Abstract
Tetrapods contain a single CD4 co-receptor with four immunoglobulin domains that likely arose from a primordial two-domain ancestor. Notably, teleost fish contain two CD4 genes. Like tetrapod CD4, CD4-1 of rainbow trout includes four immunoglobulin domains while CD4-2 contains only two. Since CD4-2 is reminiscent of the prototypic two-domain CD4 co-receptor, we hypothesized that by characterizing the cell types bearing CD4-1 and CD4-2, we would shed light into the evolution and primordial roles of CD4-bearing cells. Using newly established monoclonal antibodies against CD4-1 and CD4-2, we identified two bona fide CD4+ T-cell populations, a predominant lymphocyte population co-expressing surface CD4-1 and CD4-2 (CD4 DP), and a minor subset expressing only CD4-2 (CD4-2 SP). While both subsets produced equivalent levels of Th1, Th17, and Treg cytokines upon bacterial infection, CD4-2 SP lymphocytes were less proliferative and displayed a more restricted TCRβ repertoire. These data suggest that CD4-2 SP cells represent a functionally distinct population and may embody a vestigial CD4+ T cell subset, the roles of which reflect those of primeval CD4+ T cells. Importantly, we also describe the first CD4+ monocyte/macrophage population in a non-mammalian species. Of all myeloid subsets, we found the CD4+ population to be the most phagocytic, while CD4+ lymphocytes lacked this capacity. This study fills in an important gap in the knowledge of teleost CD4-bearing leukocytes thus revealing critical insights into the evolutionary origins and primordial roles of CD4+ lymphocytes and CD4+ monocyte/macrophages.
Introduction
The largest subset of T cells in jawed vertebrates expresses a TCR bearing α and β chains that recognizes antigens bound to MHC molecules (1). Such T cells use two main co-receptors, CD4 and CD8 which show mutually exclusive expression on naive helper T (Th) and cytotoxic T cells respectively in mammals (2). CD4+ Th cells can further differentiate into a variety of effector Th-cell subsets that produce cytokines involved in the regulation of inflammation and immune responses against different types of pathogens (3). Mammalian CD4 is also expressed on cell types other than T lymphocytes (4). CD4 expression pattern shows species disparity but often defines functionally distinct subsets in a certain myeloid cell population. Significantly, the majority of human and rat monocytes/macrophages express CD4 while in mouse and birds CD4 appears to be absent in these cells (5, 6). Whether CD4-expressing monocytes/macrophages play any particular role in immunity remains for the most part a mystery.
Although sharks contain TCR-expressing lymphocytes (7, 8), recent genome sequence and transcriptome analyses of cartilaginous fish (elephant and nurse shark) have failed to identify a molecule with classical CD4 features, thus making teleosts the oldest living species with bona fide CD4 co-receptors (9–12). In contrast to the situation of tetrapods, which possess a single CD4 gene, bony fish contain two CD4 genes, cd4-1 and cd4-2, which share low amino acid identity (~20%) (13, 14). Like tetrapod CD4, teleost CD4-1 contains four Ig domains: two V (D1 and D3) and two C (D2 and D4) domains. In contrast, CD4-2 may contain two or three Ig domains (1V and 1C or 2V [D1 and D3] and 1C) (13, 14). Similarities between D1 and D3 and between D2 and D4 of tetrapod CD4 have led to the hypothesis that the four-domain CD4 emerged through the duplication of the gene encoding an ancestral two-domain (V-C) receptor (14–18). Hence, it has been hypothesized that the teleost two-domain CD4-2 might be reminiscent of this ancestral gene (14, 18). In line with these hypotheses, a CD4-like gene encoding only two Ig domains (V-C) has been identified in lamprey, and it was proposed to represent the prototypic two-domain CD4 co-receptor in vertebrate (19). It is worth noting that lymphocyte-specific protein tyrosine kinase (LCK) in catfish binds to both CD4-1 and CD4-2, thus suggesting that both teleost CD4 co-receptors are functionally active and play a role in T-cell development and activation (20).
Thus far the concurrent surface expression of CD4-1 and CD4-2 on teleost leukocytes has not been determined due to the lack of reagents capable of detecting both molecules in a single species. In the absence of those, transcript levels have been used to assess expression patterns of cd4-1 and cd4-2, but results in teleosts have been inconclusive. For example, flounder cd4-1 and cd4-2 transcripts are expressed in mutually exclusive cell types (21) while transcript analysis of sorted CD4-1+ cells in zebrafish, ginbuna carp, and fugu suggests the existence of cells expressing CD4-1 and/or CD4-2 (22–24). Due to the poor characterization of teleost CD4+ leukocytes, very few functional studies have been carried out on these cells. More specifically, it has been reported that CD4-1+ cells in ginbuna carp and zebrafish undergo antigen-specific proliferation (25–27). With regards to cytokine expression, zebrafish and fugu CD4-1+ cells can produce T-cell related cytokines in response to stimulation with TLR ligands and specific antigen (22, 24); however the expression of such cytokines upon pathogen challenge remains to be studied. The only study performed on teleost CD4-2+ cells suggests that these leukocytes represent a regulatory T cell (Treg)-like phenotype in pufferfish (28). In the context of these functional similarities between tetrapod CD4+ T cells and teleost CD4-1+ or CD4-2+ cells, it has been well documented that teleost fish contain most of the critical genes (i.e., cytokines and their receptors) involved in T-cell function, thus supporting the potential presence of effector T-cell subsets in teleost fish (29, 30).
Since two-Ig-domain CD4 molecules are reminiscent of the prototypic CD4 co-receptor (14, 18, 19), we hypothesized that by gaining understanding into the cell types bearing CD4 with either two- or four-Ig domains, we could shed light into the evolutionary history of CD4-bearing cells as well as their primordial roles in immunity. To this end, we phenotypically and functionally characterized CD4-1- and CD4-2-expressing cells in rainbow trout, a model species in the field of evolutionary and comparative immunology (31). Our studies represent the first comprehensive phenotypic and functional characterization of two- and four-domain CD4-bearing lymphoid and myeloid cells in a vertebrate species and reveal critical insights into the evolutionary origins and functionally conserved roles of vertebrate CD4+ T cells and CD4+ monocytes/macrophages.
Materials and methods
Fish
Rainbow trout (Oncorhynchus mykiss) were provided by the National Center for Cool and Cold Water Aquaculture (NCCCWA) and Clear Springs Foods Inc. Fish were maintained in the laboratory of J.O.S. as previously described (32). Animal procedures were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania.
PCR and cDNA cloning
RNA extraction and gene expression analyses of sorted trout leukocytes were performed as described previously by us (32–34). Real-time PCR was performed with Power SYBR Green Master Mix (Thermo Fisher Scientific) and gene-specific primers in a 7500 Fast Real-Time PCR System or ViiA 7 Real-Time PCR System (Thermo Fisher Scientific) depending on sample numbers analyzed. Primer sequences are shown in Supplemental Table I.
Development of monoclonal antibodies (mAbs) against rainbow trout CD4-1 and CD4-2
Rainbow trout CD4-1, CD4-2a, CD4-2b, and LAG-3 (Accession numbers: AAY42068 [http://www.ncbi.nlm.nih.gov/protein/AAY42068], AAX09644 [http://www.ncbi.nlm.nih.gov/protein/AAX09644], AAX84789 [http://www.ncbi.nlm.nih.gov/protein/AAX84789], and ADM15538 [http://www.ncbi.nlm.nih.gov/protein/ADM15538], respectively) were cloned from cDNA generated from rainbow trout thymocytes. The full length of the coding DNA sequences except the signal peptide of CD4-1 and the cDNA sequences encoding Ig domains for CD4-2a, CD4-2b, and LAG-3 were further subcloned into pDisplay vector (Thermo Fisher Scientific) containing an HA (hemagglutinin A) tag (for both CD4s and LAG-3) and a PDGFR transmembrane region (for CD4-2a, CD4-2b, and LAG-3). The aforementioned DNA constructs were further subcloned into a pMXs-IRES-Puro Retroviral Expression Vector (Cell Biolabs). Plat-E Retroviral Packaging Cell Lines (Cell Biolabs) were thereafter transfected with the pMXs vectors by using FuGENE HD Transfection Reagent (Promega). NRK-52E (Normal Rat Kidney cells; ATCC CRL-1571) were infected with the retrovirus produced from the transfected Plat-E cells. The resulting stable NRK cells expressing rainbow trout CD4-1 (CD4-1/NRK), CD4-2a (CD4-2a/NRK), CD4-2b (CD4-2b/NRK), and LAG-3 (LAG-3/NRK) were thereafter selected with puromycin (InvivoGen). The expression of CD4-1, CD4-2a, CD4-2b, and LAG-3 on NRK cells was confirmed by flow cytometry with a murine anti-HA mAb (Sigma-Aldrich). With the goal to produce soluble CD4-2b, the cDNA sequence encoding the Ig domains of CD4-2b were also subcloned into pINFUSE-mIgG2b-Fc2 vector (InvivoGen). CD4-2b/Fc fusion protein was produced with FreeStyle 293 Expression System (Thermo Fisher Scientific) according to the manufacturer’s instructions, and then purified with HighTrap Protein G HP column (GE Healthcare). Rats were immunized with either twenty million CD4-1/NRK cells or 100 μg of CD4-2b/Fc-fusion protein emulsified in TiterMax Gold (Sigma-Aldrich). Both preparations were injected into the tail-base muscle of different Sprague Dawley Rats. Two weeks after immunization, lymph node cells from the rat iliac lymph node were isolated as previously described (25, 35). Cell fusion of SP2/0 myeloma cells with rat lymph node cells and hybridoma culture were performed in the Cell Center Service Facility at the University of Pennsylvania. Of all hybridomas stably secreting antibodies to CD4-1 or CD4-2, we chose three different hybridomas to characterize CD4-1- and CD4-2-bearing leukocytes in trout: clone 4.1.1 (recognizing CD4-1; rat IgG1 isotype), clone 4.1.2 (recognizing CD4-1; rat IgG2a isotype), and clone 4.2.12 (recognizing CD4-2b; rat IgG2b isotype). The isotypes of rat mAbs were determined with the Rat Immunoglobulin Isotyping ELISA Kit (BD Phamingen). For further validation of mAb specificity, we used the Amaxa Cell Line Nucleofector Kit L (Lonza) to transfect the trout embryo cell line (STE-137) with the same pDisplay vectors encoding rainbow trout CD4-1, CD4-2a, CD4-2b, and LAG-3. The transfection conditions were found according to the Amaxa Cell Line Nucleofector Kit instructions. Transfected STE-137 cells expressing the aforementioned molecules were then tested by flow cytometry for the reactivity of the mAbs to rainbow trout CD4-1 and CD4-2b. The expression of aforementioned molecules on the transfected STE-137 cells was confirmed with the simultaneous staining of the murine anti-HA mAb and rat anti-CD4-1 or anti-CD4-2b mAbs. Alexa Fluor 647-conjugated goat anti-mouse IgG1 and Alexa Fluor 488-conjugated donkey anti-rat IgG (H+L) (Jackson ImmunoResearch; 2 μg/ml) were used as secondary antibodies.
