Recent research highlights the importance of both starch and sucrose for guard cell osmoregulation and its integration with carboxylate metabolism and membrane ion transport during stomatal movements.
Abstract
Stomata control gaseous fluxes between the internal leaf air spaces and the external atmosphere and, therefore, play a pivotal role in regulating CO2 uptake for photosynthesis as well as water loss through transpiration. Guard cells, which flank the stomata, undergo adjustments in volume, resulting in changes in pore aperture. Stomatal opening is mediated by the complex regulation of ion transport and solute biosynthesis. Ion transport is exceptionally well understood, whereas our knowledge of guard cell metabolism remains limited, despite several decades of research. In this review, we evaluate the current literature on metabolism in guard cells, particularly the roles of starch, sucrose, and malate. We explore the possible origins of sucrose, including guard cell photosynthesis, and discuss new evidence that points to multiple processes and plasticity in guard cell metabolism that enable these cells to function effectively to maintain optimal stomatal aperture. We also discuss the new tools, techniques, and approaches available for further exploring and potentially manipulating guard cell metabolism to improve plant water use and productivity.
Stomata are microscopic, adjustable pores on the leaf surface. The evolution of stomata more than 400 million years ago (Edwards et al., 1986, 1992, 1998) helped facilitate the adaptation of plants to a terrestrial environment, where water is typically a limiting resource. Each stoma is composed of two kidney- or dumbbell-shaped guard cells, whose volume changes to adjust pore aperture, allowing plants to simultaneously regulate CO2 uptake and water loss. This facilitation of gas exchange by stomatal opening is one of the most essential processes in plant photosynthesis and transpiration, affecting plant water use efficiency and agricultural crop yields (Lawson and Blatt, 2014).
Plant physiologists have a long history of investigating the behavior of these fascinating structures, reaching back more than a century to the pioneering work of Sir Francis Darwin (Darwin, 1916) and the American botanist Francis Ernest Lloyd (Lloyd, 1908). Major contributions to stomatal research arose from inventing and improving equipment and methods for quantitatively measuring the effects of environmental factors on stomatal pore aperture. After Darwin’s work, it became clear that the stomatal aperture actively responds to changes in the environment and regulates leaf transpiration rates (Meidner, 1987). Over the past century, much has been learned about their structure, development, and physiology.
Despite the anatomical simplicity of the stomatal valve, the surrounding guard cells are highly specialized. Guard cells are morphologically distinct from general epidermal cells and possess complex signal transduction networks, elevated membrane ion transport capacity, and modified metabolic pathways. These features allow rapid modulations in guard cell turgor in response to endogenous and environmental signals, promoting the opening and closure of the stomatal pore in time scales of seconds to hours (Assmann and Wang, 2001). A variety of osmotically active solutes contribute to the buildup of stomatal turgor. Potassium (K+) and chloride (Cl−) act as the main inorganic ions, and malate2− and sucrose (Suc) function as the main organic solutes. Whereas K+ and Cl− are taken up from the apoplast, Suc and malate2− can be imported or synthesized internally using carbon skeletons deriving from starch degradation and/or CO2 fixation in the guard cell chloroplast (Roelfsema and Hedrich, 2005; Vavasseur and Raghavendra, 2005; Lawson, 2009; Kollist et al., 2014). The accumulation of these osmotica lowers the water potential, promoting the inflow of water, the swelling of guard cells, and the opening of the stomatal pore. Most of the ions taken up, or synthesized by guard cells, are sequestered into the vacuole (Barbier-Brygoo et al., 2011). As a result, the guard cell vacuoles undergo dynamic changes in volume and structure, which are crucial for achieving the full amplitude of stomatal movements (Gao et al., 2005; Tanaka et al., 2007; Andrés et al., 2014). During stomatal closure, guard cells reduce their volume through the release of ions into the cell wall and the consequent efflux of water.
The transport of osmolytes across the plasma and tonoplast guard cell membranes is energized by H+-ATPase activity, which generates a proton motive force by translocating H+ ions against their concentration gradient (Blatt, 1987a, 1987b; Thiel et al., 1992; Roelfsema and Hedrich, 2005; Gaxiola et al., 2007). After the pioneering work of Fischer demonstrated the importance of K+ uptake in stomatal opening (Fischer, 1968; Fischer and Hsiao, 1968), K+ transport became of central interest and has long been considered the essence of stomatal movement regulation. The development of the voltage clamp technique, along with the relative easy acquisition of knockout mutants and transgenics in the model plant Arabidopsis (Arabidopsis thaliana), helped to uncover the precise mechanism and function of K+ fluxes in guard cells. It is well established that changes in membrane potential in response to several stimuli (e.g. light/darkness, CO2, and abscisic acid [ABA]) alter the direction of K+ transport (Thiel et al., 1992; Blatt, 2000; Roelfsema et al., 2001, 2002, 2004). During stomatal opening, the activation of the proton pump generates a sufficiently negative electric potential to cause the uptake of K+ through the inward-rectifying K+ channels (K+in; Fig. 1). During stomatal closure, K+ outflow from outward-rectifying K+ channels (K+out) results from membrane depolarization (Fig. 2; Blatt, 1988; Schroeder, 1988; Anderson et al., 1992; Sentenac et al., 1992). Besides being gated by opposing changes in voltage, the activation of (K+out) channels is dependent on the extracellular K+ concentration, while that of K+in is not (Blatt, 1988, 1992; Roelfsema and Prins, 1997; Dreyer and Blatt, 2009). There is also strong evidence for H+-coupled K+ symport in guard cells, which could account for up to 50% of total K+ uptake during stomatal opening (Blatt and Clint, 1989; Clint and Blatt, 1989; Hills et al., 2012). At least for K+in, the loss of a single-channel gene in Arabidopsis has little or no impact on stomatal movement (Szyroki et al., 2001), showing the redundancy among the different K+in isoforms and of K+ transport in general.
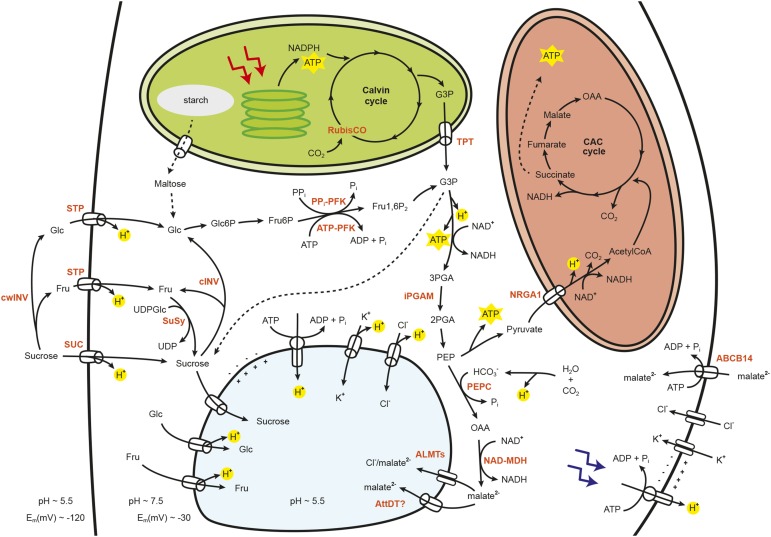
Figure 1.
Integration of guard cell carbohydrate metabolism with membrane ion transport during stomatal opening. Sugars in guard cells can be imported from the apoplast, derive from starch breakdown, or be synthesized in the Calvin cycle. These sugars then can be stored as osmotically active solutes in the vacuole or metabolized in the cytosol to yield energy, reducing equivalents, and phosphoenolpyruvate (PEP). PEP can be further metabolized to pyruvate in the mitochondrial tricarboxylic acid (CAC) cycle or used as carbon skeletons for the biosynthesis of malate via PEP carboxylase (PEPC) and NAD-dependent malate dehydrogenase (NAD-MDH). Malate (which also can be imported from the apoplast) and the inorganic ions K+ and Cl− accumulate in the vacuole, lowering the guard cell osmotic potential, thereby promoting stomatal opening. ABCB14, ATP-binding cassette transporter B14; AcetylCoA, acetyl-CoA; ALMT, aluminum-activated malate transporter; ATP-PFK, ATP-dependent phosphofructokinase; AttDT, dicarboxylate transporter; cINV, cytosolic invertase; cwINV, cell wall invertase; Fru6P, Fru-6-P; Fru1,6P2, fructose 1,6-bisphosphate; Gl6P, Glc-6-P; G3P, glyceraldehyde 3-phosphate; iPGAM, phosphoglycerate mutase isoforms; NRGA1, negative regulator of guard cell ABA signaling1; OAA, oxaloacetate; 2-PGA, 2-phosphoglycerate; 3-PGA, 3-phosphoglycerate; PPi-PFK, PPi-dependent Fru-6-P phosphotransferase; STP, monosaccharide/H+ cotransporter; SUC, Suc/H+ cotransporter; SuSy, Suc synthase; TPT, triose phosphate/phosphate translocator. Compartments are not to scale. The dotted line indicates multiple metabolic steps.
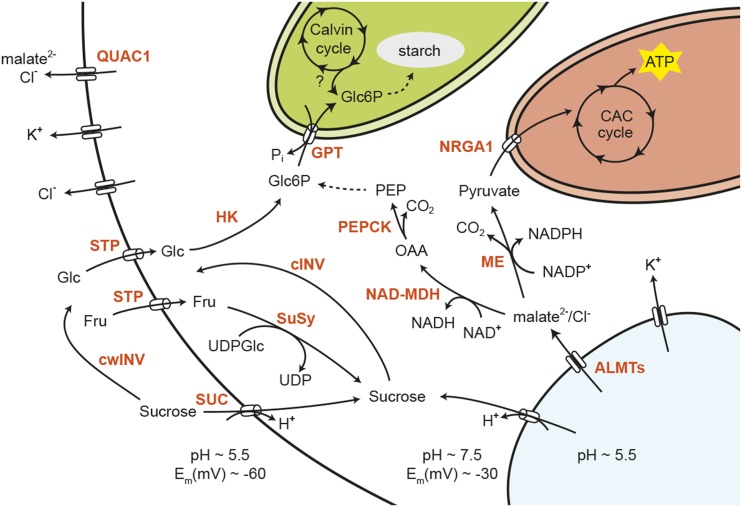
Figure 2.
Proposed pathways of osmolyte dissipation during stomatal closure. While the removal of Cl− and K+ is well described in the literature, the fate of Suc and malate during stomatal closure is unclear. Suc can be cleaved by cytosolic invertase (cINV), and the resulting hexoses can be imported into the chloroplast in the form of Glc-6-P (Glc6P). Glc6P is used subsequently for starch biosynthesis. Malate can be removed from the cell via decarboxylation to pyruvate by malic enzyme (ME) and the subsequent complete oxidation in the mitochondrial tricarboxylic acid (CAC) cycle. Alternatively, malate can be converted to PEP via NAD+-dependent malate dehydrogenase (NAD-MDH) and PEP carboxykinase (PEPCK). Gluconeogenic conversion of PEP to Glc6P establishes a possible link between malate removal and starch synthesis. Compartments are not to scale. PEP, Phosphoenolpyruvate; OAA; oxaloacetate; STP, monosaccharide/H+ cotransporter; SUC, Suc/H+ cotransporter; SuSy, Suc synthase; cINV, cytosolic invertase; NRGA1, negative regulator of guard cell ABA signaling1; ALMT, aluminum-activated malate transporter; GPT, Glc-6-P/Pi translocator; cwINV, cell wall invertase; HK, hexokinase; QUAC1, quickly activating anion channel1.
Despite the undisputed importance of K+ uptake in stomatal opening, the accumulation of K+ ions alone cannot account for the increase in osmotic pressure necessary to explain stomatal aperture. Studies from the 1980s by MacRobbie and Fischer demonstrated that Vicia faba guard cells take up approximately 2 pmol of K+ during stomatal opening. Assuming that K+ uptake is balanced by the accumulation of similar amounts of counter ions (Cl− and/or malate2−), the expected increase in stomatal turgor to approximately 3 MPa is less than the 4.5 MPa expected for fully open stomata (Fischer, 1972; MacRobbie and Lettau, 1980a, 1980b; Chen et al., 2012). The realization that other solutes must accumulate in addition to K+ salts was one of the major paradigm shifts in stomatal physiology research in the last decades, equal to the discovery of ion channels. Suc was put forward as the most likely candidate for the additional osmoticum to support stomatal opening (MacRobbie, 1987; Tallman and Zeiger, 1988; Talbott and Zeiger, 1993, 1998). Nonetheless, this research area subsequently failed to attract notice commensurate with its importance.
In the last few years, the metabolism of starch, sugars and, organic acids in guard cells has seen a rebirth, making this the perfect time to review the developments in this field. In this review, we focus on photosynthetic carbon assimilation and respiratory metabolism in guard cells and provide a historical overview of the subject that highlights the most up-to-date and novel discoveries in guard cell research. We describe the various metabolic pathways separately, but as metabolism is an integrated network, we also discuss their reciprocal and beneficial interactions. Finally, we highlight their connection with the metabolism in the subjacent mesophyll cells and how they integrate with guard cell signal transduction networks and membrane ion transport to regulate stomatal movements. The enzymes and transporters discussed in this review are listed in Table I.
Table I. Enzymes and transporters discussed in this review.
Dashes denote genes with multiple isoforms.
