Abstract
Background
Post-operative endophthalmitis is a rare and dreaded complication in ophthalmic operations because it often induces irreparable vision loss. Although many ophthalmological studies aimed at reducing the rate of endophthalmitis have been performed around the world, controversy continues to surround some issues, including the choice of antimicrobials and their route of administration, duration and timing. The aim of this study is to investigate some of these unresolved issues.
Methods
A systematic review and meta-analysis of randomized controlled trials and observational studies was performed. The PubMed, EMBASE, Cochrane Library and Clinical Trials databases were searched to identify studies published until Feb. 2016. The relative risk (RR) for each clinical outcome data is presented with 95% confidence intervals (CIs). Pooled estimates of effects were calculated using random-effect models.
Results
Thirty-four studies from twenty-four reports involving 1264797 eyes were included in this analysis. Endophthalmitis occurred, on average, in one out of 6177 eyes when intracameral vancomycin/moxifloxacin were used and in one out of 1517 eyes when intracameral vancomycin/moxifloxacin were not used. The relative risk (95% CI) of endophthalmitis was reduced to 0.20 (0.10, 0.42) when intracameral antibiotics were used (p<0.0001). The subconjunctival injection of antibiotics was not superior to other administration routes included in this study (RR = 1.67, 95% CI (0.55, 5.05), p = 0.36). A statistically significant difference was found in the rate of endophthalmitis between the use and lack of use of topical antibiotics (RR = 0.65, 95% CI (0.43, 0.99), p = 0.04). However, no statistically significant difference was found in microbial isolation rates between these groups (RR = 0.77, 95% CI (0.34, 1.75), p = 0.53). When long-term and short-term use of topical antibiotics before surgery were compared, a statistically significant difference was found in microbial isolation rates (RR = 0.57, 95% CI (0.44, 0.74), p<0.0001).
Conclusions
This meta-analysis concluded intracameral antibiotics are effective at preventing endophthalmitis in ocular surgery. A randomized controlled trial confirms the efficacy of cefuroxime but recent large cohort studies support the efficacy of vancomycin/moxifloxacin intracamerally. Intracameral antibitoics are superior to subconjunctival injections but that irrigation antibitoic data are not of enough quality to make a comparison. Different results were found in two clinical outcomes between the use or lack of use of topical antibiotic therapy, we did not find sufficient evidence to conclude that its use prevents endophthalmitis.
Introduction
Post-operative endophthalmitis is a complication that can follow all ophthalmic procedures. Endophthalmitis is a calamitous event that can result in a patient suffering the loss of sight. Since 2000, the reported frequency of endophthalmitis is low worldwide, ranging from 0% to 0.63% [1]. Coagulase-negative Staphylococcus species are the organisms that are most frequently isolated from patients who develop postoperative endophthalmitis after cataract surgery. It is followed by coagulase-positive Staphylococcus species, Streptococcus species, Enterococcus species, and Corynebacterium species [2–7]. In pars plana vitrectomies, the reported bacteria include coagulase-negative Staphylococcus species, Pseudomonas species, Propionibacterium species, Enterococcus species, and Bacillus species [8]. Preoperative, intraoperative, and postoperative risk factors have been reported by some ophthalmologists. Diabetes mellitus, an immunocompromised state, chronic blepharitis, lacrimal passage infection, contaminated eyedrops, contact lens use, contralateral prosthesis and gender are preoperative factors, while the application of 2% xylocaine gel before povidone-iodine instillation, prolonged surgery, secondary surgery, posterior capsular rupture, vitreous loss and contaminated irrigating solution are intraoperative factors. Wound leakage, vitreous incarceration and behaviors (e.g., eye rubbing and personal hygiene) are associated with the development of post-operative endophthalmitis [9]. To reduce its incidence, many measures have been employed by ophthalmologists around the world. These include the use of topical antibiotics, intracameral antibiotics, subconjunctival antibiotic injections, lash trimming, saline irrigation, and antibiotic-containing irrigating solutions. However, the most frequent measure is the use of povidone–iodine before surgery to decrease contamination by ocular microbes and prevent postoperative endophthalmitis [10–12]. Not all of these techniques have been found to influence clinical outcomes. Intracameral cephalosporin (cefazolin, cefuroxime) has been shown to be effective in randomized controlled trials and meta-analyses [13–14]. An increasing number of ophthalmologists have supported the use of intracameral cefuroxime to prevent postoperative endophthalmitis because of the ESCRS study. However, as a result of the severe drug resistance of the causal bacteria, some new intracameral antibiotics, such as vancomycin and moxifloxacin, have been suggested to avoid endophthalmitis [15–21]. The subconjunctival injection and topical application of antibiotics have also been used by some ophthalmology centers, but the results of different reports have varied. This paper focuses on the use of intracameral vancomycin/moxifloxacin and the subconjunctival injection and topical application of antibiotics. The timing of topical drops was also analyzed. We sought to use a systemic review and meta-analysis to discuss those controversial issues.
Materials and Methods
Search Strategy
A comprehensive literature search was performed to identify studies published until Feb. 2016. The PubMed, EMBASE, Cochrane Library, and Clinical Trials databases were the main sources that were searched. Other routes (e.g., hand-search and library resource sharing) were also considered. Ophthalmologic surgical procedures, cataract extractions, vitrectomies, keratoplasties, intraocular lens implantations, glaucoma procedures, strabotomies, retinal detachment repair, laser in situ keratomileusis, laser-assisted subepithelial keratectomy, antimicrobial, antibacterial agents, antibiotic prophylaxis, anti-infective agent and eye surgery were the search terms that were used. The specific searching strategy is described in S1 Table.
Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: (i) random studies and observational studies, (ii) compared endophthalmitis rates or microbial isolation rates in two comparable populations, (iii) received/did not receive intracameral vancomycin/moxifloxacin therapy, or received/did not receive subconjunctival antibiotic injection, or received/did not receive topical antibiotics or compared administration with different timing, (iv) published from January 2000-February 2016 (reduced the influence of new operations), and (v) exceeded 1000 individuals if the study reported endophthalmitis rates and 50 if it reported microbial isolation rates. Studies were excluded if they: (i) were not written in English, (ii) were incomplete or included duplicated data, (iii) did not contain any predetermined clinical outcomes, (iv) could not be pooled with other included studies, (v) did not instill topical antibiotics (in terms of the group received topical antibiotics), and (vi) had no conformity at baseline with other studies in the timing (antibiotic drops and povidone–iodine were administered before surgery) and site of the analyzed specimen (conjunctival sac).
Data extraction
The data were extracted independently by two authors (JZH and XHC). A standardized form was designed before the extraction to collect information including first author, publication date, mean age, male (%), type of surgery, study design, follow-up time, no. of eyes, therapeutic regimen, timing and clinical outcomes. If disagreements arose between these two authors, all final decisions were made by LCL and QYS after a discussion.
Quality assessment
Randomized controlled trials (RCT) and observational studies were included in our analysis, and the quality assessment of those data is described in the S1 File. Observational studies were evaluated using the Newcastle–Ottawa scale (NOS) [22]. Nine items comprised the check list, and every item accounted for 1 point in each of three parts (selection, comparability and exposure). If a score was larger than 6, the study was determined to be of high quality. RCTs were assessed using the recommendations of the Cochrane Collaboration [23].
Outcomes analyzed
In the current data set, the rate of endophthalmitis was the best and most direct clinical outcome that could be used to measure the effect of prophylactic antimicrobial agents. However, because postoperative endophthalmitis is rare, some studies also selected the microbial isolation rate as their outcome. In the analysis in this study, the rate of endophthalmitis was used as the primary outcome, and the microbial isolation rate was the secondary outcome.
Statistical analysis
In this meta-analysis, we used risk ratios (RR) with 95% confidence intervals (CIs) to present dichotomous outcomes or enumeration data and random-effect models to calculate pooled estimates of effects. In light of fact that the rate of endophthalmitis was low, in case-control studies, the odds ratio (OR) was considered to be approximately equal to the RR. The I2 statistic was used to assess heterogeneity among studies. We considered the I2 values from 0% to 24%, 25% to 50% and greater than 50% to indicate low, moderate and high heterogeneity, respectively [23]. To decrease heterogeneity and increase reliability, a subgroup analysis was performed for every comparable group. Forest plots, the risk of bias in randomized controlled trials and the above-mentioned traits were analyzed using RevMan version 5.1. Furthermore, the analyses to determine sensitivity and publication bias analysis using Stata version 12.0.
Results
Results of the search and study characteristics
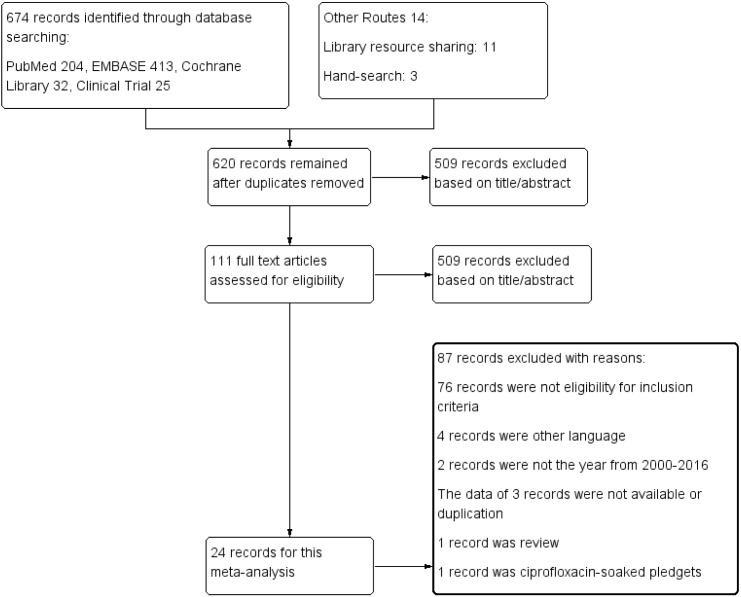
Up to February 2016, a total of 688 reports were identified through database searches, and 34 studies included in 24 reports involving 1264797 eyes were included in the final analysis [2, 13, 15–21, 24–38]. The detailed screening process is described in Fig 1. Of the remaining reports, there were nine RCTs [13, 30–37] and fourteen observational studies [2, 15–21, 24–29, 38]. A total of 21 studies reported the rate of postoperative endophthalmitis [2, 13, 15–21, 24, 27–29, 38], and 13 studies reported the microbial isolation rate [25, 26, 30–37]. Thirty studies [2, 13, 15–21, 24, 27–30, 33–37, 38] included only eyes that received cataract surgery, but an additional four studies [25–26, 31–32] included other intraocular surgeries. The characteristics of the included studies are shown in Table 1.
Fig 1. Flow diagram showing the selection process used to include studies in the meta-analysis.
