Abstract
Proteins represent a major class of therapeutic molecules with vast potential for the treatment of acute and chronic diseases and regenerative medicine applications. Hydrogels have long been investigated for their potential in carrying and delivering proteins. As compared to bulk hydrogels, hydrogel microparticles (microgels) hold promise in improving aspects of delivery owing to their less traumatic route of entry into the body and improved versatility. This review discusses common methods of fabricating microgels, including emulsion polymerization, microfluidic techniques, and lithographic techniques. Microgels synthesized from both natural and synthetic polymers are discussed, as are a series of microgels fashioned from environment-responsive materials.
Keywords: hydrogel, microgel, microparticle, protein delivery, growth factor, PEG, PNIPAM
Introduction
The delivery of proteins is a major therapeutic avenue to treat a multitude of diseases such as cancer, osteoarthritis, and diabetes. Larger than conventional small molecule drugs, proteins consist of long chains of peptides formed from amino acid building blocks. Their complex structural and functional nature means their actions in the body are likewise complex and unique, and are unable to be mimicked by small molecule drugs. Because proteins act in a very specific manner, fewer side effects are expected. In addition, the average FDA approval time for a protein therapeutic is similar or even quicker than the time required for small molecule drugs.55
One of the most common therapeutic proteins used today, insulin, is the first line of treatment for patients suffering from diabetes mellitus. Advances in biotechnology have led to improvements in protein production and purification procedures. Only a few decades ago, production of insulin required animal organs until advances in recombinant DNA technology led to the ability to use bacteria to produce insulin.19,59 These bacteria-produced proteins are easier to manufacture on a mass scale and also lower immunogenicity in patients hypersensitive to animal-derived insulin.33 Alternative protein production methods include the use of yeast,24 mammalian cells,73 and plants.44 Other major protein-based therapeutics include antibodies, hormones, hemostasis factors, metabolic enzymes, fertility-associated proteins, and many more. A selected list of FDA-approved protein therapeutics can be found in Table 1; over 130 protein-based therapeutics are approved by the U.S. Food and Drug Administration (FDA) for human use.39 As such, the use of proteins as potent drugs and their delivery in the human body are becoming increasingly important.
Table 1.
Selected FDA-approved protein therapeutics
Data obtained from Leader et al.39 and www.fda.gov
| Trade name(s) | Therapeutic use | Administration route | Description |
|---|---|---|---|
| Humulin, Novolin | Diabetes mellitus | Injection (subcutaneous) | Insulin, recombinant human Hormone that regulates blood glucose |
| Afrezza, Exubera | Diabetes mellitus | Inhalation (powder) | Insulin, recombinant human Hormone that regulates blood glucose |
| Genotropin, Humatrope, Nutropin, Saizen, Zomacton, Zorbtive | Growth deficiencies | Injection (subcutaneous or intramuscular) | Somatropin (human growth hormone) Hormone that has anabolic effect |
| Adynovate, Benefix, Bioclate, Eloctate, Novoeight, Nuwiq, Recombinate | Hemophliia | Injection (intravenous) | Antihemophilic factor (factor VIII, factor IX) Acts as a coagulation factor to stem bleeding |
| Elaprase | Hunter symdrome (MPS II) | Injection (intravenous) | Idursulfase Cleaves the GAGs heparan sulfate and dermatan sulfate, preventing their buildup |
| Follistim | Fertility | Injection (subcutaneous or intramuscular) | Human follicle stimulating hormone (FSH) Aids ovulation, generally used with HCG |
| Ovidrel | Fertility | Injection (subcutaneous or intramuscular) | Human chorionic gondadotropin (HCG) Aids ovulation, generally used with FSH |
| Avastin | Cancer, anti-angiogenesis | Injection (intravenous, intravitreal) | Bevacizumab Monoclonal antibody that targets VEGF-A |
| Herceptin | Cancer | Injection (intravenous) | Trastuzumab Monoclonal antibody that targets HER2/Neu |
| Humira | Rheumatoid Arthiritis | Injection (subcutaneous) | Adalimumab Monoclonal antibody that targets TNF-α |
Hydrogels, consisting of hydrophilic polymeric networks hydrated by large amounts of water, are commonly used biomaterial scaffolds for such delivery of proteins. These polymer networks generally make up anywhere from 1 to 10% of the swollen hydrogel by weight, with the rest of the hydrogel weight coming primarily from water. The network of polymers is connected by crosslinks, which may have a covalent, ionic, affinity or physical basis. Most often, covalent chemical crosslinking (e.g., via free radical polymerization, Michael addition) or ionic crosslinking (e.g., alginate coordinating with divalent cations) are employed. Control over crosslinking density offers control over hydrogel properties; higher crosslinking density will increase elastic modulus and decrease mesh size. The polymer weight percentage of the hydrogel is also tunable, lending control over properties such as mesh size and elastic modulus. Tuning the diffusivity of encapsulated proteins has been well-investigated in previous literature42, as described in the next section. The ability to functionalize hydrogels with biological molecules such as cell-adhesive peptide motifs or proteins (for example, the well-known arginine–glycine–aspartic acid (RGD) sequence) allows for additional control over the interaction of a hydrogel with its environment and with encapsulated cells.40
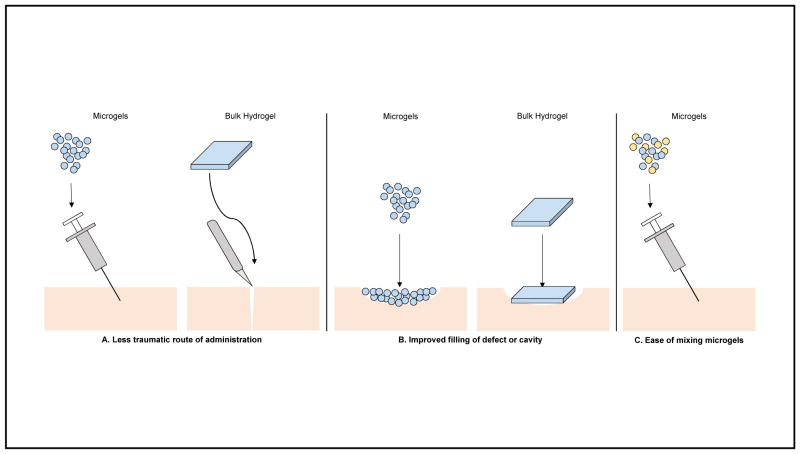
While investigation into hydrogels at the bulk scale continues, hydrogel microparticles (microgels) have also emerged as a potent delivery vehicle. Microgels are highly versatile - they have been investigated in the delivery of small molecule drugs,10 lipids,14 polymers,13 and nanoparticles;68 used as scaffolds for tissue regeneration;2,20 and used as implant coatings to modulate inflammation.7,8,22 They represent a biomaterial that requires less traumatic application methods - injection of soft, deformable microgels can be conducted with a needle or catheter as opposed to a more-involved surgery to implant a bulk hydrogel. These injectable microgels also conform to defects and cavities, resulting in superior coverage of an area as compared to preformed bulk hydrogels. In addition, multiple/repeated administrations due to the less invasive nature of microgel injections can lead to better therapeutic outcomes. The individual, discrete nature of each microgel also allows for the formulation of two or more different proteins to be delivered simultaneously by simply mixing different, separately-made microgels (Figure 1).
