The tobacco plasma membrane NADPH oxidase and the extracellular polyamine oxidase interact functionally to regulate the homeostasis of reactive oxygen species.
Abstract
The apoplastic polyamine oxidase (PAO) catalyzes the oxidation of the higher polyamines spermidine and spermine, contributing to hydrogen peroxide (H2O2) accumulation. However, it is yet unclear whether apoplastic PAO is part of a network that coordinates the accumulation of reactive oxygen species (ROS) under salinity or if it acts independently. Here, we unravel that NADPH oxidase and apoplastic PAO cooperate to control the accumulation of H2O2 and superoxides (O2·−) in tobacco (Nicotiana tabacum). To examine to what extent apoplastic PAO constitutes part of a ROS-generating network, we examined ROS accumulation in guard cells of plants overexpressing or down-regulating apoplastic PAO (lines S2.2 and A2, respectively) or down-regulating NADPH oxidase (line AS-NtRbohD/F). The H2O2-specific probe benzene sulfonyl-H2O2 showed that, under salinity, H2O2 increased in S2.2 and decreased in A2 compared with the wild type. Surprisingly, the O2·−-specific probe benzene sulfonyl-So showed that O2·− levels correlated positively with that of apoplastic PAO (i.e. showed high and low levels in S2.2 and A2, respectively). By using AS-NtRbohD/F lines and a pharmacological approach, we could show that H2O2 and O2·− accumulation at the onset of salinity stress was dependent on NADPH oxidase, indicating that NADPH oxidase is upstream of apoplastic PAO. Our results suggest that NADPH oxidase and the apoplastic PAO form a feed-forward ROS amplification loop, which impinges on oxidative state and culminates in the execution of programmed cell death. We propose that the PAO/NADPH oxidase loop is a central hub in the plethora of responses controlling salt stress tolerance, with potential functions extending beyond stress tolerance.
Several enzymatic and nonenzymatic reactions control the production of reactive oxygen species (ROS; Gilroy et al., 2014; Foyer and Noctor, 2016). Superoxide ions (O2·−) are generated mainly by the respiratory burst oxidase homologs NADPH oxidases (encoded by the Rboh genes), and O2·− dismutation by superoxide dismutase is considered one of the major routes for subsequent hydrogen peroxide (H2O2) production (Torres et al., 2002; Kwak et al., 2003; Wang et al., 2013; Baxter et al., 2014).
The homeostasis of ROS is controlled by low-Mr intermolecular and intramolecular compounds, such as the polyamines (PAs). PAs are highly reactive aliphatic polycations; the main PAs in plants are the diamine putrescine (Put) and the so-called higher PAs, spermidine (Spd; triamine) and spermine (Spm; tetramine; Tiburcio et al., 2014; Saha et al., 2015, and refs. therein). PA homeostasis affects a vast range of dynamic developmental and metabolic processes (Paschalidis and Roubelakis-Angelakis, 2005a, 2005b; Moschou et al., 2009; Wu et al., 2010; Moschou and Roubelakis-Angelakis, 2014; Tiburcio et al., 2014; Pal et al., 2015). The oxidation of PAs is catalyzed by amine oxidases (AOs). AOs, such as the diamine oxidases (DAOs; or copper-containing AOs) and the flavin-containing polyamine oxidases (PAOs), localize either intercellularly (i.e. apoplast) or intracellularly (i.e. cytoplasm and peroxisomes). DAOs oxidize mainly Put, but also Spd and Spm (with much lower efficiency), yielding H2O2 and aminoaldehydes. The apoplastic PAOs terminally oxidize Spd and Spm, yielding aminoaldehydes and H2O2, while the intracellular PAOs (also referred to as back-converting PAOs) oxidize PAs to produce H2O2, an aminoaldehyde, and a PA with one less amino group (in the order tetramine→triamine→diamine; Angelini et al., 2010; Pottosin and Shabala, 2014). Through their catabolic oxidative deamination, PAs increase the intracellular and extracellular H2O2 load.
Under physiological or stress conditions, the rate of ROS generation/scavenging determines their steady level; this rate is integrated into a multitude of vital signaling cues. ROS seem to be multifaced players: at low levels, they are efficiently scavenged by enzymatic and nonenzymatic antioxidants, present in nearly all cellular compartments (Mittler et al., 2004; Miller et al., 2010; Suzuki and Mittler, 2012; Baxter et al., 2014; Foyer and Noctor, 2015); at medium levels and up to a threshold signature, ROS participate in downstream signaling cascades that activate stress-protective effector genes/mechanisms. When a certain high level is reached, oxidative stress is established and ROS participate in a plethora of destructive pathways that culminate in the induction of programmed cell death (PCD; Moschou et al., 2008a, 2008b; Gémes et al., 2011; Moschou and Roubelakis-Angelakis, 2014).
PAOs and NADPH oxidases, major ROS generators, have been mostly studied separately, and it remains unknown whether they are functionally linked. Their involvement in similar processes points at their possible interplay. Perhaps the best example of the convergent action of PAOs and NADPH oxidases is the control of stomatal aperture. In Arabidopsis (Arabidopsis thaliana) guard cells, abscisic acid (ABA) induces the production of H2O2 arising from O2·− generated by NADPH oxidases. The produced H2O2 activates, among others, downstream ROS-dependent Ca2+ channels contributing to cytosolic Ca2+ increase (Kwak et al., 2003; Desikan et al., 2004; Baxter et al., 2014). Likewise, ABA induces the increase of H2O2 in the apoplast through the up-regulation of peroxidase and apoplastic PAO (Zhu et al., 2006).
In an attempt to increase our understanding of how PAOs can contribute to processes where NADPH oxidases are involved, we examined the interplay between these genes/enzymes. To this end, we used tobacco (Nicotiana tabacum) plants up-/down regulating apoplastic PAO (lines S2.2 and A2, respectively; Moschou et al., 2008a, 2008b) and tobacco plants down-regulating two NADPH oxidase genes (AS-NtRbohD and AS-NtRbohF; Ji and Park, 2011). We used guard cells for real-time in vivo monitoring of apoplastic PAO/NADPH oxidase-derived H2O2 and O2·− intracellularly and intercellularly (Song et al., 2014). Our results provide evidence for an interplay of PAO/NADPH oxidase that is important for balancing the ratio of intracellular and intercellular O2·− and H2O2 levels.
RESULTS
Apoplastic PAO Represents the Main Spd Oxidation Source
Considering the large number of AOs in plants (Moschou et al., 2008c), we aimed at determining the relative contribution of apoplastic versus intracellular PA oxidation to H2O2 production during salinity. We previously established that, during salt stress, Spd is secreted into the apoplast, where it is oxidized by the apoplastic PAO (Moschou et al., 2008b). However, the contribution of intracellular AOs to Spd oxidation under the same conditions was not examined. In an attempt to dissect the contribution of different AOs to H2O2 production, we used tobacco transgenic lines overexpressing or down-regulating ZmPAO (S-ZmPAO [line S2.2] and AS-ZmPAO [line A2], respectively; Moschou et al., 2008a, 2008b). Line S2.2 shows increased while A2 shows reduced apoplastic PAO activity (Moschou et al., 2008b). In contrast to our previous works, here we used leaves that were not fully expanded, in order to take into consideration the importance of PAOs in developmental processes, such as leaf expansion during salt stress (Rodríguez et al., 2009). At this stage, the profile of PAs in the wild type, A2, and S2.2 was somewhat different from what has been described previously (Supplemental Fig. S1; Moschou et al., 2008a, 2008b). However, the observed expected increase of PAs in A2 and the decrease of higher PAs (Spd and Spm) in S2.2 suggest that the apoplastic PAO controls PA levels in expanding leaves, as was the case for the fully expanded ones (Moschou et al., 2008a, 2008b).
