Abstract
Background
This study aimed to analyze the relationship of UGT2B7 and UGT1A4 polymorphisms with metabolism of valproic acid (VPA) and lamotrigine (LTG) in epileptic children.
Material/Methods
We administered VPA (102) and LTG (102) to 204 children with epilepsy. Blood samples were collected before the morning dose. Serum concentration of LTG was measured by high-performance liquid chromatography (HPLC). Serum VPA concentration was tested by fluorescence polarization immunoassay. UGT2B7 A268G, C802T, and G211T polymorphisms, as well as UGT1A4 L48V polymorphism, were assayed by direct automated DNA sequencing after PCR. Evaluation of efficacy was conducted using the Engel method.
Results
The adjusted serum concentration of VPA was 4.26 μg/mL per mg/kg and LTG was 1.56 μg/mL per mg/kg. Multiple linear regression analysis revealed that VPA or LTG adjusted concentration showed a good linear relation with sex and age. UGT2B7 A268G and C802T polymorphisms were demonstrated to affect the serum concentration of VPA (F=3.147, P=0.047; F=22.754, P=0.000). UGT1A4 L48V polymorphism was not related with the serum concentration of LTG (F=5.328, P=0.006). In the efficacy analysis, we found that C802T polymorphism exerted strong effects on efficacy of VPA (χ2=9.265, P=0.010). L48V polymorphism also showed effects on efficacy of LTG (χ2=17.397, P=0.001).
Conclusions
UGT2B7, UGT1A4 polymorphisms play crucial roles in metabolism of VPA and LTG.
MeSH Keywords: Epilepsy, Absence; Polymorphism, Genetic; Valproic Acid
Background
Epilepsy is a chronic neurological disease manifesting as frequent uncontrolled seizures [1]. According to the statistics, 40–70 persons per 100 000 develop epilepsy every year [2]. In addition to the uncontrolled seizures and complex treatments, people with epilepsy also have high risk of comorbidities (emphysema, heart disease, or cancer) [3], mental problems (psychological disorder or insomnia) [4] and poor quality of life [5,6]. Antiepileptic drugs containing valproic acid (VPA) and lamotrigine (LTG) have been widely used clinically. The goal of these antiepileptic drugs is to eliminate seizures and relieve the adverse effects caused by treatments [7].
VPA is a broad-spectrum antiepileptic drug that is used as first-line therapy for intractable epilepsy and minor epilepsy. However, it shows obvious individual variability in pharmacokinetics and pharmacodynamics, which means the serum concentration of VPA varies in the individual patient, even with the same doses of VPA. Therefore, the serum concentration of VPA should be monitored for optimizing dose usage during the course of therapy [8]. The differences in serum concentration may reflect functional consequences of genetic factors, miscellaneous conditions, and personal behavior [9–11]. Lamotrigine (LTG) is another antiepileptic drug for partial seizures and generalized seizures. Similarly, LTG also shows inter-individual variability in its pharmacokinetics and pharmacodynamics [12].
Until now, the researchers mainly investigated the possible roles of genetic polymorphisms in the pharmacokinetics and pharmacodynamics of antiepileptic drugs. Munisamy et al. reported that UGT1A6 552 A>C polymorphism played a significant role in the steady-state concentration of VPA [13]. In another study, Inoue et al. found that UGT2B7 -161C>T affects VPA concentration in pediatric epilepsy patients [14]. Chang et al. observed higher blood LTG concentration and better therapeutic efficacy in patients with UGT1A4 142TT polymorphism [15]. Singkham et al. investigated the influence of genetic and non-genetic factors in pharmacokinetics of LTG and concluded that both factors affected LTG pharmacokinetics; therefore, these factors should be considered when determining LTG dosing [16].
Our study enrolled 204 Chinese children with epilepsy from Qingzhou district and explored the relationship of UGT2B7 polymorphisms (A268G, C802T, and G211T) with metabolism of VPA and LTG. We also analyzed the association of UGT2B7 polymorphisms with efficacy of the drugs.
