Abstract
A critical comment to a recent paper claiming to have evidence for C4 photosynthesis in the wheat grain.
Research on C4 photosynthesis has seen a revival in recent years, leading to a Nature Scientific Reports article announcing the discovery of C4 photosynthesis in grains of the C3 plant wheat (Triticum aestivum; Rangan et al., 2016). The authors indicate the presence of C4 cycle enzymes, which they interpret as evidence for the existence of a C4-type carbon-concentrating mechanism in the wheat pericarp. “Extraordinary claims require extraordinary evidence,” however, and the evidence presented in this case is based entirely on transcriptome data of the entire wheat grain, with a notable absence of biochemical or physiological support, or the exact location of expression. One needs to consider the wider context of C4 photosynthesis, and this challenges the interpretation provided by Rangan et al. (2016).
It is a common misconception that in C4 photosynthesis C3’s primary CO2-fixing enzyme Rubisco is simply replaced by phosphoenolpyruvate carboxylase (PEPC). PEPC indeed initially incorporates CO2 as 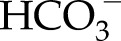 into the C4 acid oxaloacetate, but this molecule is difficult to chemically reduce beyond the level of malate to that required for sugar, cellulose, and other carbohydrates, while still recycling the substrate PEP. So the CO2 is later released to increase the CO2 concentration in a specialized compartment, such as the bundle sheath (Leegood, 2002). There, the photosynthetic assimilation of CO2 into carbohydrates occurs, via the same pathway as in C3 plants, by Rubisco, ATP, and NADPH. This C4 carbon-concentrating mechanism thus requires a PEPC:Rubisco activity ratio of roughly 1:1; a larger ratio means that ATP is spent on running a futile C4 cycle. Without Rubisco, PEPC is only able to fix CO2 via anaplerotic reactions. Two observations contradict the conclusions of Rangan et al. (2016) in this regard.
into the C4 acid oxaloacetate, but this molecule is difficult to chemically reduce beyond the level of malate to that required for sugar, cellulose, and other carbohydrates, while still recycling the substrate PEP. So the CO2 is later released to increase the CO2 concentration in a specialized compartment, such as the bundle sheath (Leegood, 2002). There, the photosynthetic assimilation of CO2 into carbohydrates occurs, via the same pathway as in C3 plants, by Rubisco, ATP, and NADPH. This C4 carbon-concentrating mechanism thus requires a PEPC:Rubisco activity ratio of roughly 1:1; a larger ratio means that ATP is spent on running a futile C4 cycle. Without Rubisco, PEPC is only able to fix CO2 via anaplerotic reactions. Two observations contradict the conclusions of Rangan et al. (2016) in this regard.
The authors show that the expression level of rbcS, the small subunit of Rubisco, is decreased by 99% relative to the expression in the leaf, and conclude that, together with an increase in PEPC expression and other C4 cycle enzymes, this indicates a shift from C3 to C4 photosynthesis. Without providing any measurements of the activity of Rubisco or PEPC to substantiate their claim, they refer to previous work showing that PEPC is 100 times as active in incorporating CO2 into organic compounds as Rubisco (Duffus and Rosie, 1973). Assuming this to be the case here, the low concentration of Rubisco would leave insufficient capacity for photosynthetic fixation of the vast majority of CO2 supplied by PEPC via a C4 pump. It implies that the uptake of CO2 by PEPC is to supply the large demand in amino and fatty acids. Support for the idea that PEPC facilitates the production of these compounds, and not carbohydrates, was earlier provided by pulse-chase experiments (Bort et al., 1995; Rolletschek et al., 2004).
The C4 cycle constitutes an energy-driven CO2 pump from a compartment of relatively low CO2 concentration to a largely gas-tight compartment in which the CO2 concentration is increased to reduce photorespiration (Leegood, 2002). Immunolabeling studies in wheat grains have shown that PEPC is not localized in the cross-cells of the pericarp, as suggested by Rangan et al. (2016), but in the aleurone layer and endosperm (Araus et al., 1993; González et al., 1998), the sites where one would expect the highest concentration of respiratory CO2. With Rubisco located in the chloroplasts of the pericarp (Tambussi et al., 2005), this would render a C4-like CO2 pump ineffective.
In our view, the claim of a C4 carbon-concentrating mechanism in wheat grains therefore cannot be upheld with the supplied data. In any such analysis it is important to distinguish production of C4 acids from the C4 photosynthetic pathway.
References
- Araus JL, Bort J, Brown RH, Bassett CL, Cortadellas N (1993) Immunocytochemical localization of phosphoenolpyruvate carboxylase and photosynthetic gas-exchange characteristics in ears of Triticum durum Desf. Planta 191: 507–514 [Google Scholar]
- Bort J, Brown RH, Araus JL (1995) Lack of C4 photosynthetic metabolism in ears of C3 cereals. Plant Cell Environ 18: 697–702 [Google Scholar]
- Duffus CM, Rosie R (1973) Some enzyme activities associated with the chlorophyll containing layers of the immature barley pericarp. Planta 114: 219–226 [DOI] [PubMed] [Google Scholar]
- González MC, Osuna L, Echevarría C, Vidal J, Cejudo FJ (1998) Expression and localization of phosphoenolpyruvate carboxylase in developing and germinating wheat grains. Plant Physiol 116: 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC. (2002) C(4) photosynthesis: principles of CO(2) concentration and prospects for its introduction into C(3) plants. J Exp Bot 53: 581–590 [DOI] [PubMed] [Google Scholar]
- Rangan P, Furtado A, Henry RJ (2016) New evidence for grain specific C4 photosynthesis in wheat. Sci Rep 6: 31721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Radchuk R, Miranda M, Heim U, Wobus U, Weber H (2004) Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy. Plant Biotechnol J 2: 211–219 [DOI] [PubMed] [Google Scholar]
- Tambussi EA, Nogués S, Araus JL (2005) Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta 221: 446–458 [DOI] [PubMed] [Google Scholar]


