Abstract
Background
Irisin, an exercise-induced myokine, is closely correlated with obesity and body mass index. Obesity is one risk factor of coronary artery disease (CAD). Therefore, the present study aimed to determine if serum irisin concentrations are correlated with the presence and severity of CAD.
Material/Methods
Serum irisin concentrations were determined in 350 patients with CAD and in 214 healthy subjects. The severity of CAD was assessed by coronary atherosclerosis index (CAI).
Results
Serum irisin concentrations were significantly lower in CAD patients compared with healthy controls. Multivariable logistic regression analysis revealed that serum irisin concentrations were an independent determinant of the presence of CAD. In addition, Pearson correlation analysis showed that serum irisin concentrations were negatively correlated with CAI in CAD patients.
Conclusions
Decreased serum irisin concentrations may be associated with the presence and severity of CAD.
MeSH Keywords: Coronary Artery Disease; Receptors, Cytokine; Severity of Illness Index
Background
Coronary artery disease (CAD) is a major cause of morbidity and mortality worldwide. Diabetes, dyslipidemia, hypertension, family history, obesity, and smoking are thought to increase the risk of developing CAD [1]. In addition, genetic factors contribute to the susceptibility of developing CAD [2]. Recent studies have demonstrated various candidate genes and epigenetics in the pathogenesis of CAD [3]. However, the exact mechanism of CAD has not been fully elucidated.
Irisin, a cleaved and secreted fragment of fibronectin type III domain containing 5 (FNDC5), contributes to energy consumption in mice [4]. Irisin reversed diet-induced obesity and diabetes by inducing the browning of subcutaneous white adipocytes and up-regulating the expression of thermogenesis-related genes [5]. In humans, serum irisin concentrations are negatively correlated with body mass index (BMI) [6]. Serum irisin concentrations were significantly reduced in obese subjects compared with lean ones [7]. Obesity is a potential risk factor of developing CAD. Therefore, irisin is speculated to play a role in the pathogenesis of CAD.
We performed this cross-sectional investigation in a population of CAD patients to determine the relation of serum irisin to the development and severity of CAD.
Material and Methods
Subjects
This cross-sectional study was performed in a population of 350 patients with angiographically proven CAD and 214 healthy subjects. The case and control groups were statistically matched for age, sex, and BMI. Subjects who had angiographic evidence of stenosis ≥50% in at least 1 major coronary artery were considered as CAD patients. Patients were excluded if they had acute coronary syndromes, history of coronary artery bypass surgery, heart failure, valvular heart disease, malignant tumor, or systemic disease. Control subjects were recruited from individuals attending routine checkups including CT angiography in our hospital. All control subjects were free of personal or family history of cardiovascular or hemorrhagic disease. The study protocol was approved by the Human Ethics Review Committee of our hospital and a signed consent form was obtained from each subject.
We used the coronary atherosclerosis index (CAI) to assess the severity of CAD. The sum of the following scores was graded as CAI: no significant stenosis, defined as a score of 0; 1–24% narrowing, defined as a score of 1; 25–49% narrowing, defined as 2; 50–74% narrowing, defined as 3; and 75–100% narrowing, defined as 4 [8].
Laboratory methods
Serum was obtained from all subjects in a fasting state. An enzyme-linked immunosorbent assay kit (Phoenix Pharmaceuticals, Inc, USA) [coefficient of variations (CVs) for intra assay: 5–7%; CVs for inter-assay: 12–15%; detection limit range: 0.1–1000 ng/mL] was used to evaluate serum irisin concentrations.
Statistical analysis
The data are exhibited as means ± standard errors (interquartile range). We used unpaired t test, chi-square tests, or Mann-Whitney U test to determine the differences in parameters between case and control groups. Univariate analysis was performed and the variables with a P<0.10 were then entered into a backward stepwise multivariate logistic regression model to examine the risk factors of CAD. The correlation of serum irisin concentrations with CAI was analyzed by Pearson correlation analysis. P value <0.05 was considered to be a statistically significant difference.
Results
Baseline clinical characteristics
CAD patients showed higher levels of triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and smoking, and decreased high-density lipoprotein cholesterol (HDL-C), compared with the controls.