Figure 1.
Advantages of microgels as a delivery vehicle as compared to bulk hydrogels. (A) Microgels have a less traumatic route of administration, as they can be injected via needle or catheter as compared to a more-involved surgery to implant a bulk hydrogel. (B) Microgels conform to the defect or cavity in which they are placed, which results in superior coverage of the defect as compared to preformed bulk hydrogels. (C) Microgels have the potential to be simultaneously delivered with other microgels carrying different therapeutics.
Protein delivery from microgels
The route of administration of proteins as a therapeutic agent is an important consideration. Proteins are sensitive to their environment; improper pH levels, chemical degradation, proteolytic degradation, and clearance by the immune system can all decrease or entirely remove the activity of proteins. As such, most delivery routes involve direct infusion or injection of the protein therapeutic, often intravenously. Delivering proteins orally, one of the most common routes for current small molecule therapeutics, results in rapid destruction of the protein and poor transport through the gastrointestinal tract to targeted tissues. Thus, the use of biomaterials to deliver proteins holds immense potential for overcoming current clinical problems with the use of protein therapeutics. Microgel-based biomaterial approaches may be used to shield proteins from unfavorable conditions, to prolong retention time in a targeted area, to co-deliver cells for augmented therapy, or to react to environmental cues to release their payload, among many other applications.
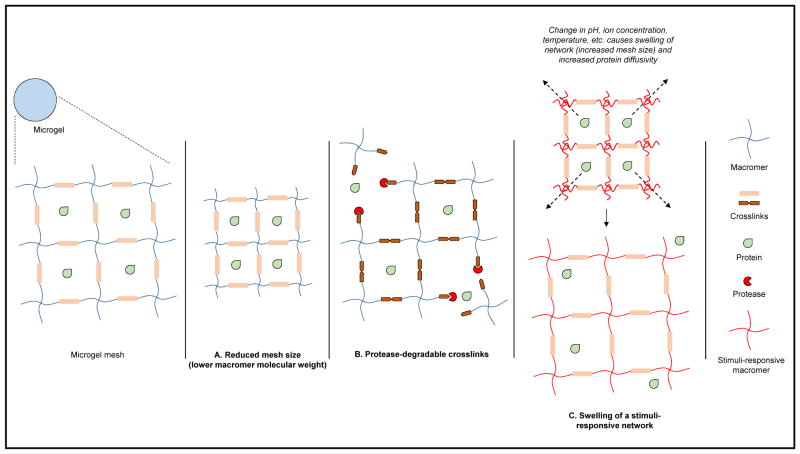
Many microgels release their payloads in a diffusion-based manner from the hydrogel network. Parameters such as mesh size can therefore be controlled to tune protein release profiles (a smaller mesh size hinders diffusion-based release of protein). Methods to control the diffusivity of encapsulated proteins by altering hydrogel network properties have been reviewed by Lin and Metters.42 Theoretically, as the mesh size decreases the diffusivity of encapsulated protein will also be reduced; once the mesh size is at or below the size of the protein, little to no diffusion is predicted to occur. Most hydrogel mesh sizes range from 5 to 100 nm42, and as such, hydrogels are primarily used to carry macromolecules such as proteins as opposed to small-molecule drugs. Additional control over diffusion-based release can be achieved by incorporating molecules such as heparin to take advantage of affinity interactions with encapsulated proteins.32
Degradation of the microgel over time can play a role in the release of protein, especially if protein diffusivity is low. Polymer weight percentage, molecular weight and crosslinking density, factors which also influence mesh size, can therefore be altered to control degradation-based protein release as well. As such, microgels with enzymatically degradable crosslinkers will have a release profile dependent on the cellular microenvironment of the microgel. External stimuli (temperature, pH, ion concentration) can also be utilized to control protein delivery when diffusivity is low by swelling (and thus expanding mesh size) and deswelling the microgel network in response to a trigger (Figure 2). Other delivery strategies may involve the combination of microgels with other elements, such as enzyme nanocapsules that respond to environmental glucose concentration for insulin delivery21 or incorporating microgels within a bulk hydrogel network to reduce the burst effect.5
Figure 2.
Strategies to control protein release from microgels. Control over mesh size, the ability to utilize protease-degradable crosslinkers, and incorporation of stimuli-responsive polymers all modulate protein release.
Fabrication methods for microgels
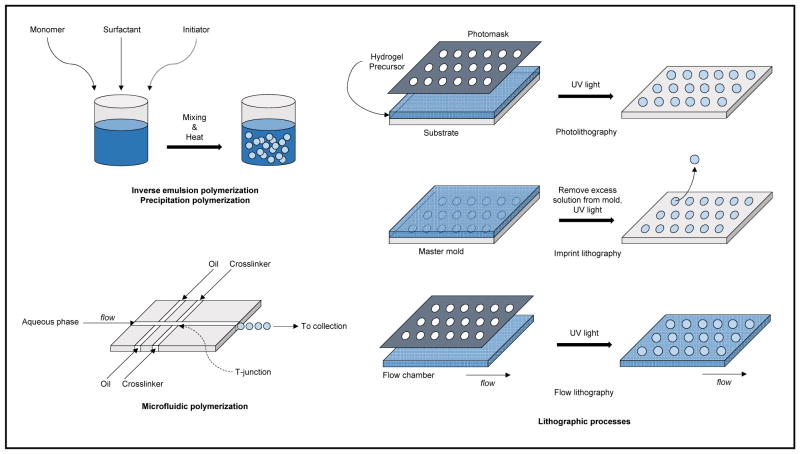
As detailed below, various synthesis routes are available to generate microgels (Figure 3). Originally, batch emulsion or precipitation polymerization techniques involving polymerization in bulk solution were the most common methods. Use of microfluidic polymerization techniques that take advantage of advances in soft lithography to generate microfluidic devices is now a common synthesis route. Microfluidic synthesis schemes are continuous as opposed to batch, and offer greater control over microgel polydispersity at the cost of a slower synthesis speed. Lithographic techniques involving a master template or mask to directly control microgel size and morphology constitute another major class of fabrication methods. Lithographic methods likewise offer good control over particle polydispersity, but are in general slower than batch polymerization methods. Microfluidic and lithographic synthesis schemes also allow for improved control over particle structure and complexity (e.g., Janus microgels,46 core-shell microgels3) as compared to batch schemes. Lastly, although less common, electrospraying techniques can also be utilized to fabricate microgels.31 It should be noted that the crosslinking method employed with the fabrication methods mentioned must be compatible. A review on hydrogel crosslinking chemistries by Hennink and van Nostrum provides a detailed discussion in this regard.27
Figure 3.
Summary of common microgel synthesis schemes.
