Abstract
Converging lines of evidence indicate that elevations in synaptic dopamine levels play a pivotal role in the reinforcing effects of cocaine, which are associated with its abuse liability. This evidence has led to the exploration of dopamine receptor blockers as pharmacotherapy for cocaine addiction. While neither D1 nor D2 receptor antagonists have proven effective, medications acting at two other potential targets, D3 and D4 receptors, have yet to be explored for this indication in the clinic. Buspirone, a 5-HT1A partial agonist approved for the treatment of anxiety, has been reported to also bind with high affinity to D3 and D4 receptors. In view of this biochemical profile, the present research was conducted to examine both the functional effects of buspirone on these receptors and, in non-human primates, its ability to modify the reinforcing effects of i.v. cocaine in a behaviourally selective manner. Radioligand binding studies confirmed that buspirone binds with high affinity to recombinant human D3 and D4 receptors (~98 and ~29 nM respectively). Live cell functional assays also revealed that buspirone, and its metabolites, function as antagonists at both D3 and D4 receptors. In behavioural studies, doses of buspirone that had inconsistent effects on food-maintained responding (0.1 or 0.3 mg/kg i.m.) produced a marked downward shift in the dose–effect function for cocaine-maintained behaviour, reflecting substantial decreases in self-administration of one or more unit doses of i.v. cocaine in each subject. These results support the further evaluation of buspirone as a candidate medication for the management of cocaine addiction.
Keywords: Buspirone, cocaine addiction, D3 receptor antagonist, D4 receptor antagonist, i.v. self-administration, medications development
Introduction
Cocaine abuse and addiction remain a major public health concern. Estimates from the National Survey on Drug Use and Health indicate that, in 2009, 4.8 million Americans abused cocaine. There are no approved medications to treat cocaine addiction and multiple therapeutic approaches have failed in double-blind, placebo-controlled trials (Anderson et al. 2009; Elkashef et al. 2006; Kahn et al. 2009; Montoya & Vocci, 2009). Given the prominent role of dopamine in the reinforcing effects of cocaine, both D1-like and D2-like dopamine receptor antagonists that might block such effects have been explored as anti-cocaine medications. Although D1-like antagonists attenuate the behavioural effects of cocaine in laboratory animals (e.g. Bergman et al. 1990), subsequent studies have reported that the D1 antagonist ecopipam (SCH-39166) fails to decrease the subjective effects of cocaine in man (Haney et al. 2001; Nann-Vernotica et al. 2001). Dopamine D2-preferring antagonists are similarly effective in laboratory animals, but have also failed to reduce the subjective effects of cocaine in the clinic. However, these findings must be interpreted cautiously because the extrapyramidal side-effects of D2 receptor blockade may have precluded a full evaluation of cocaine-antagonist dosing regimens (Brauer & de Wit, 1996; Gunne et al. 1972; Sherer et al. 1989; Wachtel et al. 2002).
Several lines of evidence suggest that the addictive effects of cocaine may preferentially involve activity mediated through the D3 and D4 subtypes of the D2- like-family of receptors. For example, D3-preferring antagonists have provided positive results in rodent and non-human primate models of cocaine self-administration and relapse (reviewed in Heidbreder & Newman, 2010). Additionally, D4 receptor blockade, using the probe compound PNU 101387G, has been shown to attenuate amphetamine-induced increases in accumbal dopamine (Feldpausch et al. 1998). The D4 receptor exists in multiple forms and a ‘long’ polymorphism in exon 3 has previously been linked to novelty seeking in humans, which may increase vulnerability to substance abuse (reviewed in Le Foll et al. 2009). While observations such as these suggest that D3 or D4 receptors might be suitable targets for the development of medications (e.g. Heidbreder & Newman, 2010; Kotler et al. 1997; Le Foll et al. 2000), neither D3 nor D4 antagonists have been available for clinical evaluation in substance use disorders.
Buspirone (Buspar®) was initially developed as an antipsychotic agent (Temple et al. 1982) based on its antagonist actions at dopamine receptors (Riblet et al. 1982). However, buspirone exhibited a low (IC50 ~260 nM) affinity for D2-like dopamine receptors in the striatum that are now recognized as predominantly the D2 subtype and its pharmacological profile differed from ‘classical ‘ neuroleptics (Riblet et al. 1982). While clear antipsychotic effects have never been demonstrated in schizophrenic patients (Brody et al. 1990; Goff et al. 1991; Sirota et al. 2001; Sumiyoshi et al. 2007; Temple et al. 1982), buspirone early on was found to reduce anxiety without producing side-effects commonly observed with benzodiazepines and other γ-aminobutyric acid (GABA)ergic anxiolytics (Goldberg & Finnerty, 1979; Rickels et al. 1982; Tompkins et al. 1980). Based on this therapeutic advantage, buspirone was approved to treat generalized anxiety disorder>25 yr ago. Its anti-anxiety effects are thought to reflect serotonergic actions and buspirone is commonly characterized as a potent and selective 5-HT1A receptor partial agonist (Wong et al. 2007). Buspirone has also been evaluated for clinical indications other than anxiety, including depression and drug addiction. For example, it has been evaluated for the management of drug addiction, including alcoholism, nicotine dependence and cocaine withdrawal and dependence (Bruno, 1989; Malec et al. 1996; Moeller et al. 2001; Schneider et al. 1996). Although encouraging effects sometimes were obtained, especially for alleviating withdrawal-related anxiety, definitive results in such trials were precluded by their small sample size.
Notwithstanding its early characterization as a D2 antagonist (Riblet et al. 1982), independent studies published in the 1990s (Kula et al. 1994; Tallman et al. 1997) reported that buspirone binds with high affinity to the D3 and D4 subtypes of dopamine receptor (ki values of 3.5 and 78 nM, respectively). The efficacy of buspirone at D3 and D4 receptors was not evaluated in those studies, but the reported affinities are within the range of values reported for buspirone at recombinant human 5-HT1A receptors (4–78 nM; NIMH, undated), suggesting that clinically relevant doses of buspirone may also occupy these dopamine receptor subtypes. These reports provided the impetus for the present studies to evaluate the relative potencies and efficacies of buspirone at D3 and D4 receptors as well as its ability to modify the reinforcing effects of i.v. cocaine in non-human primates in a behaviourally selective manner. The present studies confirm the high affinity of buspirone at human D3 and D4 receptors (ki ~98 and 29 nM, respectively) and establish its antagonist properties at both types of receptors in functional assays. Consistent with this neurochemical profile, moderate doses of buspirone (0.1 and 0.3 mg/kg i.m.), which did not consistently alter food-maintained performance, markedly attenuated the reinforcing effects of a wide range of unit doses of i.v. cocaine, resulting in a marked downward shift in the function relating unit dose and self-administration behaviour. These results provide a basis for the further evaluation of buspirone as a candidate medication for the management of cocaine addiction.