Serum irisin concentrations in CAD patients
The case group had lower serum irisin concentrations than in the control group (P<0.001) (Table 1). Both simple and multivariate logistic regression analysis indicated that serum irisin concentrations were negatively associated with the presence of CAD (Table 2).
Table 1.
Clinical and biochemical characteristics of the case and control groups.
| The controls | CAD patients | P value | |
|---|---|---|---|
| N | 214 | 350 | |
| Age (years) | 59.29±10.41 | 60.33±9.95 | 0.239 |
| Gender (M/F) | 118/96 | 190/160 | 0.843 |
| BMI (Kg/m2) | 24.43±2.65 | 24.82±3.17 | 0.129 |
| SBP (mmHg) | 138.44±26.20 | 141.10±16.95 | 0.145 |
| DBP (mmHg) | 82.04±12.73 | 83.19±10.06 | 0.238 |
| TC (mmol/L) | 5.09±0.87 | 5.18±1.11 | 0.301 |
| TG (mmol/L) | 1.21±0.71 | 1.78±1.16 | <0.001 |
| LDL-C (mmol/L) | 3.18±0.51 | 3.42±0.81 | <0.001 |
| HDL-C (mmol/L) | 1.41±0.24 | 1.18±0.28 | <0.001 |
| Irisin (ng/mL) | 146.22 (112.34–179.52) | 119.55 (99.53–139.27) | <0.001 |
| Smoking, n (%) | 33 (15.42%) | 82 (23.43%) | 0.022 |
CAD – coronary artery disease; BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; TC – total cholesterol; TG – triglycerides; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein holesterol.
Table 2.
Logistic regression Analysis for the presence of CAD.
| Simple regression | Multiple regression | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (years) | 1.010 (0.993–1.027) | 0.239 | ||
| Gender (M/F) | 1.035 (0.735–1.457) | 0.843 | ||
| BMI (Kg/m2) | 1.046 (0.987–1.108) | 0.130 | ||
| SBP (mmHg) | 1.006 (0.998–1.015) | 0.145 | ||
| DBP (mmHg) | 1.009 (0.994–1.025) | 0.238 | ||
| TC (mmol/L) | 1.093 (0.924–1.292) | 0.301 | ||
| TG (mmol/L) | 2.388 (1.810–3.151) | <0.001 | 2.507 (1.820–3.455) | <0.001 |
| LDL-C (mmol/L) | 1.643 (1.278–2.113) | <0.001 | 1.974 (1.432–2.723) | <0.001 |
| HDL-C (mmol/L) | 0.046 (0.023–0.095) | <0.001 | 0.035 (0.015–0.081) | <0.001 |
| Irisin (ng/mL) | 0.972 (0.966–0.978) | <0.001 | 0.974 (0.967–0.981) | <0.001 |
| Smoking, n (%) | 1.678 (1.074–2.621) | 0.023 | 1.687 (0.980–2.903) | 0.059 |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; TC – total cholesterol; TG – triglycerides; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein holesterol.
Serum irisin concentrations in smoking cases and controls
As shown in Figure 1, serum irisin concentrations were not significantly different between smoking and non-smoking CAD patients, or between smoking and non-smoking controls. Furthermore, both smoking and non-smoking CAD patients showed lower serum irisin concentrations than in smoking and non-smoking controls.
Figure 1.
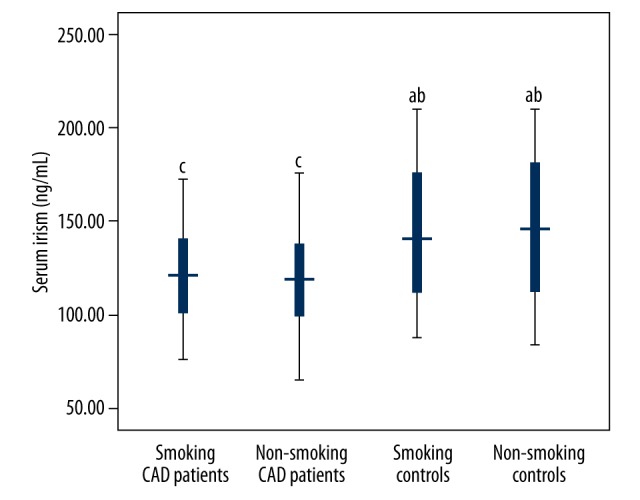
Serum irisin concentrations in smoking cases and controls. both smoking and non-smoking CAD patients showed lower serum irisin concentrations than in smoking and non-smoking controls.