Next, we determined the total cellular capacity of Spd oxidation (terminal plus back conversion) versus terminal Spd oxidation in the wild type, A2, and S2.2. To achieve this, we developed an in-gel Spd oxidation assay that determines total Spd oxidation activity. We compared the results obtained from this in-gel Spd oxidation assay with those obtained from a colorimetric assay that determines terminal Spd oxidation (Supplemental Fig. S2A). The in-gel assay is based on the fact that H2O2 produced by Spd oxidation reacts with 3,3′-diaminobenzidine (DAB), forming a brownish adduct that denotes the gel regions (bands) enriched in Spd oxidase activity. In the wild type, Spd oxidase can be visualized as multiple bands (three main ones), with a major isoenzyme (greater than 50%) showing high mobility (referred to hereafter as anodal). This isoenzyme pattern is consistent with the large number of predicted PAOs and DAOs in the tobacco genome (at least one apoplastic and four intracellular PAOs and more than 12 DAOs; Supplemental File S1). However, we could not define a large number of bands in the wild type, suggesting that some isoenzymes may show similar mobility on the gel, preventing their separation, may not be present in leaves, or could be refractory to this analytical method. In A2, the major anodal Spd oxidase isoenzymes were depleted, suggesting that they most likely correspond to apoplastic PAO isoforms (Supplemental Fig. S2, A and B). In S2.2, we observed a significant increase of the in-gel Spd oxidase potential and, in particular, the appearance of an additional fast-migrating band that could not be seen in the wild type and A2. Although we could only achieve a fair resolution of isoenzymes, we assume that the fast-migrating band corresponds to the apoplastic maize (Zea mays) PAO isoenzyme (ZmPAO), which is overexpressed in S2.2 (predicted mass approximately 53 kD). We also observed in S2.2 an increase of additional bands, which were significantly less mobile than the band that presumably corresponds to maize PAO. These isoenzymes could correspond to a posttranslationally modified maize PAO or different maize PAO fractions (Cona et al., 2006). Alternatively, the increase in apoplastic PAO may signal the up-regulation of other AOs, or simply the DAB adduct, due to its higher production in S2.2, may diffuse, producing erroneous bands. The quantification of bands in the three genotypes showed that the overall Spd oxidase activity in S2.2 increased significantly by 2-fold, mostly due to the increase of the anodal isoenzymes; A2 lines showed a 2-fold decrease due to the absence of the major anodal isoenzyme. Taken together, these results suggest that the apoplastic PAO represents the major Spd oxidase activity.
In the colorimetric assay, DAO activity (terminal oxidation of Put) was not increased significantly among the three genotypes (Supplemental Fig. S2C). On the other hand, the terminal Spd oxidase activity (mainly apoplastic PAO) was reduced significantly in A2 lines, while in S2.2 it increased by 3-fold (Supplemental Fig. S2C). In addition, apoplastic PAO activity was highly responsive to 200 mm salt treatment (referred to hereafter as NaCl treatment), exhibiting a significant increase (Moschou et al., 2008b), whereas the cathodal total Spd oxidase activity responded moderately to NaCl treatment (Supplemental Fig. S2, B and C).
To further substantiate the previous finding, we examined the Spd oxidase activity of protoplasts by the colorimetric 4-aminopterine oxidation assay used to determine the activity of both terminal and back-converting PAOs and DAOs (Tavladoraki et al., 2006). The activity of Spd oxidase in wild-type protoplasts was negligible (close to background levels [as a positive control, purified AtPAO3 was used in these assays]; Moschou et al., 2008c), suggesting that the main Spd oxidase activity resides in the apoplastic compartment. Taken together, the data produced through the in-gel and in vitro assays suggest that the apoplastic PAO accounts for at least 50% of the total Spd oxidase activity in expanding tobacco leaves; therefore, it is the major Spd oxidase activity during salinity.
Apoplastic PAO Impacts O2·− Production
Previously, we found that S2.2 plants show increased superoxide dismutase activity, suggesting that O2·− homeostasis may be compromised in these plants (Moschou et al., 2008a). NaCl treatment can be used to examine the contribution of apoplastic PAO to H2O2 levels, and the in situ ROS detection assay is a powerful tool in the estimation of PAO-derived H2O2 levels (Moschou et al., 2008a, 2008b). Under control conditions, we could not detect significant differences in the staining intensities for O2·− and H2O2 among the three genotypes (Fig. 1, A and B, 0 h). NaCl treatment induced the increase of both ROS in a time-dependent manner. One to 24 h posttreatment, A2 leaves contained lower, while S2.2 leaves contained higher, levels of O2·− and H2O2 than the wild type (Fig, 1, A and B, 1 h). These results were confirmed using an in vitro quantification assay for H2O2 (Fig. 1C) and suggest that apoplastic PAO influences the production of not only H2O2 but also of O2·− under stress conditions.
Figure 1.
In situ ROS detection in leaves of wild-type (WT), A2, and S2.2 plants post-NaCl treatment. A, In situ detection of O2·− (blue) and H2O2 (brown) levels 1, 6, and 24 h post-NaCl treatment. Images are representative of three independent experiments with six leaf images per genotype in each time point. B, Quantification of O2·− (blue) and H2O2 (brown) signal from the in situ detection. NBT, Nitroblue tetrazolium; RU, relative units. C, H2O2 levels in leaves 3 and 24 h post-NaCl treatment. FW, Fresh weight; n.d., not detected. Data in B and C are means ± se of three independent experiments with three technical replicates each. Different letters indicate significant differences of Duncan’s multiple comparisons (P < 0.05).
The Apoplastic PAO-Dependent ROS Accumulation Is Sufficient to Induce PCD within the First Few Hours of NaCl Treatment
We have shown that apoplastic PAO is critically required for PCD execution during prolonged NaCl stress (stress treatment in the range of several days; Moschou et al., 2008b). Here, we examined to what extent under short-term NaCl treatments (in the range of hours) apoplastic PAO-generated ROS are sufficient to induce PCD hallmarks. The array of events that precede PCD execution during NaCl stress are yet unclear and might be context/species specific. S2.2 showed an early accumulation of oxidized proteins (Supplemental Fig. S3, A and B; 1 h posttreatment), in contrast to A2. Significant accumulation of necrotic cells was observed 6 h posttreatment and onward (Supplemental Fig. S3C). Thus, the accumulation of oxidized proteins and ROS seems to precede PCD. Our results suggest that short NaCl treatments (i.e. less than 24 h) are enough to induce apoplastic PAO-derived ROS accumulation of sufficient amounts to induce PCD hallmarks. In addition, our results suggest that protein oxidation and the accumulation of ROS are upstream events in the execution of NaCl-induced PCD, at least under the described conditions.
Guard Cells Reflect the Real-Time ROS Accumulation Post-NaCl Treatment
Guard cells have been used to study real-time ROS accumulation (Song et al., 2014). In these cells, the NADPH oxidase genes RbohD and RbohF are involved in ABA-mediated stomatal closure (Zhang et al., 2001; Kwak et al., 2003; Song et al., 2014). Similarly, apoplastic PAO contributes to ABA-induced H2O2 production in maize under control conditions (Xue et al., 2008).