| Arabidopsis Genome Initiative Code | Gene | Protein | Function |
|---|---|---|---|
| Malate transport | |||
| AT1G28010 | ABCB14 | ATP-binding cassette transporter B14 | Import of apoplastic malate |
| AT5G47560 | tDT | Dicarboxylate transporter | Transport of carboxylates into the vacuole |
| AT3G18440 | ALMT9 | Aluminum-activated malate transporter9 | Transport of Cl−/malate2− into the vacuole |
| AT2G17470 | ALMT6 | Aluminum-activated malate transporter6 | Transport of malate2− into the vacuole |
| AT4G17970 | ALMT12/QUAC1 | Aluminum-activated malate transporter12 | Export of cytosolic Cl−/malate2− to the apoplast |
| Malate metabolism | |||
| – | PEPC | Phosphoenolpyruvate carboxylase | β-Carboxylation of PEP to OAA |
| – | NAD-MDH | NAD+-dependent malate dehydrogenase | Reduction of OAA to malate |
| – | ME | Malic enzyme | Oxidative decarboxylation of malate to pyruvate |
| AT4G37870 | PEPCK1 | PEP carboxykinase1 | Conversion of OAA to PEP |
| – | PPDK | Pyruvate, orthophosphate dikinase | Conversion of pyruvate to PEP |
| Other carboxylates | |||
| – | TPT | Triose phosphate/phosphate translocator | Export of triose phosphate from the chloroplast to the cytosol |
| AT4G05590 | NRGA1 | Negative regulator of guard cell ABA signaling1 | Putative mitochondrial pyruvate carrier |
| – | SDH2 | Succinate dehydrogenase2 | Oxidation of succinate to fumarate |
| AT2G47510 | FUM1 | Fumarase1 | Hydration of fumarate to malate |
| – | iPGAM | Phosphoglycerate mutase | Interconversion of 3-PGA to 2-PGA |
| – | PPi-PFK | PPi-dependent Fru-6-P phosphotransferase | Phosphorylation of Fru-6-P to Fru-1,6-bisphosphate |
| – | ATP-PFK | ATP-dependent phosphofructokinase | Phosphorylation of Fru-6-P to Fru-1,6-bisphosphate |
| Calvin cycle | |||
| – | Rubisco | Rubisco | Carboxylation of ribulose 1,5-bisphosphate |
| AT3G55800 | SBPase | Sedoheptulose-bisphosphatase | Dephosphorylation of sedoheptulose-1,7-bisphosphate to sedoheptulose-7-phosphate |
| Sugar metabolism | |||
| AT4G29130 | HK1 | Hexokinase1 | Phosphorylation of Glc to Glc-6-P |
| AT4G02280 | SuSy | Suc synthase3 | Interconversion of Suc to Fru and UDP-Glc |
| – | cINV | Cytosolic invertase | Hydrolysis of Suc to Fru and Glc |
| – | cwINV | Cell wall invertase | Hydrolysis of Suc to Fru and Glc |
| Sugar transport | |||
| AT1G11260 | STP1 | Monosaccharide/H+ cotransporter1 | Import of apoplastic hexose sugars |
| AT3G19930 | STP4 | Monosaccharide/H+ cotransporter4 | Import of apoplastic hexose sugars |
| AT1G71880 | SUC1 | Suc/H+ cotransporter1 | Import of apoplastic Suc |
| AT2G02860 | SUC3 | Suc/H+ cotransporter3 | Import of apoplastic Suc |
| Starch degradation | |||
| AT3G23920 | BAM1 | β-Amylase1 | Hydrolysis of α-1,4 external glucoside linkages in starch |
| AT1G69830 | AMY3 | α-Amylase3 | Hydrolysis of α-1,4 internal glucoside linkages in starch |
| Starch synthesis | |||
| – | GPT | Glc-6-P/Pi translocator | Uptake of cytosolic Glc-6-P into the chloroplast |
| AT4G24620 | PGI | Phosphoglucose isomerase | Conversion of Fru-6-P to Glc-6-P |
| AT5G51820 | PGM1 | Phosphoglucomutase1 | Conversion of Glc-6-P to Glc-1-P |
| AT5G48300 | APS1 | ADPGlc pyrophosphorylase small subunit | Conversion of Glc-1-P to ADPGlc, catalytic subunit |
| – | APL | ADPGlc pyrophosphorylase large subunit | Conversion of Glc-1-P to ADPGlc, regulatory subunit |
| Various | |||
| AT3G45780 | PHOT1 | Phototropin1 | Blue light photoreceptor |
| AT5G58140 | PHOT2 | Phototropin2 | Blue light photoreceptor |
| AT4G14480 | BLUS1 | Blue light signaling1 | Protein kinase, regulator of blue light-induced stomatal opening |
| – | PP1 | Protein phosphatase1 | Regulator of blue light-induced stomatal opening |
| AT3G01500 | CA1 | Carbonic anhydrase1 | Interconversion of CO2 and water into H2CO3 |
| AT1G70410 | CA4 | Carbonic anhydrase4 | Interconversion of CO2 and water into H2CO3 |
| AT1G62400 | HT1 | High leaf temperature1 | Protein kinase, regulator of CO2-induced stomatal closure |
CARBOXYLATE METABOLISM AND FUNCTION IN GUARD CELLS
Carboxylates are organic compounds that contain carboxylate groups (-COO−). Because of their chemical nature and their logarithmic acid dissociation constant (pKa), carboxylates are negatively charged at neutral pH and, to a lesser extent, at acidic pH. Therefore, these compounds often are transported across cellular membranes in the charged form, a process that affects cellular osmolyte concentration and pH homeostasis. Plants produce a variety of monocarboxylates, dicarboxylates, and tricarboxylates, such as citrate, malate, fumarate, and pyruvate, which are key intermediates for numerous biosynthetic pathways. Carboxylates also are major substrates for the mitochondrial reactions of the tricarboxylic acid cycle (CAC), producing energy and reducing power in the form of ATP and NADH to fuel metabolic activities. In guard cells, carboxylates, mainly malate, are important for osmoregulation and as counter ions for K+, while their function as respiratory substrates remains vague.
The importance of malate in guard cell physiology was recognized only after it was noted that Cl− influx during stomatal opening is not absolutely necessary to balance the positive charge of K+ (Raschke and Humble, 1973) and that there is a good correlation between stomatal opening and the accumulation of malate (Allaway, 1973; Pallas and Wright, 1973; Pearson, 1973; Outlaw and Lowry, 1977). It seems, however, that the contribution of malate as a counter ion for K+ depends on the availability of Cl− and can range between 50% and 90% of total cellular osmolytes (Raschke and Schnabl, 1978). In the extreme case of onion (Allium cepa), which lacks starch in guard cells, malate accumulation is not observed and Cl− is used as the exclusive counter ion (Schnabl and Raschke, 1980; Amodeo et al., 1996).
Malate Transport
Malate is found in several cellular compartments (apoplast, cytosol, chloroplast, mitochondria, and vacuole), and its transport from one compartment to another is fundamental in controlling its subcellular concentrations and defining its function in stomatal movements (Chen et al., 2012; Hills et al., 2012). Malate ions can be translocated across biological membranes passively, through anion channels, or actively, using the energy released from ATP hydrolysis.
In Arabidopsis guard cells, the uptake of apoplastic malate is mediated by the plasma membrane ATP-binding cassette transporter family member AtABCB14 (Fig. 1; Lee et al., 2008). In the leaf apoplast, malate is typically found at concentrations of 0.5 to 2 mm, but these levels rise by 50% to 100% during prolonged illumination or in response to high CO2 concentrations, leading in both cases to stomatal closure (Hedrich et al., 1994; Lohaus et al., 2001). In the absence of AtABCB14, stomatal opening is delayed and high CO2- or malate-induced stomatal closure is more efficient (Lee et al., 2008). Thus, AtABCB14 plays a critical role in modulating stomatal movements in response to light and CO2 by controlling the levels of the apoplastic malate pool. Arabidopsis contains more than 120 ABC-type proteins (Geisler and Murphy, 2006), raising the question of whether other family members related to AtABCB14 (e.g. AtABCB13) also can transport malate.
In the guard cell vacuole, malate concentrations fluctuate over the diurnal cycle, reaching levels of up to 300 mm when the stomata are fully open (Gerhardt et al., 1987; Winter et al., 1993, 1994; Martinoia and Rentsch, 1994). Vacuoles contain two different malate translocation systems, a malate transporter and a malate channel (Fig. 1). The dicarboxylate transporter AttDT from Arabidopsis is encoded by a single gene and shares homology with the renal human sodium/dicarboxylate cotransporter HsNaDC-1 (Emmerlich et al., 2003). However, unlike animal HsNaDC-1, AttDT transport activity is not sodium dependent. The driving force for this transport is thought to be the difference in electrical membrane potential between the cytosol and vacuole. AttDT has been implicated in the control of malate and fumarate levels in the vacuoles of leaf tissues and in the regulation of transpiration rates and cytosolic pH (Emmerlich et al., 2003; Hurth et al., 2005). Despite these important functions in carboxylate metabolism, Arabidopsis mutants of AttDT have no visible phenotype (Emmerlich et al., 2003). This can be explained by the finding that vacuoles from the mutants exhibit similar malate channel activities to wild-type vacuoles, which may compensate, at least in part, for the absence of AttDT (Hurth et al., 2005). Whether AttDT is present in guard cells is unclear and remains an interesting question to be studied further.
The presence of malate channels in the vacuolar membrane was first revealed by electrophysiological studies using mesophyll vacuoles isolated from C3 and Crassulacean acid metabolism (CAM) plants (Martinoia et al., 1985; Pantoja et al., 1992; Ratajczak et al., 1994; Cerana et al., 1995; Hafke et al., 2003). These studies showed that malate transport is energized by the vacuolar proton pumps and driven by the electrochemical potential gradient across the tonoplast membrane. Malate currents generally are strongly inward rectifying, thus favoring the direction of malate fluxes from the cytosol into the vacuole, with slow activation kinetics (Pantoja and Smith, 2002; Hafke et al., 2003). However, the molecular nature of the channel underlying these currents was unraveled only several years later, when Kovermann et al. (2007) hypothesized that members of the aluminum-activated malate transporter (ALMT) family could be targeted to the tonoplast and function as malate channels (Fig. 1). In Arabidopsis, the AtALMT protein family is subdivided into three clades (Delhaize et al., 2007). Members from clade II, such as AtALMT6 and AtALMT9, are aluminum insensitive and function as vacuolar membrane-localized anion channels (Kovermann et al., 2007; Meyer et al., 2011; De Angeli et al., 2013). AtALMT9 was the first vacuolar ALMT channel shown to mediate malate and fumarate inward-rectifying currents in Arabidopsis mesophyll cells (Kovermann et al., 2007). In recent years, the biophysical and physiological characteristics of AtALMT9 have been widely investigated. A reevaluation of AtALMT9-mediated currents revealed that AtALMT9 also can transport chloride and that cytosolic malate serves as an allosteric activator (Fig. 1; De Angeli et al., 2013). The same authors also reported that free cytosolic ATP at physiological concentrations acts as a voltage-dependent open channel blocker (Zhang et al., 2014). The mechanism is based on the physical obstruction of the permeation pathway of the ion channel by a charged molecule able to enter the pore but too large to permeate it (De Angeli et al., 2016). The finding that the Km of AtALMT9 for malate transport is 27 mm, a value far from cytosolic malate concentrations (De Angeli et al., 2013), suggests that, in vivo, AtALMT9 likely acts as a malate-activated vacuolar chloride channel whose activity is strictly coupled to the energetic and metabolic status of the cell through ATP/AMP balances. The situation is different in CAM plants, where early experiments revealed a Km for malate transport of 2 to 2.5 mm (Hafke et al., 2003). This finding suggests the presence of channels permeable to malate, or simply that CAM plants might have a higher affinity toward malate due to their special photosynthetic carbon metabolism. Loss of AtALMT9 results in plants with reduced stomatal aperture and slower opening kinetics, leading to reduced transpiration and greater drought tolerance (De Angeli et al., 2013). AtALMT6 is far less characterized. This protein also is expressed in guard cell vacuoles and mediates malate inward-rectifying currents, but there is no indication of Cl− permeability. Unlike AtALMT9, AtALMT6 is activated by micromolar concentrations of cytosolic calcium in a pH-dependent manner (Meyer et al., 2011). In CAM plants, endogenous malate currents are independent from cytosolic calcium concentrations (Pantoja and Smith, 2002), suggesting that different species have different regulatory mechanisms. Loss of AtALMT6 leads to no obvious phenotype, perhaps because of the complex regulation of this ALMT (Meyer et al., 2011). Whether other members of the same clade as AtALMT9 and AtALMT6 also are targeted to the vacuole and participate in the control of stomatal movements remains to be investigated.
During stomatal closure, the malate that accumulated previously in the vacuole can be dissipated metabolically (see following section) or released from the guard cells to the surrounding apoplastic space to favor the decrease in turgor and the efflux of water (Fig. 2). Evidence for malate efflux during stomatal closure dates back to the 1970s, when Van Kirk and Raschke (1978) demonstrated that isolated epidermal peels of V. faba and Commelina communis released malate into their bathing medium when the stomata closed, with the greatest release in the presence of ABA. It is now well established that malate efflux at the guard cell plasma membrane is mediated by the rapid-type anion channel. Upon depolarization, this channel type, initially named guard cell anion channel 1, activates within milliseconds, whereas it is deactivated by hyperpolarization (Keller et al., 1989; Hedrich et al., 1990; Kolb et al., 1995). Genes encoding rapid-type anion channels belong to clade III of the ALMT protein family (Dreyer et al., 2012). One such protein is AtALMT12, which is highly expressed in guard cells and targeted to the plasma membrane (Meyer et al., 2010; Sasaki et al., 2010). Loss of AtALMT12 impairs stomatal closure in response to high levels of CO2, darkness, and ABA (Meyer et al., 2010). Similar to AtALMT9, quickly activating anion channel1 (QUAC1, rapid-type) AtALMT12 is insensitive to aluminum, activated by malate, and blocked by cytosolic nucleotides at negative resting membrane potentials (Thomine et al., 1997; Colcombet et al., 2001; Meyer et al., 2010; Sasaki et al., 2010). This type of channel regulation, where cytosolic nucleotides act as voltage-dependent open channel blockers, may represent an effective way to couple plasma and vacuolar membrane ion fluxes when large changes in cellular ion concentrations are required (Barbier-Brygoo et al., 2011). Therefore, intracellular free ATP might play a critical role in defining the energetic status of the guard cell, integrating metabolic components with membrane ion transport activities during stomatal movements. In this scenario, open-pore anion channel blockage provides an original mechanism for intracellular ATP sensing.
Synthesis of Malate and Its Catabolism in Guard Cells
Since malate is at the branching point of a wide number of metabolic pathways affecting cellular pH homeostasis and osmotic balance, its cytosolic concentrations must be tightly controlled. Malate levels are kept constantly low, ranging from 1 to 3 mm in the dark and 2 to 5 mm in the light (Gerhardt et al., 1987). 13C-NMR studies demonstrated that, as soon as the threshold of cytosolic malate concentration is attained (i.e. when its rate of formation is matched by its rate of utilization), the bulk of this organic acid is transported into the vacuole. Conversely, when malate is no longer synthesized, its concentration declines in the cytosol, leading to a slow efflux of malate from the vacuole (Gout et al., 1993). Although this pattern of malate flux is generally true, it appears that both efflux and influx of malate2− ions occur simultaneously across the tonoplast and are driven by changes in membrane potential as well as malate concentrations at both sides of the membrane (Gout et al., 1993; Chen et al., 2012).
Malate synthesis and degradation in guard cells are closely linked to phosphoenolpyruvate (PEP) deriving from the glyceraldehyde 3-phosphate, triose phosphate, and pyruvate-utilizing pathways (Figs. 1 and 2). During stomatal opening, cytosolic light-stimulated PEP carboxylase (PEPC) catalyzes the irreversible β-carboxylation of PEP in the presence of bicarbonate to yield oxaloacetate (OAA) and inorganic phosphate (Pi; Willmer et al., 1973; Willmer and Dittrich, 1974; Outlaw and Kennedy, 1978; Rao and Anderson, 1983; Chollet et al., 1996). OAA is reduced subsequently to malate through the action of NAD+-dependent malate dehydrogenase (NAD-MDH; Fig. 1; Scheibe et al., 1990). OAA is a strong inhibitor of malate efflux (Ki = 100 μm), meaning that PEP carboxylation gives an intermediate in malate synthesis that promotes malate transfer to the vacuole (Wang and Blatt, 2011).
PEPC is a highly regulated enzyme, as PEP carboxylation is the pivotal step leading to malate accumulation. Changes in the cytosolic levels of positive (e.g. Glc-6-P and triose phosphate) and negative (e.g. malate) metabolite effectors contribute to the overall regulation of guard cell PEPC activity in vivo (Outlaw et al., 1979; Outlaw, 1990; Tarczynski and Outlaw, 1990, 1993). However, the diurnal reversible phosphorylation of this enzyme is probably the most important form of regulation and has received a great deal of attention in the past three decades. Phosphorylation of a Ser residue located at a highly conserved region at the N terminus of the protein increases maximal enzyme activity and considerably lessens malate inhibition (Jiao and Chollet, 1991; Cotelle et al., 1999). This correlates with the observations that PEPC is phosphorylated when stomata are stimulated to open (Du et al., 1997; Outlaw et al., 2002) and that the enzyme from open stomata is less sensitive to malate than that from closed stomata (Zhang et al., 1994). Therefore, this phosphorylation of PEPC seems to help protect it against inhibition by malate during guard cell swelling and stomatal opening, thereby synchronizing the appropriate catalytic properties with the required in situ flux through this metabolic branch point. In line with this hypothesis, transgenic potato (Solanum tuberosum) plants with decreased or elevated PEPC activity showed delayed and accelerated stomatal opening compared with the wild type, respectively (Gehlen et al., 1996). Although PEPC has been studied extensively over the years, the signal transduction network that impinges upon this highly regulated enzyme has not been fully resolved. There is clear evidence that PEPC activation during stomatal opening is linked to H+-ATPase activity at the plasma membrane, as both white light and the phytotoxin fusicoccin reduce the sensitivity of PEPC to malate and increase its phosphorylation state (Du et al., 1997; Meinhard and Schnabl, 2001; Outlaw et al., 2002). While the light-induced activation of PEPC is dependent on K+ and photosynthesis, fusicoccin-induced activation is independent of K+. However, PEPC phosphorylation is strongly reduced or abolished if stomatal opening is driven by Suc rather than K+ or if K+ uptake is associated with Cl− uptake rather than malate (Du et al., 1997; Meinhard and Schnabl, 2001). Likewise, the guard cell kinase and phosphatase involved in this process have yet to be identified.