Material and Methods
Patients and blood sampling
The study enrolled 204 epileptic children at Weifang Yidu Central Hospital. All the patients were diagnosed as having epilepsy based on their seizure history as well as bio-chemical laboratory and electroencephalogram tests. They showed no abnormal hepatic and renal function. The study was approved by the Ethics Committee of the hospital and written consent was acquired from the parents/guardians of all participating patients.
Patients were randomly divided into 2 groups treated with lamotrigine (LTG) or valproic acid (VPA). Patients in one group were treated with VPA (Deparkin; SanofiSynthelabo Minsheng Pharmaceutical, Hangzhou, China) (250–1000 mg/kg) while patients in the other group were treated with LTG (≥50mg/d). The dosing regimen was continued for at least 2 weeks (>5 half-lives) to maintain a steady-state condition with respect to drugs pharmacokinetics.
We collected 5-mL blood samples before the morning dose. These blood samples were separated into 2 tubes; one tube was centrifuged immediately to get plasma and then stored at −70°C until drug analysis, while the other tube was stored at −20°C for DNA isolation.
Plasma determination of lamotrigine and valproic acid
Serum concentrations of LTG were determined by high-performance liquid chromatography (HPLC). The blood sample was centrifuged for 5 min (3500 rpm), then 200-μl serum samples were mixed with 50 μl of 80 μg/mL chlorzoxazone and 50 μl methanol. We added 3.0 mL of diethyl ether after brief vortexing. Subsequently, the mixture was vortexed for 20 min and centrifuged at 3500 rpm for 8 min. The organic layer was transferred and put into a new glass tube, then evaporated to dryness under a stream of air at 40°C. The residue was reconstituted with a 200-μl mixture of methanol and water (3: 2, v/v), then centrifuged at 10 800 rpm for 5 min. Afterwards, 10 μl of aliquot was injected into the HPLC system with an ultraviolet (UV) detector (Agilent 1100 system; Agilent Technologies, Inc., Santa Clara, CA, USA). The analytic column was a Capcell Pak C18 column (4.6×250mm, 5 μm, Shiseido Co., Ltd., Japan). The column temperature was 25°C. The detection wavelength was 220 nm for measuring the peak areas. The mobile phase for separation consisted of acetonitrile and 0.05 mol/L NaH2PO4 (v/v, 26.5: 73.5, pH=4.5) at a flow rate of 1.0mL/min. Intraday and relative standard deviations were lower than 15% and the lower limit of quantification was 0.5 μg/mL. Due to the differences in oral doses taken by the patients, plasma concentrations of LTG were adjusted by dose and body weight of each subject.
Serum VPA concentration were measured by fluorescence polarization immunoassay using the Abbott TDx system. The test had a coefficient of variation <10% and the lower limit quantification (LOQ) was 0.7 μg/ml. Similarly, plasma concentration of VPA was adjusted by dose and body weight.
Genetic analysis of UGT2B7
Genomic DNA was extracted from peripheral blood with phenol/chloroform method. All DNA samples were stored at −70°C for detection of A268G, C802T, and G211T polymorphisms.
UGT2B7 A268G, C802T, and G211T polymorphisms were determined by direct automated DNA sequencing after PCR. The PCR primers of A268G were: forward primer (5′-TCCAACTGATTGTTATGGTAGAT-3′); reverse primer (5′-GCTGTTCCTTTCTGTCATTTCTC-3′). Primers of C802T were: forward primer (5′-TCCAACTGATTGTTATGGTAGAT-3′); reverse primer (5′-GCTGTTCCTTTCTGTCATTTCTC-3′). For G211T, the primers were: forward primer (5′-TGCTTTAGCTCTGGGAATTGT-3′); reverse primer (5′-TGCATGATGAAATTVTCCAAC-3′). The primers of UGT1A4 L48V polymorphism were: forward primer (5′-GGTGGCTCAATGACAA-3′); reverse primer (5′-TCAAAGGAAGTAGCATTCAGGT-3′). All the PCR products were analyzed by gene sequencing (ABI3730, Applied Biosystems).
