Summary
Objective
The objective of this study was to compare the efficacy of two multi-agent chemotherapeutic regiments that were previously used at the Institution for treatment of advanced and recurrent endometrial cancer.
Methods
A retrospective review of patients with Stage III, IV, and recurrent endometrial cancer who received adjuvant chemotherapy at Roswell Park Cancer Institute over a period of 21 years. Two patient groups were defined based on treatment received: cisplatin, adriamycin, and VP-16 with or without megace (PAV-M), or carboplatin and paclitaxel (CT).
Results
Forty-two patients with advanced or recurrent endometrial cancer were included in this review based on regimen received. Median duration of follow up was 55 months. Treatment with PAV-M resulted in more dose modifications compared to CT group (42% vs 11%, respectively). There were no significant differences in disease-free survival or overall survival.
Conclusions
PAV/PAV-M is active in patients with advanced or recurrent endometrial cancer. However, toxicity associated with this triplet regimen may limit clinical use.
Keywords: Advanced endometrial carcinoma, Multi-agent chemotherapies, Adriamycin, Cisplatin, Etoposide
Introduction
The optimal chemotherapeutic regimen for advanced or recurrent endometrial cancer remains unknown. There is currently little hope of a cure for patients with metastatic or recurrent endometrial cancer. The current standard chemotherapy combination was established following the publication of Gynecologic Oncology Group (GOG) 177, which recognized the regimen of doxorubicin, cisplatin, and paclitaxel (TAP) as the optimal front line therapy, with median overall survival of 15 months [1].
Doublet therapy with carboplatin and paclitaxel, used as the comparison arm in GOG 177, has activity advanced endometrial cancer [2–4]. Other modalities including hormonal and progesterone therapies are effective in the treatment of recurrent and metastatic carcinoma with response rates of 9–34% [5–9].
The authors report a retrospective review of patients with Stage III, IV, and recurrent endometrial cancer who received adjuvant chemotherapy at Roswell Park Cancer Institute over a period of 21 years. Two patient groups were defined based on treatment received: cisplatin, adriamycin, and VP-16 with or without Megace (PAV-M), or carboplatin and paclitaxel (CT).
Material and Methods
Between 1980 and 2001, 69 patients were retrospectively identified with advanced or recurrent endometrial carcinoma who received treatment with multi-agent chemotherapy. Fourty-two patients were eligible for review based on pathological diagnosis and treatment with PAV/PAV-M or CT. Treatment schedule for PAV-M consisted of cisplatin (20 mg/2) daily for three days, etoposide (75 mg/m2) daily for three days, and adriamycin (40 mg/m2) on day one, and megace 160 mg daily every three weeks until progression of disease. CT consisted of carboplatin AUC 5 and paclitaxel 175 mg/m2 every three weeks until progression of disease.
Toxicity parameters determined dose modification, a 20% dose reduction of adriamycin and etoposide occurred if white blood count of < 1,000/mm3 white blood cells or platelets < 50,000/mm3 platelets was identified. Complete blood counts and basic metabolic panels were performed weekly. Electrocardiogram and physical examination were preformed every 21 days prior to next cycle of each therapy. Baseline cardiac ejection fraction was obtained before the first course of adriamycin and prior to each additional course after five courses were administered. Cardiac toxicity was defined as a decrease in cardiac ejection fraction of > 10% or the development of congestive heart failure. Baseline renal function was established. Nephrotoxicity was defined as increased serum creatinine > 2 mg/dl.
Study outcomes included overall survival and time to progression, defined by World Health Organization (WHO) criteria. Complete response was identified as disappearance of all lesions. Partial response was > 50% reduction in index lesions, stable disease a 50% reduction compared with baseline nor 25% increase in disease. Progressive disease was defined as > 25% increase in index lesion or appearance of new lesions. The duration of overall survival was the interval between diagnosis and death. Observation time was the interval between diagnosis and last contact (death or last follow-up). Data were censored at the last follow-up for patients with no evidence of recurrence, progression, or death.
Comparison between groups was calculated using the student t-test. Kaplan Meier log rank analysis was used to estimate overall survival and disease-free progression. A p value of 0.05 was considered statistically significant.
Results
The clinical characteristics of patients included in this review are shown in Table 1. No statistical difference between patient’s age, stage, grade of tumor, histology, previous surgical intervention, or primary treatment was identified. However, patients in the CT group were older, average age 70 years compared to 62 years (p = 0.7). Patients were more likely to be treated with radiation in the PAV/PAV-M group (57% vs 17%, p = 0.01). Among the PAV-M/PAV group, 42% required dose modification compared to 11% in the CT group (p = 0.07).
Table 1.