Table 1. Main characteristics of the studies included in the meta-analysis.
| First Autdor, Date | Age | Male(%) | Type of Surgery | Study Design | Follow-up Time | No. of Eyes | Tderapeutic Regimen | Timing | Clinical Outcomes | Tde Quality of tde Study | |||
| T | C | T | C | T | C | ||||||||
| Intracameral Antibiotic VS No Intracameral | |||||||||||||
| Rush,2015 | 71.0 | 49.4 | CAS | COS | 3 months | 9386 | 11333 | IC vancomycin, PVI | PVI | PE | PE | RPE | High |
| Anijeet,2010 | NA | NA | CAS | COS | 6 weeks | 12702 | 3904 | IC vancomycin, PVI | PVI | PE | PE | RPE | Moderate |
| Rudnisky,2014 | NA | NA | CAS | CCS | 6 weeks | 11818 | 59739 | IC vancomycin | No intracameral antibiotic | PE | PE | RPE | Moderate |
| Rudnisky,2014 | NA | NA | CAS | CCS | 6 weeks | 3738 | 59739 | IC moxifloxacin | No intracameral antibiotic | PE | PE | RPE | Moderate |
| Shorstein,2013 | 74.0 | NA | CAS | CCS | 12 months | 1890 | 3655 | IC moxifloxacin, PVI | PVI | PE | PE | RPE | Moderate |
| Haripriya,2016 | NA | NA | CAS | COS | 6 weeks | 38160 | 78554 | IC moxifloxacin, PVI | PVI | PE | PE | RPE | High |
| Matsuura,2013 | NA | NA | CAS | COS | 1 month | 18794 | 15958 | IC moxifloxacin, PVI | PVI | PE | PE | RPE | Moderate |
| Friling,2013a | NA | 38.3 | CAS | COS | 10 months | 6897 | 2804 | IC moxifloxacin, PVI | PVI | PE | PE | RPE | High |
| Galvis,2014 | 67.2 | NA | CAS | COS | 2 weeks | 1618 | 1056 | IC moxifloxacin, PVI | PVI | PE | PE | RPE | Moderate |
| Subconjunctival Injection Antibiotic VS No Subconjunctival Injection | |||||||||||||
| Jabbarvand,2016b | 79.0 | NA | CAS | RCS | 6 weeks | 69120 | 25290 | SI antibiotic +PVI | IC+PVI | PE | PE | RPE | Moderate |
| Jabbarvand,2016c | 79.0 | NA | CAS | RCS | 6 weeks | 69120 | 76800 | SI antibiotic +PVI | topical antibiotic +PVI | PE | PE | RPE | Moderate |
| Jabbarvand,2016d | 79.0 | NA | CAS | RCS | 6 weeks | 69120 | 260744 | SI antibiotic +PVI | PVI | PE | PE | RPE | Moderate |
| Asencio,2015 | 71.5 | NA | CAS | CCS | 6 weeks | 5068 | 9217 | SI gentamicin +PVI | Irrigation BBS+ vancomycin+ gentamicin +PVI | PE | PE | RPE | High |
| Tan,2012 | NA | NA | CAS | COS | 1 month | 29539 | 20638 | SI gentamicin+ cefazolin +PVI | IC cefazolin +PVI | PE | PE | RPE | Moderate |
| Yu-Wai,2008 | NA | NA | CAS | COS | 3 weeks | 19425 | 17318 | SI cefuroxime +PVI | IC cefuroxime +PVI | PE | PE | RPE | Moderate |
| Colleaux,2000b | NA | NA | CAS | COS | NA | 8856 | 5030 | SI gentamicin ± cefazolin | PVI +Topical antibiotic | PE | PE | RPE | Moderate |
| Topical Antibiotic VS No Topical Antibiotic | |||||||||||||
| ESCRS,2007a | NA | NA | CAS | RCT | 6 weeks | 4000 | 3997 | Topical levofloxacin +IC cefuroxime +PVI | Placebo +IC cefuroxime +PVI | PE | PE | RPE | High |
| ESCRS,2007b | NA | NA | CAS | RCT | 6 weeks | 3984 | 3990 | Topical levofloxacin +PVI | Placebo +PVI | PE | PE | RPE | High |
| Coskun,2011a | NA | 51.2 | CAS | RCT | NA | 54 | 53 | Topical ciprofloxacin | PVI | PE | PE | MIR | Moderate |
| Coskun,2011b | NA | 51.2 | CAS | RCT | NA | 57 | 53 | Topical ofloxacin | PVI | PE | PE | MIR | Moderate |
| Eyal,2009 | 69.7 | 48.9 | OCS | RCT | 72 hours | 237 | 227 | Topical moxifloxacin +PVI | PVI | PE | PE | MIR | Moderate |
| First Author, Date | Age | Male(%) | Type of Surgery | Study Design | Follow-up Time | No. of Eyes | Therapeutic Regimen | Timing | Clinical Outcomes | The Quality of the Study | |||
| T | C | T | C | T | C | ||||||||
| Kaspar,2008 | 67.8 | 34.1 | OCS | RCT | 10 days | 67 | 65 | Topical levofloxacin +PVI | PVI | PE | PE | MIR | High |
| Colleaux,2000a | NA | NA | CAS | COS | NA | 12152 | 1734 | Topical Tobramycin or Gentamicin or Ofloxacin or Polymyxin-trimethoprim | SI antibiotic +PVI | PE | PE | RPE | Moderate |
| Friling,2013b | NA | NA | CAS | COS | 10 months | 7307 | 396894 | Topical chloramphenicol or fusidic acid | IC antibiotic +PVI | PE | PE | RPE | High |
| Jabbarvand,2016a | 79.0 | NA | CAS | RCS | 6 weeks | 76800 | 260744 | Topical ciprofloxacin +PVI | PVI | PE | PE | RPE | Moderate |
| Long time VS Short Time of Topical Antibiotic | |||||||||||||
| Bing,2015 | 70.6 | 50.4 | CAS | RCT | 5 days | 69 | 64 | Topical neomycin/polymyxin-B | Topical neomycin/polymyxin-B | 1d | 1h | MIR | Moderate |
| Inoue,2008a | 74.0 | 46.3 | CAS | RCT | 5 days | 79 | 76 | Topical levofloxacin | Topical levofloxacin | 3d | 1h | MIR | Moderate |
| Inoue,2008b | 74.0 | 46.3 | CAS | RCT | 5 days | 79 | 89 | Topical levofloxacin | Topical levofloxacin | 3d | 1d | MIR | Moderate |
| Inoue,2008c | 74.0 | 46.3 | CAS | RCT | 5 days | 89 | 76 | Topical levofloxacin | Topical levofloxacin | 1d | 1h | MIR | Moderate |
| Lingmin,2009 | 71.1 | 50.8 | OCS | RCT | 10 days | 57 | 63 | Topical moxifloxacin | Topical moxifloxacin | 3d | 1d | MIR | Moderate |
| Ta,2002 | NA | NA | CAS | RCT | 10 days | 43 | 48 | Topical ofloxacin | Topical ofloxacin | 3d | 1h | MIR | High |
| Ta,2007 | NA | NA | CAS | RCT | 6 days | 50 | 50 | Topical levofloxacin | Topical levofloxacin | 3d | 1d | MIR | High |
| Christopher,2008 | 69.3 | 68.3 | OCS | COS | 5 days | 60 | 60 | Topical moxifloxacin | Topical moxifloxacin | 1d | 1h | MIR | Moderate |
| Jason,2008 | 67.7 | 58.3 | OCS | COS | 5 days | 60 | 60 | Topical gatifloxacin | Topical gatifloxacin | 1d | 1h | MIR | Moderate |
Footnotes:Age: Mean age or median age.