Method
Radioligand binding assays
Cell lines stably expressing the D2 and D4 receptors were created using the HEK-GIRK-M4 cells as a host cell line. Cells were routinely cultured in 1:1 Dulbecco’s modified essential medium: Ham’s F-12 supplemented with 10% foetal bovine serum, 10 mM Hepes, 1×Glutamax, 1×non-essential amino acids, 1×antibiotic/antimitotic and 200 μg/ml hygromycin B. The D3 receptor-expressing CHO cell line that was used for the β-arrestin interaction assays (see below) was also used for radioligand binding assays. Membrane binding assays were performed as described previously (Namkung et al. 2009). Briefly, membranes were prepared from HEK-GIRK-D2, CHO-arrestin-D3 or HEK-GIRK-D4 cells and added to tubes containing various concentrations of buspirone or metabolite compounds and radioligand in a final volume of 1 ml. For the competition binding assays, [3H]methylspiperone was used at the following concentrations: D2, 0.5 nM; D3, 0.3 nM; D4, 1 nM; in order to use radioligand concentrations approximating the Kd of methylspiperone for these receptors. ki values were derived from the IC50 values using the Cheng–Prusoff equation (Cheng & Prusoff, 1973).
β-Arrestin functional assay
The β-arrestin interaction assays were performed as previously described (Banala et al. 2011) with minor modifications. Briefly, CHO-K1 cells expressing the D2, D3 or D4 dopamine receptors (DiscoveRx Inc., USA) were seeded into 384-well clear bottom plates using CP2 media (DiscoveRx Inc.) 24 h prior to the assay. Cells were then treated with multiple concentrations of the indicated drug and incubated for 60 min at 37 °C. DiscoveRx PathHunter reagent was then added to the cells followed by 60 min incubation at room temperature. Luminescence was measured on a Hamamatsu FDSS μ-cell plate reader (Hamamatsu, Japan). Exposure times ranged from 1 to 5 s. Data were analysed using GraphPad Prism software.
Self-administration studies
Subjects
Adult male rhesus monkeys (Macaca mulatta) weighing 6–10 kg served as subjects and were maintained at 95% free-feeding weights with a diet of chow (Purina Jumbo Monkey Chow no. 503; Purina Mills LLC, USA), fresh fruit daily and vitamins. Water was freely available at all times. Animal maintenance and research were conducted in accordance with guidelines provided by the Guide for Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 1996), as adopted and promulgated by the National Institutes of Health, and protocols were approved by the Institutional Animal Care and Use Committee at McLean Hospital. Subjects lived in well-ventilated, stainless steel chambers (60×100×76 cm), each equipped with an operant panel containing visual stimuli and response keys, an externally mounted 1-g food pellet dispenser and two syringe pumps for delivery of saline or cocaine injections (for additional details, see Negus & Mello, 2003). Each subject was surgically prepared with a double-lumen i.v. catheter that was protected by a custom-fitted nylon vest (Lomir Biomedical Inc., USA) and tether system described previously (Negus & Mello, 2003). Subjects were previously trained under the self-administration schedule contingencies described below and had participated in studies of acute treatment with drugs from several pharmacological classes. Programming equipment and software (MED Associates, USA) for operation of experimental stations and data collection were located in another room.
Procedures
Daily 2-h experimental sessions consisted of three response components separated by 5-min time-out (TO) periods. During the first and last components (food 1 and food 2 components), the centre response key was illuminated with red lights and 1 g banana-flavoured food pellets were available under a 30-response fixed ratio (FR 30); TO 10 s schedule for 5 min. During the second component (self-administration component), the centre response key was illuminated with green lights and i.v. injections of cocaine or saline were available for 100 min under an FR 30; TO 60 s schedule. This component was immediately preceded by illumination of a yellow light for 10 s together with the non-contingent delivery of a single ‘priming’ injection of saline or the dose of cocaine that was subsequently available. During the TO following each reinforcer delivery (food or i.v. injection), the centre response key was illuminated with yellow lights. During intercomponent TO periods, all lights were turned off and responding had no scheduled consequences.
The present studies began with the determination of self-administration dose–effect curves over a dose range of 0.003–0.1 mg/kg i.v. cocaine. Saline and unit doses of i.v. cocaine were available under a double alternation schedule and the order of availability varied irregularly among subjects. Next, the effects of pretreatment with buspirone (i.m., 15 min pre-session) were studied in test sessions that were routinely conducted on the second day of the double alternation schedule and following a session during which control rates and patterns of self-administration behaviour were observed. Initially, dose-ranging experiments were conducted to determine the effects of buspirone on self-administration of 0.032 mg/kg per injection i.v. cocaine. This unit dose of i.v. cocaine was selected for dose-ranging experiments because it reliably produced stable levels of self-administration behaviour throughout the four components of the experimental session. The goal of these experiments was to identify pretreatment doses of buspirone that decreased i.v. cocaine self-administration by ≥50% for further study; consequently, low or ineffective doses of buspirone (≤0.03 mg/kg) were not evaluated in 97D105 or 96D155. Subsequently, the acute effects of a single dose of buspirone (0.1 mg/kg in one subject and 0.32 mg/kg in three subjects) were evaluated when either i.v. saline or a range of unit doses of i.v. cocaine were available for self-administration.
Data analysis
The principal dependent variables were the number of reinforcers (food pellets or injections) delivered during each component of the test session. These data are presented in Figs. 4 and 5 as averaged results for the group of monkeys (mean±S.E.M) and in Tables 2 and 3 for individual subjects. Response rate data (response key presses per second) also were collected in individual subjects (data not shown). Statistical significance of the effects of buspirone on food-maintained behaviour and i.v. self-administration behaviour during saline availability was determined using paired t tests. A one-way analysis of variance (ANOVA) was used to analyse the effects of several doses of buspirone during dose-ranging experiments and a two-way repeated-measures ANOVA followed by Bonferroni’s t tests was used to analyse data from dose–effect determinations (GraphPad Prism 5.02). The criterion for significance was p<0.05 for all analyses.
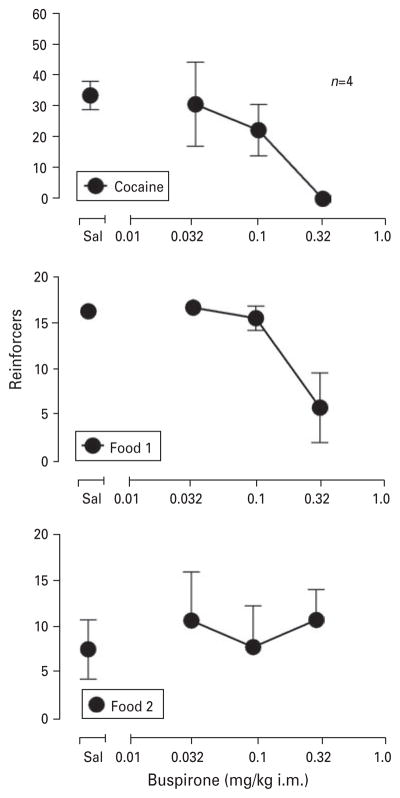
Fig. 4.
Mean effects of buspirone (0.03–0.32 mg/kg) on behaviour maintained by 0.032 mg/kg per injection cocaine. Abscissa: Pretreatment dose of buspirone. Ordinate: Number of total cocaine injections that were self-administered in the 100-min drug component or number of food pellets that were delivered during food 1 and food 2. The results shown above ‘Sal’ represent the effects of saline pretreatment and serve as baseline values for comparison with the effects of buspirone. The effects of 0.1 and 0.32 mg/kg buspirone shown here were determined in all monkeys; the effects of 0.032 mg/kg buspirone were determined in three monkeys. Data for individual subjects are shown in Table 2.
