Summary
Objective
To determine response rates (RR), progression-free survival (PFS), overall survival (OS), and toxicity in patients treated with cytotoxic chemotherapy, in combination with bevacizumab compared to cytotoxic chemotherapy alone, in the setting of recurrent ovarian cancer.
Materials and Methods
After obtaining Institutional Review Board approval, two cohorts of patients with recurrent ovarian cancer were identified: 1) patients that received cytotoxic chemotherapy with bevacizumab from January 2006 to June 2009; 2) patients that received cytotoxic chemotherapy alone. RR were measured using RECIST criteria or by CA-125 levels using modified Rustin criteria. RR, OS, and PFS were determined using Kaplan-Meier survival analysis.
Results
Thirty-two patients that received bevacizumab in combination with cytotoxic chemotherapy and 32 patients that received cytotoxic chemotherapy alone were identified. The control patients were matched for age, platinum response, histology, surgical outcome, grade, and number of previous chemotherapy regimens. There were no differences between the two cohorts in the rates of venous thromboembolism (VTE) (p = 0.39), bleeding (p = 0.15) or bowel obstruction (p = 0.40). The rate of hypertension in the bevacizumab cohort was greater than in the comparison cohort (p < 0.005). There were no differences in response rates PR/CR vs SD/PD (p = 0.46), OS (p = 0.79) or PFS (p = 0.43).
Conclusions
With increased toxicity, increased cost of therapy and no improvement in PFS or OS, the role of bevacizumab in patients with recurrent ovarian cancer warrants further investigation.
Keywords: Squamous cell carcinoma, Endometrial carcinoma, Ichthyosis uteri, Ovarian cancer, Chemotherapy, Bevacizumab
Introduction
Ovarian cancer is the fifth most common cause of death from malignancies in women in the United States and the leading cause of death of gynecologic malignancies. It is estimated that 14,560 women will die from ovarian cancer in 2011 [1]. Approximately seventy-five percent of patients with epithelial ovarian cancer (EOC) present with advanced Stage disease (III and IV). It is most commonly treated with tumor debulking followed by adjuvant taxane and platinum-based chemotherapy [2, 3]. Although 70%–80% of patients demonstrate a response to primary adjuvant therapy, most of these patients will have disease recurrence within 15 months and often die from tumor progression [3, 4]. In the treatment of recurrent disease, there is only a 15%–20% response rate (RR) with no possibility of a cure. With almost no change in disease-specific mortality in the last three decades with standard of care treatment, these patients present a challenge for treatment and thus the use of novel targeted therapy warrants further investigation [5].
Angiogenesis, a recently studied target in ovarian cancer patients, is crucial in the development and progression of ovarian cancer [6–12]. Some studies have demonstrated that increased angiogenesis activity is associated with increased aggressiveness of the tumor and may be a useful prognostic factor [8–10, 12–16]. Vascular endothelial growth factor (VEGF) is known to stimulate endothelial cell growth and promote vascular permeability leading to the formation of new blood vessels. VEGF has been shown to be a potential target for novel therapy and has been used in the treatment of recurrent ovarian cancer [17–19].
Bevacizumab, a humanized monoclonal antibody against VEGF, is FDA-approved for the treatment of metastatic colorectal cancer, unresectable non-squamous, non-small cell lung cancer, glioblastoma multiforme, and metastatic renal cell carcinoma [20–25]. Modest RR (8%–36%) and disease stabilization (8%–36%) have been reported in ovarian cancer [18, 26–28]. Bevacizumab is currently being evaluated in three phase III studies, Gynecologic Oncology Group (GOG) 218, ICON-7, and the OCEANS trial. GOG 218 and ICON-7 evaluate the use of bevacizumab with first-line adjuvant taxane/platinum-based chemotherapy for the treatment of ovarian cancer [29, 30]. Preliminary data on progression-free survival (PFS) are available for these two trials; however data on overall survival (OS) and quality of life have not yet been reported. PFS data from ICON-7 support the use of bevacizumab in combination with carboplatin/paclitaxel for front-line adjuvant therapy for the treatment of ovarian cancer, with a significant improvement in PFS of 17.3 vs 19 months (p = 0.004). Also a significant improvement in PFS with patients with Stage III/suboptimal debulking and Stage IV disease of 10.5 vs 15.9 months (p < 0.001) [31]. However the preliminary analysis of OS does not show an improvement in OS (p = 0.098). Preliminary data from GOG-218 also report an improvement in median PFS of 3.8 months (10.3 vs 14.1 months) when bevacizumab is given as maintenance therapy for an additional ten months [32]. The OCEANS trial tests the benefit of bevacizumab in addition to carboplatin and gemcitabine in patients with platinum-sensitive recurrent ovarian cancer. The patients included in the study could have only received one previous line of chemotherapy to qualify for enrollment. Preliminary results demonstrate an improvement in PFS of 12.4 vs 8.4 months for patients with bevacizumab in addition to carboplatin and gemcitabine compared to placebo with carboplatin and gemcitabine. The OS data are not mature and have not been published [33].
The most common toxicities (> 10%) associated with the use of bevacizumab include: hypertension, proteinuria, epistaxis, headache, rhinitis, dry skin, back pain, exfoliative dermatitis, and rectal hemorrhage. Documented events associated with its use include, stroke, transient ischemic attacks, myocardial infarctions and angina. Age > 65 years has also been associated with an increased risk of thromboembolic events [34]. Bevacizumab received a black box warning for gastrointestinal perforations (GIP), wound healing complications, fistula formation, and hemorrhage. National Comprehensive Cancer Network practice guidelines in oncology list the use bevacizumab as an acceptable single agent therapy or as a part of combination chemotherapy for the treatment of patients with recurrent ovarian cancer.
