A mutant in the thiamine biosynthesis gene THI1 reduces the size of root nodules and leads to high frequency of immature seeds in Lotus japonicus.
Abstract
Thiamine (vitamin B1) is essential for living organisms. Unlike animals, plants can synthesize thiamine. In Lotus japonicus, the expression of two thiamine biosynthesis genes, THI1 and THIC, was enhanced by inoculation with rhizobia but not by inoculation with arbuscular mycorrhizal fungi. THIC and THI2 (a THI1 paralog) were expressed in uninoculated leaves. THI2-knockdown plants and the transposon insertion mutant thiC had chlorotic leaves. This typical phenotype of thiamine deficiency was rescued by an exogenous supply of thiamine. In wild-type plants, THI1 was expressed mainly in roots and nodules, and the thi1 mutant had green leaves even in the absence of exogenous thiamine. THI1 was highly expressed in actively dividing cells of nodule primordia. The thi1 mutant had small nodules, and this phenotype was rescued by exogenous thiamine and by THI1 complementation. Exogenous thiamine increased nodule diameter, but the level of arbuscular mycorrhizal colonization was unaffected in the thi1 mutant or by exogenous thiamine. Expression of symbiotic marker genes was induced normally, implying that mainly nodule growth was delayed in the thi1 mutant. Furthermore, this mutant formed many immature seeds with reduced seed weight. These results indicate that thiamine biosynthesis mediated by THI1 enhances nodule enlargement and is required for seed development in L. japonicus.
Rhizobia associate with legumes and receive carbon sources from host plants via symbiotic organs (nodules), and in return they provide host plants with nitrogen sources generated by the fixation of atmospheric nitrogen (Oldroyd et al., 2011). Arbuscular mycorrhizal fungi also are plant microsymbionts and provide phosphate to host plants through symbiotic organs (arbuscules; Gutjahr and Parniske, 2013). Common genes are involved in both root nodule (RN) symbiosis and arbuscular mycorrhiza (AM), and more than 10 components are known to be involved in early symbiotic signaling or the formation of symbiotic organs (Gutjahr and Parniske, 2013). Additionally, many genes specific to each symbiosis have been identified (Oldroyd et al., 2011; Gutjahr and Parniske, 2013).
Thiamine (vitamin B1) is essential for living organisms. It is required for plant growth, but plants grow normally under thiamine-deficient conditions because, like microorganisms, they can synthesize thiamine (Jurgenson et al., 2009). The thiamine biosynthesis pathway is well studied in prokaryotes such as Escherichia coli and Bacillus subtilis but not in yeasts and plants (Jurgenson et al., 2009). Thiamine is assembled from pyrimidine and thiazole moieties synthesized independently (Jurgenson et al., 2009; Goyer, 2010). Although the condensation of pyrimidine and thiazole moieties is similar in prokaryotes and eukaryotes, the details of biosynthesis of each moiety differ. In plants, THIC and THID/E catalyze the biosynthesis of the pyrimidine moiety, whereas THI1 and THIM catalyze that of the thiazole moiety synthesized in chloroplasts (Supplemental Fig. S1; Julliard and Douce, 1991; Ogata et al., 1999; Papini-Terzi et al., 2003; Kong et al., 2008; Yazdani et al., 2013). After condensation of the two moieties by THID/E, thiamine pyrophosphate kinase (THIPPK) and thiamine triphosphatase (THITPase) catalyze thiamine conversion to its active form, thiamine pyrophosphate (TPP). Multiple enzymes require TPP, for example those essential for obtaining energy by respiration and photosynthesis (such as pyruvate dehydrogenase in mitochondria and chloroplasts and trans-ketolase in chloroplasts) and enzymes involved in amino acid synthesis (Goyer, 2010). Tissues and organs with high cell division activity require thiamine. Maize (Zea mays) THI2, a paralog of Arabidopsis (Arabidopsis thaliana) THI1, is highly expressed in dividing tissues (shoot apical meristem, immature tassel, and leaf primordium) and regulates organ formation (Woodward et al., 2010). The effects of thiamine in plants have been studied mainly in aerial tissues. Most articles have reported that thiamine deficiency leads to chlorotic leaves (Papini-Terzi et al., 2003; Ajjawi et al., 2007a, 2007b; Kong et al., 2008; Yazdani et al., 2013). However, the role of thiamine biosynthesis genes in roots remains uncertain.
In RN symbiosis, high THI1 expression in nodules of Alnus glutinosa was reported (Ribeiro et al., 1996), but the function of thiamine in nodule organogenesis remains uninvestigated. Nodule organogenesis is initiated by the RN symbiotic signals, known as Nod factors. Initial division occurs in cortical cells and continues until nodule formation (Oldroyd et al., 2011). The AM fungus Rhizophagus irregularis was reported to lack genes encoding enzymes of the thiamine biosynthesis pathway, and thiamine is considered as an essential nutrient provided by the host plant for AM fungal growth (Tisserant et al., 2013). These facts suggest that thiamine plays important roles in symbioses. In this study, we examined the functions of the THI1 gene in RN and AM symbioses and demonstrated that THI1 and thiamine promote nodule formation.
RESULTS
The Thiamine Biosynthesis Genes THI1 and THIC Are Induced during RN Symbiosis But Not AM
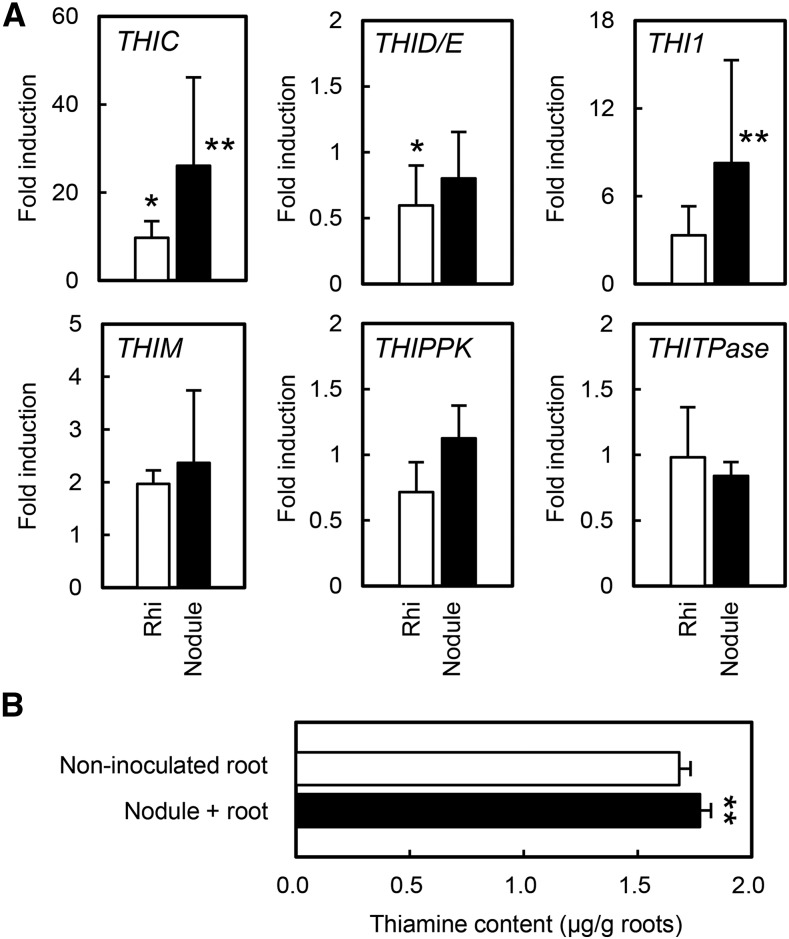
The expression of THIC, THID/E, THI1, THIM, THIPPK, and THITPase during RN symbiosis and AM was analyzed by quantitative real-time (qRT)-PCR. During RN symbiosis, the expression of THIC and THI1, but not other thiamine biosynthesis genes, was enhanced in infected roots and nodules, whereas the expression of THID/E was slightly down-regulated in whole roots but not in nodules (Fig. 1A). To confirm the effect of the induction of thiamine biosynthesis genes, we measured thiamine content in the root during RN symbiosis. Thiamine content was higher in nodulated roots than in uninoculated roots (Fig. 1B). During AM, none of the thiamine biosynthesis genes were induced, but THIC was down-regulated slightly (Supplemental Fig. S2). We focused on THIC and THI1 during RN symbiosis, because their expression was clearly induced in nodules (greater than five times). In Lotus japonicus, THIC is a single-copy gene, whereas THI1 has a paralog, THI2. According to the Lotus japonicus Gene Expression Atlas (LjGEA; Verdier et al., 2013), THIC is expressed in leaves and nodules, THI1 mainly in roots and nodules, and THI2 in flowers and leaves but not in roots (Supplemental Fig. S3).