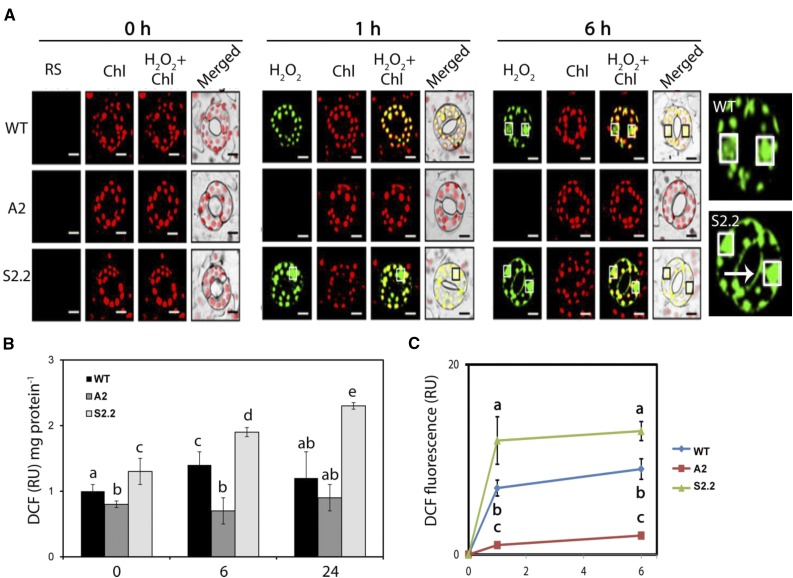
First, we used the unspecific ROS probe 2′,7′-dichlorofluorescein diacetate (DCFDA) to determine ROS production in guard cells. DCFDA is hydrolyzed by cellular esterases to form DCFH, which is oxidized in the presence of peroxidases by hydroxyl or organic peroxyl radicals and the reactive nitrogen species NO· and ONOO− to form the fluorescent dye dichlorofluorescein (DCF; Myhre et al., 2003). The intensity of DCF reflects the formation of general reactive species (RS; the sum of nitrogen and oxygen reactive species) rather than specific ones, providing a rough estimate of ROS production. In guard cells of the wild type, A2, and S2.2, the fluorescence of DCF coincided with the total H2O2 and O2·− production determined using the in situ detection method (Fig. 2A). In particular, under control conditions, no significant differences were observed in DCF fluorescence in guard cells among the three genotypes (Fig. 2, 0 h). Thus, under control conditions, apoplastic PAO does not seem to influence the RS levels. However, 1 and 6 h posttreatment, S2.2 contained higher, while A2 contained lower, DCF compared with the wild type (Fig. 2, 1 and 6 h). DCF accumulated mainly in the nucleus and chloroplasts, but also at the cell margins, of S2.2 guard cells (Fig. 2C, 6 h). This accumulation pattern does not necessarily reflect the RS production sites. In accordance, previous studies suggested that different ROS probes tend to accumulate to distinct intracellular sites that may not coincide with the ROS-producing sites (Snyrychova et al., 2009).
Figure 2.
RS detection in guard cells of wild-type (WT), A2, and S2.2 plants post-NaCl treatment. A, Confocal laser scanning microscopy (CLSM) images of DCF fluorescence (green; DCFDA staining) and chlorophyll (Chl) autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes (black in merged images) denote nuclei. Images at right show enlarged versions of wild-type and S2.2 guard cells (6 h). The arrow indicates the signal accumulation on the cell margins. Images are representative of three independent experiments with six micrographs per genotype in each time point. Bars = 20 μm. B, DCF fluorescence quantification in leaf extracts. C, Time-course quantification of DCF fluorescence in A. Data in B and C are means ± se of three independent experiments with three technical replicates each. Different letters indicate significant differences of Duncan’s multiple comparisons (P < 0.05). RU, Relative units.
Next, we used more specific dyes to estimate H2O2 levels in guard cells. To this end, we evaluated two different sets of fluorescent probes. First, we used the H2O2 probes Amplex Red (AR) and Amplex Ultra Red (AUR; Ashtamker et al., 2007), which are used to estimate H2O2 levels intracellularly and extracellularly, respectively. Under control conditions, no significant differences could be observed among the three genotypes in AR and AUR fluorescence intensities (Supplemental Fig. S4, A and B, 0 h). One and 6 h posttreatment with NaCl, an increase in AR and AUR fluorescence was detected in all three genotypes, mostly in S2.2 plants (Supplemental Fig. S4A). Significant AR and AUR fluorescent signals accumulated in chloroplasts. A2 plants showed reduced AR and AUR fluorescence (6 h), preceded by a transient increase of AUR 1 h posttreatment. This transient increase may reflect the presence of high levels of peroxidase in the apoplast of A2 plants or the interference of the probe with a cellular metabolite. Snyrychova et al. (2009) showed that AR and AUR are highly sensitive to peroxidase levels, similar to DCF and DAB, which also are highly sensitive to peroxidase (Noctor et al., 2016).
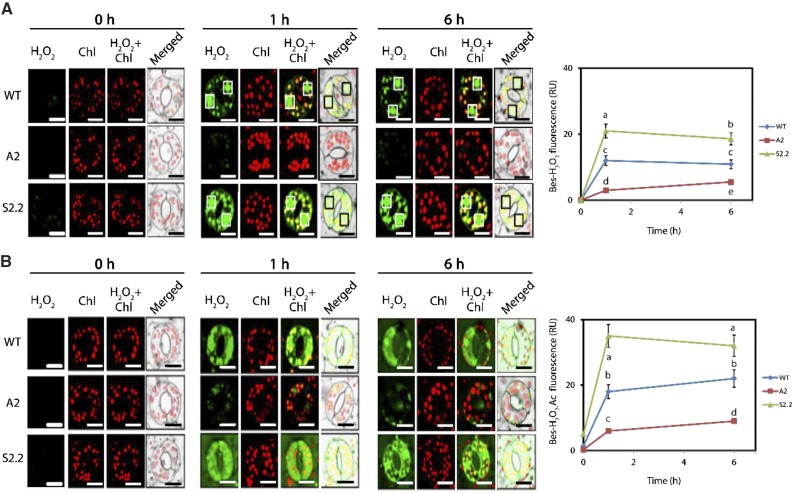
Then, we employed a peroxidase-independent method for the estimation of H2O2 levels. We used the highly specific benzene sulfonyl (BES)-H2O2 and BES-H2O2-Ac probes to estimate intracellular/extracellular H2O2 levels, respectively (Fig. 3). This probe pair is converted to fluorescent molecules in the presence of esterases and might be more specific than AR and AUR, which are used more extensively in the in vitro determinations of H2O2 where peroxidases are added in surplus (Noctor et al., 2016). By using BES-H2O2 and BES-H2O2-Ac, we observed a similar trend of H2O2 accumulation in S2.2 (Fig. 3). However, in this case, we did not observe the transient increase of H2O2 in A2 1 h posttreatment (compare Fig. 3B with Supplemental Fig. S4B). Taken together, our results confirm that guard cells can be used efficiently to monitor real-time ROS accumulation. In addition, guard cells offer some unique advantages over other cell tissues/types for ROS detection. They are homogenous, readily accessible for microscopic observation, and they show a profound physiological responsiveness to short-term NaCl treatment. In addition, we confirm that BES-H2O2 and BES-H2O2-Ac are more specific probes for the detection of H2O2 levels in plants. Nevertheless, a careful assessment of different probes might be required depending on the context/tissue.
Figure 3.
Intracellular/extracellular H2O2 in guard cells of wild-type (WT), A2, and S2.2 plants post-NaCl treatment. A, Representative CLSM images of intracellular BES-H2O2-Ac fluorescence (green) and chlorophyll (Chl) autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes (black in merged images) denote nuclei. Images are representative of three independent experiments with six micrographs per genotype in each time point. Quantification of green signal is shown at right. Bars = 20 μm. B, CLSM images of intercellular BES-H2O2 fluorescence (green) and chlorophyll autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes (black in merged images) denote nuclei. Images are representative of three independent experiments with six micrographs per genotype in each time point. Quantification of green signal is shown at right. Bars = 20 μm. Data in charts at right are means ± se of three independent experiments with three technical replicates each. Different letters indicate significant differences of Duncan’s multiple comparisons (P < 0.05). RU, Relative units.
