Ethylene participates in the regulation of glutamate dehydrogenase genes during anoxia-reoxygenation and modulates metabolism by enhancing tricarboxylic acid cycle replenishment to aid recovery in Arabidopsis.
Abstract
Ethylene is an essential hormone in plants that is involved in low-oxygen and reoxygenation responses. As a key transcription factor in ethylene signaling, ETHYLENE INSENSITIVE3 (EIN3) activates targets that trigger various responses. However, most of these targets are still poorly characterized. Through analyses of our microarray data and the published Arabidopsis (Arabidopsis thaliana) EIN3 chromatin immunoprecipitation sequencing data set, we inferred the putative targets of EIN3 during anoxia-reoxygenation. Among them, GDH2, which encodes one subunit of glutamate dehydrogenase (GDH), was chosen for further studies for its role in tricarboxylic acid cycle replenishment. We demonstrated that both GDH1 and GDH2 are induced during anoxia and reoxygenation and that this induction is mediated via ethylene signaling. In addition, the results of enzymatic assays showed that the level of GDH during anoxia-reoxygenation decreased in the ethylene-insensitive mutants ein2-5 and ein3eil1. Global metabolite analysis indicated that the deamination activity of GDH might regenerate 2-oxoglutarate, which is a cosubstrate that facilitates the breakdown of alanine by alanine aminotransferase when reoxygenation occurs. Moreover, ineffective tricarboxylic acid cycle replenishment, disturbed carbohydrate metabolism, reduced phytosterol biosynthesis, and delayed energy regeneration were found in gdh1gdh2 and ethylene mutants during reoxygenation. Taken together, these data illustrate the essential role of EIN3-regulated GDH activity in metabolic adjustment during anoxia-reoxygenation.
Irregular weather patterns are impacting agriculture more and more seriously (Wheeler and von Braun, 2013). Sudden or long-term rainfall often leads to floods in low-lying areas and reduces crop yields. Although most plants cannot survive for a long period under complete submergence, owing to the limited oxygen microenvironment, metabolic adjustment can sustain plants to survive temporarily in such an energy crisis until desubmergence provides an opportunity for recovery (Bailey-Serres et al., 2012; Voesenek and Bailey-Serres, 2013).
Plant metabolic responses to oxygen deprivation can be divided into three phases: anaerobic energy generation, reduction of energy consumption, and accumulation of compounds for stress adaptation. During anaerobiosis, plants mainly rely on limited ATP generated by glycolysis. Since each Glc molecule generates only two ATPs during glycolysis, enhanced catabolism of starch and sugars in plants provides large amounts of carbon units to increase glycolytic energy generation during oxygen deprivation (Porterfield et al., 1997; Bieniawska et al., 2007; Ismail et al., 2009). Moreover, up-regulation of genes in the glycolytic and fermentative pathways demonstrates that plants enhance the efficiency of glycolysis to boost energy yield under low-oxygen conditions (Narsai et al., 2009; Mustroph et al., 2010). Besides glycolysis, it has been proposed that parts of the tricarboxylic acid cycle can still operate during anaerobiosis, and the reaction triggered by succinate-CoA ligase produces another ATP along with succinate accumulation (Rocha et al., 2010). Although plants can generate ATP without oxygen, the increase is usually not sufficient to compensate for the loss of oxidative phosphorylation. To meet the lowest energy demand for survival, precise control of energy cost is required. Stunted growth or development is a typical result of severe oxygen deprivation, which is linked to the suppression of ATP-consuming reactions like nucleosome assembly, ribosome biogenesis, and cell wall biosynthesis during stress (Branco-Price et al., 2005; Liu et al., 2005). Selective translational repression under low-oxygen conditions leads to significant energy conservation in rice (Oryza sativa; Edwards et al., 2012). In Arabidopsis (Arabidopsis thaliana), over 65% of total cellular mRNA is not fully and efficiently translated during low-oxygen stress as an energy-saving strategy (Branco-Price et al., 2008; Juntawong et al., 2014). It has been proposed that poorly translated mRNA can be stored in cellular stress granules to be degraded during stress or reutilized during reoxygenation (Sorenson and Bailey-Serres, 2014). As well as meticulous energy management, plants also synthesize specific metabolites, such as Ala and γ-aminobutyric acid (GABA), under oxygen deprivation for stress adaptation or future recovery. Alanine aminotransferase (AlaAT; EC 2.6.1.2), a transaminase that reversibly converts pyruvate and Glu into Ala and 2-oxoglutarate (2OG), has been suggested to be a key player in Ala accumulation during low-oxygen stress (de Sousa and Sodek, 2003; Rocha et al., 2010). As the alcohol and lactic acid accumulated by fermentation are toxic, AlaAT may promote the conversion of pyruvate into harmless Ala to maintain glycolysis (Rocha et al., 2010). Furthermore, it was suggested that AlaAT also acts in the reverse direction to break down Ala and then regenerate pyruvate during reoxygenation (Miyashita et al., 2007). However, since there are two AlaAT genes, AlaAT1 and AlaAT2, in Arabidopsis, lack of an alaat1/alaat2 double mutant makes it difficult to fully examine the function of AlaAT (Mustroph et al., 2014). GABA is known to participate in the regulation of cytosolic pH, osmolality, and reactive oxygen species metabolism and accumulates in plant cells under various stress conditions, including salinity, drought, heat, and hypoxia (Bouché and Fromm, 2004). In Arabidopsis, five glutamate decarboxylase (GAD; EC 4.1.1.15) genes encode the enzyme for the conversion of Glu into GABA. When plants were subjected to oxygen deprivation, reduced GABA and Ala accumulation were observed in gad1 mutants, indicating that GABA also contributes to Ala biosynthesis during this stress (Miyashita and Good, 2008a). However, the gad1 mutants displayed no phenotype, leaving the biological roles of GABA in hypoxic stress unclear.
Ethylene plays a central role in the accommodation of plants to low-oxygen stress. It triggers responses such as adventitious root development, aerenchyma formation, petiole/stem elongation, and hyponasty response when plants are waterlogged (Voesenek and Sasidharan, 2013). In Arabidopsis, two transcription factors, HYPOXIA RESPONSIVE ERFS1 and RELATED TO AP2.2, which are responsible for various low-oxygen downstream signaling, are regulated by ethylene (Hinz et al., 2010; Yang et al., 2011). Additionally, ethylene is required, but not sufficient, for the induction of ALCOHOL DEHYDROGENASE (Peng et al., 2001). In rice, ethylene is involved in the regulation of ethylene response factor (ERF)-like genes, such as SUBMERGENCE1-A-1 (SUB1A-1) in SUB1A-1-containing cultivars and SNOKEL1 (SK1) and SK2 in deep-water rice to confer submergence tolerance (Xu et al., 2006; Hattori et al., 2009). The biosynthesis of ethylene during desubmergence also has been reported (Voesenek et al., 2003). Ethylene has been suggested to play an important role in modulating the homeostasis of hormone signaling, metabolism, and reactive oxygen species during reoxygenation (Tsai et al., 2014). Although there is molecular evidence linking hypoxia and ethylene, detailed information about ethylene signal transduction during this stress is still patchy. Nevertheless, the ethylene signaling pathway has been well characterized and begins with the reception of the ethylene molecule by ethylene receptors. Binding of ethylene to its receptors inactivates CONSTITUTIVE TRIPLE REPSPONSE, which functions to repress ETHYLENE INSENSITIVE2 (EIN2). Activation of EIN2 leads to the stabilization of EIN3 and EIN3-like (EIL) transcription factors. EIN3 and EILs induce the expression of various genes involved in vegetative growth, development, and stress responses (Zhao and Guo, 2011; Merchante et al., 2013).
In this report, we focused on EIN3-dependent molecular events in response to low-oxygen stress. To identify the direct targets of stress-regulated EIN3, we analyzed our anoxia-reoxygenation (A/R) transcriptomic data and the published EIN3 chromatin immunoprecipitation sequencing (ChIP-seq) data set (Chang et al., 2013). We found that the GDH2 gene, which encodes a subunit of glutamate dehydrogenase (GDH; EC 1.4.1.2), is a potential direct target of EIN3. GDH is a key enzyme in the interconversion between Glu and 2OG. Through metabolic profiling, we discovered that GDH participates in modulating Ala breakdown and tricarboxylic acid cycle replenishment during reoxygenation. We characterized the connection between ethylene signaling and GDH and investigated the physiological roles of GDH by metabolite profiling to illustrate ethylene-dependent metabolic adaptive responses to low-oxygen and reoxygenation stress.
RESULTS
Activation of EIN3-Dependent Signaling in Response to A/R
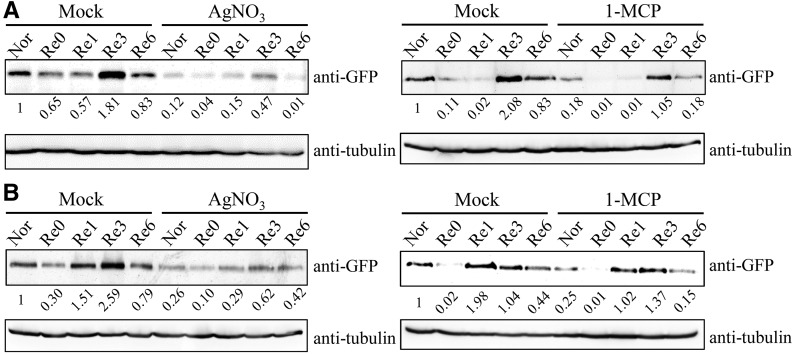
To investigate the molecular events of ethylene signaling transduction under low-oxygen stress, the protein stabilization of EIN3 during stress was examined. We adopted a previously developed anaerobic chamber system (Tsai et al., 2014) to treat 7-d-old 35S::EIN3-GFP/ein3eil1 transgenic Arabidopsis seedlings (He et al., 2011) with A/R and monitored the level of the fusion protein using anti-GFP antibody. Our results showed that EIN3 protein was destabilized slightly at the early stage of anoxic treatment (10–30 min; Supplemental Fig. S1). However, after 30 min of anoxia, the level of EIN3 protein began to stabilize and was maintained at the same level during the later stages of anoxia (30 min to 4 h), indicating that, even under anoxia, low levels of EIN3 protein are present (Supplemental Fig. S1). Interestingly, the fusion protein increased from 1 to 3 h after the onset of reoxygenation (Fig. 1A), showing the activation of EIN3 signaling at the recovery stage. Prolonged darkness could trigger ethylene production and leads to leaf senescence (van der Graaff et al., 2006). To exclude a darkness effect, we also applied reoxygenation under light conditions. The pattern under these conditions was similar to reoxygenation in the dark (Fig. 1B). To verify the signaling transduction, we treated seedlings with AgNO3 and 1-MCP (ethylene signaling inhibitors) before and after applying anoxia treatment. The results showed that the addition of AgNO3 and 1-MCP caused the reduction of the EIN3 protein level (Fig. 1). Although 1-MCP treatment did not cause the effect as strong as AgNO3, it still caused a reduction in EIN3 protein amount based on the quantification of band intensity. These data suggest that ethylene signaling is activated during reoxygenation and are consistent with the findings that EIN3-dependent signaling is critical for the recovery of plant cells during reoxygenation (Voesenek et al., 2003; Tsai et al., 2014).