Figure 1.
Expression of thiamine biosynthesis genes and thiamine content during RN symbiosis. A, Expression levels in whole roots (Rhi) and nodules (Nodule) were measured 2 weeks after inoculation with M. loti. Expression was normalized to that in noninoculated roots. Error bars indicate sd (n = 3). Asterisks indicate significant differences from noninoculated roots (Student’s t test: *, P < 0.05 and **, P < 0.01). B, Thiamine content in roots was measured 3 weeks after inoculation with (Nodule + root) or without (Non-inoculated root) M. loti. Error bars indicate sd (n = 3 or 4). Asterisks indicate significant differences from noninoculated roots (Student’s t test: **, P < 0.01).
Thiamine Promotes Nodule Growth But Does Not Affect AM Colonization
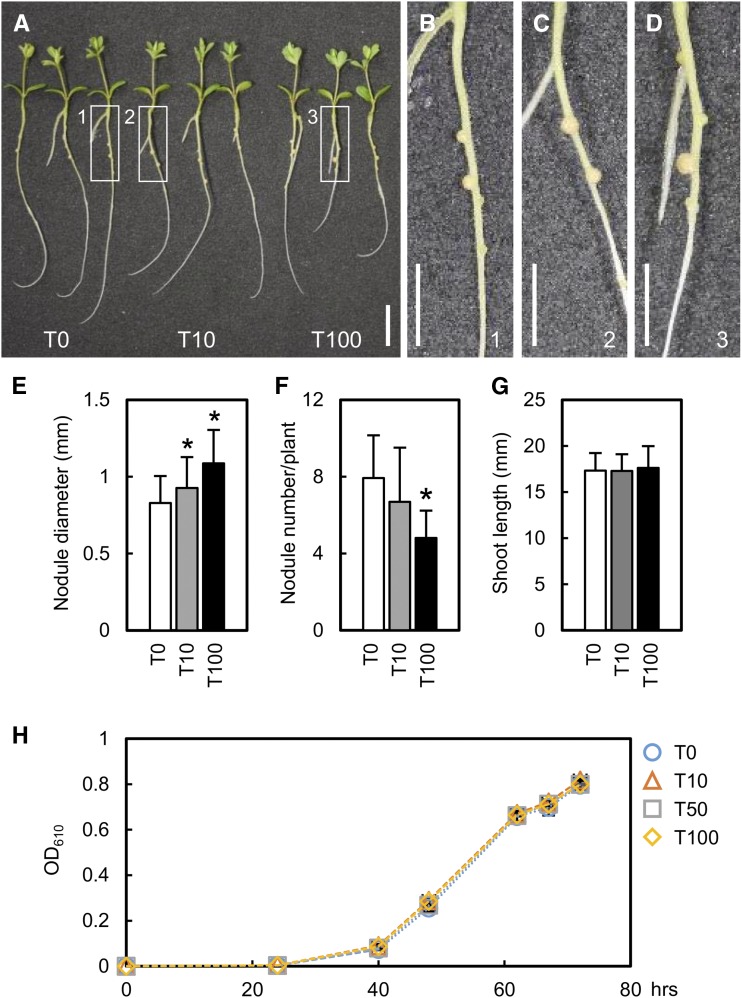
We evaluated the effect of exogenous thiamine on RN symbiosis at three concentrations, 0 (T0), 10 (T10), and 100 (T100) mg L−1. Nodules of plants treated with T10 and T100 were larger than those of the untreated plants (Fig. 2, A–D). Nodules were classified into six groups according to their diameter, as shown in Supplemental Figure S4. Group 5 nodules (with a diameter from 1 mm to 1.2 mm) were observed in thiamine-treated plants 10 d after inoculation (dai; Supplemental Fig. S4, B and C) but not in untreated plants (Supplemental Fig. S4A). In thiamine-treated plants, the frequency of large nodules (groups 4–6; diameter greater than 0.8 mm) increased further at 14 and 21 dai (Supplemental Fig. S4, D–I); both median and maximum nodule diameters were larger than those in untreated plants (Supplemental Fig. S4J). For simplicity, only the diameter of the largest nodule was measured in each plant. The maximum nodule diameter was larger in thiamine-treated plants than in untreated plants (Fig. 2E). The increase in maximum nodule diameter from 10 to 14 dai in thiamine-treated plants was faster than that in untreated plants (Supplemental Fig. S5). Thus, thiamine treatment accelerated nodule enlargement. Shoot length was not affected by thiamine treatment (Fig. 2G), but the nodule number was decreased significantly at T100 (Fig. 2F). An increase in nodule number may lead to smaller nodules (Nishimura et al., 2002), so we used the hypernodulating plenty mutant (Yoshida et al., 2010) to assess whether nodule enlargement by exogenous thiamine was caused by the decrease in nodule number. In this mutant, exogenous thiamine induced nodule enlargement (Supplemental Fig. S6A) with no significant effect on nodule number (Supplemental Fig. S6B). This result suggests that exogenous thiamine, rather than a decrease in nodule number, led to nodule enlargement. Furthermore, nodule growth in the thiamine-treated plenty mutant from 10 to 14 dai also was faster than that in the untreated plenty mutant, and the nodule size became the same as that of untreated nodules at 21 dai (Supplemental Fig. S6D), implying that exogenous thiamine promotes nodule enlargement. Rhizobial growth in liquid culture was not affected by thiamine treatment (Fig. 2H). In AM, on the contrary, thiamine treatment did not change the levels of internal hyphal and arbuscular colonization (Supplemental Fig. S7, A and B). These results indicate that thiamine promotes nodule growth but not AM colonization.
Figure 2.
Effects of exogenous thiamine on nodulation, shoot length, and rhizobial growth. A, Thiamine-treated and untreated wild-type Gifu plants 2 weeks after inoculation with M. loti. Thiamine was used at 0 (T0), 10 (T10), or 100 (T100) mg L−1. Bar = 1 cm. B to D, Enlargements of the areas in white boxes 1, 2, and 3, respectively, in A. Bars = 5 mm. E to G, Maximum nodule diameter (E), total nodule number (F), and shoot length (G) in thiamine-treated and untreated plants. Error bars indicate sd (n = 30–32). Significant differences from untreated plants are shown (Student’s t test: *, P < 0.05). H, Effect of thiamine on rhizobial growth. OD610, Optical density at 610 nm. Error bars indicate sd (n = 3).
Shoot Phenotypes in Plants with Knockdown and Knockout of Thiamine Biosynthesis Genes
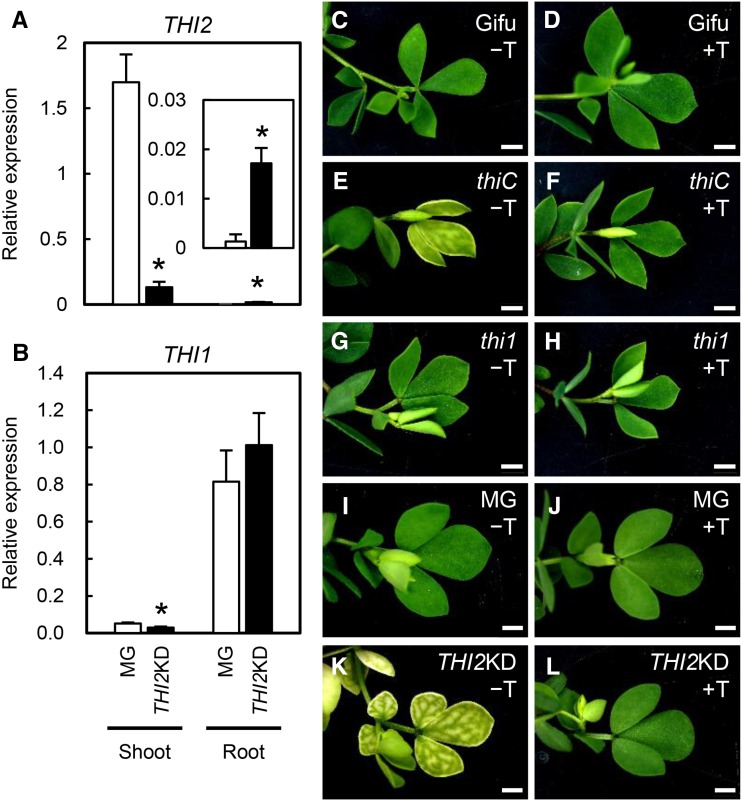
To further investigate the effects of thiamine, we used thiC and thi1 mutant plants with an inserted retrotransposon (Lotus Retrotransposon1 [LORE1]; Fukai et al., 2012; Urbański et al., 2012) and THI2-knockdown plants (THI2KD) generated in this study using RNA interference (THI2-RNAi construct). In accordance with the LjGEA data (Supplemental Fig. S3), THI2 expression was high in shoots but very low in roots (Fig. 3A), whereas THI1 expression was low in shoots but high in roots (Fig. 3B). In THI2KD shoots, THI2 expression was about 10% of that in wild-type shoots (Fig. 3A), suggesting that THI2 RNA interference effectively repressed THI2 in shoots. THI1 expression in shoots and THI2 expression in roots also were affected in THI2KD plants, but the absolute expression levels were very low in these organs.