Efficacy analysis
A 1-year follow-up was conducted to test the efficacy of VPA or LTG by monitoring the frequency of epileptic seizures. The evaluation of efficacy was according to the Engel method [15]. The mean monthly seizure frequency after VPA or LTG treatment was compared to that at 3 months before treatment. Many patients for whom VPA or LTG treatment was effective and brought about improved symptoms were regarded as good efficacy. The sum of patients for whom the treatment was ineffective and caused deteriorated symptoms was defined as poor efficacy. The effectiveness rate was computed as the number of patients with good efficacy normalized against the total number of patients.
Statistics
Hardy-Weinberg equilibrium (HWE) analysis was performed on UGT2B7 and UGT1A4 polymorphisms. Data are expressed as mean ± standard deviation (SD). Multiple linear regressions were performed to determine the relation of serum concentration of LTG or VPA with factors of sex and age. ANOVA was used to investigate the association between genotypes distribution and serum concentration of LTG or VPA. The χ2 test was used to analyze the differences in serum concentration as well as efficacy of VPA or LTG among the genotypes. A p value <0.05 was regarded as statistically significant. All the statistical analysis was completed with SPSS 18.0 software. The figure was made using GraphPad 5.0 software.
Results
Basic information of epileptic children
The children were randomly divided into 2 groups: LTG and VPA. The average age of children in the LTG group was 12.33 years, while it was 11.62 years in the VPA group. The daily dose of LTG was 2.74 mg/kg and the daily dose of VPA was 17.62 mg/kg. The steady-state serum concentration of LTG was 6.10 μg/mL. After adjustment by dose and body weight, it was changed to 2.58 μg/mL per mg/kg. The steady-state serum concentration of VPA was 66.62 μg/mL and the adjusted concentration was 4.26 μg/mL per mg/kg. Detailed information is listed in Table 1.
Table 1.
Basic information of epileptic children.
| Demographic data | VPA | LTG |
|---|---|---|
| No. of patients | 102 | 102 |
| Gender (F/M) | 68/34 | 45/57 |
| Age(years) | 11.62±7.69 | 12.33±6.13 |
| BMI | 19.85±7.18 | 18.79±7.33 |
| Daily LTG dose (mg/kg) | – | 2.74±1.17 |
| Daily VPA dose (mg/kg) | 17.62±6.43 | – |
| LTG concentration (μg/mL) | – | 6.10±2.29 |
| Adjusted LTG concentration (μg/mL per mg/kg) | – | 2.58±0.87 |
| VPA concentration (μg/mL) | 66.62±10.00 | – |
| Adjusted VPA concentration (μg/mL per mg/kg) | 4.26±1.54 | – |
Multiple linear regression analysis
Multiple linear regression was performed to elucidate the relationship of adjusted concentration of LTG and VPA with factors of sex and age. Statistical analysis revealed that LTG or VPA concentration (Y) showed a good linear relation with sex (X1) and age (X2). The results ae exhibited in Table 2.
Table 2.
Results of multiple linear regression analysis.