Figure 1.
EIN3 stabilization during A/R. 35S::EIN3-GFP seedlings (7-d-old) in the ein3eil1 mutant background (35S::EIN3GFP-OE/dm) were used to follow EIN3 levels by immunoblot detection of EIN3-GFP under A/R treatment. Anoxia treatment was performed in the dark. Reoxygenation was carried out either in the dark (A) or light (B). Nor, Plants grown under aerated conditions (normoxia); Re0, after 4 h of anoxia (beginning of reoxygenation); Re1, Re3, and Re6, 1, 3, and 6 h of reoxygenation, respectively. Treatment with AgNO3 (20 μm) or 1-methylcyclopropene (1-MCP) was used as a negative control to inhibit ethylene signal transduction. The quantified signal values were normalized to time point Nor and are shown below the gels.
EIN3-Dependent Signaling Activates Various Targets during A/R
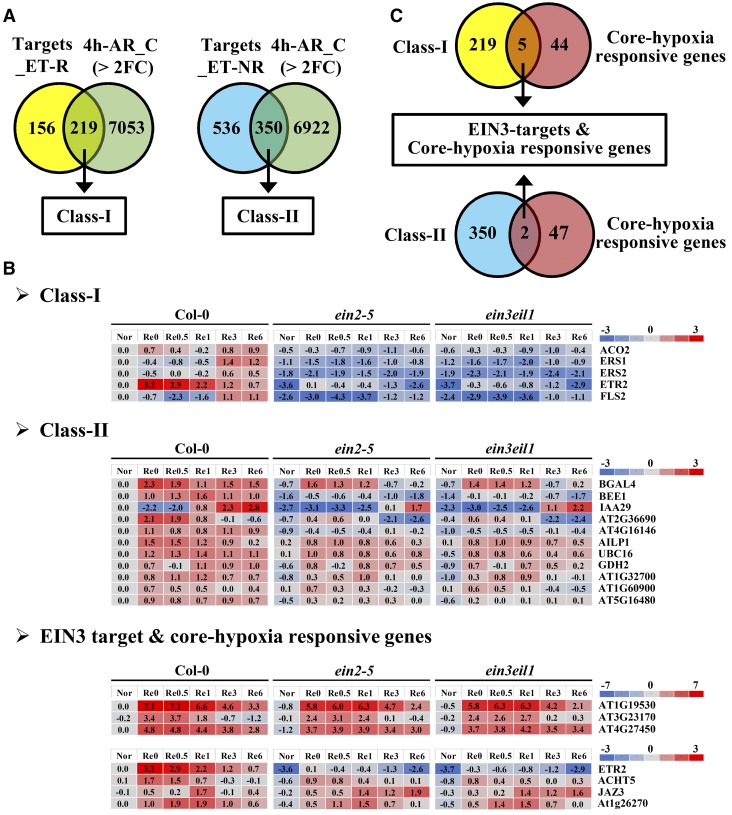
As a primary transcription factor of ethylene signaling, EIN3 activates various genes that are essential for the survival of plants under low-oxygen stress. The published EIN3 ChIP-seq data (Chang et al., 2013) provide valuable information about the direct targets of EIN3. We compared the ChIP-seq data with our own transcriptomic data (Tsai et al., 2014) for genes that are activated by EIN3 during A/R. First, we generated a list of genes that are up- or down-regulated more than 2-fold during anoxia (Re0) or each reoxygenation time point (Re0.5, Re1, Re3, and Re6) in the wild type. Then, Venn diagram analysis was performed using the group of EIN3 targets (Chang et al., 2013) and the stress-induced genes that we generated (Fig. 2A). Since there are two groups of EIN3 targets (regulated by ethylene and not regulated by ethylene) defined by their ethylene response under aerated conditions (Chang et al., 2013), we compiled two classes of genes. Class I (219 genes) represented targets that could be regulated by ethylene under both normal and stress conditions. Class II (350 genes) represented targets that were only regulated by ethylene during A/R stress (Fig. 2A; Supplemental Data Set S1). To further verify the ethylene effects on these targets, we compared the gene expression levels in Columbia-0 (Col-0) and two ethylene-insensitive mutants, ein2-5 and ein3eil1, by microarray data (Tsai et al., 2014). We set the criterion to 1.5-fold change (wild type/ein2-5 or wild type/ein3eil1) to look for EIN3 direct targets. Several notable targets in class I and class II are presented in Figure 2B. In class I, the ethylene biosynthesis gene ACO2, and the genes encoding ethylene receptors ERS1, ERS2, and ETR2, were significantly regulated by EIN3, demonstrating the feed-forward regulation reported previously (Chang et al., 2013) and indicating that the general ethylene response was still activated during A/R stress (Fig. 2B). In contrast, genes belonging to class II were referred to as specific stress-induced targets. These genes included BEE1 and IAA29, which are involved in brassinoid and auxin responses, implying that EIN3-driven cross talk between ethylene and different hormones occurs during low-oxygen stress. Moreover, BGAL4 and GDH2, two essential genes that encode enzymes in carbohydrate metabolism, also were directly regulated by EIN3 in response to stress (Fig. 2B). We also noticed that, among these ethylene-regulated targets, some already showed different expression levels during anoxia (Fig. 2B). Since we could not clearly find an increase in the EIN3 protein level during anoxia, we concluded that a basal level of EIN3 protein is still sufficient to trigger low-oxygen responses.
Figure 2.
Analyses of EIN3 direct targets and A/R-regulated genes. Venn diagrams and heat maps were used to demonstrate the EIN3-regulated targets in response to A/R. A, The gene lists of Targets_ET-R and Targets_N-ET-R, which represent EIN3 targets under ethylene regulation and EIN3 targets not under ethylene regulation, respectively, were extracted from Chang et al. (2013). The clustering of these EIN3 targets was defined according to their responses to ethylene treatment. Some of the EIN3 targets were regulated by ethylene alone, and some were not (Chang et al., 2013). The gene list of 4h-AR_C (> 2FC), which represents genes with 2-fold changes in response to 4 h of A/R (P < 0.05), was extracted from the microarray data presented by Tsai et al. (2014). B, Representative genes from each group were selected to present as a heat map by applying them to previous A/R array data. Nor, Plants grown under aerated conditions (normoxia); Re0, after 4 h of anoxia (beginning of reoxygenation); Re0.5, Re1, Re3, and Re6, after 0.5, 1, 3, and 6 h of reoxygenation, respectively. The scales at right are presented as log2 values. C, Class I and class II genes were used to perform Venn diagram analysis with the core hypoxia-responsive genes that were reported by Mustroph et al. (2009).
Although we have identified these stress-induced EIN3 targets, most of these targets are enriched and grouped into several unspecific Gene Ontology (GO) terms (Supplemental Data Set S2), and some of them are still functionally uncharacterized. To demonstrate the importance of these targets, we compared these targets with the core hypoxia-responsive genes reported by Mustroph et al. (2009). Class I and class II targets were chosen separately to make a Venn diagram with the core hypoxia-responsive genes. Surprisingly, among 49 core hypoxia-responsive genes, seven (14.3%) were direct targets of EIN3 (Fig. 2C). These results suggested that EIN3 targets might play a pivotal role in oxygen deprivation tolerance.
