Lipid remodeling of chloroplast membranes mediated by SENSITIVE TO FREEZING 2 protects freezing-sensitive plants such as tomatoes against cellular dehydration brought on by drought and salt stress.
Abstract
SENSITIVE TO FREEZING2 (SFR2) is crucial for protecting chloroplast membranes following freezing in Arabidopsis (Arabidopsis thaliana). It has been shown that SFR2 homologs are present in all land plants, including freezing-sensitive species, raising the question of SFR2 function beyond freezing tolerance. Similar to freezing, salt and drought can cause dehydration. Thus, it is hypothesized that in freezing-sensitive plants SFR2 may play roles in their resilience to salt or drought. To test this hypothesis, SlSFR2 RNAi lines were generated in the cold/freezing-sensitive species tomato (Solanum lycopersicum [M82 cv]). Hypersensitivity to salt and drought of SlSFR2-RNAi lines was observed. Higher tolerance of wild-type tomatoes was correlated with the production of trigalactosyldiacylglycerol, a product of SFR2 activity. Tomato SFR2 in vitro activity is Mg2+-dependent and its optimal pH is 7.5, similar to that of Arabidopsis SFR2, but the specific activity of tomato SFR2 in vitro is almost double that of Arabidopsis SFR2. When salt and drought stress were applied to Arabidopsis, no conditions could be identified at which SFR2 was induced prior to irreversibly impacting plant growth, suggesting that SFR2 protects Arabidopsis primarily against freezing. Discovery of tomato SFR2 function in drought and salt resilience provides further insights into general membrane lipid remodeling-based stress tolerance mechanisms and together with protection against freezing in freezing-resistant plants such as Arabidopsis, it adds lipid remodeling as a possible target for the engineering of abiotic stress-resilient crops.
As one of the major environmental plant stresses, freezing greatly afflicts the production of crops and limits the geographic distribution of naturally occurring plants (Pearce, 2001). Adaptations to freezing have been studied at the physiological, metabolic, molecular, and genetic levels (Andrews, 1996; Browse and Xin, 2001; Griffith and Yaish, 2004). The Arabidopsis (Arabidopsis thaliana) mutant sensitive to freezing2 (sfr2) was discovered as a freezing-sensitive plant during a forward genetic mutant screen (Warren et al., 1996). The SFR2 protein is associated with the outer envelope membrane of chloroplasts and is crucial for maintaining chloroplast membrane integrity after exposure to freezing temperatures (Fourrier et al., 2008; Roston et al., 2014). Despite the fact that this protein was originally classified as a family I glycosyl-hydrolase and originally described as glucosidase (Thorlby et al., 2004), SFR2 has galactosyltransferase activity (Moellering et al., 2010; Roston et al., 2014). Once activated under freezing conditions, SFR2 processively transfers galactosyl residues from the most abundant chloroplast membrane lipid monogalactosyldiacylglycerol (MGDG) to a second galactolipid acceptor, forming oligogalactolipids and diacylglycerol (DAG). The DAG is then converted to triacylglycerol (Moellering et al., 2010). This mechanism of remodeling chloroplast membrane lipids upon freezing helps adjusting envelope membranes by removing extra polar lipids as the organelle shrinks due to apoplastic ice formation and cellular dehydration. Membranes are stabilized by increasing the ratio of bilayer-forming to nonbilayer-forming lipids. In addition, through increased abundance of polar oligogalactolipid head groups, the hydration of the envelope membrane is increased during freezing-related dehydration (Moellering et al., 2010).
Phylogenetic analysis shows that SFR2 orthologs are ubiquitous in the genomes of land plants, including freezing-sensitive species. Ectopic expression of SFR2 orthologs from freezing-sensitive soybean (Glycine max) and rice (Oryza sativa) has been shown to revert the sfr2 phenotype in Arabidopsis (Fourrier et al., 2008). This finding suggests that SFR2 orthologs are functionally interchangeable regardless of their origin from freezing-tolerant or -sensitive species. Therefore, we hypothesized that SFR2 must play physiological roles beyond freezing resilience in plants that never encounter freezing temperatures in their natural habitats. Given the sessile nature of all plants, encountering unfavorable growth conditions even in lush tropical environments is at times inevitable. Unfavorable conditions might include periods of high temperature and drought, or increased salt concentrations in soils following coastal flooding. Salt and drought stress are conceptually closely related to freezing, as all are accompanied by severe dehydration of plant cells (Andrews, 1996; Verslues et al., 2006). Drought stress directly decreases the water potential in the apoplast and causes water deficiency within the symplast. Increased salt exposure can change the cellular ion and solute homeostasis, which directly limits water availability for other cellular processes such as biochemical reactions or membrane hydration. Upon freezing, cellular dehydration is caused by extracellular ice formation, which acts as a nucleation site for water drawn out of the cell (Andrews, 1996). Cellular dehydration in plants usually is accompanied by the shrinkage of the symplast and organelles, bringing different cellular membranes into close proximity, which can lead to the fusion of membranes along with membrane bilayer structure disruption. To alleviate cellular dehydration, plants have evolved common signaling pathways, transcriptional responses, and metabolic adjustments shared in response to freezing, salt, and drought exposure (Zhu, 2002; Mahajan and Tuteja, 2005; Krasensky and Jonak, 2012), further suggesting that these abiotic stresses elicit similar protective mechanisms in plants.
As outlined above, SFR2-mediated remodeling of membrane lipids was shown to protect chloroplast membrane structure during dehydration caused by freezing. The same may be true for cellular dehydration as the result of salt or drought stress. In fact, in the resurrection plant Craterostigma plantagineum MGDG is increasingly converted to digalactosyldiacylglycerol (DGDG) and DAG during desiccation (Gasulla et al., 2013). Even though the responsible enzyme has not yet been identified, this observation suggests that MGDG conversion to higher order galactolipids might be a general strategy for plants coping with dehydration. Recently, SFR2 was shown to interact in guard cells with Open Stomata1, an SnRK2-type protein kinase involved in mediating ABA responses in Arabidopsis (Waadt et al., 2015). It remains to be shown whether this interaction is physiologically relevant or whether SFR2 may be involved in remodeling of chloroplast membranes in guard cells in response to dehydration. Here, tomato (Solanum lycopersicum) was chosen as a representative cold-sensitive plant to explore possible roles of SFR2 in the protection of plants against dehydration-inducing abiotic stresses other than freezing. In addition, we tested whether SFR2 could protect Arabidopsis against other abiotic stresses than freezing.