Abbreviations: NA = not available, T = treatment group, C = control group, CAS = cataract surgery, OCS = ocular surgery, COS = cohort study, CCS = case-control study, RCT = randomized controlled trial, RCS = retrospective cross-section study, IC = intracameral, SI = subconjunctival injection, PVI = povidone-iodine, PE = perioperation, 1d = 1 day before surgery, 3d = 3 days before surgery, 1h = within 1 hour before surgery, RPE = rate of postoperative endophthalmitis, MIR = microbial isolation rate.
The Rate of Postoperative Endophthalmitis
Intracameral Antibiotic
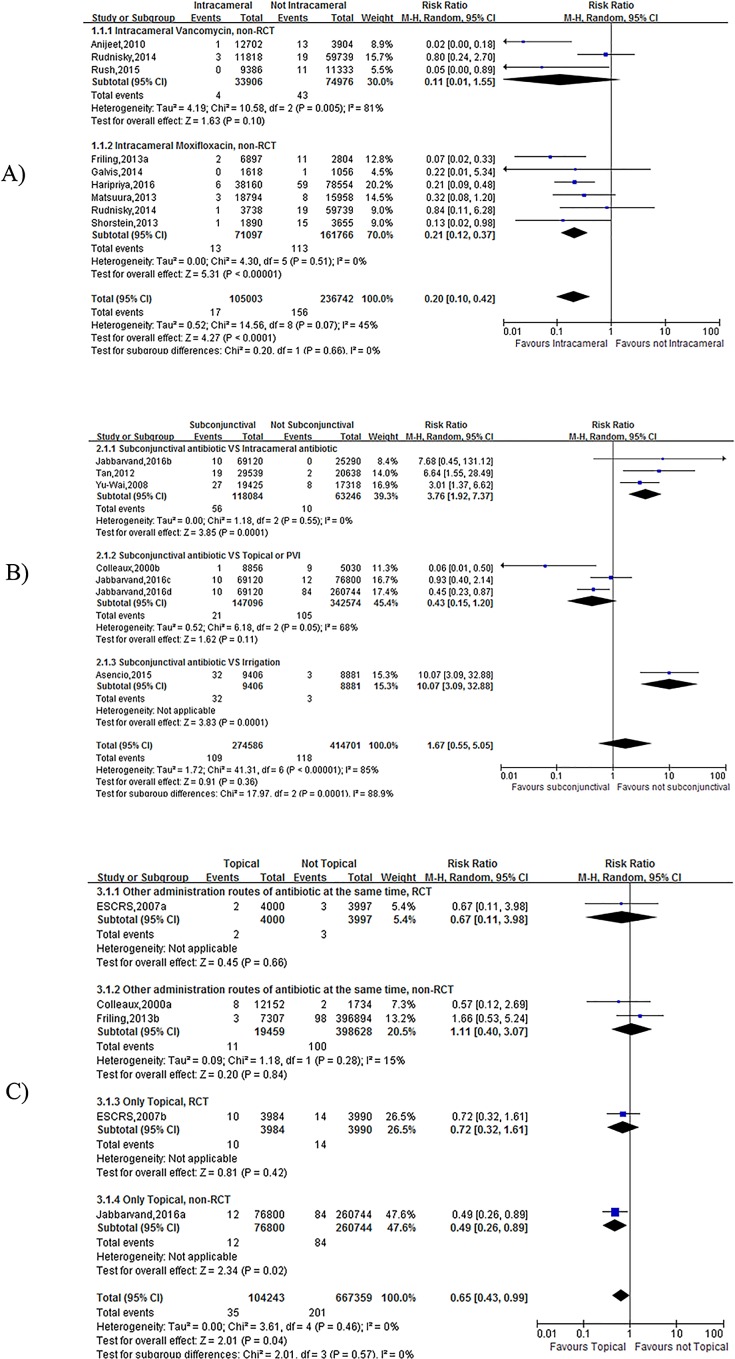
Nine studies compared patients who received/did not receive intracameral vancomycin/moxifloxacin therapy [15–21, 38]. A significant difference was found in the meta-analysis results, which suggested that the rate of postoperative endophthalmitis was lower in the intracameral vancomycin/moxifloxacin group (OR = 0.20, 95% CI (0.10, 0.42), p<0.0001, I2 = 45%) (Fig 2A). In the subgroup analysis, the results in the moxifloxacin group was homologous (OR = 0.21, 95%CI (0.12, 0.37), p<0.00001, I2 = 0%) (Fig 2A). However, there was heterogeneity in the vancomycin group (OR = 0.11, 95%CI (0.01, 1.55), p = 0.10, I2 = 81%) (Fig 2A).