Materials and Methods
After obtaining Institutional Review Board approval, two cohorts of patients receiving chemotherapy for recurrent ovarian cancer were identified; 1) 32 patients that received cytotoxic chemotherapy with bevacizumab (January 2006 to June 2009) and 2) 32 patients that received cytotoxic chemotherapy alone. The control patients were matched for age, platinum response, histology, surgical outcome, grade, and number of previous chemotherapy regimens. Patients were eligible if they had documented recurrent ovarian cancer by CA-125 or radiographic studies. All patients received taxane and platinum as front-line adjuvant therapy prior to their first recurrence. No patients received bevacizumab as part of front line therapy or as single agent therapy.
Patient demographics, clinico-pathologic data, and toxicities were extracted from patient charts. Bevacizumab was continued until disease progression or severe cytotoxic events occurred. PFS and OS were obtained using Kaplan-Meier curves. RR were calculated using response to treatment in solid tumors (RECIST) criteria or CA-125 levels according to modified Rustin criteria [35, 36]. Complete response (CR) was defined as no gross evidence of disease, resolution of measurable disease on computed tomography (CT) scan or normalization of CA-125 levels from an elevated level. Partial response (PR) was defined as a 30% reduction in lesions on CT scan or 50% reduction in CA-125. Progressive disease (PD) was defined as a 20% or greater increase in the lesions based on CT scan or doubling of CA-125 within eight weeks of starting therapy. Stable disease (SD) was any of the conditions that did not meet the above criteria. The best response for each patient was reported. CA-125 levels were routinely drawn with the pre-chemotherapy labs and imaging was not required to document a response. PFS was defined as the time from the initiation of treatment with bevacizumab or last cytotoxic chemotherapy until PD or date of last contact.
Results
A total of 64 patients were identified, 32 received cytotoxic chemotherapy in combination with bevacizumab and an additional 32 received cytotoxic chemotherapy alone for the treatment of recurrent ovarian cancer. The most commonly prescribed dose of bevacizumab was 15 mg/kg every three weeks (84%). No patients in the study group had received prior bevacizumab. The median age of patients in the bevacizumab cohort was 56.5 years and 58 years for the cytotoxic chemotherapy alone cohort (p = 0.23). Patient demographics are depicted in Table 1. Fifty-nine percent (19/32) of patients received weekly paclitaxel in combination with bevacizumab which was the most common regimen. Of the other regimens given, nine patients (28%) received cyclophosphamide, three patients (9%) received doxorubicin, and one patient (3%) received carboplatin/gemcitabine along with bevacizumab. In the chemotherapy alone cohort, nine (28%) patients received doxorubicin, eight (25%) patients received carboplatin/ gemcitabine, five (16%) patients received paclitaxel, two (6%) patients received cyclophosphamide, two (6%) patients received topotecan, two (6%) patients received etoposide, one (3%) patient received carboplatin, one (3%) patient received navelbine, one (3%) patient received pemetrexed, and one (3%) patient received carboplatin/paclitaxel (Table 2). At the conclusion of the study, all 32 patients that received bevacizumab had died.
Table 1.
Patient demographics.
| Characteristic | Bevacizumab n = 32 |
Chemotherapy n = 32 |
p value |
|---|---|---|---|
| Median age (years) | 56.5 (27–84) | 54 (36–89) | 0.23 |
| Stage | |||
| I & II | 2 | 0 | 0.15 |
| III & IV | 30 | 32 | |
| Histology | |||
| Serous | 25 | 25 | 1.0 |
| Non-serous | 7 | 7 | |
| Debulking status | |||
| Optimal | 27 | 28 | 0.72 |
| Suboptimal | 5 | 4 | |
| Overall response | |||
| CR/PR | 6 | 4 | 0.46 |
| PD/SD | 26 | 28 | |
| UTA | 1 | ||
| Platinum sensitivity | |||
| Resistant | 25 | 28 | 0.77 |
| Sensitive | 7 | 4 |
Table 2.
Chemotherapy regimen.
| Bevacizumab n = 32 |
Chemotherapy n = 32 |
|
|---|---|---|
| Paclitaxel | 19 (59%) | 5 (16%) |
| Cyclophosphamide | 9 (28%) | 2 (6%) |
| Doxorubicin | 3 (10%) | 9 (28%) |
| Carboplatin/Gemcitabine | 1 (3%) | 8 (25%) |
| Pemetrexed | 1 (3%) | |
| Carboplatin | 1 (3%) | |
| Carboplatin/Paclitaxel | 1 (3%) | |
| Etoposide | 2 (6%) | |
| Navelbine | 1 (3%) | |
| Topotecan | 2 (6%) |
The cytotoxic regimens used for the treatment of recurrent ovarian cancer prior to the initiation of bevacizumab included taxanes, platinum compounds, doxorubicin, gemcitabine, and topotecan. The mean number of previous chemotherapy regimens prior to staring bevacizumab was 3.4 and 3.3 in the comparison cohort (p = 0.48) (Table 3). The mean number of cycles of bevacizumab was seven (range 1–18) and the mean cumulative dose was 8,329 mg (range 952–33,704 mg). Mean time of treatment length was 126 days (range 21–378 days). To determine response rates, PFS and OS, CA-125 values and radiologic studies were used.