When stomata close, the accumulated malate can be dissipated metabolically via decarboxylation, either through the action of malic enzyme (NADP-ME) and the tricarboxylic acid cycle or through gluconeogenesis via PEP carboxykinase (PEPCK; Fig. 2). Evidence for the occurrence of either pathway in guard cells exists, but it is scarce or contradictory. Early experiments with radiolabeled malate showed that gluconeogenesis can proceed in guard cells and that starch formation from malic acid occurs, especially when stomata close slowly (Dittrich and Raschke, 1977a). When stomatal closure was accelerated by ABA in isolated C. communis epidermis, the guard cells lost most of the labeled malate to the medium (Dittrich and Raschke, 1977a), suggesting that gluconeogenesis may not be fast enough to remove all malate when the loss of turgor occurs quickly. By contrast, Schnabl (1981) and Outlaw et al. (1981a) failed to detect PEPCK activity in guard cells, questioning the decarboxylation of malate via this carboxykinase. The authors instead found high levels of NAD-ME and NADP-ME activity, suggesting that malate is metabolized directly to pyruvate by this enzyme and subsequently to PEP, potentially by pyruvate, orthophosphate dikinase (Outlaw et al., 1981a; Schnabl, 1981). NADP-ME also is expressed in Arabidopsis guard cells (Wheeler et al., 2005) and was implicated in the mechanism of stomatal closure when NADP-ME was overexpressed in tobacco (Nicotiana tabacum) plants (Laporte et al., 2002). Thus, a role for PEPCK was nearly forgotten until Penfield et al. (2012) revisited the importance of this metabolic step by taking advantage of an Arabidopsis mutant lacking PEPCK1, a highly expressed guard cell isoform in this plant. The pck1 mutants have increased stomatal conductance and wider stomatal aperture than wild-type plants, pointing toward a function for PCK1 in full stomatal closure in the dark (Penfield et al., 2012). Furthermore, pck1 mutants close their stomata normally in response to ABA or high [CO2], supporting previous observations that malate export may be more important than malate metabolism when stomata lose turgor within minutes.
Another seemingly important event during stomatal closure is the inhibition of PEPC, which would prevent unnecessary malate synthesis. This process is mediated by ABA, which suppresses malate accumulation and reduces the phosphorylation of PEPC (Schnabl et al., 1982; Du et al., 1997). PEPC also appears to be a target of ubiquitination, which would promote enzyme proteolysis during stomatal closure. Like phosphorylation, this posttranslational modification also is linked to ABA in a process mediated by the second messenger inositol 1,4,5-trisphosphate, which accumulates during ABA-induced stomatal closure (Lee et al., 1996; Klockenbring et al., 1998). Interestingly, inositol 1,4,5-trisphosphate also has been implicated in the regulation of K+ channels and Ca2+ signaling (Blatt et al., 1990; Gilroy et al., 1990), highlighting the key role of ABA in the coordinated activation of membrane ion transport and the associated metabolic activities required for stomatal closure. With our current knowledge, it is difficult to think about malate metabolism in a conclusive way, as genetic evidence often is based on overexpression using constitutive promoters or on knockout mutants. Of course, the observed phenotypes might result from changes in apoplastic metabolite concentrations or CO2 production by mesophyll cells, rather than from direct alterations in the guard cell intracellular malate pool. Additional experiments using guard cell-specific promoters are necessary to define their relative importance for the resulting phenotypes (see Box 1).
Emerging Roles for Other Carboxylates
While malate is unequivocally recognized as the predominant anion in most plants during stomatal opening and closure, increasing evidence also supports a role for other carboxylates. Similar to malate, the three-carbon molecule pyruvate serves as a key intersection point in several metabolic pathways. Pyruvate can be produced through glycolysis in the cytosol, converted back to carbohydrates via gluconeogenesis, or transported into mitochondria, where it can either be carboxylated to OAA or serve as a precursor of acetyl-CoA in the tricarboxylic acid cycle (Divakaruni and Murphy, 2012; Figs. 1 and 2). Furthermore, pyruvate-derived isopentenyl diphosphate can be used as a precursor of ABA biosynthesis in the chloroplast through the methyl erythritol phosphate pathway (Milborrow, 2001). Pyruvate has been implicated in the regulation of stomatal function. An early study showed that ABA-induced inhibition of stomatal opening in the light is reversed by ATP and pyruvate, suggesting that pyruvate may function as a negative regulator of ABA signaling in guard cells (Raghavendra et al., 1976). More recently, negative regulator of guard cell ABA signaling1 (NRGA1) was identified as a putative mitochondrial pyruvate carrier that negatively regulates ABA-induced guard cell signaling (Li et al., 2014). Disruption of NRGA1 in Arabidopsis results in increased ABA sensitivity of stomatal movements through increased ABA inhibition of K+in currents and ABA activation of slow-type anion currents in guard cells (Li et al., 2014). In the absence of NRGA1, the excess pyruvate may be converted to ABA through the methyl erythritol phosphate pathway (Milborrow, 2001), leading to elevated ABA levels in guard cells. Although this observation offers a putative molecular mechanism linking pyruvate to ABA signaling, a defect in pyruvate transport also might alter downstream metabolic pathways and, thus, only indirectly affect the ABA sensitivity of plasma membrane ion channels. For example, loss of NRGA1 could result in reduced levels of tricarboxylic acid cycle products (e.g. malate and fumarate), ATP, and NADH, thereby affecting guard cell physiology through effects on cellular energetics or osmoregulation. Alternatively, cytosolic pyruvate could be converted into sugar, which also has been implicated in guard cell ABA signaling (Kelly et al., 2013). In any case, the extent to which the alterations in organic acid metabolism in the neighboring mesophyll cells determine the observed nrga1 mutant phenotypes remains a matter of debate. Again, experiments using guard cell-specific promoters are needed to define the relative importance of mesophyll and guard cells for the resulting mutant phenotypes (see Box 1).
In the cytosol, the oxidation of hexoses to pyruvate through glycolysis also might contribute to stomatal movements by providing energy and reducing equivalents as well as metabolites (Fig. 1). Compared with mesophyll cells, guard cells have a high rate of glycolysis (Outlaw et al., 1979, 1985), and all of the enzymatic steps are represented by one or more proteins in the Arabidopsis guard cell proteome (Zhao et al., 2008). Glycolysis generates ATP and NADH and provides the mitochondria with substrates for the tricarboxylic acid cycle and oxidative phosphorylation for further ATP production and reducing power (Plaxton, 1996). Pharmacological studies using the respiratory poison potassium cyanide suggested that stomatal opening induced by a low-intensity blue light-specific response (Kinoshita et al., 2001; Shimazaki et al., 2007) or low CO2 concentrations under darkness (Sharkey and Raschke, 1981; Schwartz and Zeiger, 1984) mainly relies on oxidative phosphorylation as a source of ATP. A study by Zhao and Assmann (2011) supports this hypothesis and directly connects glycolysis to the tricarboxylic acid cycle in the regulation of stomatal movements. The authors showed that Arabidopsis double mutants lacking two highly similar phosphoglycerate mutases (iPGAMs), which catalyze the interconversion of 3-phosphoglycerate to 2-phosphoglycerate (3-PGA and 2-PGA, respectively), have defects in blue light-, low CO2-, and ABA-regulated stomatal movements. The simplest explanation is that glycolysis provides ATP, reducing equivalents, and malate under blue light. However, a more sophisticated, yet intriguing, explanation takes into account that disruption of the glycolytic pathway in the ipgam double mutants might affect the levels of other metabolites that have a signaling or regulatory effect. For example, an important signaling metabolite that can originate from the glycolytic intermediate 3-PGA is the amino acid Ser. Ser might function as a positive regulator of ABA signaling in guard cells (Muñoz-Bertomeu et al., 2011). Thus, the high concentrations of 3-PGA in the ipgam double mutants also may explain its impaired ABA response.
Another key regulatory metabolite of carbohydrate/carboxylate metabolism in the cytosol is Fru2,6P2, as it determines the direction of metabolic flux (i.e. glycolysis versus gluconeogenesis). Early biochemical studies showed that Fru2,6P2 is significantly more abundant in guard cells than in mesophyll cells and that its levels rise in the light by 3- to 10-fold within only 15 min (Hedrich et al., 1985). High levels of Fru2,6P2 during light-induced stomatal opening, in turn, would activate Glc breakdown in glycolysis through allosteric modulation of the enzymes involved in the phosphorylation of Fru-6-P to Fru1,6P2 (Hedrich et al., 1985; Hite et al., 1992). These enzymes are recognized as the inorganic pyrophosphate (PPi)-dependent Fru-6-P phosphotransferase (PPi-PFK) and the ATP-dependent phosphofructokinase1 (ATP-PFK1), both of which are more abundant in guard cells than in palisade cells (Hite et al., 1992; Fig. 1). The elevated activities of both enzymes and the phenotype of the ipgam mutants indicate that glycolysis in the cytosol is indeed a crucial event for stomatal opening, as it responds to the increased demand for energy and carbon skeleton accompanying this process. These findings establish a priority for future research directions.
In higher plants, glycolysis also can occur in the chloroplast through the hydrolysis of starch to dihydroxyacetone phosphate (DHAP) and 3-PGA, which then can be exported to the cytosol via the phosphoglycerate/DHAP shuttle (Heber, 1974). This highly selective transport system in the inner plastid envelope allows the carbohydrate/carboxylate metabolism between different subcellular compartments to be integrated, preventing the simultaneous occurrence of potentially incompatible metabolic processes. The parallel plastidic and cytosolic glycolytic reactions are catalyzed by isozymes encoded by distinct nuclear genes (Plaxton, 1996). However, the contribution of the plastidic isoforms to stomatal movements is currently unknown.
Carboxylates Connect the Mesophyll with Guard Cells
Evidence is accumulating that carboxylate metabolism in the adjacent mesophyll cells also plays a critical role in controlling stomatal behavior. Within the tricarboxylic acid cycle, two critical sequential steps are the oxidation of succinate to fumarate by succinate dehydrogenase (SDH) and the hydration of fumarate to malate by fumarase (FUM). These enzymatic reactions are required for both the decarboxylating energy-producing reactions of the tricarboxylic acid cycle and the carbon-conserving state during which carbon is shunted through the glyoxylate cycle (Sweetlove et al., 2010). Two recent studies from the Fernie group showed that transgenic tomato (Solanum lycopersicum) plants exhibiting antisense inhibition of FUM or the iron-sulfur subunit of SDH had impaired mitochondrial metabolism in the mesophyll cells, which affected stomatal function by regulating organic acid levels in the apoplast and the energetic status of the cell (Nunes-Nesi et al., 2007; Araújo et al., 2011). In both transgenic plants, the flux through the tricarboxylic acid cycle was reduced, with very little alteration in other aspects of leaf metabolism. However, the two antisense lines displayed somewhat opposite phenotypes. Whereas deficiency in FUM impaired CO2 assimilation and restricted growth due to reduced stomatal conductance (Nunes-Nesi et al., 2007), the SDH antisense line had an enhanced transpiration rate and stomatal conductance, resulting in elevated CO2 assimilation and aerial growth (Araújo et al., 2011). These differences correlated with the apoplastic levels of malate and fumarate, which were elevated in the FUM antisense lines and reduced in the SDH lines (Araújo et al., 2011), indicating a negative correlation between the concentrations of these metabolites and gas exchange through the stomata. According to this model, increased CO2 concentrations would inhibit the decarboxylation reactions of the tricarboxylic acid cycle, leading to pyruvate and malate accumulation in the apoplast and consequently reducing stomatal aperture. By contrast, low CO2 concentrations would favor the decarboxylation reactions and promote an increase in flux through the tricarboxylic acid cycle, and as such, a decrease in pyruvate and malate concentrations would lead to increased stomatal opening (Araújo et al., 2011). In addition to their role as osmolytes, altered levels of malate and fumarate may compromise mitochondrial oxidative phosphorylation, impinging on the production of ATP and NADH required for stomatal opening.
Further insights into the importance of carboxylates in the connection between stomatal behavior and leaf primary metabolism come from two recent studies where higher stomatal conductance was observed in plants with increased accumulation of malate (Gago et al., 2016; Medeiros et al., 2016). Plants lacking a functional AtALMT12 malate channel not only displayed slower stomatal closure, as mentioned above (Meyer et al., 2010), but also were characterized by changes in organic acid accumulation and increased stomatal and mesophyll conductance (Medeiros et al., 2016). These responses were accompanied by increased photosynthetic capacity and respiration rates, resulting in slightly elevated growth (Medeiros et al., 2016). Furthermore, a multispecies meta-analysis of 14 species grown in different experiments revealed a strong and positive correlation between net photosynthesis, stomatal conductance (gs), and mesophyll conductance (gm) with organic acid levels in the leaf (Gago et al., 2016). Altogether, these studies clearly support a role for the mesophyll in regulating guard cell aperture, whereby the import of organic acids from the mesophyll not only provides osmotic control but also plays a critical role in meeting the energetic demands of guard cells. Thus, genetic manipulation of carboxylate metabolism in mesophyll cells could lead to changes in stomatal behavior and could potentially improve photosynthesis and water use efficiency in plants.
PHOTOSYNTHETIC CARBON METABOLISM IN GUARD CELLS
Role of Guard Cell Chloroplast in Stomatal Movements
Chloroplasts are a common feature of guard cells in most plants, except for species such as the fern Polypodium vulgare (Lawson et al., 2003). Guard cells usually contain many fewer chloroplasts than the adjacent mesophyll cells (Humble and Raschke, 1971), typically 10 to 15, depending on species, compared with 30 to 70 in palisade cells. However, there are some notable exceptions. Certain fern species can have up to 100 chloroplasts per cell (Stevens and Martin, 1978), whereas some Selaginella spp. typically have only two chloroplasts per guard cell (Brown and Lemmon, 1985). Species of the orchid genus Paphiopedilum are another interesting exception, as their guard cells are completely devoid of chloroplasts (Nelson and Mayo, 1975; D’Amelio and Zeiger, 1988), indicating that these guard cells possess a particular metabolism where photosynthesis does not seem to play a role.
In general, guard cell chloroplasts tend to be smaller and have a different structure compared with mesophyll cells (Willmer and Fricker, 1983). Their thylakoid structure appears less well developed, and granal stacking is reduced (Willmer and Fricker, 1983), although functional photosystem I and II (PSI, PSII) have been reported (Outlaw et al., 1981b; Zeiger et al., 1981; Lawson et al., 2002, 2003), along with linear electron transport, oxygen evolution, and photophosphorylation (Hipkins et al., 1983; Willmer and Fricker, 1983; Shimazaki and Zeiger, 1985; Tsionsky et al., 1997). Guard cell electron transport can be moderated by [CO2] (Melis and Zeiger, 1982), suggesting that Calvin cycle activity acts as a major sink for electrons. Indeed, using high-resolution chlorophyll fluorescence imaging under controlled gas environments, Lawson et al. (2002, 2003) determined that Rubisco is a major sink for the end products of electron transport. These researchers also showed that quantum efficiency for PSII photochemistry in guard cells is 70% to 80% that of mesophyll cells across a wide range of light levels, pointing toward similar mechanisms operating in both cell types (Baker et al., 2001; Lawson et al., 2002). The argument for Rubisco as a significant sink for the products of electron transport was strengthened by the lack of a response of oxygen concentration in mesophyll cells of the C4 species Amaranthus caudatus (Lawson et al., 2003), which, because of the CO2-concentrating mechanism, virtually eliminates photorespiration. However, the quantum efficiency of PSII photochemistry did respond to changes in oxygen concentration in guard cells, which is consistent with immunogold labeling studies revealing considerable amounts of Rubisco in A. caudatus guard cells but not in mesophyll cells (Ueno, 2001). These results demonstrate that the two cell types respond differently to changes in oxygen concentration, suggesting that different metabolic processes act as sinks for the end products of electron transport. Other researchers, including Zeiger and colleagues, also detected significant Calvin cycle activity and demonstrated that it was osmotically important (Tallman and Zeiger, 1988; Talbott and Zeiger, 1993; Zeiger et al., 2002). CO2 uptake into 3-PGA and ribulose 1,5-bisphosphate (Gotow et al., 1988), along with Suc production in response to red light in epidermal peels, was observed in the absence of the mesophyll as a source of Suc (Poffenroth et al., 1992) and without starch breakdown (Talbott and Zeiger, 1993). Stomatal opening occurred in tobacco guard cells without exogenous application of K+ or Suc, with no evidence of starch breakdown, also suggesting that guard cells fix carbon via photosynthesis (Daloso et al., 2015). By contrast, Outlaw (1989) proposed that, due to the low levels of Rubisco and chlorophyll in guard cells, the Calvin-Benson cycle could not contribute significantly to the overall cell carbon balance and, hence, stomatal function. This idea was supported by Reckmann et al. (1990), who suggested that the Calvin cycle contributes only 2% to guard cell osmotica.