Emulsion polymerization
Broadly speaking, emulsion polymerization involves an aqueous phase containing a surfactant, an oil phase containing monomer, and an initiator (often thermally-activated) to start the radical polymerization process; a crosslinker may be substituted for the initiator depending on the specific polymerization scheme employed. Inverse emulsion polymerization is an often used variant of this synthesis technique to generate microgels, and simply refers to the switching of oil and aqueous phases. That is, the aqueous phase will contain the hydrophilic monomer while the initiator can be in either phase.66 A mixing or homogenization step, such as using a vortex mixer or a homogenizer, is then employed to generate droplets of monomer in aqueous phase, surrounded by the oil phase (inverse emulsion). The surfactant serves to prevent re-aggregation of the droplets. After adding the initiator and adjusting the environment correctly (e.g., heating the system to a sufficient temperature of 50 °C to 70 °C for a thermal initiator), the initiator reacts to form free radicals that start the polymerization process. The polymerized products are then collected, washed, and optionally lyophilized for storage. Tuning of particle size can be achieved by altering parameters such as mixing or homogenization speed and reaction temperature. Protein can be loaded post-synthesis by diffusion-based incubation of microgels with a concentrated protein solution.
Certain microgel emulsion polymerization schemes (e.g., emulsion polymerization of gelatin microgels crosslinked with genipin or glutaraldehyde) may allow for substitution of initiator with a chemical crosslinker, along with removal of the heating step.51 These schemes thus may also allow for loading of protein by adding the protein to the aqueous phase before emulsification and polymerization occurs, as no thermally-initiated free radical polymerization takes place. Emulsion polymerization processes that are surfactant-free have been reported by Serrano-Medina et al.61 Recently developed microemulsion schemes involving DMSO droplets suspended in a Pluronic-based continuous phase have also been reported by Rios et al.56 Aqueous two-phase emulsions are also an effective method for generating microgels. This water-in-water emulsion system has been used to produce polyethylene glycol (PEG) microgels.30,53
Precipitation polymerization
Precipitation polymerization is similar in several respects to emulsion polymerization. Both techniques proceed in a batch process, with all microgels being formed simultaneously in solution. One of the major differences lies in the fact that all the reagents - the monomer, the initiator, and the crosslinker - are dissolved in an aqueous phase. No surfactant or stabilizing agent is needed for precipitation polymerization. After addition of initiator and proper environmental control (e.g. heating the system), a spontaneous homogenous nucleation process occurs and growth of microgel particles proceeds. Instead of relying on two phases and a surfactant to prevent excess microgel particle aggregation, once the microgel particles reach a critical size they are electrostatically stabilized by initiator fragments that eventually are incorporated into polymer chains during nucleation and growth.54 While most microgels synthesized with this technique range from 100 nm to 3 μm, Meng et al. reported precipitation polymerization synthesis of microgels from 2.5 to 5 μm in diameter.47 Flake et al. have also reported precipitation polymerization synthesis of monodisperse PEG microgels between 1 to 5 μm in diameter.17
Microfluidic polymerization
Microfluidic synthesis techniques proceed in a continuous fashion, with each microgel droplet being formed one at a time at a high throughput. These techniques require a lithographically generated microfluidic device, most often fabricated from polydimethylsiloxane (PDMS). In general, either emulsion-based techniques or flow lithography-based techniques are employed. In an emulsion-based microfluidic system, multiple phases (aqueous, oil, crosslinker, etc.) meet in a junction geometry (e.g., T-junction), where droplets are formed (see Figure 3). After formation of these droplets crosslinking will occur, often via chemically or ionically induced gelation. Following crosslinking, microgels are purified from the collection phase, usually by centrifugation and supernatant removal washing steps. The encapsulated protein is often loaded by mixing with the macromer in the aqueous phase. Control over microfluidic device length scales (nozzle diameter) and flow rates offer a great depth of tunability of microgel size and monodispersity. In a flow lithography-based microfluidic system, the crosslinking takes place via photoinduced polymerization (discussed below).12
Lithographic processes
Lithographic fabrication techniques involve the templating of hydrogels at micro- and nano-scale level using photopolymerization. By affording the designer a high level of control and reproducibility, lithographic synthesis can be an appealing alternative to older, more established methods. There are three major lithographic methods - photolithography, imprint lithography, and flow lithography.26 In all three variants, some type of mask or mold is required. This template is used to control both the size and morphology of the resulting microgels. In photolithography, a template with the desired pattern is created, and used as a photomask for UV polymerization of a bulk hydrogel precursor. Imprint lithography involves a template that acts as a mold for the hydrogel precursor; UV light is then used to photopolymerize the material inside the mold. Finally, flow lithography combines principles from microfluidic synthesis with a patterned photomask and a UV light source to photopolymerize microgels inside a flow channel. In stop-flow lithography, stopping the flow of oligomer before polymerizing yields improved resolution during synthesis.15 Protein is often loaded post-synthesis via swelling/diffusion to avoid exposure to UV light. Given the ability to fine-tune the photomask template, there is a relatively high degree of control over particle size and monodispersity with this method as well. Merkel et al. have reported the fabrication of monodisperse, deformable, discoid-shaped microgels ranging from 0.8 to 8.9 μm in diameter and investigated their administration in vivo.48
Electrospraying fabrication
Hydrogels can be crosslinked by ionic gelation, such as those fabricated from naturally-derived alginate. Microfluidic methods to generate these alginate microgels are employed, as are electrospraying methods, in which liquid flows from a capillary nozzle through an electric field that results in disruption of the flow into droplets.31 While it is less commonly used than other techniques, electrospraying has been used to generate glucose-responsive21 and alginate-based microgels.35 Young et al. have used submerged electrospraying, in which the generation of droplets is conducted in an insulating fluid such as oil (as opposed to air), to generate polyvinyl alcohol (PVA) microgels as well.75
Polymers commonly used for microgels
Natural polymers
Alginate
Alginate is a natural anionic polysaccharide derived from brown seaweed. It is a linear, random copolymer of 1,4-linked β-D-mannuronic acid and α-L-guluronic acid residues that are arranged in a block pattern.69 Divalent cations such as Ca2+ are able to induce a mild, ionic gelation of alginate strands into a hydrogel material. Kim et al. developed alginate microgels carrying both live cells and protein growth factors.35 Arginine–glycine–aspartic acid (RGD), a cell-adhesive peptide motif, was incorporated into the alginate; outgrowth endothelial cells (OECs), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) were also added. Release of fluorescently labeled protein in vitro showed nearly complete cumulative release after a period of 10 days. An ex vivo aorta sprouting assay showed significantly increased sprouting of vasculature for OECs encapsulated within microgels as compared to OECs alone. In vivo results from a rat hind limb ischemia model indicated significantly improved blood flow and reperfusion in the damaged limb.
A microfluidic-based synthesis method was employed by Utech et al. to produce alginate microgels with a tight monodispersity and structural homogeneity.65 Calcium-EDTA chelated complexes were mixed with alginate in a microfluidic chamber; after droplet formation, crosslinking was induced by introduction of acetic acid, releasing the calcium ions. RGD-functionalized alginate was used to encapsulate mesenchymal stem cells (MSCs) with this process, with cell viability (70%) observed out through two weeks. Cells, as well as proteins, can thus conceivably be loaded in this manner.
Alginate-based microfibers with variable chemical composition and complex structure and morphology were reported by Kang et al.34 A fiber-spinning method based on a microfluidic device, driven by computer-controlled pneumatic valves, that deposited the microfiber onto a rotating spool was employed. Triple-helix microfiber structures could be generated via this spool rotation, and nanoporous structures within a fiber could be generated by a process of salt dissolution. These microfibers were used to encapsulate rat hepatocytes and fibroblasts, with co-encapsulation of both cell lines resulting in mutually-improved cell viability over 5 days. These highly tunable microfibers could feasibly be used to carry both live cells and proteins.