Fig 2.
Forest plot of the Rate of Postoperative Endophthalmitis (A: the effect of including and not including Intracameral Antibiotics; B: the effect of using VS not using subconjunctival antibiotic injections; C: the effect of using VS not using Topical Antibiotics). The vertical line indicates no difference between the groups. RRs are represented by diamond shapes, and 95% CIs are depicted by horizontal lines. Squares indicate point estimates, and the size of each square indicates the weight of the given study in the meta-analysis. M-H, Mantel-Haenszel random-effects model.
Subconjunctival antibiotic injections
Seven studies reported the use of subconjunctival antibiotic injections [2, 24, 27–29]. When patients who received subconjunctival antibiotic injections were compared to those who did not, no significant difference was found (OR = 1.67, 95% CI (0.55, 5.05), p = 0.36, I2 = 85%) (Fig 2B). Because I2 = 85%, three subgroup analyses were performed to increase reliability. The results suggested that a significant difference was found between the subconjunctival antibiotic injections group and the intracameral antibiotic and irrigation groups (intracameral antibiotic: OR = 3.76, 95% CI (1.92, 7.37), p = 0.0001, I2 = 0%; irrigation: OR = 10.07, 95% CI (3.09, 32.88), p = 0.0001) (Fig 2B). When the subconjunctival antibiotic injection group was compared to the topical or PVI (povidone-iodine) group, there was no significant difference (OR = 0.43, 95% CI (0.15, 1.20), p = 0.11, I2 = 68%) (Fig 2B).
Topical Antibiotic
The meta-analysis results revealed that there was a significant difference among the five studies (2, 13, 20, 24) that reported the rate of postoperative endophthalmitis (OR = 0.65, 95% CI (0.43, 0.99), p = 0.04, I2 = 0%) (Fig 2C). However, when subgroups were analyzed, no significant difference was found except for in a retrospective study (OR = 0.49, 95% CI (0.26, 0.89), p = 0.02) (Fig 2C).
Microbial Isolation Rate
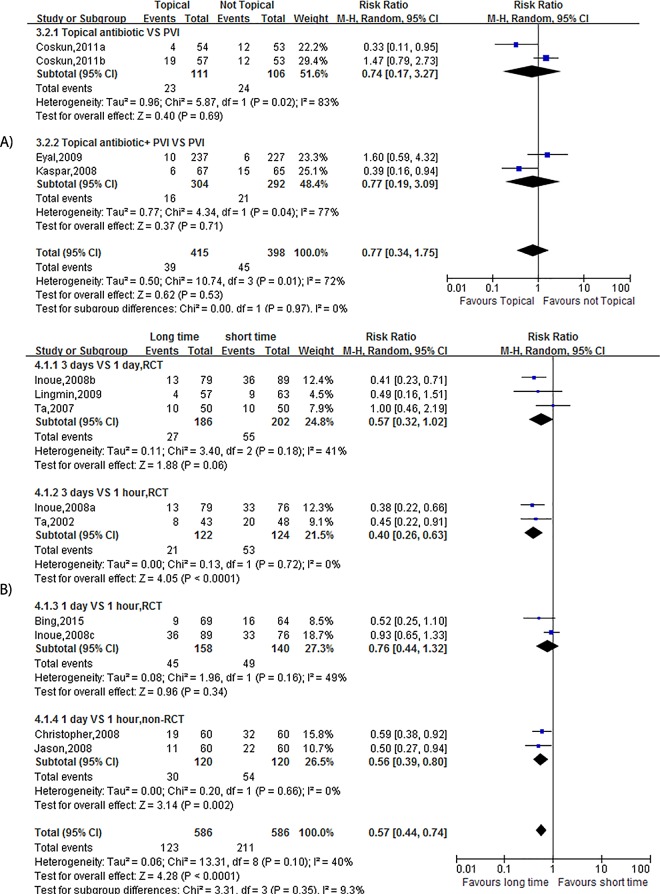
Topical Antibiotic
There were four studies [30–32] that provided a microbial isolation rate, and the results of these studies suggested that there was no significant difference (OR = 0.77, 95% CI (0.34, 1.75), p = 0.53, I2 = 72%) (Fig 3A). A subgroup analysis was performed, but the results were similar (only topical antibiotic: OR = 0.74, 95% CI (0.17, 3.27), p = 0.69, I2 = 83%; topical antibiotic + PVI: OR = 0.77, 95% CI (0.19, 3.09), p = 0.71, I2 = 77%) (Fig 3A).
Fig 3.
Forest plot of the Microbial Isolation Rate (A: the effect of using VS not using Topical Antibiotics; B: the effect of Long-term VS Short-term use). The vertical line indicates no difference between the groups. RRs are represented by diamond shapes, and 95% CIs are depicted by horizontal lines. Squares indicate point estimates, and the size of each square indicates the weight of the given study in the meta-analysis. M-H, Mantel-Haenszel random-effects model.