The positive hybridomas for trout CD4-1 and CD4-2b were injected intraperitoneally into nude mice to produce ascites (Cocalico Biologicals, PA). Alternatively, mAbs were also produced in Hybridoma-SFM (Thermo Fisher Scientific) in the Cell Center Service Facility at the University of Pennsylvania. The IgG fraction from the ascites and supernatant of the serum-free medium was purified using a HiTrap protein G and L column (GE Healthcare) according to the instructions of the manufacturer.
Flow cytometry and cell sorting
Trout leukocytes from lymphoid organs and blood were isolated as previously reported (32–34). Briefly, blood was drawn from the caudal vein with a heparinized syringe and diluted in a DMEM (Gibco) medium supplemented with 1% FBS (Serum Source International) and 1% Penicillin-Streptomycin (Gibco). Trout organs (spleen, head kidney [HK], and thymus) were removed and pressed through a 100-μm cell strainer (Corning Life Sciences) and suspended in DMEM. The diluted blood and cell suspensions from spleen, HK and thymus were layered onto 34/51% discontinuous Percoll (GE Healthcare) density gradients. After centrifugation (400×g, 30 min), cells lying at the interface of the gradient were collected and washed with DMEM twice. The cell suspensions were kept on ice until further use. To initially identify trout CD4-bearing cells, HK leukocytes were stained with biotinylated rat anti-CD4-1 (clone 4.1.1; 5 μg/ml) and rat anti-CD4-2b (clone 4.2.12; 5 μg/ml). Stained cells were detected with Brilliant Violet 421 Streptavidin (BioLegend; 1 μg/ml) and mouse anti-rat IgG2b-PE (SouthernBiotech; 1 μg/ml). To elucidate whether trout CD8α+ cells expressed CD4-1+ and/or CD4-2+, leukocytes were stained with biotinylated rat anti-CD4-1 (clone 4.1.1; 5 μg/ml), rat anti-CD4-2b (clone 4.2.12; 5 μg/ml), and rat anti-CD8α (clone 13.2D; rat IgG2a isotype; 5 μg/ml) (35) primary mAbs. Stained cells were detected with Brilliant Violet 421 Streptavidin (BioLegend; 1 μg/ml), mouse anti-rat IgG2b-PE (SouthernBiotech; 1 μg/ml), and mouse anti-rat IgG2a-Alexa Fluor 647 (SouthernBiotech; 1 μg/ml). To elucidate whether trout B cells expressed CD4-1+ and/or CD4-2+, leukocytes were stained with rat anti-CD4-1 (clone 4.1.2; 5 μg/ml), rat anti-CD4-2b primary mAbs in combination with either biotinylated mouse anti-IgM (clone 1.14; mouse IgG1 isotype; 1 μg/ml) (36) or biotinylated mouse anti-IgT (clone 41.8; mouse IgG2b isotype; 1 μg/ml) (32) primary mAbs. Stained leukocytes were thereafter detected with mouse anti-rat IgG2a PE (eBioscience; 1 μg/ml), mouse anti-rat IgG2b eFluor 660 (eBioscience; 1 μg/ml) mAbs and Brilliant Violet 421 Streptavidin. Cell suspensions were incubated on ice with primary mAbs and corresponding secondary conjugates for 30 min and 15 min, respectively, and then were washed twice with DMEM after each respective staining step. As controls, we used biotinylated rat IgG1 (clone RTK2071; BioLegend; 5 μg/ml), rat IgG2a (clone eBR2a; eBioscience; 5 μg/ml), rat IgG2b (clone RTK4530; BioLegend; 5 μg/ml), biotinylated mouse IgG1 (clone MOPC-21; BioLegend; 1 μg/ml), and biotinylated mouse IgG2b (clone MPC-11; BioLegend; 1 μg/ml) isotype-matched control mAbs. Flow cytometry was performed using a BD LSRFortessa cell analyzer (BD Biosciences). Alternatively, stained cells were sorted for cytology and gene expression analyses with BD FACSAria II flow cytometer (BD Biosciences) as previously reported (32, 33). Data on flow cytometry were analyzed using FlowJo software (Tree Star).
Cytochemical staining
Sorted cells and HK leukocytes were spun onto Poly-L-Lysine coated slides (Newcomer Supply) by using a Shandon Cytospin. Cytospin preparations were stained with Wright-Giemsa-like (WG; Hema 3 from Thermo Fisher Scientific), myeloperoxidase (MPO), Sudan Black B (SBB), naphthol AS-D chloroacetate esterase (NCAE), and β-glucuronidase (BG) staining (Sigma-Aldrich) according to the manufacturer’s instructions. The cells were imaged with Eclipse E600 (Nikon).
Immunofluorescence microscopy
Spleen leukocytes were stained with biotinylated rat anti-CD4-1 (clone 4.1.1; 5 μg/ml) and rat anti-CD4-2b (5 μg/ml) primary mAbs followed by labeling with streptavidin Alexa Fluor 488 conjugates (Jackson ImmunoResearch; 2 μg/ml) and mouse anti-rat IgG2b Alexa Fluor 647 (SouthernBiotech; 2 μg/ml) mAbs as described above. Stained cells were then fixed with Fixation buffer (Biolegend), counterstained with DAPI (Biolegend), and mounted on slides with Fluoromount-G (SouthernBiotech) according to the manufacturer’s instructions. The cells were imaged with Eclipse E600 (Nikon).
Western blot analysis
NRK cells and NRK transfectants were lysed with Laemmli sample buffer. The lysed samples were resolved on 4–15% SDS-PAGE Ready Gel (Bio-Rad) under reducing conditions. The gels were transferred onto Sequi-Blot PVDF membranes (Bio-Rad). The membranes were blocked with 8% skim milk and incubated with anti-CD4-1 (clone 4.1.2; 2 μg/ml) or anti-CD4-2b (2 μg/ml) mAbs followed by incubation with peroxidase-conjugated anti-rat IgG (GE Healthcare). Immunoreactive bands were visualized using the HyGLO Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific Products).
CDR3 length spectratyping analysis
For the repertoire analysis of CD4+ T-cell populations, we isolated whole HK leukocytes of healthy adult rainbow trout (~50g) and sorted their CD4 DP and CD4-2 SP lymphocytes. Total RNA from whole HK leukocytes was purified and DNase treated using the RNeasy Mini Kit (Qiagen). RNA (2 μg) was reverse transcribed into cDNA using Superscript II Reverse Transcriptase (Thermo Fisher Scientific) with 2.5 mM oligodT25 primer in a final volume reaction of 20 μl. For sorted CD4-2 SP and CD4 DP cells, total RNA was extracted from each cell population using RNeasy Micro Kit (Qiagen). Full-length cDNA was generated and amplified using the SMARTer PCR cDNA synthesis kit (Clontech Laboratoires), following the manufacturer’s instructions. The optimized protocol of this kit preferentially enriches for full-length cDNAs and retains true gene representation of genes in the original sample. The optimal number of PCR cycles determined for ds cDNA synthesis and amplification of CD4-2 SP and CD4 DP samples was 21 cycles.
The spectratyping of T-cell receptor beta variable region (TRBV) CDR3 length (Immunoscope analysis) were performed as previously described (37). Primer sequences for the spectratyping of TRBV CDR3 length are shown in Supplemental Table I. The repertoire diversity can be assessed by a diversity score based on the concept of Shannon entropy that provides a measure of the quantity of information encompassed in the repertoire (37, 38). Repertoire diversity scores were used to perform Principal Component Analysis (PCA) in order to compare the statistical dispersion of the samples on a multidimensional plan. Statistical and multivariate analyses were performed using R software (http://www.r-project.org/).
Importantly, we verified that low values of diversity index computed for the CD4-2 SP subset - compared to the CD4 DP - were not due to the smaller number of sorted CD4-2 SP cells. To do so, we followed two approaches: (i) cDNA used for spectratyping was synthesized from different amounts of RNA, allowing to compare profiles obtained from similar amount of RNAs from CD4-2 SP and CD4 DP cells; and (ii) spectratyping was always repeated from independent initial cDNA amplifications to verify that skewed profiles - mainly observed in CD4-2 SP subset - were not due to random picking of template molecules. Only reproducible profiles, hence corresponding to templates concentrated enough to avoid such artefact, were considered in our analysis.