RESULTS
Salt and Drought Treatment Similarly Affect Arabidopsis Wild Type and sfr2-3 Mutant
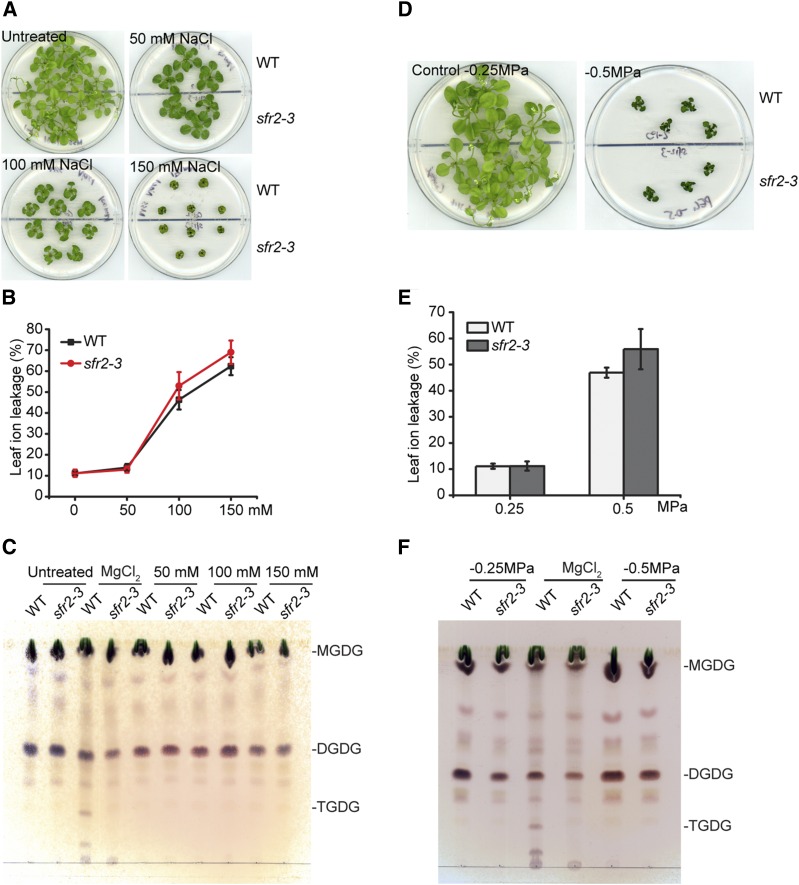
SFR2 has been shown to be essential for freezing tolerance in Arabidopsis. In principle, if SFR2 provides protection against cellular dehydration in general, one might expect that it also protects Arabidopsis against salt and drought stress. To test this hypothesis, we investigated whether salt or drought tolerance is compromised in the Arabidopsis sfr2-3 mutant, which carries an sfr2 loss-of-function allele (Moellering et al., 2010). For the salt stress assay, wild-type (Col-2) and mutant sfr2-3 seedlings were grown for 1 week on regular Murashige and Skoog (MS) medium prior to the transfer to MS plates containing different concentrations of NaCl (Fig. 1). Within 3 weeks of treatment, all plants were increasingly stunted with increasing NaCl concentrations (Fig. 1). No significant difference in growth was observed between the wild type and sfr2-3 (Fig. 1). Ion leakage of the leaf tissues also increased in parallel with NaCl concentrations with no difference observed for the wild type and sfr2-3 (Fig. 1). To check whether SFR2 was activated during the treatment, lipids were analyzed by thin-layer chromatography (TLC; Fig. 1). To activate SFR2 in vivo, leaves were treated by MgCl2 infiltration (Moellering et al., 2010) and as a result, wild-type leaves contained trigalactosyldiacylglycerol (TGDG), a specific product of SFR2, while sfr2-3 did not (Fig. 1). No TGDG was detected in all salt-treated plant leaves, suggesting that SFR2 was not activated under the conditions tested (Fig. 1). Root bending assays in response to NaCl treatment and direct salt treatment of soil-grown plants showed consistently no differences between the wild type and the sfr2-3 mutant (Supplemental Fig. S1).
Figure 1.
SFR2 is not involved in protection against salt or drought stress in Arabidopsis. A and D, Images of plants grown on MS agar plates with (A) different concentrations of NaCl or with (D) different water potentials as indicated. One-week-old, normal, MS-grown seedlings were transferred to salt or drought mimicking plates and grown for 3 weeks before the pictures were taken. B and E, Ion leakage measurements of the salt (B) or drought (E)-treated plant leaves (n = 4, ± sd). C and F, TLC of the lipids from salt-stressed leaves (C) or drought-stressed leaves (D). MgCl2-treated wild-type samples served as positive controls for SFR2 activation. Lipids were stained for sugar head groups.
For the drought assay, wild-type and sfr2-3 plants were grown on control MS plates (Ψ = −2.5 MPa) for 1 week before transfer to other plates with lower water potentials (Fig. 1). When grown on MS medium with Ψ as low as −7.5 MPa, both wild type and sfr2-3 were dead after 2 weeks. After 3 weeks of growth on MS medium with Ψ = −5 MPa, plants were alive but growth was pronouncedly compromised (Fig. 1). No differences were observed between wild type and sfr2-3 (Fig. 1). In addition, no TGDG accumulation was observed, suggesting that SFR2 was not activated during the drought treatment (Fig. 1). Similar results were also observed for soil-grown plants as shown in Supplemental Figure S2.
Apparently, for Arabidopsis no condition of salt or drought treatment could be identified that would lead to activation of SFR2 before the plants were irreversibly compromised. One interpretation is that salt and drought stress affect essential cellular processes other than chloroplast membrane stability and if those become compromised in Arabidopsis prior to the membranes, the hypothesis stated above cannot be tested in Arabidopsis. Therefore, we shifted the focus of this study to the freezing-sensitive tomato, in which we were able to study the function of SFR2 in other abiotic stresses than freezing.
Identification of an SFR2 Ortholog of Tomato
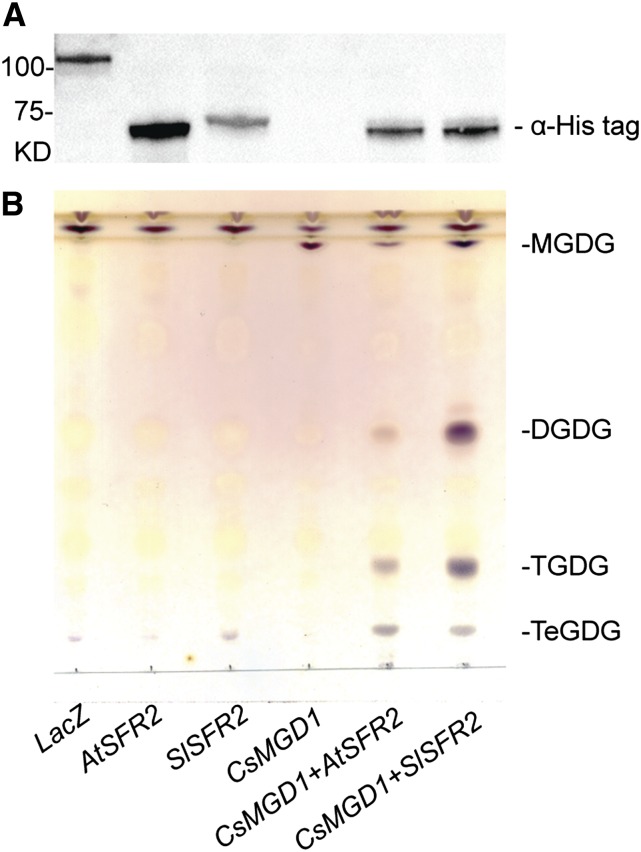
To identify the SFR2 ortholog of tomato (M82), a BLASTp search of the tomato genome protein database (http://solgenomics.net/) was performed using the Arabidopsis SFR2 sequence (AtSFR2) as the query (Fernandez-Pozo et al., 2015). Tomato gene Solyc01g058140.2.1 encoded the top-scoring protein fragment with a reported E-value of zero and the respective protein was designated SlSFR2. The two aligned protein sequences showed 59% identity overall and 72% similarity (Supplemental Fig. S3). To corroborate that SlSFR2 has galactolipid:galactolipid galactosyltransferase activity, the respective cDNA was expressed in a yeast strain that also expresses a Cucumis sativus MGDG synthase-encoding cDNA (CsMGD1; Shimojima et al., 1997) ensuring the production of MGDG, which is the substrate for SFR2. Arabidopsis and tomato SFR2 cDNAs were tested in parallel. SFR2 protein production in yeast was confirmed by immunoblot using an anti-His antibody against a C-terminal 6xHis-TAG included with both constructs (Fig. 2), and the lipids were extracted from induced cultures. When LacZ, AtSFR2, or SlSFR2 were expressed in yeast lacking MGDG, no galactolipids were detected by TLC (Fig. 2). When CsMGD1was expressed alone, only MGDG was present. Coexpression of SlSFR2 or AtSFR2 with CsMGD1 led to the formation of the oligogalactolipids DGDG, TGDG, and tetragalactosyldiacylglycerol (TeGDG). Therefore, SlSFR2 encodes an SFR2 protein with galactolipid:galactolipid galactosyl transferase activity.
Figure 2.
SlSFR2 is a galactolipid:galactolipid galactosyltransferase (GGGT). A, Immunoblot of the proteins extracted from yeast that are transformed with constructs as indicated. LacZ, AtSFR2, and SlSFR2 constructs incorporate a His-tag at the C terminus of the protein but not the CsMGD1 construct. B, TLC of the lipids extracted from yeast strains as indicated. Galactolipids were selectively stained by α-naphthol. Strains solely producing LacZ, AtSFR2, or SlSFR2 cannot produce galactolipids (left three lanes). Expression of CsMGD1 produces MGDG (the fourth lane) and coexpressing CsMGD1 with AtSFR2 or SlSFR2 results in the production of MGDG and DGDG, TGDG, and TeGDG (fifth and sixth lanes).
