Abstract
Recently, it has been reported that eriC and crcB are involved in bacterial fluoride resistance. However, the fluoride-resistance mechanism in oral streptococci remains unclear. BLAST studies showed that two types of eriCs (eriC1 and eriC2) and two types of crcBs (crcB1 and crcB2) are present across 18 oral streptococci, which were identified in ≥ 10% of 166 orally healthy subjects with ≥ 0.01% of the mean relative abundance. They were divided into three groups based on the distribution of these four genes: group I, only eriC1; group II, eriC1 and eriC2; and group III, eriC2, crcB1, and crcB2. Group I consisted of Streptococcus mutans, in which one of the two eriC1s predominantly affected fluoride resistance. Group II consisted of eight species, and eriC1 was responsible for fluoride resistance, but eriC2 was not, in Streptococcus anginosus as a representative species. Group III consisted of nine species, and both crcB1 and crcB2 were crucial for fluoride resistance, but eriC2 was not, in Streptococcus sanguinis as a representative species. Based on these results, either EriC1 or CrcBs play a role in fluoride resistance in oral streptococci. Complementation between S. mutans EriC1 and S. sanguinis CrcB1/CrcB2 was confirmed in both S. mutans and S. sanguinis. However, neither transfer of S. sanguinis CrcB1/CrcB2 into wild-type S. mutans nor S. mutans EriC1 into wild-type S. sanguinis increased the fluoride resistance of the wild-type strain. Co-existence of different F− channels (EriC and CrcB) did not cause the additive effect on fluoride resistance in oral Streptococcus species.
Introduction
Fluoride is commonly used as an effective caries-preventive agent in many countries. Organisms present in the oral cavity are frequently exposed to fluoride ions from drinking water or from the use of fluoride dentifrices and fluoride mouth rinses. Fluoride is known to interfere with metabolic processes in many organisms [1], and high-concentration fluoride shows a bactericidal effect [2, 3]. These anti-microbial effects contribute to the anti-caries effect of fluoride. However, the anti-microbial effects of fluoride may have an unexpected effect on the bacterial composition of oral microflora.
The widespread, long-term use of fluoride can result in the emergence of fluoride-resistant oral Streptococcus species, including Streptococcus mutans, a cariogenic bacterium [4–6]. These fluoride-resistant strains show clear phenotypic differences in growth, adherence, and metabolic activity compared to the fluoride-sensitive strains [7–9]. The emergence of fluoride-resistant oral Streptococcus species may not only decrease the anti-caries effects of fluoride, but also disrupt the composition of oral streptococci in the oral cavity, followed by the deterioration of oral health.
More than 700 bacterial species are present in the oral cavity, and oral streptococci are predominant. Oral streptococci account for approximately 20% of all bacteria in saliva [10] and approximately 50% of those during the early stages of dental plaque formation [11]. Oral streptococci pioneer early dental plaque formation and have a specific temporal and spatial distribution that is crucial for the development of oral biofilms [12].
Bacteria have evolved numerous strategies to alleviate the toxic effects of ions other than fluoride, and yet analogous systems for fluoride toxicity mitigation were notably absent [13]. Recently, it has been reported that the eriC gene of Pseudomonas syringae and the crcB gene of Escherichia coli are involved in bacterial fluoride resistance [14]. The eriC gene is described as a ClC chloride channel in many bacteria, and the crcB gene has previously been implicated in resistance to camphor-induced chromosome decondensation. Furthermore, Liao et al. [15] identified two single nucleotide polymorphisms (SNPs) in the gene cluster, including two eriC genes in the genome of the fluoride-resistant mutant S. mutans C180-2FR. Expression of the cluster was approximately 10-fold higher in C180-2FR than in the parent strain C180-2. These results suggested that eriC may be related to the response to fluoride in S. mutans. However, it remains unclear whether eriC and/or crcB play a role in fluoride resistance in oral streptococci. To maintain the appropriate bacterial composition of oral microflora, it is important to investigate the fluoride resistance mechanism in oral streptococci. In this study, we attempted to identify and characterize fluoride-resistance-related genes in oral streptococci.
Materials and Methods
Bacterial strains and culture conditions
The following bacterial strains were used in this study: Streptococcus mutans UA159, Streptococcus sobrinus OMZ175, Streptococcus mitis ATCC 49456, Streptococcus oralis ATCC 10557, Streptococcus gordonii ATCC 10558, Streptococcus sanguinis SK36, Streptococcus parasanguinis ATCC 15912, Streptococcus tigurinus ATCC 15914, Streptococcus australis ATCC 700641, Streptococcus infantis ATCC 700779, Streptococcus salivarius HHT, Streptococcus anginosus NCTC 10707, Streptococcus intermedius ATCC 27335, and Escherichia coli DH5α
E. coli strains were grown in 2×YT Broth (Becton Dickinson, Franklin Lakes, NJ, USA). Oral streptococci strains were grown in brain heart infusion (BHI) broth (Becton Dickinson) at 37°C in 5% CO2. Antibiotics were used at the following concentrations: 300 μg/mL erythromycin and 50 μg/mL ampicillin for E. coli, 20 μg/mL erythromycin for oral streptococci, 600 μg/mL spectinomycin for S. mutans and S. anginosus, and 150 μg/mL spectinomycin for S. sanguinis.
