Abstract
Objective
To identify linear determinants of human aquaporin 4 (hAQP4) in the context of HLA-DRB1*03:01.
Design
In this controlled study with humanized experimental animals, HLA-DRB1*03:01 transgenic mice were immunized with whole-protein hAQP4 emulsified in complete Freund adjuvant. To test T-cell responses, lymph node cells and splenocytes were cultured in vitro with synthetic peptides 20 amino acids long that overlap by 10 amino acids across the entirety of hAQP4. The frequency of interferon γ, interleukin (IL) 17, granulocyte-macrophage colony-stimulating factor, and IL-5–secreting CD4+ T cells was determined by the enzyme-linked immunosorbent sport assay. Quantitative immunofluorescence microscopy was performed to determine whether hAQP4281–300 inhibits the binding of antih-AQP4 recombinant antibody to surface full-length hAQP4.
Setting
Academic neuroimmunology laboratories.
Subjects
Humanized HLA-DRB1*03:01+/+ H-2b−/− transgenic mice on a B10 background.
Results
Peptide hAQP4281–300 generated a significantly (P<.01) greater TH1 and TH17 immune response than any of the other linear peptides screened. This 20mer peptide contains 2 dominant immunogenic 15mer peptides. hAQP4284–298 induced predominantly an IL-17 and granulocyte-macrophage colony-stimulating factor TH cell phenotype, whereas hAQP4285–299 resulted in a higher frequency of TH1 cells. hAQP4281–300 did not interfere with recombinant AQP4 autoantibody binding.
Conclusions
hAQP4281–330 is the dominant linear immunogenic determinant of hAQP4 in the context of HLA-DRB1*03:01. Within hAQP4281–330 are 2 dominant immunogenic determinants that induce differential TH phenotypes. hAQP4 determinants identified in this study can serve as diagnostic biomarkers in patients with neuromyelitis optica and may facilitate the monitoring of treatment responses to pharmacotherapies.
Neuromyelitis optica (NMO) is a demyelinating inflammatory disorder of the central nervous system (CNS) that is clinically and pathologically defined as the co-occurrence of optic neuritis and myelitis.1 NMO-IgG, an autoantibody that binds to human aquaporin 4 (hAQP4), is detectable in the serum of most patients with NMO.2,3 AQP4 plays an important role in the transportation of water across the cell membrane of multiple cell types. Within the CNS, it is highly expressed in the foot processes of astrocytes.4,5 There are 2 isoforms of hAQP4: M1 and the shorter M23 isoform, which lacks the first 22 amino acids but is otherwise identical in sequence.6 Possibly because of the identification of the NMO-IgG antibody in patients with NMO, neurologists have focused on pharmacotherapies that predominantly target the humoral immune system.7–9
There is evidence to suggest a cellular immune response in NMO. Recently, HLA haplotype analyses of patients with NMO suggest a positive association with HLA-DRB1*03:01 (HLA-DR17),10,11 a gene that codes for a major histocompatibility class (MHC) II molecule that presents linear antigens 12 to 15 amino acids in length to CD4+ T cells.12 In some patient cohorts, NMO-IgG is undetectable in a substantial number of patients with NMO.2 In patients with NMO-IgG, antibody isotype switching from IgM to IgG could not occur without CD4+ TH cell involvement.13,14 The response to B cell–depleting therapies is not consistently beneficial in patients with NMO,7–9 and CD3+ T cells are abundantly present in NMO lesions.15 We hypothesize that hAQP4-specific CD4+ T cells play an important role in the pathogenesis of NMO.
To test our hypothesis, we screened 32 peptides of 20 amino acid length that overlap by 10 amino acids and span the entirety of hAQP4 in HLA-DRB1*03:01 transgenic mice. This process led us to identify the immunodominant linear determinants that stimulate cellular immune response in the context of HLA-DRB1*03:01. After identification of 1 immunodominant 20mer peptide, we determined dominant immunogenic 15mer peptides within. Proliferating CD4+ TH cells were further defined by their expression of interferon γ (IFN-γ), interleukin (IL) 17, and granulocyte-macrophage colony-stimulating factor (GM-CSF).
METHODS
PEPTIDES AND PROTEIN
Whole-protein AQP4 M1 was donated by William Harries, PhD, of the Membrane Protein Expression Center & Center for Structures of Membrane Proteins Macromolecular Structure Group (University of California, San Francisco) (Figure 1). Synthetic peptides 20 amino acids long that overlapped by 10 amino acids across the entirety of hAQP4 (Table 1) and synthetic peptides 15 amino acids long that overlapped by a single amino acid spanning the immunodominant 20mer AQP4298–301 (Table 2) were generated by JPT Innovative Peptide Solutions.