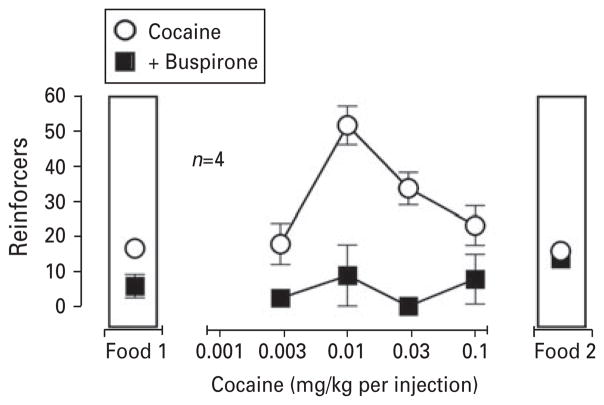
Fig. 5.
Mean effects of buspirone on behaviour maintained by different doses of i.v. cocaine or food reinforcement. Abscissa: Dose of cocaine that was available for i.v. self-administration (mg/kg per injection; log scale) or the availability of food reinforcement during the first and last components of the session (food 1 and food 2). Ordinate: Number of cocaine injections that were self-administered in the 100-min drug component during which that dose was available or number of food pellets that were delivered during the 5-min food components (food 1 and food 2). Open symbols represent data for i.v. cocaine self-administration during control sessions averaged for the group of four monkeys. Filled symbols represent average data for i.v. cocaine self-administration and food-maintained behaviour following pre-session i.m. administration of buspirone (0.32 mg/kg or, for monkey 96D155, 0.1 mg/kg) for the group of four subjects. Data for individual subjects are shown in Table 3.
Table 2.
Potencies for inhibiting D2, D3 and D4 receptor function by buspirone and its metabolites
| D2 IC50±S.E.M. (n) |
D3 IC50±S.E.M. (n) |
D4 IC50±S.E.M. (n) |
|
|---|---|---|---|
| Buspirone | 0.67±0.12 (7) | 0.44±0.18 (7) | 0.35±0.06 (6) |
| 5-OH Buspirone | 2.6±1.3 (4) | 0.93±0.46 (4) | 1.4±0.82 (3) |
| 6-OH Buspirone | 3.1±1.8 (4) | 4.9±2.8 (3) | 0.85±0.49 (3) |
| 1-PP | >10 | >10 | >10 |
Table 3.
Dose-ranging studies: effects of pretreatment with i.m. buspirone on i.v. cocaine self-administration and food-maintained behaviour
| Monkey | Buspirone (mg/kg) | 1 Inj | 2 Inj | 3 Inj | 4 Inj | Total Inj | Food 1 Pellets | Food 2 Pellets |
|---|---|---|---|---|---|---|---|---|
| RIB9 | 12 (±1) | 8 (±1) | 8 (±1) | 7 (±1) | 35 (± 1) | 14 (± 1) | 16 (±0) | |
| 0.03 | 14 | 13 | 14 | 13 | 54 | 16 | 15 | |
| 0.10 | 14 | 8 | 2 | 0 | 24 | 15 | 14 | |
| 0.32 | 0 | 0 | 0 | 0 | 0 | 16 | 13 | |
| 97D105 | 9 (±1) | 7 (±0) | 6 (±0) | 6 (±0) | 28 (± 1) | 16 (± 0) | 1 (±1) | |
| 0.10 | 0 | 10 | 10 | 5 | 25 | 12 | 0 | |
| 0.32 | 0 | 0 | 0 | 0 | 0 | 7 | 13 | |
| 97D113 | 11 (±2) | 5 (±1) | 5 (±1) | 5 (±1) | 25 (± 2) | 18 (± 0) | 4 (±3) | |
| 0.03 | 11 | 10 | 7 | 3 | 31 | 17 | 0 | |
| 0.10 | 14 | 13 | 7 | 6 | 40 | 18 | 0 | |
| 0.32 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 96D155 | 13 (±3) | 12 (±1) | 11 (±1) | 10 (±2) | 46 (± 4) | 17 (± 0) | 9 (±3) | |
| 0.03 | 1 | 0 | 0 | 6 | 7 | 17 | 17 | |
| 0.10 | 0 | 0 | 0 | 0 | 0 | 17 | 17 | |
| 0.32 | 0 | 0 | 0 | 0 | 0 | 0 | 16 |
Data under 1, 2, 3 and 4 show numbers of injections (Inj) self-administered during the availability of the unit dose of 0.03 mg/kg per injection of cocaine during the four successive 25-min segments of the self-administration component alone (shown in bold) and following a range of pretreatment doses of buspirone.
The last two columns show number of pellets obtained in components of food-maintained behaviour prior to (food 1) and following (food 2) the drug component when cocaine was available for i.v. self-administration.
Values for the number of injections for cocaine alone are averaged data (mean±S.E.M.) obtained during the present assessments.
Materials
HEK-GIRK-M4 cells were the kind gift of Lily Jan. [3H]methylspiperone was purchased from PerkinElmer Life Sciences (USA). Arrestin assay plating media and reagents were purchased from DiscoveRx. Assay plates (black 384-well tissue culture treated) were purchased from Greiner (USA). Cell culture reagents were purchased from Invitrogen (USA). Dopamine was purchased from Sigma (USA). Cocaine HCl and buspirone HCl, 1-PP, 5-hydroxybuspirone and 6-hydroxybuspirone were provided by the National Institute on Drug Abuse. For in vivo studies, cocaine was dissolved in sterile saline and delivered i.v., whereas buspirone was dissolved in sterile water for i.m. administration. Doses are expressed as the salt forms.
Results
Effects of buspirone and its metabolites at dopamine receptor subtypes
Radioligand binding studies
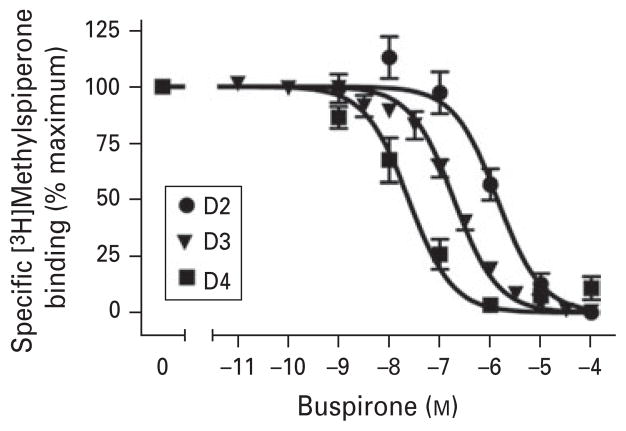
As buspirone has been reported to bind with moderate-high affinity to D2-like dopamine receptors (Kula et al. 1994), we initially evaluated its ability to compete for receptor binding in cells heterologously expressing recombinant human dopamine receptor subtypes. Figure 1 shows competition curves illustrating the ability of buspirone to compete for [3H]methyspiperone binding to human D2, D3 or D4 receptors. Notably, buspirone exhibits the highest affinity for the D4 receptor followed by the D3 then the D2 receptor. Average ki values for buspirone are shown in Table 1. Buspirone exhibits a ki of 29 nM for the D4 receptor whereas it exhibits about ~3.5-fold and ~20-fold lower affinity for the D3 and D2 receptors, respectively. Thus, the affinity of buspirone for the D3 and D4 receptors is within the range of that reported for the 5-HT1A serotonin receptor (Wong et al. 2007).