Marquis et al. used a microfluidic flow-focusing scheme to generate pectin-alginate dual-compartment Janus microgels.46 The separation of the two hemispheres was shown by confocal scanning laser microscopy, and the diffusivity of encapsulated bovine serum albumin depended on its placement in either the pectin or the alginate hemisphere of the microgel. In addition, use of specific enzymes against the two different polysaccharides that comprised the Janus microgels resulted in selective degradation of either hemisphere. Varying shapes and morphologies, ranging from spheres to oblate ellipsoids and tori, were also synthesized.
Gelatin
Gelatin, obtained by partially processing collagen, is most commonly used as a food additive. Depending on the nature of processing, derived gelatin can be either anionic or cationic. As such, this property of charge can be an additional tool to direct charged biomolecule interactions with a gelatin hydrogel matrix.76 Methacrylate-modified gelatin used to generate microgels via an emulsion polymerization scheme was reported by Nguyen et al.51 The methacrylation took place via amine substitution; by varying the degree of methacrylation, various physicomechanical properties such as elastic modulus or protein release characteristics could be tuned. Lower degrees of methacrylation resulted in larger mesh sizes and faster particle degradation; more of the growth factor BMP4 could be loaded into microgels with lower methacrylation. Microgels with lower degrees of methacrylation also had more complete growth factor release in vitro relative to either microgels with higher methacrylation or microgels crosslinked with glutaraldehyde.
Gelatin microgels embedded in injectable PEG-fumarate hydrogels were described by Holland et al.29 The microgels were fabricated with emulsion polymerization using a glutaraldehyde crosslinker. Transforming growth factor-β1 (TGF-β1) was loaded into the gelatin microparticles by mixing the former with the latter in a dried state. In vitro release studies revealed the ability to dampen burst release characteristics as compared to TGF-β1-loaded directly into PEG-fumarate hydrogels; burst release was attenuated from as high as 51.3% down to as low as 31.4%, depending on microparticle loading and crosslinking time. Cumulative release over three weeks could also be tuned by modifying PEG-fumarate hydrogel mesh size and the amount of gelatin microgels loaded.
Hyaluronic acid
Hyaluronic acid is an anionic linear glycosaminoglycan with high molecular weight that is often found in the extracellular matrix (ECM) and is comprised of two disaccharides, N-acetylglucosamine and D-glucuronic acid. Its major function in the body is to bind water and lubricate moving parts in the body; it is most often found in connective tissues, synovial fluid, and the vitreous fluid of the eye.50 Hyaluronic acid was used to create microgels by modifying it with the addition of thiol groups by Hahn et al.23 A two-step chemical reaction was used to thiolate hyaluronic acid, which was then crosslinked with the addition of sodium tetrathionate. The protein hormone drug erythropoietin was loaded into these microgels pre-gelation, and in vitro results indicated controlled release over a period of nine days. In vivo rat results showed elevated plasma concentrations of erythropoietin above the critical minimal efficacy concentration, maintained for a week.
Dextran
Dextran is a natural, branched polysaccharide consisting of repeating glucose units in the form of either α-(1,6) linkages in the main chain or α-(1,3) branch linkages.67 It has been used therapeutically as a blood thinner to decrease thrombosis.6 Microstructured dextran-based hydrogels that significantly reduced the burst effect in releasing the PEGylated protein drug interferon-α2a were reported by Bae et al.; microstructured PEG domains carrying the protein drug were dispersed throughout a bulk dextran hydrogel.5 Humanized mice (hepatitis C model) were used to show that the microstructured hydrogels resulted in an increase in sustained plasma concentration of interferon-α2a over a 40-day period; there was more than a 5-fold increase in plasma half-life. Serum levels of ALT and IFN-γ, markers for liver damage and immune response, were significantly decreased as compared to controls.
Synthetic polymers
Synthetic polymers are promising due to the tighter control of chemical structure, reproducibility, and tunability. While naturally-sourced polymers may have batch-to-batch variation, finer chemical control processes allow synthetically created polymers to be more consistent. Appropriate knowledge of chemistry allows for modification of polymer backbones and side chains to optimally tune polymer characteristics.
PEG-based polymers
Polyethylene glycol (PEG) is a synthetic, hydrophilic polymer well-investigated for hydrogels, particle “stealthing”, drug half-life extension, and more. It has a relatively cytocompatible nature, often eliciting minimal inflammatory responses. Its chemistry is well-defined, and a multitude of variants (linear, branched, star, multi-arm, multi-functionality, etc.) are commercially available. Headen et al. demonstrated the microencapsuation of both cells and proteins using 4-arm PEG-based chemical hydrogels generated with a flow-focusing microfluidic device and crosslinked via Michael addition.25 The cell-binding peptide motif RGD was incorporated into the microgel for cell adhesion; the proteins insulin, bovine serum albumin, and IgG were loaded and showed sustained release over three days. Human MSCs were encapsulated and shown to be viable for at least seven days; human islets were encapsulated and had a comparable glucose stimulation index as compared to non-encapsulated islets.
A PEG-based microgel platform based on a microfluidic synthesis approach was also reported by Allazetta et al.3 The crosslinks used in this platform were enzymatically degradable, allowing for certain proteases to cleave the hydrogel network. Mouse embryonic stem cells were encapsulated with this procedure, followed by a second microfluidic step into hydrogel beads chemically crosslinked via Michael addition, resulting in an encapsulating non-degradable hydrogel shell. ESCs escaped after 20 hours if the second microfluidic step was absent; with the additional non-degradable layer, no cells escaped after 4 days of culture. The related idea of conformally coating cells with a hydrogel shell has been reported by Tomei et al.; no significant loss of encapsulated islet function was observed.64 These approaches of generating an additional layer of hydrogel material over an existing microgel foundation holds promise for modulating both cell and protein delivery.
PEG-based microparticles that are photodegradable by single photon or two-photon irradiation were reported by Tibbitt et al.63 A PEG macromer with bivalent photodegradable acrylates was copolymerized with a PEG macromer with tetravalent thiol groups via Michael addition; the photodegradable nitrobenzyl ether moieties absorb single photon radiation at 365 nm or two-photon radiation at 740 nm. It was demonstrated that labeled bovine serum albumin could be released selectively by spatially targeting microspheres to irradiate. Bioactivity of encapsulated TGF-β1 on mink lung epithelial cells was verified with a luciferase assay in vitro. The use of fluorescent annexin V within degradable microgels to identify apoptotic cells (induced with camptothecin) was also demonstrated.
Impellitteri et al. generated PEG microgels that incorporated VEGF receptor 2 (VEGFR2) into the hydrogel matrix to carry VEGF.30 VEGFR2 was covalently linked into the matrix and served to increase binding affinity and specificity of the microgels for VEGF. An aqueous two-phase water-in-water emulsion scheme using UV crosslinking was used to fabricated the microgels. An in vitro transwell HUVEC proliferation assay showed an increase in cell count when HUVECs were incubated with microgels with VEGFR2 linked into the matrix loaded with VEGF, as compared to control (microgels with a scrambled peptide linked into the matrix). Microgels with VEGFR2 linked into the matrix were also used to show the ability to downregulate growth factor signaling. HUVECs incubated with soluble VEGF had lower cell counts when co-incubated with these microgels as compared to control, presumably due to the microgels sequestering some of the soluble VEGF.