SlSFR2 Has Higher Specific Activity than AtSFR2
It seemed possible that the specific activity and activation of SFR2 orthologs from freezing-insensitive and -sensitive plants differ. The activity of AtSFR2 is Mg2+- and pH-dependent (Moellering et al., 2010; Roston et al., 2014; Barnes et al., 2016), and we tested whether this was also the case for SlSFR2. To estimate SlSFR2- and AtSFR2-specific activities, microsomes were prepared from the respective yeast cultures, and MGDG-based galactosyl transfer activity was assayed in the presence of 8 mm MgCl2 at pH 7.5 (Moellering et al., 2010) using approximately equal amounts of SFR2 proteins as estimated by immunoblotting (Fig. 3). A LacZ expressing line was included as a background control. Under these conditions, SlSFR2 activity was nearly twice that of AtSFR2 (Fig. 3) and given that approximately equal amounts of protein were used, its specific activity must be higher than that of the Arabidopsis enzyme. Using different concentrations of MgCl2 ranging from 0 to 12 mm (Fig. 3), both enzymes showed similarly strong Mg2+ dependency of their activity. When the pH dependency of SlSFR2 was tested using a series of buffers with varying pH, it was apparent that the optimal pH for SlSFR2 is approximately 7.5 (Fig. 3), which agrees with that observed for AtSFR2 (Roston et al., 2014). Thus, aside from an observed higher specific activity of SlSFR2, both orthologs behaved essentially the same under the conditions tested.
Figure 3.
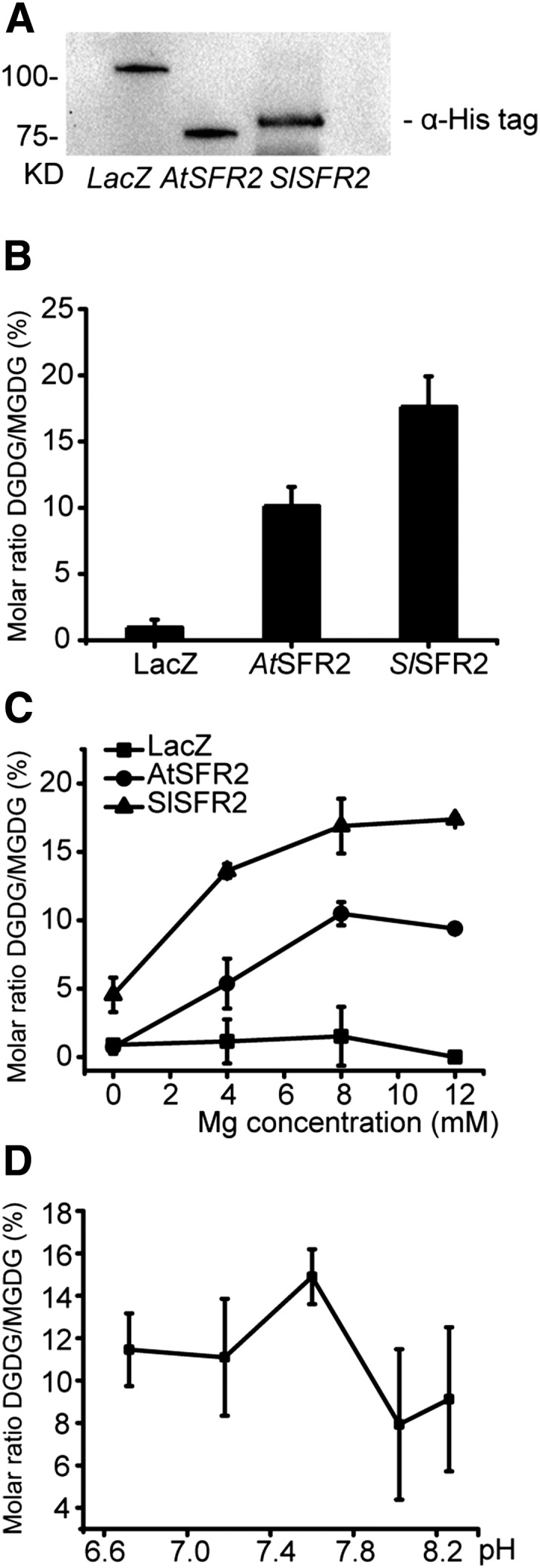
Activity of SlSFR2 in vitro. A, Immunoblot of yeast microsomes containing approximately equal amounts of recombinant His-tagged LacZ and SFR2 proteins. AtSFR2 and SlSFR2 microsomes with these comparable protein amounts were used for the assays. B, Activity comparison between AtSFR2 and SlSFR2 in a buffer with pH 7.5 and 8 mm MgCl2. Activity is shown as the molar ratio between the product DGDG and the substrate MGDG (n = 3, ±sd). C, Magnesium dependency of SFR2 activity. Assay buffers contained MgCl2 concentrations ranging from 0 to 12 mm (n = 3–4, ± sd). D, pH dependency of SlSFR2 activity. Assays buffers had a range of pHs from 6.72 to 8.26 (n = 3, ±sd).
Generation of SlSFR2 RNAi Lines in Tomato
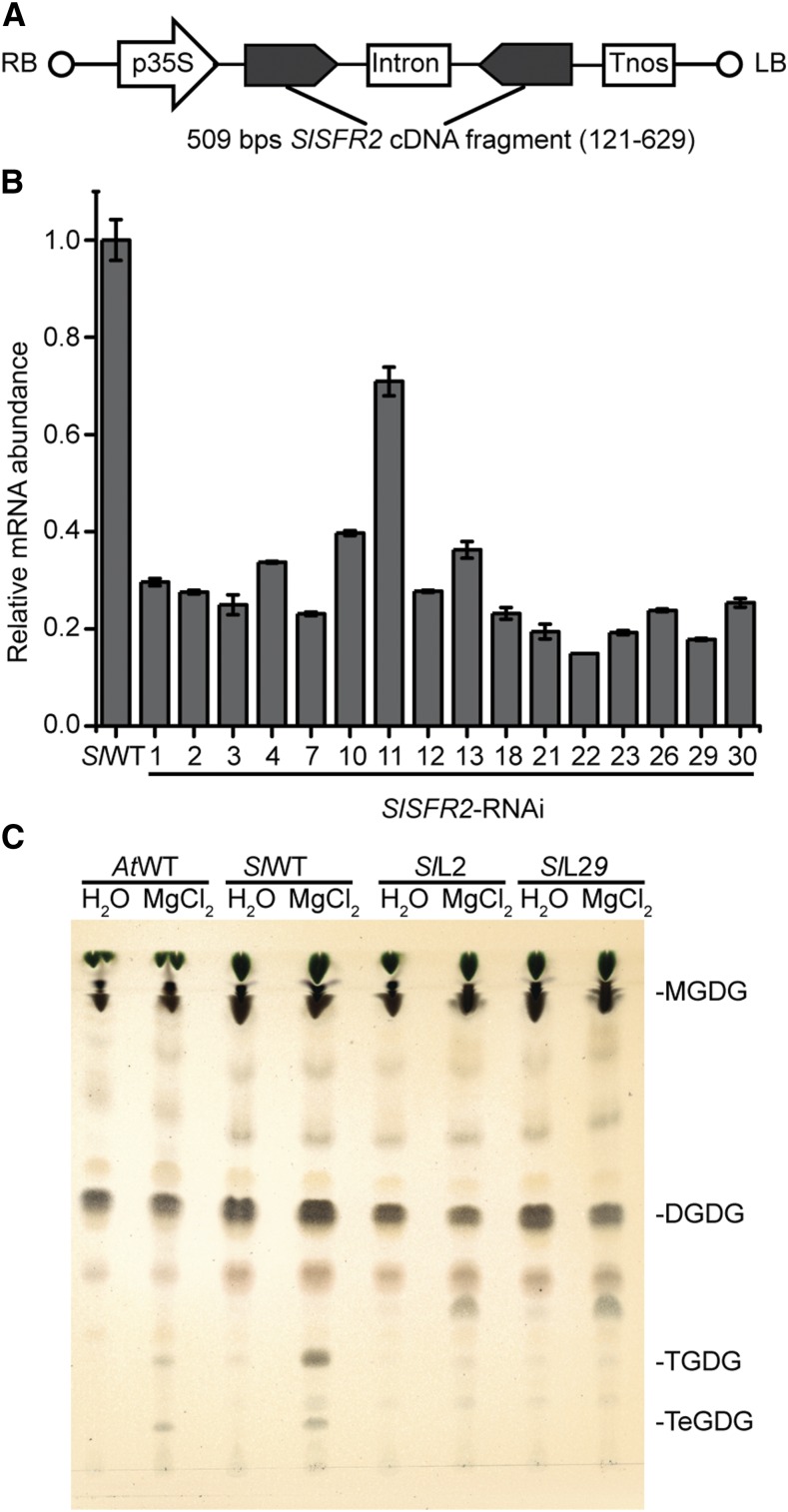
To explore physiological functions of SlSFR2 in cold-/freezing-sensitive tomato, an SlSFR2-RNAi construct was introduced into tomato M82 (Fig. 4). In total, 23 transgenic lines were generated and the presence of the SlSFR2-RNAi construct was confirmed by PCR using genomic DNA (Supplemental Fig. S4). To examine the RNAi effect, SlSFR2 transcript abundance of the transgenic lines was quantified by quantitative real-time PCR (Fig. 4), and the majority of lines showed an 80% reduction in transcript abundance.