Proliferation assay
To determine the proliferative responses of CD4+ lymphocytes to mitogens, spleen and HK leukocytes were labeled with CellTrace Violet (Life Technologies) according to manufacturer’s instruction. Labeled cells were plated in a 96-well round-bottom microplate (Corning) and cultured with either 1 μg/ml of leucoagglutinin PHA-L (Sigma-Aldrich), a mix of E. coli 0111:B4 LPS and E. coli 055:B5 LPS (Sigma-Aldrich) (1:1 ratio, dose 100 μg/ml), or DMEM containing 10% FBS, 1% Penicillin-Streptomycin (Gibco), and 0.1% Gentamicin (Gibco). To examine one-way mixed leukocytes reaction (MLR), leukocytes from blood, spleen, and HK of outbred trout from the ARS-Fp-C strain, which are produced in NCCCWA (39), were isolated as described above. With the goal of performing the MLR, we also collected blood leukocytes of outbred trout from the Fish Lake DeSmet (FLDS) strain, which are produced at the Egan State Fish Hatchery, Utah Division of Wildlife Resources (40). Spleen and HK leukocytes from ARS-Fp-C trout were labeled with CellTrace Violet and were used as responder cells. Leukocytes from blood of FLDS and ARS-Fp-C trout strain were used as allogeneic and autologous stimulator cells, respectively. To neutralize the proliferative ability of these stimulator cells, leukocytes were incubated with 50 μg/ml of Mitomycin C from Streptomyces caespitosus (Sigma-Aldrich) for 30 min, and were washed three times with DMEM. The same cell number (5×105 cells) of responder and stimulator cells was co-cultured in a 96-well round-bottom microplate. For antigen-specific proliferation of CD4+ cells, fish were first immunized with 200 μg of Hemocyanin, Keyhole Limpet (KLH), Megathura crenulata (EMD Millipore) emulsified in Freund’s complete adjuvant (Sigma-Aldrich). Three weeks after primary immunization, fish were boosted with the same amount of antigen emulsified in Freund’s incomplete adjuvant (Sigma-Aldrich). Ten days after boosting, spleen and HK leukocytes from immunized fish were isolated and labeled with CellTrace Violet. Labeled cells were incubated with either 100 μg/ml of KLH, 100 μg/ml of Ovalbumin (OVA, Sigma-Aldrich) as unrelated control protein, or DMEM alone (as culture medium control). All incubations were carried out in a humidified atmosphere of 5% CO2 at 20°C for 7 days. For all proliferation experiments, after 7 days incubation of cells with stimulants, proliferation of CD4+ cells was assessed by their staining with anti-CD4-1 (clone 4.1.2; 5 μg/ml) and anti-CD4-2b (5 μg/ml) primary mAbs followed by labeling with mouse anti-rat IgG2a PE (eBioscience; 1 μg/ml) and mouse anti-rat IgG2b eFluor 660 (eBioscience; 1 μg/ml) mAbs as described above. 7-AAD (1 μg/ml; Thermo Fisher Scientific) was added to the cell suspension for the detection and exclusion of dead cell. The percentage of proliferating CD4+ cells was analyzed by flow cytometry by the dye-dilution method according to the manufacturer’s instructions (Thermo Fisher Scientific).
Yersinia ruckeri infection
Y. ruckeri strain YRNC10 was grown in Brain heart infusion broth and agar (Becton Dickinson). Rainbow trout (~100g) were challenged by intraperitoneal injection of a dose of ~2×105 colony forming units (cfu) in PBS. Viable cfu were determined by plate counts. Control fish were injected with the same volume of PBS (200 μl). Four days after challenge, splenic leukocytes were sampled and sorted as described above.
Phagocytic assay
Phagocytosis experiments were assessed as previously reported by us (32, 33). Briefly, HK leukocytes were isolated as described above. Cells (2×105 cells/well) were plated and incubated with 1.0 μm Fluoresbrite Yellow Green (YG) Microspheres (Polysciences) at 1:10 (cell:bead) ratio. Alternatively, cells were incubated with pHrodo Red E. coli BioParticles conjugates (Thermo Fisher Scientific). The mixture of cells and the phagocytic targets were placed in a humidified atmosphere of 5% CO2 at 18°C. After 3h incubation, cells were collected and non-ingested beads and E. coli were removed by centrifuging cells over a 3% BSA-4.5% glucose gradient. After washing with cold PBS, leukocytes were stained with rat anti-CD4-1 (clone 4.1.2; 5 μg/ml) and rat anti-CD4-2b (clone 4.2.12; 5 μg/ml) mAbs followed by labeling with mouse anti-rat IgG2a PerCP-eFluor 710 (eBioscience; 1 μg/ml) and mouse anti-rat IgG2b eFluor 660 (eBioscience; 1 μg/ml) mAbs as described above, and analyzed by flow cytometry in a FACScanto (BD Biosciences). For immunofluorescence microscopy, HK leukocytes were incubated with 1.0 μm FluoSpheres crimson fluorescent microspheres (Thermo Fisher Scientific) at 1:10 (cell:bead) ratio. After 6h incubation, non-ingested beads were removed with the aforementioned method. Leukocytes were labeled with biotinylated anti-CD4-1 mAbs (clone 4.1.1; 5 μg/ml) and streptavidin Alexa Fluor 488 conjugates (Jackson ImmunoResearch; 2 μg/ml). Stained cells were then fixed and were imaged as described above.
Statistical analysis
Paired or unpaired Student’s t-test (Excel version 14.0; Microsoft), nonparametric Mann-Whitney test, and one-way ANOVA with Bonferroni correction (Prism version 6.0; GraphPad) were used for analysis of differences between groups. P values of 0.05 or less were considered statistically significant.
Results
Development of anti-CD4-1 and anti-CD4-2b mAbs
In addition to CD4-1, salmonid fish contain two cd4-2 genes (cd4-2a and cd4-2b), which are probably derived from salmonid-specific genome duplication, sharing ~80% amino acid identity (18, 41, 42). To study the surface expression of CD4-1 and CD4-2 molecules in trout leukocytes, we produced mAbs against CD4-1 and CD4-2b that specifically recognized transfected mammalian and trout cells expressing surface CD4-1 and CD4-2b, respectively (Supplemental Fig. 1A, 1C, 1D). The anti-CD4-1 mAbs did not cross-react with CD4-2-transfected rat and trout cell lines, and vice versa, the anti-CD4-2b mAbs did not cross-react with CD4-1-transfected cells (Supplemental Fig. 1A, 1C, 1D). Western blotting with mammalian transfectants further verified the specificity of the anti-CD4-1 and anti-CD4-2b mAbs, which specifically recognized the protein with expected molecular size from CD4-1- and CD4-2b-transfectants, respectively (Supplemental Fig. 1B). Moreover, none of these mAbs cross-reacted with trout CD4-2a or LAG-3 (Supplemental Fig. 1). However, since cd4-2a transcripts were exclusively expressed in all lymphocytes expressing CD4-2b molecules (see below), the cells expressing surface CD4-2b are hereafter described as CD4-2+ cells.
Identification of leukocyte populations expressing trout CD4-1 and CD4-2
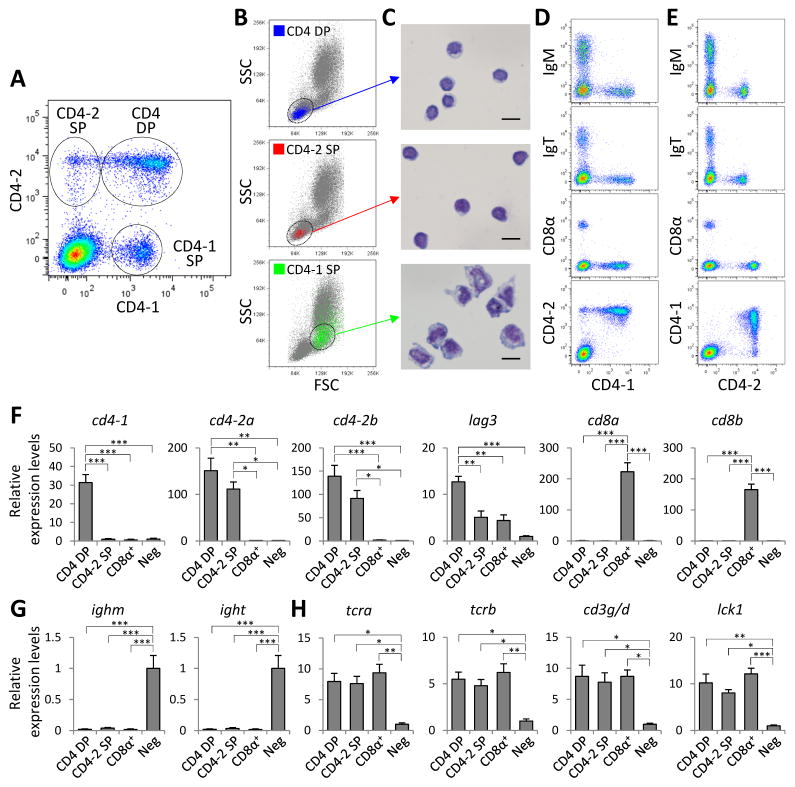
Flow cytometry of whole HK leukocytes double-stained with anti-CD4-1 and anti-CD4-2b mAbs revealed three distinct leukocyte populations: CD4-1+/CD4-2− (CD4-1 SP), CD4-1−/CD4-2+ (CD4-2 SP), and CD4-1+/CD4-2+ (CD4 DP) subsets (Fig. 1A; isotype-matched control mAbs, Supplemental Fig. 1E). CD4 DP and CD4-2 SP cells were localized within the lymphocyte population (Fig. 1B, top and middle) and displayed typical lymphocyte morphology (Fig. 1C, top and middle). In contrast, CD4-1 SP cells were found within the myeloid cell population (Fig. 1B, bottom) and displayed a morphology resembling that of monocyte/macrophage-like cells (Fig. 1C, bottom). Interestingly we could not detect lymphocytes exclusively expressing surface CD4-1.
FIGURE 1.
Characterization of leukocyte populations expressing surface CD4-1 and CD4-2. (A) Flow cytometry of HK leukocytes double-stained with anti-CD4-1 and anti-CD4-2b mAbs. Representative dot plot shows CD4-2 vs. CD4-1 expression on whole HK leukocytes. Three different cell populations are circled: CD4-1+/CD4-2+ (CD4 DP), CD4-1−/CD4-2+ (CD4-2 SP), and CD4-1+/CD4-2− (CD4-1 SP) cells. (B) Representative dot plot profiles (FSC vs. SSC) of CD4 DP, CD4-2 SP, and CD4-1 SP cells. The distributions of CD4 DP (top), CD4-2 SP (middle) and CD4-1 SP (bottom) cells are shown in blue, red and green dots respectively, while negatively stained cells are in gray dots. (C) The morphology of sorted CD4 DP (top), CD4-2 SP (middle), and CD4-1 SP cells (bottom), visualized by WG staining. Scale bar = 10 μm. (D and E) Staining of HK leukocytes with anti-CD4-1 (D) or anti-CD4-2b (E) mAbs in combination with anti-IgM, anti-IgT, or anti-CD8α mAbs. Representative dot plots show stained cells within the lymphocyte gate. Data in A–E are representative of three independent experiments (n = 12 fish). (F–H) Transcription profile of sorted lymphocyte populations. CD4 DP, CD4-2 SP, CD8α+, and CD4-1−/CD4-2−/CD8α− lymphocytes (Neg), were sorted from HK leukocytes. Gene expression analysis of these sorted lymphocyte populations was performed by real-time PCR for the genes encoding for: (F) T-cell co-receptors (cd4-1, cd4-2a, cd4-2b, lag3, cd8a, and cd8b); (G) B-cell receptors (membrane bound form of ighm and ight); and (H) TCR/CD3 complex and Lck (tcra, tcrb, cd3g/d, and lck1). The transcript levels of indicated genes in F–H are shown relative to the expression levels in the Neg lymphocyte population (set to 1) and are expressed as mean ± SEM (n = 4 fish). Data in F–H are representative of two independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 (one-way ANOVA with Bonferroni correction).