PEG-diacrylate (PEGDA) microgels were synthesized by DeVolder et al. via emulsion polymerization, with PEGDA crosslinked to an ionic monomer (either an anionic or cationic monomer) to generate charged microgels.16 These separate, charged microgels could be combined to create a colloidal gel material that resisted flow. Release of bovine serum albumin and VEGF (separately) over a seven day period was evaluated, and it was shown that release rates were mostly independent of microgel formulation. A chorioallantoic membrane in vivo model was used to assess microgel retention, inflammation response, and capillary formation. The colloidal gel formed from mixing the oppositely charged microgels (carrying VEGF) had improved retention, comparable inflammation, and improved vascular density as compared to controls.
Hybrid synthetic polymers
Zachman et al. synthesized PEG- and PCL-based microgels carrying both an angiogenic peptide (C16) and an anti-inflammatory peptide (Ac-SKDP).77 The microgels were delivered into thigh muscle adjacent to the femoral artery ligations in a murine hind limb ischemia model of peripheral artery disease. Microgels in a series of 1 μL injections, allowing for thermosensitive in situ polymerization, were tested against preformed implantable scaffolds. Dual delivery of C16 and Ac-SKDP showed improved hind himb reperfusion for both the microgel and bulk hydrogel formulation. However, the series of microgel injections resulted in reduced inflammatory responses as compared to bulk hydrogels.
Poly(acrylic acid) (PAA) and PEG were crosslinked via esterification in a DMSO/Pluronic microemulsion to form microgels.56 RAFT polymerization was used to generate the PAA, allowing for good control of molecular weight; the size of degradation products were tuned to be within the glomerular filtration size range of the kidney. Control over crosslinking density also allowed control over microgel shape and morphology; higher crosslinking resulted in spherical shapes while lower crosslinking led to less-spherical, porous microgels. Several proteins (lysozyme, hemoglobin, myoglobin, albumin) were loaded into these PAA-PEG microgels post-fabrication; lysozyme had high loading efficiency (>90%) and loading efficiencies for the other proteins were dependent on microgel formulation. Release of lysozyme over a period of thirty days was also shown to be dependent on microgel formulation, with high retention of enzymatic activity.
Sajeesh et al. reported polymethacrylic acid-PEG-chitosan microgels either functionalized with thiol groups or complexed with cyclodextrin for oral insulin delivery.58,57 The addition of thiol groups by grafting cysteine residues to the surface of of the microgels improves polymer-mucus interactions due to the formation of disulfide linkages.1 Insulin transport in vitro across Caco-2 colon adenocarcinoma cell monolayers was improved with the addition of cysteine residues; insulin transport in vivo across rat intestinal tissue was also increased. Oral administration of thiol-functionalized microgels led to an improved hypoglycemic effect over ten hours as compared to non-functionalized microgels or a subcutaneous injection of insulin. Complexation of these microgels with cyclodextrin was also evaluated, and similar improvements in insulin transport and hypoglycemic effect were observed.
Liposomes immobilized onto pNIPAM-based microgels via biotin-avidin interactions were reported by MacKinnon et al.45 Acrylic acid and NIPAM were copolymerized to form microgels that were modified to present amines,28 and then biotinylated using amine-NHS ester chemistry. Multilamellar liposomes containing biotin groups were generated, followed by conjugation of avidin. Mixing of microgels presenting biotin and liposomes presenting avidin resulted in coupling of the liposomes to the microgels via a biotin-avidin-biotin chain. The ability of the liposomes to retain loaded molecules as compared to free liposomes was verified.
Hydroxyethyl starch-based methacrylated polymers were used to generate microgels using photo-initiated emulsion polymerization.71 FITC-labeled goat anti-human IgG was loaded into microgels with and without PEG incorporated, and sustained release was observed over nine days in vitro. Subcutaneous injection of these microgels in a mouse model showed more sustained release as compared to FITC-IgG in solution, but the authors noted an inflammatory response in the microgels without PEG incorporated.
Stimulus-responsive polymers
Polymers with the ability to react in response to external stimuli have been of particular interest. Materials that respond to temperature as a driving stimulus can be used to trigger protein release. One of the most common, pNIPAM (a polymer of the subunit N-isopropylacrylamide), has a lower critical solution temperature (LCST) of 32 °C. As the environmental temperature of the pNIPAM increases and crosses this LCST, water is expunged from the hydrogel network and the remaining polymer chains collapse.43 Release of small molecule drugs from pNIPAM microgels has been demonstrated.11 pH and temperature can both simultaneously be used to modulate the swelling behavior of pNIPAM microgels18 and PAA-based microgels.9
Other external triggers such as pH and ion concentration can be used to induce these volume phase transitions as well. For example, Kiser et al. reported microgels formed from methylene-bis-acrylamide and methacrylic acid and coated with a lipid bilayer that act as a mimic of a secretory granule.36 Responsive to both pH and ion concentration, these microgels can also be triggered by another separate stimulus (electrical, ultrasound) to destroy the lipid bilayer and allow for the microgel contents to be released. Glucose-responsive microgels, as discussed in the next section, are often designed around pH-sensitivity. Incorporation of a glucose oxidase yields gluconic acid from glucose, therefore lowering the pH.72
Glucose-responsive
Chitosan-based pH- and glucose-responsive microgels fabricated by an electrospraying technique were reported by Gu et al.21 These microgels were loaded with human recombinant insulin and enzyme nanocapsules containing glucose oxidase and catalase. Upon an increase in glucose concentration, these enzymatic nanocapsules increase their activity which ultimately results in the protonation of the amine groups on the chitosan chains, leading to microgel expansion and release of insulin. In vivo mouse models showed a noticeable modulation of blood glucose level for 72 hours. Wu et al. also reported glucose-responsive microgels utilizing a glucose oxidase/catalase enzymatic reaction mechanism, generated with inverse emulsion polymerization.72 An acid-labile crosslinker (DMOPA) was used to create microgels that degrade in response to decreased pH; increased glucose concentration decreases pH via glucose oxidase. Release of insulin in vitro was shown to vary significantly as a function of glucose concentration.
Yin et al. synthesized glucose-responsive microgels using concanavalin A, a carbohydrate-binding protein that possesses a strong, reversible affinity to glucose, as the glucose-responsive agent.74 An emulsion polymerization technique was used to generate the acrylated chitosan-based microgels, followed by crosslinking with genipin and post-synthesis loading of insulin by diffusion. Insulin release in vitro was assessed, and increased insulin release over 2 hours was observed when the microgels were submitted to a 4 mg/mL glucose stimulation. Pulsatile release of insulin from the microgels in response to alternating on/off glucose signals was also demonstrated.