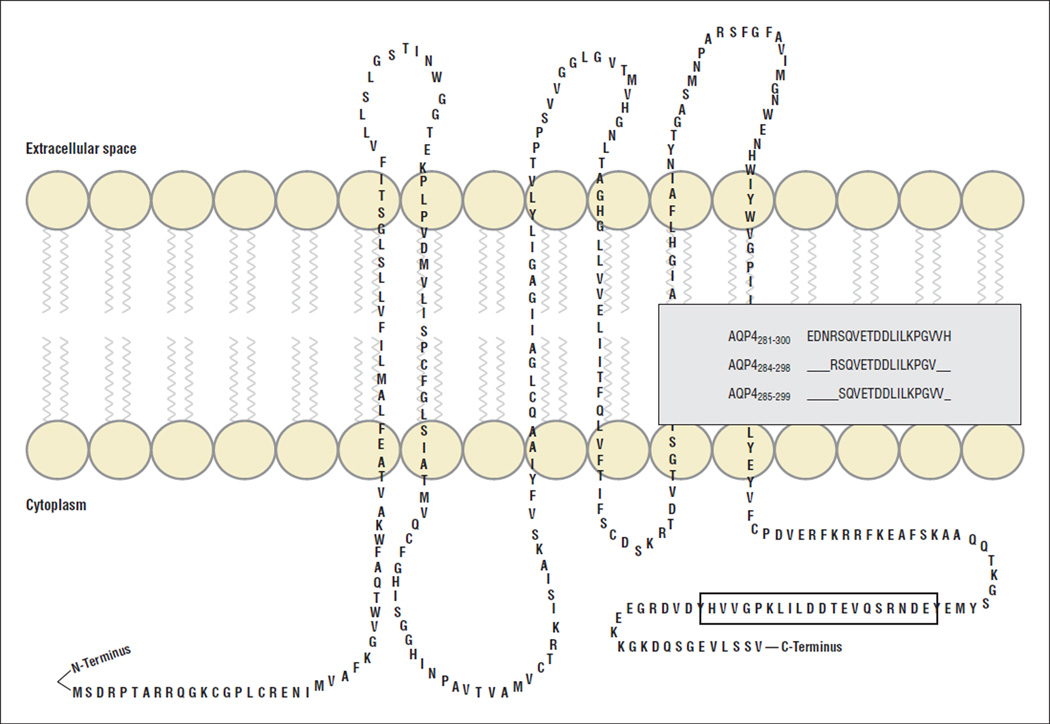
Figure 1.
The M1 isoform of human aquaporin 4 (hAQP4). hAQP4 consists of 6 transmembrane α-helices. There are 2 isoforms of hAQP4, M1 and M23, which differ in their N terminus start site. Three immunogenic linear determinants in the context of HLA-DRB1*03:01 are described in this study.
Table 1.
Synthetic Peptides 20 Amino Acids Long That Overlap by 10 Amino Acids Across the Entirety of Human Aquaporin 4 (AQP4)
| Peptide No. | Sequence | AQP4 |
|---|---|---|
| 1 | MSDRPTARRWGKCGPLCTRE | 1–20 |
| 2 | GKCGPLCTRENIMVAFKGVW | 11–30 |
| 3 | NIMVAFKGVWTQAFWKAVTA | 21–40 |
| 4 | TQAFWKAVTAEFLAMLIFVL | 31–50 |
| 5 | EFLAMLIFVLLSLGSTINWG | 41–60 |
| 6 | LSLGSTINWGGTEKPLPVDM | 51–70 |
| 7 | GTEKPLPVDMVLISLCFGLS | 61–80 |
| 8 | VLISLCFGLSIATMVQCFGH | 71–90 |
| 9 | IATMVQCFGHISGGHINPAV | 81–100 |
| 10 | ISGGHINPAVTVAMVCTRKI | 91–110 |
| 11 | TVAMVCTRKISIAKSVFYIA | 101–120 |
| 12 | SIAKSVFYIAAQCLGAIIGA | 111–130 |
| 13 | AQCLGAIIGAGILYLVTPPS | 121–140 |
| 14 | GILYLVTPPSVVGGLGVTMV | 131–150 |
| 15 | VVGGLGVTMVHGNLTAGHGL | 141–160 |
| 16 | HGNLTAGHGLLVELIITFQL | 151–170 |
| 17 | LVELIITFQLVFTIFASCDS | 161–180 |
| 18 | VFTIFASCDSKRTDVTGSIA | 171–190 |
| 19 | KRTDVTGSIALAIGFSVAIG | 181–200 |
| 20 | LAIGFSVAIGHLFAINYTGA | 191–210 |
| 21 | HLFAINYTGASMNPARSFGP | 201–220 |
| 22 | SMNPARSFGPAVIMGNWENH | 211–230 |
| 23 | AVIMGNWENHWIYWVGPIIG | 221–240 |
| 24 | WIYWVGPIIGAVLAGGLYEY | 231–250 |
| 25 | AVLAGGLYEYVFCPDVEFKR | 241–260 |
| 26 | VFCPDVEFKRRFKEAFSKAA | 251–270 |
| 27 | RFKEAFSKAAQQTKGSYMEV | 261–280 |
| 28 | QQTKGSYMEVEDNRSQVETD | 271–290 |
| 29 | EDNRSQVETDDLILKPGVVH | 281–300 |
| 30 | DLILKPGVVHVIDVDRGEEK | 291–310 |
| 31 | VIDVDRGEEKKGKDQSGEVL | 301–320 |
| 32 | VDRGEEKKGKDQSGEVLSSV | 304–323 |
Table 2.