Correlation of serum irisin concentrations with the severity of CAD
Pearson correlation analysis revealed that serum irisin concentrations were negatively correlated with CAI score, which is a characteristic for evaluating the severity of CAD (r=−0.340, P<0.001) (Figure 2).
Figure 2.
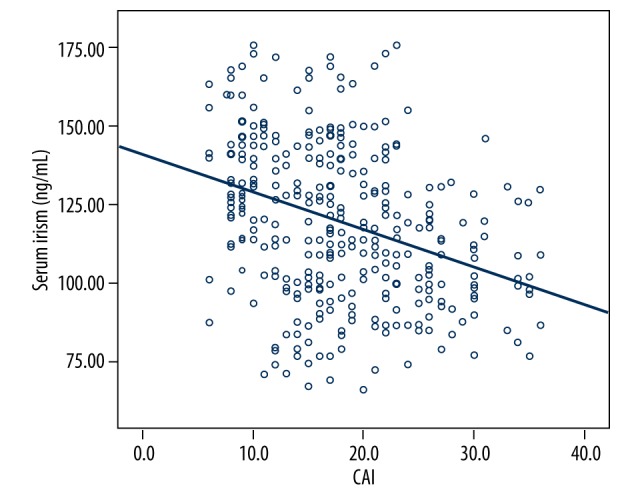
The correlation of serum irisin concentrations and CAI. Pearson correlation analysis revealed a negative correlation of serum irisin concentrations with CAI score (r=−0.340, P<0.001).
Discussion
To investigate whether serum irisin concentrations are correlated with the presence and severity of CAD, we performed this study. The current study demonstrated that serum irisin concentrations are negatively associated with the presence and severity of CAD.
Irisin was shown to regulate endothelial function in recent investigations [9–12]. Irisin treatment in obese mice and human umbilical vein endothelial cells (HUVEC) both led to NO secretion and phosphorylation of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), Akt, and endothelial nitric oxide synthase (eNOS) [9]. This demonstrates the important role of irisin in improving endothelial function [9]. In another study, diabetic aortic segments showed endothelium-dependent vasorelaxation and decreased overproduction of superoxide and peroxynitrite after irisin incubation, which suggests that irisin alleviates endothelial dysfunction through inhibiting oxidative/nitrative stresses [10]. Wu et al. reported that irisin promoted angiogenesis in human umbilical vein endothelial cells [11]. Irisin alleviated endothelial injury by inhibiting inflammation and oxidative stress in apolipoprotein E-null diabetic mice [12].
Recently, irisin has recently been implicated in the development of atherosclerosis [12]. Irisin treatment improved endothelial dysfunction, inhibited endothelial apoptosis, and decreased atherosclerotic plaque area in apoE(−/−) diabetic mice [12]. In addition, serum irisin concentrations are closely correlated with exercise [4]. Therefore, exercise should be encouraged to elevate irisin levels. Serum irisin was negatively correlated with carotid intima-media thickness (cIMT) [13]. Serum irisin was an independent predictor for carotid atherosclerosis in peritoneal dialysis patients [13]. In a study performed by Sesti et al., circulating irisin levels were associated with vascular atherosclerosis as assessed by cIMT in a cohort of white adults [14].
In this study, we found that serum irisin concentrations were negatively correlated with the occurrence and severity of CAD, supporting the potential role of irisin in the pathogenesis of CAD.
However, Aronis et al. reported that there is no association of circulating irisin levels with acute coronary syndromes [15], and serum irisin was associated with major adverse cardiovascular events in CAD patients after receiving percutaneous coronary interventions [15]. These conflicting results may be attributable to differences in disease advancement, different populations, or assays used. Therefore, irisin could serve as a therapeutic drug for ameliorating or preventing atherosclerosis and CAD.
The exact mechanism by which irisin is involved in CAD is unclear. Obesity is one of risk factors of CAD, and irisin is closely correlated with obesity. Irisin acts on white adipose cells in culture and to stimulate UCP1 expression and a broad program of brown-fat-like development [4]. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling [16]. In humans, serum irisin concentrations were found to be negatively correlated with body mass index (BMI) [6]. Considering the close correlation of irisin with obesity, we speculate that irisin may contribute to the development of CAD by a crosslink with obesity.