A recent study by Azoulay-Shemer et al. (2015) has renewed interest in guard cell photosynthesis and guard cell chloroplasts in stomatal function. Transgenic Arabidopsis plants with degraded chlorophyll in their guard cells, and therefore impaired photosynthesis, exhibited a deflated, thin phenotype, suggesting that photosynthesis in guard cells is critical for guard cell turgor. Interestingly, these plants showed typical wild-type responses to [CO2] and ABA, indicating that guard cell photosynthesis is not involved in these responses (Azoulay-Shemer et al., 2015). Similarly, transgenic tobacco plants with reduced levels of Rubisco had substantially reduced photosynthetic capacity, while stomatal responses to light and changing [CO2] were similar to those of the wild type (von Caemmerer et al., 2004; Baroli et al., 2008). However, Wang et al. (2014) found that, in an Arabidopsis crumpled leaf mutant (cr1), stomatal aperture in plants lacking guard cell chloroplasts (cr1-no chl) was 30% to 40% smaller than that of plants with guard cell chloroplasts (cr1-chl) and 40% to 50% smaller than that of the wild type, perhaps likely due to reduced ATP levels in their guard cells (and in the adjacent epidermal cells). These studies provide evidence that both guard cell chloroplasts and mesophyll contribute to ATP for H+ extrusion in guard cells and that guard cell chloroplasts play an essential role in light-induced stomatal opening (Wang et al., 2014).
Guard cell photosynthesis is a highly controversial topic, and although there is agreement that it takes place, the role it plays in guard cell function remains widely debated, mainly due to contradictory evidence from different experiments in different laboratories using different species. It is clear from recent studies that guard cell photosynthesis indeed contributes to stomatal behavior, through either the supply of energy for proton pumps or the production of osmoticum, but the extent of this contribution remains to be determined. Further studies using recent advances in molecular technology, such as cell-specific promoters and the ability to perform single-cell metabolomics, will allow us to elucidate the role of guard cell photosynthesis in stomatal function (see Box 1).
Contribution of PEPC to CO2 Fixation in Guard Cells
As an alternative to the end products of electron transport in guard cells, in the absence of CO2 fixation by Rubisco, there is evidence for CO2 fixation by PEPC into malate (Willmer and Dittrich, 1974; Raschke and Dittrich, 1977; Outlaw, 1990). Light-stimulated increases in PEPC activity have been demonstrated with enhanced malate accumulation and increased NADP- or NAD-dependent MDH activity, which facilitates the reduction of OAA to malate (Rao and Anderson, 1983; Scheibe et al., 1990). A recent study using stable isotope labeling to explore guard cell metabolism revealed that tobacco guard cells fix CO2 via both Rubisco and PEPC (Daloso et al., 2015), which agrees with earlier studies showing that guard cells have all the necessary machinery to operate the two CO2 fixation pathways (Outlaw and Manchester, 1979; Tarczynski et al., 1989; Parvathi and Raghavendra, 1997; Zeiger et al., 2002; Outlaw, 2003; Suetsugu et al., 2014; Wang et al., 2014). The importance of PEPC in stomatal behavior has been shown by Cousins et al. (2007) in PEPC-deficient mutants of the C4 plant Amaranthus edulis, which exhibited reduced stomatal opening and low final conductance compared with wild-type plants. The PEPC-deficient plants also showed a lower assimilation rate, which could account for the lower gs. This finding is in contrast to the maintained gs values observed in C3 antisense Rubisco (von Caemmerer et al., 2004) and antisense sedoheptulose-bisphosphatase tobacco plants (Lawson et al., 2008), which also had significantly reduced photosynthetic rates. Although there has been considerable support for C4 metabolism in guard cells of C3 plants (Cockburn, 1979; Vavasseur and Raghavendra, 2005), a recent study by Aubry et al. (2016) comparing gene expression in guard cells of C3 and C4 species revealed low expression of C4 genes in C3 guard cells. However, guard cells of the C4 plants showed similar gene expression patterns to those of C4 mesophyll cells, suggesting a role for C4 genes in guard cell regulation in C4 plants. These findings also agree with a role for organic acids in stomatal regulation. There was no evidence for the up-regulation of core C4 genes in guard cells of C3 plants (Aubry et al., 2016). Having said this, overexpressing PEPC in C3 potato increased the rate of stomatal opening, whereas reducing PEPC expression reduced stomatal opening rates (Gehlen et al., 1996). Both PEPC and Rubisco play roles in guard cell metabolism that affect stomatal function. However, their roles appear to be dependent on the time of day or environmental factors.
Interaction between Photosynthetic Carbon Assimilation and Mitochondrial Metabolism
Guard cells also contain numerous mitochondria (Willmer and Fricker, 1983), and evidence exists for a high respiration rate (Antunes et al., 2012), suggesting that these organelles also may represent a significant source of ATP for plasma membrane proton pumps and ion transport in guard cells (Wu and Assmann, 1993; Tominaga et al., 2001). Indeed, energy demands required for proton pumping and ion exchange for stomatal opening and closing are most likely met by a combination of photosynthesis and ATP and NADPH production during solute accumulation (Tominaga et al., 2001; Zeiger et al., 2002). This finding is in line with a recent study by Daloso et al. (2015) that provides strong evidence for respiration and sugar catabolism as a source of energy during stomatal opening. It also supports earlier work from Goh et al. (1999), who used single-cell chlorophyll fluorescence of guard cell protoplasts to show a dependency of guard cell electron transport on oxygen concentration, suggesting the involvement of the Mehler ascorbate peroxidase cycle and metabolic coupling between photosynthetic electron transport and export of reducing equivalents via the phosphoglycerate/DHAP shuttle and oxidative phosphorylation in the mitochondria. These findings clearly show that there is tight integration between the metabolism in chloroplasts and mitochondria in guard cells, which provides the energy, reducing power, and carbon skeletons necessary to support stomatal functions.
Guard Cell Chloroplasts and Light Signaling
Stomata have both a specific blue light response (for review, see Shimazaki et al., 2007) and a red light- or photosynthesis-dependent response. The blue light response saturates at low fluence rates (approximately 10 μmol m−2 s−1) and relies on the activation of a plasma membrane ATPase H+ pump (Kinoshita and Shimazaki, 1999; Shimazaki et al., 2007), while the red light response saturates at rates similar to photosynthesis and is inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU; an inhibitor of PSII), indicating that it is photosynthesis dependent (Sharkey and Raschke, 1981; Tominaga et al., 2001; Olsen et al., 2002; Messinger et al., 2006). Chlorophyll is thought to be a receptor for the red light response (Assmann and Shimazaki, 1999), and both sugar and K+ accumulation have been observed during red light-induced opening, with evidence that the sugar is derived from guard cell photosynthesis (Olsen et al., 2002). Experiments conducted with and without inhibitors of oxidative phosphorylation (oligomycin) and PSII (DCMU) demonstrated that chloroplasts supply ATP to the cytosol under red light for ATPase activity at the plasma membrane and for stomatal opening (Tominaga et al., 2001). Blue light opening is generally believed to be independent of guard cell photosynthetic electron transport and, therefore, carbon fixation metabolism, as opening is not inhibited by DCMU (Sharkey and Raschke, 1981; Schwartz and Zeiger, 1984; Roelfsema and Hedrich, 2005) but is reliant on energy from mitochondrial respiration (Shimazaki et al., 1982). Application of potassium cyanide, an inhibitor of mitochondrial respiration, inhibits blue light-induced stomatal opening (Schwartz and Zeiger, 1984). However, partial inhibition with DCMU was reported by Mawson (1983), which implies a role for ATP produced during guard cell photosynthetic electron transport. Parvathi and Raghavendra (1997) also observed increased Calvin cycle activity using 3,3-dichloro-2-dihydroxyphosphinoyl-methyl-2-propenoate (an inhibitor of PEPC and, therefore, involved in malate accumulation), suggesting that multiple osmoregulation pathways can function in guard cells and that one pathway may become important when another is restricted. Red light enhances blue light-induced stomatal opening. Suetsugu et al. (2014) explored the role of guard cell chloroplasts in this enhanced opening, showing that DCMU inhibited red light-enhanced, blue light-induced opening of Arabidopsis stomata. However, DCMU did not affect H+-ATPase in response to blue light, but it inhibited both red light- and blue light-dependent stomatal opening in intact leaves. These experiments illustrated that end products of electron transport (ATP and/or NADPH) in guard cells are essential for blue light responses.
Role of Guard Cell Chloroplasts in the Coordination between Mesophyll Photosynthesis and Stomatal Behavior
A close correlation between stomatal conductance and photosynthetic rates has been recorded over a range of CO2 concentrations and light intensities (Wong et al., 1979; Farquhar and Wong, 1984; Mansfield et al., 1990; Buckley et al., 2003). It is widely assumed that the concentration of CO2 inside the leaf (Ci) coordinates mesophyll photosynthesis with stomatal aperture. As the Ci is dependent on the consumption of CO2 through mesophyll photosynthesis and the flux of gas from the atmosphere to the inside of the leaf, which is determined by stomatal aperture and CO2 levels inside the leaf, maintaining a set Ci would provide an ideal mechanism for linking mesophyll photosynthesis with stomatal behavior. However, it is now believed that stomatal responses to changes in CO2 and Ci are too small to account for the relatively large changes observed in gs (Raschke, 1975; Farquhar et al., 1978; Farquhar and Sharkey, 1982; Morison and Jarvis, 1983). Also, several studies have demonstrated changes in gs in response to light, even when Ci was held constant (Messinger et al., 2006; Lawson et al., 2008; Wang and Song, 2008). Recently, Matrosova et al. (2015) used various Arabidopsis mutants to explore in detail the role of Ci in stomatal responses to red light. The authors showed that the protein kinase HIGH LEAF TEMPERATURE1 (HT1), whose mutation results in impaired low CO2-induced stomatal opening, is essential for red light (photosynthetic) stomatal opening, indicating that photosynthetically derived reductions in Ci contribute to stomatal opening in response to light. However, carbonic anhydrase mutants (ca1ca4), which typically exhibit slow stomatal responses to [CO2], respond more strongly to red light than to low [CO2], suggesting that other processes not reliant on low-Ci signaling are involved in red light-induced stomatal opening (Matrosova et al., 2015). The authors concluded that “red light responses can be mediated both independent and dependent of a reduction in [Ci].”
If guard cell photosynthesis responds to changing environmental cues in a similar manner to mesophyll cells, guard cell photosynthetic activity may provide the sensing mechanisms that coordinate stomatal behavior with mesophyll demands for CO2 (Jarvis et al., 1999; Lawson, 2009; Daloso et al., 2015). For example, environmental stimuli that increase or decrease mesophyll photosynthesis also will increase or decrease guard cell photosynthesis, and stomata will sense and respond to these changes by increasing or decreasing aperture. A recent study by Suetsugu et al. (2014) indicated that guard cell chloroplasts indirectly monitor photosynthetic CO2 fixation in the mesophyll by absorbing photosynthetically active radiation in the epidermis. Busch (2014) suggested that the stomatal red light response (mesophyll response) could be related to the balance between photosynthetic electron transport and carbon assimilation (Farquhar and Wong, 1984) and that the redox state of the plastoquinone pool in guard cells would be an appropriate mediator for such a signal.
Studies on stomata in isolated epidermal strips have demonstrated no (or reduced) effect of red light or [CO2] on stomatal aperture (Lee and Bowling, 1992, 1993; Roelfsema and Hedrich, 2002) compared with intact leaves (Mott et al., 2008), which indicates that a mesophyll signal must play a role in stomatal responses. Additionally, Lee and Bowling (1992, 1993) demonstrated that, when epidermis cells were incubated with mesophyll cells or chloroplasts, the stomata responded, but in their absence, no response was observed. These studies suggest that a diffusible mesophyll signal is needed for stomatal responses and that this signal could cocoordinate mesophyll photosynthesis with stomatal behavior (Wong et al., 1979; Lee and Bowling, 1992; Mott et al., 2008). However, the mechanism that links these is debated, with evidence for a mesophyll signal both in the vapor phase (Sibbernsen and Mott, 2010) and the liquid phase (Fujita et al., 2013). Several metabolites of photosynthesis (including ATP and NADPH) might balance photosynthesis between Rubisco and electron transport limitation (Wong et al., 1979; Messinger et al., 2006). Clearly, further studies are needed to elucidate the mechanisms that coordinate photosynthetic CO2 demands with stomatal behavior.
THE SUC PARADOX
Role for Suc in Guard Cell Osmoregulation
In the early 20th century, studies supported the notion that Suc was the only osmolyte required for stomatal opening and was produced by starch breakdown in the guard cells. This starch-sugar hypothesis (Lloyd, 1908) was the accepted theory until the 1960s, when it was replaced by the K+-malate theory (Imamura, 1943; Fischer, 1968; Raschke, 1975) correlating stomatal opening with K+ uptake, along with the counter ions malate2− and/or Cl− (Allaway, 1973; Schnabl and Raschke, 1980; Outlaw, 1983). As outlined above, this became accepted as the main osmoregulatory pathway and often is still considered the exclusive mechanism for regulating stomatal aperture. Therefore, a role for Suc was forgotten until MacRobbie (1987) and Talbott and Zeiger (1993) showed that soluble sugars could function as additional osmoticum for stomatal opening. The observation of a decline in K+ concentration throughout the day concomitant with an increase in Suc concentration led to the idea that K+ is important for stomatal opening early in the day, which is replaced by Suc later in the diel period (Amodeo et al., 1996; Talbott and Zeiger, 1998; Schroeder et al., 2001). These discoveries renewed interest in Suc metabolism in guard cells.
Several reports have suggested that soluble sugars are key osmotica for stomatal opening (Poffenroth et al., 1992; Lu et al., 1995; Talbott and Zeiger, 1998). Talbott and Zeiger (1993, 1996) observed an increase in Suc content in V. faba guard cells from 0.2 to 0.7 pmol, or even higher, during the light period. Stomatal opening in epidermal strips of V. faba was accompanied by a 300-fmol increase in hexose sugar levels and a 140-fmol increase in Suc levels (Outlaw and Manchester, 1979). Lu et al. (1995, 1997) also found that the symplastic guard cell Suc concentrations increased 3-fold upon the transition to light. Increasing Suc content in epidermal peels of V. faba in the afternoon also was reported by Pearson (1973); however, the correlation with stomatal aperture was weak. This research was not followed up in detail, most likely due to the lack of appropriate experimental methods and genetic resources, which sometimes led to conflicting results (see Box 1). Therefore, the role of Suc as a major osmoticum to maintain stomatal aperture in the afternoon remains to be fully elucidated, with the exact temporal changes in Suc concentrations over the day/night cycle one of the important factors to be determined in the future.