Table 3.
Number of previous chemo regimens.
| No. of previous chemo regimens |
Bevacizumab n = 32 |
Chemotherapy n = 32 |
|---|---|---|
| 1 | 3 | 5 |
| 2 | 5 | 8 |
| 3 | 10 | 6 |
| 4 | 5 | 5 |
| 5 | 7 | 5 |
| 6 | 2 | 1 |
| 7 | 0 | 0 |
| 8 | 0 | 2 |
Toxicity
Table 4 depicts the adverse events that occurred in each cohort. In the bevacizumab cohort, two patients experienced complications from bleeding, one patient developed severe epistaxis (11 cycles of bevacizumab - 19,640 mg cumulative dose), and one developed an upper gastrointestinal bleed (one cycle of bevacizumab - 1,680 mg cumulative dose), both requiring transfusions. Bevacizumab was discontinued for both patients and although there were no hemorrhagic events in the cytotoxic chemotherapy alone cohort, this difference was not statistically significant (p = 0.15). Two (6%) patients in the bevacizumab cohort developed a venous thromboembolism (VTE) compared to four (13%) in the cytotoxic chemotherapy alone cohort (p = 0.39). Seven (22%) patients in the bevacizumab cohort and ten (31%) patients in the cytotoxic chemotherapy alone cohort developed a bowel obstruction (p = 0.40). Nine (28%) patients developed hypertension requiring medical therapy during treatment with bevacizumab compared to none in the cytotoxic chemotherapy alone cohort (p < 0.005). One patient receiving bevacizumab developed an enterocutaneous fistula (after first cycle of bevacizumab, 705 mg) and one (3%) patient in the cytotoxic chemotherapy alone developed a fistula (p = 0.55).
Table 4.
Adverse events documented during treatment
| Bevacizumab n = 32 |
Chemotherapy n = 32 |
p value | |
|---|---|---|---|
| Bleeding | 2 (6.3%) | 0 | 0.15 |