Fig. 1.
Inhibition of [3H]methylspiperone binding to human recombinant D2, D3 or D4 dopamine receptors by buspirone. Membranes from HEK293 cells were prepared and used for radioligand binding assays as described in Method. Specific binding is expressed as a percentage of the maximum observed in each experiment. Results are the average of three independent experiments, each done in triplicate. The average ki values for buspirone and its metabolites are shown in Table 1.
Table 1.
Affinities of buspirone and its metabolites for the D2-like receptors as determined using radioligand binding assays
| D2 ki ± S.E.M (nM) |
D3 ki ± S.E.M. (nM) |
D4 ki ± S.E.M. (nM) |
|
|---|---|---|---|
| Buspirone | 484 ± 114 | 98 ± 16 | 29.2 ± 11.3 |
| 5-OH Buspirone | 4010 ± 792 | 261 ± 46 | 107 ± 44.8 |
| 6-OH Buspirone | 5390 ± 425 | 795 ± 84 | 40.4 ± 17.5 |
| 1-PP | >10 μM | >10 μM | >10 μM |
Competition binding assays were performed as described in Fig. 1.
Data are expressed as mean±S.E.M.
N=6 for all buspirone values whereas N=3 for all metabolite values.
Since buspirone is extensively metabolized in humans (Dockens et al. 2006, 2007; Gammans et al. 1986), we also examined the affinities of three major metabolites for all three D2-like receptors (Table 1). Notably, the 1-PP metabolite does not bind to any of the receptors tested. However, the 5-OH and 6-OH metabolites exhibit varying affinities for D2-like dopamine receptors. Compared to buspirone, the 6-OH and 5-OH metabolites exhibit, respectively, ~4-fold and ~2-fold lower affinities for the D4 receptor and ~2.5-fold and ~8-fold lower affinities for the D3 receptor. The affinities of both the 5-OH and 6-OH metabolites for the D2 receptor decreased by ~10-fold.
Live cell functional assays
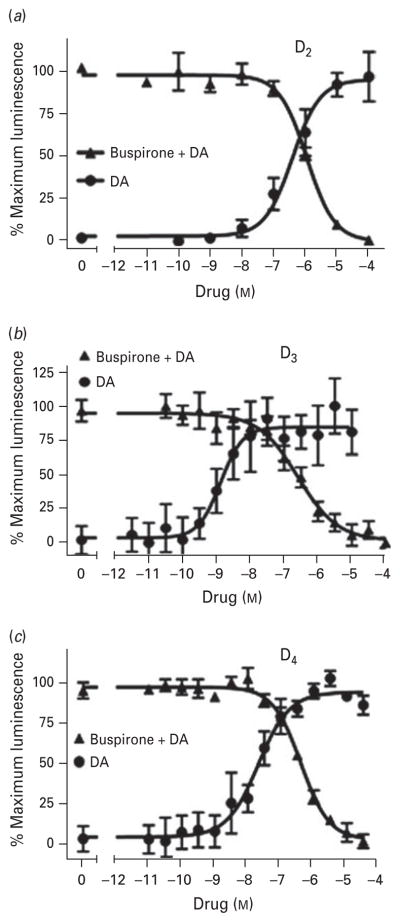
We next evaluated the functional activity of buspirone on D2-like receptors. In order to do this, we used an assay that measures the ability of agonists to induce receptor coupling to the scaffolding protein β-arrestin. β-arrestin×G protein-coupled receptors interaction is a normal consequence of agonist activation and is involved in both receptor internalization and activation of intracellular signalling pathways (Shenoy & Lefkowitz, 2011). This assay is particularly useful to assess D3 receptor activity, for which few functional assays are available. Specifically, this assay involves the expression of a receptor construct that is fused to an N-terminal fragment of β-galactosidase and a β-arrestin construct that is fused to a C-terminal fragment of β-galactosidase. Upon receptor activation, the receptor and β-arrestin interact, which results in complementation of the β-galactosidase enzyme and can be measured by the addition of a luminescent substrate (Zhao et al. 2008). Figure 2a shows that, using this assay, buspirone is able to completely antagonize dopamine-induced β-arrestin association with the D2 receptor. The average IC50 for this effect is 0.67 μM (Table 2). Notably, when tested alone, buspirone did not exhibit any agonist activity at the D2 receptor (data not shown).
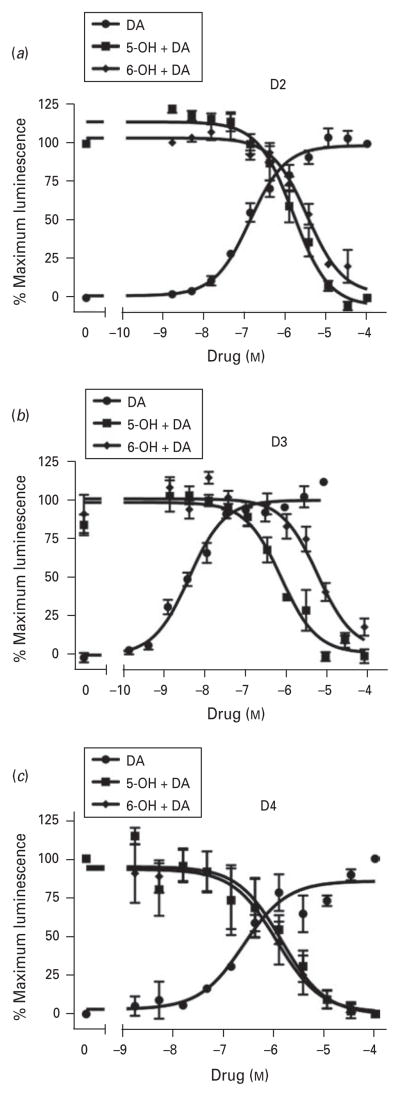
Fig. 2.
Buspirone antagonizes dopamine (DA)-induced receptor-β-arrestin interactions at human recombinant D2, D3 or D4 DA receptors. CHO-K1 cells stably expressing (a) D2, (b) D3, or (c) D4 receptors were incubated with the indicated concentrations of either DA or buspirone plus an EC90 concentration of DA for 60 min. Following treatment with assay reagent (DiscoveRx Inc.), luminescence was measured as described in Method. Data are normalized to the maximum response in each experiment and are plotted as mean±S.E.M. of three independent experiments, each performed in triplicate. The average IC50 values for buspirone are shown in Table 2. The average EC50 values (mean±S.E.M.) for DA are: (a) 150±30 nM; (b) 1.8±0.17 nM; (c) 400±57 nM.
Figures 2b, c show that buspirone also fully antagonizes dopamine activation of both the D3 and D4 receptors with average IC50 values of 0.44 and 0.35 μM, respectively (Table 2). It should be noted that the functional IC50 values appear to be lower than those observed in the binding assays. This is because the functional assays were performed using a saturating concentration of dopamine that competes with buspirone for binding to the receptors whereas the radioligand binding assays are performed with a sub-saturating concentration of radioligand. Nonetheless, from a functional standpoint, buspirone exhibits the identical rank order of potency for the receptors, D4>D3>D2, as observed with the radioligand binding assays. As with the D2 receptor, buspirone has no activity as an agonist at the D3 and D4 receptors (data not shown).