| Variables | Regression coefficient | t | P |
|---|---|---|---|
| VPA | |||
| Gender | 0.225 | 2.518 | 0.013 |
| Age | −0.396 | −4.432 | 0.000 |
| LTG | |||
| Age | 0.140 | 73.253 | 0.000 |
| Gender | 0.043 | 1.819 | 0.072 |
Influence of UGT2B7 and UGT1A4 polymorphisms on serum concentration of VPA and LTG
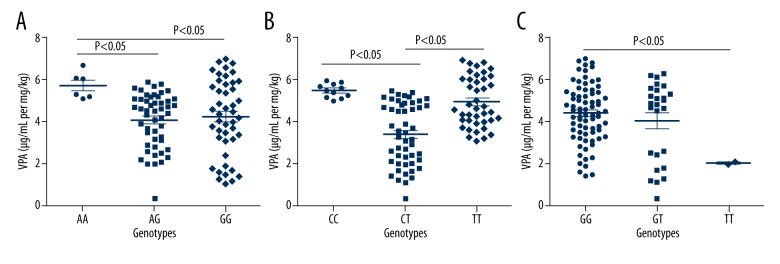
As shown in Table 3 and Figure 1, serum concentration of VPA of each child was assayed. The children with AA genotype of A268G polymorphism had higher serum concentration of VPA compared to the other 2 genotypes (P<0.05 for both). In terms of C802T polymorphism, CC genotype carriers showed the highest serum content of VPA among the 3 genotypes, while CT genotype carriers showed the lowest serum content of VPA. In the analysis of G211T polymorphism, the children with GG genotype showed higher content of VPA than that of TT genotype (P<0.05). As for LTG, we found significant relation of UGT1A4 polymorphism with its serum concentration (F=5.328, P=0.006) (Table 4, Figure 2).
Table 3.
Influence of UGT2B7 polymorphisms on serum concentration of VPA.
| Genotype | No. | Adjusted VPA concentration (μg/mL per mg/kg) | F | P |
|---|---|---|---|---|
| A268G | 3.147 | 0.047 | ||
| AA | 6 | 5.75±0.64 | ||
| AG | 50 | 4.09±1.28 | ||
| GG | 46 | 4.25±1.78 | ||
| C802T | 22.754 | 0.000 | ||
| CC | 11 | 5.50±0.33 | ||
| CT | 50 | 3.40±1.47 | ||
| TT | 41 | 4.98±1.19 | ||
| G211T | 2.671 | 0.074 | ||
| GG | 74 | 4.39±1.38 | ||
| GT | 26 | 4.05±1.88 | ||
| TT | 2 | 2.05±0.07 |
Figure 1.
(A–C) UGT2B7 polymorphisms affected serum concentration of VPA.
Table 4.
Influence of UGT1A4 polymorphism on serum concentration of LTG.
| Genotype | No. | Adjusted LTG concentration (μg/mL per mg/kg) | F | P |
|---|---|---|---|---|
| L48V (142T>G) | 5.328 | 0.006 | ||
| TT | 74 | 2.77±0.87 | ||
| TG | 26 | 2.18±0.74 | ||
| GG | 2 | 1.95±0.49 |
Figure 2.
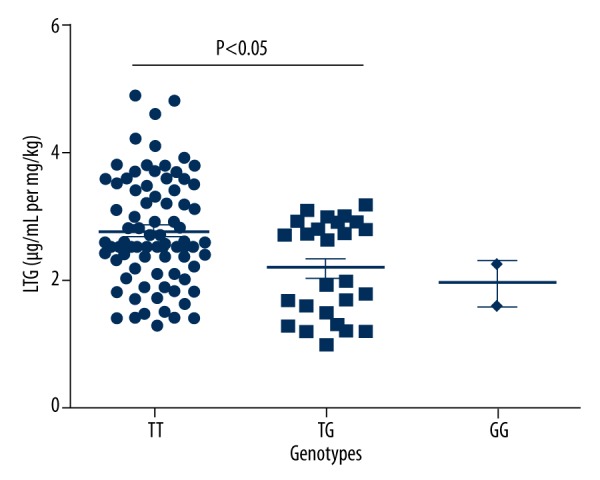
UGT1A4 polymorphism affected serum concentration of LTG.
Influence of UGT2B7 and UGT1A4 polymorphisms on efficacy of VPA and LTG
The children were divided into 3 subgroups according to genotypes of C802T polymorphism: CC, CT, and TT subgroup. As shown in Table 5, in Group CC 9 children had good efficacy and 2 children had poor efficacy, resulting in an effectiveness rate of 81.8%. In Group CT, 23 children showed good efficacy and 27 children showed poor efficacy, resulting in an effectiveness rate of 46.0%. In Group TT, 30 children had good efficacy and 11 children had poor efficacy, resulting in an effectiveness rate of 73.2%. Similarly, UGT1A4 L48V exerted strong effects on efficacy of LTG (χ2=17.397, P=0.001) (Table 6).
