boundaries (Fig. 7, K and L). These results indicate that the formation of elaioplasts and tapetosomes in the atsec31b tapetum is notably impaired, which may have potential effects on pollen coat deposition.
Figure 7.
Ultrastructure of tapetal cells in wild-type (WT) and atsec31b plants during anther stages 7 to 11. TEM analysis revealed the defective elaioplast (El) and tapetosome (Te) developmental process, especially the fragmented tapetosomes. I and L show enlarged views of the dotted boxes in H and K, respectively. G, Golgi body; Mt, mitochondria; N, nucleus; P, plastid; Ve, vesicle. Bars = 500 nm.
The Expression of sGFP:ABCG9 Protein Is Altered in the atsec31b Mutant
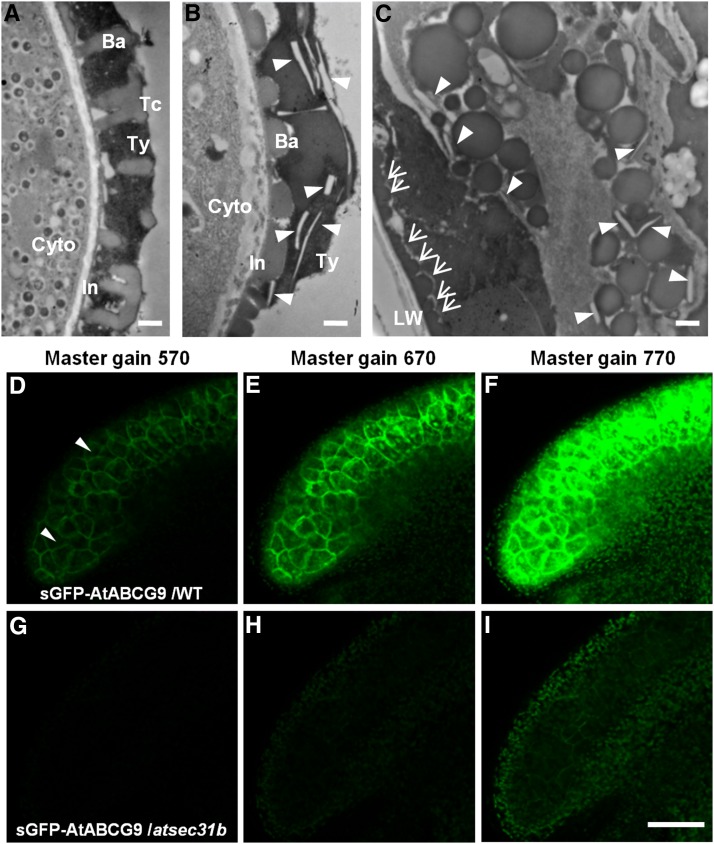
To find potential candidate proteins that are transported by AtSEC31B-mediated COPII vesicle trafficking, we screened nearly all proteins reported to participate in pollen wall development. ABCG9 was selected for further investigation because sGFP:ABCG9 localizes to the plasma membrane of tapetal cells and is expressed specifically at anther stage 10 (Choi et al., 2014). ABCG9 and ABCG31 also harbor several potential ER export signal sites, such as the diacid motifs and dibasic motifs in the C terminus of ABCG9 (Supplemental Table S2). Moreover, the plasma membrane localization of sGFP:ABCG9 could accumulate in Brefeldin A (BFA) compartments in the tapetal cells (Supplemental Fig. S10) after BFA treatment, which inhibits transport events between the ER and Golgi stacks (Nebenführ et al., 2002). These findings imply that sGFP:ABCG9 is very likely transported from the ER to the Golgi. In addition, the pollen coat of atsec31b mature pollen grains also harbored numerous vesicular, linear, and electron-lucent structures (Fig. 8, A–C), which is similar to that of abcg9 abcg31 plants as reported previously (Choi et al., 2014). Then, we examined the distribution of sGFP:ABCG9 in wild-type and atsec31b plants by laser confocal scanning microscopy (Fig. 8, D–I). In the wild-type background, the signal of sGFP:ABCG9 is clearly detected in plasma membrane in the tapetal cells in master gain 550, which is consistent with the previous report (Fig. 8D). In the same condition, the signal of sGFP:ABCG9 could not be detected in atsec31b (Fig. 8G). In master gain 670, the signal of sGFP:ABCG9 still could not be detected in atsec31b (Fig. 8H). In master gain 770, the signal of sGFP:ABCG9 was weakly detected in the plasma membrane of tapetum in atsec31b (Fig. 8I). Therefore, the expression of sGFP:ABCG9 in the atsec31b background was remarkably decreased compared with that in wild-type plants.
Figure 8.
Distribution and expression of sGFP-AtABCG9 in wild-type and atsec31b mutant plants. A to C, TEM observation of mature pollen grains from wild-type (A) and atsec31b (B and C) dehiscent anthers. The white arrowheads indicate the irregular structures in the pollen coat of atsec31b (B) or in the debris of the atsec31b anther locule (C). The white arrows show the random deposition of sporopollenin on the locule wall (LW) of anthers. Ba, Baculae; Cytop, cytoplasm; In, intine; Tc, tectum; Ty, tryphine. D to I, Subcellular localization pattern of sGFP-AtABCG9 in the wild-type (WT) background (D–F) and the atsec31b mutant background (G–I). For A to C, bars = 500 nm; and for D to I, bars = 50 μm.
DISCUSSION
The Arabidopsis tapetum is of the secretory type, and its position is maintained during microspore development (Liu and Fan, 2013). The secretory role played by the tapetum in microspore development is widely recognized, but the molecular mechanism of the secretory pathway in tapetal cells is largely unknown. Here, we found that AtSEC31B plays a crucial role in pollen wall formation by regulating the secretory pathway in the tapetum in Arabidopsis.