PAO-Derived H2O2 Coincides with O2·− Production in Guard Cells
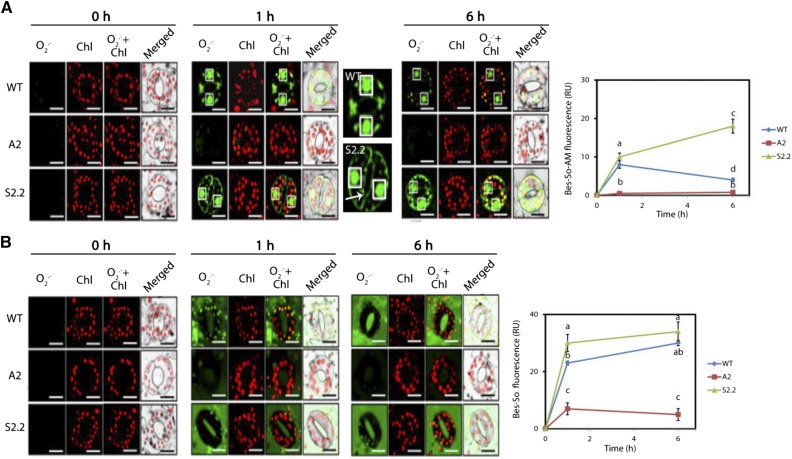
Intracellular generation of O2·− was detected using BES-So-AM, a highly specific fluorescent probe for O2·− (Maeda et al., 2007). Under control conditions, no significant accumulation of O2·− could be detected in the three genotypes (Fig. 4A, 0 h). One and 6 h posttreatment, the levels of intracellular O2·− were increased significantly in guard cells of wild-type and S2.2 plants compared with A2 plants (Fig. 4A, 1 and 6 h). Particularly, fluorescent BES-So-AM accumulated in the nucleus and chloroplasts of the wild type. BES-So-AM also was detected in cell margins of S2.2 guard cells. Thus, although the 1-h post-NaCl treatment pixel intensity of BES-So-AM fluorescence differed marginally between the wild type and S2.2, the difference in the total intracellular levels of fluorescent BES-So-AM was very big, as estimated by counting the total number of pixels pseudocolored green (in arbitrary units: 50 ± 10 for the wild type, 10 ± 2 for A2, and 153 ± 32 for S2.2; see “Materials and Methods”). The previous result is due to additional BES-So-AM in the cell margins of S2.2 plants.
Figure 4.
Intracellular/extracellular O2·− in guard cells of wild-type (WT), A2, and S2.2 plants post-NaCl treatment. A, CLSM images of intracellular BES-So-Am fluorescence (green) and chlorophyll (Chl) autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes (black in merged images) denote nuclei. Images next to the 1-h time point show enlarged versions of wild-type and S2.2 guard cells. The arrow indicates the signal accumulation on the cell margins. Images are representative of three independent experiments with six micrographs per genotype in each time point. Quantification of green signal is shown at right. Bars = 20 μm. B, CLSM images of intercellular BES-So fluorescence (green) and chlorophyll autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes (black in merged images) denote nuclei. Images are representative of three independent experiments with six micrographs per genotype in each time point. Quantification of green signal is shown at right. Bars = 20 μm. Data in charts at right are means ± se of three independent experiments with three technical replicates each. Different letters indicate significant differences of Duncan’s multiple comparisons (P < 0.05). RU, Relative units.
Next, we used BES-So to detect extracellular O2·−. Similar to the intracellular O2·−, no significant accumulation of BES-So could be detected under control conditions in the three genotypes (Fig. 4B, 0 h). One hour posttreatment, the extracellular BES-So fluorescence increased significantly in S2.2 and the wild type, while it increased moderately in A2 (Fig. 4B, 1 h). Six hours posttreatment, BES-So fluorescence increased further in the wild type and mainly in S2.2, but not in A2 (Fig. 4B, 6 h). Our results indicate that apoplastic PAO levels positively correlate with O2·− levels in guard cells.
Apoplastic PAO Levels Correlate with NADPH Oxidase Activity
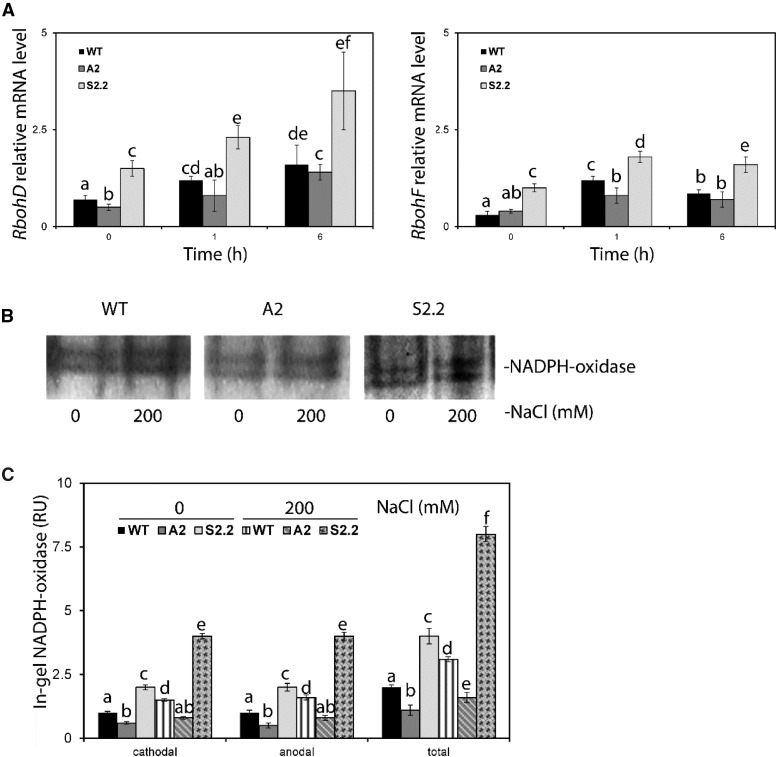
Τhe correlation between PAO and O2·− levels in our experiments prompted us to examine the genetic interaction between PAO and two of the major NADPH oxidase genes in guard cells, RbohD and RbohF (Song et al., 2014). Under control conditions, mRNA levels of RbohD/F were increased significantly in S2.2 compared with the wild type, but not in A2 (Fig. 5A). One and 6 h post-NaCl treatment, the mRNA levels of RbohD tended to increase in all genotypes (Fig. 5A, 1 and 6 h). The same trend, although to a lesser extent, was observed in all genotypes for mRNA levels of RbohF. However, 6 h post-NaCl treatment, the mRNA levels of RbohF decreased slightly in all genotypes compared with 1 h. Under both control and NaCl treatments, the higher mRNA levels of RbohD/F in S2.2 were accompanied by increased in-gel activity of NADPH oxidase, while A2 showed a marked decrease (Fig. 5, B and C, 1 h).
Figure 5.
mRNA levels and activity of NADPH oxidase in wild-type (WT), A2, and S2.2 leaves post-NaCl treatment. A, Abundance of mRNA levels of RbohD (left) and RbohF (right) in leaves post-NaCl treatment with 200 mm NaCl. B, Gel images showing the in gel activity assay of NADPH oxidase 1 h post-NaCl treatment with 200 mm NaCl. Images are representative of three independent experiments with one technical replicate in each (one gel). C, Quantification of anodal and cathodal isoenzymes of NADPH oxidase. A similar isoenzyme pattern has been reported previously in tobacco (Sagi and Fluhr, 2001). Data in A and C are means ± se of three independent experiments with three technical replicates. Different letters indicate significant differences of Duncan’s multiple comparisons relative to the wild type (P < 0.05). RU, Relative units.
PAO-Mediated ROS Production Depends on NADPH Oxidase
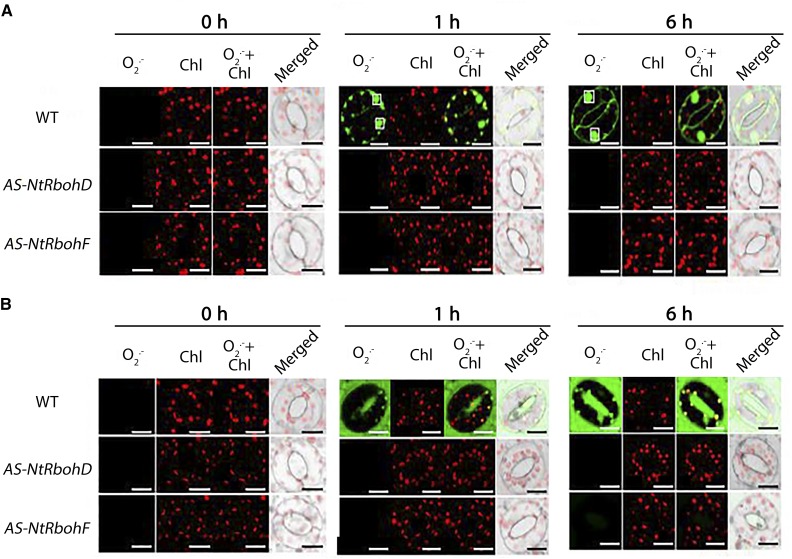
Furthermore, we examined the physiological effect of RbohD/F down-regulation in ROS production using plants with silenced RbohD or RbohF (AS-NtRbohD and AS-NtRbohF; Ji and Park, 2011). We observed that RbohF and RbohD mRNA also were reduced in AS-NtRbohD and AS-NtRbohF (Supplemental Fig. S5), respectively. The mRNA of RbohD and RbohF share high sequence similarity (81%; query coverage, 89%), suggesting that the antisense cDNA of RbohD and RbohF can down-regulate RbohF and RbohD, respectively. Therefore, we refer to these transgenics hereafter as AS-NtRbohD/F. Importantly, under control and post-NaCl treatment conditions, the AS-NtRbohD/F plants showed apoplastic NtPAO similar to the wild type (Supplemental Fig. S5). Interestingly, neither O2·−, as expected, nor H2O2 significantly accumulated post-NaCl treatment in the two transgenic genotypes under control and stress conditions (Figs. 6 and 7). These results point to the importance of NADPH oxidase in the production of ROS under short-term NaCl treatment.