Table 5.
UGT2B7 polymorphisms affect efficacy of VPA.
| Genotype | Good efficacy, n (%) | Poor efficacy, n (%) | χ2 | P |
|---|---|---|---|---|
| A268G | 1.759 | 0.415 | ||
| AA | 4 | 2 | ||
| AG | 24 | 26 | ||
| GG | 18 | 27 | ||
| C802T | 9.265 | 0.010 | ||
| CC | 9 | 2 | ||
| CT | 23 | 27 | ||
| TT | 30 | 11 | ||
| G211T | 0.565 | 0.754 | ||
| GG | 48 | 26 | ||
| GT | 15 | 11 | ||
| TT | 1 | 1 |
Table 6.
UGT1A4 polymorphism affect efficacy of LTG.
| Genotype | Good efficacy, n (%) | Poor efficacy, n (%) | χ2 | P |
|---|---|---|---|---|
| L48V (142T>G) | 17.397 | 0.001 | ||
| TT 74 | 56 | 18 | ||
| TG 26 | 9 | 17 | ||
| GG 2 | 1 | 1 |
Discussion
It is commonly thought that cytochrome P450 (CYP) enzymes, mitochondrion-mediated β-oxidation, and glucuronosyltransferases (UGTs) are the 3 main metabolic routes of VPA [17]. Glucuronide metabolites account for 50% of the VPA dose [18,19]; therefore, it plays an important role in metabolism of VPA. UGT1A3, UGT1A6, and UGT2B7 are all UGT isozymes that exhibit pivotal roles in the production of VPA glucuronides [20,21]. The UGT2B7 gene is approximately 16 kb and contains 6 exons. This gene shows multiple polymorphisms in the proximal promoter region [22,23]. Unlike other isoforms in the 2B family, UGT2B7 promotes the glucuronidation of carboxyl, hydroxyls, amino, and methoxyl [24]. Existing evidence suggests that UGT2B7 isoforms have high activity in VPA glucuronidation. Nevertheless, UGT2B7*2 polymorphism was observed to have little effect on the metabolism of VPA in healthy individuals [24]. Ma et al. reported that UGT2B7 A268G polymorphism was involved in the metabolism of VPA and affects serum concentration of VPA [25]. Xiang et al. analyzed C802T and G211T polymorphisms in epilepsy patients and concluded that C802T is an important determinant of individual variability in the pharmacokinetics of VPA, while G211T had no influence on the serum concentration of VPA [26].
In our study we investigated the influences of UGT2B7 polymorphisms (A268G, C802T, and G211T) on the serum concentration of VPA in epileptic children in Qingzhou district. The results show that A268G and C802T polymorphisms exert influences on the metabolism of VPA, which is consistent with previous research [25,26]. Further analysis on efficacy indicated that C802T polymorphism could affect the efficacy of VPA.
In terms of LTG, the main metabolic pathway is N-glucuronidation catalyzed by UGT1A4 [27]. It has been reported that there are many polymorphisms in the 5′-upstream regions and coding regions of the UGT1A4 gene; 70C>A and 142T>G are the most common SNPs. Corresponding catalytic activities of these SNPs are changed in a substrate-dependent manner [28–30]. A previous report in China suggested that patients with UGT1A4 142TT genotype had a higher serum concentration of LTG [15], while 142T>G was found to decrease the serum concentration of LTG in another report [31]. Our results indicate that TT genotype of 142T>G (L48V) is related to high serum content of LTG. Moreover, L48V polymorphism showed effects on efficacy of LTG, which is also consistent with the results of Chang et al. [15].
Our results investigated the influences of related genetic polymorphisms on the pharmacokinetics and pharmacodynamics of VPA and LTG. These findings contribute to improving treatments for use in epileptic children. However, the results cannot fully elaborate the regulatory mechanism. There are interactions between genes that may play certain roles in the metabolism of these drugs. Moreover, the differences in constitution, diet, and behavior of patients could affect the metabolism and efficacy of drugs. Further analysis is needed on this topic.