EIN3 Regulates GDHs during A/R
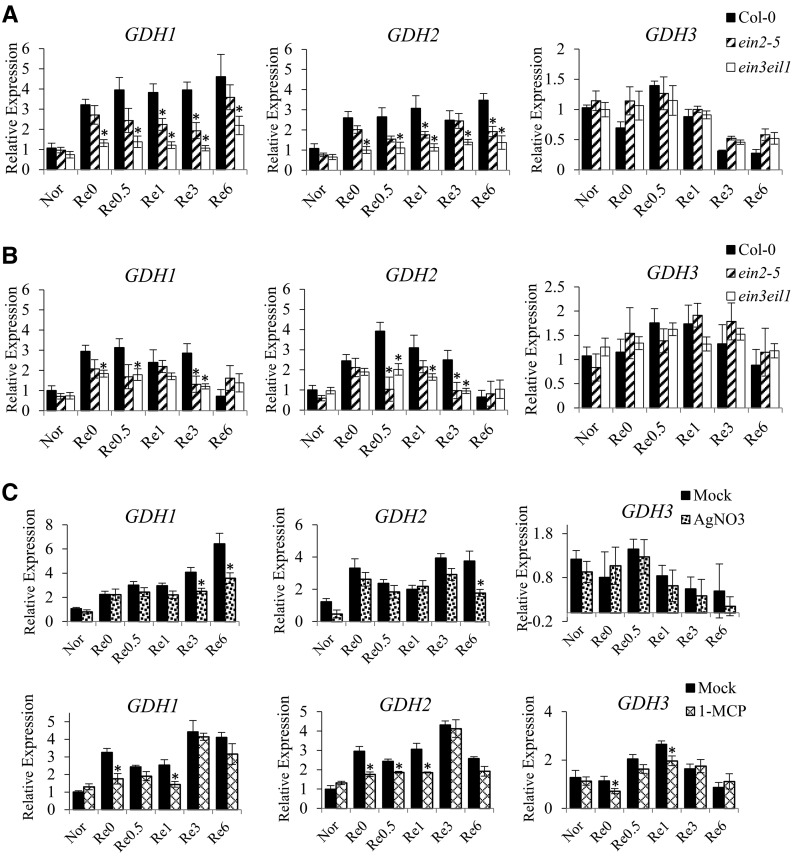
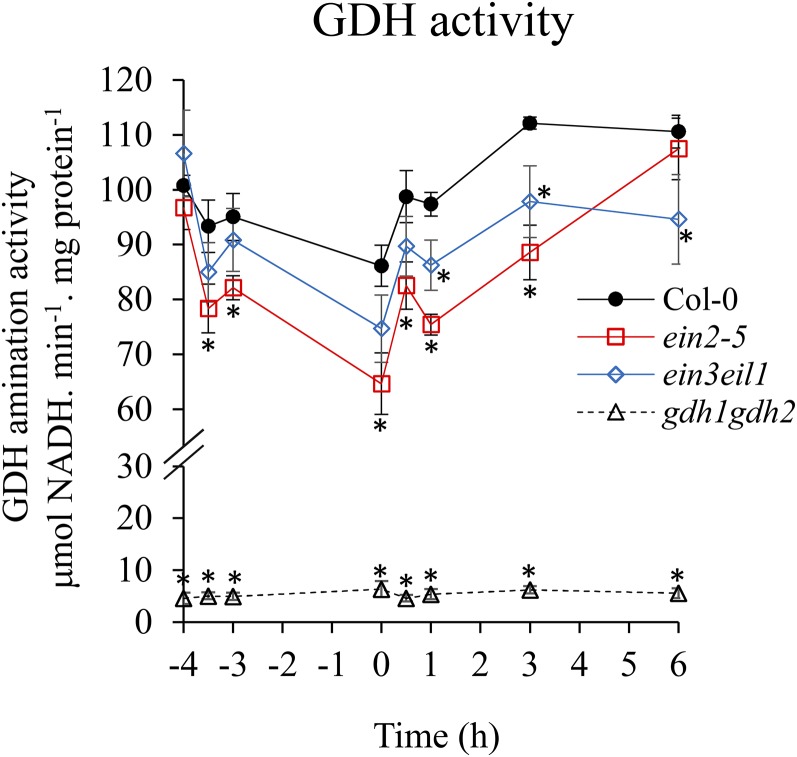
One of the stress-induced EIN3 targets found was GDH2, which encodes a subunit of GDH. Since GDH plays an essential role in maintaining the balance of carbon and nitrogen (C/N), we decided to characterize the relationship between ethylene signaling and GDH. The transcripts of GDH1, GDH2, and GDH3 were measured by quantitative real-time PCR in the wild type and two ethylene-insensitive mutants, ein2-5 and ein3eil1. The results showed a reduction of GDH1 and GDH2 transcripts in ein2-5 and ein3eil1 in response to A/R (Fig. 3A). However, GDH3 had a much lower level of transcripts in comparison with GDH1 and GDH2 (based on raw cycle threshold value) and was not significantly regulated by ethylene (Fig. 3). It was reported that GDHs could be induced in dark conditions (Miyashita and Good, 2008b). Since we applied A/R in the dark, we also inspected the transcripts during the stress under light conditions, and the expression of GDH1 and GDH2 was still under ethylene regulation (Fig. 3B). Interestingly, when we further applied the ethylene signaling inhibitor AgNO3 or 1-MCP to seedlings before and after anoxia stress, the transcripts of GDH1 and GDH2 were decreased (Fig. 3C). The fact that AgNO3 or 1-MCP treatment, which inhibits ethylene function, resulted in a decrease in the induction of GDH1 and GDH2 indicates that ethylene signaling is necessary. An in vitro enzyme activity assay was applied to determine if the reduced transcripts of GDH1 and GDH2 led to lower enzyme activity. The amination of GDH enzyme activity was measured under A/R stress. We observed that the enzyme activity dropped under low oxygen but recovered during reaeration (Fig. 4). In addition, the activity was lower in ein2-5 and ein3eil1 mutants compared with the wild type, indicating that ethylene signaling contributed to regulating GDH enzyme activity (Fig. 4). Taken together, these results demonstrated that GDH is regulated specifically by EIN3-dependent signaling during A/R.
Figure 3.
Ethylene participates in the regulation of GDH genes during A/R. Quantitative real-time PCR was used to verify the effects of ethylene signaling on the GDHs. A, Transcript levels of GDH1, GDH2, and GDH3 in 7-d-old wild-type, ein2-5, and ein3eil1 seedlings in response to A/R in the dark. B, Transcript levels were determined in plants under A/R in light. C, Transcript levels in 7-d-old wild-type seedlings treated with AgNO3 or 1-MCP before and after anoxia treatment in the dark. Nor, Plants grown under aerated conditions (normoxia); Re0, after 4 h of anoxia (beginning of reoxygenation); Re0.5, Re1, Re3, and Re6, after 0.5, 1, 3, and 6 h of reoxygenation, respectively. The results are mean values of at least four biological replicates with se. Asterisks indicate P < 0.05 by Student’s t test.
Figure 4.
Enzyme activity of GDH during A/R. An in vitro enzyme activity assay was used to measure GDH activity in 7-d-old wild-type, ein2-5, ein3eil1, and gdh1gdh2 seedlings during A/R in the dark. The x axis shows the treatment time course, and the zero point was set as the beginning of reoxygenation. The time points −4, −3.5, and −3 represent normoxia, 30 min, and 1 h of anoxia treatment, respectively. The time points 0.5, 1, 3, and 6 represent 0.5, 1, 3, and 6 h of reoxygenation, respectively. The results are mean values of at least four biological replicates with se. Asterisks indicate P < 0.05 by Student’s t test.
GDH Plays an Essential Role in Facilitating Ala Breakdown during A/R
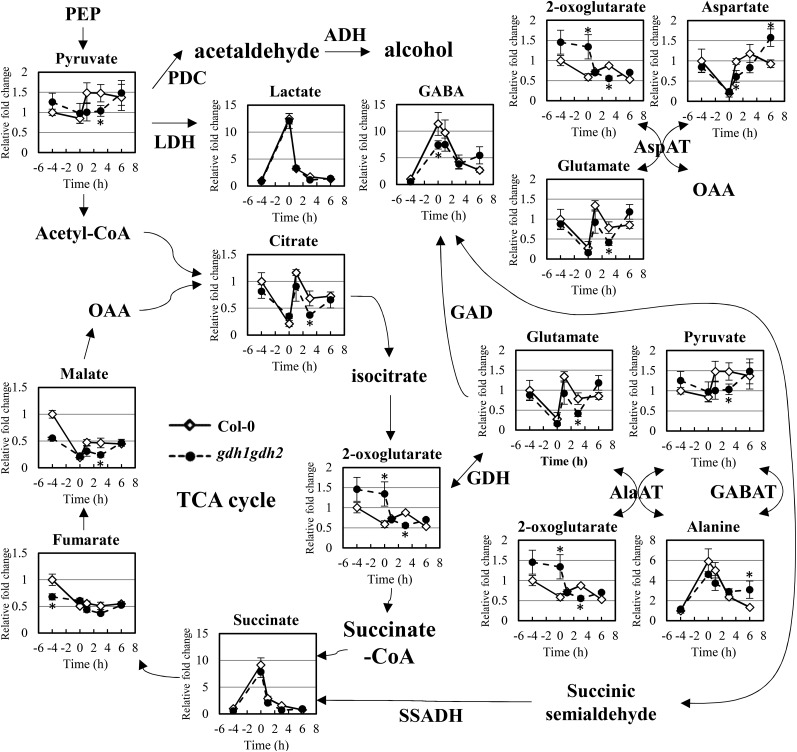
Previously, GDH was hypothesized to play a critical role in tricarboxylic acid cycle replenishment during the recovery stage of low-oxygen stress (Limami et al., 2008; Tsai et al., 2014). To evaluate this, we used the gdh1gdh2 double mutant, which had no significant GDH enzyme activity (Miyashita and Good, 2008b; Fig. 4), to validate the metabolic function of GDH under the stress. We applied gas chromatography-time-of-flight-mass spectrometry (GC-TOF-MS) assays to globally monitor various metabolites in response to A/R. In the wild type, we observed notable accumulation of lactic acid, succinate, GABA, and Ala in response to anoxia treatment (Fig. 5). However, when reoxygenation started, all of these compounds declined to basal levels. It has been hypothesized that Ala accumulated during oxygen deprivation is reutilized by being converted into pyruvate and Glu (Miyashita et al., 2007). Consistent with this hypothesis, we noticed that a sudden increase in pyruvate and Glu in response to reaeration occurred in concurrence with Ala breakdown (Fig. 5). Additionally, we found a rapid increase in citric acid during the reoxygenation stage, implying that the tricarboxylic acid cycle may reoperate through the increase in pyruvate and citric acid.
Figure 5.
Changes of metabolites in the wild type and gdh1gdh2 in response to A/R. GC-TOF-MS was applied to investigate the metabolite profiles during anoxia and reoxygenation. Black lines with diamonds correspond to the wild type, and dotted lines with circles correspond to the gdh1gdh2 mutant. The x axis shows the treatment time course. The time point −4 corresponds to normoxia, and the zero point is set as the beginning of reoxygenation. The time points 1, 3, and 6 represent 1, 3, and 6 h of reoxygenation, respectively. The level of metabolites is expressed as relative fold change calculated by normalizing values of the wild type (normoxia). The results are mean values of at least four biological replicates with se. Asterisks indicate P < 0.05 by Student’s t test. ADH, Alcohol dehydrogenase; GABAT, GABA aminotransferase; LDH, lactate dehydrogenase; PDC, pyruvate decarboxylase; SSADH, succinic semialdehyde dehydrogenase; TCA, tricarboxylic acid.
We next compared the metabolite profiles of the wild type and the gdh1gdh2 mutant. Compared with the wild type, a reduced level of GABA accumulation was observed in gdh1gdh2 under anoxic conditions (Fig. 5). We reasoned that the disturbance of Glu homeostasis in gdh1gdh2 might affect the accumulation of GABA. However, the level of Glu did not display any notable difference between the wild type and gdh1gdh2 during anoxia (Fig. 5). Thus, we further checked the level of 2OG and found that it was present at a higher level in the gdh1gdh2 mutant under oxygen deprivation (Fig. 5), showing that the conversion between 2OG and Glu was blocked in the mutant and that GDH was favored to perform amination activity under anoxia. Nevertheless, the accumulation of Ala was reduced only slightly in gdh1gdh2, indicating that even if GDH tends to synthesize Glu under anoxia, its enzyme activity plays a minor role in Ala accumulation. On the other hand, a previous report also mentioned that supplementation of Glu with another enzyme, aspartate aminotransferase (AspAT), might occur under hypoxic conditions (Rocha et al., 2010). AspAT catalyzes the interconversion of Asp and 2OG to oxaloacetate (OAA) and Glu. Therefore, we further inspected the level of Asp and found that it was decreased significantly in response to anoxia. Compared with GDH, AspAT seemed to be more dominant in Glu recycling under low-oxygen conditions.
In contrast to under anoxia, there were many profound differences in metabolites between the wild type and the gdh1gdh2 mutant during reoxygenation. In gdh1gdh2, Ala did not decrease to the basal level as it did in the wild type during reoxygenation (Fig. 5). The inefficient breakdown of Ala seemed to result in lower levels of pyruvate and Glu in the gdh1gdh2 mutant, as shown in the GC-TOF-MS data (Fig. 5). Compared with the wild type, the level of 2OG was reduced at the recovery stage in the gdh1gdh2 mutant, implying that the reduction was caused by loss of GDH deamination activity in the mutant. Since AlaAT was responsible for the conversion of Ala and 2OG to pyruvate and Glu, we suggest that insufficient amounts of 2OG affect the action of AlaAT. Interestingly, the levels of several tricarboxylic acid cycle intermediates, like citric acid and malate, recovered less during reoxygenation in gdh1gdh2 (Fig. 5). This may be attributed to the reduced regeneration of pyruvate or lower 2OG resupply in gdh1gdh2.