We next focused our studies on the two CD4+ lymphocyte populations (CD4 DP and CD4-2 SP). Both lymphocyte subsets lacked the expression of surface IgM, IgT, and CD8α, indicating that CD4 DP and CD4-2 SP lymphocytes are neither B nor CD8α+ T cells (Fig. 1D, 1E). Importantly, CD4 DP cells comprised the main CD4+ lymphocyte subset (Fig. 1D, 1E, bottom). To characterize further the phenotype of the CD4 DP and CD4-2 SP lymphocyte subsets and confirm their identity as T cells, we assessed the expression of a variety of B- and T-cell-related genes in four sorted lymphocyte populations: CD4 DP, CD4-2 SP, CD8α+, and CD4-1−/CD4-2−/CD8α− negative lymphocytes (Neg). In agreement with the staining by the anti-CD4-1 and anti-CD4-2b mAbs (Fig. 1A, 1D, 1E), we confirmed that cd4-1 transcripts were only detected in CD4 DP lymphocytes while cd4-2 transcripts (cd4-2a and cd4-2b) were expressed in both CD4 DP and CD4-2 SP subsets (Fig. 1F). As expected, transcripts defining CD8+ T cells (cd8a and cd8b) and B cells (membrane bound form of ighm and ight) were absent in both CD4 DP and CD4-2 SP lymphocytes (Fig. 1F, 1G). Furthermore, CD4 DP, CD4-2 SP, and CD8α+ lymphocytes expressed similar degrees of key T-cell marker genes (tcra, tcrb, cd3g/d, and lck1), whereas as expected, the Neg population expressed very low levels of these transcripts (Fig. 1H).
Distribution of CD4 DP and CD4-2 SP lymphocytes in systemic lymphoid tissues
We found CD4 DP and CD4-2 SP lymphocytes in all tissues examined while negligible numbers of CD4-1 SP lymphocytes were detected (Fig. 2A). As expected, there was a large percentage of total CD4+ lymphocytes (including CD4 DP and CD4-2 SP cells) amongst thymic lymphocytes (~74.5%) while a significant percentage was also observed in the lymphocyte gate from spleen (~10.5%) and HK (~22.6%) (Fig. 2A, 2D). The frequency of CD4 DP and CD4-2 SP lymphocytes in blood leukocytes was very low (~1.4%) (Fig. 2A, 2D). CD4 DP cells were the predominant population of CD4+ lymphocytes in all tissues examined and accounted for ~83–91% of the total CD4+ lymphocyte count (Fig. 2E). Similar to HK (Fig. 1D, 1E), both CD4 DP and CD4-2 SP cells in blood, spleen, and thymus were devoid of surface IgM and IgT expressions (data not shown). As observed in chicken and most mammals, except for the thymus, no CD8α surface expression was detected on CD4+ lymphocytes in any organs analyzed (Fig. 2B, 2C). In contrast, ~74% and ~35% of CD4 DP and CD4-2 SP thymocytes respectively displayed CD8α expression on their surface (Fig. 2B, 2C, rightmost panel). Overall, eight distinct thymocyte populations could be detected: CD4 DP/CD8α+ (~46.4%); CD4 DP/CD8α− (~16.3%); CD4-2 SP/CD8α+ (~4.9%); CD4-2 SP/CD8α− (8.9%); CD4-1SP/CD8α+ (~2.1%); CD4-1SP/CD8α− (~1.3%); CD4 double-negative (DN)/CD8α+ (~9.8%); and CD4 DN/CD8α− (~10.3%) thymocytes (Fig. 2F). Immunofluorescence microscopy studies showed that most of the CD4-1 and CD4-2 staining in both splenic CD4 DP (Fig. 2G) and CD4-2 SP (Fig. 2H) lymphocytes appeared punctuated and unevenly distributed in vesicle-like structures not present in cells stained with isotype-matched control mAbs (Fig. 2I). More importantly, for the most part we did not observe co-localization of CD4-1 and CD4-2 staining in CD4 DP cells, thus indicating the lack of a potential association or complex formation between surface CD4-1 and CD4-2 molecules (Fig. 2G).
FIGURE 2.
Distribution of CD4+ lymphocyte subsets in lymphoid tissues. (A-C) Flow cytometry of leukocytes from blood (PBL), spleen (SPL), HK (HKL), and thymus (THY) stained with anti-CD4-1, anti-CD4-2b, and anti-CD8α mAbs. Representative dot plots show CD4-2 vs. CD4-1 (A), CD4-1 vs. CD8α (B), and CD4-2 vs. CD8α (C) expressions within the lymphocyte population. The values adjacent to outlined areas indicate percentage of each subset within the lymphocyte gate. (D) The percentage of CD4 DP and CD4-2 SP lymphocytes within the lymphocyte gate of indicated tissues. (E) Frequency of CD4 DP and CD4-2 SP lymphocytes among total CD4+ lymphocytes of indicated tissues. (F) The percentage of indicated lymphocyte subsets in thymus. The values shown in D–F are expressed as mean ± s.d. (n = 8 fish). (G–I) Immunofluorescence microscopy of CD4-1 (green) and CD4-2 (red) expressions on CD4 DP (G) and CD4-2 SP (H) lymphocytes from spleen. Spleen lymphocytes stained with isotype-matched control mAbs are shown in I. Nuclei are counterstained with DAPI (blue). Scale bar = 5 μm. Data are representative of two independent experiments.
CD4 DP and CD4-2 SP lymphocyte subsets express distinct TCRβ repertoires
To gain insight into the TCRβ diversity expressed by the CD4+CD8α− T-cell subsets identified in this study, CD4 DP and CD4-2 SP lymphocytes were sorted from HK leukocytes of healthy adult trout, and their TCRβ repertoires were analyzed using CDR3 spectratyping. Similar analyses were also carried out on whole HK leukocytes from the same individual fish to discern the potential TCR peculiarities of the two CD4+ T-cell subsets. To this end, TCRβ CDR3 length profiles were produced and analyzed for the four trout TCRVβ families that have previously shown to have the highest expression levels (i.e., TCRVβ-1, -2, -3, and -7) (43).
Overall, bell-shaped profiles typically consisting of 5–8 peaks were observed for the four TCRVβ families in CD4 DP T cells as well as in whole HK leukocytes (Fig. 3A). In contrast, skewed distributions were observed for CD4-2 SP cells, indicating that the repertoires of the sorted CD4-2 SP and CD4 DP subpopulations were distinct. A diversity index based on a modified Shannon index (38) was computed from each Vβ-Jβ profile, and a PCA was performed (Fig. 3B). The PCA projection according to the first two components (40.39% and 15.96% global variability, respectively) clearly distinguishes CD4-2 SP from whole HK leukocytes and CD4 DP lymphocytes. The projection also indicates that inter-individual variability was much higher for CD4-2 SP cells. The heatmap of diversity index computed from Vβ-Jβ profiles (Fig. 3C) reveals a higher diversity index for TCRβ profiles from whole HK and CD4 DP cells. In that regard, for all four tested Vβ, the left panel of Fig. 3C corresponding to CD4-2 SP cells is significantly lighter (i.e., less red) when compared to the other two panels, thus illustrating the lower diversity of the TCRβ repertoire expressed by this subset.
FIGURE 3.
CDR3 length analysis of TRVβ transcripts from CD4+ T cells. (A) CDR3 length profiles from sorted CD4-2 SP and CD4 DP lymphocytes and from whole HK leukocytes for selected TRBV-TRBC combinations. Data are representative of three healthy fish. X-axis: length of run-off products (in bp); Y-axis: fluorescence arbitrary units. (B) PCA projection of CD4-2 SP, CD4 DP, and whole HK leukocyte samples according to the first two components using diversity scores computed from all TRBV-TRBC combinations. (C) Heatmap of diversity scores for selected TRBV-TRBJ combinations from three healthy animals. Scores are represented on pale-yellow to red scale color, corresponding to increasing diversity of TRBV-TRBJ profiles.
CD4 DP and CD4-2 SP subsets exhibit differential proliferative responses to various stimuli
To further investigate the functional differences between CD4 DP and CD4-2 SP lymphocytes, we studied their proliferative responses to mitogens, MLR, and antigen-specific stimulation. There was a remarkable proliferation of both CD4 DP and CD4-2 SP subsets in spleen and HK in response to the T-cell mitogen PHA when compared to the negligible proliferation observed upon stimulation with PBS or the B-cell mitogen LPS (Fig. A–C). Similarly, significant proliferative responses of both CD4 DP and CD4-2 SP cells were observed in response to alloantigen stimulation (Fig. 4D, 4E). Importantly, the extent of proliferation in response to PHA and alloantigen stimulation was significantly higher in splenic CD4 DP cells than in CD4-2 SP cells (Fig. 4C, 4E). For antigen-specific proliferative responses, whole leukocytes isolated from spleen and HK of fish immunized with KLH were incubated with either KLH or OVA. Both splenic CD4 DP and CD4-2 SP cells showed significant proliferative responses in the presence of KLH, but not OVA or culture medium alone (Fig. 4F, 4G). In contrast, no antigen-specific proliferative responses were detected in the CD4+ cell subsets of the HK (Fig. 4G).
FIGURE 4.