Figure 4.
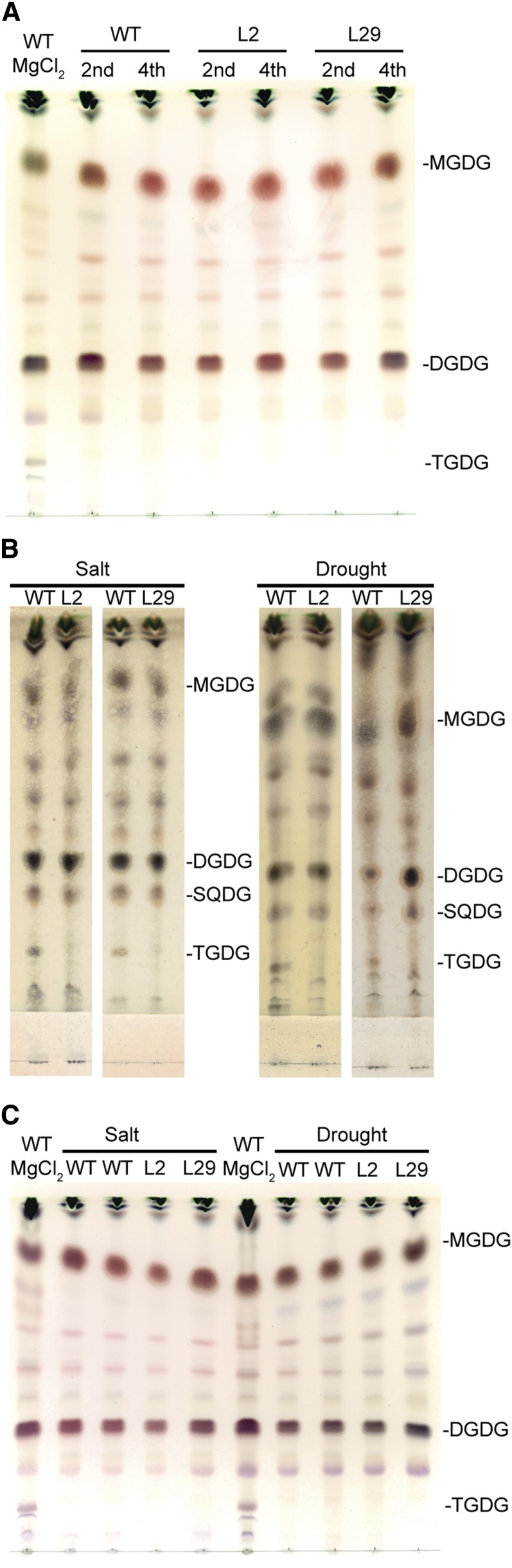
Generation the SlSFR2-RNAi lines. A, Simplified diagram of the SlSFR2-RNAi construct. RB, Right border; p35S, Cauliflower mosaic virus (CaMV) 35S promoter; Intron, pyruvate dehydrogenase kinase (PDK) intron; Tnos, NOS-terminator; LB, left border. B, Quantitative RT-PCR probing SlSFR2 mRNA abundance in wild-type (SlWT) and SlSFR2-RNAi lines. C, TLC of the lipids from Arabidopsis wild-type plants (AtWT), tomato wild-type plants (SlWT), or select transgenic lines (SlL2 and SlL29). Lipids were stained for sugar head groups. The leaves were incubated in either water or 0.5 m MgCl2 aqueous solution for 6 h before lipid extraction.
To estimate the maximal in vivo SlSFR2 activity in the RNAi transgenic lines, we incubated detached tomato leaves in a 0.5 m MgCl2 aqueous solution for 6 h, which fully activates the enzyme, or water only (mock) followed by lipid extraction and TLC of the polar lipids (Fig. 4; Supplemental Fig. S5 for additional lines). Arabidopsis and tomato wild type produced TGDG in the MgCl2-treated leaves only. However, most of the 13 lines with low SlSFR2 RNA abundance showed a decreased ability to produce TGDG following MgCl2 treatment (Fig. 4; Supplemental Fig. S5). Based on the extent of SlSFR2 transcript levels, TGDG reduction and number of seeds recovered from individual transgenic plants, the two transgenic lines 2 and 29 (L2 and L29) were selected for further study. As shown in Figure 4, L2 and L29 produced very little TGDG or TeGDG in response to MgCl2 treatment showing the effect of RNAi suppression of SlSFR2.
SlSFR2 RNAi Transgenic Lines Show Increased Salt and Drought Sensitivity
When grown under normal conditions, SlSFR2 RNAi transgenic lines L2 and L29 had no obvious growth defects at all ages tested (Fig. 5; Supplemental Figs. S6 and S7). To detect responses to cellular dehydration, SlSFR2 RNAi lines and M82 wild-type plants were subjected to salt and drought stress conditions. To apply salt stress, 2- (Supplemental Fig. S6) or 3-week-old plants (Fig. 5) were watered over a period of time with an aqueous nutrient solution containing an increasing amount of NaCl to allow slow acclimatization. Plants of two different ages were included in the analysis to observe the robustness of the response during at least two different developmental stages. Precautions were taken that at the end of the treatment, both SlSFR2 RNAi lines and M82 wild-type plants were able to recover and resume normal growth after rewatering with nutrient solution, from which NaCl was omitted. At the end of the treatment with NaCl, SlSFR2 RNAi lines and M82 wild-type plants showed a visible reduction in growth compared to nutrient solution only-watered plants (Fig. 5; Supplemental Fig. S6). Fresh weight measured for the 2-week-old L2 and L29 lines was significantly reduced (P < 0.01; t test) compared to the wild-type plants (Supplemental Fig. S6). The 3-week-old L2 and L29 plants showed increased chlorosis and decreased size of older leaves, such as the second leaf, which is the second oldest true leaf (Fig. 5). On the 2-week-old plants, the cotyledons were most chlorotic (Supplemental Fig. S6). On the 3-week-old plants, the second and the fourth true leaves were compared as representatives of older and younger leaves, respectively (Fig. 5). While the fourth leaves were slightly chlorotic on all plants but did not show much difference between wild-type and SlSFR2 RNAi transgenic lines (Fig. 5), the second leaves of L2 and L29 were more chlorotic and had visibly reduced leaf sizes (Fig. 5). Hence in general, the older leaves on either plant showed the most severe response to salt treatment, which could be described as accelerated senescence.
Figure 5.
SlSFR2-RNAi lines are sensitive to salt and drought. A, Whole-plant images of wild-type (WT) and SlSFR2-RNAi lines L2 and L29 grown under normal condition or with salt treated (B) or drought treated (C). The plants were 3 weeks old at the beginning of the treatment, and images were taken at 5 weeks of age. At right, leaf appearances of the representative fourth and second leaves are shown.