Metabolite profiling also was applied with ein2-5 and the ein3eil1 double mutant. Notably, the level of Ala in ein2-5 and ein3eil1 was lower than in the wild type during anoxia (Supplemental Figs. S2 and S3), indicating that ethylene signaling may affect other metabolic responses and disturb Ala accumulation. Interestingly, when we checked the enzyme activity of AlaAT and AspAT in ein2-5 and ein3eil1, lower activities were observed in both mutants (Supplemental Fig. S4). Reduced induction of AlaAT1 and ASPARTATE AMINOTRANSFERASE 2 (ASP2) transcripts during A/R also were found in the mutants (Supplemental Data Set S3). These results indicated that ethylene signaling not only regulates GDH activity but also targets multiple metabolic reactions during A/R. Owing to the versatile effects on metabolism derived from ethylene signaling, irregular postanoxia recovery in ein2-5 and ein3eil1 mutants was shown, including reduced levels of Glu, pyruvate, citric acid, and several tricarboxylic acid cycle intermediates (Supplemental Figs. S2 and S3). In addition, overall metabolite changes in ein2-5 and ein3eil1 also indicated poor recoveries of metabolites in comparison with the wild type (Supplemental Data Set S4). In summary, ethylene signaling shows broad effects on metabolic events during A/R, and its specific target, GDH, functions to regenerate 2OG to ensure that efficient tricarboxylic acid cycle replenishment happens during postanoxia reoxygenation.
GDH Plays Roles in Energy Regeneration during A/R
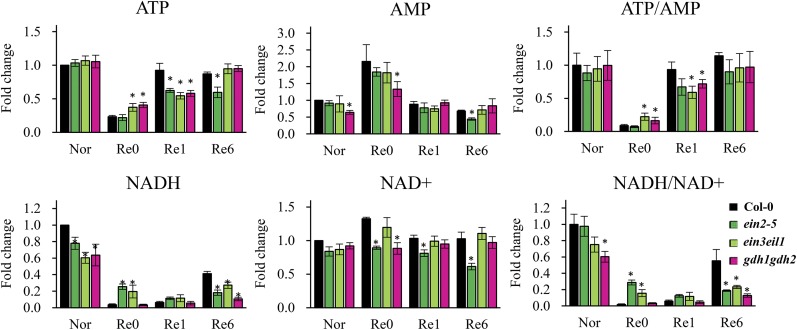
To determine whether the incomplete replenishment of the tricarboxylic acid cycle intermediates in gdh1gdh2 and ethylene mutants cause any negative effect on plants during reoxygenation, we measured the energy-related compounds via liquid chromatography-tandem mass spectrometry (LC-MS/MS). Relative quantification of ATP, AMP, NADH, and NAD+ is shown in Figure 6. The ratio of cellular ATP to AMP was used as an index of energy status in plant cells. The anaerobic environment led to a profound decrease in ATP level as well as ATP-AMP ratio, and this phenomenon was recovered within 6 h of reoxygenation (Fig. 6). Anoxia caused an energy crisis in ein2-5, ein3eil1, and gdh1gdh2, although the recovery of energy status during reoxygenation was less efficient in these mutants (Fig. 6). Consumption of NADH without passing through the electron transport chain is a classical feature of oxygen deprivation that provides cells with sufficient NAD+. Interestingly, we found that the level of NADH displayed differences in ethylene mutants and gdh1gdh2. After 4 h of anoxia, the amount of NADH in the wild type dropped to an almost undetectable level, but in ein2-5 and ein3eil1 mutants, a higher level of NADH was detected (Fig. 6). This suggests that the loss of ethylene signaling impacted NAD+ regeneration under anoxia. In addition, a slower NADH recovery was observed in ein2-5, ein3eil1, and gdh1gdh2 during reoxygenation (Fig. 6). These results not only revealed the metabolic roles of ethylene-regulated GDH in the recovery of plants from anaerobic conditions but also showed the dynamic energy adjustment during A/R.
Figure 6.
Energy-related metabolites in response to A/R treatment. Nor, Seven-day-old plants grown under aerated conditions (normoxia); Re0, after 4 h of anoxia (beginning of reoxygenation); Re1 and Re6, after 1 and 6 h of reoxygenation, respectively. Black bars correspond to the wild type, dark green bars correspond to ein2-5, light green bars correspond to ein3eil1, and magenta bars correspond to gdh1gdh2. The level of metabolites is expressed as fold change that was calculated by normalizing values of the wild type (Nor). The results are mean values of at least four biological replicates with se. Asterisks indicate P < 0.05 by Student’s t test.
GDH Deficiency Causes Misregulation in Carbohydrate Metabolism and Phytosterol Synthesis during Reoxygenation
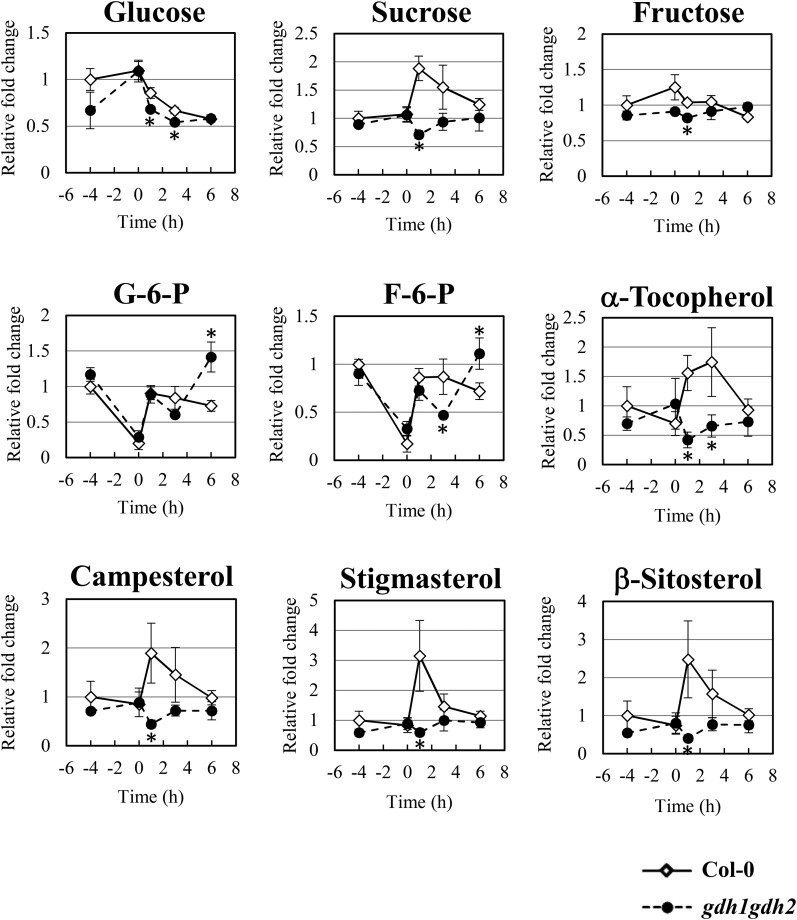
The metabolic change of various sugars was then surveyed in the wild type and the gdh1gdh2 mutant through GC-TOF-MS. The cellular level of Glc increased under anoxia but decreased rapidly during reoxygenation (Fig. 7). This pattern suggested that a restricted utilization of Glc occurred in response to anoxia and that the accumulated Glc was reused when plant cells were reaerated. Compared with Glc, the level of Suc did not alter during anoxia, but it increased dramatically when reoxygenation began and declined at later stages of recovery (Fig. 7). A sudden rise in Suc level during the beginning of reoxygenation might represent a transient stage when sugar metabolism shifts from Suc hydrolysis to Glc catabolism, resulting in a temporal accumulation of Suc. Furthermore, two glycolytic pathway intermediates, Glc-6-P and Fru-6-P, were inspected as indexes of glycolysis and gluconeogenesis proceeding, respectively. Under anoxic conditions, a significant reduction of Glc-6-P and Fru-6-P was observed in both the wild type and the gdh1gdh2 mutant. Nevertheless, within 30 min of reoxygenation, the levels of Glc-6-P and Fru-6-P were recovered to the levels under normal conditions (Fig. 7). These data along with the Glc metabolic profile suggest that both Glc-6-P and Fru-6-P were exhausted at the end of anoxia, and oxygen resupply may enhance the Glc breakdown to fuel glycolysis. In addition, the tricarboxylic acid cycle replenishment during reoxygenation also may increase gluconeogenic flux and thus regenerate Glc-6-P and Fru-6-P. Compared with the wild type, gdh1gdh2 had reduced amounts of Suc, Glc, Fru, Glc-6-P, and Fru-6-P at 1 and 3 h after the onset of reoxygenation, but with Glc-6-P and Fru-6-P it increased to higher levels after 6 h of reoxygenation (Fig. 7), suggesting that the inefficient tricarboxylic acid cycle replenishment in the mutant led to abnormal recovery in central carbohydrate metabolism after anoxic stress. In contrast, the sugar contents in ein2-5 and ein3eil1 did not follow the pattern in the gdh1gdh2 mutant (Supplemental Figs. S5 and S6). The relative levels of Glc and Fru were higher in ein2-5 or increased during reoxygenation in ein3eil1, showing that, in addition to GDH, ethylene signaling may regulate multiple steps in sugar metabolism. Nevertheless, the lower recovered levels of Glc-6-P and Fru-6-P in ein2-5 and ein3eil1 illustrated an inefficient recovery when ethylene signaling is lacking (Supplemental Figs. S5 and S6).
Figure 7.
Changes of carbohydrates, α-tocopherol, and phytosterols in wild-type and gdh1gdh2 plants in response to A/R. GC-TOF-MS was applied to investigate carbohydrates, α-tocopherol, and phytosterols during A/R. Black lines with diamonds correspond to the wild type, and dotted lines with circles correspond to the gdh1gdh2 mutant. The x axis shows the treatment time course. The time point −4 corresponds to normoxia, and the zero point is set as the beginning of reoxygenation. The time points 1, 3, and 6 represent 1, 3, and 6 h of reoxygenation, respectively. The level of metabolites is expressed as relative fold change that is calculated by normalizing values of the wild type (normoxia). The results are mean values of at least four biological replicates with se. Asterisks indicate P < 0.05 by Student’s t test. F-6-P, Fru-6-P; G-6-P, Glc-6-P. Campesterol, stigmasterol, and sitosterol are common sterols in plants.