Role for Suc Other Than Osmoregulation
A nonosmoregulatory role for Suc in guard cell function also was suggested recently (Kelly et al., 2013). Transgenic plants overexpressing hexokinase (HK, a sugar-phosphorylating enzyme involved in sugar sensing) specifically in guard cells exhibited accelerated stomatal closure induced by sugar. This observation supports the idea of photosynthesis feedback inhibition of stomatal conductance by Suc (Kelly et al., 2013) and agrees with the earlier suggestion by Outlaw and colleagues that Suc produced by mesophyll photosynthesis is loaded into the apoplast and carried to the vicinity of the guard cells, where an extracellular osmotic effect closes the stomata (Lu et al., 1995, 1997; Ewert et al., 2000; Outlaw and De Vlieghere-He, 2001; Kang et al., 2007). This might provide a mechanism for coordinating photosynthetic rates with transpiration. When the production of Suc in the mesophyll exceeds phloem loading, the excess Suc is carried to the guard cells and stimulates stomatal closure via hexokinase, thereby reducing water loss when photosynthesis is saturated (Kelly et al., 2013). Although this process may provide long-term coordination between transpiration and photosynthesis, such a mechanism could not account for short-term coordination between photosynthesis and stomatal behavior, as reduced stomatal conductance is not usually observed under conditions of high photosynthetic rates (Wong et al., 1979; Lawson et al., 2010, 2014). Kelly et al. (2013) speculated that the accumulation of Suc late in the day is not a reflection of diurnal changes in guard cell osmoregulation, as suggested by Zeiger et al. (2002), but instead reflects the uptake of apoplastic Suc by the guard cells, which eventually induces stomatal closure. Although the molecular mechanism by which Suc promotes stomatal closure is not fully understood, it appears to be mediated by ABA signaling (Kelly et al., 2013). A role for Suc in stomatal closure explains the findings of a recent multispecies meta-analysis revealing an opposite correlation for leaf sugar content with net photosynthesis and stomatal conductance (Gago et al., 2016), suggesting that the tradeoff between carbon assimilation and water loss through stomata is at least partially regulated by Suc level.
Suc also might function as a respiratory substrate for mitochondrial energy supply and the production of carbon skeletons (Dittrich and Raschke, 1977b). Potato plants overexpressing yeast acid invertase (INV, a Suc-cleaving enzyme) in guard cells had higher stomatal conductance than wild-type control plants (Antunes et al., 2012). Conversely, plants with reduced levels of Suc synthase (SuSy), which catalyzes the reversible conversion of Suc to Fru and UDP-Glc, had lower stomatal conductance (Antunes et al., 2012). The importance of Suc breakdown was further highlighted by Daloso et al. (2016), who reported that tobacco plants overexpressing SuSy under the control of the guard cell-specific promoter KST1 had increased stomatal conductance, greater photosynthetic rates, and higher biomass than wild-type plants. Following a dark-to-light transition, the Suc content was lower in transgenic plants than in wild-type plants, and metabolite analysis showed a reduction in the ratio of disaccharides to monosaccharides and a greater capacity to degrade Suc (Daloso et al., 2016). It seems, therefore, that during stomatal opening, SuSy functions in the Suc hydrolysis direction rather than in Suc biosynthesis. Suc breakdown may provide substrates for respiration (Fig. 1), providing further evidence for a link between Suc and the tricarboxylic acid cycle for stomatal opening and highlighting the importance of glycolysis and mitochondrial respiration in stomatal function.
In conclusion, our current knowledge suggests that both Suc breakdown and Suc accumulation play roles in stomatal function, indicating that the exact mechanism and role of Suc may be far more complex than originally thought. To disentangle this paradox of the role of Suc in stomatal function, highly targeted manipulation of guard cell metabolism will be required (see Box 1).
Sources of Suc in Guard Cells
An additional unresolved issue concerns the potential sources of Suc in guard cells. Several routes for Suc accumulation in guard cells exist: starch breakdown produces Suc; Suc produced by mesophyll photosynthesis is transported to the guard cells via the apoplast; and Suc is produced autonomously from Calvin cycle activity in the guard cells (Fig. 1). Evidence exists for all three of these pathways. However, it seems that starch degradation and guard cell photosynthesis can provide only limited amounts of Suc, implying that guard cell apoplastic Suc is the most important source (Tarczynski et al., 1989; Reckmann et al., 1990; Vavasseur and Raghavendra, 2005). Outlaw and colleagues (Lu et al., 1995; Ritte et al., 1999; Outlaw and De Vlieghere-He, 2001; Kang et al., 2007) suggested that Suc produced by mesophyll photosynthesis is transported to the guard cells via the apoplast and is taken up into the guard cell symplast. A few studies have documented sugar uptake into guard cells, apparently in symport with protons (Dittrich and Raschke, 1977b; Ritte et al., 1999). Suc in the apoplast is either degraded to Glc and Fru by cell wall invertase and taken up by monosaccharide transporters or is imported directly as Suc (Fig. 1). Guard cell transcriptomic analyses, along with localization studies using reporter genes, suggested that the major sugar transporters at the guard cell plasma membrane are the Suc/H+ cotransporters SUC1 and SUC3 and the monosaccharide/H+ cotransporters STP1 and STP4 (Stadler et al., 2003; Meyer et al., 2004; Bates et al., 2012; Bauer et al., 2013). STP1 and STP4 transcript levels are 8- to 16-fold higher than those of SUC1, suggesting that Suc in the guard cell apoplast is first converted to monosaccharides. Interestingly, STP1 is expressed mostly in the dark but shows a peak of expression at midday (Stadler et al., 2003), matching the time at which guard cells accumulate high amounts of sugars (Talbott and Zeiger, 1996). However, the functional analysis of these transporters is currently limited. Future studies using transgenic plants with guard cell-specific overexpression or silencing of these transporters will be invaluable for increasing our understanding of the role of Suc metabolism in stomatal function (see Box 1).
GUARD CELL STARCH METABOLISM
Starch Is a Versatile d-Glc Polymer
Starch is synthesized inside plastids of both photosynthetic and nonphotosynthetic cells, but its metabolism and function depend upon the cell type from which it is derived. In the leaves, starch typically accumulates gradually during the day using a fraction of the carbon assimilated through photosynthesis. This transitory starch is degraded at night in a nearly linear manner for continued Suc synthesis and energy production when photosynthesis does not occur, a process vital for plant growth (Smith and Stitt, 2007; Stitt and Zeeman, 2012). In most species, transitory starch also is present in guard cells. In this cell type, however, starch turnover differs markedly from that of the rest of the leaf, as starch is present at night and degraded in the light, helping to generate organic acids and sugars to promote stomatal opening (Vavasseur and Raghavendra, 2005; Lawson, 2009). Although botanists have known at least since the formulation of the starch-osmoticum hypothesis by Lloyd (1908) that starch granules in guard cells may disappear during stomatal opening and reappear during or after closure, the genetic and molecular bases of this process have remained obscure. Research in the past few years has progressed remarkably and has led to a steep increase in our understanding of this topic.
Carbon Sources for Guard Cell Starch
Theoretically, starch in the guard cell can be synthesized from CO2 fixed via the Calvin cycle or from sugars and/or organic acids that have accumulated early in the day (synthesized by the guard cell itself or imported from neighboring cells). There is some experimental evidence for all of these mechanisms, but their relative contributions to the pool of accumulated starch, as well as their timing, remain a matter of debate. As mentioned above, guard cells can produce malic acid by the carboxylation of PEP using CO2, as demonstrated by experiments with isolated epidermis exposed to 14CO2 (Willmer et al., 1973; Raschke and Dittrich, 1977; Daloso et al., 2015). Furthermore, Schnabl (1980) showed that 14C-starch was synthesized by isolated guard cell protoplasts supplied with 14CO2 or [U-14C]malate, whereas only a small amount of radioactive starch was synthesized from exogenously supplied [4-14C]malate, indicating that the labeled 4C of malic acid was lost as CO2 and not refixed. Lastly, the hexose phosphate needed for guard cell starch accumulation also can be derived from the metabolism of Suc stored in the vacuole or from sugars imported directly from the apoplast. Indeed, starch formation was observed when isolated epidermis with open or closed stomata was incubated with 14C-labeled Glc or Suc or simply floated on sugar solutions (Pallas, 1964; Dittrich and Raschke, 1977b). It is possible that Suc, or its degradation products, is metabolized to hexose phosphate, which may then move into the chloroplast and be converted to starch (Fig. 2). If so, hexose phosphate transport would reduce the phosphate concentration in the chloroplast and provide carbon for starch synthesis in the form of ADPglucose (ADPGlc). The energy required for starch synthesis may be derived from the oxidative phosphorylation of malate that accumulates from stomatal opening, as malate levels decrease during starch accumulation.
Function, Pathway, and Regulation of Guard Cell Starch Degradation
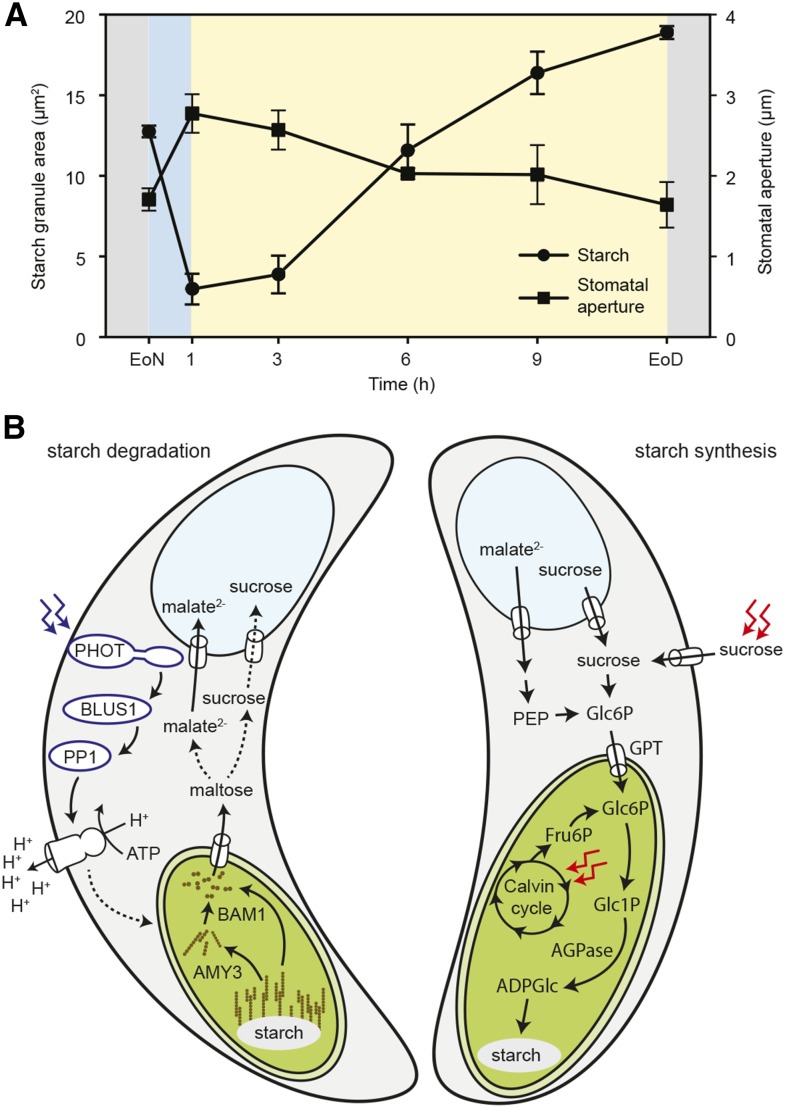
After the initial blush of research leading to the K+-malate hypothesis (Fischer and Hsiao, 1968; Allaway, 1973; Outlaw and Manchester, 1979; Donkin and Martin, 1980; Schnabl, 1980; Schnabl et al., 1982), attention shifted to other questions about stomatal physiology. The development of voltage clamp technology and molecular genetics led to the use of Arabidopsis as a preeminent model for studying guard cell membrane ion transport and signaling, whereas research in guard cell starch metabolism has suffered from the lack of suitable analytical methods. Because guard cells constitute only a minute fraction of the total leaf, it is impossible to study starch metabolism using conventional methods (see Box 1). In most cases, starch content in guard cells has been estimated on a relative value scale by comparing the intensity of iodine-stained guard cell chloroplasts (Tallman and Zeiger, 1988; Kang et al., 2007; Valerio et al., 2011; Prasch et al., 2015). In a few studies, starch content was determined quantitatively using the oil-well technique on freeze-dried leaflets (Outlaw and Manchester, 1979; Ding et al., 2014) or spectrophotometrically using guard cell-enriched epidermal fragments (Daloso et al., 2015), but, in these cases, no temporal dynamics were reported. Thus, our knowledge of starch metabolism in guard cells has remained fragmentary, mostly correlative in nature, and even contradictory. Until very recently, it was still a matter of debate whether starch is present in Arabidopsis guard cells at the end of the night or, indeed, whether it is required for stomatal opening (Lasceve et al., 1997; Stadler et al., 2003; Daloso et al., 2015), to the point that starch metabolism in guard cells was considered to differ between species (Lawson et al., 2014). Only early this year was starch metabolism in Arabidopsis guard cells unequivocally shown to be similar to that of other species. Horrer et al. (2016) developed a new method for quantifying starch in guard cells of intact leaves using the fluorophore propidium iodide and high-resolution confocal microscopy on a cell-by-cell basis, at last overcoming a technically challenging problem that deterred research in this area for many years. Using this newly developed method, Horrer et al. (2016) showed that starch in Arabidopsis guard cells is very rapidly degraded within the first 30 min of light, which is correlated with an increase in stomatal aperture (Fig. 3A). Molecular genetics analysis further demonstrated that starch in guard cells is mobilized by a specific set of enzymes that are not required for starch degradation in other leaf tissues (Fig. 3B). Blocking this pathway (i.e. by knocking out the β-amylase BAM1 and/or the α-amylase AMY3) resulted in elevated guard cell starch levels, while starch was metabolized normally in the rest of the leaf, severely affecting stomatal function, transpiration, and biomass production (Valerio et al., 2011; Prasch et al., 2015; Horrer et al., 2016). Impaired guard cell starch breakdown in bam1 mutant plants leads to improved drought tolerance, likely due to reduced water uptake and limited cell wall extension associated with the closed state of stomata of bam1 mutants compared with wild-type plants (Prasch et al., 2015).
Figure 3.
Guard cell starch breakdown and synthesis during stomatal movements in Arabidopsis. A, Guard cell starch content and stomatal aperture over the 12-h light phase (n = 110 ± se). EoD, End of day; EoN, end of night. Redrawn from Horrer et al. (2016). B, During stomatal opening, blue light-dependent activation of the plasma membrane proton pump leads to starch degradation in the guard cell chloroplast by amylases. The degradation product maltose can be stored in the form of malate and/or Suc in the vacuole, helping increase guard cell turgor for stomatal opening. During stomatal closure, starch is resynthesized in response to red light using carbon fixed via the Calvin cycle or from sugars and/or organic acids synthesized by the guard cells itself or imported from neighboring cells. AGPase, ADPGlc pyrophosphorylase; AMY3, α-Amylase3; BAM1, β-amylase1; BLUS1, blue light signaling1; Fru6P, Fru-6-P; Glc1P, Glc-1-P; Gl6P, Glc-6-P; GPT, Glc-6-P/Pi translocator; PEP, Phosphoenolpyruvate; PHOT, phototropins 1 and 2; PP1, protein phosphatase1. Compartments are not to scale. Dotted lines represent suggested steps.
Early histochemical studies demonstrated that illuminating isolated V. faba epidermal peels with blue light at a low fluence rate was accompanied by a marked disappearance of guard cell starch (Tallman and Zeiger, 1988). Furthermore, Arabidopsis mutants devoid of starch showed a reduced rate of stomatal opening specifically under blue light (Lasceve et al., 1997). Horrer et al. (2016) disentangled the molecular basis of this blue light-dependent response, showing that, in Arabidopsis, starch degradation in guard cells is triggered by blue light through the action of the blue light photoreceptors phototropin 1 and 2 (PHOT) and their downstream signaling components (Fig. 3B). These authors also provided evidence for a direct molecular link between proton pumping and starch degradation in guard cells (Horrer et al., 2016). The signal linking plasma membrane H+-ATPase activity to starch degradation in the chloroplast remains unknown, but it is unlikely that the simple degradation of starch leading to malate accumulation provides the energy required for this transport process. Only two ATPs are synthesized for each glucosyl moiety catabolized. Thus, it is more likely that starch in guard cells is degraded to provide carbon skeletons for the synthesis of malate and/or Suc to increase guard cell turgor and to promote stomatal opening.