Proliferative capacities of CD4 DP and CD4-2 SP cells upon mitogen, MLR, and antigen-specific stimulations. (A) Gating strategy used to identify proliferating and non-proliferating CD4 DP and CD4-2 SP cells. We first selected singlets using forward scatter area (FSC-A) versus forward scatter height (FSC-H) parameters (left dot plot). From the singlet population, we selected all CD4-2+ leukocytes from living cells (leukocytes were stained with the anti-CD4-1 anti- CD4-2b mAbs in combination with 7-AAD). Thereafter CD4-2+ singlets were gated (middle dot plot), and the living and single CD4-2+ cells were further separated into CD4 DP and CD4-2 SP cells (right dot plot). The percentage of dividing CD4 DP and CD4-2 SP cells was determined by analyzing their degree of CellTrace Violet staining (upper and lower histogram, respectively). (B) Representative histograms of splenic CD4 DP (left) and CD4-2 SP (right) cell proliferation 7 days after incubation with PHA (1 μg/ml), LPS (100 μg/ml), or medium alone (Control). (C) The percentage of dividing CD4 DP and CD4-2 SP cells from experiments shown in B. (D) Representative histograms of splenic CD4 DP (left) and CD4-2 SP (right) cell proliferation 7 days after MLR cultures upon stimulation with allogenic PBLs (Allo-PBL), autologous PBLs (Self-PBL), or no PBLs (Control). (E) The percentage of dividing CD4 DP and CD4-2 SP cells from experiments shown in D. (F) Representative histograms of splenic CD4 DP (left) and CD4-2 SP (right) cell proliferation upon antigen-specific stimulation for 7 days with KLH (100 μg/ml). Controls included irrelevant protein (OVA, 100 μg/ml) or culture medium alone (Medium). (G) The percentage of dividing CD4 DP and CD4-2 SP cells from experiments shown in F. The percentage of dividing CD4 DP and CD4-2 SP cells was determined by the dye-dilution method with CellTrace Violet stain and measured by flow cytometry as shown in A, B, D and F. Data are representative of three independent experiments and are expressed as mean ± s.e.m. (n = 11–13 fish/group). *P < 0.05, **P < 0.01, and ***P < 0.001 (repeated-measures one-way ANOVA with Bonferroni correction was used for comparison among different stimulants for each CD4+ cell population; and one-way ANOVA with Bonferroni correction was used for comparison between CD4+ cell population incubated with PHA or alloantigen).
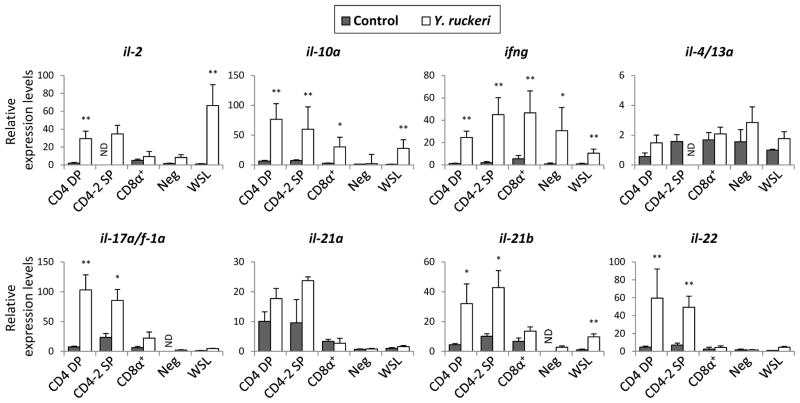
Induction of Th cytokines in CD4+ lymphocytes upon Y. ruckeri infection
To assess the ability of trout CD4 DP and CD4-2 SP cells to produce cytokines, we evaluated the mRNA expression of T-cell cytokines in fish infected with Y. ruckeri (44). At the pathogen dose used here, maximal inflammation can be detected 3–5 days post-infection (dpi) (45). Thus, spleen leukocytes from control and infected fish were isolated 4 dpi and sorted into four lymphocyte populations, CD4 DP, CD4-2 SP, CD8α+, and CD4-1−/CD4-2−/CD8α− lymphocytes (Neg). In line with previous reports (29, 46), whole spleen leukocytes (WSLs) in infected fish showed higher expression levels of il-2, il-10, ifng, il-17a/f-1a, il-21b, and il-22 cytokines when compared to those in control fish, whereas the expression levels of il-4/13a and il-21a remained unchanged (Fig. 5). Transcripts levels of both CD4 DP and CD4-2 SP lymphocytes in infected fish showed a similar expression pattern to WSLs although the overall levels (except for il-2) were much higher (Fig. 5). Moreover, except for ifng, the highest expression levels of these up-regulated cytokines were consistently detected in the sorted CD4+ lymphocyte populations. In fact, most of the analyzed cytokines were not upregulated in either CD8α+ or Neg lymphocytes except for il-10a and ifng which were upregulated in CD8α+ lymphocytes. Importantly, no differences in the cytokine expression pattern were found between CD4 DP and CD4-2 SP lymphocytes.
FIGURE 5.
Expression analysis of cytokine transcripts in CD4+ lymphocytes after Y. ruckeri infection. CD4 DP, CD4-2 SP, CD8α+, and CD4-1−/CD4-2−/CD8α− (Neg) lymphocytes were sorted from spleen leukocytes of PBS-injected control fish and Y. ruckeri-injected fish at day 4 post-injection. Transcript levels of indicated cytokine genes were analyzed by real-time PCR in the sorted lymphocyte populations as well as the unsorted whole spleen leukocytes (WSL). Data are shown relative to the expression levels of WSL from control fish (set to 1). Data are representative of two independent experiments and are expressed as mean ± s.e.m. (n = 5~6 fish/group). *P < 0.05 and **P < 0.01 (Mann-Whitney test). ND, not detected.
CD4-1+ myeloid cells consist of monocytes/macrophages
Although the presence of CD4-1 SP lymphocytes was negligible (Figs. 1A–E and 2A-C), we were able to observe a significant population of myeloid cells expressing surface CD4-1, but not CD4-2 (Fig. 1A–C). To further characterize the CD4-1+ myeloid cells, we sorted them and compared their expression of myeloid and T-cell lineage genes with that of CD4-1− myeloid cells, as well as with CD4+ and CD4− lymphocyte populations. Expression analysis of myeloid cell markers revealed that CD4-1+ myeloid cells expressed high transcript levels of monocyte/macrophage markers, including mcsfra/b (alias csf1ra/b), mpeg1, and lyz (Fig. 6A). In contrast, the same cells expressed negligible amounts of mpo, a neutrophil marker (alias mpx). Conversely, CD4-1− myeloid cells expressed high levels of mpo while expressing very low to negligible transcript levels of the macrophage markers. Instead, gcsfr (alias csf3r), which is present on a variety of mammalian myeloid cells, was highly expressed in both CD4-1+ and CD4-1− myeloid cells. Importantly, the expression of high levels of cd4-1 transcripts was detected in both CD4-1+ myeloid cells and CD4+ lymphocytes (Fig. 6A), being in agreement with their cell-surface staining by the anti-CD4-1 mAb (Fig. 1A–C). However, CD4-1+ and CD4-1− myeloid cells completely lacked the expression of TCR co-receptors (cd4-2a, cd4-2b, cd8a, cd8b, and lag3) and pan T-cell markers (tcra, tcrb, cd3g/d, lck1, and lck2) (Supplemental Fig. 2). These expression analyses strongly suggest that CD4-1+ myeloid cells are monocytes/macrophages while CD4-1− myeloid cells comprise neutrophils.
FIGURE 6.
Gene expression and cytochemical staining analyses of CD4-1+ and CD4-1− myeloid cells. (A) Transcription profile of sorted CD4-1+ and CD4-1− myeloid cells. CD4-1+ and CD4-1− myeloid cells (Mye) as well as CD4+ (comprising of CD4 DP and CD4-2 SP) and CD4− lymphocytes (Lym) were sorted from HK leukocytes. Gene expression analysis of these sorted leukocyte populations were performed by real-time PCR for the indicated genes. The transcript levels of indicated genes are shown relative to the expression levels in the CD4-1− myeloid cell population (set to 1) and are expressed as mean ± s.e.m. (n = 4 fish). Data are representative of two independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 (one-way ANOVA with Bonferroni correction). ND, not detected. (B and C) Cytochemical staining of CD4-1+ (B) and CD4-1− (C) myeloid cells sorted from HK leukocytes. Cytospin preparations of sorted CD4-1+ and CD4-1− myeloid cells were stained with Wright-Giemsa-like (WG), β-glucuronidase (BG), naphthol AS-D chloroacetate esterase (NCAE), myeloperoxidase (MPO), and Sudan Black B (SBB) stains (From left to right). Scale bar = 10 μm. (D) The percentage of sorted CD4-1+ and CD4-1− myeloid cells (Mye) positive for BG, NCAE, MPO, and SBB stains. At least 200 cells were counted per preparation to obtain the percentage of cells positive for each cytochemical staining. The values shown are expressed as mean ± s.e.m. (n = 4 fish). (E) The percentage of CD4-1+ myeloid cells within the myeloid cell population (gray bars) and the whole leukocyte population (white bars) from indicated tissues. Data are expressed as mean ± s.e.m. (n = 4 fish). Data in D and E are representative of two independent experiments.
Similar to mammalian monocytes/macrophages and neutrophils, trout CD4-1+ and CD4-1− myeloid subsets are composed of large cells (FSChigh) with low and high granularity (SSClow and SSChigh), respectively (Fig. 1B, bottom). To further validate the identity of these two myeloid cell populations, we sorted CD4-1+ and CD4-1− myeloid cells from HK and subjected them to several cytochemical stains, including WG, MPO, SBB, NCAE, and BG stains. Sorted CD4-1+ myeloid cells mainly consisted of cells with kidney-shaped nucleus typical of monocytes as well as cells with typical macrophage morphology (Fig. 6B, WG). Almost all CD4-1+ myeloid cells were positive for BG (~97%) and NCAE (~95%) staining while negative for MPO and SBB (Fig. 6B, 6D). In contrast, CD4-1− myeloid cells included mostly polymorphonuclear cells that stained positively with MPO and SBB (~80%), but also contained some cells with round or oval nuclei and dark blue cytoplasm (i.e., immature myeloblast cells) that stained negatively with MPO and SBB (Fig. 6C, 6D). Moreover, the CD4-1− myeloid cells did not stain for BG and NCAE while immature cells (blue cytoplasm) were very weakly positive for these stains (Fig. 6C, 6D). These results are consistent with reports that salmonid monocytes/macrophages are positive for BG and NCAE stains while neutrophils are positively stained with MPO and SBB stains (47, 48). The distribution of CD4-1+ myeloid cells was determined by flow cytometry and revealed that significant percentages of CD4-1+ myeloid cells were found in the myeloid cell population of blood (~14%) and HK (~14.9%), whereas relatively low percentages of these cells were observed in spleen (~6.4%) and thymus (~2.8%) (Fig. 6E, gray bars). Since the percentage of all myeloid cells is lower among leukocytes from spleen and thymus when compared to that of blood and HK, the percentage of CD4-1+ myeloid cells among whole leukocytes is much higher in the HK (~4.9%) and blood (~0.9%) than in the spleen (~0.14%) and thymus (~0.12%) (Fig. 6E, white bars).