Timing
When long-term and short-term use of a topical antibiotic before ocular surgery was analyzed, nine studies that had consistent baselines were included (25–26, 33–37). The meta-analysis revealed that short-term use was associated with an increased incidence of microbial isolation (OR = 0.57, 95% CI (0.44, 0.74), p<0.0001, I2 = 40%) (Fig 3B). Because there was moderate heterogeneity (I2 = 40%), a subgroup analysis was performed. A significance difference was found in the group that included two RCTs and that compared application between three days and within one hour before surgery (OR = 0.40, 95% CI (0.26, 0.63), p<0.0001, I2 = 0%) (Fig 3B). A significance difference was also found in the group that included two non-RCTs and that compared results between groups that were administered at one day and within one hour before surgery (OR = 0.56, 95% CI (0.39, 0.80), p = 0.002, I2 = 0%) (Fig 3B). There was no difference between the other two subgroups (3 days VS 1 day, RCT: OR = 0.57, 95% CI (0.32, 1.02), p = 0.06, I2 = 41%; 3 days VS 1 day, RCT: OR = 0.76, 95% CI (0.44, 1.32), p = 0.36, I2 = 49%) (Fig 3B).
Publication Bias and Sensitivity Analysis
There was no significant publication bias according to the Begg’s and Egger’s funnel plot asymmetry tests that were performed in the meta-analysis (S2 File). The results of the sensitivity analysis were diverse, and the details are described in S3 File. In the group comparison between using and not using intracameral antibiotics, sensitivity was not influenced by the studies that were omitted obviously. The sensitivity analysis for receiving or not receiving subconjunctival antibiotic injections demonstrated that three studies (Colleaux,2000b, Jabbarvand,2016c and Jabbarvand,2016d) influenced the pooled effect size, and these studies were therefore deleted. New results that were similar to those of the subgroup analysis revealed that intracameral and irrigation antibiotics were superior to subconjunctival injections. In the case of topical antibiotics, we excluded the study (Jabbarvand, 2016a) because it influenced the sensitivity in the group that used RPE as a clinical outcome. When we then reanalyzed the data, we found that there was no statistical difference. Hence, that study should be deleted to increase the conclusion’s reliability. In the group in which MIR was used as the outcome, we re-conducted a subgroup analysis according to classifications of quinolone, but the result was similar to the previous result. The sensitivity analysis for the timing of application of topical antibiotics demonstrated that the study (Inoue, 2008c) exhibited slightly more influence on the pooled effect size than other studies, and this study was therefore deleted. The new result was almost the same as the initial result, but the heterogeneity was smaller. Because of the variety of available antibiotics, we also reanalyzed new subgroups according to the classifications of the drugs that were used. The result was similar to the previous result, but the heterogeneity was larger.
Discussion
The low rate of postoperative endophthalmitis makes it difficult to conduct a large RCT to investigate the optimal method for preventing it [39]. Based on the currently available clinical evidence, preoperative preparation with 5% povidone-iodine solution has consensus approval among ophthalmologists. However, no consensus has been reached regarding the agent of choice, the administration route or the timing of antimicrobial prophylaxis, and no agent has been FDA-approved for this indication in ophthalmic procedures [1]. For these reasons, we performed this meta-analysis. However, because of the absence of relevant RCTs, we also included observational studies to resolve the problem mentioned above in this analysis. Since the ESCRS study, intracameral antibiotics have gradually gained acceptance around the world. Of those antibiotics, cefuroxime has been shown to be effective at reducing the rate of postoperative endophthalmitis. However, with the increase in resistant microorganisms, moxifloxacin and vancomycin have received an increasing amount of attention. The results of this meta-analysis show that intracameral moxifloxacin/vancomycin can prevent endophthalmitis. Unfortunately, no RCTs were included to address this point. Additionally, no study compared the use of cephalosporin with moxifloxacin or vancomycin. In addition to intracameral moxifloxacin/vancomycin, we also compared the results of administering subconjunctival antibiotic injections via other routes. According to our analysis, intracameral antibiotics were superior to subconjunctival injections, but subconjunctival injections were not superior to topical antibiotics or PVI. There is only one case-control study to compare irrigation antibiotics with subconjunctival injections, so the data is not enough quality to make a comparison. According to the results of this meta-analysis, we do not recommend subconjunctival antibiotic injections as a routine method for preventing endophthalmitis. Interestingly, when the timing of administration was not considered, topical antibiotics seemed to lose their value. The results were the same whether RPE or MIR was used as the clinical outcome. But there was a higher incidence of microbial isolation in short-term use than long-term use patients. The reason for this contradictory result might be that the efficiency of bacterial eradication varied with differences in the timing of topical antibiotics use. However, reducing the number of bacteria on the ocular surface is not the same as preventing endophthalmitis. Additionally, many topical antibiotics are used in combination, which makes it difficult to determine optimal timing [1]. The timing reported in the papers ranged from one hour to three days before the surgery [4, 11, 40–41]. Further investigations should be performed to optimize the best regimens. Given the results we obtained in the meta-analysis and the results from the ESCRS study, the use of topical antibiotics was not recommended.There are several limitations to our meta-analysis. First, few RCTs were included. Second, although we performed subgroup and sensitivity analyses, the I2 values remained large, especially in the topical antibiotic group, demonstrating that heterogeneity was high. The fact that we did not identify the reason for the heterogeneity may make the results unreliable. Third, some results were obtained from small sample studies. In addition, difficulties in diagnosing and defining endophthalmitis could also influence the primary data. For the studies that selected MIR as the clinical outcome, the method and duration of specimen cultivation might have made the results in the primary studies more diverse, which could have influenced our results.In conclusion, intracameral vancomycin/moxifloxacin therapy is effective for preventing postoperative endophthalmitis. Intracameral antibiotics are superior to subconjunctival injections, but that irrigation antibitoic data are not of enough quality to make a conclusion. Different results were found for topical antibiotic therapies between two clinical outcomes, and we did not find sufficient evidence to conclude that this technique prevents endophthalmitis. Long-term use of topical antibiotics before surgery appears to be more effective, but clearance the number of pathogens on the ocular surface is not the same as preventing endophthalmitis. So, topical antibiotics before surgery to prevent endophthalmitis were not recommended by this meta-analysis.