To apply drought stress, water was withheld from 3-week-old plants for 3 d followed by watering for 2 d to acclimatize the plants to drought. This acclimatization treatment was followed by withholding water for 9 d followed by rewatering for 2 d. Before rewatering, water potential and relative water content of the wild-type plants were measured as indicated in Supplemental Figure S7. Even though SlSFR2 RNAi lines were not affected at the level of the whole plant with regard to fresh weight or plant height, the older leaves as represented by the second leaves were smaller and had less fresh weight at the end of the drought period (P < 0.01; t test; Supplemental Fig. S7). After 2 d of rewatering, most severe chlorosis of older leaves was observed (Fig. 5), similar as was seen after salt treatment (Fig. 5). It should be noted that both wild-type and SlSFR2 RNAi transgenic lines were able to recover from this drought treatment following rewatering.
Chlorophyll and Protein Content of SlSFR2 RNAi Transgenic Lines
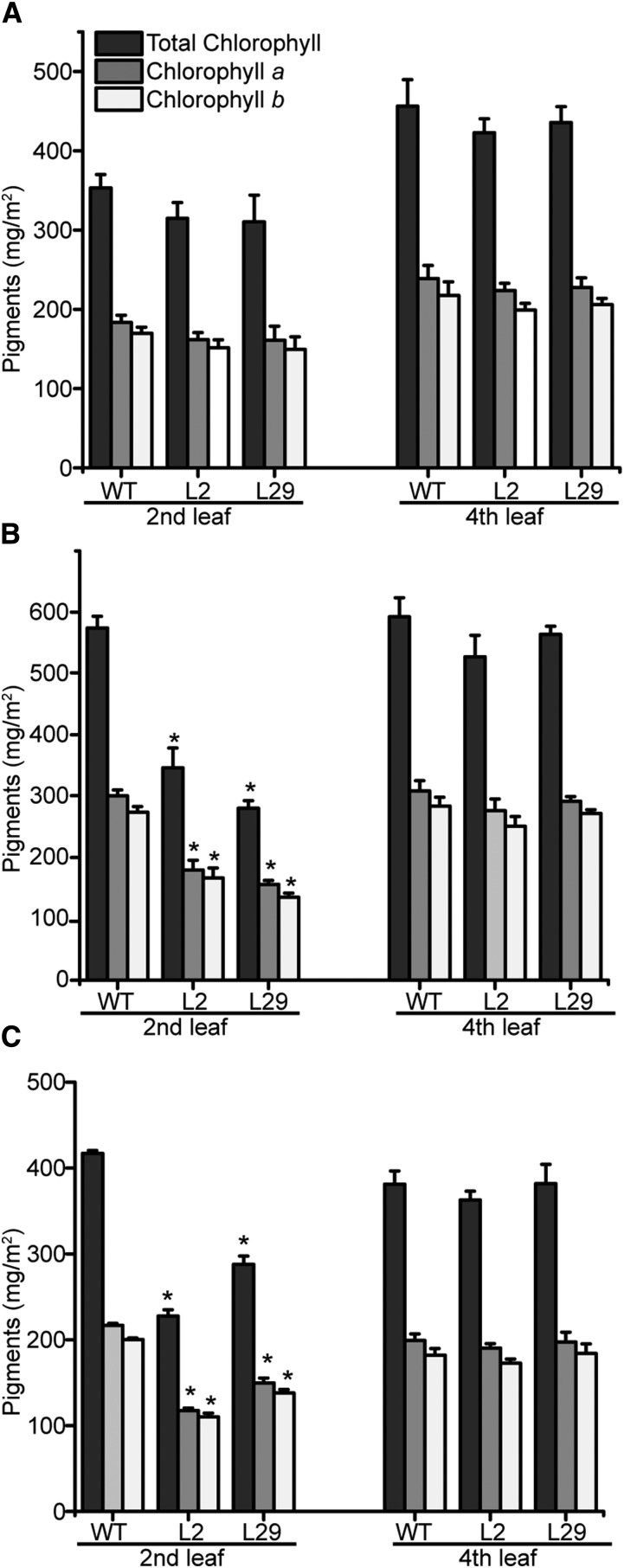
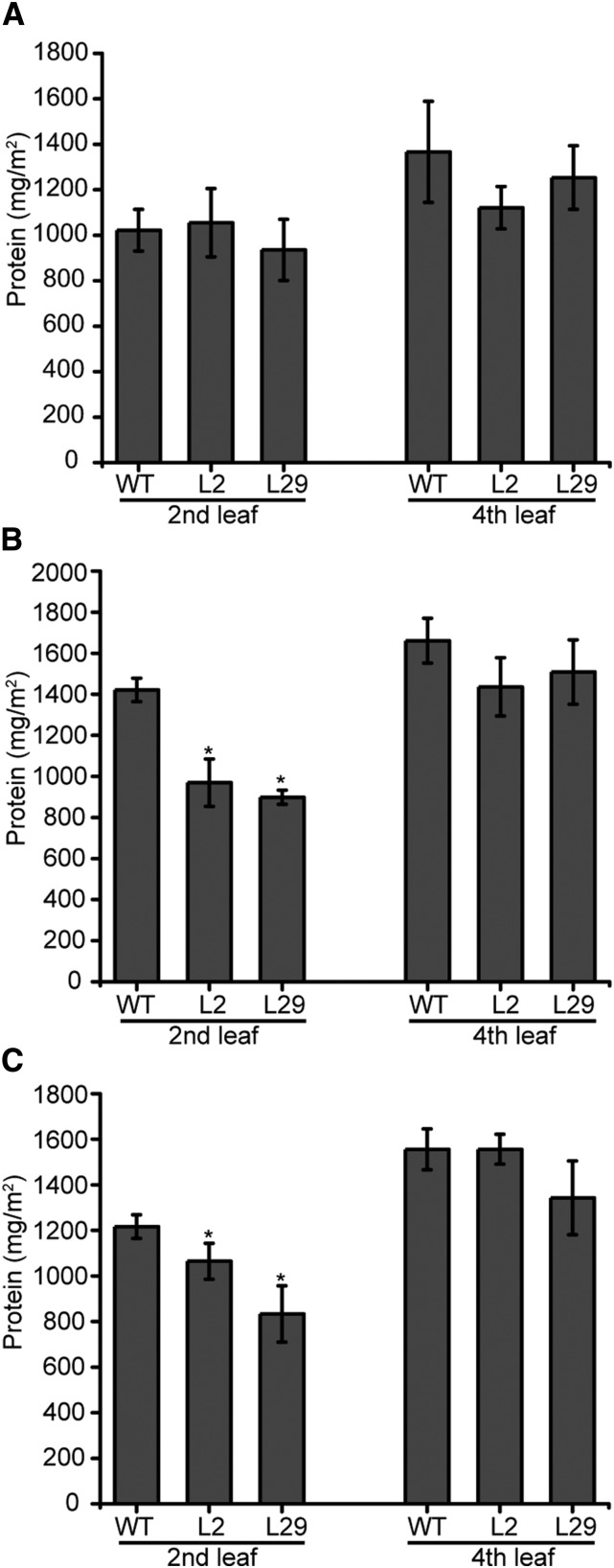
Leaf protein and chlorophyll content are sensitive markers for abiotic stresses, including salt and drought. Thus, chlorophyll contents were quantified on both the fourth and second leaves of 3-week-old-plants (Fig. 6) or the cotyledons and second leaves of 2-week-old plants (Supplemental Fig. S8) undergoing stress treatment to corroborate the visual phenotypes. Consistent with the observed chlorosis of 3-week-old plants, a decrease in chlorophyll was observed for only the second leaves during salt (Fig. 6) and drought stress (Fig. 6) or the cotyledons of 2-week-old plants following salt treatment (Supplemental Fig. S8). Similarly, measuring the total leaf protein content of 3-week-old-plants (Fig. 7), the second but not the fourth leaves of the transgenic lines showed a decrease in protein content in response to both stress treatments.
Figure 6.
Chlorophyll in the leaves of untreated and stressed 3-week-old tomato plants. A, Total chlorophyll and chlorophyll a, b content in untreated and salt-treated (B), and of drought-treated (C) wild-type (WT) and transgenic L2 and L29 lines plants as shown in Figure 5. Statistics: n = 3, ±sd. *P < 0.01 (t test).
Figure 7.
Total protein in leaves from untreated and stress treated 3-week-old tomato plants. A, Total protein in untreated and salt-treated (B) and of drought-treated (C) wild-type (WT) and transgenic L2 and L29 lines plants as shown in Figure 5. Statistics: n = 3 to 5, ±sd. *P < 0.01 (t test).