| VTE (during treatment) | 2 (6.3%) | 4 (13%) | 0.39 |
| Bowel obstruction | 9 (28%) | 10 (31.2%) | 0.40 |
| Hypertension | 7 (22%) | 0 | 0.005 |
| Fistula | 1 (3%) | 2 (6.3%) | 0.55 |
Bevacizumab was discontinued for the following reasons: disease progression (66%) bowel obstruction (13%), change in care (3%), hemorrhage (6%), congestive heart failure/myocardial infarction (CHF/MI, 6%), and enterocutaneous fistula (3%).
Response data
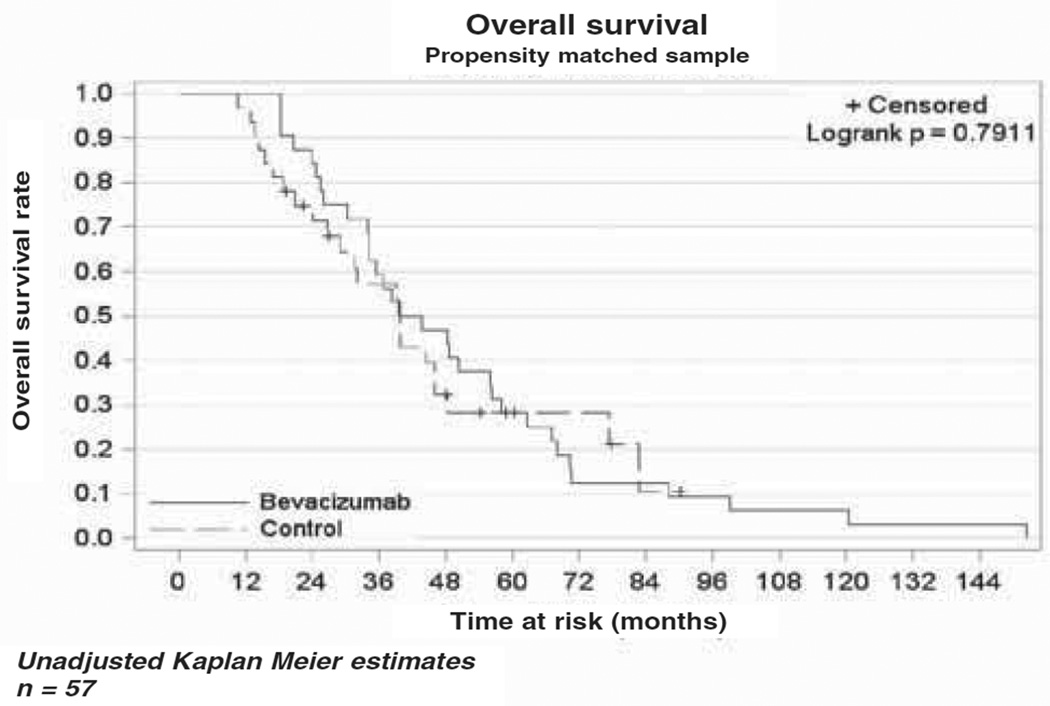
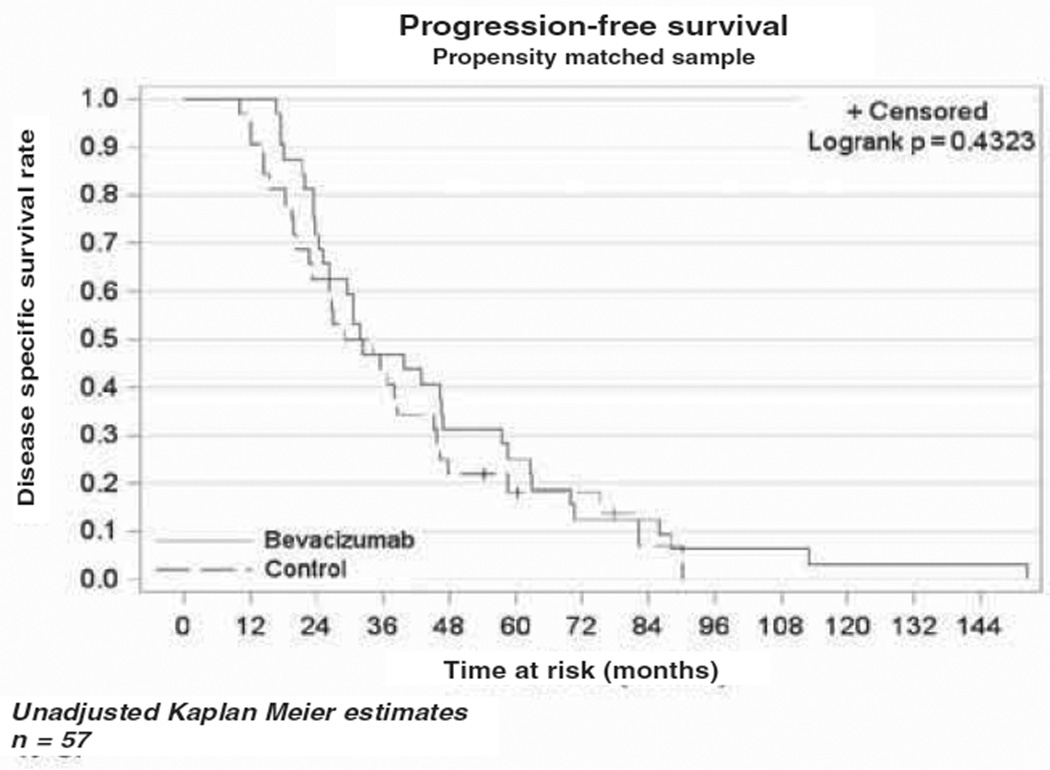
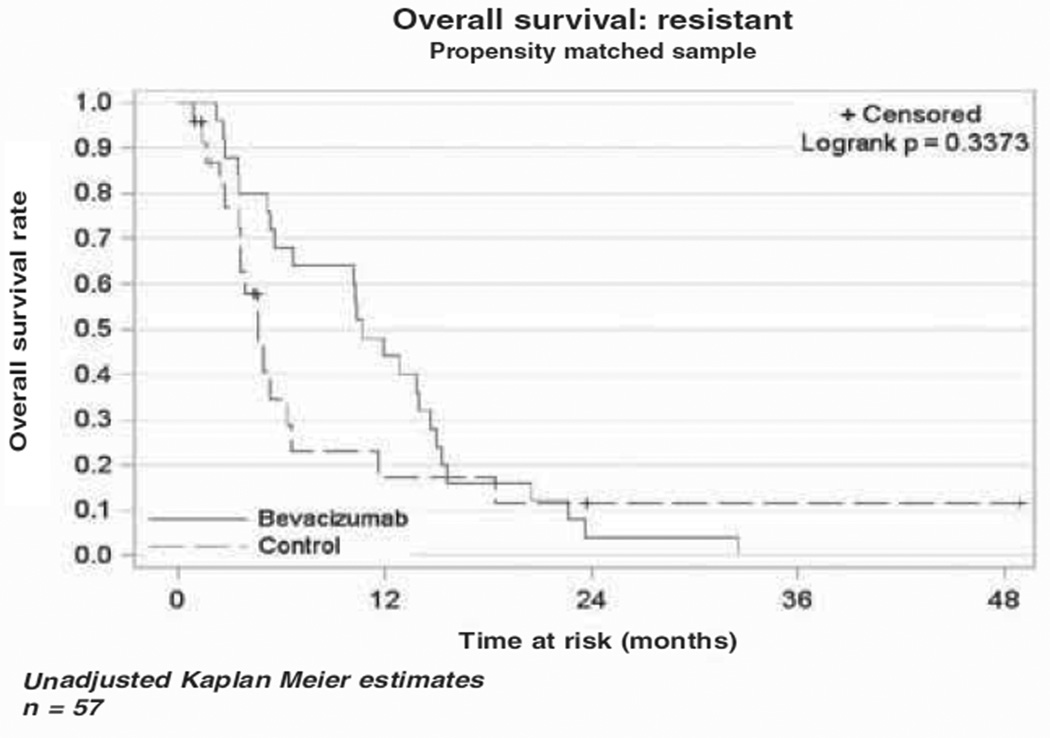
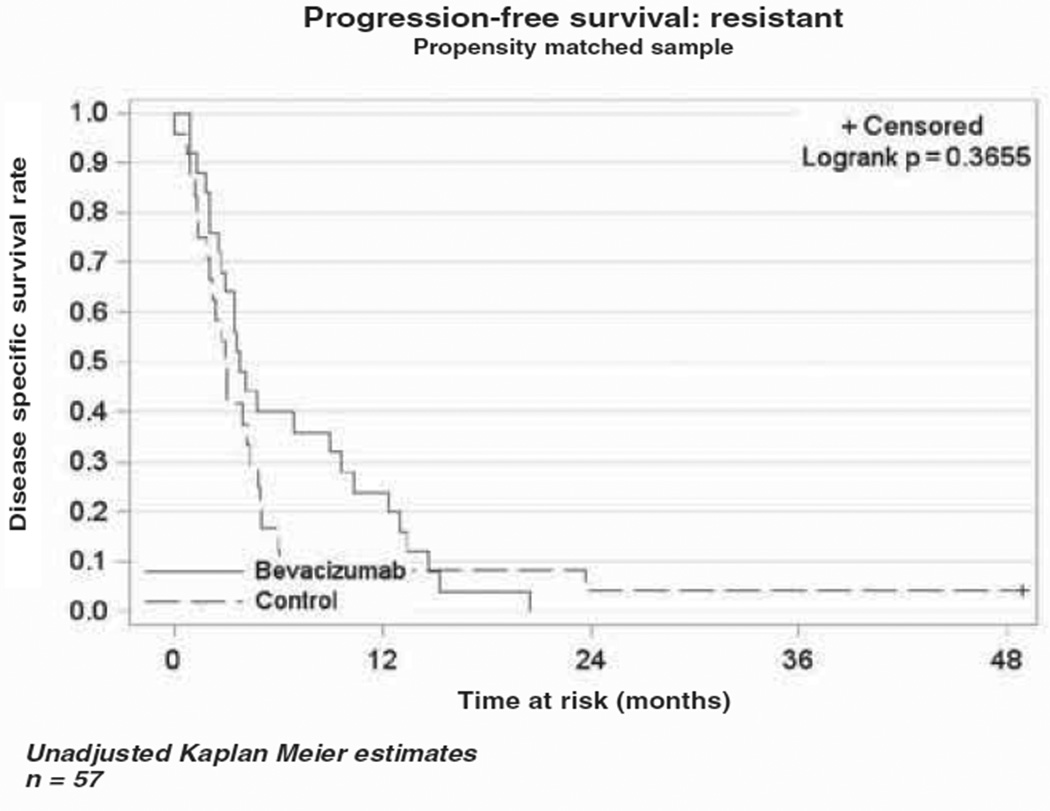
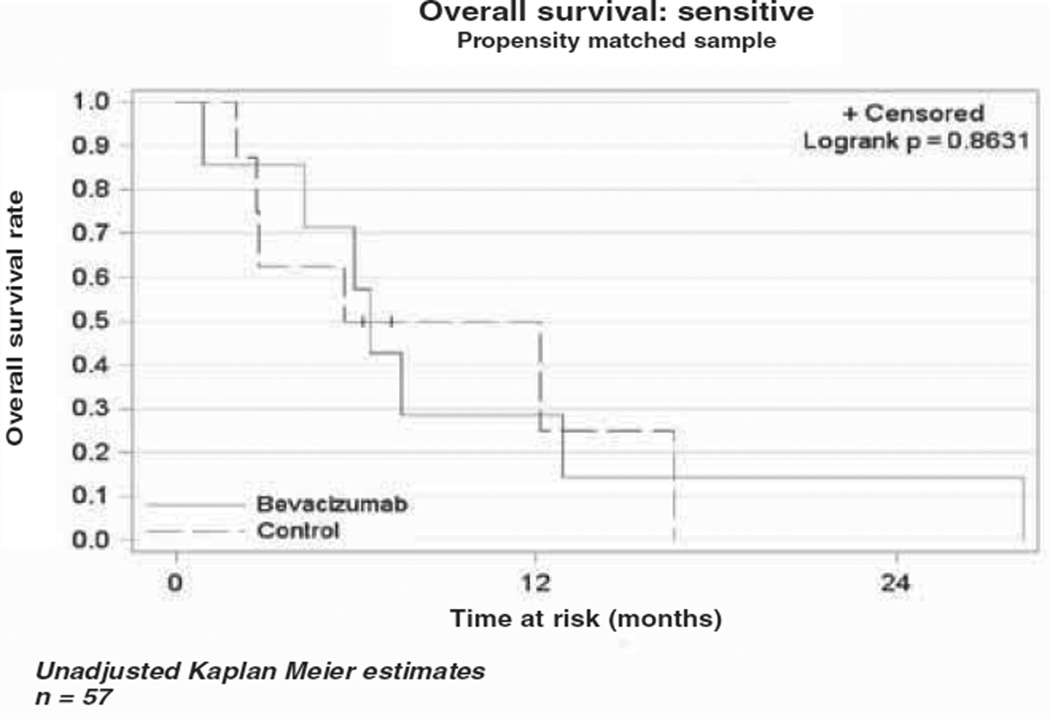
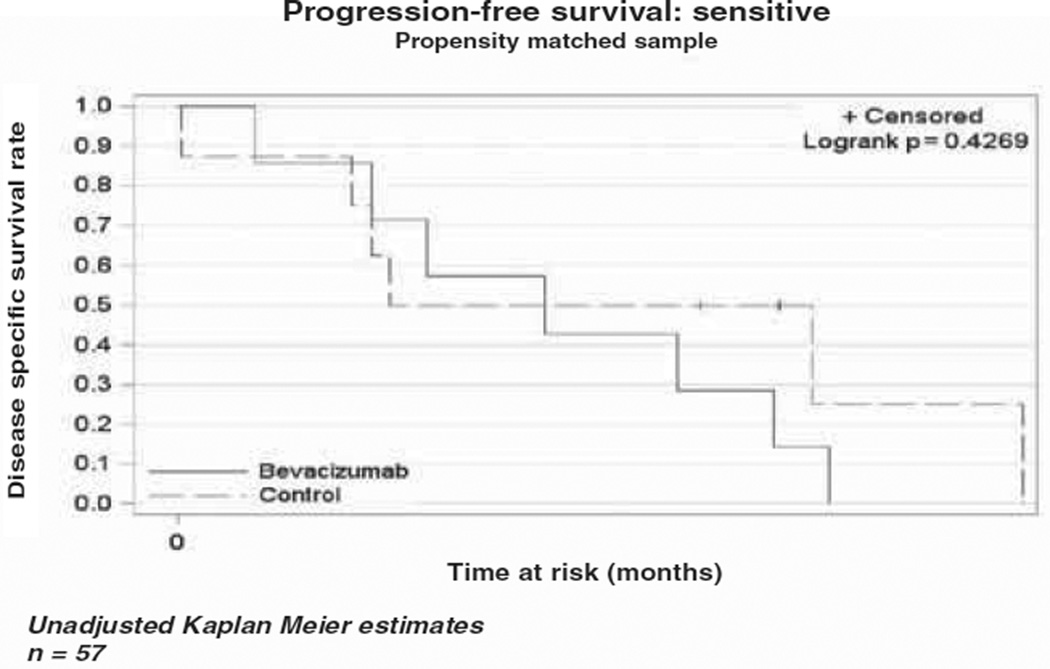
The median OS in the bevacizumab cohort was 10.4 months (range 5.6–13.8), while the median OS in the cytotoxic chemotherapy alone cohort was 4.9 months (range 3.5–6.6) as demonstrated in the Kaplan-Meier curve (Figure 1). This difference was not statistically different (p = 0.79). The median PFS was four months (range 2.8–7.1) in the bevacizumab cohort vs three months (range 2–4.9) in the comparison cohort (Figure 2). As in the OS, there was no statistical difference in PFS between the two cohorts (p = 0.43). In a subset analysis of platinum resistant patients there was no difference in OS (p = 0.34) or PFS (p = 0.37) between the two cohorts (Figures 3 and 4). In the platinum sensitive group there was no difference in OS (p = 0.87) or PFS (p = 0.43) between the two cohorts (Figures 5 and 6).
Figure 1.
Kaplan-Meier plot demonstrating overall survival in the two cohorts.
Figure 2.
Kaplan-Meier plot demonstrating progression-free survival in the two cohorts.
Figure 3.