Conclusions
In conclusion, UGT2B7 and UGT1A4 polymorphisms are important determinants of metabolism of VPA and LTG.
Footnotes
Source of support: Departmental sources
References
- 1.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 2.Kotsopoulos IA, van Merode T, Kessels FG, et al. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia. 2002;43(11):1402–9. doi: 10.1046/j.1528-1157.2002.t01-1-26901.x. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Comorbidity in adults with epilepsy – United States, 2010. Morb Mortal Wkly Rep. 2013;62(43):849–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Tellez-Zenteno JF, Patten SB, Jetté N, et al. Psychiatric comorbidity in epilepsy: A population-based analysis. Epilepsia. 2007;48(12):2336–44. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 5.Kobau R, Zahran H, Thurman DJ, et al. Epilepsy surveillance among adults – 19 States, Behavioral Risk Factor Surveillance System, 2005. Surveill Summ. 2008;57(6):1–20. [PubMed] [Google Scholar]
- 6.Kobau R, Luncheon C, Zack MM, et al. Satisfaction with life domains in people with epilepsy. Epilepsy Behav. 2012;25(4):546–51. doi: 10.1016/j.yebeh.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Louis EK. Minimizing AED adverse effects: Improving quality of life in the interictal state in epilepsy care. Curr Neuropharmacol. 2009;7(2):106–14. doi: 10.2174/157015909788848857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chadwick DW. Concentration-effect relationships of valproic acid. Clin Pharmacokinet. 1985;10(2):155–63. doi: 10.2165/00003088-198510020-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hung CC, Ho JL, Chang WL, et al. Association of genetic variants in six candidate genes with valproic acid therapy optimization. Pharmacogenomics. 2011;12(8):1107–17. doi: 10.2217/pgs.11.64. [DOI] [PubMed] [Google Scholar]
- 10.Downing C, Biers J, Larson C, et al. Genetic and maternal effects on valproic acid teratogenesis in C57BL/6J and DBA/2J mice. Toxicol Sci. 2010;116(2):632–39. doi: 10.1093/toxsci/kfq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franciotta D, Kwan P, Perucca E. Genetic basis for idiosyncratic reactions to antiepileptic drugs. Curr Opin Neurol. 2009;22(2):144–49. doi: 10.1097/WCO.0b013e328328f276. [DOI] [PubMed] [Google Scholar]
- 12.Johannessen SI, Tomson T. Pharmacokinetic variability of newer antiepileptic drugs: When is monitoring needed? Clin Pharmacokinet. 2006;45(11):1061–75. doi: 10.2165/00003088-200645110-00002. [DOI] [PubMed] [Google Scholar]
- 13.Munisamy M, Tripathi M, Behari M, et al. The effect of uridine diphosphate glucuronosyltransferase (UGT)1A6 genetic polymorphism on valproic acid pharmacokinetics in Indian patients with epilepsy: a pharmacogenetic approach. Mol Diagn Ther. 2013;17(5):319–26. doi: 10.1007/s40291-013-0041-8. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K, Suzuki E, Yazawa R, et al. Influence of uridine diphosphate glucuronosyltransferase 2B7-161C>T polymorphism on the concentration of valproic acid in pediatric epilepsy patients. Ther Drug Monit. 2014;36(3):406–9. doi: 10.1097/FTD.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Yang LY, Zhang MC, Liu SY, et al. Correlation of the UGT1A4 gene polymorphism with serum concentration and therapeutic efficacy of lamotrigine in Han Chinese of Northern China. Eur J Clin Pharmacol. 2014;70(8):941–46. doi: 10.1007/s00228-014-1690-1. [DOI] [PubMed] [Google Scholar]
- 16.Singkham N, Towanabut S, Lertkachatarn S, et al. Influence of the UGT2B7-161C>T polymorphism on the population pharmacokinetics of lamotrigine in Thai patients. Eur J Clin Pharmacol. 2013;69(6):1285–91. doi: 10.1007/s00228-012-1449-5. [DOI] [PubMed] [Google Scholar]