AtSEC31B is a putative ortholog of ScSEC31p. We provide several lines of evidence indicating that AtSEC31B is a functional COPII protein. First, AtSEC31B is targeted to ERESs (Fig. 6A). ERESs are the ER region at which the COPII complex assembles (Marti et al., 2010). Second, the interaction of AtSEC31B and AtSEC13s has been established by previous studies, but those studies were performed in either a heterogenous background or in vitro (Hino et al., 2011; Takagi et al., 2013). Here, we performed Co-IP and BiFC analyses to demonstrate the interaction of AtSEC31B and AtSEC13A in vivo (Fig. 6, B–D). Third, the FRAP assay demonstrates that the disruption of AtSEC31B leads to retarded protein trafficking between the ER and Golgi (Fig. 6, E–L). Therefore, AtSEC31B is implicated as an important functional COPII component.
The only homozygous atsec31b mutant exhibits significantly reduced viable pollen grains and severe seed abortion (Figs. 1, D and F, and 2, C–H), suggesting that the male-sterile phenotype of atsec31b is under sporophytic control. The abnormal selfing segregation ratio, the decreased male transmission efficiency (Supplemental Table S1), and the compromised pollen germination rate in heterozygous plants (Supplemental Fig. S4A) indicate that AtSEC31B also functions in the male gametophyte. Therefore, AtSEC31B functions both in sporophytes and gametophytes. We also found that pollen tubes grow dramatically slowly in the atsec31b mutant at 4 and 10 hap but could reach the bottom of the pistil at 24 hap (Supplemental Fig. S4C). Excessive pollination in the atsec31b self-cross plant fully recovered the abortion phenotype (Supplemental Fig. S2). Therefore, the deceased pollen grain in pistils is responsible for the severe pod abortion. Although 40% of pollen grains in the atsec31b mutant anther appear normal by SEM analysis, most of them stick to pollen debris in the anther locule and could not be released successfully to the pistil.
Numerous works demonstrate that the tapetum plays an important role in the exine formation of microspores (Ting et al., 1998; Ariizumi and Toriyama, 2011). In atsec31b mutants, exine development was impaired dramatically (Fig. 3), which is controlled by sporophytes. These results imply the abnormal functioning of the sporophytic tapetum in the atsec31b mutant. Additionally, we found that AtSEC31B is expressed universally, but particularly strong GUS and AtSEC31B-EGFP signals were detected in tapetal cells (Figs. 4 and 5). Furthermore, we used AtSEC31B driven by the tapetum-specific A9 promoter to successfully rescue the atsec31b phenotype (Fig. 5, E–U). Thus, AtSEC31B functions primarily in tapetal cells sporophytically.
However, the morphology and programmed cell death process of the tapetum in mutants is similar to that of wild-type plants (Fig. 2). By TEM analysis, the mutant tapetum also exhibits normal ER and Golgi, different from the point mutation in SEC24A, which results in aberrant ER structure. However, the development of tapetosomes is dramatically defective in atsec31b mutants (Fig. 7). Tapetosomes are unique organelles found in tapetal cells; they accumulate lipid materials released to the locule, forming the pollen coat (Piffanelli et al., 1998; Hsieh et al., 2003; Hsieh and Huang, 2007; Shi et al., 2015). Tapetosomes are derived from the ER, but the mechanism of their formation is still unclear (Boavida and McCormick, 2007). The fragmented shape of the tapetosomes may result from some inconspicuous alteration of the tapetal ER. Alternatively, AtSEC31 may mediate some important type of cargo export that may be required for normal tapetosome formation. Also, we cannot exclude that impaired tapetosome development is the indirect result of the loss of function of AtSEC31B. How COPII-coated vesicle transport is involved in tapetosome formation requires further investigation.
Moreover, the development of elaioplast in the tapetal cells in the atsec31b mutant also is retarded significantly compared with that in wild-type plants. As reported, elaioplast in tapetum is derived from proplastids (Piffanelli et al., 1998). Recent bioinformatics analysis and experimental evidence supported that vesicle transport also exists in different kinds of plastids, including proplastids (Lindquist et al., 2016). COPII-related proteins may mediate vesicle trafficking in chloroplasts (Lindquist et al., 2016). For instance, the chloroplast protein CPSAR1, which shows similarity to the AtSAR1 protein in cytoplasm, and CPRabA5e may participate in thylakoid biogenesis and vesicle trafficking in Arabidopsis (Garcia et al., 2010; Khan et al., 2013; Karim et al., 2014; Karim and Aronsson, 2014; Lindquist and Aronsson, 2014). But whether other COPII proteins are involved in vesicle traffic in chloroplasts is still unknown (Lindquist et al., 2016). Therefore, one possible explanation for the retarded development of elaioplast in atsec31b is that the disruption of AtSEC31B leads to the lower efficiency of COPII protein transport in proplastids, and then the differentiation from proplastid to elaioplast is postponed in the atsec31b mutant. However, this question needs further investigation.
It has been accepted that the undulation of the microspore plasma membrane and primexine formation play vital roles in pollen exine formation (Ariizumi and Toriyama, 2011). The pollen coat also is vital for pollen adhesion and hydration in the stigma (Ariizumi and Toriyama, 2011). We found that primexine formation and pollen coat deposition are both disrupted significantly in the atsec31b mutant (Figs. 3 and 8, A–C). Through TEM observations, we found that exine formation is impaired significantly in the atsec31b mutant, which ultimately causes pollen abortion. The atsec31b mutant exhibited several changes in the process of pollen exine formation: (1) the plasma membrane undulation was not evident, and the plasma membrane was less invaginated than in the wild type; (2) the sporopollenin was deposited randomly on a very thin primexine; (3) some of the microspores formed thick baculae and exine-like structures; and (4) the pollen protoplasts were separated from the aberrant exine and eventually degenerated (Fig. 3).
Plasma membrane undulation occurs in the tetrad stage, which has been found in many species, including Arabidopsis, and is considered to determine the final exine patterning in different species (Anger and Weber, 2006; Ariizumi and Toriyama, 2011). The primexine deposited between the plasma membrane and the callose wall serves as an ideal sporopollenin receptor (Gabarayeva and Grigorjeva, 2004; Ariizumi and Toriyama, 2011). Additionally, the processes of plasma membrane undulation and primexine deposition are closely related. Abnormal primexine formation often leads to severely defective pollen exine development (Ariizumi and Toriyama, 2011). Therefore, the defective primexine formation in the atsec31b mutant may be the primary cause of the subsequent defective exine formation, defective pollen coat, even the collapsed pollen grains, although we could not exclude the possibility that AtSEC31B mediates the transporting events of unidentified proteins or members that are involved in the subsequent development of pollen grains and the pollen coat.