Aquaporin 4281–300 Overlapping Peptides
| Peptide No. | Amino Acids | Sequence |
|---|---|---|
| 1 | 280–294 | VEDNRSQVETDDLIL |
| 2 | 281–295 | EDNRSQVETDDLILK |
| 3 | 282–296 | DNRSQVETDDLILKP |
| 4 | 283–297 | NRSQVETDDLILKPG |
| 5 | 284–298 | RSQVETDDLILKPGV |
| 6 | 285–299 | SQVETDDLILKPGVV |
| 7 | 286–300 | QVETDDLILKPGVVH |
| 8 | 287–301 | VETDDLILKPGVVHV |
MICE
Generation of transgenic mice expressing HLA-DRB1*03:01 was previously described.16 Briefly, DRBI*0301 (DR3) transgenic mice were generated by coinjection of an HLADRoL genomic fragment and a DRB1*030113 gene fragment into (C57BL/6 × DBA/2) F1 C57BL/6 embryos and backcrossed to B10 mice.15 Subsequently, the DR3 gene was introduced into the class II–negative H2b−/− strain16 by mating the B10.M-DRBI*0301 line with the B10.MHCII−/− line. All mice were bred and maintained in a pathogen-free mouse colony at The University of Texas Southwestern Medical Center at Dallas according to the guidelines set forth by the National Institutes of Health and the institution. All experiments were approved by the Institutional Animal Care and Use Committee at The University of Texas Southwestern Medical Center at Dallas.
ENZYME-LINKED IMMUNOSORBENT SPOT ASSAY
The frequency of IFN-γ, IL-17–, GM-CSF–, and IL-5–secreting CD4+ T cells was determined by the enzyme-linked immunosorbent spot (ELISpot) assay. Groups of 3 male HLA-DRB1*03:01 mice were inoculated in the inguinal and axillary regions with 100 µg of whole-protein hAQP4 emulsified in complete Freund adjuvant in a 1:1 ratio. On day 10, lymph nodes and spleens were collected to generate single-cell suspensions. Next, cells (2.5–5.0 × 105 cells per well) were incubated with a single overlapping hAQP4 peptide (50 µg/mL), whole-length hAQP4 (50 µg/mL), media only, or concanavalin A (1 µg/mL) for 48 hours in 96-well ELISpot plates (MultiScreen 96-Well Plates; Millipore). Capture and detection of cytokines were accomplished by using monoclonal antibodies (eBiosciences) specific for mouse IFN-γ (clone AN-18 [capture] and R4-6A2 [detection]), IL-17 (clone eBio17CK15A5 [capture] and eBio17B7 [detection]), GM-CSF (clone MP1-22E9 [capture] and MP1-2231G6 [detection]), or IL-5 (clone TRFK5 [capture] and JES1-5A10 [detection]). All experiments were repeated at least twice. To identify dominant 15mer determinants within immunodominant 20mer determinants, mice were immunized with the 20mer peptide, and immune recall was performed with overlapping 15mers within that peptide. Spots were counted with an automated ELISpot plate reader (Bioreader 5000; Biosys).
GENERATION OF NMO RECOMBINANT ANTIBODY AND QUANTITATIVE IMMUNOFLUORESCENCE MICROSCOPY
Recombinant monoclonal anti-hAQP4 antibody (NMO-rAb) and isotype control were generated from clonally expanded plasmablasts recovered from the cerebrospinal fluid of a seropositive patient with NMO as described previously.17 U87MG cells stably transfected with M23 hAQP4 were grown on coverslips and fixed with 4% paraformaldehyde for 15 minutes and then rinsed with 1× phosphate-buffered saline. Coverslips were subsequently blocked with 10% normal goat serum and then incubated with recombinant antibody18 (10 µg/mL) with or without T-cell peptide (5 µg/mL; 40-fold molar excess) in 5% normal goat serum overnight at 4°C. Coverslips were washed 5 times with 1× phosphate-buffered saline and then incubated with rabbit polyclonal anti-AQP4 (sc-20812; Santa Cruz Biotechnology) (4 µg/mL) in 2% goat serum and 0.1% Triton X100 for 1 hour at room temperature. Coverslips were subsequently washed and then incubated with goat antihuman AlexaFluor 488 (A-11013; Invitrogen) and goat antirabbit AlexaFluor 594 (DI-1594; Vector) in 2% goat serum for 1.5 hours at room temperature in the dark. Samples were then washed, fixed, and mounted with mounting media containing DAPI (H-1500; Vector).