DNA Manipulation
Standard recombinant DNA procedures such as DNA isolation, endonuclease restriction, ligation, and agarose gel electrophoresis were performed as described by Sambrook & Russell [16]. Transformations of oral streptococci and E. coli were performed as described previously [17, 18]. Protein sequence similarity searches were performed with the BLAST program via the National Center for Biotechnology Information server.
Construction of mutant forms of the genes in Streptococcus species
Various deletion mutants were constructed by replacing the target gene with an erythromycin resistance (Emr) or spectinomycin resistance (Spcr) gene using double cross-over homologous recombination, as described previously [19]. As an example, we describe a strategy for construction of the eriC1a/eriC1b double mutant. An 869-bp fragment upstream of eriC1a and a 767-bp fragment downstream of eriC1b were amplified from S. mutans UA159 genomic DNA and inserted upstream and downstream, respectively, of the Emr gene in pBSSKII-Emr [20], in which the Emr fragment was cloned into HindIII- and EcoRV-digested pBluescript SK II (+). The resultant plasmid (pBSSKII-Emr-eriC1a/eriC1b -UD) was digested with KpnI and SacII, and the assembled fragment was transformed into S. mutans UA159. Correct insertions or replacements of transformants were confirmed by PCR.
Complementation between Streptococcus species
Complementation between Streptococcus species was performed using the shuttle vector, pSEP, which consisted of an Emr gene, pC194ori for replication in Gram-positive organisms, pUCori for replication in E. coli, and the promoter region of the Emr gene derived from Gram-positive organisms inserted within the multi-cloning site (MCS). A target gene was amplified by PCR from the parent strain genomic DNA and cloned downstream of the promoter region within the MCS. Prior to complementation, Emr within plasmids for construction of the mutant strains were replaces with Spcr. Complementing plasmid was introduced into Spcr-mutant and transformants were selected on BHI agar plates containing appropriate concentration of erythromycin and spectinomycin. The existence of complementing plasmid was confirmed by plasmid extraction from transformants.
Evaluation of the fluoride sensitivity of strains
The mutant strains and wild-type control strain cells were grown in BHI broth overnight at 37°C in 5% CO2. The cultures were then diluted 1:10 into fresh BHI and grown to an OD550 of ~0.5. Aliquots (20 μL) of cell suspensions with the same turbidity were inoculated into wells containing 200 μL of fresh BHI medium with several different sodium fluoride (NaF) concentrations. The ranges of NaF concentrations tested were selected based on preliminary experiments in which growth rates of oral streptococci were examined in dilution series of NaF using planktonic culture. Growth was evaluated after incubation for 16 h by measuring OD550 using a SpectraMax 340PC384 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Wells containing only BHI were used as controls. Growth yields were estimated based on the means of the data obtained from three independent experiments.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The Bonferroni test was used to determine the significance of differences in multiple comparisons. Differences were considered significant only for values of P < 0.05. All statistical analyses were performed using SPSS for Windows version 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Distribution of eriC and crcB genes in oral streptococci
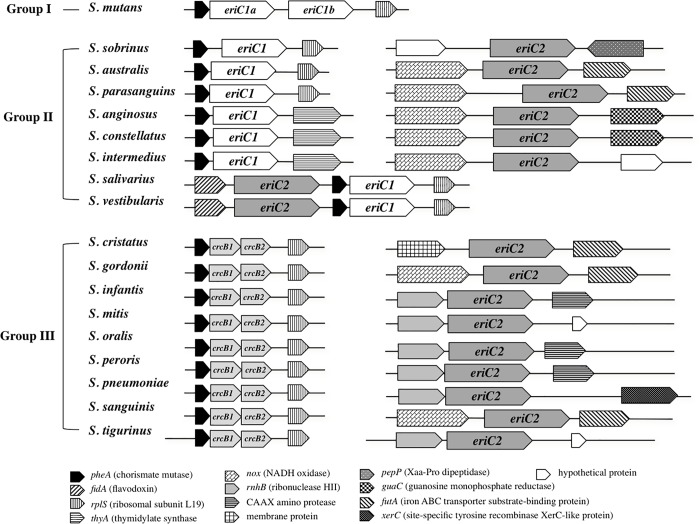
To examine the distribution of eriC and crcB genes in oral streptococci, we performed sequence homology analysis for the complete genome sequences of 18 types of oral streptococci. These species were selected based on the following criteria: identification in ≥ 10% of 166 orally healthy subjects and ≥ 0.01% of the mean relative abundance [10]. There were two types of eriC genes (eriC1 and eriC2) and two types of crcB genes (crcB1 and crcB2) in oral streptococci (Fig 1). The eriC1 gene product showed about 50% similarity with EriC of P. syringae DC3000, which was identified as a fluoride channel protein in a previous report [14]. On the other hand, another EriC2 had no significant similarity with P. syringae EriC. Both crcB products showed about 50% similarity with CrcB of E. coli K-12, which was involved in fluoride resistance in a previous report [14]. As shown in Fig 1, these oral streptococci were divided into three groups based on the distribution of eriC and crcB genes: group I with only eriC1, group II with eriC1 and eriC2, and group III with eriC2, crcB1, and crcB2. The eriC1 and crcB genes were flanked by highly similar gene arrangements.