Evaluation of the functional activities of the buspirone metabolites demonstrates that both the 5-OH and 6-OH metabolites are also full antagonists at the D2, D3 and D4 receptors (Fig. 3 and Table 2). In contrast, none of the metabolites demonstrated any agonist activities at the receptors tested (data not shown). All the metabolites exhibited decreased potencies when compared to buspirone, in agreement with binding data shown in Table 1. In general, the metabolites, like buspirone, exhibit the highest potencies for the D3 and D4 receptors.
Fig. 3.
The active metabolites of buspirone antagonize dopamine (DA)-induced receptor-β-arrestin interactions at human recombinant D2, D3 or D4 DA receptors. Details are shown in Fig. 2.
We also used a β-arrestin interaction assay to evaluate the functional efficacy of buspirone at the D1 receptor and found that it functioned as a weak antagonist (IC50>10 μM), indicating that it has low affinity for the D1-like family of dopamine receptors. Taken together, these data indicate that buspirone is a functional antagonist at the D2-like family of dopamine receptors, specifically with high affinities/potencies for the D3 and D4 receptor subtypes.
Cocaine self-administration studies
Baseline cocaine self-administration
Cocaine self-administration generally was dose-related and described by an inverted U-shaped function that is characteristic for cocaine-maintained behaviour under FR schedules. Saline extinction was characterized in all monkeys by a rapid decline within the experimental session to low levels of responding. Peak levels of cocaine self-administration (~52 injections per session) were maintained by the i.v. dose of 0.01 mg/kg per injection, whereas the highest i.v. dose of cocaine (0.1 mg/kg per injection) produced a >50% decrease from peak levels of self-administration.
Initial dose-ranging experiments with buspirone
Pretreatment with buspirone generally produced dose-related and significant (F3,12=4.83; p=0.02) decreases in the intake of 0.03 mg/kg per injection i.v. cocaine (Fig. 4, Table 3). Averaged for the group of monkeys, the lowest dose of buspirone (0.032 mg/kg) did not greatly (<20%) alter response rates, the intermediate pretreatment dose (0.1 mg/kg) decreased responding to approximately 65% of control values and the highest dose of buspirone (0.32 mg/kg) completely eliminated cocaine-maintained responding. As shown in Table 3, the effects of 0.03 and 0.1 mg/kg buspirone differed among subjects. One or both doses of buspirone produced moderate (≤60%) increases in cocaine-maintained behaviour in two monkeys (RIB9 and 97D113), nearly or completely eliminated i.v. cocaine self-administration in one monkey (96D155) and did not greatly alter i.v. self-administration in the fourth monkey (97D105). Experiments were not conducted to determine whether yet lower doses of buspirone might increase cocaine-maintained responding in 96D155 or 97D105 (see Method). In contrast to the differing effects of low and intermediate doses of buspirone, the effects of the highest dose of buspirone (0.32 mg/kg) were highly consistent among monkeys, eliminating i.v. cocaine self-administration behaviour in all four subjects.
Averaged data also reveal that buspirone produced statistically significant decreases in food-maintained responding prior to (food 1) the i.v. cocaine self-administration component of the session (F3,12=6.34, p=0.008). Effects of buspirone on low levels of food-maintained responding following the i.v. cocaine self-administration component (food 2) were not significant. These effects of buspirone on food-maintained responding were generally consistent among subjects (Table 3). Thus, pretreatment with the lower two doses of buspirone (0.032 and 0.1 mg/kg) did not markedly decrease (≤25%) food-maintained responding in the food 1 or food 2 session components in any subject. In contrast, the highest dose of buspirone (0.32 mg/kg) either markedly decreased (~50%) or eliminated food-maintained behaviour during the food 1 session component in three of four subjects. This dose of buspirone was without effect on responding during the food 1 component in the fourth subject or during the food 2 component in any subject.
Effects of buspirone on the cocaine dose effect function
Further studies were conducted to determine how pretreatment with the lowest dose of buspirone that eliminated cocaine-maintained responding in dose-ranging experiments (0.32 or, in monkey 96D155, 0.1 mg/kg) modified intake of i.v. saline or self-administration of a range of doses of i.v. cocaine (Fig. 5, Table 4). Averaged for the group, buspirone produced non-significant and minimal (15%) alterations in responding during i.v. saline availability, but a highly significant change in the dose–effect function for i.v. cocaine self-administration (F1,12=104, p<0.0001). Thus, buspirone dramatically reduced behaviour maintained by all unit doses of i.v. cocaine, reflected by a flattening of the dose–effect curve for i.v. cocaine self-administration (Fig. 5). Data for individual subjects (Table 4) show that buspirone eliminated responding during i.v. saline availability in two monkeys and increased responding in the remaining two monkeys (11 vs. eight injections in 97D113 and 19 vs. nine injections in 96D155, respectively). Notwithstanding such inter-subject variability, pretreatment with 0.32 mg/kg buspirone nearly eliminated i.v. self-administration behaviour during the availability of all doses of i.v. cocaine (0.003–0.1 mg/kg per injection) in three monkeys whereas pretreatment with the lower dose of 0.1 mg/kg buspirone eliminated i.v. self-administration of two doses of i.v. cocaine (0.003 and 0.03 mg/kg per injection) in the fourth monkey.
Table 4.
Number of injections: effects of pre-session administration of buspirone on total number of i.v. cocaine injections during the self-administration component
| f | Buspirone (mg/kg) | Sal | Cocaine (mg/kg per injection)
|
Food 1 | Food 2 | |||
|---|---|---|---|---|---|---|---|---|
| 0.003 | 0.01 | 0.03 | 0.1 | |||||
| RIB9 | 10 (±2) | 28 (±13) | 50 (±2) | 35 (±1) | 28 (±3) | 16 (±0) | 15 (±0) | |
| 0.32 | 0 | 2 | 0 | 0 | 0 | 13 | 12 | |
| 97D105 | 8 (±2) | 14 (±7) | 56 (±4) | 28 (±1) | 11 (±0) | 16 (±0) | 14 (±0) | |
| 0.32 | 1 | 0 | 0 | 1 | 0 | 9 | ||
| 97D113 | 8 (±1) | 3 (±3) | 37 (±4) | 25 (±2) | 17 (±1) | 17 (±0) | 17 (±0) | |
| 0.32 | 11 | 2 | 0 | 0 | 1 | 10 | 16 | |
| 96D155 | 9 (±1) | 26 (±11) | 63 (±1) | 46 (±4) | 36 (±7) | 17 (±0) | 17 (±0) | |
| 0.10 | 19 | 3 | 35 | 0 | 29 | 0 | 17 | |
The numbers of self-administered i.v. injections alone (mean±S.E.M.) are shown for saline (Sal) and for each unit dose of i.v. cocaine (shown in bold) for each monkey.
The last two columns show number of pellets obtained in components of food-maintained behaviour prior to (food 1) and following (food 2) the drug component when saline was available for i.v. self-administration.
The rows in non-bold type show the number of injections and food pellet deliveries in single determinations of the effects of pretreatment with 0.32 mg/kg buspirone in RIB9, 97D105 and 97D113, and with 0.1 mg/kg buspirone in 96D155.