Supporting Information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Am J Health Syst Pharm. Clinical practice guidelines for antimicrobial prophylaxis in surgery. 2013; 70(3):195–283. [DOI] [PubMed] [Google Scholar]
- 2.Colleaux KM, Hamilton WK. Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can J Ophthalmol. 2000;35: 373–378. 10.1016/S0008-4182(00)80124-6 [DOI] [PubMed] [Google Scholar]
- 3.Garat M, Moser CL, Martín-Baranera M, Alonso-Tarrés C, Alvarez-Rubio L. Prophylactic intracameral cefazolin after cataract surgery: endophthalmitis risk reduction and safety results in a 6-year study. J Cataract Refract Surg. 2009;35: 637–642. 10.1016/j.jcrs.2008.12.023 [DOI] [PubMed] [Google Scholar]
- 4.Jensen MK, Fiscella RG, Moshirfar M, Mooney B. Third- and fourth-generation fluoroquinolones: retrospective comparison of endophthalmitis after cataract surgery performed over 10 years. J Cataract Refract Surg. 2008;34: 1460–1467. 10.1016/j.jcrs.2008.05.045 [DOI] [PubMed] [Google Scholar]
- 5.Moshirfar M, Feiz V, Vitale AT, Wegelin JA, Basavanthappa S, Wolsey DH. Endophthalmitis after uncomplicated cataract surgery with the use of fourth-generation fluoroquinolones: a retrospective observational case series. Ophthalmology. 2007;114: 686–691. 10.1016/j.ophtha.2006.08.038 [DOI] [PubMed] [Google Scholar]
- 6.Recchia FM, Busbee BG, Pearlman RB, Carvalho-Recchia CA, Ho AC. Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch Ophthalmol. 2005;123: 341–346. 10.1001/archopht.123.3.341 [DOI] [PubMed] [Google Scholar]
- 7.Wu PC, Li M, Chang SJ, Teng MC, Yow SG, Shin SJ, et al. Risk of endophthalmitis after cataract surgery using different protocols for povidone-iodine preoperative disinfection. J Ocul Pharmacol Ther. 2006;22: 54–61. 10.1089/jop.2006.22.54 [DOI] [PubMed] [Google Scholar]
- 8.Dave VP, Pathengay A, Schwartz SG, Flynn HW Jr. Endophthalmitis following pars plana vitrectomy: a literature review of incidence, causative organisms, and treatment outcomes. Clin Ophthalmol. 2014;8: 2183–2188. 10.2147/OPTH.S71293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland EJ, McDonald MB, Parekh JG, Sheppard JD. Antibiotic resistance in acute postoperative endophthalmitis. Ophthalmology. 2014;121(11 Suppl): S1–S9. 10.1016/j.ophtha.2014.06.049 [DOI] [PubMed] [Google Scholar]
- 10.Grzybowski A, Kuklo P, Pieczynski J, Beiko G. A review of preoperative manoeuvres for prophylaxis of endophthalmitis in intraocular surgery: topical application of antibiotics, disinfectants, or both? Current Opinion in Ophthalmology. 2016; 27(1); 9–23. 10.1097/ICU.0000000000000216 [DOI] [PubMed] [Google Scholar]
- 11.Gordon-Bennett P, Karas A, Flanagan D, Stephenson C, Hingorani M. A survey of measures used for the prevention of postoperative endophthalmitis after cataract surgery in the United Kingdom. Eye. 2008;22: 620–627. 10.1038/sj.eye.6702675 [DOI] [PubMed] [Google Scholar]
- 12.Vazirani J, Basu S. Role of topical, subconjunctival, intracameral, and irrigative antibiotics in cataract surgery. Curr Opin Ophthalmol. 2013;24: 60–65. 10.1097/ICU.0b013e32835a93be [DOI] [PubMed] [Google Scholar]
- 13.Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33: 978–988. 10.1016/j.jcrs.2007.02.032 [DOI] [PubMed] [Google Scholar]
- 14.Kessel L, Flesner P, Andresen J, Erngaard D, Tendal B, Hjortdal J. Antibiotic prevention of postcataract endophthalmitis: a systematic review and meta-analysis. Acta Ophthalmol. 2015;93: 303–317. 10.1111/aos.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rush SW, Vu D, Rush RB. The safety and efficacy of routine administration of intracameral vancomycin during cataract surgery. J Ophthalmol. 2015; Article ID: 813697. 10.1155/2015/813697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anijeet DR, Palimar P, Peckar CO. Intracameral vancomycin following cataract surgery: an eleven-year study, Clin Ophthalmol. 2010;4: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39: 8–14. 10.1016/j.jcrs.2012.07.031 [DOI] [PubMed] [Google Scholar]
- 18.Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123: 302–308. 10.1016/j.ophtha.2015.09.037 [DOI] [PubMed] [Google Scholar]
- 19.Matsuura K, Miyoshi T, Suto C, Akura J, Inoue Y. Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J Cataract Refract Surg. 2013;39: 1702–1706. 10.1016/j.jcrs.2013.05.036 [DOI] [PubMed] [Google Scholar]
- 20.Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39: 15–21. 10.1016/j.jcrs.2012.10.037 [DOI] [PubMed] [Google Scholar]
- 21.Galvis V, Tello A, Sánchez MA, Camacho PA. Cohort study of intracameral moxifloxacin in postoperative endophthalmitis prophylaxis. Ophthalmol Eye Dis. 2014;6: 1–4. 10.4137/OED.S13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, et al. Testing the Newcastle Ottawa scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013;66: 982–993. 10.1016/j.jclinepi.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0 (updated March 2011), The Cochrane Collaboration 2011. Available: http://www.cochrane-handbook.org/.