Fig 1. Locations of eriC and crcB genes in 18 oral streptococci.
These species were selected based on the following criteria: identification in ≥ 10% of 166 orally healthy subjects and ≥ 0.01% of the mean relative abundance [10].
Fluoride resistance of oral streptococci
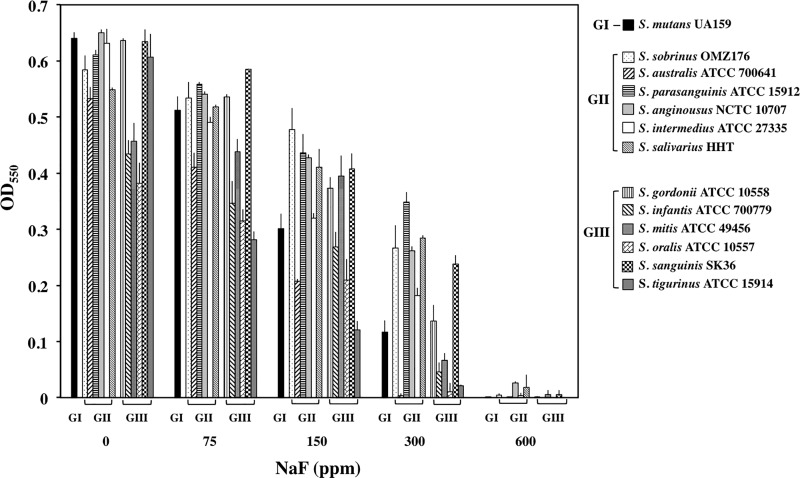
To examine the fluoride resistance of oral streptococci, 13 available species among the above 18 oral Streptococcus species were grown in BHI broth with various concentrations of NaF. These oral streptococci showed various resistances to fluoride (Fig 2). S. parasanguinis, S. sanguinis, S. sobrinus, S. salivarius, and S. anginosus grew fairly well, even in the presence of 300 ppm NaF. S. australis, S. oralis, S. infantis, S. mitis, and S. tigurinus grew poorly under the same conditions. In the presence of 600 ppm NaF, all 13 oral streptococci did not grow. Among species with the higher fluoride-resistance, S. parasanguinis, S. sobrinus, S. salivarius, and S. anginosus belong to group II and S. sanguinis belongs to group III. On the other hand, among species with the lower fluoride-resistance, S. oralis, S. infantis, S. mitis, and S. tigurinus belonged to group III and S. australis belonged to group II. S. mutans, which belonged to group I, showed medium fluoride-resistance. No strong relationship was observed between the distribution of eriC and crcB genes and fluoride resistance. Next, we explored the related gene(s) for fluoride resistance in S. mutans, S. anginosus, and S. sanguinis, which possess a fair fluoride resistance, as representative species of the three groups, respectively.
Fig 2. Growth yields of 13 oral streptococci at various NaF concentrations.
The y-axis represents the OD550 after incubation for 16 h. The data represent the mean standard deviations of three independent experiments.