Figure 6.
Intracellular/extracellular H2O2 in guard cells of wild-type (WT), AS-NtRbohD, and AS-NtRbohF plants post-NaCl treatment. A, CLSM images of intracellular BES-H2O2-Ac fluorescence (green) and chlorophyll (Chl) autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes denote nuclei. Images are representative of three independent experiments with six micrographs per genotype in each time point. Bars = 20 μm. B, CLSM images of intercellular AUR fluorescence (red) at 0, 1, and 6 h post-NaCl treatment. Images are representative of three independent experiments with six micrographs per genotype in each time point. Bars = 20 μm.
Figure 7.
Intracellular/extracellular O2·− in guard cells of wild-type (WT), AS-NtRbohD, and AS-NtRbohF plants post-NaCl treatment. A, CLSM images of intracellular BES-So-Am fluorescence (green) and chlorophyll (Chl) autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes (black in merged images) denote nuclei. Images are representative of three independent experiments with six micrographs per genotype in each time point. Quantification of green signal is shown at right. Bars = 20 μm. B, CLSM images of intercellular BES-So fluorescence (green) and chlorophyll autofluorescence (red) at 0, 1, and 6 h post-NaCl treatment. White boxes denote nuclei. Images are representative of three independent experiments with six micrographs per genotype in each time point.
In order to confirm the previous result and examine the contribution of PAO/NADPH oxidase to a presumably sustained H2O2 accumulation, we used a pharmacological approach. We used the potent inhibitors diphenyleneiodonium (DPI; 50 μm) and guazatine (Guaz; 5 μm) to inhibit NADPH oxidase and PAO, respectively. Our guard cell assay cannot be used to assay sustained H2O2 accumulation, since even the untreated leaf strips die out after approximately 12 h. In order to estimate H2O2 for a prolonged time (up to 72 h), we used whole leaves. In all genotypes, DPI ameliorated NaCl-induced H2O2 production (Supplemental Fig. S6). These data point out that NADPH oxidase contributes significantly to the accumulation of H2O2. In the presence of Guaz and NaCl, H2O2 accumulation was induced relative to the control, albeit to a lesser extent. The strong effect of DPI at early time points (compare 6 h with 72 h) indicates the importance of NADPH oxidase for ROS homeostasis at the onset of stress. As expected, the accumulation of H2O2 was further inhibited by the simultaneous addition of both DPI and Guaz, supporting the notion that the two enzymes cooperate in constituting a feed-forward ROS amplification loop.
We should note that DPI inhibits PAO activity among others; however, the potency of this inhibition is much weaker than that of Guaz (Moschou et al., 2008c). We estimated the activity of PAO in the presence of DPI or Guaz. Under our experimental conditions, in the wild type and S2.2, DPI slightly inhibited the apoplastic PAO activity (approximately 15%; Supplemental Fig. S7). However, Guaz nullified the activity of PAO in both genotypes within 6 h. We assume that the weak inhibitory effect of DPI on PAO is not significant.
DISCUSSION
In this work, we studied the contribution of the apoplastic PAO and the plasma membrane NADPH oxidase to ROS accumulation and how their cross talk regulates ROS homeostasis. Building on the unexpected observation that PAO regulates O2·− accumulation, the results presented here allow us to propose a model in which a feed-forward amplification loop that involves apoplastic PAO and NADPH oxidase controls ROS accumulation. Our model integrates the observations that apoplastic PAO positively influences the activity of NADPH oxidase and that NADPH oxidase is upstream of PAO in the relay of events that control ROS accumulation. By detailing the relationship between PAO and NADPH oxidase, we could show the absolute requirement of NADPH oxidase for ROS production within the first few hours of NaCl treatment. The apoplastic PAO functions as an amplifier of the initial ROS accumulation controlled by NADPH oxidase. Taken together, our model suggests that the apoplastic PAO feeds a stress-inducible ROS amplification loop that can lead to ROS accumulation above a toxicity threshold, culminating in PCD. Our findings allow us to extend our understanding of how apoplastic PAOs control tolerance responses during stresses. Notably, the tissue-wide role of NADPH oxidase and apoplastic PAO in ROS regulation can be detailed in a single-cell context, the guard cells, by the careful selection of specific ROS probes. The observed positive correlation between O2·− and apoplastic PAO levels upon short-term NaCl treatment at an organ level (leaf; Fig. 1) could be extrapolated in guard cells (Figs. 2–4). This finding simplifies analyses of ROS accumulation, considering the unique advantages of guard cells as a study system: accessibility for microscopic studies and homogenicity. The latter reason can be quite important considering that different cell types can have different contributions to ROS levels.
But to what extent are the NADPH oxidase and apoplastic PAO important for guard cell physiology? It is well established that both of them contribute to the regulation of stomatal aperture, and this role is executed through their intrinsic relation to ROS (Zhang et al., 2009; Fincato et al., 2012, and refs. therein). Loss of RBOHF in Arabidopsis leads to the partial impairment of ABA-induced stomatal closure, which is further reduced, and ROS production is abolished in an AtRbohD/F mutant, suggesting that the two genes act redundantly in the control of stomatal aperture (Chater et al., 2015). In addition, AOs positively contribute to stomatal closure in grapevine (Vitis vinifera; Paschalidis et al., 2010). In contrast, the acetylation of 1,3-diaminopropane, a product of apoplastic PAO by N-ACETYLTRANSFERASE ACTIVITY1 in Arabidopsis, can result in the slowing of stomatal closure (Jammes et al., 2014). Thus, both enzymes are of critical importance to the physiology of stomatal aperture and may act redundantly or cooperatively in the same ROS network.
Feed-forward loops offer an evolutionarily conserved solution to the problem of signal amplification (Cordero and Hogeweg, 2006). Their overabundance in signaling networks most likely reflects their incremental acquisition of adaptive single interactions between different components within the network. Plants have evolved a wide array of feedback loops to control a variety of physiological responses upon various exogenous or endogenous signals. For example, salicylate operates in a feed-forward ROS loop that culminates in cell death (Yun and Chen, 2006). Feed-forward loops for ROS amplification have been described in nonplants as well, between NADPH oxidase and mitochondria-derived ROS (Graham et al., 2012). These loops are subordinate to additional signals, such as metabolic perturbations (e.g. Glc deprivation). Likewise, the PAO/NADPH oxidase loop is subordinate to exogenous stress; activation of this loop requires NaCl treatment. In the absence of NaCl, the loop could not be initiated, even though in S2.2, NADPH oxidase was increased in the controls (Fig. 5). Indeed, under control conditions, the cellular content of O2·− and H2O2 does not differ significantly among the wild type, A2, and S2.2, as well among the wild type and AS-NtRbohD/F (Figs. 1–4). On the contrary, NaCl treatment dramatically increases both H2O2 and O2·− in S2.2; these ROS increase moderately in the wild type and at very low levels in A2, both intracellularly and extracellularly. Taken together, these findings suggest that the PAO/NADPH oxidase loop is subordinate to yet unidentified signals.