Figure 3.
Leaf phenotypes in THI2 knockdown plants and the LORE1 insertion mutants thiC and thi1. A and B, Expression of THI2 (A) and its paralog THI1 (B) in MG (control) and THI2KD plants 3 weeks after inoculation with rhizobia. THI2 expression in roots is shown in the inset. Expression levels were normalized to that of the ubiquitin gene. Error bars indicate sd (n = 3–5). Significant differences from MG plants are shown (Student’s t test: *, P < 0.05). C to L, LORE1 insertion thiC and thi1 mutants and THI2KD were either untreated (−T) or treated with thiamine at 50 mg L−1 (+T). Gifu, L. japonicus B129 Gifu (the wild type for LORE1 insertion mutants); MG, L. japonicus MG-20 Miyakojima (the wild type for THI2KD). Bars = 2 mm.
The thiC mutant and THI2KD plants were chlorotic, but their veins were still green (Fig. 3, E and K). Their shoot growth was inhibited and their shoots became withered. Lateral buds appeared but soon died, and no flower initiation (and, therefore, no seed production) was observed. These phenotypes were rescued by exogenous thiamine treatment (Fig. 3, F and L). These results suggest that THIC and THI2 function in shoots and that the loss of function was lethal. We confirmed the nodulation phenotype of the thiC mutant, which has a LORE1 insertion in the N-terminal domain (Supplemental Fig. S8A). Nodulation was lower (Supplemental Fig. S8B) and shoots were shorter in the thiC mutant than in wild-type segregant (WTseg) plants (Supplemental Fig. S8C); the latter effect was presumably caused by chlorosis (Supplemental Fig. S8, D and E). Therefore, it is not clear whether the nodulation defect in the thiC mutant is caused by the loss of THIC function in nodule development or by the lethal deficiency in nutrients derived from shoots. In contrast, the thi1 mutant had green leaves regardless of whether exogenous thiamine was used (Fig. 3, G and H). Analyses of the THI1 expression pattern and the thi1 shoot and leaf phenotype implied that THI1 regulates thiamine biosynthesis in roots but not in aboveground parts.
THI1 Is Highly Expressed in Cortical Cells of Nodule Primordia
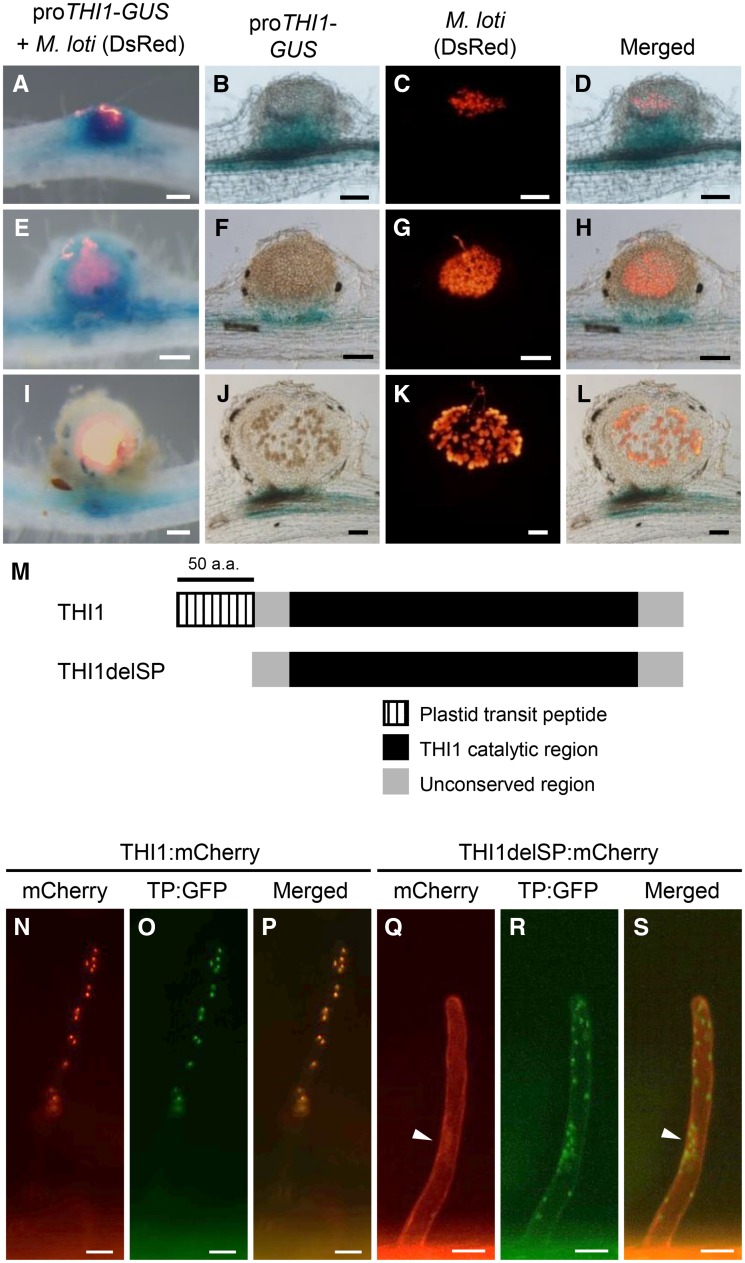
To reveal the spatiotemporal expression patterns of THI1, we analyzed GUS activity in the roots of wild-type plants transfected with the proTHI1-GUS construct, which contained the THI1 promoter region fused to the GUS gene, and inoculated with Mesorhizobium loti. Strong GUS activity was detected in nodule primordia and in whole young nodules (Fig. 4, A and E), but no GUS activity was observed in mature nodules except for their basal region (Fig. 4I). In sliced nodules, GUS activity was detected in dividing cortical cells but not in infected cells, in which the rhizobia differentiated into bacteroids (Fig. 4, B–D and F–H). In mature nodules, the region of GUS staining was smaller and restricted to the basal region of the nodules (Fig. 4, J–L).
Figure 4.
Spatiotemporal expression of THI1 during RN symbiosis and subcellular localization of THI1. GUS staining is shown for transgenic hairy roots carrying the proTHI1-GUS construct inoculated with M. loti expressing DsRed. A, E, and I, Whole nodules. B to D, F to H, and J to L, Nodule sections of 100 μm. A to D, Nodule primordia. E to H, Young nodules. I to L, Mature nodules. Bars = 100 μm. M, Schematic diagram of the THI1 structure. The truncated protein THI1delSP has a deletion of the plastid transit peptide (50 amino acids [a.a.]). N to P, Coexpression of THI1:mCherry and GFP fused with a fragment encoding the plastid transit peptide of Arabidopsis Rubisco (TP:GFP) in L. japonicus hairy roots. Q to S, Coexpression of THI1delSP:mCherry and TP:GFP. Arrowheads indicate the nucleus. Bars = 20 μm.