α-Tocopherol and plant sterols, including campesterol, stigmasterol, and sitosterol, were detected through GC-TOF-MS. The levels of α-tocopherol and phytosterols were increased immediately in the wild type when reoxygenation occurred (Fig. 7). Interestingly, in gdh1gdh2, ein2-5, and ein3eil1, the increased levels of α-tocopherol and phytosterols during reoxygenation were decreased notably (Fig. 7; Supplemental Figs. S5 and S6). These results suggest that the impairment of tricarboxylic acid cycle replenishment during reoxygenation might lead to insufficient substrates or reduced energy generation for α-tocopherol and phytosterol production. These results suggest that ethylene signaling and GDH are required to maintain metabolic responses for the recovery and biogenesis during reoxygenation.
Loss of GDH Activity Causes Damage to Plants during Reoxygenation
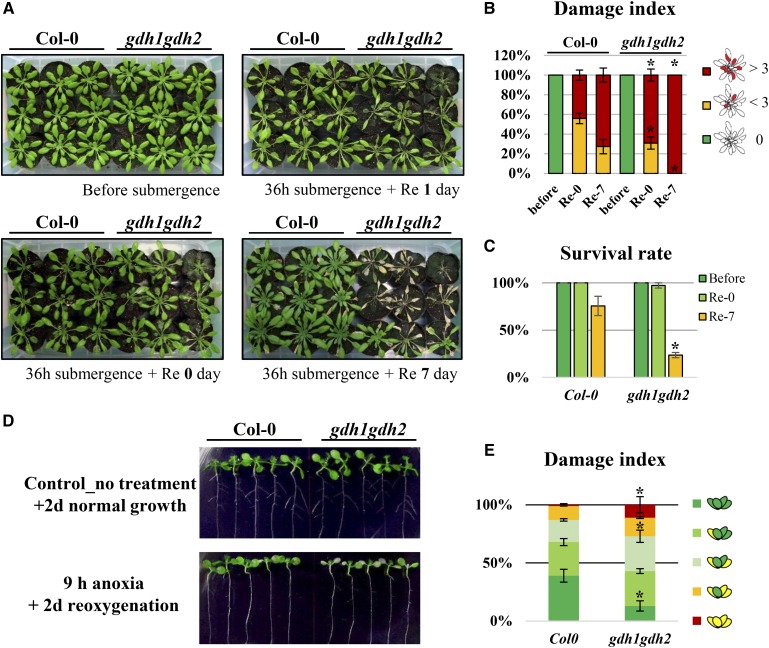
We next investigated whether the loss of GDH activity affects plant survival in response to low-oxygen stress via phenotypic resistance testing. Two well-established methods, complete submergence (Vashisht et al., 2011) and an anaerobic chamber system, were applied (see “Materials and Methods”). During submergence treatment, plants were kept under dark conditions, and postsubmerged plants were replaced in a regular short-day growth chamber after the treatment. Interestingly, except for a few partially damaged leaves, there was no obvious damage to the wild type and the gdh1gdh2 mutant after submergence. In contrast, both wild-type and gdh1gdh2 plants sustained damage during reoxygenation, and more rosette leaves became wilted each day in gdh1gdh2 than in the wild type (Fig. 8, A and B). Also, the survival rate at day 7 postsubmergence showed that the gdh1gdh2 mutant did not recover well during reoxygenation (Fig. 8C). In the anaerobic chamber system, we applied anoxia to 7-d-old seedlings and reaerated them with oxygen for 2 d. Young seedlings of gdh1gdh2, the sensitive phenotype, had more wilted leaves in response to A/R (Fig. 8, D and E). Our results demonstrate that GDH benefits recovery from limited oxygen stress.
Figure 8.
Phenotypes of the gdh1gdh2 mutant in response to oxygen deprivation. A, Five-week-old Arabidopsis plants were submerged in water for 36 h and reaerated for 7 d. B and C, Quantification of damage was performed based on the number of wilted leaves (B), and survival rate was determined by observing growth (growing corresponds to survival) after submergence (C). D, Seven-day-old seedlings were treated with 9 h of anoxia and reaerated for 2 d with the anaerobic chamber. E, Quantification of damage was measured based on the number of wilted leaves. The results are mean values of at least four biological replicates with se. Asterisks indicate P < 0.05 by Student’s t test.
DISCUSSION
EIN3-Driven Targets May Play Diverse Roles in Stress Adaption
The stabilization of EIN3 is a key step in the ethylene signaling pathway in plant responses to various abiotic and biotic stresses (Shi et al., 2012; Peng et al., 2014). Stabilization of EIN3 in response to submergence also was verified recently. EIN3 protein accumulated when plants were submerged in water (Xie et al., 2015). However, during A/R by anaerobic chamber, the level of EIN3 was much lower during anoxia, suggesting a deficiency of ethylene production in anoxic environments. The difference between submergence and the anaerobic chamber may be explained by the theory of trapping ethylene under water (Jackson, 1985; Voesenek et al., 1993; Vandenbussche et al., 2012). Ethylene is slightly soluble in water, so water restricts and traps ethylene in plant tissues during submergence. In contrast, in the anaerobic chamber system, pure nitrogen replacement may not lead to ethylene trapping. However, when we analyzed microarray data of the wild type and ein3eil1 under anoxia, we found that, even though the level of EIN3 did not increase during anoxia in the wild type, the basal level of EIN3 was able to trigger partial downstream anoxia responses, including several EIN3 targets (Fig. 2B). In contrast, the accumulation of EIN3 (Fig. 1) and decreased inductions of GDH1 and GDH2 in ein2-5 and ein3eil1 (Fig. 3) during reoxygenation demonstrate the involvement of ethylene signaling during this stage. Taken together, these results indicate that ethylene signaling should contribute its effects during both anoxia and reoxygenation conditions.
Several genes that encode metabolic enzymes also were found to be EIN3 targets, such as ARGININE DECARBOXYLASE2 (class I), which encodes an enzyme involved in Arg catabolism and polyamine biosynthesis (Hanfrey et al., 2001); FORMATE DEHYDROGENASE (class II), which encodes an enzyme involved in formate catabolism that was suggested to play an important role during reoxygenation (Tsai et al., 2014); TREHALOSE PHOSPHATE SYNTHASE10 (class II), which encodes an enzyme involved in trehalose biosynthesis; and GDH2 (class II), which plays an key role in maintaining C/N homeostasis (Fig. 2B; Supplemental Data Set S1). Interestingly, these metabolic targets illustrate that EIN3 not only functions as a central signaling hub to transduce signals but also controls the enzyme reactions that lead to stress adaptation.
Distinct Functions of GDH during Anoxia and Reoxygenation
GDH is a key enzyme in maintaining C/N. The isotopic tracing experiment and in vitro enzyme activity assays suggest that GDH might contribute to the regeneration of 2OG during the period of reoxygenation (Limami et al., 2008). The Arabidopsis mutants gdh1gdh2 and gdh1gdh2gdh3 have been used in functional studies of GDH under long-term dark stress (Miyashita and Good, 2008b; Fontaine et al., 2012). Global metabolite profiling and phenotypic testing in these mutants reveal an essential role of GDH in the adaptation of carbon starvation under long-term dark conditions. GDH plays roles in tricarboxylic acid cycle replenishment via its deamination enzyme activity (Miyashita and Good, 2008b; Fontaine et al., 2012). The majority of metabolic studies in higher plants suggest that GDH operates mainly in the direction of deamination (Robinson et al., 1992; Fox et al., 1995; Stewart et al., 1995; Glevarec et al., 2004; Labboun et al., 2009). However, GDH has been proposed to be involved in ammonium assimilation through the activity of amination in some specific organs or under certain physiological conditions, such as external ammonium application and salt stress (Yamaya and Oaks, 1987; Oaks, 1995; Melo-Oliveira et al., 1996; Skopelitis et al., 2006; Limami et al., 2008; Ferraro et al., 2015). An increased concentration of ammonium ions in cells may push the chemical reaction toward Glu synthesis. It is hypothesized that such a flexible and dynamic regulation by GDH of C/N may confer plant tolerance in response to fluctuating environments.
In this study, we examined the function of GDH in response to low oxygen using the gdh1gdh2 mutant. We found a reduction of GABA and a higher level of 2OG in gdh1gdh2 compared with the wild type during anoxia (Fig. 5). Although this implies that GDH might operate in the direction of amination to convert 2OG into Glu during anoxia, the levels of Glu did not show a clear difference between the wild type and gdh1gdh2. In addition, there was no significant difference in Ala accumulation between the wild type and gdh1gdh2 (Fig. 5). Therefore, we concluded that GDH might have limited or repressed amination activity to regenerate Glu during anoxia. This conclusion is supported by prior studies that involved isotope-labeling experiments to verify the direction of enzymatic reactions in vivo (Robinson et al., 1991; Limami et al., 2008; Labboun et al., 2009). On the other hand, AspAT has been considered to be the other key enzyme to provide Glu through degrading Asp during hypoxic stress. Increased AspAT activity under hypoxia has been verified (Rocha et al., 2010). Interestingly, in our metabolic analysis, a reduction of Asp was found during anoxia (Fig. 5; Supplemental Figs. S2 and S3), which suggests that AspAT might regenerate Glu that is required for the Ala accumulation. In contrast, our metabolic data showed a pronounced metabolic change in the gdh1gdh2 mutant during reoxygenation. Inefficient tricarboxylic acid cycle reoperation caused by insufficient replenishment of intermediates was observed in the gdh1gdh2 mutant (Fig. 5). To demonstrate that the changes of metabolites in gdh1gdh2 are specifically attributed to the loss of GDH enzyme activity, we checked the expression of genes that encode enzymes in the tricarboxylic acid cycle and determined the enzyme activities of AlaAT and AspAT in the wild type and gdh1gdh2 (Supplemental Fig. S4; Supplemental Data Set S3). Our results showed that the expression of genes involved in multiple metabolic reactions was almost the same as in the wild type, indicating that a lack of GDH activity does not dramatically influence transcriptional events related to anaerobic metabolism (Supplemental Data Set S3). For the enzyme activity assays, only slight reductions of AlaAT and AspAT could be observed in gdh1gdh2, and they were not as profound as GDH activity, which was almost abolished (Supplemental Fig. S4). Based on the metabolic flow, we propose that GDH might play a key role in refilling 2OG that could facilitate the Ala breakdown during reoxygenation. Loss of GDH activity resulted in the interruption of this metabolic cycle and led to a reduction in the chain for the regeneration of pyruvate, citric acid, and malate (Fig. 5; Supplemental Figs. S2 and S3). Moreover, misregulated metabolism in gdh1gdh2 eventually led to disturbances of carbohydrate metabolism, phytosterol biosynthesis, and energy regeneration (Figs. 6 and 7; Supplemental Figs. S5 and S6). α-Tocopherol and phytosterols are essential components for the stability of cell membranes, including chloroplast and mitochondria membranes (Hincha, 2008; Roche et al., 2008; Mlayeh et al., 2010). An increase of phytosterols is known to be an important adaptive mechanism to maintain membrane integrity and fluidity in response to large temperature variations (Dufourc, 2008). Thus, we suggested that a membrane repair system is activated at the recovery stage and that newly synthesized α-tocopherol and phytosterols could be utilized to fix and reinforce the damaged membranes caused by reoxygenation. Irregular recovery of phytosterols might cause cellular membrane damage. Taken together, this negative impact caused insufficient recovery from oxygen deprivation and resulted in the sensitive phenotype of gdh1gdh2 (Fig. 8).