Buspirone had effects on food-maintained behaviour during sessions of i.v. saline availability that did not differ meaningfully from its effects during dose-ranging experiments. On average, responding was decreased to~35% of control values during the food 1 component of the session but recovered to ~85% of control values during the food 2 component of the session (see symbols above food 1 and food 2 in Fig. 5). The effects of buspirone on food-maintained responding did not achieve significance during the food 1 component (p=0.052), likely due to variability in results among individual subjects, and were clearly non-significant during the food 2 component of the session. As with dose-ranging data, averaged results reflect varying effects of buspirone among subjects (Table 4). For example, responding during the food 1 component of the session was eliminated in two monkeys, 97D113 and 96D155, but decreased by <50% in the remaining two monkeys (RIB9 and 97D105). In contrast, food-maintained responding during the food 2 component of the session was either unaffected (two monkeys) or decreased only by 25–35% from control values (two monkeys).
Discussion
The present results demonstrate that buspirone acts as an antagonist at the D2, D3 and D4 subtypes of dopamine receptor and, in i.v. cocaine self-administration studies in nonhuman primates, can potently reduce cocaine intake. Although dose extrapolation between species is problematic, especially when comparing different routes of administration, behaviourally effective doses of buspirone (0.1–0.32 mg/kg i.m.) in the present studies are well within the range of oral doses routinely used to treat generalized anxiety disorder (20–60 mg). This consideration suggests that the present findings in non-human primates should have direct relevance for buspirone’s potential utility in the treatment of cocaine abuse and addiction.
Acute treatment with buspirone either greatly decreased or eliminated self-administration maintained by two or more i.v. doses of cocaine in each subject. It is noteworthy that the effects of buspirone on food-maintained behaviour in the present studies might suggest non-selective effects on schedule-controlled behaviour. However, disruptions in food-maintained responding were not as consistent among subjects as reductions in the reinforcing effects of i.v. cocaine, even though both effects were measured during the same experimental sessions. This is especially evident when comparing the effects of buspirone in the fourth quartile of the self-administration component of dose-ranging studies with its effects 5–10 min later in the food 2 component of the session. Thus, doses of buspirone that were studied further (0.32 mg/kg and, in 96D155, 0.1 mg/kg) eliminated self-administration of 0.032 mg/kg per injection cocaine in the fourth quartile but had limited, if any, effect on food 2 responding in three of four monkeys. Although such differences can be difficult to interpret, these findings are in line with previous findings that comparable doses of buspirone modified i.v. cocaine self-administration without greatly altering food consumption (Gold & Balster, 1992). They also are consistent with other, more recent findings that buspirone can surmountably antagonize the discriminative-stimulus effects of cocaine in non-human primates at doses that do not greatly influence operant behaviour maintained under a schedule of stimulus-termination rather than food presentation (Bergman, unpublished results).
The doses of buspirone that decreased i.v. cocaine self-administration in the present studies were previously found to increase cocaine intake by rhesus monkeys studied under a comparable FR schedule (Gold & Balster, 1992). Increases in cocaine self-administration behaviour were also noted in two subjects following low and intermediate doses of buspirone in the present dose-ranging experiments. As suggested previously, increased i.v. cocaine self-administration in the present and previous studies suggest that buspirone may attenuate the reinforcing or, alternatively, behaviourally disruptive effects of cocaine (Gold & Balster, 1992). An additional consideration in the present experiments is that i.v. self-administration behaviour was strongly influenced by pre-session priming injections that signalled availability of either a reinforcing dose or, for saline or low doses of cocaine, injections that were not reinforcing. As a result of this cueing, subjects typically exhibited rapid within-session extinction (i.e. without overall increases in responding) when saline or low unit doses of cocaine were available for i.v. self-administration. Thus, in the present experiments, the highest doses of buspirone may have decreased the cueing effects of priming injections of cocaine to the extent that they were no longer identified as reinforcing, leading to rapid within-session extinction. This view is consistent with previous findings that buspirone can decrease the discriminative-stimulus, or cueing, effects of dopaminergic agonists including cocaine (Bergman, unpublished results; Callahan & Cunningham, 1997; Kamien & Woolverton, 1990).
Buspirone is considered to be a potent and selective 5-HT1A receptor partial agonist (Wong et al. 2007). In view of the pivotal role ascribed to 5-HT1A receptors in many aspects of stimulant abuse (reviewed in Müller et al. 2007), it may be hypothesized that 5-HT1A partial agonist actions of buspirone contributed to the reductions in cocaine self-administration described herein. However, early behavioural studies of buspirone in monkeys do not support this idea. For example, the 5-HT1A agonist, gepirone, which does not possess dopamine-related activity, does not produce buspirone- like changes in i.v. self-administration behaviour (Gold & Balster, 1992). Also, buspirone can surmountably antagonize the discriminative stimulus effects of direct and indirect dopamine agonists including, respectively, apomorphine and D-amphetamine, consistent with antagonist properties of buspirone at the D2-like family of dopamine receptors (Kamien & Woolverton, 1990; Nader & Woolverton, 1994). Thus, although dopamine×5-HT1A interactions cannot be excluded, it is unlikely that the effects of buspirone on i.v. cocaine self-administration behaviour are directly related to its actions at 5-HT1A receptors.
The relatively low affinity of buspirone (>450 nM) and its metabolites (>4 μM) at the D2 subtype of dopamine D2-like receptors suggests that the doses of buspirone used in this study do not meaningfully engage D2 receptors. However, two early reports also indicated that buspirone binds with high affinity at D3 and D4 receptors, comparable to its affinity at 5-HT1A receptors (Kula et al. 1994; Tallman et al. 1997). The present results documenting the high affinity of buspirone at D3 and D4 receptors are consistent with those reports. The affinities for buspirone at D3 and D4 receptors (98 and 29 nM) reported herein are within the range of values reported for buspirone at 5-HT1A receptors. However, these affinities also are somewhat lower and higher (3.5 and 78 nM, respectively) than values in earlier reports (Kula et al. 1994; Tallman et al. 1997). Such differences most likely result from the use of different radioligands across studies. Consistent with this hypothesis, Tallman et al. (1997) reported significant differences in ki values for a variety of compounds, including buspirone, at recombinant D4 receptors, depending on whether [3H]NGD 94-1 or [3H]YM-09151-2 was used as radioligand. In the Kula study, [3H]YM-09151-2 was used as a radioligand whereas the present studies employed [3H]methylspiperone at both D3 and D4 receptors. The ki values of buspirone at D2-like receptors determined by radioligand binding may not reflect the true affinity of buspirone for this family of receptors in vivo. However, as the ki values were estimated under the same conditions, the relative affinities of buspirone (~17 and ~5-fold higher at D4 and D3 compared to D2 receptors) are likely to reflect relative differences in vivo. In contrast, the potencies of buspirone obtained in functional assays are even less likely to reflect the in vivo situation because saturating concentrations of dopamine are used to optimize sensitivity. Nonetheless, buspirone exhibited the identical rank order of potency in the functional assay, D4>D3>D2, as observed in radioligand binding assays, strengthening the idea that this rank order accurately reflects the in vivo situation.