SFR2 Is Activated in Older Leaves in Response to Salt and Drought Treatment
To assess a direct correlation of the observed phenotypes of SlSFR2 RNAi transgenic lines with SFR2 function, lipids were extracted and analyzed for oligogalactolipids as a measure of SFR2 activation. The second and fourth leaves of 3-week-old (Fig. 8), and the cotyledon and second leaf of 2-week-old plants (Supplemental Figure S9) of wild-type and SlSFR2 RNAi transgenic lines treated as described above were assayed. Unstressed wild-type and SlSFR2 RNAi transgenic lines did not produce detectable amounts of TGDG or TeGDG (Fig. 8; Supplemental Fig. S9). However, following salt treatment, TGDG was detected in the second leaves in 3-week-old (Fig. 8) and cotyledons of 2-week-old (Supplemental Fig. S9) wild-type plants, but not in the SlSFR2 RNAi transgenic lines L2 and L29. Younger leaves on both sets of plants did not show an induction of SlSFR2 in wild-type or transgenic lines (Fig. 8; Supplemental Fig. S9). It should be noted that similar to AtSFR2, the SlSFR2 transcript level did not change during the salt treatment (Supplemental Fig. S10), suggesting that the tomato SFR2 protein is present at all times and directly activated.
Figure 8.
SFR2 activation in 3-week-old tomato plants subjected to salt or drought stress. A, Lipids were extracted from the second and fourth leaves of untreated wild-type (WT) and SlSFR2-RNAi plants (L2, L9) and analyzed by TLC. B, Lipids were extracted from the second leaves and (C) from the fourth leaves of salt- and drought-treated plants as indicated. Lipids were stained for sugar head groups. Extracts of MgCl2-treated WT leaves (MgCl2; SFR2 induced) were included (A and C) to detect the diagnostic TGDG following SFR2 induction. SQDG, Sulfoquinovosyldiacylglycerol. A representative result is shown.
Lipid analysis following drought stress was done for 3-week-old plants (Fig. 8). Lipids were extracted from the second and fourth leaves directly following the 9-d drought treatment before the recovery phase to avoid the turnover of potential oligogalactolipids during recovery. Similar to the salt treatment, no TGDG was detected in the second leaves of the SlSFR2 RNAi transgenic lines L2 and L29, but was detected in the second leaves of the wild-type plants (Fig. 8). Therefore, the growth and chlorosis phenotypes observed for older leaves of the SlSFR2 RNAi transgenic lines above coincided with the absence of SFR2 activity following salt and drought treatment.
DISCUSSION
AtSFR2 and SlSFR2 Are Genuine Orthologs
This study was based on the premise that all plant genomes characterized harbor a presumed SFR2 ortholog, including plants that never encounter freezing in their natural environments. Functionality of different predicted orthologs including those from freezing-sensitive plants was indirectly shown by heterologously restoring the freezing tolerance of the Arabidopsis sfr2 mutant (Fourrier et al., 2008). At that time, the biochemical activity of AtSFR2 was not accurately known but was later shown to be the processive transfer of galactosyl residues from MGDG to a galactolipid acceptor to form oligogalactolipids and DAG (Moellering et al., 2010; Roston et al., 2014). Here, we conclusively determined that the recombinant SFR2 ortholog from tomato, SlSFR2, when produced in a suitable yeast host containing the MGDG substrate has galactolipid:galactolipid galactosyltransferase activity comparable to its Arabidopsis ortholog AtSFR2 (Fig. 3). Further biochemical comparison of the two recombinant proteins in yeast-derived microsomal fractions indicated that both enzymes respond qualitatively similarly to changes in pH and MgCl2 concentrations in the buffer (Fig. 3). Thus, the two enzymes from a freezing-tolerant and a -sensitive species appear to function fundamentally in similar ways at the biochemical level. However, it should be noted that SlSFR2 exhibited about 2-fold higher galactolipid:galactolipid galactosyltransferase activity than AtSFR2 (Fig. 3).
AtSFR2 was shown to contain multiple sequence regions that are important for its galactosyl transferase activity (Roston et al., 2014). The SlSFR2 sequence is only 72% similar to that of AtSFR2 (Supplemental Fig. S3), allowing room for sufficient diversity to explain these subtle differences. Hence, surveying a larger range of SFR2 orthologs from diverse plants for their specific SFR2 activity might yield enzymes with different activation profiles or extent of activities that might be better suited for future efforts to engineer tolerance to cellular dehydration based on SFR2 for increasingly stress-tolerant crops. For example, overexpression of a cDNA encoding a tightly regulated SFR2 enzyme may not lead to crop protection, while a constitutively activated SFR2 ortholog or one with higher sensitivity to MgCl2 might be more suitable for engineering purposes.
In Arabidopsis, the AtSFR2 transcript and protein levels are kept constant in the tissues and under all conditions tested (Thorlby et al., 2004). Similarly, we observed a constant abundance of SlSFR2 transcripts at all instances tested in tomato (Supplemental Fig. S10), suggesting that as in Arabidopsis, activation of SFR2 occurs posttranscriptional/translational.
Aside from the direct determination of the activity of SlSFR2, reducing the abundance of SlSFR2 transcripts in SlSFR2 RNAi lines decreased the formation of the oligogalactolipids TGDG and TeGDG in response to MgCl2 (Fig. 4), providing further corroboration that SlSFR2 is involved in the formation of these oligogalactolipids in vivo. It should be noted that 0.5 m MgCl2 used in this experiment constitutes a much higher concentration than found under physiological conditions, which is usually 2 to 10 mm (Shaul, 2002). We used this concentration of MgCl2 here as a simple method to induce the enzyme in vivo and estimate the maximal enzyme activity.
Oligogalactolipid Formation by SlSFR2 Protects against Salt and Drought Stress
Having established in multiple ways that SlSFR2 is a genuine ortholog of AtSFR2 with a similar activity profile, we can pursue the question whether SlSFR2 protects tomato, a generally cold-/freezing-sensitive plant, against other forms of cellular dehydration induced by osmotic stress: either high salt or drought. A set of SlSFR2 RNAi lines with up to 80% reduction in mRNA abundance was generated and analyzed to answer this question. Exposing different wild-type and transgenic plants at different ages to salt and drought stress using generally accepted protocols (Conroy et al., 1988; Shalata and Tal, 1998; Umezawa et al., 2000), we were able to show that older leaves on the SlSFR2 RNAi plants remain smaller and senesce earlier than corresponding leaves on wild-type plants (Fig. 5; Supplemental Figs. S6 and S7). Most importantly, this phenotype strictly correlated with the inability to generate oligogalacotlipids in those leaves in the transgenic lines (Fig. 8; Supplemental Fig. S9). Hence, we conclude that the ability to produce oligogalcotlipids as a result of induction of SlSFR2 activity under salt and drought stress allows wild-type tomato plants to continue to produce biomass under these adverse conditions.
The fact that older leaves are most severely affected by different abiotic stress regimes while young leaves stay relatively protected has been previously observed. For example, the drought-tolerant plant Senecio medley-woodii responds to drought stresses with a gradual dehydration of leaves progressing from the oldest to the young leaves until the older leaves are eventually shed, while the younger leaves are still able to maintain or even increase transpiration (Donatz and Eller, 1993). In Arabidopsis during drought stress, enzymes protecting against oxidative stress including catalase, peroxidase, superoxide dismutase, and glutathione reductase accumulate in mature but not in young leaves, suggesting indirectly that older leaves may be more susceptible to drought-induced oxidative damage (Jung, 2004). Even though the concept that water translocation from older to younger leaves during dehydration stress may still be debatable, it is well known that younger photosynthetically active tissues are preferably protected (Munns and Tester, 2008; Chaves et al., 2009). In rice, young leaves are protected from salt stress as NaCl accumulates preferably in the older leaves (Wang et al., 2012). Similarly, younger leaves are more effective than older in adjusting the osmotic potential by increasing solute concentrations, which helps maintain water status and turgor pressure during dehydration conditions (Hajlaoui et al., 2010). It is possible that the salt stress phenotype of the older tomato leaves observed in our hands is caused by a combination of NaCl toxicity and cellular dehydration. However, dehydration must be at least partially responsible, because a similar phenotype was observed during drought stress alone.