THI1 Is Targeted to Plastids by the N-Terminal Plastid Transit Peptide
L. japonicus THI1 contains a predicted N-terminal plastid transit peptide (50 amino acids) and a catalytic domain homologous to that of Arabidopsis THI1 (Fig. 4M). To investigate the localization of THI1, we coexpressed the THI1:mCherry construct (THI1 fused with mCherry driven by the 35S promoter) and the TP:GFP construct (GFP fused with the plastid transit peptide of Arabidopsis Rubisco [Niwa et al., 1999]) in transgenic hairy roots (Fig. 4, N–P). Merged mCherry and GFP images demonstrated that the two proteins were colocalized in punctate structures, presumably plastids (Fig. 4P). Deletion of the N-terminal 50 amino acids of the THI1 protein (THI1delSP; Fig. 4M) caused the loss of plastid localization, and the truncated THI1:mCherry fluorescence (THI1delSP:mCherry) was observed in the nucleus and cytosol (Fig. 4, Q–S). These results indicate that THI1 is localized in the plastids and that its localization is mediated by the N-terminal domain.
The thi1 Mutant Has Small Nodules But Normal Mycorrhizal Colonization
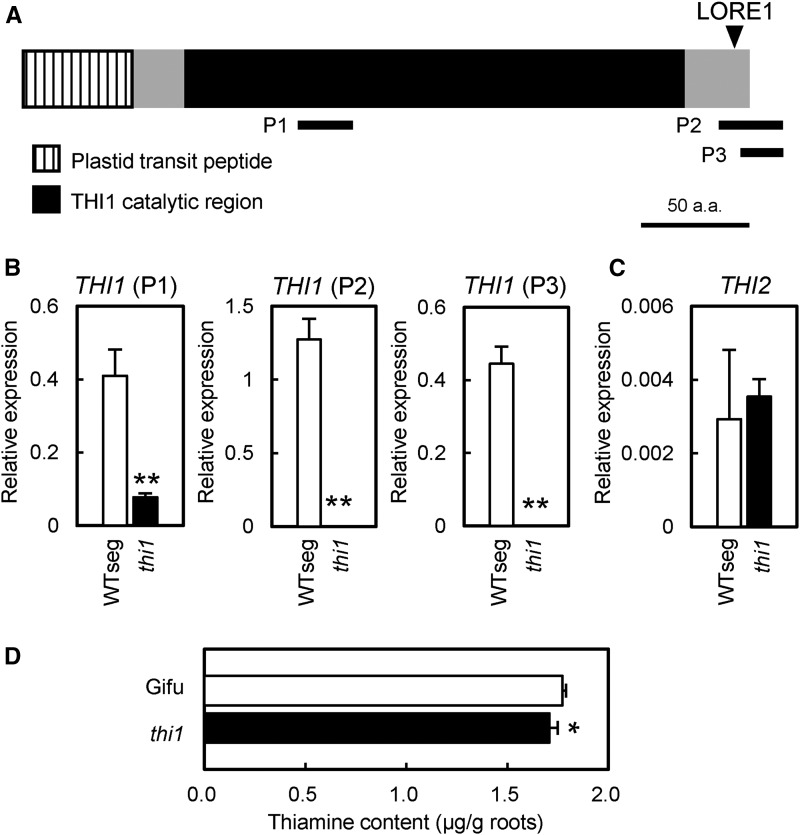
The site of LORE1 insertion in the thi1 mutant corresponds to the C-terminal nonconserved domain of THI1 (Fig. 5A). L. japonicus THI1 has a highly conserved catalytic domain (Supplemental Fig. S9; Godoi et al., 2006). LORE1 insertion is downstream of any conserved amino acid residues important for THI1 structure and, thus, may not completely disrupt THI1 function. We examined THI1 transcript levels in roots of the thi1 mutant by qRT-PCR using THI1-specific primer sets positioned upstream (P1) or downstream (P2 and P3) of the LORE1 insertion site (Fig. 5A). No THI1 expression was detected with P2 and P3, whereas the transcript level detected with P1 was approximately 25% of that in a WTseg plant selected from thi1 F2 populations backcrossed to wild-type Gifu (Fig. 5B). The expression of the paralogous gene THI2 was unchanged (Fig. 5C), indicating that the defect in THI1 did not affect THI2 expression. These results suggest that LORE1 at least considerably decreased the THI1 transcript level, possibly because of transcriptional repression or loss of mRNA stability. To confirm the defect in THI1 function in the thi1 mutant, we measured thiamine content in whole roots. During RN symbiosis, thiamine content was lower in the thi1 mutant than in the wild type (Fig. 5D). There is a possibility that the reduced nodule size in the thi1 mutant affected thiamine content. However, the contribution of nodule biomass to total biomass is very small; therefore, the effect of the nodule size difference on whole-root thiamine content should be negligibly small. Therefore, we conclude that the thi1 mutant has reduced thiamine biosynthesis in roots during RN symbiosis.
Figure 5.
THI1 expression and thiamine content in thi1 mutant roots. A, THI1 structure and position of the LORE1 insertion in the thi1 mutant. P1, P2, and P3 indicate the positions of fragments amplified with primer sets for qRT-PCR. a.a., Amino acids. B, THI1 expression in the thi1 mutant 2 weeks after inoculation with M. loti. P1, P2, and P3 indicate data obtained with the respective primer sets shown in A. Error bars indicate sd (n = 3). Significant differences from WTseg are shown (Student’s t test: **, P < 0.01). C, THI2 expression in the thi1 mutant. Expression levels were normalized to that of ubiquitin. Error bars indicate sd (n = 3). D, Thiamine content in thi1 mutant roots 3 weeks after inoculation with M. loti. Error bars indicate sd (n = 4). A significant difference from wild-type Gifu is shown (Student’s t test: *, P < 0.05).
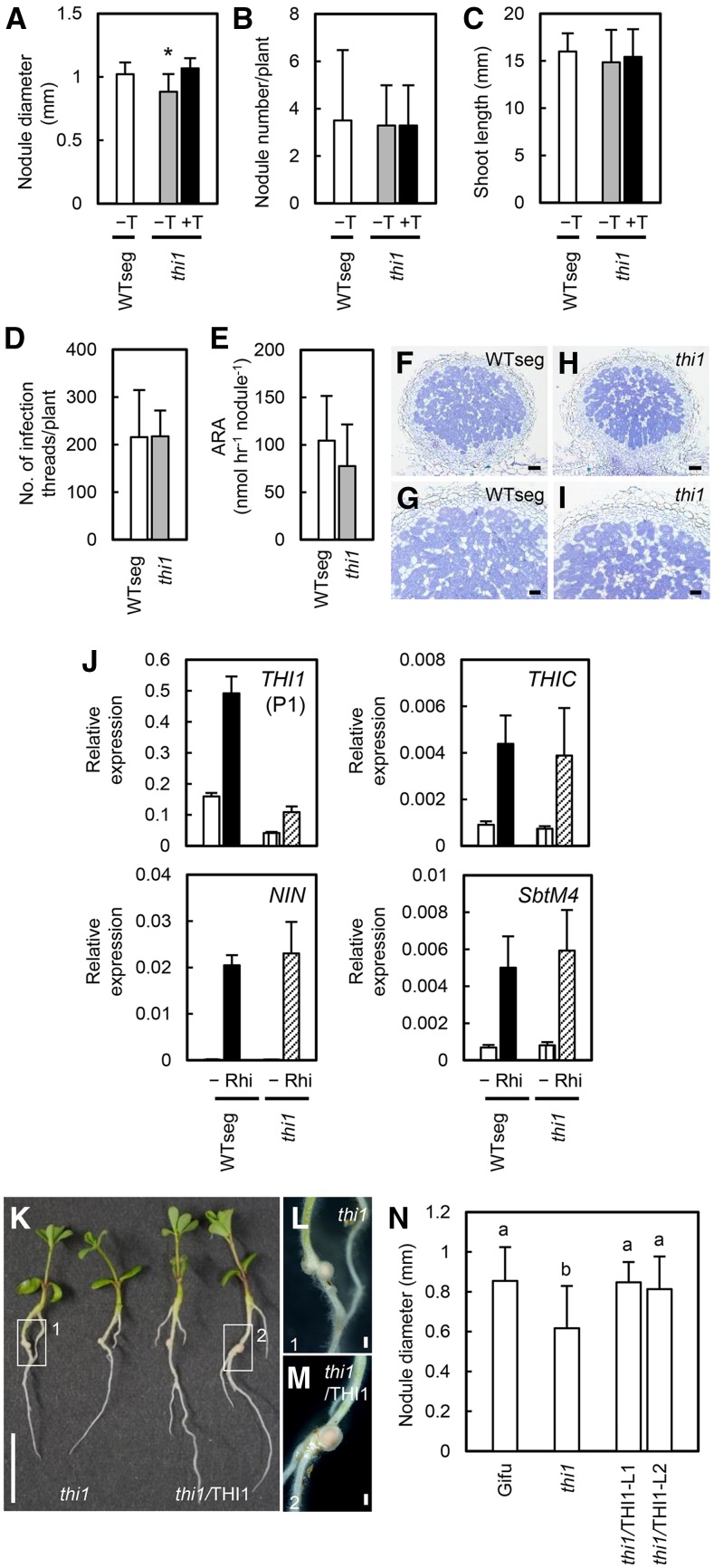
To investigate the role of THI1 during RN symbiosis, we examined the nodule size in the thi1 mutant. The maximum nodule diameter was smaller in the thi1 mutant than in WTseg; this phenotype was rescued by exogenous thiamine treatment (Fig. 6A). In agreement with the growth promotion by thiamine treatment (Supplemental Figs. S5 and S6D), nodule growth from 10 to 14 dai was slower in the thi1 mutant than in both WTseg and the thiamine-treated thi1 mutant (Supplemental Fig. S10). No differences in nodule number (Fig. 6B), shoot length (Fig. 6C), infection thread number (Fig. 6D), acetylene reduction activity (ARA; Fig. 6E), or nodule development (Fig. 6, F–I) were detected between the thi1 mutant and WTseg. The expression of THIC and RN-responsive genes was induced normally in the thi1 mutant (Fig. 6J), implying that THI1 regulates nodule enlargement but not other developmental processes. In mutant nodules, infected cells (Fig. 6, H and I) and ARA (Fig. 6E) were normal, suggesting that the development of symbiotic rhizobia was not affected by thiamine outside the rhizobia. To verify the contribution of THI1, we produced THI1-complemented transgenic plants. The nodules of these plants were significantly larger than those of the thi1 mutant and similar to wild-type nodules (Fig. 6, K–N).