Buspirone is rapidly metabolized in humans (Gammans et al. 1986) and its pharmacological effects mediated by 5-HT1A receptors have been attributed, at least in part, to the metabolite 6-hydroxybuspirone (Wong et al. 2007). The concentrations of this metabolite are much higher than the parent compound after oral administration in humans, with maximal plasma concentrations after clinically relevant doses in excess of 100 nM (compared to ~6.5 nM for buspirone; Dockens et al. 2006, 2007) and a total plasma exposure ~40-fold higher than for buspirone. The affinity of this metabolite is similar to the parent compound at D4 receptors and ~8-fold lower than the parent at D3 receptors (Table 1). Thus, the high concentrations of 6-hydroxybuspirone formed in vivo suggest that pharmacologically relevant doses of buspirone yield levels of this metabolite that likely occupy D4 receptors and, perhaps to a lesser extent, D3 receptors. In contrast, the lower potency of 5-hydroxybuspirone (with plasma levels similar to the parent compound after oral dosing; Gammans et al. 1986) may well be out of the concentration range that meaningfully occupies D3 and D4 receptors at pharmacologically relevant doses. The other major metabolite of buspirone, 1-PP is inactive at dopamine receptors and likely did not play a role in the effects of buspirone in these studies. The present results are consistent with the view that the ability of buspirone to reduce cocaine self-administration behaviour in non-human primates may reflect the engagement of multiple neurochemical targets (D3, D4, 5-HT1A receptors) by both buspirone and selected metabolites.
The demonstration that buspirone and its 5-and 6-hydroxy metabolites bind to both D3 and D4 receptors (Table 1) and are antagonists at these receptors (Figs. 2 and 3) suggests mechanisms that may alternatively, or in conjunction, contribute to the reductions in cocaine self-administration in the present experiments. Thus, selective D3 antagonists such as SB-277011A are effective in decreasing discriminativestimulus and – under selected schedule conditions – reinforcing effects of cocaine that likely contribute to its self-administration and D3 antagonists are considered ‘high value targets’ in the search for medications to treat cocaine abuse (Heidbreder & Newman, 2010; Xi et al. 2005). While there has been less evidence for a role of D4 receptors in reinforcing effects of cocaine and, more generally, substance use disorders (Le Foll et al. 2009), polymorphisms in the D4 receptor have been linked to novelty seeking (Laucht et al. 2007; Lusher et al. 2001), a personality trait associated with an increased risk of substance abuse. Recent data provide additional support for the involvement of D4 mechanisms in addiction-related processes. For example, the D4 antagonist L-745,870 has been reported to attenuate reinstatement of nicotine-seeking behaviour in rats by either nicotine or nicotine-associated cues (Yan et al. 2012). Also, the D4 antagonist NGD 94-1 (Tallman et al. 1997) can markedly reduce the reinforcing effects of cocaine in monkeys at doses that do not substantively affect responding for food (J. Bergman, unpublished observations). Thus, based on the high affinity of buspirone and its 6-hydroxy metabolite at D4 receptors (Table 1), such D4 antagonist actions may make a prominent contribution to the reductions in i.v. cocaine self-administration observed in the present studies.
In summary, the present studies indicate that buspirone can produce marked and consistent reductions in i.v. cocaine self-administration behaviour without similar consistent effects on food-maintained behaviour and that such effects may involve actions of buspirone and metabolites at D3 and/or D4 subtypes of D2-like receptors. Buspirone has been clinically available for>25 yr and appears relatively free of toxicity or major side-effects (Jann, 1988). It produces neither the marked sedation associated with other anti-anxiety agents such as benzodiazepines or neuroleptic-like catalepsy (Jann et al. 1990). Notwithstanding the ability of buspirone to serve as a dopamine antagonist and its favourable safety profile, there has been only one trial so far examining its effects in a cocaine-abusing population (Moeller et al. 2001). While no significant effects were reported, both the small sample size (n<20) and lack of a compliance measure (Czobor & Skolnick, 2011) could well contribute to a type II error. Based upon the ability of buspirone to potently suppress i.v. cocaine self-administration in non-human primates, this compound should be further evaluated for the management of cocaine addiction.
Acknowledgments
The authors acknowledge support of the present work, in part, by contract DA8-8876 (N. K. Mello, P. I.) as well as the Intramural Program of NINDS/NIH. The authors also acknowledge the invaluable assistance toward completing this project that has been provided by Dr Jane Acri at NIDA/NIH.
Footnotes
Statement of Interest
None.
References
- Anderson AL, Reid MS, Li SH, Holmes T, et al. Modafinil for the treatment of cocaine dependence. Drug and Alcohol Dependence. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banala AK, Levy BA, Khatri SS, Furman CA, et al. N-(3-Fluoro-4-(4-(2-methoxy or 2,3-dichlorophenyl) piperazine-1-yl)butyl)arylcarboxamides as selective dopamine D3 receptor ligands: critical role of the carboxamide linker for D3 receptor selectivity. Journal of Medicinal Chemistry. 2011;54:581–594. doi: 10.1021/jm200288r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D1 and D2 antagonists. Behavioural Pharmacology. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after primozide pretreatment in normal, healthy volunteers. Biological Psychiatry. 1996;39:26–32. doi: 10.1016/0006-3223(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Brody D, Adler LA, Kim T, Angrist B, et al. Effects of buspirone in seven schizophrenic subjects. Journal of Clinical Psychopharmacology. 1990;10:68–69. doi: 10.1097/00004714-199002000-00025. [DOI] [PubMed] [Google Scholar]
- Bruno F. Buspirone in the treatment of alcoholic patients. Psychopathology. 1989;22(Suppl 1):49–59. doi: 10.1159/000284626. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Modulation of the discriminative stimulus properties of cocaine: comparison of the effects of fluoxetine with 5-HT1A and 5-HT1B receptor agonists. Neuropharmacology. 1997;36:373–381. doi: 10.1016/s0028-3908(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochemical Pharmacology. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Czobor P, Skolnick P. The secrets of a successful clinical trial: compliance, compliance, and compliance. Molecular Interventions. 2011;11:107–110. doi: 10.1124/mi.11.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockens RC, Salazar DE, Fulmor IE, Croop R. Pharmacokinetics of a newly identified metabolite of buspirone following administration of buspirone over its therapeutic dose range. Journal of Clinical Pharmacology. 2006;46:1308–1312. doi: 10.1177/0091270006292250. [DOI] [PubMed] [Google Scholar]
- Dockens RC, Tran AQ, Zeng J, Croop R. Pharmacokinetics of 6-hydroxybuspirone and its enantiomers administered individually or following buspirone administration in humans. Biopharmaceutics and Drug Disposition. 2007;28:393–402. doi: 10.1002/bdd.566. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Fudala PJ, Gorgon L, Li SH, et al. Double-blind, placebo-controlled trial of selegiline transdermal system (STS) for the treatment of cocaine dependence. Drug and Alcohol Dependence. 2006;85:191–197. doi: 10.1016/j.drugalcdep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Feldpausch DL, Needham LM, Stone MP, Althaus JS, et al. The role of dopamine D4 receptor in the induction of behavioral sensitization to amphetamine and accompanying biochemical and molecular adaptations. Journal of Pharmacology and Experimental Therapeutics. 1998;286:497–508. [PubMed] [Google Scholar]