What is the nature of the signals that bring about the activation of the PAO/NADPH oxidase loop? Considering that this loop is activated early after the onset of salinity, it is highly unlikely that it is activated by time-consuming pathways, such as lengthy transcriptional cascade(s). In fact, accumulating evidence supports that NADPH oxidase is amenable to several regulatory posttranslational modifications (Li et al., 2014). Likewise, apoplastic PAO activity also may be controlled by posttranslational modifications. In maize, apoplastic PAO activity is controlled by its phosphorylation status (Cona et al., 2006). An alternative scenario would be that the loop is not induced at all but its effect is masked by the ROS-scavenging machinery. In accordance, an adaptive regulation of the ROS-scavenging machinery has been suggested to dispose of surplus H2O2 produced by apoplastic PAO during development (Moschou et al., 2008a). This is supported by the absence of significant ROS accumulation in S2.2, although NADPH oxidase is preinduced in this line (Fig. 5). During stress, a transient decrease of the antioxidant machinery may lead to the unmasking of the effect of the PAO/NADPH oxidase loop that is further enhanced by additional signaling pathways. These two scenarios are not mutually exclusive and may both be plausible, perhaps at different times/phases.
Taking into consideration the potency of the PAO/NADPH oxidase loop to the overall ROS contribution, the next question is to what extent these ROS signal downstream events. A dedicated set of sensor proteins is involved in the perception of ROS signals (Bosch et al., 2014). These proteins are clustered in networks that mediate signaling events leading to downstream responses, including changes in gene expression and the activation of cell death programs. Our work highlights that the PAO/NADPH oxidase loop has the potential to trigger cell death. Indeed, this loop produces ROS of sufficient quantity to drive protein oxidation and to reach a level of cellular toxicity (Supplemental Fig. S3). Protein oxidation might be the tip of the iceberg in myriad additional cell-wide consequences brought about by the PAO/NADPH oxidase loop, which is set in motion by NaCl treatment and may affect many downstream processes that culminate in cell death execution. Certainly, this loop might just be a hub in a plethora of additional pathways that refine the decision toward cell death. However, it seems likely that the PAO/NADPH oxidase loop possesses a central regulatory role in the execution of cell death, taking into consideration the tight association between apoplastic PAO levels and cell death levels.
An interesting twist to our story is the possible temporal dependence for a PAO/NADPH oxidase loop. The application of DPI significantly affected H2O2 levels mostly at early time points (6 h), while Guaz had a minor effect that was escalated with time (more than 24 h; Supplemental Fig. S6). We speculate that this time-resolved effect of the two inhibitors may indicate the initial importance of the PAO/NADPH oxidase loop; then, PAO is uncoupled from NADPH oxidase and is required to sustain ROS levels. In support of this, AS-NtRbohD/F failed to accumulate O2·− and H2O2 (Figs. 6 and 7) during the early stages of salinity, although they contained wild-type-like levels of apoplastic PAO. This finding suggests that NADPH oxidase is upstream of the apoplastic PAO in ROS regulation and that an initial ROS accumulation by NADPH oxidase might be important for triggering the activation of the apoplastic PAO pathway. However, we should note that the interaction between PAO/NADPH oxidases and their feed-forward relationships do not allow us at this stage to efficiently disentangle their distinct contribution to ROS levels. Considering that inhibitors may be imposed to differential uptake during different stages of stress, our model regarding the temporal emergence of the loop requires further refinement.
Overall, our data suggest that NADPH oxidase and the apoplastic PAO are not parallel pathways for ROS production. Instead, they form a nexus and cross talk in the frame of the strategy of plant cells to regulate ROS homeostasis. In addition, NADPH oxidase and apoplastic PAO show a feed-forward relationship in which high PAO levels correlate with high NADPH oxidase activity. Therefore, the two proteins are part of the same ROS homeostatic regulatory module, which affects the extracellular and intracellular cross talk of ROS regulatory mechanisms. However, it is still unclear to what extent intracellular PAOs affect this module. We previously established that, in Arabidopsis, a peroxisomal PAO cross talks with NADPH oxidase to activate the mitochondrial alternative oxidase pathway (Andronis et al., 2014). To advance our understanding of PAO/NADPH oxidase cross talk, the next critical step could be to explore how ROS signals are transduced/perceived for the fine orchestration of this cross talk and the relationship between apoplastic and intracellular PAOs in this regulation.
MATERIALS AND METHODS
Preparation of Transgenic Plants and Growth Conditions
The preparation of transgenic tobacco (Nicotiana tabacum ‘Xanthi’) plants with altered expression of the maize (Zea mays) POLYAMINE OXIDASE (ZmPAO) gene (lines A2 and S2.2) has been described previously (Moschou et al., 2008a, 2008b). The preparation of transgenic tobacco specifically down-regulating the two genes coding NADPH oxidase, RbohD and RbohF, was described by Ji and Park (2011). Surface-sterilized transgenic seeds (T3 homozygous) were cultured on solid Murashige and Skoog medium (pH 5.8) and then transferred to soil under light (16/8-h photoperiod and 100 μmol photons m−2 s−1) at 25°C ± 5°C. Two- to 3-week old-plants were used.
RNA Extraction Quantitative PCR
Total RNA preparation was performed as described previously (Wi and Park, 2002). The primers used (Bionics) are shown in Supplemental Table S1. One microgram of total RNA from leaves was reverse transcribed for 30 min at 42°C in a 20-μL reaction volume using the High Fidelity PrimeScript RT-PCR kit (Takara) according to the manufacturer’s instructions. Quantitative PCR was carried in the Chromo 4 Continuous Fluorescence Detector (Bio-Rad). Cycle threshold values were analyzed using MJ Opticon Monitor Software version 3.1 (Bio-Rad) and then exported to Microsoft Excel for further analysis. The reference gene β-ACTIN was used.
Protein Extraction, Western Blotting, in Gel Enzymatic Assays, and Electrophoresis
Proteins were extracted and treated as described by Papadakis and Roubelakis-Angelakis (2005). For NADPH oxidase activity staining, the procedure was carried out according to Carter et al. (2007). An aliquot containing 100 µg of protein from each tissue homogenate was electrophoresed on a 10% native PAGE gel. The gel was then incubated in 0.5 mg mL−1 NBT in 10 mm Tris, pH 7.4, supplied with 134 mm NADPH until bands were detected. For PAO activity staining, 50 μg of protein extracts was electrophoretically resolved on a 10% polyacrylamide gel. Subsequently, the gel was incubated in 50 mm phosphate buffer (pH 7) for 30 min, to which 10 mm Spd was added for a further 10 min. The gel was rinsed and then incubated in 50 mm phosphate buffer (pH 7) containing 1 mg mL−1 DAB. Protein samples that were incubated with 1 μm Guaz prior to electrophoresis were used as negative controls.
PAO and DAO Enzymatic Assay
The spectrophotometric method developed by Federico et al. (1985) was used to determine apoplastic PAO and DAO activities. Absorbance was read at 460 nm.
Determination of Endogenous PAs
PAs were analyzed as described by Goren et al. (1982). Leaves (0.2 g) were homogenized in 0.5 mL of 5% (v/v) perchloric acid and centrifuged at 15,000 rpm for 20 min. Then, 0.2 mL of saturated sodium carbonate and 0.4 mL of dimethylaminonaphthalene-1-sulfonyl chloride (1 mg mL−1 in acetone) were added to 0.2 mL of the supernatant, and the mixture was incubated at room temperature for 24 h in the dark. The dansylated products were extracted with benzene and separated by thin-layer chromatography in chloroform:triethylamine (25:2, v/v). The separated PAs were scraped off and quantified using a spectrophotofluorimeter (RF-1501; Shimadzu; http://www.shimadzu.com), by which the emission at 495 nm was recorded after excitation at 350 nm. Alternatively, PAs were determined as described previously (Kotzabasis et al., 1993) using an HP 1100 high-performance liquid chromatograph (Hewlett-Packard).
Photometric Determination of H2O2 Levels
The endogenous levels of H2O2 content of the tissues were determined as described by Sahebani and Hadavi (2009). Fresh leaf material (100 mg) was homogenized in an ice bath with 0.375 mL of 0.1% (w/v) TCA. The homogenate was centrifuged at 7,000 rpm for 20 min, and 0.25 mL of the supernatant was added to 0.25 mL of 10 mm potassium phosphate buffer (pH 7) and 0.5 mL of 1 m KI. The absorbance of the supernatant was read at 390 nm. The content of H2O2 was determined using a standard curve.