This discovery highlights the importance of starch metabolism in stomatal movement and shows that Arabidopsis is an ideal system for investigating starch metabolism in guard cells, laying the foundation for future studies integrating light signaling and membrane ion transport with guard cell metabolism.
Guard Cell Starch Biosynthesis
Starch biosynthesis in guard cells begins only after 1 to 3 h of light, when the stomata have fully opened, and proceeds slowly until the end of the day (Fig. 3A; Horrer et al., 2016). This unusual pattern of starch deposition in guard cells suggests that specific regulatory factors are involved that act independently of the transitions between light and dark. Light quality is one such factor. Horrer et al. (2016) demonstrated that red light promotes efficient starch synthesis in guard cells, whereas blue light promotes starch degradation. It is conceivable that red light-induced CO2 fixation provides the precursors needed for starch synthesis.
It is tempting to speculate that guard cell starch synthesis also follows a distinct pathway. In mesophyll cells, the supply of ADPGlc, the activated glucosyl donor for starch biosynthesis, is linked directly to the Calvin cycle via three sequential enzymatic reactions. Fru-6-P, an intermediate of the Calvin cycle, is converted to Glc-6-P by plastidial phosphoglucose isomerase (PGI; Yu et al., 2000). Plastidial phosphoglucomutase (PGM1) converts Glc-6-P into Glc-1-P (Caspar et al., 1985). The last step is catalyzed by ADP-Glc pyrophosphorylase (AGPase), which converts Glc-1-P and ATP into ADPGlc and PPi (Lin et al., 1988). Arabidopsis plants lacking PGM are devoid of starch in both mesophyll and guard cells (Caspar et al., 1985; Lasceve et al., 1997; Horrer et al., 2016), showing that stromal Glc-6-P to Glc-1-P conversion is required for guard cell starch synthesis (Fig. 3B). By contrast, PGI seems not to be required; the Arabidopsis pgi mutant lacks starch in mesophyll cells but not in guard cells (Yu et al., 2000; Tsai et al., 2009). A likely explanation for this phenotype is that, in the absence of plastidial PGI, guard cell chloroplasts can presumably take up Glc-6-P from the cytosol into chloroplasts via Glc-6-P/Pi translocator (GPT) and use it for starch synthesis, bypassing the PGI reaction (Fig. 3B). In support of this hypothesis, an early study showed that envelope membranes of guard cell chloroplasts have GPT transport activity (Overlach et al., 1993). This appears to be a specific feature of guard cells, as mesophyll cells cannot import Glc-6-P efficiently for starch synthesis unless GPT translocators are overexpressed (Niewiadomski et al., 2005).
AGPase activity in guard cells is comparable to that of palisade and spongy cells (Outlaw and Tarczynski, 1984). AGPase is finely tuned through allosteric and redox control: it is activated by 3-PGA, an intermediate of the Calvin cycle (and an indicator of photosynthetic carbon assimilation) and inhibited by Pi (Iglesias et al., 1993). AGPase is a heterotetrameric enzyme composed of two small subunits (encoded by a single APS gene) and two large subunits (encoded by one of four genes, APL1–APL4; Morell et al., 1987). The small subunit is catalytic, whereas the large subunit is primarily regulatory. The combination of APS with different large subunits differs between autotrophic and heterotrophic tissues and confers distinct kinetic and regulatory properties to the enzyme, modulating its activity in response to allosteric effectors and its own substrates (Crevillén et al., 2003, 2005). APL1 would be the main isoform in mesophyll cell chloroplasts, whereas other APLs may participate in the establishment of the AGPase heterotetramer in different cell types, including guard cells (Crevillén et al., 2005). Another seemingly important enzyme for guard cell starch biosynthesis could be SuSy. Similar to what happens in potato tubers, UPDGlc originating from the hydrolysis of Suc could be channeled into glycolysis and converted into Glc-1-P. Glc-1-P could be taken up subsequently by the chloroplast and further processed by AGPase. Together, these observations suggest that an alternative starch biosynthetic pathway may well exist in guard cells, requiring the activities of specialized proteins such as GPT, SuSy, or specific combinations of different APL isoforms (Fig. 3B).
While we have begun to understand the function of guard cell starch degradation in stomatal movements, the role of starch synthesis remains mostly unexplored. A very recent study suggested that starch biosynthesis in guard cells, but not in mesophyll cells, is involved in high-CO2-induced stomatal closure (Azoulay-Shemer et al., 2016). The authors compared stomatal responses to [CO2] shifts in pgi and aps1 Arabidopsis mutants and demonstrated that aps1 but not pgi exhibited impaired CO2-induced stomatal closure. It is plausible that, during stomatal closure, starch synthesis functions as a sink for sugars and malate, thereby promoting the necessary changes in guard cell turgor for water efflux.
CONCLUSION
Guard cell metabolism during stomatal movements is highly complex. Furthermore, there is strong evidence for tight coordination between guard cell metabolism and the adjacent mesophyll, as well as for its integration with membrane ion transport and light signaling.
The motor that drives stomatal movements is the strongly electrogenic H+-extruding ATPase of the guard cell plasma membrane. When this pump hyperpolarizes the membrane potential, voltage-sensitive K+ channels open, which results in stomatal opening as the internal K+ concentration increases nominally to 300 mm (Outlaw, 1983). During stomatal opening, a pair of guard cells extrudes approximately 3 pmol of H+, which exceeds the expected pH-buffering capacity (Blatt et al., 1983; Chen et al., 2012). This leaves the guard cell with a significant electrical imbalance and cytosolic alkalinization. K+ uptake compensates directly for H+ efflux, at least for the charge. Likewise, H+-coupled K+ and Cl− uptake compensates for the charge and, to a small extent, for the alkaline load on the cytosol (Raschke and Schnabl, 1978). However, metabolism is the major source of H+ protons to compensate for the alkaline load. Most of the extruded protons are replaced by the protons that are released during the accumulation of carboxylates, the synthesis of which releases protons (Robinson and Preiss, 1985; Outlaw et al., 2002). The accumulation of carboxylates, both through synthesis within the guard cells and import from the adjacent mesophyll, also compensates electrically for K+ uptake and promotes changes in the water potential for stomatal turgor.
Proton pumping across the plasma membrane requires a significant amount of energy in the form of ATP. One of the roles of guard cell metabolism is to meet the energetic demands of stomatal movements. Mitochondrial oxidative phosphorylation, along with photophosphorylation and glycolysis, are important sources of ATP. The pathways within the mitochondria, chloroplasts, and cytosol are in a delicate metabolic balance; the rapid exchange of metabolites between these subcellular compartments is an important channel of communication, ultimately coordinating the energetic and metabolic status of the cell with membrane ion transport activity. The synthesis and degradation of metabolites such as malate and Suc, therefore, is a cardinal event in guard cells, not only for osmoregulation but also for ATP generation. To what extent energy demand is distinct from carbon skeleton demand is not clear (see “Outstanding Questions”). An integrated systemic approach based on the guard cell-specific manipulation of metabolism will allow us to disentangle this mystery.
Acknowledgments
We thank Daniel Horrer for help with figure preparation; Alexis De Angeli, Cornelia Eisenach, Enrico Martinoia, Arianna Nigro, and Sabrina Flütsch for helpful discussion during article preparation; and Mike Blatt and our reviewers, whose comments helped to greatly improve the article.
Glossary
- ABA
abscisic acid
- CAM
Crassulacean acid metabolism
- PEP
phosphoenolpyruvate
- OAA
oxaloacetate
- Pi
inorganic phosphate
- 3-PGA
3-phosphoglycerate
- 2-PGA
2-phosphoglycerate
- PPi
inorganic pyrophosphate
- DHAP
dihydroxyacetone phosphate
- DCMU
3-(3,4-dichlorophenyl)-1,1-dimethylurea
Footnotes
This work was supported by the Swiss National Science Foundation (grant no. 31003A_147074 to D.S.), by Vontobel Stiftung (grant no. F–74503–12 to D.S.), and by the Biotechnology and Biological Sciences Research Council (grant no. BB/1001187_1 to T.L.).
Articles can be viewed without a subscription.
References
- Allaway WG. (1973) Accumulation of malate in guard cells of Vicia faba during stomatal opening. Planta 110: 63–70 [DOI] [PubMed] [Google Scholar]
- Amodeo G, Talbott LD, Zeiger E (1996) Use of potassium and sucrose by onion guard cells during a daily cycle of osmoregulation. Plant Cell Physiol 37: 575–579 [Google Scholar]
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 89: 3736–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B, et al. (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc Natl Acad Sci USA 111: E1806–E1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes WC, Provart NJ, Williams TCR, Loureiro ME (2012) Changes in stomatal function and water use efficiency in potato plants with altered sucrolytic activity. Plant Cell Environ 35: 747–759 [DOI] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Osorio S, Usadel B, Fuentes D, Nagy R, Balbo I, Lehmann M, Studart-Witkowski C, Tohge T, et al. (2011) Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid-mediated effect on stomatal aperture. Plant Cell 23: 600–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki Ki (1999) The multisensory guard cell: stomatal responses to blue light and abscisic acid. Plant Physiol 119: 809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Wang XQ (2001) From milliseconds to millions of years: guard cells and environmental responses. Curr Opin Plant Biol 4: 421–428 [DOI] [PubMed] [Google Scholar]
- Aubry S, Aresheva O, Reyna-Llorens I, Smith-Unna RD, Hibberd JM, Genty B (2016) A specific transcriptome signature for guard cells from the C4 plant Gynandropsis gynandra. Plant Physiol 170: 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay-Shemer T, Bagheri A, Wang C, Palomares A, Stephan AB, Kunz HH, Schroeder JI (2016) Starch biosynthesis in guard cells but not in mesophyll cells is involved in CO2-induced stomatal closing. Plant Physiol 171: 788–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay-Shemer T, Palomares A, Bagheri A, Israelsson-Nordstrom M, Engineer CB, Bargmann BOR, Stephan AB, Schroeder JI (2015) Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2- and ABA-induced stomatal closing. Plant J 83: 567–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR, Oxborough K, Lawson T, Morison JI (2001) High resolution imaging of photosynthetic activities of tissues, cells and chloroplasts in leaves. J Exp Bot 52: 615–621 [PubMed] [Google Scholar]
- Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S (2011) Anion channels/transporters in plants: from molecular bases to regulatory networks. Annu Rev Plant Biol 62: 25–51 [DOI] [PubMed] [Google Scholar]
- Baroli I, Price GD, Badger MR, von Caemmerer S (2008) The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiol 146: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GW, Rosenthal DM, Sun J, Chattopadhyay M, Peffer E, Yang J, Ort DR, Jones AM (2012) A comparative study of the Arabidopsis thaliana guard-cell transcriptome and its modulation by sucrose. PLoS ONE 7: e49641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KAS, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Blatt MR. (1987a) Electrical characteristics of stomatal guard cells: the contribution of ATP-dependent, “electrogenic” transport revealed by current-voltage and difference-current-voltage analysis. J Membr Biol 98: 257–274 [Google Scholar]
- Blatt MR. (1987b) Electrical characteristics of stomatal guard cells: the ionic basis of the membrane potential and the consequence of potassium chlorides leakage from microelectrodes. Planta 170: 272–287 [DOI] [PubMed] [Google Scholar]
- Blatt MR. (1988) Potassium-dependent, bipolar gating of K+ channels in guard cells. J Membr Biol 102: 235–246 [Google Scholar]
- Blatt MR. (1992) K+ channels of stomatal guard cells: characteristics of the inward rectifier and its control by pH. J Gen Physiol 99: 615–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR. (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Blatt MR, Clint GM (1989) Mechanisms of fusicoccin action: kinetic modification and inactivation of K+ channels in guard cells. Planta 178: 509–523 [DOI] [PubMed] [Google Scholar]
- Blatt MR, Slayman CL (1983) KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. J Membr Biol 72: 223–234 [DOI] [PubMed] [Google Scholar]
- Blatt MR, Thiel G, Trentham DR (1990) Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature 346: 766–769 [DOI] [PubMed] [Google Scholar]
- Brown R, Lemmon BE (1985) Development of stomata in Selaginella: division polarity and plastid movements. Am J Bot 72: 1914–1925 [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26: 1767–1785 [Google Scholar]
- Busch FA. (2014) Opinion: the red-light response of stomatal movements is sensed by the redox state of the photosynthetic electron transport chain. Photosynth Res 119: 131–140 [DOI] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerana R, Giromini L, Colombo R (1995) Malate-regulated channels permeable to anions in vacuoles of Arabidopsis thaliana. Aust J Plant Physiol 22: 115 [Google Scholar]
- Chen ZH, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O’Leary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 273–298 [DOI] [PubMed] [Google Scholar]
- Clint GM, Blatt MR (1989) Mechanisms of fusicoccin action: evidence for concerted modulations of secondary K+ transport in a higher plant cell. Planta 178: 495–508 [DOI] [PubMed] [Google Scholar]
- Cockburn W. (1979) Relationships between stomatal behavior and internal carbon dioxide concentration in Crassulacean acid metabolism plants. Plant Physiol 63: 1029–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombet J, Thomine S, Guern J, Frachisse JM, Barbier-Brygoo H (2001) Nucleotides provide a voltage-sensitive gate for the rapid anion channel of Arabidopsis hypocotyl cells. J Biol Chem 276: 36139–36145 [DOI] [PubMed] [Google Scholar]
- Cotelle V, Pierre JN, Vavasseur A (1999) Potential strong regulation of guard cell phosphoenolpyruvate carboxylase through phosphorylation. J Exp Bot 50: 777–783 [Google Scholar]
- Cousins AB, Baroli I, Badger MR, Ivakov A, Lea PJ, Leegood RC, von Caemmerer S (2007) The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiol 145: 1006–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevillén P, Ballicora MA, Mérida A, Preiss J, Romero JM (2003) The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J Biol Chem 278: 28508–28515 [DOI] [PubMed] [Google Scholar]
- Crevillén P, Ventriglia T, Pinto F, Orea A, Mérida A, Romero JM (2005) Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP-glucose pyrophosphorylase-encoding genes. J Biol Chem 280: 8143–8149 [DOI] [PubMed] [Google Scholar]
- Daloso DM, Antunes WC, Pinheiro DP, Waquim JP, Araújo WL, Loureiro ME, Fernie AR, Williams TCR (2015) Tobacco guard cells fix CO2 by both Rubisco and PEPcase while sucrose acts as a substrate during light-induced stomatal opening. Plant Cell Environ 38: 2353–2371 [DOI] [PubMed] [Google Scholar]
- Daloso DM, Williams TCR, Antunes WC, Pinheiro DP, Müller C, Loureiro ME, Fernie AR (2016) Guard cell-specific upregulation of sucrose synthase 3 reveals that the role of sucrose in stomatal function is primarily energetic. New Phytol 209: 1470–1483 [DOI] [PubMed] [Google Scholar]
- D’Amelio DE, Zeiger E (1988) Diversity in guard cell plastids of the Orchidaceae: a structural and functional study. Can J Bot 66: 257–271 [Google Scholar]
- Darwin F. (1916) On the relation between transpiration and stomatal aperture. Philos Trans R Soc Lond B 207: 413–437 [Google Scholar]
- De Angeli A, Thomine S, Frachisse JM (2016) Anion channel blockage by ATP as a means for membranes to perceive the energy status of the cell. Mol Plant 9: 320–322 [DOI] [PubMed] [Google Scholar]
- De Angeli A, Zhang J, Meyer S, Martinoia E (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4: 1804–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Ryan PR (2007) The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett 581: 2255–2262 [DOI] [PubMed] [Google Scholar]
- Ding ZJ, Yan JY, Xu XY, Yu DQ, Li GX, Zhang SQ, Zheng SJ (2014) Transcription factor WRKY46 regulates osmotic stress responses and stomatal movement independently in Arabidopsis. Plant J 79: 13–27 [DOI] [PubMed] [Google Scholar]
- Dittrich P, Raschke K (1977a) Malate metabolism in isolated epidermis of Commelina communis L. in relation to stomatal functioning. Planta 134: 77–81 [DOI] [PubMed] [Google Scholar]