Kaplan-Meier plot for overall survival for platinum-resistant patients.
Figure 4.
Kaplan-Meier plot for progression-free survival for platinum-resistant patients.
Figure 5.
Kaplan-Meier plot for overall survival for platinum-sensitive patients.
Figure 6.
Kaplan-Meier plot for progression-free survival for platinum-sensitive patients.
The overall RR was 19% in the bevacizumab group as compared to 16% in the control group. In the bevacizumab group, twenty-three (72%) patients had PD, six (19%) had a PR, and two (6%) had SD (one (3%) was unable to be assessed). In the control group, four (13%) had a CR, 24 (75%) had PD, one (3%) had a PR, three (9%) had SD.
Discussion
Bevacizumab, a humanized monoclonal antibody against VEGF, has shown activity in several solid tumors, including ovarian carcinoma. Despite the fact that most patients with ovarian cancer have good RR to adjuvant cytotoxic chemotherapy, a majority of these patients will recur within five years. Recurrent and platinum-resistant ovarian cancer continue to present treatment dilemmas, since RR in platinum-resistant patients are reported to be 15%. Five phase II studies have been performed to examine the use of bevacizumab in the setting of recurrent ovarian cancer: two single agent trials, two in combination with cytotoxic therapy, and one in combination with biologic chemotherapy. RR of 8%–36% and disease stabilization in 55%–75% of patients receiving bevacizumab were reported in these studies [18, 26–28, 37]. These results are promising for the treatment of recurrent ovarian cancer.