Identification of fluoride-resistance-related genes in S. mutans
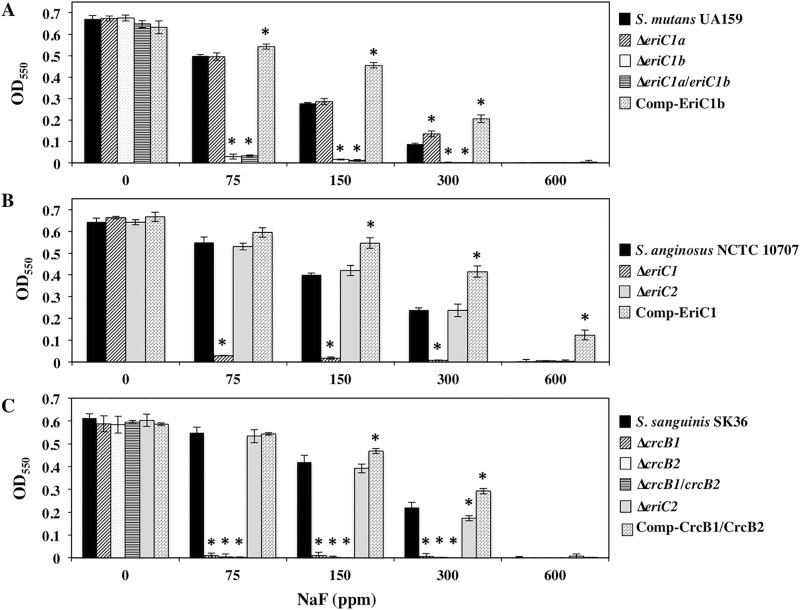
Sequence homology analysis revealed two eriC1 genes [SMU_1290 (eriC1a) and SMU_1289 (eriC1b)] in S. mutans UA159. eriC1a and eriC1b exist in tandem and in the same orientation. Both eriC1a and eriC1b encode a protein of 406 amino acids, named the chloride channel permease. The amino acid sequences deduced from eriC1a and eriC1b showed 52.6 and 52.5% similarity, respectively, to P. syringae EriC. The amino acid sequence similarity between EriC1a and EriC1b was 74.4%. To examine whether inactivation of eriC1a or/and eriC1b resulted in the loss of fluoride resistance, eriC1a and eriC1b single mutants, and the eriC1a/eriC1b double mutant were constructed. As shown in Fig 3A, the eriC1b mutant showed extremely limited growth in the presence of 75 ppm NaF, while it grew similarly to the wild-type UA159 in the absence of NaF. In contrast, the eriC1a mutant showed a growth rate similar to wild-type UA159 in both the presence and absence of NaF. Furthermore, the growth rates of the eriC1a/eriC1b double mutant and the eriC1b single mutant were similar in the presence of NaF. Next, gene complementation analysis was performed using the pSEP shuttle vector in the eriC1a/eriC1b double mutant. Introduction of eriC1b into the eriC1a/eriC1b double mutant restored fluoride resistance (Fig 3A). On the other hand, the complementation by eriC1a did not rescued fluoride resistance (data not shown). These results demonstrated that EriC1b was responsible for the fluoride resistance in S. mutans.
Fig 3.
Growth yields of the parent strains, eriC or crcB deletion mutants, and complemented strains in S. mutans (A), S. anginosus (B), and S. sanguinis (C) at various NaF concentrations. The y-axis represents the OD550 after incubation for 16 h. Data represent the mean standard deviations of three independent experiments. *, significant differences against the OD550 value of the wild-type strain within the same NaF concentration.
Identification of fluoride-resistance-related genes in S. anginosus
S. anginosus possesses two types of eriC genes (eriC1 and eriC2). eriC1 encodes a protein of 405 amino acids with amino acid sequence similarity of 52% to the P. syringae EriC, and is named the voltage-gated chloride channel protein. eriC2 encodes a protein of 518 amino acids with no significant amino acid sequence similarity to the P. syringae EriC. To examine whether the inactivation of eriC1 or eriC2 leads to the loss of fluoride resistance, the eriC1 and eriC2 single mutants were constructed in S. anginosus NCTC 10707. The eriC1 mutant hardly grew in the presence of 75 ppm NaF, while the eriC2 mutant showed a growth rate similar to that of wild-type NCTC 10707 in the presence of NaF (Fig 3B). Furthermore, complementation of eriC1 rescued fluoride resistance (Fig 3B), while the introduction of eriC2 did not (data not shown). These results demonstrated that EriC1 was responsible for the fluoride resistance in S. anginosus.
Identification of fluoride-resistance-related genes in S. sanguinis
The eriC2, crcB1, and crcB2 genes are present in S. sanguinis. The eriC2 gene product is a protein of 518 amino acids with no significant similarity to P. syringae EriC, but with a similarity of 71% to S. anginosus EriC2, which is not involved in fluoride resistance in S. anginosus. The crcB1 gene encodes a protein of 124 amino acids with amino acid sequence similarity of 51% to E. coli CrcB (127-aa protein), which is involved in fluoride resistance in E. coli, and the crcB2 gene product encodes a protein of 116 amino acids with a similarity of 62.2% to E. coli CrcB. Amino acid sequence similarity between CrcB1 and CrcB2 was 58.4%. The crcB1 and crcB2 genes exist in tandem and in the same orientation. To examine whether the inactivation of eriC2, crcB1, or crcB2 leads to the loss of fluoride resistance, eriC2, crcB1, and crcB2 single mutants were constructed in S. sanguinis SK36. Inactivation of eriC2 did not affect fluoride resistance of wild-type SK36 (Fig 3C). On the other hand, both crcB1 and crcB2 single mutants showed extremely limited growth in the presence of 75 ppm NaF, while they grew similarly to wild-type SK36 in the absence of NaF (Fig 3C). The crcB1/crcB2 double mutant showed a growth rate similar to that of the crcB1 or crcB2 single mutants in both the absence and presence of NaF (Fig 3C). Complementation of crcB1/crcB2 in the crcB1/crcB2 double mutant restored fluoride resistance to wild-type levels (Fig 3C), while the introduction of eriC2 did not (data not shown). Furthermore, the crcB1/crcB2 mutant complemented by either crcB1 or crcB2 also restored fluoride resistance, while the fluoride resistance of these strains did not reach the level of wild type strain (data not shown). These results demonstrated that both crcB1 and crcB2 genes were critical for fluoride resistance in S. sanguinis.