- Dittrich P, Raschke K (1977b) Uptake and metabolism of carbohydrates by epidermal tissue. Planta 134: 83–90 [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Murphy AN (2012) A mitochondrial mystery, solved. Science 337: 41–43 [DOI] [PubMed] [Google Scholar]
- Donkin ME, Martin ES (1980) Changes in starch and glucose levels in the epidermis of Commelina communis in relation to stomatal movements. Plant Cell Environ 3: 409–414 [Google Scholar]
- Dreyer I, Blatt MR (2009) What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci 14: 383–390 [DOI] [PubMed] [Google Scholar]
- Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D (2012) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci 3: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Aghoram K, Outlaw WH Jr (1997) In vivo phosphorylation of phosphoenolpyruvate carboxylase in guard cells of Vicia faba L. is enhanced by fusicoccin and suppressed by abscisic acid. Arch Biochem Biophys 337: 345–350 [DOI] [PubMed] [Google Scholar]
- Edwards D, Davies KL, Axe L (1992) A vascular conducting strand in the early land plant Cooksonia. Nature 357: 683–685 [Google Scholar]
- Edwards D, Fanning U, Richardson JB (1986) Stomata and sterome in early land plants. Nature 323: 438–440 [Google Scholar]
- Edwards D, Kerp H, Hass H (1998) Stomata in early land plants: an anatomical and ecophysiological approach. J Exp Bot 49: 255–278 [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE (2003) The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 100: 11122–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert MS, Outlaw WH, Zhang S, Aghoram K, Riddle KA, Outlaw B (2000) Accumulation of an apoplastic solute in the guard-cell wall is sufficient to exert a significant effect on transpiration in Vicia faba leaflets. Plant Cell Environ 23: 195–203 [Google Scholar]
- Farquhar GD, Dubbe DR, Raschke K (1978) Gain of the feedback loop involving carbon dioxide and stomata: theory and measurement. Plant Physiol 62: 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345 [Google Scholar]
- Farquhar GD, Wong SC (1984) An empirical model of stomatal conductance. Aust J Plant Physiol 11: 191–210 [Google Scholar]
- Fischer RA. (1968) Stomatal opening: role of potassium uptake by guard cells. Science 160: 784–785 [DOI] [PubMed] [Google Scholar]
- Fischer RA. (1972) Aspects of potassium accumulation by stomata of Vicia faba. Aust J Biol Sci 25: 1107–1123 [Google Scholar]
- Fischer RA, Hsiao TC (1968) Stomatal opening in isolated epidermal strips of Vicia faba. II. Responses to KCl concentration and the role of potassium absorption. Plant Physiol 43: 1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Noguchi K, Terashima I (2013) Apoplastic mesophyll signals induce rapid stomatal responses to CO2 in Commelina communis. New Phytol 199: 395–406 [DOI] [PubMed] [Google Scholar]
- Gago J, Daloso DM, Figueroa CM, Flexas J, Fernie AR, Nikoloski Z (2016) Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary plant metabolism: a multispecies meta-analysis approach. Plant Physiol 171: 265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XQ, Li CG, Wei PC, Zhang XY, Chen J, Wang XC (2005) The dynamic changes of tonoplasts in guard cells are important for stomatal movement in Vicia faba. Plant Physiol 139: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581: 2204–2214 [DOI] [PubMed] [Google Scholar]
- Gehlen J, Panstruga R, Smets H, Merkelbach S, Kleines M, Porsch P, Fladung M, Becker I, Rademacher T, Häusler RE, et al. (1996) Effects of altered phosphoenolpyruvate carboxylase activities on transgenic C3 plant Solanum tuberosum. Plant Mol Biol 32: 831–848 [DOI] [PubMed] [Google Scholar]
- Geisler M, Murphy AS (2006) The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett 580: 1094–1102 [DOI] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW (1987) Subcellular metabolite levels in spinach leaves: regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiol 83: 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ (1990) Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature 346: 769–771 [DOI] [PubMed] [Google Scholar]
- Goh CH, Schreiber U, Hedrich R (1999) New approach of monitoring changes in chlorophyll a fluorescence of single guard cells and protoplasts in response to physiological stimuli. Plant Cell Environ 22: 1057–1070 [Google Scholar]
- Gotow K, Taylor S, Zeiger E (1988) Photosynthetic carbon fixation in guard cell protoplasts of Vicia faba L.: evidence from radiolabel experiments. Plant Physiol 86: 700–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Pascal N, Douce R (1993) 13C nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J Biol Chem 268: 3986–3992 [PubMed] [Google Scholar]
- Hafke JB, Hafke Y, Smith JAC, Lüttge U, Thiel G (2003) Vacuolar malate uptake is mediated by an anion-selective inward rectifier. Plant J 35: 116–128 [DOI] [PubMed] [Google Scholar]
- Heber U. (1974) Metabolite exchange between chloroplasts and cytoplasm. Annu Rev Plant Physiol 25: 393–421 [Google Scholar]
- Hedrich R, Busch H, Raschke K (1990) Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J 9: 3889–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Marten I, Lohse G, Dietrich P, Winter H, Lohaus G, Heldt HW (1994) Malate-sensitive anion channels enable guard cells to sense changes in the ambient CO2 concentration. Plant J 6: 741–748 [Google Scholar]
- Hedrich R, Raschke K, Stitt M (1985) A role for fructose 2,6-bisphosphate in regulating carbohydrate metabolism in guard cells. Plant Physiol 79: 977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills A, Chen ZH, Amtmann A, Blatt MR, Lew VL (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkins MF, Fitzsimons PJ, Weyers JDB (1983) The primary processes of photosystem II in purified guard-cell protoplasts and mesophyll-cell protoplasts from Commelina communis L. Planta 159: 554–560 [DOI] [PubMed] [Google Scholar]
- Hite DRC, Bodson MJ, Outlaw WH Jr (1992) Enzymic potential for fructose 6-phosphate phosphorylation by guard cells and by palisade cells in leaves of the broad bean Vicia faba L. Histochem J 24: 368–374 [DOI] [PubMed] [Google Scholar]
- Horrer D, Flütsch S, Pazmino D, Matthews JSA, Thalmann M, Nigro A, Leonhardt N, Lawson T, Santelia D (2016) Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr Biol 26: 362–370 [DOI] [PubMed] [Google Scholar]
- Humble GD, Raschke K (1971) Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol 48: 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurth MA, Suh SJ, Kretzschmar T, Geis T, Bregante M, Gambale F, Martinoia E, Neuhaus HE (2005) Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol 137: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias AA, Barry GF, Meyer C, Bloksberg L, Nakata PA, Greene T, Laughlin MJ, Okita TW, Kishore GM, Preiss J (1993) Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem 268: 1081–1086 [PubMed] [Google Scholar]
- Imamura S. (1943) Untersuchungen uber den mechanismus der turgorschwandung der spaltoffnungesschliesszellen. Jpn J Bot 12: 82–88 [Google Scholar]
- Jarvis AJ, Mansfield TA, Davies WJ (1999) Stomatal behaviour, photosynthesis and transpiration under rising CO2. Plant Cell Environ 22: 639–648 [Google Scholar]
- Jiao JA, Chollet R (1991) Posttranslational regulation of phosphoenolpyruvate carboxylase in C4 and Crassulacean acid metabolism plants. Plant Physiol 95: 981–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Outlaw WH Jr, Fiore GB, Riddle KA (2007) Guard cell apoplastic photosynthate accumulation corresponds to a phloem-loading mechanism. J Exp Bot 58: 4061–4070 [DOI] [PubMed] [Google Scholar]
- Keller B, Hedrich R, Raschke K (1989) Voltage-dependent anion channels in the plasma membrane of guard cells. Nature 341: 450–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G, Moshelion M, David-Schwartz R, Halperin O, Wallach R, Attia Z, Belausov E, Granot D (2013) Hexokinase mediates stomatal closure. Plant J 75: 977–988 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki Ki (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockenbring T, Meinhard M, Schnabl H (1998) The stomatal phosphoenolpyruvate carboxylase: a potential target for selective proteolysis during stomatal closure? J Plant Physiol 152: 222–229 [Google Scholar]
- Kolb HA, Marten I, Hedrich R (1995) Hodgkin-Huxley analysis of a GCAC1 anion channel in the plasma membrane of guard cells. J Membr Biol 146: 273–282 [DOI] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Kovermann P, Meyer S, Hörtensteiner S, Picco C, Scholz-Starke J, Ravera S, Lee Y, Martinoia E (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J 52: 1169–1180 [DOI] [PubMed] [Google Scholar]
- Laporte MM, Shen B, Tarczynski MC (2002) Engineering for drought avoidance: expression of maize NADP-malic enzyme in tobacco results in altered stomatal function. J Exp Bot 53: 699–705 [DOI] [PubMed] [Google Scholar]
- Lasceve G, Leymarie J, Vavasseur A (1997) Alterations in light-induced stomatal opening in a starch-deficient mutant of Arabidopsis thaliana L. deficient in chloroplast phosphoglucomutase activity. Plant Physiol 20: 350–358 [Google Scholar]
- Lawson T. (2009) Guard cell photosynthesis and stomatal function. New Phytol 181: 13–34 [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164: 1556–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Lefebvre S, Baker NR, Morison JIL, Raines CA (2008) Reductions in mesophyll and guard cell photosynthesis impact on the control of stomatal responses to light and CO2. J Exp Bot 59: 3609–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR (2002) Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiol 128: 52–62 [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR (2003) The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J Exp Bot 54: 1743–1752 [DOI] [PubMed] [Google Scholar]
- Lawson T, Simkin AJ, Kelly G, Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol 203: 1064–1081 [DOI] [PubMed] [Google Scholar]
- Lawson T, von Caemmerer S, Baroli I (2010) Photosynthesis and stomatal behaviour. Prog Bot 72: 265–304 [Google Scholar]
- Lee J, Bowling DJF (1993) The effect of a mesophyll factor on the swelling of guard cell protoplasts of Commelina communis L. J Plant Physiol 142: 203–207 [Google Scholar]
- Lee JS, Bowling DJF (1992) Influence of the mesophyll on stomatal opening in Commelina communis. J Exp Bot 43: 951–957 [Google Scholar]
- Lee M, Choi Y, Burla B, Kim YY, Jeon B, Maeshima M, Yoo JY, Martinoia E, Lee Y (2008) The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol 10: 1217–1223 [DOI] [PubMed] [Google Scholar]
- Lee Y, Choi YB, Suh S, Lee J, Assmann SM, Joe CO, Kelleher JF, Crain RC (1996) Abscisic acid-induced phosphoinositide turnover in guard cell protoplasts of Vicia faba. Plant Physiol 110: 987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CL, Wang M, Ma XY, Zhang W (2014) NRGA1, a putative mitochondrial pyruvate carrier, mediates ABA regulation of guard cell ion channels and drought stress responses in Arabidopsis. Mol Plant 7: 1508–1521 [DOI] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J (1988) Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol 86: 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd F. (1908) The behaviour of stomata. Carnegie Inst Wash Publ 82
- Lohaus G, Pennewiss K, Sattelmacher B, Hussmann M, Hermann Muehling K (2001) Is the infiltration-centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiol Plant 111: 457–465 [DOI] [PubMed] [Google Scholar]
- Lu P, Outlaw WH Jr, Smith BG, Freed GA (1997) A new mechanism for the regulation of stomatal aperture size in intact leaves: accumulation of mesophyll-derived sucrose in the guard-cell wall of Vicia faba. Plant Physiol 114: 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Zhang SQ, Outlaw WH Jr, Riddle KA (1995) Sucrose: a solute that accumulates in the guard-cell apoplast and guard-cell symplast of open stomata. FEBS Lett 362: 180–184 [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. (1987) Ionic relations of guard cells. In Zeiger E, Farquhar GD, Cowan IR, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 125–162 [Google Scholar]
- MacRobbie EAC, Lettau J (1980a) Ion content and aperture in “isolated” guard cells of Commelina communis L. J Membr Biol 53: 199–205 [Google Scholar]
- MacRobbie EAC, Lettau J (1980b) Potassium content and aperture in “intact” stomatal and epidermal cells of Commelina communis L. J Membr Biol 56: 249–256 [Google Scholar]
- Mansfield TA, Hetherington AM, Atkinson CJ (1990) Some current aspects of stomatal physiology. Annu Rev Plant Physiol Plant Mol Biol 41: 55–75 [Google Scholar]
- Martinoia E, Flügge UI, Kaiser G, Heber U, Heldt HW (1985) Energy-dependent uptake of malate into vacuoles isolated from barley mesophyll protoplasts. Biochim Biophys Acta 806: 311–319 [Google Scholar]
- Martinoia E, Rentsch D (1994) Malate compartmentation: responses to a complex metabolism. Annu Rev Plant Physiol Plant Mol Biol 45: 447–467 [Google Scholar]
- Matrosova A, Bogireddi H, Mateo-Peñas A, Hashimoto-Sugimoto M, Iba K, Schroeder JI, Israelsson-Nordström M (2015) The HT1 protein kinase is essential for red light-induced stomatal opening and genetically interacts with OST1 in red light and CO2-induced stomatal movement responses. New Phytol 208: 1126–1137 [DOI] [PubMed] [Google Scholar]
- Mawson BT. (1983) Regulation of blue-light-induced proton pumping by Vicia faba L. guard-cell protoplasts: energetic contributions by chloroplastic and mitochondrial activities. Planta 191: 293–301 [Google Scholar]
- Medeiros DB, Martins SCV, Cavalcanti JHF, Daloso DM, Martinoia E, Nunes-Nesi A, DaMatta FM, Fernie AR, Araújo WL (2016) Enhanced photosynthesis and growth in atquac1 knockout mutants are due to altered organic acid accumulation and an increase in both stomatal and mesophyll conductance. Plant Physiol 170: 86–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meidner H. (1987) Three hundred years of research into stomata. In Zeiger E, Farquhar GD, Cowan IR, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 7–27 [Google Scholar]
- Meinhard M, Schnabl H (2001) Fusicoccin- and light-induced activation and in vivo phosphorylation of phosphoenolpyruvate carboxylase in Vicia guard cell protoplasts. Plant Sci 160: 635–646 [DOI] [PubMed] [Google Scholar]
- Melis A, Zeiger E (1982) Chlorophyll a fluorescence transients in mesophyll and guard cells: modulation of guard cell photophosphorylation by CO2. Plant Physiol 69: 642–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger SM, Buckley TN, Mott KA (2006) Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol 140: 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N (2004) Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiol 134: 684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, Geiger D, Marten I, Martinoia E, Hedrich R (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63: 1054–1062 [DOI] [PubMed] [Google Scholar]
- Meyer S, Scholz-Starke J, De Angeli A, Kovermann P, Burla B, Gambale F, Martinoia E (2011) Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J 67: 247–257 [DOI] [PubMed] [Google Scholar]
- Milborrow BV. (2001) The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J Exp Bot 52: 1145–1164 [PubMed] [Google Scholar]
- Morell MK, Bloom M, Knowles V, Preiss J (1987) Subunit structure of spinach leaf ADPglucose pyrophosphorylase. Plant Physiol 85: 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL, Jarvis PG (1983) Direct and indirect effects of light on stomata. II. In Commelina communis L. Plant Cell Environ 6: 103–109 [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306 [DOI] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Bermúdez MA, Segura J, Ros R (2011) Arabidopsis plants deficient in plastidial glyceraldehyde-3-phosphate dehydrogenase show alterations in abscisic acid (ABA) signal transduction: interaction between ABA and primary metabolism. J Exp Bot 62: 1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SD, Mayo JM (1975) The occurrence of functional non-chlorophyllous guard cells in Paphiodedilum spp. Can J Bot 53: 1–7 [Google Scholar]
- Niewiadomski P, Knappe S, Geimer S, Fischer K, Schulz B, Unte US, Rosso MG, Ache P, Flügge UI, Schneider A (2005) The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell 17: 760–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50: 1093–1106 [DOI] [PubMed] [Google Scholar]
- Olsen RL, Pratt RB, Gump P, Kemper A, Tallman G (2002) Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under reduced CO2 concentrations in Vicia spp. New Phytol 153: 497–508 [DOI] [PubMed] [Google Scholar]
- Outlaw WH. (1983) Current concepts on the role of potassium in stomatal movements. Physiol Plant 59: 302–311 [Google Scholar]
- Outlaw WH. (1989) Critical examination of the quantitative evidence for and against photosynthetic CO2 fixation by guard cells. Physiol Plant 77: 275–281 [Google Scholar]
- Outlaw WH. (1990) Kinetic properties of guard-cell phosphoenolpyruvate carboxylase. Biochem Physiol Pflanz 186: 317–325 [Google Scholar]