- 24.Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123: 295–301. 10.1016/j.ophtha.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 25.Ta CN, Chan I, Dhatt HS, Paterno J, Fisher E, Singh K, et al. Prospective comparison of topical moxifloxacin in eliminating conjunctival baterial flora following a one-day or one-hour application. J Ocul Pharmacol Ther. 2008;24: 427–431. 10.1089/jop.2008.0018 [DOI] [PubMed] [Google Scholar]
- 26.Moss JM, Nguyen D, Liu YI, Singh K, Montague A, Egbert PR et al. Comparison of one-day versus one-hour application of topical gatifloxacin in eliminating conjunctival bacterial flora. Ophthalmol. 2008;115: 2013–2016. 10.1016/j.ophtha.2008.06.024 [DOI] [PubMed] [Google Scholar]
- 27.Yu-Wai-Man P, Morgan SJ, Hildreth AJ, Steel DH, Allen D. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34: 447–451. 10.1016/j.jcrs.2007.10.041 [DOI] [PubMed] [Google Scholar]
- 28.Tan CSH, Wong HK, Yang FP. Epidemiology of postoperative endophthalmitis in an Asian population: 11-year incidence and effect of intracameral antibiotic agents. J Cataract Refract Surg. 2012;38: 425–430. 10.1016/j.jcrs.2011.09.040 [DOI] [PubMed] [Google Scholar]
- 29.Asencio MA, Huertas M, Carranza R, Tenias JM, Celis J, Gonzalez-del Valle F. Impact of changes in antibiotic prophylaxis on postoperative endophthalmitis in a Spanish hospital. Ophthal Epidemiol. 2014;21: 45–50. 10.3109/09286586.2013.867511 [DOI] [PubMed] [Google Scholar]
- 30.Coskun M, Altintas AG, Anayol MA, Raza S, Celikbilek N, Simsek S. Evaluation of efficacy of topical povidone-iodine and different types of fluoroquinolones in the sterilization of bacterial flora on the conjunctiva. J Ocul Pharmacol Ther. 2011;27: 589–592. 10.1089/jop.2010.0192 [DOI] [PubMed] [Google Scholar]
- 31.Halachmi-Eyal O, Lang Y, Keness Y, Miron D. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg. 2009;35: 2109–2114. 10.1016/j.jcrs.2009.06.038 [DOI] [PubMed] [Google Scholar]
- 32.Miño de Kaspar H, Kreutzer TC, Aguirre-Romo I, Ta CN, Dudichum J, Bayrhof M, et al. A prospective randomized study to determine the efficacy of preoperative topical levofloxacin in reducing conjunctival bacterial flora. Am J Ophthalmol. 2008;145: 136–142. 10.1016/j.ajo.2007.08.031 [DOI] [PubMed] [Google Scholar]
- 33.Li B, Miño de Kaspar H, Haritoglou C, Kook D, Kampik A, Sheng M et al. Comparison of 1-day versus 1-hour application of topical neomycin/polymyxin-B before cataract surgery. J Cataract Refract Surg. 2015;41: 724–731. 10.1016/j.jcrs.2014.06.042 [DOI] [PubMed] [Google Scholar]
- 34.Inoue Y, Usui M, Ohashi Y, Shiota H, Yamazaki T, Preoperative Disinfection Study Group. Preoperative disinfection of the conjunctival sac with antibiotics and iodine compounds: a prospective randomized multicenter study. Jpn J Ophthalmol. 2008;52: 151–161. 10.1007/s10384-008-0517-y [DOI] [PubMed] [Google Scholar]
- 35.He L, Ta CN, Hu N, Sinnar S, Miño de Kaspar H. Prospective randomized comparison of 1-day and 3-day application of topical 0.5% moxifloxacin in eliminating preoperative conjunctival bacteria. J Ocul Pharmacol Ther. 2009;25: 373–378. 10.1089/jop.2008.0102 [DOI] [PubMed] [Google Scholar]
- 36.Ta CN, Egbert PR, Singh K, Shriver EM, Blumenkranz MS, Miño De Kaspar H. Prospective randomized comparison of 3-day versus 1-hour preoperative ofloxacin prophylaxis for cataract surgery. Ophthalmol. 2002;109: 2036–2040. 10.1016/S0161-6420(02)01236-8 [DOI] [PubMed] [Google Scholar]
- 37.Ta CN, Sinnar S, He L, Myung D, Miño De Kaspar H. Prospective randomized comparison of 1-day versus 3-day application of topical levofloxacin in eliminating conjunctival flora. Eur J Ophthalmol. 2007;17: 689–695. [DOI] [PubMed] [Google Scholar]
- 38.Rudnisky CJ, Wan D, Weis E. Antibiotic choice for the prophylaxis of post-cataract extraction endophthalmitis. Ophthalmology. 2014; 121(4):835–41. 10.1016/j.ophtha.2013.08.046 [DOI] [PubMed] [Google Scholar]
- 39.Cao H, Zhang L, Li L, Lo S. Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysis. PLoS One. 2013;8: e71731 10.1371/journal.pone.0071731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moshirfar M, Feiz V, Vitale AT, Wegelin JA, Basavanthappa S, Wolsey DH. Endophthalmitis after uncomplicated cataract surgery with the use of fourth-generation fluoroquinolones: a retrospective observational case series Ophthalmology. 2007;114: 686–691. 10.1016/j.ophtha.2006.08.038 [DOI] [PubMed] [Google Scholar]
- 41.Bucci FA, Amico LM, Evans RE. Antimicrobial efficacy of prophylactic gatifloxacin 0.3% and moxifloxacin 0.5% in patients undergoing phacoemulsification surgery. Eye Contact Lens. 2008;34: 39–42. 10.1097/ICL.0b013e3180645d01 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.