- Gammans RE, Mayol RF, LaBudde JA. Metabolism and disposition of buspirone. American Journal of Medicine. 1986;80(Suppl 3B):41–51. doi: 10.1016/0002-9343(86)90331-1. [DOI] [PubMed] [Google Scholar]
- Goff DC, Midha KK, Brotman AW, McCormick S, et al. An open trial of buspirone added to neuroleptics in schizophrenic patients. Journal of Clinical Psychopharmacology. 1991;11:193–197. [PubMed] [Google Scholar]
- Gold LH, Balster RL. Effects of buspirone and gepirone on IV cocaine self-administration in rhesus monkeys. Psychopharmacology. 1992;108:289–294. doi: 10.1007/BF02245114. [DOI] [PubMed] [Google Scholar]
- Goldberg HL, Finnerty RJ. The comparative efficacy of buspirone and diazepam in the treatment of anxiety. American Journal of Psychiatry. 1979;136:1184–1187. doi: 10.1176/ajp.136.9.1184. [DOI] [PubMed] [Google Scholar]
- Gunne LM, Anggard E, Jonsson LE. Clinical trials with amphetamine-blocking drugs. Psychiatria Neurologia Neurochirurgia. 1972;75:225–226. [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effect of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration in humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Annals of New York Academy of Sciences – Addiction Reviews. 2010;2:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Jann MW. Buspirone: an update on a unique anxiolytic agent. Pharmacotherapy. 1988;8:100–116. doi: 10.1002/j.1875-9114.1988.tb03543.x. [DOI] [PubMed] [Google Scholar]
- Jann MW, Froemming JH, Borison RL. Movement disorders and new azapirone anxiolytic drugs. Journal of the American Board of Family Practice. 1990;3:111–119. [PubMed] [Google Scholar]
- Kahn R, Biswas K, Childress AR, Shoptaw S, et al. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug and Alcohol Dependence. 2009;103:59–64. doi: 10.1016/j.drugalcdep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamien JB, Woolverton WL. Buspirone blocks the discriminative stimulus effects of apomorphine in monkeys. Pharmacology Biochemistry and Behavior. 1990;35:117–120. doi: 10.1016/0091-3057(90)90214-3. [DOI] [PubMed] [Google Scholar]
- Kotler M, Cohen H, Segman R, Gritsenko I, et al. Excess dopamine D4 receptor (D4DR) exon III seven repeat allele in opioid-dependent subjects. Molecular Psychiatry. 1997;2:251–254. doi: 10.1038/sj.mp.4000248. [DOI] [PubMed] [Google Scholar]
- Kula NS, Baldessarini RJ, Kebabian JW, Neumeyer JL. S-(+)-aporphines are not selective for human D3 dopamine receptors. Cell and Molecular Neurobiology. 1994;14:185–191. doi: 10.1007/BF02090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucht M, Becker K, Blomeyer D, Schmidt MH. Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: results from a high-risk community sample. Biological Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Schwartz JC, Sokoloff P. Dopamine D3 receptor agents as potential new medications for drug addiction. European Psychiatry. 2000;15:140–146. doi: 10.1016/s0924-9338(00)00219-4. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, et al. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behavioural Pharmacology. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Lusher JM, Chandler C, Ball D. Dopamine D4 receptor gene (DRD4) is associated with novelty seeking (NS) and substance abuse: the saga continues. Molecular Psychiatry. 2001;6:497–499. doi: 10.1038/sj.mp.4000918. [DOI] [PubMed] [Google Scholar]
- Malec TS, Malec EA, Dongier M. Efficacy of buspirone in alcohol dependence: a review. Alcoholism: Clinical and Experimental Research. 1996;20:853–858. doi: 10.1111/j.1530-0277.1996.tb05263.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, et al. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Progress in Neurobiology. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Montoya ID, Vocci F. Novel medications to treat addictive disorders. Current Opinions in Psychiatry. 2009;22:263–268. doi: 10.1007/s11920-008-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Blockade of the discriminative stimulus effects of d-amphetamine in rhesus monkeys with serotonin 5-HT(1A) agonists. Behavioural Pharmacology. 1994;5:591–598. doi: 10.1097/00008877-199410000-00004. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Urizar E, Javitch JA, et al. G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. Journal of Biological Chemistry. 2009;284:34103–34115. doi: 10.1074/jbc.M109.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology. 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug and Alcohol Dependence. 2003;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- NIMH. [Accessed 31 May 2012];Psychoactive Drug Screening Program. undated. ( http://pdsp.med.unc.edu/indexR.html)
- Riblet LA, Taylor DP, Eison MS, Stanton HC. Pharmacology and neurochemistry of buspirone. Journal of Clinical Psychiatry. 1982;43(12):11–18. [PubMed] [Google Scholar]
- Rickels K, Weisman K, Norstad N, Singer M, et al. Buspirone and diazepam in anxiety: a controlled study. Journal of Clinical Psychiatry. 1982;43:81–86. [PubMed] [Google Scholar]
- Schneider NG, Olmstead RE, Steinberg C, Sloan K, et al. Efficacy of buspirone in smoking cessation: a placebo-controlled trial. Clinical Pharmacology and Therapeutics. 1996;60:568–575. doi: 10.1016/S0009-9236(96)90153-8. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends in Pharmacological Sciences. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer MA, Kumor KM, Jaffe JH. Effects of intravenous cocaine are partially attenuated by haloperidol. Psychiatry Research. 1989;27:117–125. doi: 10.1016/0165-1781(89)90127-3. [DOI] [PubMed] [Google Scholar]
- Sirota P, Epstein B, Benatov R, Sousnostzky M, et al. An open study of buspirone augmentation of neuroleptics in patients with schizophrenia. Journal of Clinical Psychopharmacology. 2001;21:454–455. doi: 10.1097/00004714-200108000-00015. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Park S, Jayathilake K, Roy A, et al. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophrenia Research. 2007;95:158–168. doi: 10.1016/j.schres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Tallman JF, Primus RJ, Brodbeck R, Cornfield L, et al. I. NGD 94–1: identification of a novel, high-affinity antagonist at the human dopamine D4 receptor. Journal of Pharmacology and Experimental Therapeutics. 1997;282:1011–1019. [PubMed] [Google Scholar]
- Temple DL, Jr, Yevich JP, New JS. Buspirone: chemical profile of a new class of anxioselective agents. Journal of Clinical Psychiatry. 1982;43(12):4–10. [PubMed] [Google Scholar]
- Tompkins EC, Clemento AJ, Taylor DP, Perhach JL. Inhibition of aggressive behavior in rhesus monkeys by buspirone. Research Communications in Psychology, Psychiatry and Behavior. 1980;5:337–352. [Google Scholar]
- Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug and Alcohol Dependence. 2002;68:23–33. doi: 10.1016/s0376-8716(02)00104-7. [DOI] [PubMed] [Google Scholar]
- Wong H, Dockens RC, Pajor L, Yeola S, et al. 6-hydroxybuspirone is a major active metabolite of buspirone: assessment of pharmacokinetics and 5-hydroxytryptamine1A receptor occupancy in rats. Drug Metabolism and Disposition. 2007;35:1387–1392. doi: 10.1124/dmd.107.015768. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, et al. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. European Journal of Neuroscience. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, et al. Blockade of dopamine D4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology. 2012;37:685–696. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Jones A, Olsen KR, Peng K, et al. A homogeneous enzyme fragment complementationbased beta-arrestin translocation assay for high-throughput screening of G-protein-coupled receptors. Journal of Biomolecular Screening. 2008;13:737–747. doi: 10.1177/1087057108321531. [DOI] [PubMed] [Google Scholar]