Complementation of the fluoride-resistance-related genes between S. mutans and S. anginosus or S. mutans and S. sanguinis
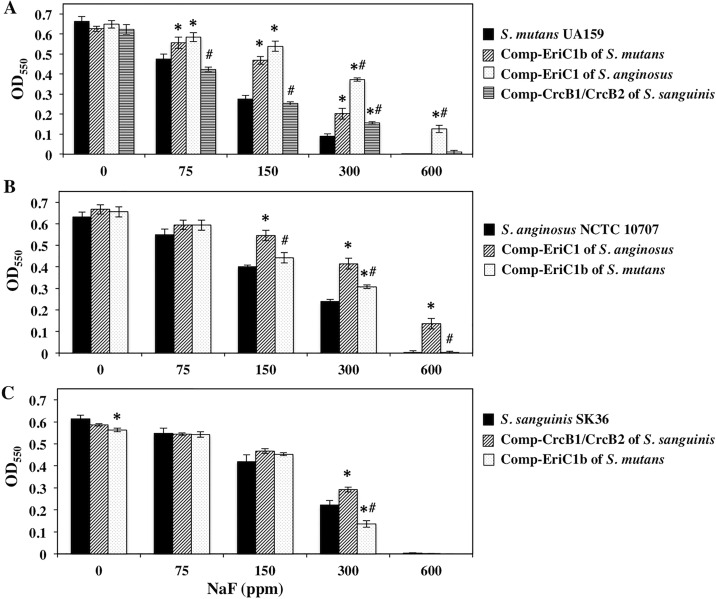
We explored whether fluoride-resistance-related genes were able to complement each other between S. mutans and S. anginosus or S. mutans and S. sanguinis. As shown in Fig 4A, complementation of eriC1a/eriC1b by S. anginosus eriC1 in the eriC1a/eriC1b double mutant rescued fluoride resistance, and the fluoride resistance ability of this complemented strain was higher than that complemented by its own eriC1b. Complementation of eriC1 by eriC1b in S. anginosus also restored fluoride resistance, and the fluoride resistance ability of this transformant was lower than that complemented by its own eriC1 (Fig 4B). Next, we performed complementation of eriC and crcB between S. mutans and S. sanguinis. Both the eriC1a/eriC1b mutant complemented by crcB1/crcB2 and the crcB1/crcB2 mutant complemented by eriC1b showed almost restored fluoride resistance (Fig 4A and 4C), as well as that E. coli crcB knock-out mutant rescued fluoride resistance by introduction of the fluoride-resistance-related EriCs of other bacteria [21]. The fluoride resistance ability of the eriC1a/eriC1b mutant complemented by crcB1/crcB2 was lower than that complemented by its own eriC1b, while the fluoride resistance ability of S. sanguinis wild-type SK36 tended to be higher than that of S. mutans wild-type UA159. Furthermore, we predicted that the co-existence of different F− channels (EriC and CrcB) may cause the additive effect on fluoride resistance in oral Streptococcus species. It was examined whether introductions of eriC1b and crcB1/crcB2 into wild-type S. sanguinis and S. mutans, respectively, affects fluoride resistance in the wild-type strains. Neither introduction of eriC1b nor crcB1/crcB2 increased the fluoride resistance of the wild-type strain (data not shown).
Fig 4. Growth yields of the parent strains and the complemented strains.
(A) Complementation by S. mutans EriC1b, S. anginosus EriC1, or S. sanguinis CrcB1/CrcB2 in the S. mutans EriC1a/EriC1b double mutant. (B) Complementation by S. anginosus EriC1 or S. mutans EriC1b in the S. anginosus EriC1 mutant. (C) Complementation by S. sanguinis CrcB1/CrcB2 or S. mutans EriC1b in the S. sanguinis CrcB1/CrcB2 double mutant. The y-axis represents the OD550 after incubation for 16 h. The data represent the mean standard deviations of three independent experiments. *, significant differences against the OD550 value of the wild-type strain within the same NaF concentration; #, significant differences against the OD550 value of the strain complemented by its own fluoride-resistance gene(s) within the same NaF concentration.
Discussion
ClC-type anion-selective channels are widespread throughout eukaryotic organisms and play a crucial role in controlling the ionic composition of the cytoplasm and the volume of cells, as well as the regulation of membrane electrical excitability. Although these channels may conduct other anions (e.g., I− or NO3−) better than Cl−, they are often called chloride channels because Cl− is the most abundant anion in organisms [22]. BLAST homology searches revealed that many microbial genomes contain members of the ClC family, and the prokaryotic ClC channel was designated an E. coli-derived ClC chloride channel homolog (EriC) [23]. E. coli EriC has been confirmed experimentally to function as a ClC Cl− channel. On the other hand, P. syringae EriC has been reported to be associated with fluoride resistance [14]. The crcB (confers resistance to camphor B) gene has previously been implicated in resistance to camphor-induced chromosome decondensation. However, it has recently been reported that an E. coli crcB knock-out mutant became sensitive to fluoride, and crcB is predicted to code for membrane proteins belonging to a superfamily composed predominantly of transporters [14]. Furthermore, it was shown that the eukaryotic crcB homolog [renamed FEX (fluoride exporter)] encodes a previously unrecognized class of fluoride exporters required for survival under standard environmental conditions [24].