Images were obtained using a spinning disc confocal microscope (Olympus Ix81; Olympus), and the amount of red and green fluorescence was quantified using Image J software (National Institutes of Health). The ratio of green to red fluorescence was measured in multiple independent fields, and the binding percentage was subsequently calculated by comparing the green to red fluorescence ratio in the presence and absence of AQP4 peptide.
STATISTICAL ANALYSIS
A 1-way analysis of variance test was used to compare the 32 different treatment groups. If the analysis of variance was found to be significant, the Bonferroni test, a pairwise post hoc test, was performed to determine which pairs of treatments were significantly different. After reviewing the graphic results for these data, only 1 peptide in this group of 32, peptide 29 (hAQP4281–300), was compared with all antigen recalls. SPSS statistical software, version 19 (SPSS Inc), was used in these statistical analyses; all statistical tests were 2-sided, and P < .05 indicated significance.
RESULTS
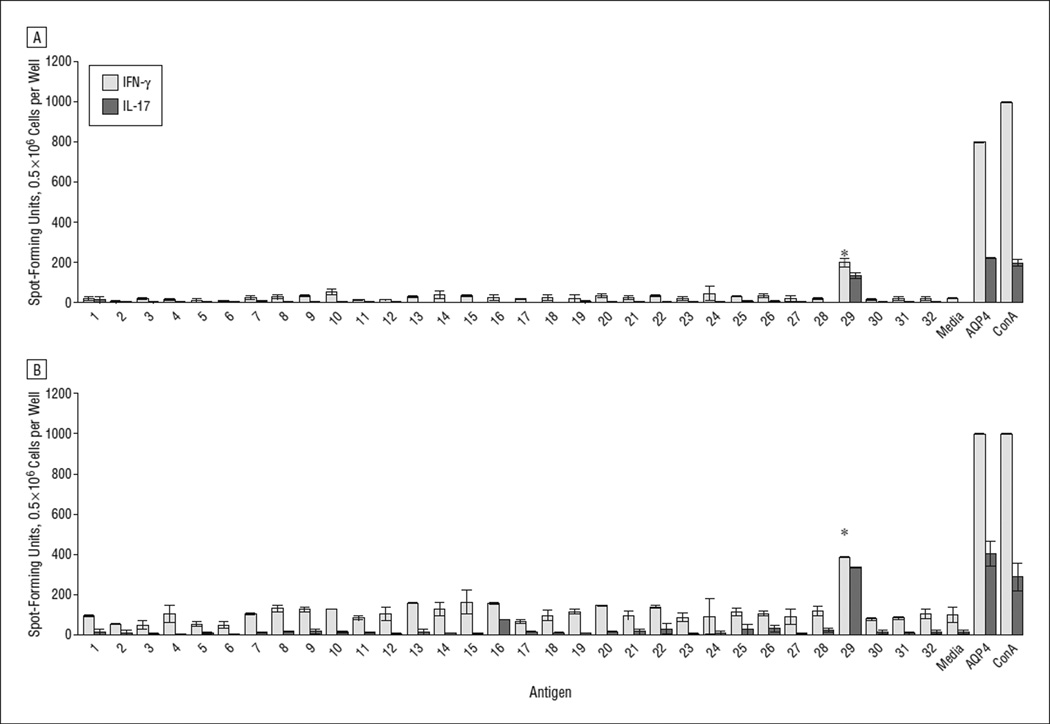
AQP4281–300 is the immunodominant linear determinant of hAQP4 in the context of HLA-DRB1*03:01. ELISpot assays were used to characterize the T-cell repertoire of HLA-DRB1*03:01 mice immunized with whole-protein hAQP4. The IFN-γ and IL-17 ELISpot assays identified hAQP4281–300 (peptide 29) as the immunodominant linear determinant in lymph node cells and splenocytes (Figure 1 and Figure 2A and B). TH17 cellular immune responses by splenocytes against hAQP4281–300 were not significantly different from those against full-length hAQP4 (Figure 1 and Figure 2B). None of the overlapping hAQP4 or full-length hAQP4 peptides induced an IL-5–driven TH2 response (data not shown).
Figure 2.
Aquaporin 4281–300 is the immunodominant linear determinant of human aquaporin 4 (hAQP4). HLA-DRB1*03:01 transgenic mice were immunized with a full-length hAQP4 complete Freund adjuvant emulsion, resulting in each mouse receiving 100 µg of antigen. On day 10, cells taken from the lymph nodes (A) and spleens (B) were collected from mice to generate single-cell suspensions. Thereafter, cells (0.5 × 106 cells) were incubated for 48 hours in 96-well enzyme-linked immunosorbent spot assay plates coated with anti–interferon-γ (IFN-γ) or anti–interleukin-17 (IL-17) with single overlapping hAQP4 peptides (50 µg/mL) (Table 1), media only, full-length hAQP4 (50 µg/mL), or concanavalin A (ConA) (1 µg/mL). Spot-forming units represent the absolute number of cells that are secreting a specific cytokine in response to antigen in the well (*P < .001). Error bars indicate SE.