GDH Is Involved in a Comprehensive Metabolic Network in Response to A/R
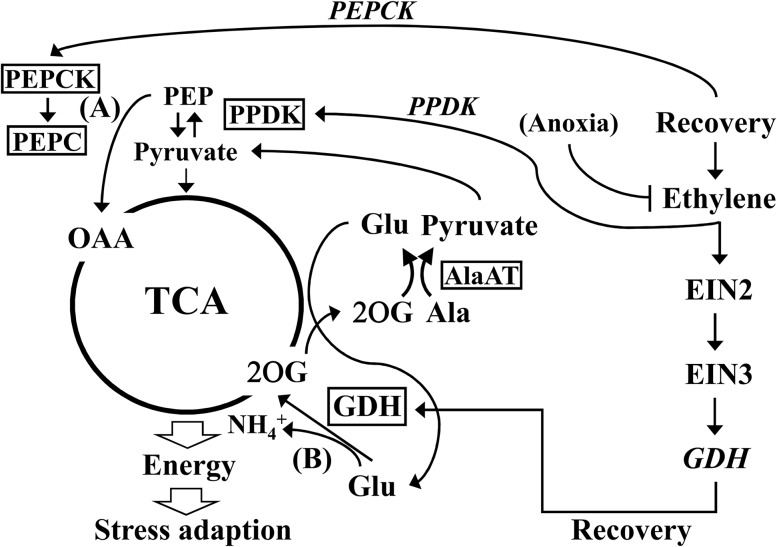
Metabolite profiling in response to hypoxic stress and reoxygenation has been performed in waterlogged Lotus japonicus (Rocha et al., 2010). The results suggest that Ala accumulation may serve as a sink that prevents the overaccumulation of pyruvate to further ensure that glycolysis proceeds. Ala accumulation also has been linked to the modular operation of a split tricarboxylic acid cycle, leading to additional ATP production under hypoxia (Rocha et al., 2010). In a recent study (António et al., 2016), 13C and 15N isotope-labeling experiments were conducted to evaluate the steady-state redistribution of metabolites throughout the metabolic network. The results strongly support the bifurcation of the tricarboxylic acid cycle. The isotope-labeling method also was applied in a study (Limami et al., 2008) to investigate the Ala and Glu metabolism in Medicago truncatula seedlings during hypoxia. That study sheds light on the connection between nitrogen and carbon metabolism during hypoxia and suggests the role of GDH during posthypoxia. Although the isotope-labeling experiment provides an overall picture of a reasonable metabolic flow, it is still difficult to directly detect the effects of specific enzymes. Through analyses of mutants, two studies (Miyashita et al., 2007; Miyashita and Good, 2008a) have shown that AlaAT and GAD enzymes contributed to Ala and GABA metabolism during hypoxia and recovery. Here, in our study, we further improved this metabolic model by including the role of GDH in response to low oxygen and recovery. By linking the molecular connection between ethylene signaling and the GDHs (Figs. 3 and 4), metabolic profiles (Figs. 5 and 6; Supplemental Figs. S2, S3, S5, and S6), and physiological data (Figs. 7 and 8), we proposed a comprehensive metabolic flow and connected it to ethylene signaling regulation (Fig. 9). This model highlights the Ala breakdown and tricarboxylic acid cycle replenishment. GDH plays a supportive role in resynthesizing 2OG that facilitates Ala degradation and further increases the tricarboxylic acid cycle flux for rapid recovery. We showed previously that, during the recovery period, ethylene signaling also is involved in the regulation of PPDK, which might participate in the alternative tricarboxylic acid cycle regeneration pathway (from pyruvate and then through PEP to OAA; Tsai et al., 2014). Therefore, we propose that ethylene signaling contributes to the regulation of tricarboxylic acid cycle replenishment through two entry points, OAA and 2OG (Fig. 9), and demonstrated the importance of GDH in response to A/R.
Figure 9.
Model of tricarboxylic acid (TCA) cycle replenishment in response to reoxygenation. To rapidly recover energy generation, tricarboxylic acid cycle replenishment is proposed to be essential during reoxygenation. Two metabolic routes for tricarboxylic acid cycle replenishment are suggested in this model. One was performed by Phosphoenolpyruvate carboxylase (PEPC), which converts Phosphoenolpyruvate (PEP) into OAA (A), and the other one was performed by GDH, which converts Glu into 2OG (B). In addition, PYRUVATE PHOSPHATE DIKINASE (PPDK), an enzyme that converts pyruvate into PEP during glycogenesis, might play a role in providing PEP at the recovery stage. Transcripts of PPDK and GDH are regulated by ethylene signaling during A/R. Increased transcripts of GDH lead to the enhanced enzymatic reaction in the direction of deamination to regenerate 2OG. 2OG can further react with Ala and facilitate Ala breakdown by the enzyme AlaAT and produce Glu and pyruvate. Pyruvate further reenters the tricarboxylic acid cycle, and Glu can be recycled through the enzyme GDH again. PEPCK, PEP carboxylase kinase.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and A/R Treatment
Arabidopsis (Arabidopsis thaliana) accession Columbia-0 and ein2-5, ein3eil1, and gdh1gdh2 mutants were used in this study. The mutant ein2-5 has a 7-bp deletion in the coding sequence at 939 to 945 that causes a frame-shift mutation (Alonso et al., 1999). ein3eil1 is a double mutant that is composed of two substitution mutants, ein3-1 and eil1-1 (Alonso et al., 2003). Both mutants present an ethylene-insensitive phenotype. gdh1gdh2 is a double mutant that is composed of gdh1-2 (SALK_042736) and gdh2-1 (SALK_102711), and the enzyme activity of GDH in the mutant is almost abolished (Miyashita and Good, 2008b). These mutant seeds were obtained from the Arabidopsis Biological Resource Center and were confirmed by PCR-based genotyping. The 35S::EIN3-GFP/ein3eil1 transgenic line was obtained from the laboratory of Dr. Hong-Wei Kuo (He et al., 2011). Seeds were sterilized by 0.5% sodium hypochlorite for 20 min and then sown on plates containing one-half-strength Murashige and Skoog (MS) medium (Duchefa Biochemie) with 0.55% phytagel and 0.5% Suc. The plates were further stored at 4°C in the dark for 3 d. After cold stratification, the plates were incubated at 22°C and placed in a vertical position in a growth chamber under a 16-h-light/8-h-dark photoperiod for 5 d with fluorescent lighting levels of 90 μE. Five-day-old seedlings were then transferred onto fresh plates for another 2 d before treatment. For A/R treatment, we followed the procedure described by Tsai et al. (2014). Seven-day-old Arabidopsis seedlings were placed into an anaerobic chamber with an anaerobic bag (MGC AnaeroPack; Mitsubishi Gas Chemical), which was then filled with pure nitrogen (100% N2) to replace the air in the chamber for 4 h. For reaeration, the lids of chambers were opened to replace the nitrogen atmosphere with air. For AgNO3 treatment, 7-d-old Arabidopsis seedlings were transferred onto fresh one-half-strength MS plates that contained 20 μm AgNO3 for 6 h pretreatment, and then seedlings were treated with anoxia and reoxygenation. For 1-MCP treatment, 7-d-old Arabidopsis seedlings were placed into an anaerobic chamber filled with 1-MCP gas (Lytone AnsiP) for 12 h pretreatment. Then, seedlings were further treated with anoxia and again put into an anaerobic chamber filled with 1-MCP under normoxia. All the A/R treatments were carried out in the dark at 22°C and were initiated in the morning (2 h after lights on).
Cross-Analysis of ChIP-seq Data and the A/R Transcriptome
The EIN3 candidate targets were extracted from Supplemental File S1 of Chang et al. (2013). Three groups of targets were defined in that report: EIN3-R (EIN3 targets, ethylene regulated), EIN3-NR (EIN3 targets, not ethylene regulated), and EIN3-ND (EIN3 targets, expression not detected). EIN3-R and EIN3-NR were selected for cross-analysis in this study. The A/R microarray (Tsai et al., 2014) was reanalyzed, and a comparison of transcripts at each time point with the normoxia was applied through statistical analysis (unpaired Student’s t test) and fold change selection (fold change of 2 or greater; P < 0.05). Venn diagram analysis was conducted using the software GeneSpring 11.5 (Agilent Technologies). The accessions of classified genes from the Venn diagram analysis were input into the agriGO analysis tool kit (Du et al., 2010) on the Web site http://bioinfo.cau.edu.cn/agriGO. The raw annotation data for Arabidopsis in agriGO were obtained from The Arabidopsis Information Resource (TAIR). Analyses were performed using the settings suggested by the singular enrichment analysis. Genes represented in significantly enriched GO terms (P < 0.05) are presented in Supplemental Data Set S2.
Protein Extraction and Immunoblotting
Total proteins from treated samples were extracted with the extraction buffer (4 m urea, 5% SDS, 15% glycerol, and 100 mm Tris-HCl, pH 7.8) with the addition of fresh proteinase inhibitor. Protein quantification was performed using the DC Protein Assay Kit (Bio-Rad). Eighty micrograms of total proteins was loaded onto an SDS-PAGE gel, and electrophoresis separation was performed (120 V, 90 min). Proteins on gels were transferred onto nitrocellulose membrane (Protran Nitrocellulose Transfer Membranes [PerkinElmer]; 250 mA, 90 min). Following transfer, 1% skim milk in Tris-buffered saline plus Tween 20 (TBST) buffer was used for 30 min of blocking. Then, the membrane was hybridized with anti-GFP antibody (catalog no. 11 814 460 001; Roche) for 90 min. After 15 min of TBST wash, anti-mouse horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) was used for another 90-min hybridization. The membrane was further developed using enhanced chemiluminescence substrates (SuperSignal West Dura Extended Duration Substrate; Thermo) after 15 min of TBST wash and detected through the FluorChem HD2 System (ProteinSimple). The signals were further quantified by the software GelQuant.NET (http://biochemlabsolutions.com/GelQuantNET.html).