- Outlaw WH. (2003) Integration of cellular and physiological functions of guard cells. Crit Rev Plant Sci 22: 503–529 [Google Scholar]
- Outlaw WH Jr, De Vlieghere-He X (2001) Transpiration rate: an important factor controlling the sucrose content of the guard cell apoplast of broad bean. Plant Physiol 126: 1716–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH Jr, Du Z, Xia Meng F, Aghoram K, Riddle KA, Chollet R (2002) Requirements for activation of the signal-transduction network that leads to regulatory phosphorylation of leaf guard-cell phosphoenolpyruvate carboxylase during fusicoccin-stimulated stomatal opening. Arch Biochem Biophys 407: 63–71 [DOI] [PubMed] [Google Scholar]
- Outlaw WH, Kennedy J (1978) Enzymic and substrate basis for the anaplerotic step in guard cells. Plant Physiol 62: 648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Lowry OH (1977) Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci USA 74: 4434–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Manchester J (1979) Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol 64: 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Manchester J, Brown PH (1981a) High levels of malic enzyme activities in Vicia faba L. epidermal tissue. Plant Physiol 68: 1047–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Manchester J, Dicamelli CA (1979) Histochemical approach to properties of Vicia faba guard cell phosphoenolpyruvate carboxylase. Plant Physiol 64: 269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Mayne BC, Zenger VE, Manchester J (1981b) Presence of both photosystems in guard cells of Vicia faba L: implications for environmental signal processing. Plant Physiol 67: 12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Springer SA, Tarczynski MC (1985) Histochemical technique: a general method for quantitative enzyme assays of single cell ‘extracts’ with a time resolution of seconds and a reading precision of femtomoles. Plant Physiol 77: 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Tarczynski MC (1984) Guard cell starch biosynthesis regulated by effectors of ADP-glucose pyrophosphorylase. Plant Physiol 74: 424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overlach S, Diekmann W, Raschke K (1993) Phosphate translocator of isolated guard-cell chloroplasts from Pisum sativum L. transports glucose-6-phosphate. Plant Physiol 101: 1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas JE. (1964) Guard-cell starch retention and accumulation in the dark. Bot Gaz 125: 102–107 [Google Scholar]
- Pallas JE, Wright BG (1973) Organic acid changes in the epidermis of Vicia faba and their implication in stomatal movement. Plant Physiol 51: 588–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja O, Gelli A, Blumwald E (1992) Characterization of vacuolar malate and K+ channels under physiological conditions. Plant Physiol 100: 1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja O, Smith JAC (2002) Sensitivity of the plant vacuolar malate channel to pH, Ca2+ and anion-channel blockers. J Membr Biol 186: 31–42 [DOI] [PubMed] [Google Scholar]
- Parvathi K, Raghavendra AS (1997) Both Rubisco and phosphoenolpyruvate carboxylase are beneficial for stomatal function in epidermal strips of Commelina benghalensis. Plant Sci 124: 153–157 [Google Scholar]
- Pearson C. (1973) Daily changes in stomatal aperture and in carbohydrates and malate within epidermis and mesophyll of leaves of Commelina cyanea and Vicia faba. Aust J Biol Sci 26: 1035–1044 [Google Scholar]
- Penfield S, Clements S, Bailey KJ, Gilday AD, Leegood RC, Gray JE, Graham IA (2012) Expression and manipulation of PHOSPHOENOLPYRUVATE CARBOXYKINASE 1 identifies a role for malate metabolism in stomatal closure. Plant J 69: 679–688 [DOI] [PubMed] [Google Scholar]
- Plaxton WC. (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47: 185–214 [DOI] [PubMed] [Google Scholar]
- Poffenroth M, Green DB, Tallman G (1992) Sugar concentrations in guard cells of Vicia faba illuminated with red or blue light: analysis by high performance liquid chromatography. Plant Physiol 98: 1460–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch CM, Ott KV, Bauer H, Ache P, Hedrich R, Sonnewald U (2015) β-Amylase1 mutant Arabidopsis plants show improved drought tolerance due to reduced starch breakdown in guard cells. J Exp Bot 66: 6059–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra AS, Rao IM, Das VSR (1976) Characterization of abscisic acid inhibition of stomatal opening in isolated epidermal strips. Plant Sci Lett 6: 111–115 [Google Scholar]
- Rao IM, Anderson LE (1983) Light and stomatal metabolism. II. Effects of sulfite and arsenite on stomatal opening and light modulation of enzymes in epidermis. Plant Physiol 71: 456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke K. (1975) Stomatal action. Annu Rev Plant Physiol 26: 309–340 [Google Scholar]
- Raschke K, Dittrich P (1977) [14C]Carbon-dioxide fixation by isolated leaf epidermes with stomata closed or open. Planta 134: 69–75 [DOI] [PubMed] [Google Scholar]
- Raschke K, Humble GD (1973) No uptake of anions required by opening stomata of Vicia faba: guard cells release hydrogen ions. Planta 115: 47–57 [DOI] [PubMed] [Google Scholar]
- Raschke K, Schnabl H (1978) Availability of chloride affects the balance between potassium chloride and potassium malate in guard cells of Vicia faba L. Plant Physiol 62: 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak R, Kemna I, Luettge U (1994) Characteristics, partial purification and reconstitution of the vacuolar malate transporter of the CAM plant Kalanchoe daigremontiana Hamet et Perrier de la Bathie. Planta 195: 226–236 [Google Scholar]
- Reckmann U, Scheibe R, Raschke K (1990) Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol 92: 246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Rosenfeld J, Rohrig K, Raschke K (1999) Rates of sugar uptake by guard cell protoplasts of Pisum sativum L. related to the solute requirement for stomatal opening. Plant Physiol 121: 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N, Preiss J (1985) Biochemical phenomena associated with stomatal function. Physiol Plant 64: 141–146 [Google Scholar]
- Roelfsema MR, Prins HBA (1997) Ion channels in guard cells of Arabidopsis thaliana (L.) Heynh. Planta 202: 18–27 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle HH, Hedrich R (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32: 65–75 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R (2002) Studying guard cells in the intact plant: modulation of stomatal movement by apoplastic factors. New Phytol 153: 425–431 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R (2005) In the light of stomatal opening: new insights into ‘the Watergate.’ New Phytol 167: 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Levchenko V, Hedrich R (2004) ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J 37: 578–588 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Steinmeyer R, Staal M, Hedrich R (2001) Single guard cell recordings in intact plants: light-induced hyperpolarization of the plasma membrane. Plant J 26: 1–13 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, Murata Y, Yamamoto Y (2010) Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol 51: 354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R, Reckmann U, Hedrich R, Raschke K (1990) Malate dehydrogenases in guard cells of Pisum sativum. Plant Physiol 93: 1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl H. (1980) CO2 and malate metabolism in starch-containing and starch-lacking guard-cell protoplasts. Planta 149: 52–58 [DOI] [PubMed] [Google Scholar]
- Schnabl H. (1981) The compartmentation of carboxylating and decarboxylating enzymes in guard cell protoplasts. Planta 152: 307–313 [DOI] [PubMed] [Google Scholar]
- Schnabl H, Elbert C, Krämer G (1982) The regulation of the starch-malate balances during volume changes of guard cell protoplasts. J Exp Bot 33: 996–1003 [Google Scholar]
- Schnabl H, Raschke K (1980) Potassium chloride as stomatal osmoticum in Allium cepa L., a species devoid of starch in guard cells. Plant Physiol 65: 88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI. (1988) K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J Gen Physiol 92: 667–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schwartz A, Zeiger E (1984) Metabolic energy for stomatal opening: roles of photophosphorylation and oxidative phosphorylation. Planta 161: 129–136 [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science 256: 663–665 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K (1981) Effect of light quality on stomatal opening in leaves of Xanthium strumarium L. Plant Physiol 68: 1170–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Zeiger E (1985) Cyclic and noncyclic photophosphorylation in isolated guard cell chloroplasts from Vicia faba L. Plant Physiol 78: 211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki KI, Gotow K, Kondo N (1982) Photosynthetic properties of guard cell protoplasts from Vicia faba L. Guard Cell Physiol 23: 871–879 [Google Scholar]
- Sibbernsen E, Mott KA (2010) Stomatal responses to flooding of the intercellular air spaces suggest a vapor-phase signal between the mesophyll and the guard cells. Plant Physiol 153: 1435–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Stadler R, Büttner M, Ache P, Hedrich R, Ivashikina N, Melzer M, Shearson SM, Smith SM, Sauer N (2003) Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol 133: 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RA, Martin ES (1978) Structural and functional aspects of stomata. I. Developmental studies in Polypodium vulgare. Planta 142: 307–316 [DOI] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC (2012) Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol 15: 282–292 [DOI] [PubMed] [Google Scholar]
- Suetsugu N, Takami T, Ebisu Y, Watanabe H, Iiboshi C, Doi M, Shimazaki K (2014) Guard cell chloroplasts are essential for blue light-dependent stomatal opening in Arabidopsis. PLoS ONE 9: e108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Beard KFM, Nunes-Nesi A, Fernie AR, Ratcliffe RG (2010) Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci 15: 462–470 [DOI] [PubMed] [Google Scholar]
- Szyroki A, Ivashikina N, Dietrich P, Roelfsema MRG, Ache P, Reintanz B, Deeken R, Godde M, Felle H, Steinmeyer R, et al. (2001) KAT1 is not essential for stomatal opening. Proc Natl Acad Sci USA 98: 2917–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E (1993) Sugar and organic acid accumulation in guard cells of Vicia faba in response to red and blue light. Plant Physiol 102: 1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E (1996) Central roles for potassium and sucrose in guard-cell osmoregulation. Plant Physiol 111: 1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E (1998) The role of sucrose in guard cell osmoregulation. J Exp Bot 49: 329–337 [Google Scholar]
- Tallman G, Zeiger E (1988) Light quality and osmoregulation in Vicia guard cells: evidence for involvement of three metabolic pathways. Plant Physiol 88: 887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kutsuna N, Kanazawa Y, Kondo N, Hasezawa S, Sano T (2007) Intra-vacuolar reserves of membranes during stomatal closure: the possible role of guard cell vacuoles estimated by 3-D reconstruction. Plant Cell Physiol 48: 1159–1169 [DOI] [PubMed] [Google Scholar]
- Tarczynski MC, Outlaw WH Jr (1990) Partial characterization of guard-cell phosphoenolpyruvate carboxylase: kinetic datum collection in real time from single-cell activities. Arch Biochem Biophys 280: 153–158 [DOI] [PubMed] [Google Scholar]
- Tarczynski MC, Outlaw WH Jr (1993) The interactive effects of pH, L-malate, and glucose-6-phosphate on guard-cell phosphoenolpyruvate carboxylase. Plant Physiol 103: 1189–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarczynski MC, Outlaw WH, Arold N, Neuhoff V, Hampp R (1989) Electrophoretic assay for ribulose 1,5-bisphosphate carboxylase/oxygenase in guard cells and other leaf cells of Vicia faba L. Plant Physiol 89: 1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, MacRobbie EAC, Blatt MR (1992) Membrane transport in stomatal guard cells: the importance of voltage control. J Membr Biol 126: 1–18 [DOI] [PubMed] [Google Scholar]
- Thomine S, Guern J, Barbier-Brygoo H (1997) Voltage-dependent anion channel of Arabidopsis hypocotyls: nucleotide regulation and pharmacological properties. J Membr Biol 159: 71–82 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kinoshita T, Shimazaki K (2001) Guard-cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening. Plant Cell Physiol 42: 795–802 [DOI] [PubMed] [Google Scholar]
- Tsai HL, Lue WL, Lu KJ, Hsieh MH, Wang SM, Chen J (2009) Starch synthesis in Arabidopsis is achieved by spatial cotranscription of core starch metabolism genes. Plant Physiol 151: 1582–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsionsky M, Cardon ZG, Bard AJ, Jackson RB (1997) Photosynthetic electron transport in single guard cells as measured by scanning electrochemical microscopy. Plant Physiol 113: 895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno O. (2001) Ultrastructural localization of photosynthetic and photorespiratory enzymes in epidermal, mesophyll, bundle sheath, and vascular bundle cells of the C4 dicot Amaranthus viridis. J Exp Bot 52: 1003–1013 [DOI] [PubMed] [Google Scholar]
- Valerio C, Costa A, Marri L, Issakidis-Bourguet E, Pupillo P, Trost P, Sparla F (2011) Thioredoxin-regulated beta-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J Exp Bot 62: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kirk CA, Raschke K (1978) Release of malate from epidermal strips during stomatal closure. Plant Physiol 61: 474–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS (2005) Guard cell metabolism and CO2 sensing. New Phytol 165: 665–682 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55: 1157–1166 [DOI] [PubMed] [Google Scholar]
- Wang P, Song CP (2008) Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 178: 703–718 [DOI] [PubMed] [Google Scholar]
- Wang SW, Li Y, Zhang XL, Yang HQ, Han XF, Liu ZH, Shang ZL, Asano T, Yoshioka Y, Zhang CG, et al. (2014) Lacking chloroplasts in guard cells of crumpled leaf attenuates stomatal opening: both guard cell chloroplasts and mesophyll contribute to guard cell ATP levels. Plant Cell Environ 37: 2201–2210 [DOI] [PubMed] [Google Scholar]
- Wang Y, Blatt MR (2011) Anion channel sensitivity to cytosolic organic acids implicates a central role for oxaloacetate in integrating ion flux with metabolism in stomatal guard cells. Biochem J 439: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MCG, Tronconi MA, Drincovich MF, Andreo CS, Flügge UI, Maurino VG (2005) A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol 139: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer CM, Dittrich P (1974) Carbon dioxide fixation by epidermal and mesophyll tissues of Tulipa and Commelina. Planta 117: 123–132 [DOI] [PubMed] [Google Scholar]
- Willmer CM, Fricker M (1983) Stomata. Chapman & Hall, London [Google Scholar]
- Willmer CM, Pallas JE, Black CC (1973) Carbon dioxide metabolism in leaf epidermal tissue. Plant Physiol 52: 448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1993) Subcellular volume and metabolic concentrations in barley leaves. Planta 191: 180–190 [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193: 530–535 [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Wu W, Assmann SM (1993) Photosynthesis by guard cell chloroplasts of Vicia faba L.: effects of factors associated with stomatal movement. Plant Cell Physiol 34: 1015–1022 [Google Scholar]
- Yu TS, Lue WL, Wang SM, Chen J (2000) Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiol 123: 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Armond P, Melis A (1981) Fluorescence properties of guard cell chloroplasts: evidence for linear electron transport and light-harvesting pigments of photosystems I and II. Plant Physiol 67: 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Talbott LD, Frechilla S, Srivastava A, Zhu J (2002) The guard cell chloroplast: a perspective for the twenty-first century. New Phytol 153: 415–424 [DOI] [PubMed] [Google Scholar]
- Zhang J, Martinoia E, De Angeli A (2014) Cytosolic nucleotides block and regulate the Arabidopsis vacuolar anion channel AtALMT9. J Biol Chem 289: 25581–25589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SQ, Outlaw WH Jr, Chollet R (1994) Lessened malate inhibition of guard-cell phosphoenolpyruvate carboxylase velocity during stomatal opening. FEBS Lett 352: 45–48 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Assmann SM (2011) The glycolytic enzyme, phosphoglycerate mutase, has critical roles in stomatal movement, vegetative growth, and pollen production in Arabidopsis thaliana. J Exp Bot 62: 5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang W, Stanley BA, Assmann SM (2008) Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell 20: 3210–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]