Patient demographics.
| PAV/PAV-M (n = 30) | CT (n = 12) | p | |
|---|---|---|---|
| Age | 62 (± 10) | 70 (± 10) | 0.7 |
| Stage | |||
| III | 4 (13%) | 0 | 0.1 |
| IV | 26 (87%) | 12 (100%) | |
| Histology | |||
| Endometroid | 9 (30%) | 3 (25%) | 0.1 |
| UPSC | 7 (24%) | 7 (58%) | |
| Clear cell | 1 (3%) | 0 | |
| Mixed | 13 (43%) | 2 (17%) | |
| Surgery | 29 (97%) | 12 (100%) | 0.5 |
| RXT | 17 (57%) | 2 (17%) | 0.01 |
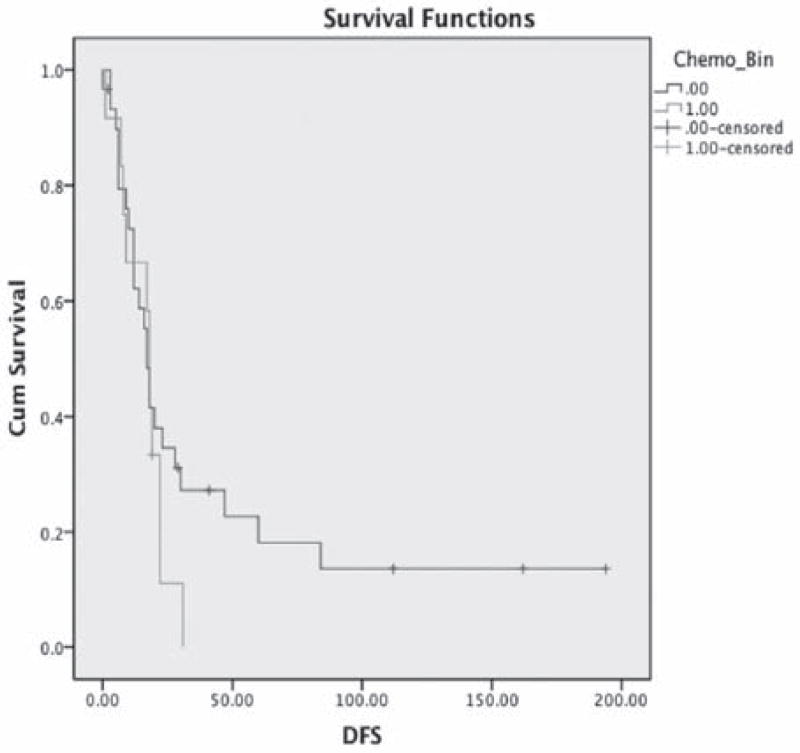
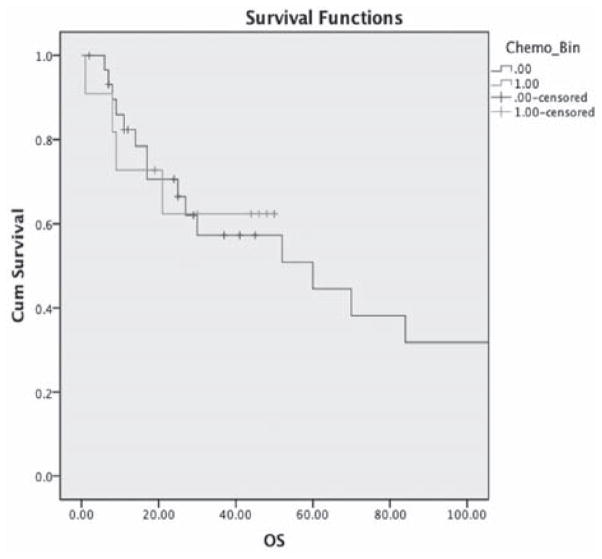
The median time to follow-up was 52 months. Disease-free progression appeared longer in the PAV/PAV-M group compared to CT group, but was not statistically significant (44 months vs 16 months p = 0.03) (Figure 1). No difference in overall survival between the two groups was identified (84 months vs 34 months p = 0.9) (Figure 2).
Figure 1.
Mean DFS PAV/PAV-M: 44 months (95% CI, 21 – 68). Carbo/Taxol: 16 months (95% CI, 11–21) (p = 0.3).
Figure 2.
Mean OS PAV/PAV-M: 84 months (95% CI, 52 – 119). Carbo/Taxol: 34 months (95% CI, 23–46) (p = 0.9).
Discussion
The standard chemotherapy regimen for advanced or recurrent endometrial cancer is cisplatin, paclitaxel, and adriamycin (TAP). This regimen was shown to be superior in a large GOG group phase III clinical trial (GOG 177). However, this regimen has significant hematologic and non-hematologic toxicities. Hematological toxicity was noted in 3% of patients; however, grade 3 neurologic toxicity was seen in 12% of patients, grade 2 neurologic toxicity was seen in 27% of patients [1]. Due to significant toxicities with TAP regimen, CT have been increasingly adopted in the treatment of women with advanced and recurrent endometrial cancer.