Temperature-responsive
Thermoresponsive release of insulin from pNIPAM-based microgel films was reported by Nolan et al.52 NIPAM and acrylic acid monomers underwent precipitation polymerization to form microgels, were loaded with FITC-labeled insulin by diffusion, and then subjected to layer-by-layer deposition with polyalylamine hydrochloride as the polycationic layer and the microgels as the polyanionic layer. The release of insulin was shown to be controllable via temperature, with pulsatile release of insulin corresponding to temperature changes between 25 °C and 40 °C. An et al. also reported pNIPAM-based microgels that demonstrated swelling and deswelling below and above the LCST of the hydrogel network based on particle diameter measurements.4
Sung et al. used water-in-oil emulsion polymerization to create thermoresponsive microgel constructs by physically entrapping p(NIPAM-co-acrylamide) chains inside genipin-crosslinked gelatin networks.62 Release of the model protein BSA due to temperature-induced deswelling could be tuned by modifying the percentage of poly(NIPAM-co-acrylamide) incorporated into the gelatin matrix. Enzymatic degradation of the microgels by collagenase was also demonstrated, resulting in free poly(NIPAM-co-acrylamide) chains that could be excreted by the kidney.
pH- and ion concentration-responsive
Hydrophobic nanoscale hydrogels within hydrophilic microscale hydrogels have been investigated for the delivery of small molecule chemotherapeutics.60 Knipe et al. showed the facile synthesis of polyanionic microgels containing polycatonic nanoscale hydrogels for protein delivery.37 Methacrylic acid and N-vinyl pyrrolidone in solution were used to synthesize microgels via photoinitiated free radical polymerization to form a film, which was subsequently dried under vacuum and then crushed to form microgels. Nanoscale hydrogels containing t-butyl methacrylate and 2-(t-butylamino)ethyl methacrylate were synthesized with emulsion polymerization41 and mixed with the solution of methacrylic acid and N-vinyl pyrrolidone prior to polymerization into a film. The swelling ratio of these microgels was shown to vary with pH. Loading of bovine serum albumin and FITC-labeled dextran was confirmed, with loading efficiencies ranging from 40% to 60%; relatively fast release profiles over 2 hours were observed. Caco-2 colon adenocarcinoma human cells incubated with microgels for 2 hours showed high viability, dependent on the weight percentage of the hydrogel material.
Murthy et al. developed microgels sensitive to acidic environments by synthesizing a crosslinker compound with an acetal linkage that is acid-labile.49 Acrylamide was crosslinked with this compound with inverse emulsion polymerization, with loaded protein (ovalbumin) incorporated before polymerization. pH-dependent protein release in vitro over a period of five hours was demonstrated, and incubation for 6 hours with RAW 309.1CR antigen-presenting cells yielded acceptable toxicity, dependent on microgel concentration. Compared to incubation with free ovalbumin, these antigen-presenting cells incubated with microgels carrying ovalbumin showed a significantly higher ability to activate B3Z cytotoxic T lymphocytes.
Hollow-core polymethacrylic acid (PMAA) hydrogel capsules used to investigated the interplay between pH and microgel swelling and macromolecule loading were reported by Kozlovskaya et al.38 PMAA and polyvinylpyrrolidone (PVPON) were deposited layer-by-layer onto a silica core, crosslinked with ethylenediamine, and then submerged in HF to dissolve the cores. Dialysis in a basic buffer removed the PVPON, yielding hollow PMAA capsules. The diameter of the PMAA capsules was shown to vary from a pH of 2 to 11, with the smallest size obtained at pH 5.5 and larger sizes obtained as the pH either increased or decreased. Loading of FITC-dextran was shown at pH 2 and pH 8, followed by entrapment of the loaded dextran by shifting the pH to 5.5. Increased crosslinking time, which reduced mesh size, affected the permeability of the PMAA microgel capsule to different-sized macromolecules.
The adsorption of the enzymatic protein lysozyme onto core-shell microgels was investigated by Welsch et al.70 The core of the microgel consisted of polystyrene, while the negatively-charged shell contained crosslinked NIPAM, methylenebisacrylamide, and acrylic acid. As ion concentration is increased, binding affinity of the lysozme to the microgel decreases. Adsorption of lysozyme results in an activity enhancement factor of roughly 3.5, and results indicate protonation of lysozyme upon adsorption.
Outlook
Microgels represent an exciting developing area in hydrogel-based therapeutics for protein delivery. Because of their versatility in fabrication methods and base materials, delivery formulation, and size, these carriers offer tremendous opportunities for the controlled delivery of protein therapeutics as well as cells. Advances in fabrication methodologies, including those yielding non-spherical morphologies, polymer formulations, incorporation of novel and/or multiple bioactive components will lead to new classes of microgels with new properties and functionalities. Because of its flexibility and versatility, this material platform will have significant impact in biomedical applications such as cancer, inflammatory disorders, immunoengineering, and regenerative medicine.
Acknowledgments
AJG is supported by the NIH (R01 AR062368, R01 AR062920, R21 EB020107) and the Juvenile Diabetes Research Foundation (2-SRA-2014-287-Q-R, 3-SRA-2015-38-Q-R). AL is supported by the NIH-sponsored Program on Graduate Training for Rationally Designed, Integrative Biomaterials (T32 EB006343).
Footnotes
Conflict of Interest Statement
No conflicts of interest.
References
- 1.Albrecht K, Bernkop-Schnurch A. Thiomers: forms, functions and applications to nanomedicine. Nanomedicine (Lond) 2007;2:41–50. doi: 10.2217/17435889.2.1.41. [DOI] [PubMed] [Google Scholar]
- 2.Allazetta S, Hausherr TC, Lutolf MP. Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules. 2013;14:1122–1131. doi: 10.1021/bm4000162. [DOI] [PubMed] [Google Scholar]
- 3.Allazetta S, Kolb L, Zerbib S, Bardy J, Lutolf MP. Cell-Instructive Microgels with Tailor-Made Physicochemical Properties. Small. 2015;11:5647–5656. doi: 10.1002/smll.201501001. [DOI] [PubMed] [Google Scholar]
- 4.An Y, Zhang L, Xiong S, Wu S, Xu M, Xu Z. Fluorine-containing thermo-sensitive microgels as carrier systems for biomacromolecules. Colloids and Surfaces B: Biointerfaces. 2012;92:246–253. doi: 10.1016/j.colsurfb.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Bae KH, Lee F, Xu K, Keng CT, Tan SY, Tan YJ, Chen Q, Kurisawa M. Microstructured dextran hydrogels for burst-free sustained release of PEGylated protein drugs. Biomaterials. 2015;63:146–157. doi: 10.1016/j.biomaterials.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Bonnar J, Walsh J. Prevention of thrombosis after pelvic surgery by British dextran 70. Lancet. 1972;1:614–616. doi: 10.1016/s0140-6736(72)90410-2. [DOI] [PubMed] [Google Scholar]