GOG conducted the largest single agent study, GOG 170D, which reported a 21% overall response rate (ORR) and a 40% six-month PFS in 62 women when treated with bevacizumab. Of these 62 women, 66% had received two prior regimens and 42% were considered platinum-resistant [26]. AVF2797, another single-agent industry sponsored trial reported an ORR of 16% in an 84% platinum-resistant population and 48% had received three prior regimens [18]. The two bevacizumab trials in combination with cytotoxic chemotherapy both reported ORR of 24% [27, 38]. The bevacizumab trial in combination with biologic chemotherapy (erlotinib, a biologic anti-epidermal growth factor receptor agent) reported a RR of 15% [37].
The bevacizumab cohort had a RR of 19% compared to a RR of 16% in the chemotherapy alone cohort. The RRs for the bevacizumab cohort is similar to what has been reported by the previous phase II studies (15.9–24%). The OS and PFS in the bevacizumab cohort, 10.4 and four months respectively, are consistent with what Loizzi et al. previously reported in patients with recurrent ovarian cancer (OS and PFS, 13 and five months, respectively) [39].
Bevacizumab is generally tolerated but is known to be associated with certain toxicities including: hypertension, proteinuria, thromboembolic events, hemorrhage, GIP, wound-healing complications, and fistula formation [34]. Hypertension, new onset or exacerbation of existing hypertension, is the most common adverse event associated with bevacizumab. In randomized trials, grade 3/4 hypertension incidence ranged from 3%–15% compared to 0%–2% for controls [23, 24, 34, 40–46]. The patients receiving bevacizumab had a higher incidence of new onset hypertension or exacerbation of existing hypertension (28%) compared what has previously been reported.