Oral streptococci are classified into four groups; namely, the mutans, the salivarius, the mitis, and the anginosus groups, according to their 16S rRNA sequences [25]. The present study suggests that oral streptococci possess either eriC1 or crcB, which are associated with fluoride resistance. Among oral streptococci examined in this study, all Streptococcus species belonging to the mutans and the salivarius groups contained EriC1. Nine of 11 Streptococcus species belonging to the mitis group possessed CrcB and the other 2 species possessed EriC1. Although a distinct relationship between the type of genes (eriC1 or crcB) and the fluoride-resistance level was not observed, oral streptococci with eriC1 were prone to higher fluoride resistance than those with crcB. The difference in the fluoride-resistance level related to the gene types suggests that the mechanisms through EriC1 and CrcB are not identical.
Baker et al. [14] showed that P. syringae EriC and C. difficile EriC associated with fluoride resistance commonly carry a specific set of amino acids in the conserved anion selectivity filter region [26], which are distinct from validated chloride-specific EriC proteins (Table 1). In this study, two novel genes encoding EriC1b of S. mutans and EriC1 of S. anginosus were shown to be involved in fluoride-resistance, and subsequently seven EriC1s in other oral streptococci were predicted to be responsible for fluoride resistance of these organisms based on amino acid sequence homology. Our table shows amino acid sequences of the conserved anion selectivity filter region of the 18 EriC homologs; 3 EriCs are Cl− channels and 15 EriCs are predicted to be F− channels. All EriC1s of oral streptococci possessed a similar set of amino acid sequences to those of the F− channel EriCs, but they were distinct from those of Cl− channel EriCs. Although it is possible that EriC1s in oral streptococci may be generally responsible for the fluoride resistance, the role of EriC1s from oral streptococci other than S. mutans and S. anginosus in fluoride resistance should be examined. The amino acid sequences of the region in the streptococci were more similar to those of C. difficile, Eubacterium ventriosum, and Lactococcus lactis EriCs than those of P. syringae, Pirellula staleyi, and Ralstonia picketti EriCs, possibly due to the difference between Gram-positive and Gram-negative bacteria. On the other hand, EriC2s identified in all oral streptococci examined in this study, except for S. mutans, possessed amino acid sequences with conserved Cl− channels (data not shown), suggesting that EriC2 may be related to Cl− channels.
Table 1. Amino acid sequences of the conserved anion selectivity filter region of several EriCs.
Eight EriCs (dotted box) were shown to be involved in fluoride resistance [14, 21]. Solid boxes show the amino acid sequences associated with fluoride resistance. Related EriCs commonly contain a specific set of amino acids, which are distinct from validated chloride-specific EriCs.
| Substrate | Organism | Cl− selectivity filter residues |
|---|---|---|
| Cl− | human (ClC-1) | GSGIP……GKEGP……GGFMP……Y |
| Cl− | Escherichia coli | GSGIP……GREGP……GIFAP……Y |
| Cl− | Salmonella typhimurium | GSGIP……GREGP……GIFAP……Y |
| F− | Pseudomonas syringae | GNNLI……GREGT……GEVTP……Y |
| F− | Pirellula staleyi | GNNLL……GREGT……GEVTP……Y |
| F− | Ralstonia picketti | GNNLL……GREGT……GEVTP……Y |
| F− | Clostridium difficile | GMNLI……GREGV……GEVTP……Y |
| F− | Eubacterium ventriosum | GMNLV……GREGV……GEVTP……Y |
| F− | Lactococcus lactis | GMGLI……GREGV……GEVTP……Y |
| F− | Streptococcus mutans | GMGLI……GREGV……GEVTP……Y |
| F− | Streptococcus anginosus | GMTLI……GREGV……GEVTP……Y |
| F− | Streptococcus sobrinus | GMGLV……GREGV……GEVTP……Y |
| F− | Streptococcus australis | GMELL……GREGV……GEVTP……Y |
| F− | Streptococcus parasanguinis | GMGLI……GREGV……GEVTP……Y |
| F− | Streptococcus salivarius | GMGLI……GREGV……GEVTP……Y |
| F− | Streptococcus vestibularis | GMGLI……GREGV……GEVTP……Y |
| F− | Streptococcus constellatus | GMTLI……GREGV……GEVTP……Y |
| F− | Streptococcus intermedius | GMTLI……GREGV……GEVTP……Y |
Liao et al. [15] have reported that 2 SNPs related to fluoride resistance were identified in the genome of the fluoride resistant strain S. mutans C180-2FR. These were located in the region of the gene cluster composed of SMU_1291, eriC1a, and eriC1b; one in its promoter region and the other in eriC1b. The expression of the cluster comprised of three genes was approximately 10-fold higher in C180-2FR than in the parent strain C180-2. Murata and Hanada [27] have shown that both eriC1a and eriC1b were involved in the fluoride resistance in S. mutans. We demonstrated that eriC1b was involved in the fluoride resistance of S. mutans, but deletion of eriC1a did not affect the fluoride resistance. Contribution of EriC1a in fluoride resistance of S. mutans in the previous study [27] was inconsistent with our result. We are not able to explain such a difference between these two studies. The discrepancy might be caused by different cultural conditions: that is, the previous study utilized anaerobic condition, while the present study did aerobic condition with 5% CO2. It is unknown why S. mutans possesses two EriC1s unlike other oral streptococci and also why inactivation of eriC1a does not result in loss of fluoride resistance even though EriC1a and EriC1b showed amino acid sequence similarity of 74% that is higher than the similarity of 52.5% between EriC1b and P. syringae EriC1 actually contributing to fluoride resistance. However, higher homology of total amino acid sequence does not necessarily guarantee the function of protein.