CAD patients had relatively higher LDL-C and TG, lower HDL-C, and decreased serum irisin, but it is unclear if these are related. Irisin has an effect on lipid metabolism. Irisin was reported to regulate the expression of lipolysis-related genes and contributed to increased secretion of glycerol and decreased lipid accumulation in 3T3-L1 adipocytes [17]. In obese mice, FNDC5 overexpression led to enhanced lipolysis and reduced hyperlipidemia [18]. These results indicate the important role of irisin in lipid metabolism. This may explain the hyperlipidemia and decreased serum irisin in CAD patients.
This study has several potential limitations. First, the sample size was not large enough to reach definitive conclusions. Secondly, our study had a cross-sectional design, and the causative relation must be confirmed by future longitudinal studies.
Conclusions
Our results suggest that decreased serum irisin concentrations is associated with the presence and severity of CAD.
Footnotes
Source of support: Departmental sources
Conflict of interests
Author have no conflict of interests to declare.
References
- 1.Lefkowitz RJ, Willerson JT. Prospects for cardiovascular research. JAMA. 2001;285:581–87. doi: 10.1001/jama.285.5.581. [DOI] [PubMed] [Google Scholar]
- 2.Starčević JN, Petrovič D. Carotid intima media-thickness and genes involved in lipid metabolism in diabetic patients using statins – a pathway toward personalized medicine. Cardiovasc Hematol Agents Med Chem. 2013;11:3–8. doi: 10.2174/1871525711311010003. [DOI] [PubMed] [Google Scholar]
- 3.Nikolajević-Starčević J, Pleskovič A, Santl Letonja M, et al. Polymorphisms 45T>G and 276G>T of the adiponectin gene does not affect plasma adiponectin level and carotid intima-media thickness in patients with diabetes mellitus type 2. Int Angiol. 2014;33:434–40. [PubMed] [Google Scholar]
- 4.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–68. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–78. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 7.Polyzos SA, Kountouras J, Anastasilakis AD, et al. Irisin in patients with nonalcoholic fatty liver disease. Metabolism. 2014;63:207–17. doi: 10.1016/j.metabol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Tatami R, Mabuchi H, Ueda K, et al. Intermediate-density lipoprotein and cholesterol-rich very low density lipoprotein in angiographically determined coronary artery disease. Circulation. 1981;64:1174–84. doi: 10.1161/01.cir.64.6.1174. [DOI] [PubMed] [Google Scholar]
- 9.Han F, Zhang S, Hou N, et al. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309:H1501–8. doi: 10.1152/ajpheart.00443.2015. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D, Wang H, Zhang J, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–47. doi: 10.1016/j.yjmcc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Wu F, Song H, Zhang Y, et al. Irisin induces angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish embryos in vivo via activation of the ERK signaling pathway. PLoS One. 2015;10:e0134662. doi: 10.1371/journal.pone.0134662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Xiang G, Liu M, et al. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis. 2015;243:438–48. doi: 10.1016/j.atherosclerosis.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Lee MJ, Lee SA, Nam BY, et al. Irisin, a novel myokine is an independent predictor for sarcopenia and carotid atherosclerosis in dialysis patients. Atherosclerosis. 2015;242:476–82. doi: 10.1016/j.atherosclerosis.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Sesti G, Andreozzi F, Fiorentino TV, et al. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014;51:705–13. doi: 10.1007/s00592-014-0576-0. [DOI] [PubMed] [Google Scholar]
- 15.Aronis KN, Moreno M, Polyzos SA, et al. Circulating irisin levels and coronary heart disease: Association with future acute coronary syndrome and major adverse cardiovascular events. Int J Obes (Lond) 2015;39:156–61. doi: 10.1038/ijo.2014.101. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Li R, Meng Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–25. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 17.Gao S, Li F, Li H, et al. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS One. 2016;11:e0147480. doi: 10.1371/journal.pone.0147480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong XQ, Chen D, Sun HJ, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis inobesity. Biochim Biophys Acta. 2015;1852:1867–75. doi: 10.1016/j.bbadis.2015.06.017. [DOI] [PubMed] [Google Scholar]