The mutant phenotype of atsec31b is clearly distinguishable from the reported primexine-defective mutants. In npu1, auxin response factor17 (arf17), and dex1 mutants, the undulation of the plasma membrane is not observed (Paxson-Sowders et al., 2001; Chang et al., 2012; Ma et al., 2013). Unlike npu1 and arf17 mutants, which have no primexine formation, the dex1 mutant has a thin primexine similar to that of the atsec31b mutant (Paxson-Sowders et al., 2001; Chang et al., 2012; Ma et al., 2013). However, sporopollenin never anchors on the plasma membrane, and no exine-like structure is formed in the dex1 mutant (Paxson-Sowders et al., 2001). Similar to atsec31b, the undulation of the plasma membrane also is reduced in nef1, but nef1 displays coarse primexine and no sporopollenin deposition (Ariizumi et al., 2004). In contrast to other primexine-defective mutants, hkm/ms1 and rpg1 mutants have more evident undulated plasma membranes but also result in thin primexine deposition (Ariizumi et al., 2005; Guan et al., 2008). Moreover, transient defective exine1 and exine formation defect (efd) mutants exhibit normal plasma membrane undulation but still produce thin and no primexine deposition, respectively (Ariizumi et al., 2008; Hu et al., 2014). However, except for efd and arf17 mutants, which lack the information about sporophytically or gametophytically controlled mutant phenotypes (Yang et al., 2013; Hu et al., 2014), all of the published primexine mutant phenotypes are under sporophytic control. AtSEC31B also functions sporophytically in primexine formation, which supports that proper primexine deposition is likely dependent on sporophytic tissue (Ariizumi and Toriyama, 2011).
The impaired exine formation and pollen coat deposition in atsec31b may result from the mislocation or less efficient trafficking of several of the reported or other unidentified proteins that participate in primexine formation and pollen coat deposition. The primexine is a matrix largely composed of neutral and acidic polysaccharides, cellulose, and proteins (Ariizumi and Toriyama, 2011). More recently, SPG2 and UPEX1, which are glycosyltransferases that participate in the biosynthesis of xylan backbone and the galactosylation of arabinogalactan proteins (AGPs), respectively, were shown to play roles in primexine formation sporophytically (Li et al., 2016). Moreover, the trafficking of cell wall polysaccharides and glycoproteins largely depends on the secretory pathway in plants (Kim and Brandizzi, 2016). Many enzymes involved in the synthesis and modification of cell wall polysaccharides and glycoproteins (e.g. AGPs) are likely transported via COPII-coated vesicles. Additionally, DEX1 and RPG1 are predicted to be membrane-associated proteins, and NPU1 is located in the plasma membrane in Arabidopsis protoplasts (Guan et al., 2008; Chang et al., 2012; Ma et al., 2013). These proteins are all likely transported to membrane organelles through COPII-coated vesicle trafficking. Although a lack of specific antibodies and XFP-fused protein could fully rescue transgenic lines, the actual subcellular locations of these proteins still need to be confirmed.
AtABCG9 and AtABCG31 are reported to be involved in pollen coat deposition (Choi et al., 2014). We found that atsec31b plants show similar pollen coat defects to those of abcg9 abcg31 plants (Fig. 8, A–C). sGFP:ABCG9 is localized in the plasma membrane of tapetal cells and harbors several potential ER export signal sites; it also accumulates in the BFA compartment following BFA treatment, which suggested that sGFP:ABCG9 is very likely transported via the ER-Golgi interface (Supplemental Table S2; Supplemental Fig. S10). sGFP-ABCG9 is expressed in tapetal cells exclusively at anther stage 10 (Choi et al., 2014), and AtSEC31B-EGFP appears from floral stages 9 to 11 (Fig. 5, A–D), which suggests that these proteins may present overlapping expression. However, in the atsec31b mutant background, the whole fluorescence intensity of sGFP:ABCG9 is reduced dramatically compared with that of wild-type plants (Fig. 8, D–I). sGFP:ABCG9 signal is weakly detected in the plasma membrane of the tapetum, and ER retention is not determined in atsec31b. It is hypothesized that, after blocking of the anterograde transport from the ER to the Golgi, the premature ABCG9 may be overaccumulated in the ER, and subsequently, excessive sGFP:ABCG9 proteins in the ER may trigger ER stress. The ER stress response may attenuate sGFP:ABCG9 translation to release the stress on the ER, or sGFP:ABCG9 may be subjected to proteasome-associated degradation (Pety de Thozée and Ghislain, 2006; Kakoi et al., 2013; Wan and Jiang, 2016), which also needs further investigation. Moreover, the identification of other candidates also needs to be explored.
Taken together, our findings show that AtSEC31B, as a functional COPII protein in tapetal cells, may mediate the membrane trafficking of many crucial proteins (e.g. membrane proteins, glycoproteins including AGPs, and enzymes involved in the biosynthesis and modification of cell wall polysaccharides), ultimately ensuring normal pollen exine development in Arabidopsis (Supplemental Fig. S11). This work characterizes a key factor participating in pollen wall development and also provides a regulation mechanism of secretory activity in tapetal cells at the molecular level.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
T-DNA insertion lines in ecotype Columbia-0 of Arabidopsis (Arabidopsis thaliana) were supplied by the ABRC (http://www.arabidopsis.org/) at Ohio State University. Seeds were sterilized (1 min in 75% ethanol, two rinses in sterile water, 12 min in 2.5% bleach, and four rinses in sterile water) and planted on growth medium (Murashige and Skoog basal salt mixture, 4.33 g L−1, supplied with 0.75% agar; PhytoTechnology Laboratories), then they were cold treated for 3 to 5 d. The medium plates were then transferred to a growth chamber (Percival) at 22°C and 50% humidity under a long-day photoperiod (16 h of light/8 h of dark) for approximately 14 d prior to planting in soil.
Phylogenic and Sequence Analyses
The amino acid sequences were aligned using the ClustalX2 program (http://www.clustal.org/; Sievers et al., 2011) with default settings. Using MEGA 6.06 software (http://www.megasoftware.net/; Tamura et al., 2013), a rectangular phylogenetic tree was constructed with full-length sequences by the neighbor-joining method with 1,000 replicates for bootstrap and default settings for other options.