- 17.Reith DM, Andrews J, Parker-Scott S, Eadie MJ, et al. Urinary excretion of valproate metabolites in children and adolescents. Biopharm Drug Dispos. 2000;21(8):327–30. doi: 10.1002/bdd.247. [DOI] [PubMed] [Google Scholar]
- 18.Dickinson RG, Hooper WD, Dunstan PR, Eadie MJ, et al. Urinary excretion of valproate and some metabolites in chronically treated patients. Ther Drug Monit. 1989;11(2):127–33. doi: 10.1097/00007691-198903000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Argikar UA, Remmel RP. Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab Dispos. 2009;37(1):229–36. doi: 10.1124/dmd.108.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green MD, King CD, Mojarrabi B, et al. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab Dispos. 1998;26(6):507–12. [PubMed] [Google Scholar]
- 21.Krishnaswamy S, Hao Q, Al-Rohaimi A, et al. UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: II. Functional impact of the three most common nonsynonymous UGT1A6 polymorphisms (S7A, T181A, and R184S) J Pharmacol Exp Ther. 2005;313(3):1340–46. doi: 10.1124/jpet.104.081968. [DOI] [PubMed] [Google Scholar]
- 22.Hirota T, Ieiri I, Takane H, et al. Sequence variability and candidate gene analysis in two cancer patients with complex clinical outcomes during morphine therapy. Drug Metab Dispos. 2003;31(5):677–80. doi: 10.1124/dmd.31.5.677. [DOI] [PubMed] [Google Scholar]
- 23.Bhasker CR, McKinnon W, Stone A, et al. Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: Ethnic diversity of alleles and potential clinical significance. Pharmacogenetics. 2000;10(8):679–85. doi: 10.1097/00008571-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Chung JY, Cho JY, Yu KS, et al. Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clin Pharmacol Ther. 2008;83(4):595–600. doi: 10.1038/sj.clpt.6100324. [DOI] [PubMed] [Google Scholar]
- 25.Ma H, Zhang T, Gong Z, et al. Effect of UGT2B7 genetic variants on serum valproic acid concentration. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38(8):766–72. doi: 10.3969/j.issn.1672-7347.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Sun YX, Zhuo WY, Lin H, et al. The influence of UGT2B7 genotype on valproic acid pharmacokinetics in Chinese epilepsy patients. Epilepsy Res. 2015;114:78–80. doi: 10.1016/j.eplepsyres.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Rowland A, Elliot DJ, Williams JA, et al. In vitro characterization of lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metab Dispos. 2006;34(6):1055–62. doi: 10.1124/dmd.106.009340. [DOI] [PubMed] [Google Scholar]
- 28.Ehmer U, Vogel A, Schütte JK, et al. Variation of hepatic glucuronidation: Novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology. 2004;39(4):970–77. doi: 10.1002/hep.20131. [DOI] [PubMed] [Google Scholar]
- 29.Benoit-Biancamano MO, Adam JP, Bernard O, et al. A pharmacogenetics study of the human glucuronosyltransferase UGT1A4. Pharmacogenet Genomics. 2009;19(12):945–54. doi: 10.1097/FPC.0b013e3283331637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiener D, Fang JL, Dossett N, Lazarus P. Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer Res. 2004;64(3):1190–96. doi: 10.1158/0008-5472.can-03-3219. [DOI] [PubMed] [Google Scholar]
- 31.Gulcebi MI, Ozkaynakcı A, Goren MZ, et al. The relationship between UGT1A4 polymorphism and serum concentration of lamotrigine in patients with epilepsy. Epilepsy Res. 2011;95(1–2):1–8. doi: 10.1016/j.eplepsyres.2011.01.016. [DOI] [PubMed] [Google Scholar]