THE DOMINANT IMMUNOGENIC REGIONS WITHIN hAQP4281–300
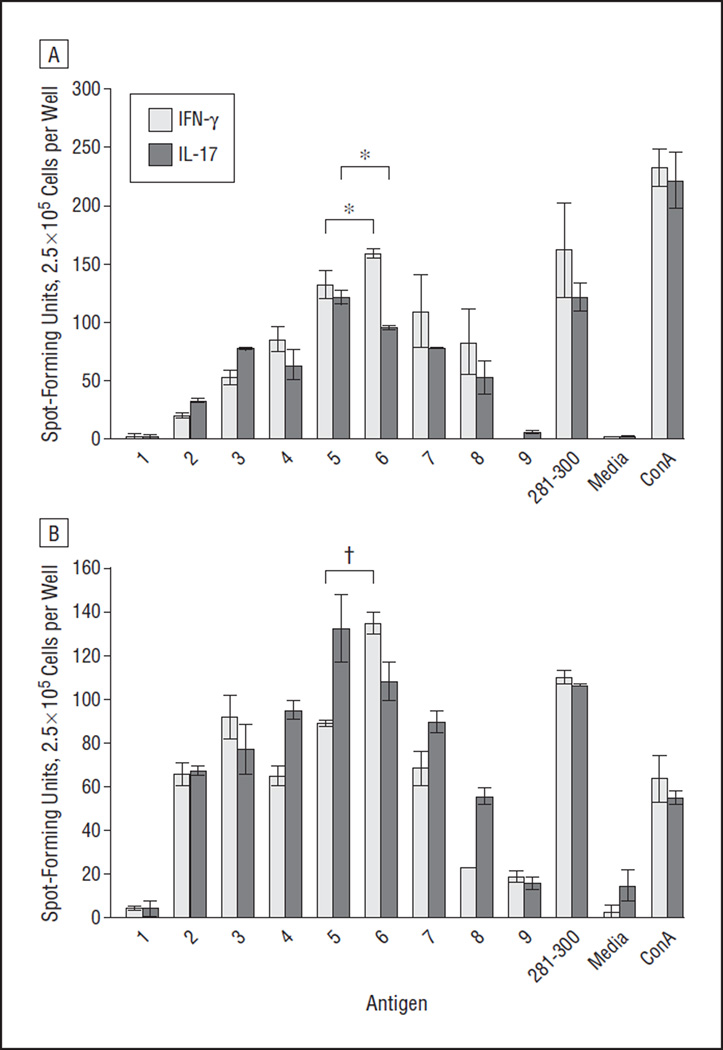
Because of their biophysical properties, linear peptides that are bound in the antigen-binding groove of the MHC class II molecule to be presented to CD4+ T cells are ideally 12 to 15 amino acids in length.12 Thus, the immunodominant determinants within hAQP4281–300 were identified by performing IFN-γ, IL-17, and GM-CSF ELISpot assays with 15mer peptides spanning hAQP4281–300 (Table 2).
In lymph node cells and splenocytes from HLA-DRB1*03:01 mice immunized with AQP4281–300, AQP4284–298 induced a significantly higher TH17 response than other 15mers (Figure 1 and Figure 3A). AQP4284–298 also induced the strongest GM-CSF–driven TH response in splenocytes significant from other 15mers (data not shown). Because of the insufficient number of lymph node cells, GM-CSF ELISpot assays could not be performed. AQP4285–298 resulted in a significantly higher TH1 response than other 15mers in lymph nodes cells (Figure 1 and Figure 3B). There was also a trend toward higher IFN-γ secretion in splenocytes after recall with AQP4285–298.
Figure 3.
Aquaporin 4284–298 (AQP4284–298) and AQP4285–299 are the immunogenic regions of AQP4281–300. HLA-DRB1*03:01 transgenic mice were immunized with AQP4281–300. On day 10, cells (2.5 × 105 cells) taken from the lymph nodes (A) and spleens (B) were incubated for 48 hours in enzyme-linked immunosorbent spot assay plates coated with anti–interferon-γ (IFN-γ) or anti–interleukin-17 (IL-17) with a single overlapping peptide of AQP4 (50 µg/mL) (Table 2), human AQP4281–300 50 µg/mL), media only, or concanavalin A (ConA) (1 µg/mL). Spot-forming units represent the absolute number of cells that are secreting a specific cytokine in response to antigen in the well (*P < .05 and †P < .01). Error bars indicate SE.