CT has a favorable side-effect profile as demonstrated in epithelial ovarian carcinoma [10, 11]. This has also been studied in prior phase II studies evaluating the efficacy and toxicity of CT in endometrial cancer [3, 12, 13]. Consequently, the GOG recently concluded a phase III trial of CT vs TAP (GOG 209) and results are pending. Comparative studies evaluating CT against previously utilized regimens are lacking.
This retrospective study was conducted to assess the response and toxicity associated with PAV/PAV-M, an established regimen in the Institution compared to CT [14, 15]. The authors found a trend towards a longer DFS and OS with the PAV/PAV-M regimen compared to CT group, although the differences did not achieve statistical significance most likely due to small sample size. Dose modification was performed in 42% of patients received PAV/PAV-M combination compared to 11% in the CT group. However, all the patients in the PAV/PAV-M group completed at least seven cycles of chemotherapy.
This study is limited by the small sample size and overall poor outcomes associated with advanced or recurrent endometrial cancer. The small sample size could account for the non-significant values found in overall survival and disease-free progression. If the awaited results of GOG 209 show CT to be equivalent or superior to TAP, triplet-based therapy will be discontinued due to increased toxicity with little benefit. However, if the regimen of CT is inferior, other triple or quadruple complications such as PAV and PAV-M need to be re-evaluated in a prospective setting.
Conclusion
In conclusion, as progress is being made in the treatment of advanced and recurrent endometrial cancer, older multi-agent chemotherapy regiments such as PAV or PAV-M need to be re-evaluated since they may be as effective and similarly tolerated as other triplet therapy. Furthermore, future efforts are necessary to identify the subset of patients that will more likely respond to PAV/PAV-M as compared to CT or newer regimens in advanced or recurrent endometrial cancer.
References
- 1.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 2.Akram T, Maseelall P, Fanning J. Carboplatin and paclitaxel for the treatment of advanced or recurrent endometrial cancer. Am J Obstet Gynecol. 2005;192:1365. doi: 10.1016/j.ajog.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Hoskins PJ, Swenerton KD, Pike JA, Wong F, Lim P, Acquino-Parsons C, et al. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol. 2001;19:4048. doi: 10.1200/JCO.2001.19.20.4048. [DOI] [PubMed] [Google Scholar]
- 4.Michener CM, Peterson G, Kulp B, Webster KD, Markman M. Carboplatin plus paclitaxel in the treatment of advanced or recurrent endometrial carcinoma. J Cancer Res Clin Oncol. 2005;131:581. doi: 10.1007/s00432-005-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley RM, Baker WH. Progestational agents in the treatment of carcinoma of the endometrium. N Engl J Med. 1961;264:216. doi: 10.1056/NEJM196102022640503. [DOI] [PubMed] [Google Scholar]
- 6.Lentz SS, Brady MF, Major FJ, Reid GC, Soper JT. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 1996;14:357. doi: 10.1200/JCO.1996.14.2.357. [DOI] [PubMed] [Google Scholar]
- 7.Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 8.Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19:364. doi: 10.1200/JCO.2001.19.2.364. [DOI] [PubMed] [Google Scholar]
- 9.Whitney CW, Brunetto VL, Zaino RJ, Lentz SS, Sorosky J, Armstrong DK, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:4. doi: 10.1016/j.ygyno.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected Stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 11.Piccart MJ, Stuart GC, Cassidy J, Bertelsen K, Parmar MK, Eisenhauer EA, et al. Intergroup collaboration in ovarian cancer: a giant step forward. Ann Oncol. 1999;10(suppl 1):83. doi: 10.1023/a:1008371821148. [DOI] [PubMed] [Google Scholar]
- 12.Price FV, Edwards RP, Kelley JL, Kunschner AJ, Hart LA. A trial of outpatient paclitaxel and carboplatin for advanced, recurrent, and histologic high-risk endometrial carcinoma: preliminary report. Semin Oncol. 1997;24(5 suppl 15):S15–78. [PubMed] [Google Scholar]
- 13.Nakamura T, Onishi Y, Yamamoto F, Kouno S, Maeda Y, Hatae M. Evaluation of paclitaxel and carboplatin in patients with endometrial cancer. Gan To Kagaku Ryoho. 2000;27:257. [PubMed] [Google Scholar]
- 14.Cornelison TL, Baker TR, Piver MS, Driscoll DL. Cisplatin, adriamycin, etoposide, megestrol acetate versus melphalan, 5-fluorouracil, medroxyprogesterone acetate in the treatment of endometrial carcinoma. Gynecol Oncol. 1995;59:243. doi: 10.1006/gyno.1995.0015. [DOI] [PubMed] [Google Scholar]
- 15.Piver MS, Fanning J, Baker TR. Phase II trial of cisplatin, adriamycin, and etoposide for metastatic endometrial adenocarcinoma. Am J Clin Oncol. 1991;14:200. doi: 10.1097/00000421-199106000-00006. [DOI] [PubMed] [Google Scholar]