Potential domains, motifs, and phosphorylation sites were predicted by SMART (http://smart.embl-heidelberg.de/; Schultz et al., 1998; Letunic et al., 2015), epestfind (http://emboss.bioinformatics.nl/; Kyte and Doolittle, 1982; Rogers et al., 1986; Rechsteiner et al., 1987; Rechsteiner and Rogers, 1996), and CBS Prediction Server (http://www.cbs.dtu.dk/services/; Blom et al., 1999), respectively.
Genetic Methods
Transgenic plants were generated via Agrobacterium tumefaciens (strain GV3101)-mediated flower-dip transformation (Clough and Bent, 1998) and selected on Murashige and Skoog solid medium containing 25 mg L−1 hygromycin B (Calbiochem) for segregation screening.
To determine plant genotypes, leaf genomic DNA was extracted via a rapid preparation method as described with some modifications (Edwards et al., 1991).
PCR genotyping was performed using three primers, one primer annealing to the T-DNA insertion site (LBb1.3) and a pair of primers designed to amplify the fragment of DNA spanning the insertion site, which were obtained from the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu/).
Outcrossing was conducted by applying pollen grains from newly dehiscing flowers onto the stigmas of flowers that had been surgically emasculated 1 d prior to dehiscence (Cole et al., 2005).
Total RNA Extraction and RT Reaction
Total RNAs were extracted using RNAiso Plus (TaKaRa) as described by the supplier. The first-strand cDNAs were synthesized from 1 μg of total RNA by the Prime Script RT Reagent Kit (TaKaRa) according to the manufacturer’s instructions.
SEM, Cross Sections, and TEM
For SEM, newly opened flowers were dissected using a dissection microscope, and the dehiscent anthers were mounted on stubs over carbon double-sided tape (Nisshin EM) and coated with gold particles using a sputter coater. Specimens were observed with a field emission scanning electron microscope (S-4800; Hitachi).
For cross sections, wild-type and atsec31b mutant inflorescences were fixed in Formalin-acetic acid-alcohol fixative (FAA) and embedded in Spurr’s resin as described (Sanders et al., 1999), then sectioned (2–3 μm) with a microtome (LKB Ultratome V; LKB). Anther sections were stained in 1% Toluidine Blue. Bright-field photographs of the cross sections were taken using a Zeiss microscope.
For ultrastructural analysis, Arabidopsis floral buds were fixed in 4% glutaraldehyde on ice, rinsed in 0.1 m phosphate-buffered saline (PBS), and postfixed in 1% OsO4 (dissolved in 0.1 m PBS). Flowers were embedded in Spurr’s resin as described for the cross-section procedure. Ultrathin sections (80 nm) were cut using a diamond knife on a Leica Ultracut ultramicrotome. Sections were double stained with saturated uranyl acetate and lead citrate and examined with a transmission electron microscope (H-7650; Hitachi).
Alexander’s Staining, Fluorescein Diacetate Staining, and 4′,6-Diamidino-2-Phenylindole Staining
Pollen grains were photographed with a Zeiss digital camera. Flower and plant images were taken with a Leica dissection microscope. Pollen viability was determined using Alexander solution (Alexander, 1969) and fluorescein diacetate (Heslop-Harrison and Heslop-Harrison, 1970; Pinney and Polito, 1990). 4′,6-Diamidino-2-phenylindole staining was performed as described (Ross et al., 1996).
GUS Staining
Tissues of ProAtSEC31B:GUS plants were stained in a solution of 1 mg mL−1 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid (GOLDBO COM), 10 mm EDTA·2Na, 0.5 mm K3FeC6N6, 0.5 mm K4Fe(CN)6·3H2O, 50 mm NaH2PO4, 50 mm Na2HPO4, and 0.1% (v/v) Triton X-100 at 37°C after treatment in 90% precooling acetone. After GUS staining, chlorophyll was removed using absolute ethanol and the tissues were treated with 2% (v/v) HCl in 20% (v/v) methanol and 7% (w/v) NaOH in 60% (v/v) ethanol (Yang et al., 1999).
For cross sections after GUS staining, the inflorescences were fixed in FAA on ice, then transferred to 4°C overnight, embedded in Paraplast (Pathologic), sectioned at 15 μm thickness, and observed with a Zeiss microscope (Wang et al., 2013).
Pollen Germination in Vitro and in Vivo
Pollen germination in vitro was performed as described (Boavida and McCormick, 2007) except at 28°C. The Aniline Blue staining of pollen tubes in vivo was conducted as described (Ishiguro et al., 2001). The pollinated pistils were collected 4, 10, and 24 hap and the procedure was continued. The stained pistils were observed and photographed using the Zeiss LSM-710 confocal microscope.
Protoplast Transformation
Protoplast preparation was performed as described (Yoo et al., 2007; Li et al., 2009). A total of 20 μg per construct was transformed into a 100-μL protoplast suspension. After culturing at 22°C for approximately 22 h, fluorescence images were taken using the Zeiss LSM-710 confocal microscope.
Confocal Laser Scanning Microscopy and Subcellular Localization
All confocal images were captured using the Zeiss LSM-710 confocal laser scanning microscope with a 40× or 63× water-immersion objective.
For subcellular analysis of AtSEC31B-GFP in transformed inflorescences, the buds were dissected using a stereoscopic microscope, then the anthers at different developmental stages were picked out, immersed in distilled, deionized water, and captured on a confocal microscope. GFP and chlorophyll signals in samples were detected using the settings as described (Huang et al., 2013).
For colocalization, HDEL-mCherry, Sed5-YFP, and ST-YFP were introduced into wild-type plants. The T1 generations selected by hygromycin B were crossed with rescue lines expressing AtSEC31B-GFP, then the progeny seeds were cultured vertically in darkness for 7 d. Cotyledon cells were analyzed for colocalization with HDEL-mCherry, and hypocotyl cells were analyzed for colocalization with Sed5-YFP or ST-YFP. Fluorescence imaging was performed using line-switching mode. GFP and mCherry in samples were excited with the 488-nm argon laser line and 543-nm helium-neon laser line, respectively, and steps were taken to subtract background noise. For imaging the coexpression of GFP and YFP, settings matched the previous report to avoid channel cross talk (Brandizzi et al., 2002).