- 7.Bridges AW, Singh N, Burns KL, Babensee JE, Andrew Lyon L, Garcia AJ. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials. 2008;29:4605–4615. doi: 10.1016/j.biomaterials.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges AW, Whitmire RE, Singh N, Templeman KL, Babensee JE, Lyon LA, Garcia AJ. Chronic inflammatory responses to microgel-based implant coatings. J Biomed Mater Res A. 2010;94:252–258. doi: 10.1002/jbm.a.32669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberg L, Temchenko M, Hatton TA. Dually Responsive Microgels from Polyether-Modified Poly(acrylic acid): Swelling and Drug Loading. Langmuir. 2002;18:4944–4952. [Google Scholar]
- 10.Cavalieri F, Chiessi E, Villa R, Vigano L, Zaffaroni N, Telling MF, Paradossi G. Novel PVA-based hydrogel microparticles for doxorubicin delivery. Biomacromolecules. 2008;9:1967–1973. doi: 10.1021/bm800225v. [DOI] [PubMed] [Google Scholar]
- 11.Chun S-W, Kim J-D. A novel hydrogel-dispersed composite membrane of poly(N-isopropylacrylamide) in a gelatin matrix and its thermally actuated permeation of 4-acetamidophen. Journal of Controlled Release. 1996;38:39–47. [Google Scholar]
- 12.Chung BG, Lee KH, Khademhosseini A, Lee SH. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip. 2012;12:45–59. doi: 10.1039/c1lc20859d. [DOI] [PubMed] [Google Scholar]
- 13.Clark MR, Aliyar HA, Lee C-w, Jay JI, Gupta KM, Watson KM, Stewart RJ, Buckheit RW, Kiser PF. Enzymatic triggered release of an HIV-1 entry inhibitor from prostate specific antigen degradable microparticles. International Journal of Pharmaceutics. 2011;413:10–18. doi: 10.1016/j.ijpharm.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Barker DA, Wang T, Lau CM, Lin Y, Botchwey EA. Delivery of bioactive lipids from composite microgel-microsphere injectable scaffolds enhances stem cell recruitment and skeletal repair. PLoS One. 2014;9:e101276. doi: 10.1371/journal.pone.0101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dendukuri D, Gu SS, Pregibon DC, Hatton TA, Doyle PS. Stop-flow lithography in a microfluidic device. Lab on a Chip. 2007;7:818–828. doi: 10.1039/b703457a. [DOI] [PubMed] [Google Scholar]
- 16.DeVolder RJ, Kong H-J. Three dimensionally flocculated proangiogenic microgels for neovascularization. Biomaterials. 2010;31:6494–6501. doi: 10.1016/j.biomaterials.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Flake MM, Nguyen PK, Scott RA, Vandiver LR, Willits RK, Elbert DL. Poly(ethylene glycol) microparticles produced by precipitation polymerization in aqueous solution. Biomacromolecules. 2011;12:844–850. doi: 10.1021/bm1011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fucinos C, Fucinos P, Miguez M, Katime I, Pastrana LM, Rua ML. Temperature- and pH-sensitive nanohydrogels of poly(N-Isopropylacrylamide) for food packaging applications: modelling the swelling-collapse behaviour. PLoS One. 2014;9:e87190. doi: 10.1371/journal.pone.0087190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, Riggs AD. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979;76:106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, Dong Y, Zhang Y, Anderson DG. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. ACS Nano. 2013;7:6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 22.Gutowski SM, Templeman KL, South AB, Gaulding JC, Shoemaker JT, LaPlaca MC, Bellamkonda RV, Lyon LA, Garcia AJ. Host response to microgel coatings on neural electrodes implanted in the brain. J Biomed Mater Res A. 2014;102:1486–1499. doi: 10.1002/jbm.a.34799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn SK, Kim JS, Shimobouji T. Injectable hyaluronic acid microhydrogels for controlled release formulation of erythropoietin. J Biomed Mater Res A. 2007;80:916–924. doi: 10.1002/jbm.a.30997. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton SR, Davidson RC, Sethuraman N, Nett JH, Jiang Y, Rios S, Bobrowicz P, Stadheim TA, Li H, Choi B-K, Hopkins D, Wischnewski H, Roser J, Mitchell T, Strawbridge RR, Hoopes J, Wildt S, Gerngross TU. Humanization of Yeast to Produce Complex Terminally Sialylated Glycoproteins. Science. 2006;313:1441–1443. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]
- 25.Headen DM, Aubry G, Lu H, Garcia AJ. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv Mater. 2014;26:3003–3008. doi: 10.1002/adma.201304880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helgeson ME, Chapin SC, Doyle PS. Hydrogel microparticles from lithographic processes: novel materials for fundamental and applied colloid science. Curr Opin Colloid Interface Sci. 2011;16:106–117. doi: 10.1016/j.cocis.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54:13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 28.Hoare T, Pelton R. Highly pH and Temperature Responsive Microgels Functionalized with Vinylacetic Acid. Macromolecules. 2004;37:2544–2550. [Google Scholar]
- 29.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 30.Impellitteri NA, Toepke MW, Lan Levengood SK, Murphy WL. Specific VEGF sequestering and release using peptide-functionalized hydrogel microspheres. Biomaterials. 2012;33:3475–3484. doi: 10.1016/j.biomaterials.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaworek A. Micro- and nanoparticle production by electrospraying. Powder Technology. 2007;176:18–35. [Google Scholar]
- 32.Jha AK, Mathur A, Svedlund FL, Ye J, Yeghiazarians Y, Healy KE. Molecular weight and concentration of heparin in hyaluronic acid-based matrices modulates growth factor retention kinetics and stem cell fate. J Control Release. 2015;209:308–316. doi: 10.1016/j.jconrel.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson I. Human insulin from recombinant DNA technology. Science. 1983;219:632–637. doi: 10.1126/science.6337396. [DOI] [PubMed] [Google Scholar]
- 34.Kang E, Jeong GS, Choi YY, Lee KH, Khademhosseini A, Lee SH. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat Mater. 2011;10:877–883. doi: 10.1038/nmat3108. [DOI] [PubMed] [Google Scholar]
- 35.Kim P-H, Yim H-G, Choi Y-J, Kang B-J, Kim J, Kwon S-M, Kim B-S, Hwang NS, Cho J-Y. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. Journal of Controlled Release. 2014;187:1–13. doi: 10.1016/j.jconrel.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Kiser PF, Wilson G, Needham D. A synthetic mimic of the secretory granule for drug delivery. Nature. 1998;394:459–462. doi: 10.1038/28822. [DOI] [PubMed] [Google Scholar]
- 37.Knipe JM, Chen F, Peppas NA. Multiresponsive polyanionic microgels with inverse pH responsive behavior by encapsulation of polycationic nanogels. Journal of Applied Polymer Science. 2014;131 n/a-n/a. [Google Scholar]
- 38.Kozlovskaya V, Sukhishvili SA. Amphoteric Hydrogel Capsules: Multiple Encapsulation and Release Routes. Macromolecules. 2006;39:6191–6199. [Google Scholar]
- 39.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nature Reviews Drug Discovery. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 40.Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, del Campo A, García AJ. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14:352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liechty WB, Scheuerle RL, Peppas NA. Tunable, responsive nanogels containing t-butyl methacrylate and 2-(t-butylamino)ethyl methacrylate. Polymer. 2013;54:3784–3795. [Google Scholar]
- 42.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Lyon LA, Meng Z, Singh N, Sorrell CD, St John A. Thermoresponsive microgel-based materials. Chemical Society Reviews. 2009;38:865–874. doi: 10.1039/b715522k. [DOI] [PubMed] [Google Scholar]