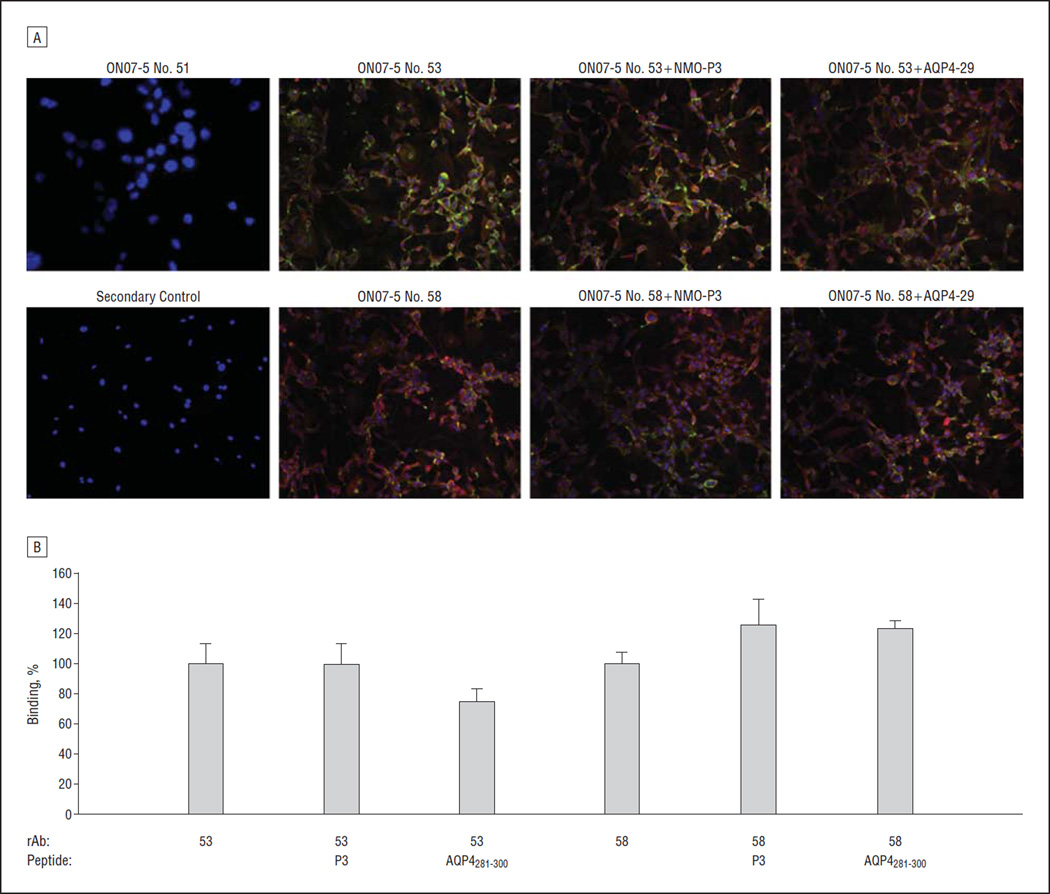
BINDING OF NMO-rAb TO SURFACE FULL-LENGTH hAQP4
We subsequently examined whether hAQP4281–300 could inhibit the binding of NMO-rAb to surface AQP4 (Figure 4).17 Using a quantitative immunofluorescence-binding assay, we observed no significant inhibition of the binding of 2 NMO-rAbs in the presence of a 40-fold molar excess of hAQP4281–300.
Figure 4.
Aquaporin 4281–300 (AQP281–300) does not inhibit AQP4 antibody binding. AQP4-specific and isotype control recombinant antibodies (rAbs) were incubated with a U87MG glial cell line stably transfected with the M23 isoform of human AQP4 in the presence or absence of AQP4 peptide. A, Fluorescence micrographs demonstrate robust binding of AQP4-specific rAbs 53 and 58 (green) to the transfected cell line. No staining was observed in the absence of rAb (secondary control) or when using an isotype control rAb (ON7-5 No. 51). A rabbit polyclonal anti-AQP4 antibody against the intracellular C-terminal portion of AQP4 was used as an internal control for AQP4 expression. B, Percentage binding of AQP4-specific rAbs 53 and 58 in the presence and absence of AQP4 peptides. NMO indicates neuromyelitis optica. Error bars indicate SE.
COMMENT
The identification of immunodominant determinants of hAQP4 may have important implications for understanding the origin of NMO and monitoring disease activity in patients with this disorder. As previously stated, there is accumulating evidence to suggest a cellular immune response against hAQP4 in NMO. Other investigators recently identified dominant determinants of hAQP4 in different wild-type mouse strains, including C57BL/6 (H-2b) and SJL/J (H-2s).19,20 One group of investigators found a dominant determinant that overlaps with hAQP4281–300, namely, hAQP4289–306, in C57BL/6 mice.20 However, it is difficult to compare this observation with ours for 2 reasons: (1) hAQP4 was obtained from different sources and (2) the C57BL/6 genetic background does not express H-2-IEα, the equivalent gene of the human class II MHC molecule HLA-DRα.