Hemorrhage is another known adverse event associated with the use of bevacizumab. The etiology of the hemorrhage is thought to be due to lack of endothelial repair in areas where subendothelial tissues are violated by processes that may or may not be associated with malignancy [34]. Two patients in the bevacizumab group experienced hemorrhage, one had severe epistaxis, and the other had an upper gastrointestinal bleed (p = 0.15). VTE events have been reported in many trials using bevacizumab, but it is not clear whether this is greater than the baseline increase from the malignancy. Randomized trials have reported VTE rates to be no different in patients receiving bevacizumab compared to controls [23, 24, 40–46]. The rates of VTE in the two cohorts (6% vs 13%) was not statistically different (p = 0.39), consistent with what has been reported.
While the use of bevacizumab has also been associated with fistula formation, this adverse event tends to be rare. Garcia et al. reported an enterovaginal fistula in a recent phase II study [27]. The present authors report one (3%) case of enterocutaneous fistula after two cycles of bevacizumab (every three weeks, 15 mg/kg) in a platinum - resistant patient that had been treated with three prior lines of chemotherapy. However one patient in the comparison cohort also developed a fistula (p = 0.55). Bevacizumab has received a black box warning for its association with GIP. Cannistra et al. reported five (11%) GIP in forty-four patients and the study was terminated early due GIP [18]. GIP rates of 2%–4% have been reported. However in our group of patients receiving bevacizumab, no GIP occurred.
Current phase III studies (GOG-218 and ICON-7) have initially shown an improvement in PFS. GOG 218 showed an improvement in PFS of only four months for patients on standard therapy plus bevacizumab during standard therapy and up to ten months after completion of standard therapy compared to standard therapy alone [47]. However, these data cannot be extrapolated to the recurrent setting. The present RR, survival, and toxicity data are consistent with what has recently been reported. The reported toxicities were greater in the bevacizumab cohort compared to patients receiving cytotoxic chemotherapy for recurrent ovarian cancer, however only significant for hypertension (p < 0.005). There was no difference in RR (p = 0.46) between the two cohorts. Similar to what Garcia et al. reported, the two patients with clear cell histology that received bevacizumab had PR and SD [27]. Of the patients that received bevacizumab, it is important to note that eight patients had improvement in symptomatic ascites and two patients had improvement in symptomatic pleural effusions. However these patients also had PD based on CT scan and/or rising CA-125. Clinically these patients had improvement in their symptoms, yet all ten of these patients had PD. It is difficult to justify the use of bevacizumab in the setting of recurrent ovarian cancer with no change in RR, PFS or OS with an increase in cost and toxicity.
Currently there are no reliable markers that determine patients’ response to VEGF-targeted therapy. The results of this retrospective study show that in recurrent ovarian cancer, bevacizumab is tolerated with increased toxicity without an increase in RR, OS or PFS. However with inferior RR, increased toxicity and increased cost, the use of bevacizumab for the treatment of recurrent ovarian cancer, especially in heavily pre-treated patients and clear cell histology, warrants further investigation.
Some limitations of the study include: study design, limited number of patients, variability in treatment regimens, non-consistent radiologic follow-up, and lack of documentation of possible toxicities including proteinuria. Baseline radiologic studies were not available for all patients at the initiation of treatment with bevacizumab, thus limiting the ability to use radiologic studies to assess response to bevacizumab. It is difficult to assess hematologic toxicity of bevacizumab since many of the patients have been treated with multiple cytotoxic agents prior to the initiation of bevacizumab and the patients were receiving additional cytotoxic therapies during treatment with bevacizumab.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer. J. Clin. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Young RC, Decker DG, Wharton JT, Piver MS, Sindelar WF, Edwards BK, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983;250:3072. [PubMed] [Google Scholar]
- 3.Hurt JD, Richardson DL, Seamon LG, Fowler JF, Copeland LJ, Cohn DE, et al. Sustained progression-free survival with weekly paclitaxel and bevacizumab in recurrent ovarian cancer. Gynecol. Oncol. 2009;115:396. doi: 10.1016/j.ygyno.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Markman M. Antineoplastic agents in the management of ovarian cancer: current status and emerging therapeutic strategies. Trends Pharmacol. Sci. 2008;29:515. doi: 10.1016/j.tips.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Stone RL, Sood AK, Coleman RL. Collateral damage: toxic effects of targeted antiangiogenic therapies in ovarian cancer. Lancet Oncol. 2010;11:465. doi: 10.1016/S1470-2045(09)70362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santin AD, Hermonat PL, Ravaggi A, Cannon MJ, Pecorelli S, Parham GP. Secretion of vascular endothelial growth factor in ovarian cancer. Eur. J. Gynaecol. Oncol. 1999;20:177. [PubMed] [Google Scholar]
- 7.Penson RT, Dizon DS, Cannistra SA, Roche MR, Krasner CN, Berlin ST, et al. Phase II study of carboplatin, paclitaxel, and bevacizumab with maintenance bevacizumab as first-line chemotherapy for advanced mullerian tumors. J. Clin. Oncol. 2010;28:154. doi: 10.1200/JCO.2009.22.7900. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin. Cancer. Res. 1999;5:587. [PubMed] [Google Scholar]
- 9.Gasparini G, Weidner N, Bevilacqua P, Maluta S, Dalla Palma P, Caffo O, et al. Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma. J. Clin. Oncol. 1994;12:454. doi: 10.1200/JCO.1994.12.3.454. [DOI] [PubMed] [Google Scholar]