CD4-1+ myeloid cells have the highest phagocytic activity amongst myeloid cells
Both CD4-1+ and CD4-1− myeloid cells were able to phagocytose latex beads and E. coli (Fig. 7A and Supplemental Fig. 3A). However, a higher percentage of phagocytic cells were found within the CD4-1+ myeloid subset (~87.5% and ~77% of the cells ingested beads and E. coli, respectively) when compared to CD4-1− myeloid cells (~56.9% and ~42% of the cells ingested beads and E. coli, respectively) (Fig. 7B and Supplemental Fig. 3B). Internalization of beads by CD4-1+ myeloid cells was further confirmed by immunofluorescence microscopy (Fig. 7C; isotype-matched control mAbs, Fig. 7D). Moreover, it is worth noting that the phagocytic capacity of CD4-1+ myeloid cells was higher than that of the CD4-1− myeloid population since a higher percentage of CD4-1+ myeloid cells (~47.9%) were able to ingest a large number of beads (6 or more) when compared to CD4-1− myeloid cells (~19.7%) (Fig. 7E). Similarly, CD4-1+ myeloid cells engulfed more pHrodo E. coli when compared to phagocytic CD4-1− myeloid cells (Supplemental Fig. 3C). In contrast, we found that CD4+ lymphocytes had a negligible phagocytic capacity (~3.3% and ~2.2% of the cells ingested beads and E. coli, respectively) (Fig. 7A, 7B and Supplemental Fig. 3A, 3B).
FIGURE 7.
Phagocytosis by CD4-1+ and CD4-1− myeloid cells with fluorescent latex beads. (A) Phagocytosis of 1.0 μm green fluorescent latex beads by CD4-1+ myeloid (Mye) cells (top), CD4-1− myeloid cells (middle), and CD4+ lymphocytes (Lym) (bottom) from HK. HK leukocytes were incubated in vitro with the beads for 3h and then stained with anti-CD4-1 and anti-CD4-2b mAbs. Bead phagocytosis was thereafter measured by flow cytometry. The figure shows histograms of cell number (y-axis) vs. fluorescence intensity (x-axis) representative of uptake activity by the indicated cell populations. Increased peak fluorescence denotes an increased number of ingested fluorescent beads. The values within histograms represent the percentage of phagocytic cells in each cell population. Phag−, non-phagocytic; Phag+, phagocytic. (B) The percentage of phagocytic cells in CD4-1+ myeloid (Mye), CD4-1− myeloid, and CD4+ lymphocyte (Lym) populations from HK leukocytes incubated with the beads (n = 6 fish). (C and D) Immunofluorescence microscopy of HK leukocytes incubated in vitro with 1.0 μm red fluorescent latex beads and then stained with anti-CD4-1 mAb (C) or isotype-matched control mAb (D). Nuclei are counterstained with DAPI (blue). From top to bottom: bright field; CD4-1 (green) or isotype-matched control antibody staining (Control mAb); beads (red); nuclei (blue); and merged fluorescence images (Merge). Scale bar = 5 μm. (E) The percentage of CD4-1+ and CD4-1− myeloid cells ingesting various number (1–6+) of beads (n = 6 fish). Data are representative of at least two independent experiments. Data shown in B and E are expressed as mean ± s.e.m. *P < 0.05, **P < 0.01, and ***P < 0.001 (one-way ANOVA with Bonferroni correction [B] or unpaired Student’s t-test [E]).
DISCUSSION
CD4+ cells are well characterized in mammals and birds, however the origins and primordial roles of CD4+ cells in non-tetrapod species are not well defined. In contrast to the single CD4 gene present in tetrapods, two CD4 genes have been identified in teleost fish (13, 14, 30). Here we generated and validated mAbs against rainbow trout CD4-1 and CD4-2 that were used to identify three previously unrecognized fish CD4+ leukocyte populations in peripheral lymphoid organs: a predominant lymphocyte subset co-expressing surface CD4-1 and CD4-2 (CD4 DP lymphocytes), a minor lymphocyte population uniquely expressing surface CD4-2 (CD4-2 SP lymphocytes), and a significant myeloid population with CD4-1 surface expression. In stark contrast with all other analyzed vertebrates, the concurrent or unique presence of CD4-1 and CD4-2 molecules on trout CD4+ T cells defines novel subpopulations of CD4+ T cells.
The percentage of total trout CD4+ (CD4 DP and CD4-2 SP) cells in the lymphocyte gate was very low in the blood (~1.4%) while moderate in systemic lymphoid organs (spleen [~10.5%] and HK [~22.6%]). At this point, the abundance of trout CD4+ T cell subsets cannot be compared with that of other fish because of the lack of reagents to detect both surface CD4-1 and CD4-2 molecules in all other teleost species. In humans, CD4+ T cells comprise ~50%, ~20%, and ~20% of lymphocytes in blood, spleen, and bone marrow respectively (49) while in chicken they represent ~45% and ~9% of their blood and spleen lymphoid cells, respectively (5). Thus, when comparing trout and tetrapods, the percentages of CD4+ T cells in central lymphoid organs fall within a similar range, but their proportions in blood is significantly larger in tetrapods than in trout (50). Nevertheless, it is worth pointing that the low percentage of CD4+ cells in blood leukocytes shown here is in agreement with a report describing the reactivity of an anti-trout CD3ε antibody that stained only ~2.5% of all trout blood leukocytes (51).
The thymus is the primary lymphoid organ specialized in T-cell development in jawed vertebrates (52). In all studied tetrapods, the majority of thymocytes consist of CD4+/CD8α+ cells while all other lymphoid organs mainly harbor T cells with single CD4 or CD8α surface expression (2, 5, 53). Here, we identified up to eight trout thymocyte subpopulations on the basis of their CD4-1, CD4-2, and CD8α surface expression. Overall the subset with surface CD4 (CD4-1+ and/or CD4-2+) and CD8α represented the majority of trout thymocytes. Therefore, like in mammals, trout CD4+/CD8α+ cells are likely to represent the main thymic T-cell progenitors. In other teleost fish, the presence of CD4+/CD8α+ cells has only been reported in ginbuna carp, where CD4-1+/CD8α+ cells comprise 15–35% of all thymocytes (25). Since ginbuna carp thymocytes are also likely to contain CD4-2+/CD8α+ cells, this would increase the overall percentage of the CD4+/CD8α+ population.
Teleosts express most of the cytokines involved in Th responses, although some relevant Th cytokines have not yet been found (i.e., IL-9 and IL-25) (29, 30). In support, teleost fish CD4-1+ cells have been found to express Th cytokines upon stimulation with TLR ligands, mitogens, or specific antigen (22, 24). However, our study is the first to show that upon challenge with a pathogen (Y. ruckeri), CD4+ lymphocytes produce high transcript levels of cytokines involved in Th1, Th17, and Treg responses. Interestingly, both CD4 DP and CD4-2 SP cells expressed similar levels of cytokine transcripts, suggesting that the activation mechanisms involved in their induction are similar. Importantly, of all leukocyte populations tested, the CD4 DP and CD4-2 SP subsets had the highest expression of Th17, Treg, and il-2 cytokines, whereas ifng was similarly produced by both the CD4+ and CD8α+ lymphocytes. Thus, as in mammals (3, 54), these data strongly suggest that CD4+ lymphocytes in trout are the major producers of the cytokines in response to bacterial challenge.
The few studies assessing the teleost TCR repertoire have generally been undertaken from whole tissues (i.e., spleen), making it impossible to distinguish the respective features of the repertoires expressed by CD4+ or CD8+ T cells (13). Here we performed the first repertoire analysis of CD4+ T-cell populations in a non-tetrapod species. Our data show that the repertoire of the CD4-2 SP subset is significantly less diverse than that of CD4 DP T cells. Overall, this observation supports the idea that clones belonging to these two subsets may have been subjected to different selection pressures. More particularly, our repertoire data could be explained by mechanistic constraints linked to the CD4/MHC class II interaction, or by a particular developmental pathway of CD4-2 SP T cells that selects clones with lower diversity and possibly predefined specificities. With regards to the latter possibility, CD4-2 SP lymphocytes may be functionally similar to mammalian T cells with invariant TCRs (i.e., invariant natural killer T cells and mucosa-associated invariant T cells) (55). Accordingly, a subpopulation of CD4-2 SP T cells might harbor limited or invariant TCRs which can interact with teleost non-classical MHC class I molecules. In addition, we cannot rule out the possibility that the CD4-2 SP subset consists of a compendium of amplified clones from previous immune responses that have been kept in a non-activated state. Future work is warranted to analyze the aforementioned hypotheses.
The proliferative responses of CD4+ T cells by T-cell mitogen, alloantigen, and specific antigen are a common readout of T-cell function in tetrapods (e.g. mammals and birds). In this study, we found that both splenic and HK CD4 DP and CD4-2 SP subsets proliferated significantly in response to PHA, alloantigen, and antigen-specific stimulations, with the critical exception of HK CD4+ subsets which were unable to proliferate upon antigen-specific stimulation. Thus, our T-cell proliferation data provide strong support for the notion that, as in mammals (56), the trout spleen is the key systemic lymphoid organ where T-cell-dependent adaptive immunity develops. In agreement with our finding, the spleen has long been hypothesized to be the main teleost secondary lymphoid organ (52). This belief is supported further by past findings showing that both clonal differentiation of teleost B cells following antigen encounter (38, 57) and early antiviral T-cell responses (58) occur in the spleen. The capacity of CD4-2 SP cells to proliferate in an antigen-dependent manner suggests the capability of CD4-2 to interact with trout MHC class II. Future studies are warranted to analyze potential differences in the interaction of trout CD4-1 and CD4-2 with MHC class II. Importantly, here we show that trout CD4 DP cells in spleen showed higher proliferative responses to alloantigen and PHA stimulations than did CD4-2 SP cells. The lower proliferative capacity of CD4-2 SP cells in response to alloantigen might correspond to a distinct state of differentiation, as suggested also by the restricted diversity of their TCRβ V repertoire. Moreover, because of their aforementioned distinct functional properties, it is conceivable that CD4-2 SP cells embody a vestigial CD4+ T-cell subset, a hypothesis that is in line with their unique expression of CD4-2, a molecule reminiscent of the prototypic two-Ig-domain CD4 co-receptor.