Figure 6.
RN symbiotic phenotype of the thi1 mutant. A, Maximum nodule diameter. B, Total nodule number. C, Shoot length. D, Infection thread number. E, ARA. F to I, Nodule sections of WTseg and the thi1 mutant 1 week (D) or 3 weeks (A–C and E–I) after inoculation with M. loti without (−T) or with thiamine treatment (+T; final concentration, 100 mg L−1). Nodule sections were stained with Toluidine Blue. Error bars indicate sd (n = 6–12). Significant differences are shown (Student’s t test: *, P < 0.05). Bars = 100 μm in F and H and 50 μm in G and I. J, Expression levels of THI1, THIC, and RN marker genes (NIN and SbtM4) 2 weeks after inoculation with M. loti determined by qRT-PCR in noninoculated (−) and inoculated (Rhi) roots of WTseg and the thi1 mutant. Expression levels were normalized to that of ubiquitin. Error bars indicate sd (n = 3). K, The thi1 mutant and THI1-complemented plants (thi1/THI1) 10 d after inoculation. Bar = 1 cm. L and M, Enlargements of the areas in boxes 1 and 2, respectively, in K. Bars = 500 μm. N, Maximum nodule diameter in wild-type (Gifu), thi1, and thi1/THI1 plants. L1 and L2 indicate independent transgenic lines. Error bars indicate sd (n = 9–13). Different lowercase letters show significant differences in the Tukey-Kramer test (P < 0.05).
We also evaluated the AM phenotype of the thi1 mutant. The morphology of symbiotic structures (Supplemental Fig. S11, A–D) and both hyphal and arbuscular colonization levels (Supplemental Fig. S11, E and F) remained unchanged in comparison with those in WTseg.
The thi1 Mutant Has Defective Seed Maturation
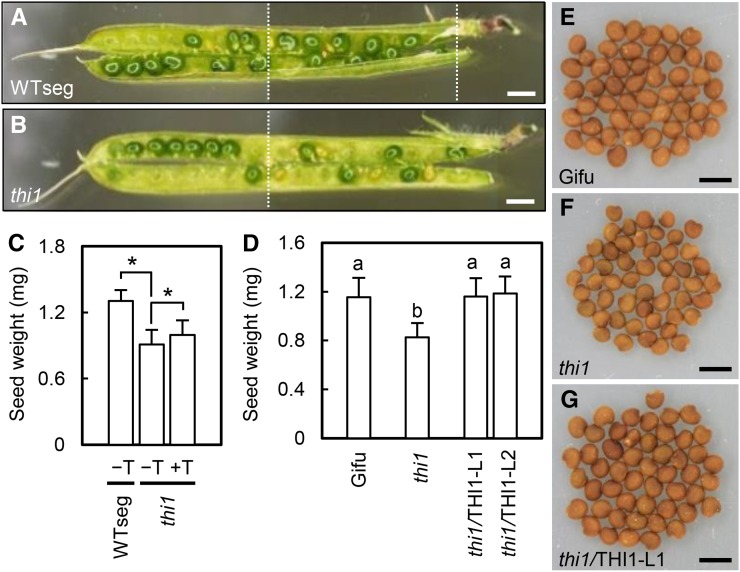
Exogenous thiamine treatment was required to obtain mature seeds of the thi1 mutant (Fig. 7). Genotyping of F2 progeny of backcrossed F1 populations (heterozygous lines 1 and 2) identified 10 wild-type, 19 heterozygous, and two homozygous mutant plants in line 1 and 10 wild-type, 18 heterozygous, and four homozygous mutant plants in line 2. The numbers of wild-type, heterozygous, and homozygous plants were expected to show a 1:2:1 ratio in the F2 population, but the number of homozygous mutants was greater than 50% lower than expected for both lines. Although the THI1 gene is expressed mainly in roots and nodules (Supplemental Fig. S3), the level of the THI1 transcript in seeds is higher than that of THI2, according to LjGEA (Supplemental Fig. S12). Immature seeds were more frequently observed in pods of the thi1 mutant than in those of WTseg (Fig. 7, A and B), although the seed number per pod was not affected (Supplemental Fig. S13). We compared mature seeds between the thi1 mutant and WTseg. Seed weight was lower in the thi1 mutant than in WTseg, and exogenous thiamine rescued this defect (Fig. 7C). In addition, seed weight was increased by THI1 complementation (Fig. 7, D–G). These results suggest that thiamine biosynthesis mediated by THI1 is required for seed development.
Figure 7.
Seed phenotype of the thi1 mutant. A and B, Pods of WTseg and the thi1 mutant. The images were assembled from partial images (the borders are indicated by dotted lines). Bars = 2 mm. C, Dry weight per seed of WTseg and the thi1 mutant with (+T) or without (−T) thiamine treatment at 50 mg L−1. Error bars indicate sd (n = 15–20). Significant differences are shown (Student’s t test: *, P < 0.05). D, Dry weight per seed of control plants (wild-type Gifu and thi1 mutant) and THI1-complemented plants (thi1/THI1). Error bars indicate sd (n = 20). Different lowercase letters show significant differences in the Tukey-Kramer test (P < 0.05). E to G, Seeds of wild-type Gifu (E), the thi1 mutant (F), and thi1/THI1 (G). Bars = 2 mm.
DISCUSSION
In plants, thiamine deficiency causes a lack of chlorophylls and carotenoids and results in chlorotic leaves (McHale et al., 1988; Ajjawi et al., 2007a, 2007b; Kong et al., 2008). In line with these reports, we observed that knockout or knockdown of the thiamine biosynthesis genes THIC and THI2 expressed in aerial tissues led to chlorotic leaves in L. japonicus (Fig. 3; Supplemental Fig. S3). The thiC mutant had low nodulation (Supplemental Fig. S8B), but we cannot exclude that a severe growth defect caused by chlorosis reduced nodulation (Supplemental Fig. S8, D and E). Therefore, we focused on THI1, which is expressed in different tissues from those where THI2 is expressed and presumably plays a different role. Our results provide new insights into the function of thiamine biosynthesis in the nodule development of L. japonicus.
Nodule Enlargement Is Enhanced by THI1 or Thiamine
Here, we demonstrated that exogenous thiamine promotes nodule enlargement during early stages of nodule growth (Fig. 2; Supplemental Figs. S5 and S6). We also showed that nodule growth was slower in the thi1 mutant than in wild-type plants and thiamine-treated thi1 mutant plants (Fig. 6A; Supplemental Fig. S10), but the formation of infection threads, ARA, infected cells, and RN marker gene expression in the thi1 mutant were normal (Fig. 6, D–J). These results suggest that thiamine is involved in nodule enlargement after infection.