In Situ Detection of ROS
In situ accumulation of H2O2 was detected using the method of Thordal-Christensen et al. (1997) and that of O2·− according to Jabs et al. (1996). In addition, NaCl-treated tobacco leaves were incubated for 2 h in NBT staining solution (1 mg mL−1, pH 7.8, 10 mm potassium phosphate buffer) at room temperature. To detect the in situ accumulation of H2O2, NaCl-treated tobacco leaves were incubated for 2 h in DAB staining solution (1 mg mL−1, pH 3.8) at room temperature. Tobacco leaves were destained by boiling in 96% (v/v) ethanol and then photographed using a digital camera.
Confocal Microscopy Detection of ROS in Guard Cells
For fluorescent detection of ROS, leaf epidermal strips were used. For DCFDA (Sigma Chemical), strips were floated on a solution of 10 mm in 20 mm potassium phosphate buffer (pH 6) for 10 min (excitation, 450 ± 490 nm; barrier, 520 ± 560 nm). AR and AUR (Invitrogen) were used at a concentration of 50 mm in 50 mm sodium phosphate buffer (pH 6) for 1 h in the dark (for AR, excitation, 571 nm; emission, 585 nm; for AUR, excitation, 568 nm; emission, 581 nm). BES-H2O2-Ac and BES-H2O2 (WAKO Chemicals) were used at a concentration of 50 mm in 20 mm potassium phosphate buffer (pH 6) for 1 h in the dark (excitation, 485 nm; emission, 530 nm). BES-So-Am and BES-So (WAKO Chemicals) were used at a concentration of 20 mm potassium phosphate buffer (pH 6) for 1 h in the dark (excitation, 505 nm; emission, 544 nm). Fluorescence was observed using the confocal laser scanning microscope FluoView 300 (FV 300; Olympus).
Quantification of DCF in Plant Extracts
Plant leaves were homogenized with 10 mm Tris buffer (pH 7.2) and then centrifuged at 2,000g for 5 min. The supernatant was incubated with DCFDA at room temperature for 10 min in the dark. DCF fluorescence was detected by a spectrofluorophotometer (excitation, 485 nm; emission, 525 nm; RF-1501; Shimadzu). Data were expressed as relative fluorescence per milligram of protein.
Detection of Carbonylated Proteins
Total proteins from tobacco leaves were extracted from frozen samples by grinding the tissue to a fine powder and resuspended in protein extraction buffer (50 mm Tris-HCl [pH 7.5], 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitor cocktail [Sigma Chemical]). The OxyBlot procedure (Millipore) was used to perform immunoblot detection of oxidatively modified proteins by the generation of carbonyl groups. Carbonylated proteins were detected and analyzed following the derivatization of protein carbonyl groups with 2,4-dinitrophenylhydrazine (DNP). Total proteins from tobacco leaves post-NaCl treatment (10 μg) were separated by SDS-PAGE. Following transfer to a nitrocellulose membrane, DNP-derivatized proteins were detected by an anti-DNP antibody. Oxidation index was calculated by the ratio between total proteins and standard protein of pixel-based integrated densitometric values using the OxyBlot procedure.
Trypan Blue Staining
To monitor cell death, NaCl-treated tobacco leaf discs were immersed for 1 min in a boiling solution of 10 mL of lactic acid, 10 mL of glycerol, 10 g of phenol, and 0.4% (w/v) Trypan Blue. After leaf discs had cooled down to room temperature, the solution was replaced with 70% (w/v) chloral hydrate. Leaf discs were destained overnight and then photographed using a digital camera.
Statistical and Image Analyses
Statistical analysis was carried out with SigmaPlot 12.0 statistical software. After ANOVA, Duncan’s multiple comparisons were performed. Image analysis was done using FIJI software (Schindelin et al., 2012). For image quantifications of NBT and DAB, we selected 10 regions of interest (five on each leaf side) of the same area (rectangular) and quantified the integrated density in inverted color images. These 10 measurements corresponded to a technical replicate. For quantification of fluorescent signals, the same approach was used. For the total green pixel count, we used the Adjust > Color Threshold in FIJI and regions of interest that included the guard cells. For FIJI analyses, the methods described by Moschou et al. (2013, 2016) were used.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: Zea mays POLYAMINE OXIDASE, NC_024468; Nicotiana tabacum POLYAMINE OXIDASE, NP_001313211; Nicotiana tabacum RESPIRATORY BURST OXIDASE HOMOLOG F, EU072744; and Nicotiana tabacum RESPIRATORY BURST OXIDASE HOMOLOG D, EF366670.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Endogenous PA levels in the leaves of wild-type, A2, and S2.2 transgenic plants under control and 24 h post-NaCl treatment.
Supplemental Figure S2. PA catalytic genes/enzymes in wild-type, A2, and S2.2 transgenic plants under control and post-NaCl treatment.
Supplemental Figure S3. PCD hallmarks in wild-type, A2, and S2.2 leaves post-NaCl treatment.
Supplemental Figure S4. Intracellular/extracellular H2O2 in guard cells of wild-type, A2, and S2.2 plants post-NaCl treatment.
Supplemental Figure S5. Relative mRNA levels of PAO, RbohD, and RbohF genes in AS-NtRbohD and AS-NtRbohF plants post-NaCl treatment.
Supplemental Figure S6. H2O2 levels in leaves 6, 24, 48, and 72 h post-NaCl treatment in the absence or presence of DPI, Guaz, or both.
Supplemental Figure S7. Apoplastic PAO activity in the presence of DPI post-NaCl treatment.
Supplemental File S1. PAO and DAO genes in tobacco.
Supplementary Material
Acknowledgments
We thank Dr. Imene Toumi for assistance in the laboratory of K.A.R.-A.
Glossary
- ROS
reactive oxygen species
- PA
polyamine
- Put
putrescine
- Spd
spermidine
- Spm
spermine
- PCD
programmed cell death
- ABA
abscisic acid
- DAB
3,3′-diaminobenzidine
- DCFDA
2′,7′-dichlorofluorescein diacetate
- DCF
dichlorofluorescein
- RS
general reactive species
- AR
Amplex Red
- AUR
Amplex Ultra Red
- DPI
diphenyleneiodonium
- Guaz
guazatine
- NBT
nitroblue tetrazolium
- DNP
2,4-dinitrophenylhydrazine
- CLSM
confocal laser scanning microscopy
Footnotes
This work was supported by European Union and Greek National funds, by the Research Funding Program THALES (grant no. MIS 377281 to K.A.R.-A.), by the Korea Research Institute of Bioscience and Biotechnology (to K.Y.P.), and by the Swedish Research Council V.R. and Carl Tryggers Stiftelse för Vetenskaplig Forskning (to P.N.M.), and it was implemented in the frame of COST Actions FA1106 and BM1307.