- 44.Ma JKC, Drake PMW, Christou P. The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet. 2003;4:794–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- 45.MacKinnon N, Guerin G, Liu B, Gradinaru CC, Macdonald PM. Liposome-hydrogel bead complexes prepared via biotin-avidin conjugation. Langmuir. 2009;25:9413–9423. doi: 10.1021/la900163r. [DOI] [PubMed] [Google Scholar]
- 46.Marquis M, Davy J, Cathala B, Fang A, Renard D. Microfluidics assisted generation of innovative polysaccharide hydrogel microparticles. Carbohydr Polym. 2015;116:189–199. doi: 10.1016/j.carbpol.2014.01.083. [DOI] [PubMed] [Google Scholar]
- 47.Meng Z, Smith MH, Lyon LA. Temperature-programmed synthesis of micron-sized multi-responsive microgels. Colloid and Polymer Science. 2009;287:277–285. [Google Scholar]
- 48.Merkel TJ, Chen K, Jones SW, Pandya AA, Tian S, Napier ME, Zamboni WE, DeSimone JM. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J Control Release. 2012;162:37–44. doi: 10.1016/j.jconrel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Frechet JM. A macromolecular delivery vehicle for protein-based vaccines: acid-degradable protein-loaded microgels. Proc Natl Acad Sci U S A. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Necas J, Bartosikova L, Brauner P, Kolar J. Hyaluronic acid (hyaluronan): a review. Veterinarni medicina. 2008;53:397–411. [Google Scholar]
- 51.Nguyen AH, McKinney J, Miller T, Bongiorno T, McDevitt TC. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater. 2015;13:101–110. doi: 10.1016/j.actbio.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nolan CM, Serpe MJ, Lyon LA. Thermally Modulated Insulin Release from Microgel Thin Films. Biomacromolecules. 2004;5:1940–1946. doi: 10.1021/bm049750h. [DOI] [PubMed] [Google Scholar]
- 53.Parlato M, Johnson A, Hudalla GA, Murphy WL. Adaptable poly(ethylene glycol) microspheres capable of mixed-mode degradation. Acta Biomater. 2013;9:9270–9280. doi: 10.1016/j.actbio.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pich A, Richtering W. Microgels by Precipitation Polymerization: Synthesis, Characterization, and Functionalization. In: Pich A, Richtering W, editors. Chemical Design of Responsive Microgels. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 1–37. [Google Scholar]
- 55.Reichert JM. Trends in development and approval times for new therapeutics in the United States. Nat Rev Drug Discov. 2003;2:695–702. doi: 10.1038/nrd1178. [DOI] [PubMed] [Google Scholar]
- 56.Rios JL, Lu G, Seo NE, Lambert T, Putnam D. Prolonged Release of Bioactive Model Proteins from Anionic Microgels Fabricated with a New Microemulsion Approach. Pharm Res. 2015 doi: 10.1007/s11095-015-1834-8. [DOI] [PubMed] [Google Scholar]
- 57.Sajeesh S, Bouchemal K, Marsaud V, Vauthier C, Sharma CP. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: An oral delivery system for insulin. J Control Release. 2010;147:377–384. doi: 10.1016/j.jconrel.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Sajeesh S, Vauthier C, Gueutin C, Ponchel G, Sharma CP. Thiol functionalized polymethacrylic acid-based hydrogel microparticles for oral insulin delivery. Acta Biomater. 2010;6:3072–3080. doi: 10.1016/j.actbio.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Schein CH. Production of Soluble Recombinant Proteins in Bacteria. Nat Biotech. 1989;7:1141–1149. [Google Scholar]
- 60.Schoener CA, Hutson HN, Peppas NA. pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the oral delivery of chemotherapeutics. J Biomed Mater Res A. 2013;101:2229–2236. doi: 10.1002/jbm.a.34532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serrano-Medina A, Cornejo-Bravo JM, Licea-Claveríe A. Synthesis of pH and temperature sensitive, core–shell nano/microgels, by one pot, soap-free emulsion polymerization. J Colloid Interface Sci. 2012;369:82–90. doi: 10.1016/j.jcis.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 62.Sung B, Kim C, Kim M-H. Biodegradable colloidal microgels with tunable thermosensitive volume phase transitions for controllable drug delivery. J Colloid Interface Sci. 2015;450:26–33. doi: 10.1016/j.jcis.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 63.Tibbitt MW, Han BW, Kloxin AM, Anseth KS. Synthesis and application of photodegradable microspheres for spatiotemporal control of protein delivery. J Biomed Mater Res A. 2012;100:1647–1654. doi: 10.1002/jbm.a.34107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A, Molano RD, Ricordi C, Stabler CL, Hubbell JA. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci U S A. 2014;111:10514–10519. doi: 10.1073/pnas.1402216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Utech S, Prodanovic R, Mao AS, Ostafe R, Mooney DJ, Weitz DA. Microfluidic Generation of Monodisperse, Structurally Homogeneous Alginate Microgels for Cell Encapsulation and 3D Cell Culture. Adv Healthc Mater. 2015;4:1628–1633. doi: 10.1002/adhm.201500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderhoff JW, Bradford EB, Tarkowski HL, Shaffer JB, Wiley RM. Polymerization and Polycondensation Processes. American Chemical Society; 1962. Inverse Emulsion Polymerization; pp. 32–51. [Google Scholar]
- 67.Vettori MHPB, Franchetti SMM, Contiero J. Structural characterization of a new dextran with a low degree of branching produced by Leuconostoc mesenteroides FT045B dextransucrase. Carbohydr Polym. 2012;88:1440–1444. [Google Scholar]
- 68.Wanakule P, Liu GW, Fleury AT, Roy K. Nano-inside-micro: Disease-responsive microgels with encapsulated nanoparticles for intracellular drug delivery to the deep lung. Journal of Controlled Release. 2012;162:429–437. doi: 10.1016/j.jconrel.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 69.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 70.Welsch N, Becker AL, Dzubiella J, Ballauff M. Core-shell microgels as “smart” carriers for enzymes. Soft Matter. 2012;8:1428–1436. [Google Scholar]
- 71.Wohl-Bruhn S, Badar M, Bertz A, Tiersch B, Koetz J, Menzel H, Mueller PP, Bunjes H. Comparison of in vitro and in vivo protein release from hydrogel systems. J Control Release. 2012;162:127–133. doi: 10.1016/j.jconrel.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y, Hu H, Hu J, Liu S. Glucose-regulated insulin release from acid-disintegrable microgels covalently immobilized with glucose oxidase and catalase. Macromol Rapid Commun. 2012;33:1852–1860. doi: 10.1002/marc.201200411. [DOI] [PubMed] [Google Scholar]
- 73.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotech. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 74.Yin R, Wang K, Du S, Chen L, Nie J, Zhang W. Design of genipin-crosslinked microgels from concanavalin A and glucosyloxyethyl acrylated chitosan for glucose-responsive insulin delivery. Carbohydr Polym. 2014;103:369–376. doi: 10.1016/j.carbpol.2013.12.067. [DOI] [PubMed] [Google Scholar]
- 75.Young CJ, Poole-Warren LA, Martens PJ. Combining submerged electrospray and UV photopolymerization for production of synthetic hydrogel microspheres for cell encapsulation. Biotechnol Bioeng. 2012;109:1561–1570. doi: 10.1002/bit.24430. [DOI] [PubMed] [Google Scholar]
- 76.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 77.Zachman AL, Wang X, Tucker-Schwartz JM, Fitzpatrick ST, Lee SH, Guelcher SA, Skala MC, Sung HJ. Uncoupling angiogenesis and inflammation in peripheral artery disease with therapeutic peptide-loaded microgels. Biomaterials. 2014;35:9635–9648. doi: 10.1016/j.biomaterials.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]