In animals and plants, thiamine is essential to provide energy for organs with active cell division (Comín-Anduix et al., 2001; Liu et al., 2010; Woodward et al., 2010). For example, the proliferation of animal cancer cells is promoted by thiamine treatment (Comín-Anduix et al., 2001). On the other hand, thiaminase I, which catalyzes the degradation of thiamine, enhances the effect of an anticancer agent (Liu et al., 2010). In leguminous plants, nodules developed de novo are formed from nodule primordia by cell division and differentiation (Oldroyd et al., 2011). In L. japonicus, the expression of THI1 was enhanced in nodule primordia (Fig. 4) and thiamine content was increased in nodulated roots (Fig. 1B); therefore, we speculate that the de novo biosynthesis of thiamine is required for nodule formation. We also hypothesize that cortical cell division may be more active in thiamine-treated plants than in untreated plants, leading to early enlargement of nodules in treated plants (Fig. 2). If the presence of thiamine in roots and nodules is determined only by THI1, a far more severe phenotype should be expected. In fact, although the thiamine content in the thi1 mutant roots was decreased, this reduction was small (Fig. 5D). One explanation could be that a small amount of the truncated THI1 protein can catalyze thiazole production in the thi1 mutant. Another could be the translocation of thiamine or the thiazole moiety produced by THI2 from aerial tissues (Supplemental Fig. S3) to root tissues. The latter explanation is plausible because thiamine is water soluble and treatment of the soil surface with thiamine rescued the chlorotic leaf phenotype in the thiC mutant and THI2KD plants (Fig. 3). The additional steps required to transport thiamine in mutant plants would delay nodule growth. Because nodule formation is energy consuming, the level of thiamine without infection might be insufficient for nodule development. Therefore, de novo thiamine biosynthesis mediated by THI1, the production of which is enhanced in plants infected by rhizobia, might be required for normal nodule formation.
Functions of THI1 during Symbioses and Seed Development
The effect of thiamine on seed development has not been investigated (Ajjawi et al., 2007a, 2007b; Kong et al., 2008; Yazdani et al., 2013). Here, we report a novel role of THI1 in seed maturation (Fig. 7). The THI1 gene was induced in seeds (Supplemental Fig. S12), and its suppression decreased seed weight (Fig. 7). Common regulatory pathways involved in symbioses and seed formation have been suggested, because symbiotic mutants (nup133, nup85, and crinkle in L. japonicus and ram2 in Medicago truncatula) have defects in seed formation (Tansengco et al., 2003; Kanamori et al., 2006; Saito et al., 2007; Wang et al., 2012). The defects in crinkle and nup85 are caused by the failure of pollen tube elongation (Tansengco et al., 2004; Saito et al., 2007). The defect in the thi1 mutant might involve embryo development but not elongation of the pollen tube, because the seed number per pod was not affected (Supplemental Fig. S13). These results indicate that symbiotic genes also are involved in reproductive organ formation from fertilization to seed development.
The absence of thiamine biosynthesis genes in AM fungi seems to be an important aspect of their obligatory biotrophic status (Tisserant et al., 2013). We showed that AM infection was not affected by thiamine or THI1, because exogenous thiamine treatment or a defect in the THI1 gene did not affect colonization levels (Supplemental Figs. S7 and S11). A low level of thiamine in host plants might be sufficient for AM infection, because, unlike RN symbiosis, AM does not require de novo dynamic organogenesis. Nevertheless, we cannot exclude that thiamine is important for AM; for example, it may be necessary during spore formation, the most important step in the AM fungal life cycle. Further investigation might clarify the effects of thiamine on AM.
Thiamine studies in plants are gaining attention, including those on thiamine effects on plant morphogenesis (Woodward et al., 2010), plant growth coordination by circadian expression of THIC (Bocobza et al., 2013), and the identification of plant thiamine transporters (Frelin et al., 2012). Moreover, we expect that THI1 may play some other roles besides that in thiazole biosynthesis. Although the activity of THI1 is completely lost after the reaction, the accumulation of inactive THI1 in Saccharomyces cerevisiae (Chatterjee et al., 2011) implies the existence of some additional roles of this protein. Disruption of rice (Oryza sativa) DR8 (a homolog of L. japonicus THI1) enhances resistance against a pathogen (Wang et al., 2006). In Arabidopsis, THI1 protects DNA from damage caused by UV radiation (Machado et al., 1997). These findings and our results indicate some THI1 functions in nonsymbiotic and RN symbiosis processes.
MATERIALS AND METHODS
Plant Growth and Inoculation of Symbiotic Microbes
The LORE1 insertion mutants thi1 (30006491) and thiC (30008726) in the Lotus japonicus B129 Gifu background were obtained from Lotus Base (http://users-mb.au.dk/pmgrp/). Seeds of wild-type (B129 and MG-20), LORE1 insertion mutant, and THI2KD plants in the L. japonicus MG-20 Miyakojima background were scarified and sterilized with 2% (v/v) sodium hypochlorite for 7 to 10 min and then germinated on 0.8% agar plates (Wako). The plants were grown in a growth chamber (16 h of light/8 h of dark, 24°C) for 2 to 3 d. For the evaluation of nodule phenotypes, plants were transferred onto new 0.8% agar plates containing B&D solution (Broughton and Dilworth, 1971) supplemented with 0.1 mm KNO3 and 1 μm aminoethoxyvinylglycine to inhibit ethylene synthesis and promote nodule formation (Penmetsa and Cook, 1997) without or with various concentrations of thiamine; plants were simultaneously inoculated with Mesorhizobium loti MAFF303099. For the evaluation of leaf phenotypes, plants were transferred into an autoclaved pot filled with a 1:1 mixture of vermiculite and fine vermiculite, and B&D solution supplemented with 0.1 mm KNO3 was added for 2 to 3 weeks. For spatiotemporal expression analysis, plants were infected with Agrobacterium rhizogenes containing the proTHI1-GUS construct by hairy root transformation. Plants were then transplanted into vermiculite pots, and B&D solution supplemented with 0.1 mm KNO3 was added for 1 to 2 weeks after inoculation with M. loti carrying DsRed. To evaluate the recovery of the seed phenotype, thiamine was sprayed onto soil and aerial tissues every 2 or 3 d.
Genotyping of thi1 and thiC Mutants
Genotypes of the thi1 and thiC mutants were determined by PCR on leaf DNA with the primers THI1_LORE_F, THI1_LORE_R, and LORE1_F for the thi1 mutant and THIC_LORE_F, THIC_LORE_R, and LORE1_F for the thiC mutant. Primer sets are listed in Supplemental Table S1.
Binary Vector Construction
For spatiotemporal expression analysis, the THI1 promoter (1,032 bp) was amplified using PrimeSTAR GXL (Takara) with the proTHI1_F and proTHI1_R primers. The amplified fragment was cloned into pENTR D/TOPO (pENTR D/TOPO cloning kit; Invitrogen). To create the proTHI1-GUS fusion construct, the entry clone (D/TOPO-THI1 promoter) was converted into the destination vector pKGWFS7 (Karimi et al., 2002) using in vitro site-directed recombination (Gateway LR Clonase Enzyme mix; Invitrogen).
For colocalization analysis, the THI1 coding region without its stop codon was amplified by PCR with the THI1_F_Met and THI1_R_less primers. The amplified fragment was cloned into pENTR D/TOPO and used as a template for PCR with the TOPO_F and TOPO_THI1_R primers to amplify the D/TOPO-THI1 fragment. An mCherry fragment was amplified using pCYCLOPS::mCherry-NLS (Suzaki et al., 2012) as a template with the mCherry_F and mCherry_R_less primers. The blunt-end mCherry fragment was phosphorylated with T4 polynucleotide kinase (Toyobo) and ligated to the D/TOPO-THI1 fragment to obtain the D/TOPO-THI1:mCherry fusion entry vector. The THI1-mCherry fragment was then transferred into p35S-GW-GFP (Yano et al., 2008) by Gateway LR reaction (Invitrogen). Finally, p35S-GW-GFP-derived GFP was removed using XhoI. To obtain a construct with a deleted plastid transit peptide (predicted by the TargetP program; http://www.cbs.dtu.dk/services/TargetP/), the THI1delSP fragment was amplified using D/TOPO-THI1:mCherry as a template with the THI1_delSP_F and TOPO_R primers. This fragment was phosphorylated and self-ligated, resulting in D/TOPO-THI1delSP:mCherry. THI1delSP:mCherry was transferred into p35S-GW-GFP (Yano et al., 2008) by Gateway LR reaction, and GFP was then removed using XhoI.