References
- Andronis EA, Moschou PN, Toumi I, Roubelakis-Angelakis KA (2014) Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Front Plant Sci 5: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A (2010) Plant amine oxidases “on the move”: an update. Plant Physiol Biochem 48: 560–564 [DOI] [PubMed] [Google Scholar]
- Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R (2007) Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells. Plant Physiol 143: 1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signaling. J Exp Bot 65: 1229–1240 [DOI] [PubMed] [Google Scholar]
- Bosch M, Berger S, Schaller A, Stintzi A (2014) Jasmonate-dependent induction of polyphenol oxidase activity in tomato foliage is important for defense against Spodoptera exigua but not against Manduca sexta. BMC Plant Biol 14: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Healy R, O’Tool NM, Naqvi SMS, Ren G, Park S, Beattie GA, Horner HT, Thornburg RW (2007) Tobacco nectaries express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms. Plant Physiol 143: 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang YK, McLachlan DH, Casson S, Isner JC, Wilson I, et al. (2015) Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr Biol 25: 2709–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona A, Rea G, Botta M, Corelli F, Federico R, Angelini R (2006) Flavin-containing polyamine oxidase is a hydrogen peroxide source in the oxidative response to the protein phosphatase inhibitor cantharidin in Zea mays L. J Exp Bot 57: 2277–2289 [DOI] [PubMed] [Google Scholar]
- Cordero OX, Hogeweg P (2006) Feed-forward loop circuits as a side effect of genome evolution. Mol Biol Evol 23: 1931–1936 [DOI] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot 55: 205–212 [DOI] [PubMed] [Google Scholar]
- Federico R, Angelini R, Cesta A, Pini C (1985) Determination of diamine oxidase in lentil seedlings by enzymic activity and immunoreactivity. Plant Physiol 79: 62–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincato P, Moschou PN, Ahou A, Angelini R, Roubelakis-Angelakis KA, Federico R, Tavladoraki P (2012) The members of Arabidopsis thaliana PAO gene family exhibit distinct tissue- and organ-specific expression pattern during seedling growth and flower development. Amino Acids 42: 831–841 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2016) Stress-triggered redox signaling: what’s in pROSpect? Plant Cell Environ 39: 951–964 [DOI] [PubMed] [Google Scholar]
- Gémes K, Poór P, Horváth E, Kolbert ZS, Szopkó D, Szepesi Á, Tari I (2011) Cross-talk between salicylic acid and NaCl-generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiol Plant 142: 179–192 [DOI] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19: 623–630 [DOI] [PubMed] [Google Scholar]
- Goren R, Palavan N, Flores H, Galston AW (1982) Changes in polyamine titer in etiolated pea seedlings following red light treatment. Plant Cell Physiol 23: 19–26 [Google Scholar]
- Graham NA, Tahmasian M, Kohli B, Komisopoulou E, Zhu M, Vivanco I, Teitell MA, Wu H, Ribas A, Lo RS, et al. (2012) Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol Syst Biol 26: 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273: 1853–1856 [DOI] [PubMed] [Google Scholar]
- Jammes F, Leonhardt N, Tran D, Bousserouel H, Véry AA, Renou JP, Vavasseur A, Kwak JM, Sentenac H, Bouteau F, et al. (2014) Acetylated 1,3-diaminopropane antagonizes abscisic acid-mediated stomatal closing in Arabidopsis. Plant J 79: 322–333 [DOI] [PubMed] [Google Scholar]
- Ji NR, Park KY (2011) Stress-induced biphasic ethylene and ROS biosynthesis are synergistically interacted in cell damage. J Plant Biotechnol 38: 22–29 [Google Scholar]
- Kotzabasis K, Christakis-Hampsas MD, Roubelakis-Angelakis KA (1993) A narrow-bore HPLC method for the identification and quantification of free, conjugated, and bound polyamines. Anal Biochem 214: 484–489 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres M, Dangl JL (2003) NADPH oxidase Atrbohd and Atrbohf genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. (2014) The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338 [DOI] [PubMed] [Google Scholar]
- Maeda H, Yamamoto K, Kohno I, Hafsi L, Itoh N, Nakagawa S, Kanagawa N, Suzuki K, Uno T (2007) Design of a practical fluorescent probe for superoxide based on protection-deprotection chemistry of fluoresceins with benzenesulfonyl protecting groups. Chemistry 3: 1946–1954 [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33: 453–467 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Delis ID, Paschalidis KA, Roubelakis-Angelakis KA (2008a) Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol Plant 133: 140–156 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Gutierrez-Beltran E, Bozhkov PV, Smertenko A (2016) Separase promotes microtubule polymerization by activating CENP-E-related kinesin Kin7. Dev Cell 37: 350–361 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA (2008b) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20: 1708–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou PN, Roubelakis-Angelakis KA (2014) Polyamines and programmed cell death. J Exp Bot 65: 1285–1296 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Sanmartin M, Andriopoulou AH, Rojo E, Sanchez-Serrano JJ, Roubelakis-Angelakis KA (2008c) Bridging the gap between plant and mammalian polyamine catabolism: a novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol 147: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou PN, Sarris PF, Skandalis N, Andriopoulou AH, Paschalidis KA, Panopoulos NJ, Roubelakis-Angelakis KA (2009) Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiol 149: 1970–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou PN, Smertenko AP, Minina EA, Fukada K, Savenkov EI, Robert S, Hussey PJ, Bozhkov PV (2013) The caspase-related protease separase (extra spindle poles) regulates cell polarity and cytokinesis in Arabidopsis. Plant Cell 25: 2171–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65: 1575–1582 [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39: 1140–1160 [DOI] [PubMed] [Google Scholar]
- Pal M, Szalai G, Janda T (2015) Speculation: polyamines are important in abiotic stress signaling. Plant Sci 237: 16–23 [DOI] [PubMed] [Google Scholar]
- Papadakis AK, Roubelakis-Angelakis KA (2005) Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase-generated hydrogen peroxide. Planta 220: 826–837 [DOI] [PubMed] [Google Scholar]
- Paschalidis KA, Roubelakis-Angelakis KA (2005a) Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant: correlations with age, cell division/expansion, and differentiation. Plant Physiol 138: 142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschalidis KA, Roubelakis-Angelakis KA (2005b) Sites and regulation of polyamine catabolism in the tobacco plant: correlations with cell division/expansion, cell cycle progression, and vascular development. Plant Physiol 138: 2174–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschalidis KA, Toumi I, Moschou PN, Roubelakis-Angelakis KA (2010) ABA-dependent amine oxidases-derived H2O2 affects stomata conductance. Plant Signal Behav 5: 1153–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez AA, Maiale SJ, Menéndez AB, Ruiz OA (2009) Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J Exp Bot 60: 4249–4262 [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase: modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha J, Brauer EK, Sengupta A, Popescu SC, Gupta K, Gupta B (2015) Polyamines as redox homeostasis regulators during salt stress in plants. Front Environ Sci 3: 21 [Google Scholar]
- Sahebani N, Hadavi NS (2009) Induction of H2O2 and related enzymes in tomato roots infected with root knot nematode (M. javanica) by several chemical and microbial elicitors. Biocontrol Sci Technol 19: 301–313 [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyrychova I, Ayaydin F, Hideg E (2009) Detecting hydrogen peroxide in leaves in vivo: a comparison of methods. Physiol Plant 135: 1–18 [DOI] [PubMed] [Google Scholar]
- Song Y, Miao Y, Song CP (2014) Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol 201: 1121–1140 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Mittler R (2012) Reactive oxygen species-dependent wound responses in animals and plants. Free Radic Biol Med 53: 2269–2276 [DOI] [PubMed] [Google Scholar]
- Tavladoraki P, Rossi MN, Saccuti G, Perez-Amador MA, Polticelli F, Angelini R, Federico R (2006) Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Physiol 141: 1519–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Tiburcio AF, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240: 1–18 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GF, Li WQ, Li WY, Wu GL, Zhou CY, Chen KM (2013) Characterization of rice NADPH oxidase genes and their expression under various environmental conditions. J Mol Sci 14: 9440–9458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wi SJ, Park KY (2002) Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Mol Cells 13: 209–220 [PubMed] [Google Scholar]
- Wu JY, Shang ZL, Wu J, Jiang XT, Moschou PN, Sun WD, Roubelakis-Angelakis KA, Zhang SL (2010) Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J 63: 1042–1053 [DOI] [PubMed] [Google Scholar]
- Xue B, Zhang A, Jiang M (2008) Involvement of polyamine oxidase in abscisic acid-induced cytosolic antioxidant defense in leaves of maize. J Integr Plant Biol 51: 225–234 [DOI] [PubMed] [Google Scholar]
- Yun LJ, Chen WL (2006) SA and ROS are involved in methyl salicylate-induced programmed cell death in Arabidopsis thaliana. Plant Cell Rep 30: 1231–1239 [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong FC, Gao JF, Song CP (2001) Hydrogen peroxide-induced changes in intracellular pH of guard cells precede stomatal closure. Cell Res 11: 37–43 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X (2009) Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21: 2357–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Ding GH, Fang K, Zhao FG, Qin P (2006) New perspective on the mechanism of alleviating salt stress by spermidine in barley seedlings. Plant Growth Regul 49: 147–156 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.