For subcellular colocalization between AtSEC31B and other COPII components, GFP and mCherry signals were detected as described above. For the BiFC assay, YFP and chlorophyll were excited with the 514-nm argon laser line and emission was detected at 519 to 580 nm and 630 to 697 nm, respectively.
Postcapture processing were performed using ZEN 2010B SP1 software and Adobe Photoshop CS3.
BiFC Assay
The BiFC assay was conducted as described with modifications (Hino et al., 2011). The plasmids were transformed into protoplasts using the method described in “Protoplast Transformation,” and YFP fluorescence images were captured after culturing for approximately 22 h.
Co-IP
The wild-type plants stably expressing AtSEC13A-MH were crossed with AtSEC31B-GFP/atsec31b-3 plants, and the whole plants of offspring were collected at about 4 weeks for Co-IP analysis. Whole plants expressing both AtSEC13A-MH and AtSEC31B-GFP were homogenized with liquid nitrogen, then 2 g of powder was added to 10 mL of lysis buffer containing 50 mm Tris-HCl, pH 8, 150 mm NaCl, 1% (v/v) Triton X-100, and protease inhibitor cocktail (freshly add before use; Songon Biotech Co.; Takagi et al., 2013). Homogenates were centrifuged at 20,000g for 15 min and then repeated twice to remove cellular debris. The supernatants were mixed with 30 μL of anti-c-Myc agarose beads (Sigma-Aldrich) and incubated on a slow rotating wheel at 4°C for 1.5 h. Then, the anti-c-Myc agarose beads were transferred into a micro bio-spin chromatography column (Bio-Rad Laboratories) and washed with 600 μL of washing buffer containing 50 mm Tris-HCl, pH 8, 150 mm NaCl, and 0.1% (v/v) Triton X-100 three times. Immunoprecipitation complexes were eluted with 50 μL of SDS sample buffer and subjected to SDS-PAGE, then to immunoblot analysis with anti-GFP antibody (TongBio Pacific). When using AtSEC31B-GFP as bait, GFP-Trap A (Chromo Tek) was applied. The procedure was according to that described above for when AtSEC13A-MH was used as bait.
FRAP
FRAP analysis was conducted as described previously (Brandizzi et al., 2002). The optimized conditions for bleaching were determined by materials fixed with 2% paraformaldehyde. Before the FRAP assay, the Golgi movement was stopped by treatment of 25 μm latrunculin B. Postacquisition and data analyses were performed with Zeiss AIM software.
BFA Treatment
BFA treatment was performed as described (Brandizzi et al., 2002; Huang et al., 2013). Anthers at stage 10 were incubated with or without 20 μg mL−1 BFA for 3 h, which was prepared using dimethyl sulfoxide and diluted with 1× PBS, while dimethyl sulfoxide was taken as a negative control. The same settings (laser power and detection gain) were used for direct comparison of the anthers before and after treatment.
Molecular Manipulation
For expression pattern analysis using GUS staining, a genomic DNA fragment containing nucleotide sequence from 2.2 kb upstream of the AtSEC31B start codon was amplified by PCR from genomic DNA of Arabidopsis ecotype Columbia-0 plants and cloned into pCambia1300-Pro-35S:GUS binary vector using PstI/XbaI, which hold GUS coding sequence behind the promoter. To generate the AtSEC31B complementation construct, the AtSEC31B promoter obtained as described above was inserted into pCambia1300-Pro-35S:TerNOS binary vector by PstI/XbaI to take the place of the 35S promoter, then three fragments of AtSEC31B genomic DNA, which were amplified by PCR from genomic DNA of Arabidopsis ecotype Columbia-0 plants using specific primers, were cloned into the plasmid at the same time by the combination of XbaI/ApaI, ApaI/SmaI, and SmaI/BamHI. For subcellular localization, the AtSEC31B promoter obtained as described above was cloned into pB35S:GFPBS-2 binary vector with EGFP fused with the C-terminal end of the target protein, and the two cDNA fragments of AtSEC31B were amplified by PCR from mRNA of the inflorescences of Arabidopsis ecotype Columbia-0 plants and then inserted into the plasmid by XbaI/ApaI and ApaI/BamHI. For investigation of whether the AtSEC31B cDNA fragment driven by the tapetum-specific A9 promoter could complement the mutant phenotype, the A9 promoter fragment was cloned by specific primers from genomic DNA of wild-type plants to take the place of the AtSEC31B promoter. For subcellular colocalization with COPII components in the Arabidopsis protoplast, the Pro-35S:TerNOS fragment was inserted into pUC19 vector by HindIII/EcoRI, then the EGFP fragment was cloned into the plasmid by BamHI/SacI followed by the insertion of AtSEC31B cDNA by XbaI/BamHI. The cDNA fragments of COPII components, including AtSAR1, AtSEC13A, and AtSEC13B, were amplified by PCR using specific primers from mRNA of wild-type plants and cloned into pUC19 vector, which fused mCherry to the C-terminal end of the protein driven by the 35S promoter.
The ER marker was provided by Andreas Nebenführ of the University of Oklahoma Health Sciences Center (Nelson et al., 2007) and modified by Yi-hua Zhou of the Chinese Academy of Sciences (Li et al., 2009). The Golgi markers, including Sed5-YFP and ST-YFP, were provided by Patrick Moreau of the University of Bordeaux (Chatre et al., 2005). The plasmids used for the BiFC assay were provided by Tsuyoshi Nakagawa of Shimane University (Hino et al., 2011).
Primers Used in This Study
Primers and constructs used in this study are listed in Supplemental Tables S3 and S4.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: AtSEC31A (At1g18830), AtSEC31B (At3g63460), AtSAR1 (At1g56330), AtSEC13A (At3g01340), AtSEC13B (At2g30050), AtSEC24A (At3g07100), ABCG9 (At4g27420), ABCG31 (At2g29940), and ACTIN7 (At5g098103).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Sequence alignment and phylogenetic analysis of SEC31 amino acid sequences from different species.
Supplemental Figure S2. Reciprocal cross analysis.