In mammals, CD4 is also expressed on some cell populations of myeloid-lineage cells although there exist large species-to-species disparities in terms of which myeloid types and what percentage of these cells express CD4 (6, 59, 60). The most consistent expression of CD4 in myeloid subsets across species is that observed on monocytes/macrophages and dendritic cells (DCs). Thus, it has been shown that varying percentages of monocytes/macrophages express CD4 in human (~65–90%) and rat (~97%), while mouse and chicken do not contain these cells (5, 6). On the other hand, the presence of CD4+ DCs has been shown in several mammals although they consistently represent a very small percentage of their leukocytes (0.1–1%) (60, 61). Importantly, since CD4 expression in monocytes/macrophages lacks in birds, the question remains on whether the presence of CD4 in myeloid-lineage cells is the result of a recent evolutionary event that took place either in mammals or in species preceding the emergence of tetrapods. In this study, cytological and gene expression analyses led to the conclusion that myeloid cells expressing surface CD4-1 were monocytes/macrophages. Interestingly, no myeloid cells with CD4-2 expression were identified. CD4-1 expression in ginbuna carp and fugu was only found in the lymphocyte population although the possibility still exists that CD4-2 in these species may be found in myeloid cells (22, 23, 25). However, zebrafish cd4-1 and cd4-2 transcripts appear to be expressed at similar levels in lymphocyte and myeloid cell populations (24). Our data represent the first description of monocytes/macrophages with surface CD4 expression in a non-mammalian species, thus suggesting that CD4 expression on the surface of these cells is the result of an ancient evolutionary event preceding the emergence of tetrapods.
To date, the specific roles of mammalian CD4+ monocytes/macrophages remain to be fully elucidated. Here we found that CD4-1+ myeloid cells represented the myeloid population with the highest phagocytic activity and capacity. It will be interesting in the future to analyze whether mammalian CD4+ monocytes/macrophages contain also such high phagocytic capabilities. On the other hand, it is worth noting that we found the vast majority of CD4+ lymphocytes to be non-phagocytic. Similarly, it has been reported that ginbuna carp CD4+ T cells are also non-phagocytic (62). In contrast, we and others have reported large subsets of phagocytic B cells in fish and several tetrapod species, including mammals (31–33, 63, 64). Thus, it would appear that from an evolutionary viewpoint, the phagocytic capacity of lymphocytes is mostly restricted to B cells.
In conclusion, this study fills in an important gap in the knowledge of teleost CD4-bearing leukocytes, thus revealing critical insights into the evolutionary origins and primordial roles of CD4+ T cells and CD4+ monocyte/macrophages (summarized in Fig. 8). The current and future studies on these lymphoid and myeloid CD4-bearing cells are also likely to provide clues for identifying new roles of these cells not only in fish but also in higher vertebrate species, and thus, contribute to the development of therapies involving these critical cell types. Importantly, as our knowledge on CD4+ T-cell responses in teleosts is very scarce, our findings will be critical for the design of more effective vaccines for fish that induce strong effector CD4+ T cell responses.
Figure 8.
Evolution of CD4 molecules and CD4-bearing leukocytes in vertebrates. (A) Evolution of CD4 molecules and CD4-bearing leukocytes. Tetrapods contain a single CD4 co-receptor with four Ig domains that is thought to have arisen from a primordial two Ig-domain ancestor. In support of this hypothesis, a CD4-like gene encoding only two Ig domains has been identified in lamprey, and thus, it was proposed to represent the prototypic two Ig-domain CD4 co-receptor. This molecule however, lacks a CXC motif (critical for T-cell function and development). While lamprey lymphocytes express transcripts of CD4-like, the protein characterization of this molecule as well as the role of the cells bearing it remain to be elucidated. Recent genome sequence and transcriptome analyses of cartilaginous fish have failed to identify a molecule with classical CD4 features, thus making teleosts the oldest living species with bona fide CD4 co-receptors. In contrast to the situation of tetrapods, which possess a single CD4 gene, rainbow trout and other teleost fish contain two CD4 genes, cd4-1 and cd4-2. Like tetrapod CD4, trout CD4-1 contains four Ig domains while trout CD4-2 contains only two. In this study, we have found two CD4+ lymphocyte populations and one CD4+ myeloid subset (described in B). Amphibians appear to contain only a four Ig-domain CD4 co-receptor, although the lack of antibodies against this molecule has precluded the phenotypic and functional characterization of amphibian CD4+ T cells. Thus far, nothing has been reported on the characterization of CD4 and CD4-bearing cells in reptiles. Birds and mammals contain a single four-Ig-domain CD4 co-receptor. Both birds and mammals contain T-cell subsets expressing surface CD4. However, while mammalian (e.g., human and rat) monocytes/macrophages express CD4, such cells have not been identified in birds. (B) Main findings of this study. Using newly generated mAbs against trout CD4-1 and CD4-2, we identified a predominant trout lymphocyte population co-expressing both CD4 molecules (CD4 DP), and a minor subset expressing only CD4-2 (CD4-2 SP). While both subsets exhibited conserved CD4+ T-cell functions (i.e., production of Th1, Th17, and Treg cytokines), CD4-2 SP lymphocytes were less proliferative and displayed a more restricted TCRβ repertoire than CD4 DP cells. Here we also identified the first non-mammalian CD4+ monocyte/macrophage population, which represented the leukocyte subset with the highest phagocytic capacity.
Supplementary Material
Acknowledgments
We thank Jeffrey S. Faust and the staff of the Flow Cytometry Facility (The Wistar Institute) for the cell-sorting procedures; Sabine Baxter of the Cell Center Service Facility (University of Pennsylvania) for the production and maintenance of hybridoma cell lines; Dr. Gregory D. Wiens (USDA-ARS-NCCCWA) for the provision of rainbow trout and Y. ruckeri; Scott LaPatra (Clear Springs Foods Inc.) for the provision of rainbow trout; Dr. Bruce Freedman and Dr. Gordon Ruthel (Penn Vet Imaging Core, University of Pennsylvania) for technical assistance and advice provided for the immunofluorescence microscopy. We would like to thank Dr. Uwe Fischer (Friedrich-Loeffler-Institut) for the provision of the anti-trout CD8α mAb and Dr. John D. Hansen (Western Fisheries Research Center) for the provision of STE-137 cells. The Fish Lake Desmet line of rainbow trout was generously provided to the NCCCWA by Don Bone from Egan State Fish Hatchery, Utah Division of Wildlife Resources.
This work was supported by the US Department of Agriculture Grant USDA-NRI-2013-01107 to J.O.S., the National Science Foundation Grant NSF-IOS-1457282 to J.O.S., the National Institutes of Health Grant 2R01GM085207-05 to J.O.S., a JSPS Postdoctoral Fellowships for Research Abroad to F.T., an institutional grant of the Institut National de la Recherche Agronomique to P.B, and by the European Commission under the Work Programme 2012 of the 7th Framework Programme for Research and Technological Development of the European Union (Grant Agreement 311993 TARGETFISH).
References
- 1.Davis MM, Chien YH. T cell antigen receptors. In: Paul WE, editor. Fundamental immunology. 7. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 279–305. [Google Scholar]
- 2.De Obaldia ME, Bhandoola A. Transcriptional regulation of innate and adaptive lymphocyte lineages. Annu Rev Immunol. 2015;33:607–642. doi: 10.1146/annurev-immunol-032414-112032. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbings D, Befus AD. CD4 and CD8: an inside-out coreceptor model for innate immune cells. J Leukoc Biol. 2009;86:251–259. doi: 10.1189/jlb.0109040. [DOI] [PubMed] [Google Scholar]
- 5.Chan MM, Chen CL, Ager LL, Cooper MD. Identification of the avian homologues of mammalian CD4 and CD8 antigens. J Immunol. 1988;140:2133–2138. [PubMed] [Google Scholar]
- 6.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 7.Criscitiello MF, Ohta Y, Saltis M, McKinney EC, Flajnik MF. Evolutionarily conserved TCR binding sites, identification of T cells in primary lymphoid tissues, and surprising trans-rearrangements in nurse shark. J Immunol. 2010;184:6950–6960. doi: 10.4049/jimmunol.0902774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scapigliati G. Functional aspects of fish lymphocytes. Dev Comp Immunol. 2013;41:200–208. doi: 10.1016/j.dci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, Hoon S, Gangu V, Roy SW, Irimia M, Korzh V, Kondrychyn I, Lim ZW, Tay BH, Tohari S, Kong KW, Ho S, Lorente-Galdos B, Quilez J, Marques-Bonet T, Raney BJ, Ingham PW, Tay A, Hillier LW, Minx P, Boehm T, Wilson RK, Brenner S, Warren WC. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174–179. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkstra JM. TH2 and Treg candidate genes in elephant shark. Nature. 2014;511:E7–9. doi: 10.1038/nature13446. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh B, Lee AP, Swann JB, Ohta Y, Flajnik MF, Kasahara M, Boehm T, Warren WC. Venkatesh et al. reply. Nature. 2014;511:E9–10. doi: 10.1038/nature13447. [DOI] [PubMed] [Google Scholar]
- 12.Flajnik MF. Re-evaluation of the immunological Big Bang. Curr Biol. 2014;24:R1060–1065. doi: 10.1016/j.cub.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro R, Bernard D, Lefranc MP, Six A, Benmansour A, Boudinot P. T cell diversity and TcR repertoires in teleost fish. Fish Shellfish Immunol. 2011;31:644–654. doi: 10.1016/j.fsi.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Laing KJ, Hansen JD. Fish T cells: recent advances through genomics. Dev Comp Immunol. 2011;35:1282–1295. doi: 10.1016/j.dci.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Williams AF, Davis SJ, He Q, Barclay AN. Structural diversity in domains of the immunoglobulin superfamily. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):637–647. doi: 10.1101/sqb.1989.054.01.075. [DOI] [PubMed] [Google Scholar]