Lipid Remodeling by SFR2 Protects against Different Abiotic Stresses in Different Plants
SFR2 has been shown to play a crucial role in freezing tolerance in Arabidopsis but is not activated during salt or drought stress, at least prior to the occurrence of irreversible damage (Fig. 1; Supplemental Figs. S1 and S2). While Arabidopsis is freezing tolerant, it is much more sensitive to salt and drought stress, and effects of salt or drought become lethal before the SFR2-based lipid remodeling system is activated. However, in freezing-intolerant tomato, SFR2 is activated during salt and drought stress before the plants are irreversibly damaged and, hence, is able to protect old leaves from stress-induced senescence (Figs. 5 and 8).
What are the common principles by which SFR2 potentially protects against cellular dehydration in different plants? Given that SFR2 mRNA levels are not regulated in different plants, the enzyme itself must be activated by posttranscriptional mechanisms. We recently showed that SFR2 in Arabidopsis is activated upon freezing by a decrease in cytosolic pH and an increase in the concentration of MgCl2 (Barnes et al., 2016). Typically, chloroplasts contain up to 10 mm MgCl2, a higher concentration than found in the cytosol (Shaul, 2002). The SFR2 protein is likely ubiquitously present in the outer envelope membranes of plant chloroplasts as has been directly shown for Arabidopsis (Fourrier et al., 2008). It should be noted that we tested the available antibody raised against AtSFR2 but failed to detect cross reactivity with SlSFR2. In addition, we were unable to assay SlSFR2 in isolated tomato chloroplasts, perhaps due to interference with secondary metabolites present in the tomato extracts. However, as in vivo activation in tomato shows (Fig. 8; Supplemental Fig. S9), SlSFR2 has access to its substrate MGDG, which is exclusively present in the plastid. Hence, we assume that SlSFR2 is likely also present in the outer envelopes of chloroplasts as shown for Arabidopsis. Therefore, a simple hypothesis for the protective function of SlSFR2 during drought and salt stress causing severe cellular dehydration is that SlSFR2 acts as a first line of defense being present at all times in the chloroplast envelopes. It is activated when the chloroplast membranes are directly disrupted during dehydration allowing leakage of MgCl2 from the chloroplast. Alternatively, the MgCl2 concentration increases due to loss of water from the cytosol during dehydration. Once activated by the cytosolic increase in MgCl2, the removal of the nonbilayer-forming lipid MGDG and the synthesis of higher order oligogalactolipids stabilize the chloroplast membranes similarly as proposed for AtSFR2 function during the response to freezing in Arabidopsis (Moellering et al., 2010; Moellering and Benning, 2011). Therefore, SFR2 constitutes a first line of defense providing a common molecular membrane lipid-remodeling mechanism against cellular dehydration accompanying different abiotic stresses such as drought, increased salt, or freezing, thereby explaining its presence in all plants currently tested.
However, other mechanisms than disruption of the chloroplast membrane raising the cytosolic MgCl2 concentration and lowering the pH to activate SFR2 cannot be excluded. It is possible that MgCl2 or protons released from vacuoles are sensed by SFR2 upon salt or drought stress in tomato, and MgCl2 and protons could enter the cytosol through specific channels that may be differently regulated in different plants in response to different abiotic stresses. Therefore, understanding the mechanisms of SFR2 activation in different plants in response to different abiotic stresses may provide new plant-specific avenues to engineer crops to be more tolerant to specific abiotic stresses.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum) cultivar M82 seeds were generously provided by Dr. Cornelius Barry, Michigan State University. Seed were germinated by being plated on a moisturized filter paper for 1 week, then seedlings were transferred to soil and kept in a growth chamber with the condition set as 200 to 300 μE m−2s−1 at 16 h light/8 h dark and 24°C/20°C (day/night). Plants were watered with tomato nutrient formula water, specifically, 12.2 g 15-30-15 (N-P-K, %weight) fertilizer, 0.13 g Fe, and 0.05 g calcium nitrate in 1 gallon deionized water.
Arabidopsis (Arabidopsis thaliana) Col-2 and sfr2-3 seeds (Moellering et al., 2010) were sterilized and plated on MS medium (Murashige and Skoog, 1962) containing 1% (w/v) Suc. Plants were grown under 100 µE m−2s−1 with a 16-h-light/8-h-dark cycle and 22°C.
RNAi Plasmid Constructions and Tomato Transformation
Total RNA was extracted from tomato M82 leaves using the Plant RNeasy RNA extraction kit (Qiagen); 600 ng RNA was reverse transcribed using SuperScript III Reverse Transcriptase (life technology) and oligo(dT)18 following the manufacturer’s protocols. The SlSFR2 RNAi construct was built by first amplifying the 509-bp (121–629 bp) cDNA of SlSFR2 using primers as shown in Supplemental Table S1. PCR products were purified by QIAquick PCR Purification Kit (Qiagen) and then inserted into the pENRT/D vector according to the pENTR/D-TOPO Cloning kit instruction (Life Technologies). The 509-bp SlSFR2 fragment was finally recombined into pHELLSGATE12 vector by the LR reaction using Gateway LR Clonase II Enzyme Mix (Life technologies). Tomato transformation was done essentially as described (McCormick, 1991).
Quantitative Real-Time PCR
Total RNA from the fourth leaves of 8-week-old tomatoes was isolated and reverse transcribed as described above. Quantitative RT-PCR was performed using the SYBR Green PCR Core Reagents mix (Life Technologies) based on the manufacturer's instructions. Supplemental Table S1 lists the primers used. The 2−ΔΔCt calculation was used to determine the relative mRNA levels.
Lipid Analysis
Lipids were extracted from approximately 50 mg 4-week-old tomato second and fourth leaves as previously described (Moellering et al., 2010). Polar lipids were analyzed on activated ammonium sulfate-impregnated silica gel TLC plates (Si250; Mallinckrodt Baker, Phillipsburg, NJ, which are no longer available and which were later substituted by DC-Fertigplatten SIL G-25; MACHEREY-NAGEL, Germany) using a solvent system consisting of chloroform:methanol:acetic acid: water (85:20:10:4, v/v/v/v). Lipids were visualized by brief exposure to iodine vapor or staining with α-naphthol to detect glycolipids.
Protein Production
An SlSFR2 yeast expression construct was built by amplifying the full SlSFR2 coding sequence from a cDNA, which was inserted into the pYES2.1 vector according to the manufacturer’s instructions (Invitrogen). The recombinant protein contained a C-terminal 6XHis tag. Arabidopsis AtSFR2 and cucumber CsMGD1 constructs are described in (Roston et al., 2014). Protein production was essentially done as described in (Roston et al., 2014). Microsomes were harvested from yeast cells post induction as described in (Dahlqvist et al., 2000) and stored at −80°C until use. Protein extraction was done according to (Kushnirov, 2000), and protein production was analyzed by SDS-PAGE and immunoblotting as described in (Roston et al., 2014). SFR2 assays were performed as described in (Roston et al., 2014), except that the MgCl2 concentration was elevated to 8 mm.
Stress Assays
For Arabidopsis, after 7 d of growth in regular medium, seedlings were transferred to MS plates with extra concentrations of salts for salt treatment or of PEG-8000 to generate different water potentials for drought treatment. For the drought assay, the detailed recipe for preparing polyethylene glycol-infused plates is descried in (van der Weele et al., 2000). Plants were grown for 3 weeks before ion leakage or lipid analysis.
Tomato seeds were first germinated on moisturized filter paper for 1 week under standard conditions as described above and then transferred to soil and grown for 2 weeks before application. For the salt assay, 3-week-old plants were acclimated by daily watering with nutrient solution, to which 50 mm NaCl was added, for 5 d and then followed by increasing NaCl to 100 mm for 1 week and 150 mm for another 2 d. Plant and leaf images were taken at the end of the salt treatment. Plants were watered daily with normal nutrient solution for one week after the end of the salt treatment to ensure they resume normal growth.
For the drought assay, 3-week-old tomato plants were not watered for 3 d to acclimate the plants followed by watering with nutrient solution for 4 d. Then 3-week-old plants were not watered for another 9 d, followed by daily watering. Lipids were extracted and analyzed at the end of the drought treatment. Plant and leaf images were taken 2 d after resuming watering.