Supplemental Figure S3. Identification and phenotype analysis of atsec31b-n and wild-type plants.
Supplemental Figure S4. Pollen germination and pollen tube growth of the atsec31b and wild-type plants.
Supplemental Figure S5. Random deposition of sporopollenin on the locule wall in atsec31b plants.
Supplemental Figure S6. Expression pattern of AtSEC31B revealed by GUS staining.
Supplemental Figure S7. ProAtSEC31B:AtSEC31Bc:EGFP could rescue the mutant phenotype of atsec31b.
Supplemental Figure S8. Subcellular localization analysis of AtSEC31B-GFP.
Supplemental Figure S9. Negative control groups from the BiFC assay showing no interaction between them.
Supplemental Figure S10. Subcellular location of sGFP-AtABCG9 after BFA treatment in tapetal cells.
Supplemental Figure S11. Putative model of AtSEC31B function in the secretary pathway of the tapetal cells.
Supplemental Table S1. Transmission efficiency analysis using reciprocal crosses.
Supplemental Table S2. Potential ER export signals for AtABCG9 and AtABCG31.
Supplemental Table S3. Primers used in this study.
Supplemental Table S4. Constructs used in this study.
Supplementary Material
Acknowledgments
We thank Patrick Moreau (University of Bordeaux) for providing the ST-YFP, Sed5-YFP, and Erd2-CFP plasmids; Tsuyoshi Nakagawa (Shimane University) for providing the plasmids used in the BiFC assay; Randy W. Schekman (University of California, Berkeley) for providing yeast mutant strains RSY255 and RSY952; Bob Lesch for guidance on the yeast functional complementation experiment; Youngsook Lee for providing seeds stably expressing sGFP-ABCG9; and the ABRC for providing seeds of Arabidopsis T-DNA insertion mutants.
Glossary
- ER
endoplasmic reticulum
- TEM
transmission electron microscopy
- Co-IP
coimmunoprecipitation
- FRAP
fluorescence recovery after photobleaching
- ABRC
Arabidopsis Biological Resource Center
- SEM
scanning electron microscopy
- hap
hours after pollination
- RT
reverse transcription
- ERES
endoplasmic reticulum exit site
- BiFC
bimolecular fluorescence complementation
- AGPs
arabinogalactan proteins
- PBS
phosphate-buffered saline
Footnotes
This work was supported by the National Science Foundation of China (grant nos. 31540005 and 31270357 to Y.G. and grant no. 31300265 to R.L.), the Program for New Century Excellent Talents in University (grant no. NECT–12–0687), the Key Project of the Chinese Ministry of Education (grant no. 211019 to Y.G.), the Natural Science Foundation of Hebei Province (grant no. C2013205160 to R.L.). the Foundation for High-Level Talents in Higher Education of Hebei, and the 100 Innovative Talents Program of Higher Education of Hebei (to Y.G.).
Articles can be viewed without a subscription.
References
- Alexander MP. (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Anger EM, Weber M (2006) Pollen-wall formation in Arum alpinum. Ann Bot (Lond) 97: 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39: 170–181 [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K (2005) The HKM gene, which is identical to the MS1 gene of Arabidopsis thaliana, is essential for primexine formation and exine pattern formation. Sex Plant Reprod 18: 1–7 [Google Scholar]
- Ariizumi T, Kawanabe T, Hatakeyama K, Sato S, Kato T, Tabata S, Toriyama K (2008) Ultrastructural characterization of exine development of the transient defective exine 1 mutant suggests the existence of a factor involved in constructing reticulate exine architecture from sporopollenin aggregates. Plant Cell Physiol 49: 58–67 [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62: 437–460 [DOI] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362 [DOI] [PubMed] [Google Scholar]
- Boavida LC, McCormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Snapp EL, Roberts AG, Lippincott-Schwartz J, Hawes C (2002) Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell 14: 1293–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HS, Zhang C, Chang YH, Zhu J, Xu XF, Shi ZH, Zhang XL, Xu L, Huang H, Zhang S, et al. (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol 158: 264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatre L, Brandizzi F, Hocquellet A, Hawes C, Moreau P (2005) Sec22 and Memb11 are v-SNAREs of the anterograde endoplasmic reticulum-Golgi pathway in tobacco leaf epidermal cells. Plant Physiol 139: 1244–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yu XH, Zhang K, Shi J, De Oliveira S, Schreiber L, Shanklin J, Zhang D (2011) Male Sterile2 encodes a plastid-localized fatty acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol 157: 842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Jin JY, Choi S, Hwang JU, Kim YY, Suh MC, Lee Y (2011) An ABCG/WBC-type ABC transporter is essential for transport of sporopollenin precursors for exine formation in developing pollen. Plant J 65: 181–193 [DOI] [PubMed] [Google Scholar]
- Choi H, Ohyama K, Kim YY, Jin JY, Lee SB, Yamaoka Y, Muranaka T, Suh MC, Fujioka S, Lee Y (2014) The role of Arabidopsis ABCG9 and ABCG31 ATP binding cassette transporters in pollen fitness and the deposition of steryl glycosides on the pollen coat. Plant Cell 26: 310–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cole RA, Synek L, Zarsky V, Fowler JE (2005) SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol 138: 2005–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo Souza C, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, Haughn GW, Kombrink E, Douglas CJ (2009) A novel fatty acyl-CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell 21: 507–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobritsa AA, Shrestha J, Morant M, Pinot F, Matsuno M, Swanson R, Møller BL, Preuss D (2009) CYP704B1 is a long-chain fatty acid ω-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol 151: 574–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Yang C, Klisch D, Ferguson A, Bhaellero RP, Niu X, Wilson ZA (2014) ECHIDNA protein impacts on male fertility in Arabidopsis by mediating trans-Golgi network secretory trafficking during anther and pollen development. Plant Physiol 164: 1338–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faso C, Chen YN, Tamura K, Held M, Zemelis S, Marti L, Saravanan R, Hummel E, Kung L, Miller E, et al. (2009) A missense mutation in the Arabidopsis COPII coat protein Sec24A induces the formation of clusters of the endoplasmic reticulum and Golgi apparatus. Plant Cell 21: 3655–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabarayeva NI, Grigorjeva VV (2004) Exine development in Encephalartos altensteinii (Cycadaceae): ultrastructure, substructure and the modes of sporopollenin accumulation. Rev Palaeobot Palynol 132: 175–193 [Google Scholar]