RNA Extraction and Quantitative Reverse Transcription PCR
Total RNAs from treated samples were isolated using TRIzol reagent (Invitrogen). According to the manufacturer’s instruction, a 1:2 ratio of sample:TRIzol reagent was used to reach higher extraction efficiency. Five micrograms of extracted total RNA was converted to cDNA using M-MLV reverse transcriptase (Invitrogen) and oligo(dT) primer to generate 20 μL of cDNA stock. The cDNA stock was then diluted 50-fold. Quantitative reverse transcription PCR was performed with 9 μL of diluted cDNA, 0.5 μm of each primer, and SYBR Green PCR Master Mix (Applied Biosystems) on an ABI 7500 Real-Time PCR Machine (Applied Biosystems). Primers used for quantitative reverse transcription PCR are provided in Supplemental Data Set S3. At least four biological repeats were performed for each time point. We adopted a comparative cycle threshold method to measure the expression levels of each gene (Schmittgen and Livak, 2008). To normalize the data, TUB3 (AT5G62700) was used as an internal control. The expression level of target genes at normoxia in Col-0 was set as a reference (fold change = 1) to calculate the relative expression fold change at other time points or in different mutant strains.
In Vitro Enzyme Activity Assay
Protein samples were extracted with extraction buffer (for GDH and AlaAT, 50 mm Tris-HCl [pH 8]; for AspAT, 20 mm potassium phosphate buffer [pH 7.5], 1 mm EDTA, 5% [v/v] glycerol, 0.05% [v/v] Triton X-100, and 0.5% [w/v] polyvinylpyrrolidone 40), and fresh protease inhibitor was added before use. Fresh samples (100 mg) were ground in 200 μL of extraction buffer as described above. The debris was removed by centrifugation at 13,000g for 15 min. Protein concentrations were measured using the DC Protein Assay Kit (Bio-Rad). GDH amination activity was measured using substrate containing 100 mm Tris-HCl (pH 8), 1 mm CaCl2, 13 mm 2-oxoglutarate, 50 mm (NH4)2SO4, and 0.25 mm NADH (Glevarec et al., 2004). AlaAT activity was determined using substrates containing 100 mm Tris-HCl (pH 8), 25 mm Ala, 13 mm 2-oxoglutarate, 0.25 mm NADH, and 5 units of lactate dehydrogenase (Sigma L2375; modified from Good and Muench, 1992). AspAT activity was assayed with 50 mm potassium phosphate buffer (pH 7.5), 25 mm Asp, 13 mm 2-oxoglutarate, 0.25 mm NADH, and 5 units of L-malate dehydrogenase (Roche; modified from Griffith and Vance, 1989). Detection of the NADH at 340 nm was conducted using a microplate spectrophotometer (ENOC; BioTek). The reduction of 340-nm absorbance per minute and the molar extinction coefficient of NADH (∑340 = 6220 m−1 cm−1) were adopted to calculate the aminating activity per mg of total proteins.
Global Metabolite Profiling Assay by GC-TOF-MS
GC-TOF-MS was performed based on the protocol built by the Metabolomics Core Facility, Agricultural Biotechnology Research Center, Academia Sinica. Whole seedling tissues (80 mg) from 7-d-old seedlings of Col-0, gdh1gdh2, ein2-5, and ein3eil1 treated with A/R for 4 h were collected at different time points and ground in liquid nitrogen. Each sample was dissolved in 1 mL of 80% methanol with 12 μg mL−1 ribitol (internal standard) and sonicated for 30 min at 4°C. Samples were centrifuged at 13,000 rpm for 15 min at 4°C, and cell debris was discarded. Equal amounts of supernatant from each sample were collected and vacuum dried. Derivatization was performed with bis(trimethylsilyl)-trifluoroacetamide containing 1% trimethylchlorosilane. GC-TOF-MS analysis was performed using the Pegasus 4D GCxGC-TOF-MS system (LECO). The first dimension column was a Resteck RTX-5MS (30 m, 0.25 mm i.d., 0.25 mm d.f.), and the second dimension column was a Resteck RTX-200 (1.6 m, 0.18 mm i.d., 0.2 mm d.f.). Samples were run in one-dimensional mode, so the second dimension separation time was set to 0 s. Sample injection volume was 1 μL with helium as the carrier gas at a flow rate of 1 mL min−1. The gas chromatography oven temperature was held at 40°C for 5 min, programmed at 300°C, and held at 300°C for 8 min. The mass range collected was mass-to-charge ratio 50 to 800. Data processing and GC-TOF-MS parameter analysis were performed using LECO ChromaTOF software (version 4.43.3.0). Mass spectra were compared against the National Institute of Standards and Technology, LECO/Fiehn, and Wiley Registry Ninth Edition mass spectrometry libraries. Peak heights of the mass (mass-to-charge ratio) fragments were normalized to the internal standard (ribitol) and fresh weight of the samples. Metabolite profiles determined by GC-TOF-MS are presented in Supplemental Data Set S4.
Energy-Related Metabolite Quantification by LC-MS/MS
Whole seedlings (80 mg) from 7-d-old Col-0, ein2-5, ein3eil1, and gdh1gdh2 treated with A/R were collected and ground with liquid nitrogen. Subsequently, each sample was resuspended with 200 μL of 2.3% TCA with 40 μg mL−1 ribitol and centrifuged at 4°C for 15 min at the highest speed. The supernatants were recovered and neutralized to pH 6.5 to 7 with a few drops of 1 m KOH, then put in the ice bath for 30 min. After 30 min in the ice bath, the samples were centrifuged for 15 min at the highest speed at 4°C, and then the supernatants were collected for LC-MS/MS quantification. The liquid chromatography system used for analysis was the ultra-performance liquid chromatography system ACQUITY UPLC (Waters). The sample was separated with an ACQUITY UPLC BEH Amide column (particle size of 1.7 μm, 1 × 150 mm; Waters) at 80 μL min−1 flow rate using a 13-min gradient for analysis. Ammonium acetate (50 mm) in water was used as mobile phase A and acetonitrile was used as mobile phase B. The ultra-performance liquid chromatography system was coupled online to the TSQ Quantum Access Max triple quadrupole mass spectrometer (Thermo Fisher Scientific). The instrument was operated in multiple reaction monitoring mode. The signals of NAD+, NADH, AMP, ADP, and ATP were detected in negative ion mode. Ribitol was used as the internal standard. The chromatogram acquisition, detection of mass spectral peaks, and waveform processing were performed using Thermo Xcalibur 2.1 SP1 software (Thermo Scientific).
Phenotype of Low-Oxygen and Reoxygenation Stress
Two phenotypic tests were applied: submergence and the anaerobic chamber system. For submergence treatment, we followed the conditions stipulated in the report by Vashisht et al. (2011) with minor modifications. Short-day-grown (9 h of light/15 h of dark at 22°C), 5-week-old mature plants were used for submergence. The height of the water was 5 cm over the top of the shoot part, and submergence was performed and initiated in the morning (2 h after lights on) in the dark. Different durations of submergence were applied for the preliminary test. We selected 36 h as the treatment time, at which point a distinct phenotype could be observed. After submergence for 36 h, the plants were taken out and placed back into a growth chamber under 8 h of light/16 h of dark for reoxygenation. The phenotypes were photographed at different times after the onset of reoxygenation. For the anaerobic chamber system, we adopted the method that was used in our previous study (Tsai et al., 2014). Five-day-old seedlings on vertical one-half-strength MS plates were transferred to fresh plates before the treatment. After 2 d of recovery, 7-d-old seedlings were subjected to anoxia using the anaerobic chamber with anaerobic bags (MGC AnaeroPack; Mitsubishi Gas Chemical) in the dark. After testing different durations of treatment time, 9 h of anoxia treatment was chosen as being suitable for phenotype observation. After treatment, plates with seedlings were placed back into a growth chamber under long-day conditions for 2 d of reoxygenation and photographed. To quantify the damage in these two systems, wilting and necrotic leaves were counted.
Accession Numbers
Accession numbers of the genes used in this study are as follows: EIN2, AT5G03280; EIN3, AT3G20770; EIL1, AT2G27050; GDH1, AT5G18170; GDH2, AT5G07440; GDH3, AT3G03910; ACO2, AT1G62380; ERS1, AT2G40940; ERS2, AT1G04310; ETR2, AT3G23150; BEE1, AT1G18400; IAA29, AT4G32280; BGAL4, AT5G56870; AlaAT1, AT1G17290; ASP2, AT5G19550; ARGININE DECARBOXYLASE 2, AT4G34710; FORMATE DEHYDROGENASE, AT5G14780; TREHALOSE PHOSPHATE SYNTHASE 10, AT1G60140.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. EIN3 protein abundance during anoxia.
Supplemental Figure S2. Changes of metabolites in the wild type and ein2-5 in response to A/R.
Supplemental Figure S3. Changes of metabolites in the wild type and ein3eil1 in response to A/R.
Supplemental Figure S4. Enzyme activities of AlaAT and AspAT during A/R.
Supplemental Figure S5. Changes of carbohydrates, α-tocopherol, and phytosterols in the wild type and ein2-5 in response to A/R.
Supplemental Figure S6. Changes of carbohydrates, α-tocopherol, and phytosterols in the wild type and ein3eil1 in response to A/R.
Supplemental Data Set S1. Class II EIN3 targets and their 4-h A/R microarray normalized data.
Supplemental Data Set S2. Class I GO analysis by agriGO.
Supplemental Data Set S3. Gene expression of metabolic enzymes involved in tricarboxylic acid cycle and anaerobic metabolism in ein2-5 and ein3eil1.
Supplemental Data Set S4. Metabolic profiling using GC-TOF-MS.
Supplementary Material
Acknowledgments
We thank Dr. Hong-Wei Kuo of Peking University for providing the 35S::EIN3-GFP/ein3eil1 transgenic plants.
Glossary
- GABA
γ-aminobutyric acid
- 2OG
2-oxoglutarate
- A/R
anoxia-reoxygenation
- ChIP-seq
chromatin immunoprecipitation sequencing
- 1-MCP
1-methylcyclopropene
- Col-0
Columbia-0
- GO
Gene Ontology
- C/N
balance of carbon and nitrogen
- GC-TOF-MS
gas chromatography-time of flight-mass spectrometry
- OAA
oxaloacetate
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- PEP
phosphoenolpyruvate
- TBST
Tris-buffered saline plus Tween 20
Footnotes
This work was supported by the Ministry of Science and Technology, Taiwan (grant nos. 103–2321–B–001–012 and 106–2321–B–001–022).