In the present study, we demonstrated that both crcB1 and crcB2 genes were critical for fluoride resistance in S. sanguinis, although a single crcB is responsible for the fluoride resistance in E. coli. In addition, all oral streptococci classified in group III in accordance with genes associated with anion channels possessed both crcB1 and crcB2. Using the KEGG (Kyoto Encyclopedia of Genes and Genomes) Organisms database (http://www.genome.jp/kegg/catalog/org_list.html), we found that Bacillus subtilis and Staphylococcus aureus, Gram-positive bacteria, possess two types of crcBs, while only a single crcB gene was found in genomes of E. coli and Salmonella enterica, Gram-negative bacteria. CrcBs of E. coli was characterized to be a fluoride efflux channel [14]. However, the function of CrcBs in the other bacteria has not yet well elucidated. Although, in this study, we aimed to broadly identify genes involved in the fluoride resistance in oral streptococci, the reason why two crcBs exist in Gram-positive bacteria in contrast to Gram-negative bacteria, in addition to a unique genotype composed of two genes encoding EriC1 in S. mutans, would be a promising research target in future.
Complementation between S. mutans EriC1b and S. anginosus EriC1 and that between S. mutans EriC1b and S. sanguinis CrcB1/CrcB2 were confirmed in this study. Introduction of S. anginosus EriC1 into an EriC1s-knockout mutant of S. mutans increased fluoride resistance when compared with the same complementation experiment with EriC1b. On the other hand, when EriC1b was transferred into the EriC1-knockout mutant of S. anginosus, fluoride resistance of the complemented strain was lower than that complemented with its own EriC1. The fluoride resistance level of S. anginosus NCTC10707 was higher than that of S. mutans UA159, and the differences in fluoride resistance levels between the above species may be attributed to differences in the kinetics of ion transport between EriC1s. Moreover, both the EriC1s-knockout mutant of S. mutans complemented by S. sanguinis CrcB1/CrcB2 and the CrcB1/CrcB2-knockout mutant complemented by S. mutans EriCb showed restored fluoride resistance. However, neither introduction of S. sanguinis CrcB1/CrcB2 into wild-type S. mutans nor S. mutans EriC1b into wild-type S. sanguinis increased the fluoride resistance level of the wild-type strain. Co-existence of different F− channels (EriC and CrcB) did not cause the additive effect on fluoride resistance in oral Streptococcus species.
Fluoride application is highly effective in preventing dental caries. On the other hand, we must consider that fluoride has anti-bacterial effects. The frequent application of fluoride results in the emergence of fluoride resistant strains, leading to the dysbiosis of oral microflora. This disturbance may affect not only oral health conditions, but also general health conditions. Exploring the mechanism of fluoride resistance may contribute to countermeasures against the risk of emergence of the fluoride-resistant strains in oral microflora.