- 10.Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ. Tumor angiogenesis in advanced stage ovarian carcinoma. Am. J. Pathol. 1995;147:33. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi Y, Kodama J, Yoshinouchi M, Tokumo K, Kamimura S, Okuda H, et al. The expression of vascular endothelial growth factor and transforming growth factor-beta associates with angiogenesis in epithelial ovarian cancer. Int. J. Gynecol. Pathol. 1997;16:256. doi: 10.1097/00004347-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997;80:98. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Cooper BC, Ritchie JM, Broghammer CL, Coffin J, Sorosky JK, Buller RE, et al. Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer. Clin. Cancer. Res. 2002;8:3193. [PubMed] [Google Scholar]
- 14.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999;77:527. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 16.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J. Natl. Cancer Inst. 1998;90:447. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 17.Richardson DL, Backes FJ, Seamon LG, Zanagnolo V, O’Malley DM, Cohn DE, et al. Combination gemcitabine, platinum, and bevacizumab for the treatment of recurrent ovarian cancer. Gynecol. Oncol. 2008;111:461. doi: 10.1016/j.ygyno.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 2007;25:5180. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 19.Cohn DE, Valmadre S, Resnick KE, Eaton LA, Copeland LJ, Fowler JM. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gynecol. Oncol. 2006;102:134. doi: 10.1016/j.ygyno.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Sfakianos GP, Numnum TM, Halverson CB, Panjeti D, Kendrick JEt, Straughn JM., Jr The risk of gastrointestinal perforation and/or fistula in patients with recurrent ovarian cancer receiving bevacizumab compared to standard chemotherapy: a retrospective cohort study. Gynecol. Oncol. 2009;114:424. doi: 10.1016/j.ygyno.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 22.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003;21:60. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 23.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 24.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 25.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003;349:427. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2007;25:5165. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 27.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J. Clin. Oncol. 2008;26:76. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 28.Wright JD, Secord AA, Numnum TM, Rocconi RP, Powell MA, Berchuck A, et al. A multi-institutional evaluation of factors predictive of toxicity and efficacy of bevacizumab for recurrent ovarian cancer. Int. J. Gynecol. Cancer. 2008;18:400. doi: 10.1111/j.1525-1438.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 29.Burger R, Brady M, Bookman M, Walker J, Homesley H, Fowler J, et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): A Gynecologic Oncology Group study. J. Clin. Oncol. 2010;28:18s. [Google Scholar]
- 30.Pfisterer J, Perren T, Smart AM, Ledermann J, Selle F, Kristensen G, et al. On behalf of ICON7 GCIG collaborators. A randomised controlled trial of bevacizumab in women with newly diagnosed epithelial ovarian, primary peritoneal or fallopian tube cancer. Int. J. Gynecol. Oncol. 2010;20:2. [Google Scholar]
- 31.Perren T, Swart AM, Pfisterer J, Ledermann J, Lortholary A, Kristensen G, et al. On behalf of GCIG ICON7 collaborators. A phase III randomized gynecologic cancer intergroup trial of concurrent bevacizumab and chemotherapy followed by maintenance bevacizumab, versus chemotherapy alone in women with newly diagnosed epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC) ESMO. 2010 Abstract LBA4. [Google Scholar]
- 32.Burger RA, Brady MF, Bookman MA, et al. Phase II trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC) or fallopian tube cancer (FTC): A Gynecologic Oncology Group study. J. Clin. Oncol. 2010;28:5s. doi: 10.1016/j.ygyno.2013.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghajanian C, Finkler N, Rutherford T, Smith D, Yi J, Parmar H, et al. OCEANS: A randomized, double-blinded, placebo-controlled phase III trial of chemotherapy with or without bevacizumab (BEV) in patients with platinum-sensitive recurrent epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC) J. Clin. Oncol. 2011;29:LBA5007. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol. Oncol. 2010;117:497. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 36.Guppy AE, Rustin GJ. CA125 response: can it replace the traditional response criteria in ovarian cancer. Oncologist. 2002;7:437. doi: 10.1634/theoncologist.7-5-437. [DOI] [PubMed] [Google Scholar]
- 37.Nimeiri HS, Oza AM, Morgan RJ, Friberg G, Kasza K, Faoro L, et al. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol. Oncol. 2008;110:49. doi: 10.1016/j.ygyno.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGonigle KF, Muntz HG, Vuky JL, Paley PJ, VD S, Gray HJ, et al. Phase II prospective study of weekly topotecan and bevacizumab in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. Gynecol. Oncol. 2009;112:145. [Google Scholar]
- 39.Loizzi V, Cormio G, Resta L, Rossi CA, Di Gilio AR, Cuccovillo A, et al. Neoadjuvant chemotherapy in advanced ovarian cancer: a case-control study. Int. J. Gynecol. Cancer. 2005;15:217. doi: 10.1111/j.1525-1438.2005.15206.x. [DOI] [PubMed] [Google Scholar]
- 40.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Colangelo LH, Lopa SH, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J. Clin. Oncol. 2009;27:3385. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 42.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25:1539. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 43.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 44.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 2005;23:792. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 45.Reck M, von Pavel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J. Clin. Oncol. 2009;27:1227. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 46.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 47.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011;365:2473. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]