- Garcia C, Khan NZ, Nannmark U, Aronsson H (2010) The chloroplast protein CPSAR1, dually localized in the stroma and the inner envelope membrane, is involved in thylakoid biogenesis. Plant J 63: 73–85 [DOI] [PubMed] [Google Scholar]
- Ge W, Song Y, Zhang C, Zhang Y, Burlingame AL, Guo Y (2011) Proteomic analyses of apoplastic proteins from germinating Arabidopsis thaliana pollen. Biochim Biophys Acta 1814: 1964–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN (2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147: 852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Renna L, Matheson LA, Brandizzi F (2007) De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated. Plant Physiol 143: 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technol 45: 115–120 [DOI] [PubMed] [Google Scholar]
- Hino T, Tanaka Y, Kawamukai M, Nishimura K, Mano S, Nakagawa T (2011) Two Sec13p homologs, AtSec13A and AtSec13B, redundantly contribute to the formation of COPII transport vesicles in Arabidopsis thaliana. Biosci Biotechnol Biochem 75: 1848–1852 [DOI] [PubMed] [Google Scholar]
- Hsieh K, Huang AH (2007) Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 19: 582–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K, Wu SS, Ratnayake C, Huang AH (2003) Tapetosomes and elaioplasts in Brassica and Arabidopsis floral tapetum. In N Murata, M Yamada, I Nishida, H Okuyama, J. Sekiya, W Hajime, eds, Advanced Research on Plant Lipids. Springer, the Netherlands, pp 215–218 [Google Scholar]
- Hu J, Wang Z, Zhang L, Sun MX (2014) The Arabidopsis Exine Formation Defect (EFD) gene is required for primexine patterning and is critical for pollen fertility. New Phytol 203: 140–154 [DOI] [PubMed] [Google Scholar]
- Huang MD, Chen TLL, Huang AH (2013) Abundant type III lipid transfer proteins in Arabidopsis tapetum are secreted to the locule and become a constituent of the pollen exine. Plant Physiol 163: 1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K (2007) Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 19: 3549–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen MK, Poulsen LR, Schulz A, Fleurat-Lessard P, Møller A, Husted S, Schiøtt M, Amtmann A, Palmgren MG (2005) Pollen development and fertilization in Arabidopsis is dependent on the MALE GAMETOGENESIS IMPAIRED ANTHERS gene encoding a type V P-type ATPase. Genes Dev 19: 2757–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoi S, Yorimitsu T, Sato K (2013) COPII machinery cooperates with ER-localized Hsp40 to sequester misfolded membrane proteins into ER-associated compartments. Mol Biol Cell 24: 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Alezzawi M, Garcia-Petit C, Solymosi K, Khan NZ, Lindquist E, Dahl P, Hohmann S, Aronsson H (2014) A novel chloroplast localized Rab GTPase protein CPRabA5e is involved in stress, development, thylakoid biogenesis and vesicle transport in Arabidopsis. Plant Mol Biol 84: 675–692 [DOI] [PubMed] [Google Scholar]
- Karim S, Aronsson H (2014) The puzzle of chloroplast vesicle transport: involvement of GTPases. Front Plant Sci 5: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NZ, Lindquist E, Aronsson H (2013) New putative chloroplast vesicle transport components and cargo proteins revealed using a bioinformatics approach: an Arabidopsis model. PLoS ONE 8: e59898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Brandizzi F (2016) The plant secretory pathway for the trafficking of cell wall polysaccharides and glycoproteins. Glycobiology doi/10.1007/s00709-016-0952-4 [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157: 105–132 [DOI] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P (2015) SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 43: D257–D260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xiong G, Li R, Cui J, Tang D, Zhang B, Pauly M, Cheng Z, Zhou Y (2009) Rice cellulose synthase-like D4 is essential for normal cell-wall biosynthesis and plant growth. Plant J 60: 1055–1069 [DOI] [PubMed] [Google Scholar]
- Li WL, Liu Y, Douglas CJ (2016) Role of glycosyl transferases in pollen wall primexine formation and exine patterning. Plant Physiol (in press) pp.00471.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC, Zhu J, Yang J, Zhang GR, Xing WF, Zhang S, Yang ZN (2012) Glycerol-3-phosphate acyltransferase 6 (GPAT6) is important for tapetum development in Arabidopsis and plays multiple roles in plant fertility. Mol Plant 5: 131–142 [DOI] [PubMed] [Google Scholar]
- Lindquist E, Aronsson H (2014) Proteins affecting thylakoid morphology: the key to understanding vesicle transport in chloroplasts? Plant Signal Behav 9: e977205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist E, Solymosi K, Aronsson H (2016) Vesicles are persistent features of different plastids. Traffic 17: 1125–1138 [DOI] [PubMed] [Google Scholar]
- Liu L, Fan XD (2013) Tapetum: regulation and role in sporopollenin biosynthesis in Arabidopsis. Plant Mol Biol 83: 165–175 [DOI] [PubMed] [Google Scholar]
- Ma LJ, Yang ZN, Zhang S (2013) DEX1, a plasma membrane-localized protein, functions in microspore development by affecting CalS5 expression in Arabidopsis thaliana. Chin Sci Bull 58: 2855–2861 [Google Scholar]
- Marti L, Fornaciari S, Renna L, Stefano G, Brandizzi F (2010) COPII-mediated traffic in plants. Trends Plant Sci 15: 522–528 [DOI] [PubMed] [Google Scholar]
- McFarlane HE, Shin JJ, Bird DA, Samuels AL (2010) Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different combinations. Plant Cell 22: 3066–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morant M, Jørgensen K, Schaller H, Pinot F, Møller BL, Werck-Reichhart D, Bak S (2007) CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 19: 1473–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano RT, Matsushima R, Ueda H, Tamura K, Shimada T, Li L, Hayashi Y, Kondo M, Nishimura M, Hara-Nishimura I (2009) GNOM-LIKE1/ERMO1 and SEC24a/ERMO2 are required for maintenance of endoplasmic reticulum morphology in Arabidopsis thaliana. Plant Cell 21: 3672–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Pacini E, Franchi G, Hesse M (1985) The tapetum: its form, function, and possible phylogeny in Embryophyta. Plant Syst Evol 149: 155–185 [Google Scholar]