For the complementation test, the THI1 genomic sequence (a 1,535-bp promoter, a 2,423-bp THI1 coding region with introns, and a 1,000-bp 3′ untranslated region) was amplified using PrimeSTAR GXL (Takara) with the proTHI1_F_EcoRI and THI1_ter_R_EcoRI primers. The amplified fragment was cloned into pENTR D/TOPO. To create a complementation construct, the THI1 fragment was digested with EcoRI and inserted into the pCAMBIA1300/CFP vector, which contained CaMV35S promoter-CFP-NOS terminator as an EcoRI-HindIII fragment.
To obtain the THI2-RNAi construct for THI2KD, the THI2-RNAi fragment was amplified by PCR on genomic DNA with THI2KD_F and THI2KD_R primers, cloned into pENTR D/TOPO, and transferred into pUB-GWS-Hyg (Maekawa et al., 2008). All primer sets are listed in Supplemental Table S1.
Expression Analysis
Total RNA was extracted from roots using the Purelink Plant RNA reagent (Invitrogen). First-strand cDNA was prepared using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo). Real-time PCR was performed using a LightCycler 96 real-time PCR machine (Roche Diagnostics) with Thunderbird SYBR qPCR Mix (Toyobo) according to the manufacturer’s protocol. The expression of ubiquitin was used as the reference. Primer sets are listed in Supplemental Table S1.
Transformation of Hairy Roots of L. japonicus
Transgenic hairy roots were induced using A. rhizogenes AR1193. Bacterial colonies were suspended in sterilized water. Roots of 6-d-old seedlings (grown for 4 d in the dark and for 2 d in 16 h of light/8 h of dark, 24°C) were cut off below the hypocotyls while immersed in the A. rhizogenes suspension. For colocalization analysis, a mixture of A. rhizogenes suspensions transformed with each construct was used. The infected shoots were incubated for 5 d in a growth chamber (16 h of light/8 h of dark, 24°C) on one-half-strength B5 medium (Wako) solidified with 0.8% agar and subsequently transferred to the same medium containing 125 μg mL−1 meropenem (Sumitomo Pharmaceuticals) for 10 to 14 d.
Transformation of L. japonicus
To obtain THI2KD and the THI1-complemented transformants, wild-type MG-20 and the thi1 mutant were transformed with Agrobacterium tumefaciens AGL1 containing the THI2-RNAi construct and the THI1 complementation construct, respectively. To obtain stable transformants, callus formation from infected hypocotyls and redifferentiation were induced (Stiller et al., 1997).
Measurement of Thiamine Content
Root samples (0.25 g) collected 3 weeks after inoculation with or without M. loti were homogenized and incubated with 20 mL of citric acid buffer (pH 4.5) in the presence of 300 mg of Taka-Diastase (Sigma) for 1 h at 37°C and then for 30 min at 95°C, quickly cooled at 30°C, and centrifuged at 9,200g for 10 min. The supernatants were passed through 0.22-μm filters. Bioassay was performed using the VitaFast Vitamin B1 kit (Biopharm) according to the manufacturer’s instructions.
GUS Staining
Transgenic hairy roots containing the proTHI1-GUS construct and infected with M. loti carrying DsRed were stained with GUS staining buffer (0.5 mm X-Gluc, 0.5 mm K3[Fe(CN)6], 0.5 mm K4[Fe(CN)6], 5 mm NaH2PO4, pH 7, and 0.01% [v/v] Triton X-100) at 37°C for 1 to 6 h after vacuum infiltration for 15 min. Semithin sections (100 μm) of fresh unfixed primordia and nodules were cut with a VT1200S vibrating-blade microtome (Leica).
Quantification of Infection Threads
At 3 d after germination, plants were inoculated with M. loti expressing DsRed. At 7 dai, infection threads in the roots were counted using an SZX16 fluorescence stereomicroscope (Olympus).
Acetylene Reduction Assay
The assay was performed as described by Suganuma et al. (2003). Nodulated roots detached from plants cultured on plates were incubated in 20-mL vials at 25°C for 30 min, and the amount of ethylene produced was determined using a GC-8A gas chromatograph (Shimazu) equipped with a glass column (2 mm i.d. × 2 m) packed with Porapak N (80–100 mesh; Waters).
Nodule Sections
Nodule samples were fixed in 0.25% (v/v) glutaraldehyde and 4% (w/v) paraformaldehyde in 0.05 m sodium phosphate buffer (pH 7.2) at room temperature using vacuum infiltration three times for 15 min and then overnight at 4°C. The samples were washed in the same buffer and dehydrated through an alcohol series (50%, 70%, 90%, and 100%). The samples were infiltrated with a 50:50 mix of ethanol and Technovit 7100 (Heraeus Kulzer) containing hardener I for 2 h, then with Technovit 7100 containing hardener I twice for 3 h or overnight at 4°C, and embedded in Technovit 7100 and hardener II according to the manufacturer’s instructions. Thin sections (5 μm) were cut on a Microtome RM2255 (Leica) and stained with 0.05% Toluidine Blue.
Microscopy
Bright-field and fluorescence images were viewed with an SZX16 stereomicroscope (Olympus) or a BX50 microscope (Olympus). Images were acquired and nodule diameters were measured with a DP Controller (Olympus).
Accession Numbers
The accession numbers are as follows: LjTHI1, Lj3g3v1010900.1 (Miyakogusa.jp 3.0; http://www.kazusa.or.jp/lotus/); LjTHI2, Lj5g3v2060670.1 (Miyakogusa.jp 3.0); AtTHI1, At5g54770 (The Arabidopsis Information Resource; https://www.arabidopsis.org/); ZmTHI1, U17350 (DNA Data Bank of Japan); ZmTHI2, U17351 (DNA Data Bank of Japan); and OsTHI1, Os07g0529600 (Rice Annotation Project Database; http://rapdb.dna.affrc.go.jp/).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Outline of the thiamine biosynthesis pathway in plants.
Supplemental Figure S2. Expression of thiamine biosynthesis genes during AM.
Supplemental Figure S3. Expression of THIC, THI1, and THI2 (a THI1 paralog) according to LjGEA.
Supplemental Figure S4. Effects of exogenous thiamine on nodulation evaluated by nodule size.
Supplemental Figure S5. Time course of maximum nodule diameter by exogenous thiamine treatment.
Supplemental Figure S6. Effects of exogenous thiamine on nodulation in the hypernodulating mutant plenty.
Supplemental Figure S7. Effect of exogenous thiamine on AM.
Supplemental Figure S8. RN symbiotic phenotype of the thiC mutant.
Supplemental Figure S9. Amino acid sequence alignment of THI1 homologs in various plants.
Supplemental Figure S10. Time course of maximum nodule diameter in the thi1 mutant.
Supplemental Figure S11. AM phenotype of the thiamine-untreated thi1 mutant.
Supplemental Figure S12. THI1 and THI2 expression patterns according to LjGEA.
Supplemental Figure S13. Seed number per pod in WTseg and two lines of the thi1 mutant.
Supplemental Table S1. Primers used in this study.
Supplemental Materials and Methods S1. Inoculation, staining, and quantification of AM fungi.
Supplementary Material
Acknowledgments
We thank Dr. Stephan Dräxl for cooperation and Dr. Takuya Suzaki for providing the pCYCLOPS::mCherry-NLS plasmid; Aarhus University for the thi1 mutant seeds; and the National Institute for Basic Biology Core Research Facilities and National Institute for Basic Biology Bioresource Center for sequencing, measurement of thiamine content, and plant cultivation.
Glossary
- RN
root nodule
- AM
arbuscular mycorrhiza
- qRT
quantitative real-time
- LjGEA
Lotus japonicus Gene Expression Atlas
- dai
days after inoculation
- WTseg
wild-type segregant
- ARA
acetylene reduction activity
Footnotes
This work was supported by the Japan Society for the Promotion of Science (grant nos. 24770050 and 15KT0122) and the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (grant no. 250023A to N.T.).