- 16.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–337. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laing KJ, Zou JJ, Purcell MK, Phillips R, Secombes CJ, Hansen JD. Evolution of the CD4 family: teleost fish possess two divergent forms of CD4 in addition to lymphocyte activation gene-3. J Immunol. 2006;177:3939–3951. doi: 10.4049/jimmunol.177.6.3939. [DOI] [PubMed] [Google Scholar]
- 19.Pancer Z, Mayer WE, Klein J, Cooper MD. Prototypic T cell receptor and CD4-like coreceptor are expressed by lymphocytes in the agnathan sea lamprey. Proc Natl Acad Sci U S A. 2004;101:13273–13278. doi: 10.1073/pnas.0405529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor EB, Wilson M, Bengten E. The Src tyrosine kinase Lck binds to CD2, CD4-1, and CD4-2 T cell co-receptors in channel catfish, Ictalurus punctatus. Mol Immunol. 2015;66:126–138. doi: 10.1016/j.molimm.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Kato G, Goto K, Akune I, Aoka S, Kondo H, Hirono I. CD4 and CD8 homologues in Japanese flounder, Paralichthys olivaceus: Differences in the expressions and localizations of CD4-1, CD4-2, CD8alpha and CD8beta. Dev Comp Immunol. 2013;39:293–301. doi: 10.1016/j.dci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kono T, Korenaga H. Cytokine Gene Expression in CD4 Positive Cells of the Japanese Pufferfish. PLoS ONE. 2013;8:e66364. doi: 10.1371/journal.pone.0066364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somamoto T, Kondo M, Nakanishi T, Nakao M. Helper function of CD4(+) lymphocytes in antiviral immunity in ginbuna crucian carp, Carassius auratus langsdorfii. Dev Comp Immunol. 2014;44:111–115. doi: 10.1016/j.dci.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Yoon S, Mitra S, Wyse C, Alnabulsi A, Zou J, Weerdenburg EM, Wang MvdSAD, Secombes CJ, Bird S. First Demonstration of Antigen Induced Cytokine Expression by CD4-1+ Lymphocytes in a Poikilotherm: Studies in Zebrafish (Danio rerio) PLoS ONE. 2015;10:e0126378. doi: 10.1371/journal.pone.0126378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toda H, Saito Y, Koike T, Takizawa F, Araki K, Yabu T, Somamoto T, Suetake H, Suzuki Y, Ototake M, Moritomo T, Nakanishi T. Conservation of characteristics and functions of CD4 positive lymphocytes in a teleost fish. Dev Comp Immunol. 2011;35:650–660. doi: 10.1016/j.dci.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Zhu LY, Lin AF, Shao T, Nie L, Dong WR, Xiang LX, Shao JZ. B cells in teleost fish act as pivotal initiating APCs in priming adaptive immunity: an evolutionary perspective on the origin of the B-1 cell subset and B7 molecules. J Immunol. 2014;192:2699–2714. doi: 10.4049/jimmunol.1301312. [DOI] [PubMed] [Google Scholar]
- 27.Shao T, Zhu LY, Nie L, Shi W, Dong WR, Xiang LX, Shao JZ. Characterization of surface phenotypic molecules of teleost dendritic cells. Dev Comp Immunol. 2015;49:38–43. doi: 10.1016/j.dci.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Wen Y, Fang W, Xiang LX, Pan RL, Shao JZ. Identification of Treg-like cells in Tetraodon: insight into the origin of regulatory T subsets during early vertebrate evolution. Cell Mol Life Sci. 2011;68:2615–2626. doi: 10.1007/s00018-010-0574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Secombes CJ. The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol. 2013;35:1703–1718. doi: 10.1016/j.fsi.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Takizawa F, Fischer U, Dijkstra JM. Along the Axis between Type 1 and Type 2 Immunity; Principles Conserved in Evolution from Fish to Mammals. Biology. 2015;4:814–859. doi: 10.3390/biology4040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunyer JO. Fishing for mammalian paradigms in the teleost immune system. Nat Immunol. 2013;14:320–326. doi: 10.1038/ni.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, LaPatra SE, Bartholomew J, Sunyer JO. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YA, Hikima J, Li J, LaPatra SE, Luo YP, Sunyer JO. Conservation of structural and functional features in a primordial CD80/86 molecule from rainbow trout (Oncorhynchus mykiss), a primitive teleost fish. J Immunol. 2009;183:83–96. doi: 10.4049/jimmunol.0900605. [DOI] [PubMed] [Google Scholar]
- 35.Takizawa F, Dijkstra JM, Kotterba P, Korytar T, Kock H, Kollner B, Jaureguiberry B, Nakanishi T, Fischer U. The expression of CD8alpha discriminates distinct T cell subsets in teleost fish. Dev Comp Immunol. 2011;35:752–763. doi: 10.1016/j.dci.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 36.DeLuca D, Wilson M, Warr GW. Lymphocyte heterogeneity in the trout, Salmo gairdneri, defined with monoclonal antibodies to IgM. Eur J Immunol. 1983;13:546–551. doi: 10.1002/eji.1830130706. [DOI] [PubMed] [Google Scholar]
- 37.Castro R, Takizawa F, Chaara W, Lunazzi A, Dang TH, Koellner B, Quillet E, Six A, Fischer U, Boudinot P. Contrasted TCRbeta diversity of CD8+ and CD8− T cells in rainbow trout. PLoS ONE. 2013;8:e60175. doi: 10.1371/journal.pone.0060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro R, Jouneau L, Pham HP, Bouchez O, Giudicelli V, Lefranc MP, Quillet E, Benmansour A, Cazals F, Six A, Fillatreau S, Sunyer O, Boudinot P. Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PLoS pathogens. 2013;9:e1003098. doi: 10.1371/journal.ppat.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiens GD, LaPatra SE, Welch TJ, Evenhuis JP, Rexroad CE, Leeds TD. On-farm performance of rainbow trout (Oncorhynchus mykiss) selectively bred for resistance to bacterial cold water disease: Effect of rearing environment on survival phenotype. Aquaculture. 2013;388:128–136. [Google Scholar]
- 40.Kincaid HL, Mengel LJ, Brimm S. National Fish Strain Registry-Trout Species Tables on Reported Strains and Broodstocks. 2002. [Google Scholar]
- 41.Dijkstra JM, Somamoto T, Moore L, Hordvik I, Ototake M, Fischer U. Identification and characterization of a second CD4-like gene in teleost fish. Mol Immunol. 2006;43:410–419. doi: 10.1016/j.molimm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Moore LJ, Dijkstra JM, Koppang EO, Hordvik I. CD4 homologues in Atlantic salmon. Fish Shellfish Immunol. 2009;26:10–18. doi: 10.1016/j.fsi.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Boudinot P, Boubekeur S, Benmansour A. Primary structure and complementarity-determining region (CDR) 3 spectratyping of rainbow trout TCRbeta transcripts identify ten Vbeta families with Vbeta6 displaying unusual CDR2 and differently spliced forms. J Immunol. 2002;169:6244–6252. doi: 10.4049/jimmunol.169.11.6244. [DOI] [PubMed] [Google Scholar]
- 44.Kumar G, Menanteau-Ledouble S, Saleh M, El-Matbouli M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet Res. 2015;46:103. doi: 10.1186/s13567-015-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiens GD, Vallejo RL. Temporal and pathogen-load dependent changes in rainbow trout (Oncorhynchus mykiss) immune response traits following challenge with biotype 2 Yersinia ruckeri. Fish Shellfish Immunol. 2010;29:639–647. doi: 10.1016/j.fsi.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Jiang Y, Wang A, Husain M, Xu Q, Secombes CJ. Identification of the salmonid IL-17A/F1a/b, IL-17A/F2b, IL-17A/F3 and IL-17N genes and analysis of their expression following in vitro stimulation and infection. Immunogenetics. 2015;67:395–412. doi: 10.1007/s00251-015-0838-1. [DOI] [PubMed] [Google Scholar]
- 47.Moritomo T, Anderson D, Schill W. Establishment of a cell line with reticulo-endothelial characteristics from a rainbow trout spleen explant. Fish Pathol. 1990;25:165–170. [Google Scholar]
- 48.Pettersen EF, Ingerslev HC, Stavang V, Egenberg M, Wergeland HI. A highly phagocytic cell line TO from Atlantic salmon is CD83 positive and M-CSFR negative, indicating a dendritic-like cell type. Fish Shellfish Immunol. 2008;25:809–819. doi: 10.1016/j.fsi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Westermann J, Pabst R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig. 1992;70:539–544. doi: 10.1007/BF00184787. [DOI] [PubMed] [Google Scholar]
- 50.Tizard IR. In: Veterinary immunology. 9. Tizard IR, editor. Elsevier/Saunders; St. Louis, Mo: 2013. pp. 127–136. [Google Scholar]
- 51.Boardman T, Warner C, Ramirez-Gomez F, Matrisciano J, Bromage E. Characterization of an anti-rainbow trout (Oncorhynchus mykiss) CD3epsilon monoclonal antibody. Vet Immunol Immunopathol. 2012;145:511–515. doi: 10.1016/j.vetimm.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 52.Boehm T, Swann JB. Origin and evolution of adaptive immunity. Annu Rev Anim Biosci. 2014;2:259–283. doi: 10.1146/annurev-animal-022513-114201. [DOI] [PubMed] [Google Scholar]
- 53.Alarcon B, van Santen HM. Two receptors, two kinases, and T cell lineage determination. Sci Signal. 2010;3:pe11. doi: 10.1126/scisignal.3114pe11. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gapin L. Check MAIT. J Immunol. 2014;192:4475–4480. doi: 10.4049/jimmunol.1400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye J, I, Kaattari M, Ma C, Kaattari S. The teleost humoral immune response. Fish Shellfish Immunol. 2013;35:1719–1728. doi: 10.1016/j.fsi.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Boudinot P, Boubekeur S, Benmansour A. Rhabdovirus infection induces public and private T cell responses in teleost fish. J Immunol. 2001;167:6202–6209. doi: 10.4049/jimmunol.167.11.6202. [DOI] [PubMed] [Google Scholar]
- 59.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 60.Summerfield A, Auray G, Ricklin M. Comparative dendritic cell biology of veterinary mammals. Annu Rev Anim Biosci. 2015;3:533–557. doi: 10.1146/annurev-animal-022114-111009. [DOI] [PubMed] [Google Scholar]
- 61.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagasawa T, Nakayasu C, Rieger AM, Barreda DR, Somamoto T, Nakao M. Phagocytosis by Thrombocytes is a Conserved Innate Immune Mechanism in Lower Vertebrates. Front Immunol. 2014;5:445. doi: 10.3389/fimmu.2014.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, Barreda DR, Sunyer JO. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91:525–536. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu LY, Shao T, Nie L, Xiang LX, Shao JZ. Evolutionary implication of B-1 lineage cells from innate to adaptive immunity. Mol Immunol. 2016;69:123–130. doi: 10.1016/j.molimm.2015.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.