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- António C, Päpke C, Rocha M, Diab H, Limami AM, Obata T, Fernie AR, van Dongen JT (2016) Regulation of primary metabolism in response to low oxygen availability as revealed by carbon and nitrogen isotope redistribution. Plant Physiol 170: 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Lee SC, Brinton E (2012) Waterproofing crops: effective flooding survival strategies. Plant Physiol 160: 1698–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniawska Z, Barratt DHP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49: 810–828 [DOI] [PubMed] [Google Scholar]
- Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9: 110–115 [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Kaiser KA, Jang CJH, Larive CK, Bailey-Serres J (2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J 56: 743–755 [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J (2005) Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot (Lond) 96: 647–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KN, Zhong S, Weirauch MT, Hon G, Pelizzola M, Li H, Huang SS, Schmitz RJ, Urich MA, Kuo D, et al. (2013) Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. eLife 2: e00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa CAF, Sodek L (2003) Alanine metabolism and alanine aminotransferase activity in soybean (Glycine max) during hypoxia of the root system and subsequent return to normoxia. Environ Exp Bot 50: 1–8 [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourc EJ. (2008) The role of phytosterols in plant adaptation to temperature. Plant Signal Behav 3: 133–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JM, Roberts TH, Atwell BJ (2012) Quantifying ATP turnover in anoxic coleoptiles of rice (Oryza sativa) demonstrates preferential allocation of energy to protein synthesis. J Exp Bot 63: 4389–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro G, D’Angelo M, Sulpice R, Stitt M, Valle EM (2015) Reduced levels of NADH-dependent glutamate dehydrogenase decrease the glutamate content of ripe tomato fruit but have no effect on green fruit or leaves. J Exp Bot 66: 3381–3389 [DOI] [PubMed] [Google Scholar]
- Fontaine JX, Tercé-Laforgue T, Armengaud P, Clément G, Renou JP, Pelletier S, Catterou M, Azzopardi M, Gibon Y, Lea PJ, et al. (2012) Characterization of a NADH-dependent glutamate dehydrogenase mutant of Arabidopsis demonstrates the key role of this enzyme in root carbon and nitrogen metabolism. Plant Cell 24: 4044–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GG, Ratcliffe RG, Robinson SA, Stewart GR (1995) Evidence for deamination by glutamate dehydrogenase in higher plants: commentary. Can J Bot 73: 1112–1115 [Google Scholar]
- Glevarec G, Bouton S, Jaspard E, Riou MT, Cliquet JB, Suzuki A, Limami AM (2004) Respective roles of the glutamine synthetase/glutamate synthase cycle and glutamate dehydrogenase in ammonium and amino acid metabolism during germination and post-germinative growth in the model legume Medicago truncatula. Planta 219: 286–297 [DOI] [PubMed] [Google Scholar]
- Good AG, Muench DG (1992) Purification and characterization of an anaerobically induced alanine aminotransferase from barley roots. Plant Physiol 99: 1520–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith SM, Vance CP (1989) Aspartate aminotransferase in alfalfa root nodules. I. Purification and partial characterization. Plant Physiol 90: 1622–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanfrey C, Sommer S, Mayer MJ, Burtin D, Michael AJ (2001) Arabidopsis polyamine biosynthesis: absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J 27: 551–560 [DOI] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, et al. (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460: 1026–1030 [DOI] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, et al. (2011) A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincha DK. (2008) Effects of alpha-tocopherol (vitamin E) on the stability and lipid dynamics of model membranes mimicking the lipid composition of plant chloroplast membranes. FEBS Lett 582: 3687–3692 [DOI] [PubMed] [Google Scholar]
- Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol 153: 757–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Ella ES, Vergara GV, Mackill DJ (2009) Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Ann Bot (Lond) 103: 197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB. (1985) Ethylene and responses of plants to soil waterlogging and submergence. Annu Rev Plant Physiol 36: 145–174 [Google Scholar]
- Juntawong P, Girke T, Bazin J, Bailey-Serres J (2014) Translational dynamics revealed by genome-wide profiling of ribosome footprints in Arabidopsis. Proc Natl Acad Sci USA 111: E203–E212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labboun S, Tercé-Laforgue T, Roscher A, Bedu M, Restivo FM, Velanis CN, Skopelitis DS, Moschou PN, Roubelakis-Angelakis KA, Suzuki A, et al. (2009) Resolving the role of plant glutamate dehydrogenase. I. In vivo real time nuclear magnetic resonance spectroscopy experiments. Plant Cell Physiol 50: 1761–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limami AM, Glévarec G, Ricoult C, Cliquet JB, Planchet E (2008) Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedlings under hypoxic stress. J Exp Bot 59: 2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Vantoai T, Moy LP, Bock G, Linford LD, Quackenbush J (2005) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137: 1115–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Oliveira R, Oliveira IC, Coruzzi GM (1996) Arabidopsis mutant analysis and gene regulation define a nonredundant role for glutamate dehydrogenase in nitrogen assimilation. Proc Natl Acad Sci USA 93: 4718–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchante C, Alonso JM, Stepanova AN (2013) Ethylene signaling: simple ligand, complex regulation. Curr Opin Plant Biol 16: 554–560 [DOI] [PubMed] [Google Scholar]
- Mlayeh L, Chatkaew S, Léonetti M, Homblé F (2010) Modulation of plant mitochondrial VDAC by phytosterols. Biophys J 99: 2097–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y, Dolferus R, Ismond KP, Good AG (2007) Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. Plant J 49: 1108–1121 [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Good AG (2008a) Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol 49: 92–102 [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Good AG (2008b) NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J Exp Bot 59: 667–680 [DOI] [PubMed] [Google Scholar]
- Mustroph A, Barding GA Jr, Kaiser KA, Larive CK, Bailey-Serres J (2014) Characterization of distinct root and shoot responses to low-oxygen stress in Arabidopsis with a focus on primary C- and N-metabolism. Plant Cell Environ 37: 2366–2380 [DOI] [PubMed] [Google Scholar]
- Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J (2010) Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol 152: 1484–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Zanetti ME, Jang CJ, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J (2009) Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Howell KA, Carroll A, Ivanova A, Millar AH, Whelan J (2009) Defining core metabolic and transcriptomic responses to oxygen availability in rice embryos and young seedlings. Plant Physiol 151: 306–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A. (1995) Evidence for deamination by glutamate dehydrogenase in higher plants: reply. Can J Bot 73: 1116–1117 [Google Scholar]
- Peng HP, Chan CS, Shih MC, Yang SF (2001) Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis. Plant Physiol 126: 742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Li Z, Wen X, Li W, Shi H, Yang L, Zhu H, Guo H (2014) Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet 10: e1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield DM, Crispi ML, Musgrave ME (1997) Changes in soluble sugar, starch, and alcohol dehydrogenase in Arabidopsis thaliana exposed to N2 diluted atmospheres. Plant Cell Physiol 38: 1354–1358 [DOI] [PubMed] [Google Scholar]
- Robinson SA, Slade AP, Fox GG, Phillips R, Ratcliffe RG, Stewart GR (1991) The role of glutamate dehydrogenase in plant nitrogen metabolism. Plant Physiol 95: 509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA, Stewart GR, Phillips R (1992) Regulation of glutamate dehydrogenase activity in relation to carbon limitation and protein catabolism in carrot cell suspension cultures. Plant Physiol 98: 1190–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M, Licausi F, Araújo WL, Nunes-Nesi A, Sodek L, Fernie AR, van Dongen JT (2010) Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol 152: 1501–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche Y, Gerbeau-Pissot P, Buhot B, Thomas D, Bonneau L, Gresti J, Mongrand S, Perrier-Cornet JM, Simon-Plas F (2008) Depletion of phytosterols from the plant plasma membrane provides evidence for disruption of lipid rafts. FASEB J 22: 3980–3991 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108 [DOI] [PubMed] [Google Scholar]
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S (2012) Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24: 2578–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopelitis DS, Paranychianakis NV, Paschalidis KA, Pliakonis ED, Delis ID, Yakoumakis DI, Kouvarakis A, Papadakis AK, Stephanou EG, Roubelakis-Angelakis KA (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18: 2767–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson R, Bailey-Serres J (2014) Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 111: 2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G, Shatilov V, Turnbull M, Robinson S, Goodall R (1995) Evidence that glutamate dehydrogenase plays a role in the oxidative deamination of glutamate in seedlings of Zea mays. Funct Plant Biol 22: 805–809 [Google Scholar]
- Tsai KJ, Chou SJ, Shih MC (2014) Ethylene plays an essential role in the recovery of Arabidopsis during post-anaerobiosis reoxygenation. Plant Cell Environ 37: 2391–2405 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vaseva I, Vissenberg K, Van Der Straeten D (2012) Ethylene in vegetative development: a tale with a riddle. New Phytol 194: 895–909 [DOI] [PubMed] [Google Scholar]
- van der Graaff E, Schwacke R, Schneider A, Desimone M, Flügge UI, Kunze R (2006) Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141: 776–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashisht D, Hesselink A, Pierik R, Ammerlaan JM, Bailey-Serres J, Visser EJ, Pedersen O, van Zanten M, Vreugdenhil D, Jamar DC, et al. (2011) Natural variation of submergence tolerance among Arabidopsis thaliana accessions. New Phytol 190: 299–310 [DOI] [PubMed] [Google Scholar]
- Voesenek L, Banga M, Thier RH, Mudde CM, Harren F, Barendse G, Blom C (1993) Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiol 103: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Bailey-Serres J (2013) Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol 16: 647–653 [DOI] [PubMed] [Google Scholar]
- Voesenek LA, Jackson MB, Toebes AHW, Huibers W, Vriezen WH, Colmer TD (2003) De-submergence-induced ethylene production in Rumex palustris: regulation and ecophysiological significance. Plant J 33: 341–352 [DOI] [PubMed] [Google Scholar]
- Voesenek LA, Sasidharan R (2013) Ethylene—and oxygen signalling—drive plant survival during flooding. Plant Biol (Stuttg) 15: 426–435 [DOI] [PubMed] [Google Scholar]
- Wheeler T, von Braun J (2013) Climate change impacts on global food security. Science 341: 508–513 [DOI] [PubMed] [Google Scholar]
- Xie LJ, Chen QF, Chen MX, Yu LJ, Huang L, Chen L, Wang FZ, Xia FN, Zhu TR, Wu JX, et al. (2015) Unsaturation of very-long-chain ceramides protects plant from hypoxia-induced damages by modulating ethylene signaling in Arabidopsis. PLoS Genet 11: e1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Yamaya T, Oaks A (1987) Synthesis of glutamate by mitochondria: an anaplerotic function for glutamate dehydrogenase. Physiol Plant 70: 749–756 [Google Scholar]
- Yang CY, Hsu FC, Li JP, Wang NN, Shih MC (2011) The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiol 156: 202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Guo HW (2011) Paradigms and paradox in the ethylene signaling pathway and interaction network. Mol Plant 4: 626–634 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.