Acknowledgments
S. sanguinis SK36 was kindly provided by M. Kilian, Department of Biomedicine, Aarhus University, Aarhus, Denmark and S. Kawabata, Department of Oral and Molecular Microbiology, Osaka University Graduate School of Dentistry, Osaka, Japan. S. anginosus NCTC 10707 was kindly provided by A. Yoshida, Department of Oral Microbiology, Matsumoto Dental University, Shiojiri, Japan. This study was supported in part by Grants-in Aid for Scientific Research 25463251 (YS) and 25293428 (YY) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported in part by Grants-in Aid for Scientific Research 25463251 (YS) and 25293428 (YY) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The funders had no role in study design and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1.Marquis RE, Clock SA, Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003; 26: 493–510. [DOI] [PubMed] [Google Scholar]
- 2.Mayhew RR, Brown LR. Comparative effect of SnF2, NaF, and SnCl2 on the growth of Streptococcus mutans. J Dent Res. 1981; 60: 1809–1814. [DOI] [PubMed] [Google Scholar]
- 3.Maltz M, Emilson CG. Susceptibility of oral bacteria to various fluoride salts. J Dent Res. 1982; 61: 786–790. [DOI] [PubMed] [Google Scholar]
- 4.Streckfuss JL, Perkins D, Horton IM, Brown LR, Dreizen S, Graves L. Fluoride resistance and adherence of selected strains of Streptococcus mutans to smooth surfaces after exposure to fluoride. J Dent Res. 1980; 59: 151–158. [DOI] [PubMed] [Google Scholar]
- 5.Bunick FR, Kashket S. Enolases from fluoride-sensitive and fluoride-resistant streptococci. Infect Immun. 1981; 34: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown LR, White JO, Horton IM, Dreizen S, Streckfuss JL. Effect of continuous fluoride gel use on plaque fluoride retention and microbial activity. J Dent Res. 1983; 62: 746–751. [DOI] [PubMed] [Google Scholar]
- 7.Van Loveren C, Spitz LM, Buijs JF, Ten Cate JM, Eisenberg AD. In vitro demineralization of enamel by F-sensitive and F-resistant mutans streptococci in the presence of 0, 0.05, or 0.5 mmol/L NaF. J Dent Res. 1991; 70: 1491–1496. [DOI] [PubMed] [Google Scholar]
- 8.Van Loveren C, Van de Plassche-Simons YM, De Soet JJ, De Graaff J, Ten Cate JM. Acidogenesis in relation to fluoride resistance of Streptococcus mutans. Oral Microbiol Immunol. 1991; 6: 288–291. [DOI] [PubMed] [Google Scholar]
- 9.Hoelscher GL, Hudson MC. Characterization of an unusual fluoride-resistant Streptococcus mutans isolate. Curr microbiol. 1996; 32: 156–161. [DOI] [PubMed] [Google Scholar]
- 10.Moritani K, Takeshita T, Shibata Y, Ninomiya T, Kiyohara Y, Yamashita Y. Acetaldehyde production by major oral microbes. Oral Dis. 2015; 21: 748–754. 10.1111/odi.12341 [DOI] [PubMed] [Google Scholar]
- 11.Takeshita T, Yasui M, Shibata Y, Furuta M. Saeki Y. Eshima N, et al. Dental plaque development on a hydroxyapatite disk in young adults observed by using a barcoded pyrosequencing approach. Sci Rep. 2015; 5: 8136 10.1038/srep08136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003; 11: 94–100. [DOI] [PubMed] [Google Scholar]
- 13.Silver S. Bacterial resistances to toxic metal ions–a review. Gene. 1996; 179: 9–19. [DOI] [PubMed] [Google Scholar]
- 14.Baker JL, Sudarsan N, Weinberg Z, Roth A, Stockbridge RB, Breaker RR. Widespread genetic switches and toxicity resistance proteins for fluoride. Science. 2012; 35: 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao Y, Chen J, Brandt BW, Zhu Y, Li J, van Loveren C, et al. Identification and functional analysis of genome mutations in a fluoride-resistant Streptococcus mutans strain. PloS One. 2015; 10: e0122630 10.1371/journal.pone.0122630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Russell DW. Molecularcloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 17.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983; 166: 557–580. [DOI] [PubMed] [Google Scholar]
- 18.Perry D, Wondrack LM, Kuramitsu HK. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983; 41: 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Shibata Y, Takeshita T, Yamashita Y. A novel gene involved in the survival of Streptococcus mutans under stress conditions. Appl Environ Microbiol. 2012; 80: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawada-Matsuo M, Shibata Y, Yamashita Y. Role of two component signaling response regulators in acid tolerance of Streptococcus mutans. Oral Microbiol Immunol. 2009; 24: 173–176. 10.1111/j.1399-302X.2008.00485.x [DOI] [PubMed] [Google Scholar]
- 21.Stockbridge RB, Lim HH, Otten R, Williams C, Shane T, Weinberg Z, et al. Fluoride resistance and transport by riboswitch-controlled CLC antiporters. Proc Natl Acad Sci USA. 2012; 109: 15289–15294. 10.1073/pnas.1210896109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol rev. 2002; 82: 503–568. 10.1152/physrev.00029.2001 [DOI] [PubMed] [Google Scholar]
- 23.Maduke M, Pheasant DJ, Miller C. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic ClC-type chloride channel. J Gen Physiol. 1999; 114: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Smith KD, Davis JH, Gordon PB, Breaker RR, Strobel SA. Eukaryotic resistance to fluoride toxicity mediated by a widespread family of fluoride export proteins. Proc Natl Acad Sci USA. 2013; 110: 19018–19023. 10.1073/pnas.1310439110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Sys Bacteriol. 1995; 45: 406–408. [DOI] [PubMed] [Google Scholar]
- 26.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002; 415: 287–294. 10.1038/415287a [DOI] [PubMed] [Google Scholar]
- 27.Murata T, Hanada N. Contribution of chloride channel permease to fluorideresistance in Streptococcus mutans. FEMS Microbiol. 2016; 363 (11): fnw101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.