- Paul W, Hodge R, Smartt S, Draper J, Scott R (1992) The isolation and characterisation of the tapetum-specific Arabidopsis thaliana A9 gene. Plant Mol Biol 19: 611–622 [DOI] [PubMed] [Google Scholar]
- Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127: 1739–1749 [PMC free article] [PubMed] [Google Scholar]
- Pety de Thozée C, Ghislain M (2006) ER-associated degradation of membrane proteins in yeast. ScientificWorldJournal 6: 967–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffanelli P, Ross EJH, Murphy JD (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11: 65–80 [Google Scholar]
- Pinney K, Polito V (1990) Olive pollen storage and in vitro germination. Acta Hortic 286: 207–210 [Google Scholar]
- Qu X, Chatty PR, Roeder AH (2014) Endomembrane trafficking protein SEC24A regulates cell size patterning in Arabidopsis. Plant Physiol 166: 1877–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilichini TD, Friedmann MC, Samuels AL, Douglas CJ (2010) ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis. Plant Physiol 154: 678–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilichini TD, Samuels AL, Douglas CJ (2014) ABCG26-mediated polyketide trafficking and hydroxycinnamoyl spermidines contribute to pollen wall exine formation in Arabidopsis. Plant Cell 26: 4483–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers S, Rote K (1987) Protein-structure and intracellular stability. Trends Biochem Sci 12: 390–394 [Google Scholar]
- Rechsteiner M, Rogers SW (1996) PEST sequences and regulation by proteolysis. Trends Biochem Sci 21: 267–271 [PubMed] [Google Scholar]
- Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C (2007) Membrane dynamics in the early secretory pathway. CRC Crit Rev Plant Sci 26: 199–225 [Google Scholar]
- Rogers S, Wells R, Rechsteiner M (1986) Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234: 364–368 [DOI] [PubMed] [Google Scholar]
- Ross KJ, Fransz P, Jones GH (1996) A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome Res 4: 507–516 [DOI] [PubMed] [Google Scholar]
- Salama NR, Chuang JS, Schekman RW (1997) Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell 8: 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire K, Hsu YC, Lee PY, Truong MT, Beals T, Goldberg R (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11: 297–322 [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95: 5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell (Suppl) 16: S46–S60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Cui M, Yang L, Kim YJ, Zhang D (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci 20: 741–753 [DOI] [PubMed] [Google Scholar]
- Shugrue CA, Kolen ER, Peters H, Czernik A, Kaiser C, Matovcik L, Hubbard AL, Gorelick F (1999) Identification of the putative mammalian orthologue of Sec31P, a component of the COPII coat. J Cell Sci 112: 4547–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz H. (1977) Role of β-1,3-glucanase in postmeiotic microspore release. Dev Biol 57: 87–97 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tsunekawa S, Koizuka C, Yamamoto K, Imamura J, Nakamura K, Ishiguro S (2013) Development and disintegration of tapetum-specific lipid-accumulating organelles, elaioplasts and tapetosomes, in Arabidopsis thaliana and Brassica napus. Plant Sci 207: 25–36 [DOI] [PubMed] [Google Scholar]
- Takagi J, Renna L, Takahashi H, Koumoto Y, Tamura K, Stefano G, Fukao Y, Kondo M, Nishimura M, Shimada T, et al. (2013) MAIGO5 functions in protein export from Golgi-associated endoplasmic reticulum exit sites in Arabidopsis. Plant Cell 25: 4658–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Nishimura K, Kawamukai M, Oshima A, Nakagawa T (2013) Redundant function of two Arabidopsis COPII components, AtSec24B and AtSec24C, is essential for male and female gametogenesis. Planta 238: 561–575 [DOI] [PubMed] [Google Scholar]
- Ting JT, Wu SS, Ratnayake C, Huang AH (1998) Constituents of the tapetosomes and elaioplasts in Brassica campestris tapetum and their degradation and retention during microsporogenesis. Plant J 16: 541–551 [DOI] [PubMed] [Google Scholar]
- Wan S, Jiang L (2016) Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in plants. Protoplasma 253: 753–764 [DOI] [PubMed] [Google Scholar]
- Wang H, Lu Y, Jiang T, Berg H, Li C, Xia Y (2013) The Arabidopsis U-box/ARM repeat E3 ligase AtPUB4 influences growth and degeneration of tapetal cells, and its mutation leads to conditional male sterility. Plant J 74: 511–523 [DOI] [PubMed] [Google Scholar]
- Wei T, Wang A (2008) Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J Virol 82: 12252–12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuestehube LJ, Duden R, Eun A, Hamamoto S, Korn P, Ram R, Schekman R (1996) New mutants of Saccharomyces cerevisiae affected in the transport of proteins from the endoplasmic reticulum to the Golgi complex. Genetics 142: 393–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Shi J, Rautengarten C, Yang L, Qian X, Uzair M, Zhu L, Luo Q, An G, Wassmann F, et al. (2016) Defective Pollen Wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol (in press) doi/10.1104/pp.16.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tian L, Sun MX, Huang XY, Zhu J, Guan YF, Jia QS, Yang ZN (2013) AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol 162: 720–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WC, Ye D, Xu J, Sundaresan V (1999) The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev 13: 2108–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Chung KP, Li B, Lai CM, Lam SK, Wang X, Cui Y, Gao C, Luo M, Wong KB, et al. (2015) Unique COPII component AtSar1a/AtSec23a pair is required for the distinct function of protein ER export in Arabidopsis thaliana. Proc Natl Acad Sci USA 112: 14360–14365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Shi J, Liang W, Xue F, Luo Q, Zhu L, Qu G, Chen M, Schreiber L, Zhang D (2015) Two ATP binding cassette G transporters, rice ATP Binding Cassette G26 and ATP Binding Cassette G15, collaboratively regulate rice male reproduction. Plant Physiol 169: 2064–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou J (2003) Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15: 1872–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.