Water potential and relative water content measurements were conducted before the rewatering phase. Water potential was measured with a pressure chamber (PMS Instrument Company, model 600) following the equipment manual. Water content measurement was performed according to (Barrs and Weatherley, 1962).
The electrolyte assays were performed as described (Gilmour et al., 1988). Briefly, three to five leaf blades were immersed in 3 mL of deionized water and the samples were gently agitated for 3 h. Conductivity was measured using a conductivity meter (YSI model 35). One hundred percent leakage was defined by placing the leaves in −80°C for 10 h and then agitating them for 3 h before conductivity of the solution was measured. Electrolyte leakage was expressed as a percentage of the final conductivity.
Chlorophyll Measurements
Chlorophyll was quantified as described (Lichtenthaler and Wellburn, 1983). In brief, leaf pigments were extracted in 80% (v/v) acetone from punched discs and measured spectrophotometrically on an Uvikon 930 spectrophotometer (Kontron Instruments). Chlorophyll a, chlorophyll b, and total carotenoids were calculated from leaf A663 and 646 nm with the following equations: chlorophyll a (mg/L) = 12.21A663 − 2.81A646; chlorophyll b (mg/L) = 20.13A646 − 5.03A663; total chlorophyll (mg/L) = chlorophyll a + chlorophyll b.
Protein Measurement
Leaf disks were ground with liquid nitrogen to which 100 µL extraction buffer (0.1 M Tris HCl, pH 6.8; 1% SDS; 15% glycerol; 5% [v/v] β-mercaptoethanol) was added, followed by vigorous mixing. Samples were boiled at 100°C for 10 min followed with centrifugation at 13,000 g for 5 min, and the proteins were quantified using a Bradford assay (BioRad) following the manufacturer’s instructions.
Accession Numbers
Sequence data for SlSFR2 from this article can be found in the GenBank/EMBL data libraries under accession number 778260 [uid].
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Root bending assay on salt-treated Arabidopsis plants and salt assay on soil grown plants.
Supplemental Figure S2. Effects of drought treatment on soil grown Arabidopsis plants.
Supplemental Figure S3. Protein sequence alignment between AtSFR2 and SlSFR2.
Supplemental Figure S4. Genotyping of wild-type and SlSFR2-RNAi lines.
Supplemental Figure S5. Activation of SlSFR2 in additional SlSFR2-RNAi lines.
Supplemental Figure S6. Effects of salt treatment on 2-week-old tomato plants.
Supplemental Figure S7. Physiological effects of drought treatment to wild-type and SlSFR2-RNAi lines.
Supplemental Figure S8. SFR2 activation in 2-week-old tomato plants subjected to salt stress.
Supplemental Figure S9. Chlorophyll in the leaves of untreated and salt-treated 2-week-old tomato plants.
Supplemental Figure S10. SlSFR2 transcript levels during salt treatment.
Supplemental Table S1. Primers used in this study.
Acknowledgments
We thank Dr. Cornelius Barry (Michigan State University) for generously providing tomato M82 seeds, Ms. Kathleen Imre and Dr. Rob Last (Michigan State University) for the tomato transformation protocol, Dr. Wayne Loescher (Michigan State University) for advice on salt and drought assays and providing the pressure chamber, Dr. Sarah J. Gilmour (Michigan State University) for providing the conductivity meter, and Dr. Rebecca Roston (University of Nebraska) for scientific discussions.
Glossary
Footnotes
This work was supported by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the United States Department of Energy Grant DE-FG02-98ER20305 (to C.B.) and Michigan State University AgBioResearch (to C.B.).
Articles can be viewed without a subscription.
References
- Andrews CJ. (1996) How do plants survive ice? Ann Bot (Lond) 78: 529–536 [Google Scholar]
- Barnes AC, Benning C, Roston R (2016) Chloroplast membrane remodeling during freezing stress is accompanied by cytoplasmic acidification activating SENSITIVE TO FREEZING 2. Plant Physiol 171: 2140–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15: 413–428 [Google Scholar]
- Browse J, Xin Z (2001) Temperature sensing and cold acclimation. Curr Opin Plant Biol 4: 241–246 [DOI] [PubMed] [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot (Lond) 103: 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy JP, Virgona JM, Smillie RM, Barlow EW (1988) Influence of drought acclimation and CO(2) enrichment on osmotic adjustment and chlorophyll a fluorescence of sunflower during drought. Plant Physiol 86: 1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97: 6487–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatz M, Eller BM (1993) Plant water status and water translocation in the drought deciduous CAM-succulent Senecio-Medley-Woodii. J Plant Physiol 141: 750–756 [Google Scholar]
- Fernandez-Pozo N, Menda N, Edwards JD, Saha S, Tecle IY, Strickler SR, Bombarely A, Fisher-York T, Pujar A, Foerster H, et al. (2015) The Sol Genomics Network (SGN)--from genotype to phenotype to breeding. Nucleic Acids Res 43: D1036–D1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourrier N, Bédard J, Lopez-Juez E, Barbrook A, Bowyer J, Jarvis P, Warren G, Thorlby G (2008) A role for SENSITIVE TO FREEZING2 in protecting chloroplasts against freeze-induced damage in Arabidopsis. Plant J 55: 734–745 [DOI] [PubMed] [Google Scholar]
- Gasulla F, Vom Dorp K, Dombrink I, Zähringer U, Gisch N, Dörmann P, Bartels D (2013) The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J 75: 726–741 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Hajela RK, Thomashow MF (1988) Cold acclimation in Arabidopsis thaliana. Plant Physiol 87: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M, Yaish MW (2004) Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci 9: 399–405 [DOI] [PubMed] [Google Scholar]
- Hajlaoui H, El Ayeb N, Garrec JP, Denden M (2010) Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind Crops Prod 31: 122–130 [Google Scholar]
- Jung SY. (2004) Variation in antioxidant metabolism of young and mature leaves of Arabidopsis thaliana subjected to drought. Plant Sci 166: 459–466 [Google Scholar]
- Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63: 1593–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnirov VV. (2000) Rapid and reliable protein extraction from yeast. Yeast 16: 857–860 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11: 591–592 [Google Scholar]
- Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444: 139–158 [DOI] [PubMed] [Google Scholar]
- McCormick S. (1991) Transformation of tomato with Agrobacterium tumefaciens. In Lindsey K, ed, Plant Tissue Culture Manual. Kluwer Academic, Dordrecht, The Netherlands, pp 1–9 [Google Scholar]
- Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci 16: 98–107 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Muthan B, Benning C (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330: 226–228 [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Pearce RS. (2001) Plant freezing and damage. Ann Bot (Lond) 87: 417–424 [Google Scholar]
- Roston RL, Wang K, Kuhn LA, Benning C (2014) Structural determinants allowing transferase activity in SENSITIVE TO FREEZING 2, classified as a family I glycosyl hydrolase. J Biol Chem 289: 26089–26106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104: 169–174 [DOI] [PubMed] [Google Scholar]
- Shaul O. (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15: 309–323 [DOI] [PubMed] [Google Scholar]
- Shimojima M, Ohta H, Iwamatsu A, Masuda T, Shioi Y, Takamiya K (1997) Cloning of the gene for monogalactosyldiacylglycerol synthase and its evolutionary origin. Proc Natl Acad Sci USA 94: 333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorlby G, Fourrier N, Warren G (2004) The SENSITIVE TO FREEZING2 gene, required for freezing tolerance in Arabidopsis thaliana, encodes a beta-glucosidase. Plant Cell 16: 2192–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Shimizu K, Kato M, Ueda T (2000) Enhancement of salt tolerance in soybean with NaCl pretreatment. Physiol Plant 110: 59–63 [Google Scholar]
- van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51: 1555–1562 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45: 523–539 [DOI] [PubMed] [Google Scholar]
- Waadt R, Manalansan B, Rauniyar N, Munemasa S, Booker MA, Brandt B, Waadt C, Nusinow DA, Kay SA, Kunz HH, et al. (2015) Identification of open stomatal-interacting proteins reveals interactions with Sucrose Non-fermenting1-Related Protein Kinases2 and with Type 2A protein phosphatases that function in abscisic acid responses. Plant Physiol 169: 760–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang M, Guo R, Shi D, Liu B, Lin X, Yang C (2012) Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol 12: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, McKown R, Marin AL, Teutonico R (1996) Isolation of mutations affecting the development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Plant Physiol 111: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]