References
- Ajjawi I, Rodriguez Milla MA, Cushman J, Shintani DK (2007a) Thiamin pyrophosphokinase is required for thiamin cofactor activation in Arabidopsis. Plant Mol Biol 65: 151–162 [DOI] [PubMed] [Google Scholar]
- Ajjawi I, Tsegaye Y, Shintani D (2007b) Determination of the genetic, molecular, and biochemical basis of the Arabidopsis thaliana thiamin auxotroph th1. Arch Biochem Biophys 459: 107–114 [DOI] [PubMed] [Google Scholar]
- Bocobza SE, Malitsky S, Araújo WL, Nunes-Nesi A, Meir S, Shapira M, Fernie AR, Aharoni A (2013) Orchestration of thiamin biosynthesis and central metabolism by combined action of the thiamin pyrophosphate riboswitch and the circadian clock in Arabidopsis. Plant Cell 25: 288–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Abeydeera ND, Bale S, Pai PJ, Dorrestein PC, Russell DH, Ealick SE, Begley TP (2011) Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase. Nature 478: 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comín-Anduix B, Boren J, Martinez S, Moro C, Centelles JJ, Trebukhina R, Petushok N, Lee WN, Boros LG, Cascante M (2001) The effect of thiamine supplementation on tumour proliferation: a metabolic control analysis study. Eur J Biochem 268: 4177–4182 [DOI] [PubMed] [Google Scholar]
- Frelin O, Agrimi G, Laera VL, Castegna A, Richardson LG, Mullen RT, Lerma-Ortiz C, Palmieri F, Hanson AD (2012) Identification of mitochondrial thiamin diphosphate carriers from Arabidopsis and maize. Funct Integr Genomics 12: 317–326 [DOI] [PubMed] [Google Scholar]
- Fukai E, Soyano T, Umehara Y, Nakayama S, Hirakawa H, Tabata S, Sato S, Hayashi M (2012) Establishment of a Lotus japonicus gene tagging population using the exon-targeting endogenous retrotransposon LORE1. Plant J 69: 720–730 [DOI] [PubMed] [Google Scholar]
- Godoi PH, Galhardo RS, Luche DD, Van Sluys MA, Menck CF, Oliva G (2006) Structure of the thiazole biosynthetic enzyme THI1 from Arabidopsis thaliana. J Biol Chem 281: 30957–30966 [DOI] [PubMed] [Google Scholar]
- Goyer A. (2010) Thiamine in plants: aspects of its metabolism and functions. Phytochemistry 71: 1615–1624 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Parniske M (2013) Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol 29: 593–617 [DOI] [PubMed] [Google Scholar]
- Julliard JH, Douce R (1991) Biosynthesis of the thiazole moiety of thiamin (vitamin B1) in higher plant chloroplasts. Proc Natl Acad Sci USA 88: 2042–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgenson CT, Begley TP, Ealick SE (2009) The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 78: 569–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EM, Miwa H, Downie JA, James EK, Felle HH, Haaning LL, et al. (2006) A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA 103: 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kong D, Zhu Y, Wu H, Cheng X, Liang H, Ling HQ (2008) AtTHIC, a gene involved in thiamine biosynthesis in Arabidopsis thaliana. Cell Res 18: 566–576 [DOI] [PubMed] [Google Scholar]
- Liu S, Monks NR, Hanes JW, Begley TP, Yu H, Moscow JA (2010) Sensitivity of breast cancer cell lines to recombinant thiaminase I. Cancer Chemother Pharmacol 66: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CR, Praekelt UM, de Oliveira RC, Barbosa AC, Byrne KL, Meacock PA, Menck CF (1997) Dual role for the yeast THI4 gene in thiamine biosynthesis and DNA damage tolerance. J Mol Biol 273: 114–121 [DOI] [PubMed] [Google Scholar]
- Maekawa T, Kusakabe M, Shimoda Y, Sato S, Tabata S, Murooka Y, Hayashi M (2008) Polyubiquitin promoter-based binary vectors for overexpression and gene silencing in Lotus japonicus. Mol Plant Microbe Interact 21: 375–382 [DOI] [PubMed] [Google Scholar]
- McHale NA, Hanson KR, Zelitch I (1988) A nuclear mutation in Nicotiana sylvestris causing a thiamine-reversible defect in synthesis of chloroplast pigments. Plant Physiol 88: 930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al. 2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429 [DOI] [PubMed] [Google Scholar]
- Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H (1999) Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J 18: 455–463 [DOI] [PubMed] [Google Scholar]
- Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M (1999) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 27: 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GE, Murray JD, Poole PS, Downie JA (2011) The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet 45: 119–144 [DOI] [PubMed] [Google Scholar]
- Papini-Terzi FS, Galhardo RS, Farias LP, Menck CF, Van Sluys MA (2003) Point mutation is responsible for Arabidopsis tz-201 mutant phenotype affecting thiamin biosynthesis. Plant Cell Physiol 44: 856–860 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR (1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275: 527–530 [DOI] [PubMed] [Google Scholar]
- Ribeiro A, Praekelt U, Akkermans ADL, Meacock PA, van Kammen A, Bisseling T, Pawlowski K (1996) Identification of agthi1, whose product is involved in biosynthesis of the thiamine precursor thiazole, in actinorhizal nodules of Alnus glutinosa. Plant J 10: 361–368 [DOI] [PubMed] [Google Scholar]
- Saito K, Yoshikawa M, Yano K, Miwa H, Uchida H, Asamizu E, Sato S, Tabata S, Imaizumi-Anraku H, Umehara Y, et al. (2007) NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19: 610–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller J, Martirani L, Tuppale S, Chian R, Chiurazzi M, Gresshoff P (1997) High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus. J Exp Bot 48: 1357–1365 [Google Scholar]
- Suganuma N, Nakamura Y, Yamamoto M, Ohta T, Koiwa H, Akao S, Kawaguchi M (2003) The Lotus japonicus Sen1 gene controls rhizobial differentiation into nitrogen-fixing bacteroids in nodules. Mol Genet Genomics 269: 312–320 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M (2012) Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139: 3997–4006 [DOI] [PubMed] [Google Scholar]
- Tansengco ML, Hayashi M, Kawaguchi M, Imaizumi-Anraku H, Murooka Y (2003) crinkle, a novel symbiotic mutant that affects the infection thread growth and alters the root hair, trichome, and seed development in Lotus japonicus. Plant Physiol 131: 1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansengco ML, Imaizumi-Anraku H, Yoshikawa M, Takagi S, Kawaguchi M, Hayashi M, Murooka Y (2004) Pollen development and tube growth are affected in the symbiotic mutant of Lotus japonicus, crinkle. Plant Cell Physiol 45: 511–520 [DOI] [PubMed] [Google Scholar]
- Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frei dit Frey N, Gianinazzi-Pearson V, et al. (2013) Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc Natl Acad Sci USA 110: 20117–20122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbański DF, Małolepszy A, Stougaard J, Andersen SU (2012) Genome-wide LORE1 retrotransposon mutagenesis and high-throughput insertion detection in Lotus japonicus. Plant J 69: 731–741 [DOI] [PubMed] [Google Scholar]
- Verdier J, Torres-Jerez I, Wang M, Andriankaja A, Allen SN, He J, Tang Y, Murray JD, Udvardi MK (2013) Establishment of the Lotus japonicus Gene Expression Atlas (LjGEA) and its use to explore legume seed maturation. Plant J 74: 351–362 [DOI] [PubMed] [Google Scholar]
- Wang E, Schornack S, Marsh JF, Gobbato E, Schwessinger B, Eastmond P, Schultze M, Kamoun S, Oldroyd GE (2012) A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr Biol 22: 2242–2246 [DOI] [PubMed] [Google Scholar]
- Wang G, Ding X, Yuan M, Qiu D, Li X, Xu C, Wang S (2006) Dual function of rice OsDR8 gene in disease resistance and thiamine accumulation. Plant Mol Biol 60: 437–449 [DOI] [PubMed] [Google Scholar]
- Woodward JB, Abeydeera ND, Paul D, Phillips K, Rapala-Kozik M, Freeling M, Begley TP, Ealick SE, McSteen P, Scanlon MJ (2010) A maize thiamine auxotroph is defective in shoot meristem maintenance. Plant Cell 22: 3305–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Yoshida S, Müller J, Singh S, Banba M, Vickers K, Markmann K, White C, Schuller B, Sato S, et al. (2008) CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc Natl Acad Sci USA 105: 20540–20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani M, Zallot R, Tunc-Ozdemir M, de Crécy-Lagard V, Shintani DK, Hanson AD (2013) Identification of the thiamin salvage enzyme thiazole kinase in Arabidopsis and maize. Phytochemistry 94: 68–73 [DOI] [PubMed] [Google Scholar]
- Yoshida C, Funayama-Noguchi S, Kawaguchi M (2010) plenty, a novel hypernodulation mutant in Lotus japonicus. Plant Cell Physiol 51: 1425–1435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.