This study specifically aimed to identify immunodominant linear determinants of hAQP4 in the context of HLA-DRB1*03:01 because this HLA haplotype was recently associated with NMO in several patient cohorts.10,11 ELISpot assays allowed us to determine the frequency of antigen-specific T cells specific for hAQP4 peptide determinants and to characterize their cytokine profiles. This is relevant because encephalitogenicity of T cells in another autoimmune disorder of the CNS, multiple sclerosis,21 is largely defined by cytokine phenotype.
TH1 cells, defined by the signature cytokine IFN-γ, were initially implicated in CNS autoimmunity.22 Perhaps the most convincing evidence to support a pathogenic role of IFN-γ in patients with multiple sclerosis was generated in a clinical study in which 7 of 18 patients who received recombinant IFN-γ therapy experienced a disease exacerbation.23,24 In the last decade, another subclass of pathogenic CD4+ TH cells was characterized by the production of IL-17. TH17 cells appear to facilitate the initiation and perpetuation of CNS autoimmune diseases25 and mediate proinflammatory and allergic responses. IL-17 mediates the localization of neutrophils to the sites of infection.26 TH17 cells have also been shown to play a critical role in the production of GM-CSF in the periphery and CNS.27,28 Our group and other investigators recently found that GM-CSF may play a critical role in different models of active and passive experimental autoimmune encephalomyelitis.28–31 Our own results indicated that GM-CSF is secreted by lymph node cells and splenocytes after antigen restimulation in the presence of IL-12. In an adoptive transfer model of experimental autoimmune encephalomyelitis, we also found that GM-CSF is highly expressed by encephalitogenic T cells.
The identification of AQP4284–298 infers that the cellular immune response may play a critical role in NMO disease development and progression because of its ability to stimulate a pronounced TH17 immune response in the context of HLA-DRB1*03:01. The level of IL-17 is increased in patients with NMO during disease relapses.32,33 This finding would explain the presence of neutrophils at sites of tissue damage.15 It is now recognized that TH17 cells possess substantial plasticity compared with other TH cells.34 In the setting of NMO, however, the increased levels of IL-6 found in the cerebrospinal fluid of patients with NMO may allow for the survival of hAQP4-specific TH17 cells while inhibiting FOXp3+ T-regulatory cells.35–37 In addition, in a Chinese patient cohort, a polymorphism in the IL-17 gene was recently associated with anti-AQP4 antibody–positive NMO.33 Uzawa et al35 did not find elevated GM-CSF levels in the cerebrospinal fluid of patients with NMO and active clinical disease. However, the accumulation of eosinophils and granulocytes in the NMO lesion may suggest that this cytokine also plays a pathogenic role.15
An animal model of NMO with the hAQP4 determinants identified in this study is currently under development in our laboratory. Perhaps more important, our observations may have immediate human applications. We are developing assays to determine a potentially low frequency of hAQP4284–298– and hAQP4285–299–specific CD4+ T cells in patients with NMO and controls together with other investigators. The biological relevance of linear hAQP4 determinants identified in this study in NMO disease activity and in response of patients with NMO to pharmacotherapies will ultimately have to be evaluated in controlled clinical trials.
Acknowledgments
Funding/Support: Dr Stüve is a recipient of a Doris Duke Clinical Scientist Development Award. Work related to this study was supported by grant 2009036 from the Doris Duke Charitable Foundation. Dr Bennett is supported by research grant RG4320 from the Guthy-Jackson Charitable Foundation and the National Multiple Sclerosis Society.
Additional Contributions: The Guthy-Jackson Charitable Foundation made whole-length hAQP4 available to our laboratory. We also thank Linda S. Hynan, PhD, in the Department of Biostatistics at the University of Texas Southwestern Medical Center at Dallas for her support.
Footnotes
Author Contributions: Study concept and design: Arellano, Hussain, Zein, Steinman, Greenberg, Lambracht-Washington, Bennett, and Stüve. Acquisition of data: Arellano, Zacharias, Yoon, David, Zein, Ritchie, Bennett, and Stüve. Analysis and interpretation of data: Arellano, Zacharias, Steinman, Forsthuber, Lambracht-Washington, Ritchie, Bennett, and Stüve. Drafting of the manuscript: Arellano, Yoon, Zein, Ritchie, Bennett, and Stüve. Critical revision of the manuscript for important intellectual content: Hussain, Zacharias, David, Steinman, Forsthuber, Greenberg, Lambracht-Washington, and Stüve. Statistical analysis: Arellano, Zacharias, Lambracht-Washington, Ritchie, Bennett, and Stüve. Obtained funding: Steinman, Greenberg, and Stüve. Administrative, technical, and material support: Hussain, Yoon, David, Zein, Steinman, Forsthuber, Greenberg, Lambracht-Washington, and Stüve. Study supervision: Zein, Steinman, and Stüve.
Financial Disclosure: None reported.
REFERENCES
- 1.Cree BA, Goodin DS, Hauser SL. Neuromyelitis optica. Semin Neurol. 2002;22(2):105–122. doi: 10.1055/s-2002-36534. [DOI] [PubMed] [Google Scholar]
- 2.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of opticspinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly DL, Shanahan CM, Weissberg PL. The aquaporins: a family of water channel proteins. Int J Biochem Cell Biol. 1998;30(2):169–172. doi: 10.1016/s1357-2725(97)00124-6. [DOI] [PubMed] [Google Scholar]
- 5.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63(7):964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 6.Furman CS, Gorelick-Feldman DA, Davidson KG, et al. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci U S A. 2003;100(23):13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob A, Weinshenker BG, Violich I, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol. 2008;65(11):1443–1448. doi: 10.1001/archneur.65.11.noc80069. [DOI] [PubMed] [Google Scholar]
- 8.Pellkofer HL, Krumbholz M, Berthele A, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology. 2011;76(15):1310–1315. doi: 10.1212/WNL.0b013e3182152881. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68(11):1412–1420. doi: 10.1001/archneurol.2011.154. [DOI] [PubMed] [Google Scholar]
- 10.Brum DG, Barreira AA, dos Santos AC, et al. HLA-DRB association in neuromyelitis optica is different from that observed in multiple sclerosis. Mult Scler. 2010;16(1):21–29. doi: 10.1177/1352458509350741. [DOI] [PubMed] [Google Scholar]
- 11.Zéphir H, Fajardy I, Outteryck O, et al. Is neuromyelitis optica associated with human leukocyte antigen? Mult Scler. 2009;15(5):571–579. doi: 10.1177/1352458508102085. [DOI] [PubMed] [Google Scholar]
- 12.Geluk A, Fu XT, van Meijgaarden KE, et al. T cell receptor and peptide-contacting residues in the HLA-DR17(3) β1 chain. Eur J Immunol. 1994;24(12):3241–3244. doi: 10.1002/eji.1830241251. [DOI] [PubMed] [Google Scholar]
- 13.Cocks BG, de Waal Malefyt R, Galizzi JP, de Vries JE, Aversa G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993;5(6):657–663. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- 14.Shapira SK, Jabara HH, Thienes CP, et al. Deletional switch recombination occurs in interleukin-4-induced isotype switching to IgE expression by human B cells. Proc Natl Acad Sci U S A. 1991;88(17):7528–7532. doi: 10.1073/pnas.88.17.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125(pt 7):1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss G, Vignali DA, Schönrich G, Hämmerling GJ. Negative and positive selection by HLA-DR3(DRw17) molecules in transgenic mice. Immunogenetics. 1994;40(2):104–108. [PubMed] [Google Scholar]
- 17.Bennett JL, Lam C, Kalluri SR, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66(5):617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graber DJ, Levy M, Kerr D, Wade WF. Neuromyelitis optica pathogenesis and aquaporin 4. J Neuroinflammation. 2008;5:22. doi: 10.1186/1742-2094-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson PA, Khodadoust M, Prodhomme T, et al. Immunodominant T cell determinants of aquaporin-4, the autoantigen associated with neuromyelitis optica. PLoS One. 2010;5(11):e15050. doi: 10.1371/journal.pone.0015050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalluri SR, Rothhammer V, Staszewski O, et al. Functional characterization of aquaporin-4 specific T cells: towards a model for neuromyelitis optica. PLoS One. 2011;6(1):e16083. doi: 10.1371/journal.pone.0016083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone, I. definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 23.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37(7):1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 24.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1(8538):893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 25.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto M, Prause O, Sjöstrand M, Laan M, Lötvall J, Lindén A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170(9):4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 27.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1– and IL-23–induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Codarri L, Gyülvészi G, Tosevski V, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 29.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(1):39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 30.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113(14):3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cravens PD, Hussain RZ, Zacharias TE, et al. Lymph node-derived donor encephalitogenic CD4+ T cells in C57BL/6 mice adoptive transfer experimental autoimmune encephalomyelitis highly express GM-CSF and T-bet. J Neuroinflammation. 2011;8:73. doi: 10.1186/1742-2094-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang HH, Dai YQ, Qiu W, et al. Interleukin-17–secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 2011;18(10):1313–1317. doi: 10.1016/j.jocn.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Zhong X, Wang K, et al. Interleukin 17 gene polymorphism is associated with anti-aquaporin 4 antibody-positive neuromyelitis optica in the Southern Han Chinese: a case control study. J Neurol Sci. 2012;314(1–2):26–28. doi: 10.1016/j.jns.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uzawa A, Mori M, Arai K, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16(12):1443–1452. doi: 10.1177/1352458510379247. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Mitsdoerffer M, Croxford AL, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183(5):3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]