Abstract
Zinc is an essential dietary element that has been implicated in the pathogenesis of prostate cancer, a cancer that disproportionately affects men of African descent. Studies assessing the association of zinc intake and prostate cancer have yielded inconsistent results. Furthermore, very little is known about the relationship between zinc intake and prostate cancer among African Americans. We examined the association between self-reported zinc intake and prostate cancer in a hospital-based case-control study of African Americans. We then compared our results with previous studies by performing a meta-analysis to summarize the evidence regarding the association between zinc and prostate cancer. Newly diagnosed African American men with histologically confirmed prostate cancer (n = 127) and controls (n = 81) were recruited from an urban academic urology clinic in Washington, DC. Controls had higher zinc intake, with a mean of 14 mg/day versus 11 mg/day for cases. We observed a non-significant, non-linear increase in prostate cancer when comparing tertiles of zinc intake (OR <6.5 vs 6.5–12.5mg/day 1.8, 95% CI: 0.6,5.6; OR <6.5 vs >12.5mg/day 1.3, 95% CI: 0.2,6.5). The pooled estimate from 17 studies (including 3 cohorts, 2 nested case-control, 11 case-control studies, and 1 randomized clinical trial, with a total of 111,199 participants and 11,689 cases of prostate cancer) was 1.07hi vs lo 95% CI: 0.98–1.16. Using a dose-response meta-analysis, we observed a non-linear trend in the relationship between zinc intake and prostate cancer (p for nonlinearity = 0.0022). This is the first study to examine the relationship between zinc intake in black men and risk of prostate cancer and systematically evaluate available epidemiologic evidence about the magnitude of the relationship between zinc intake and prostate cancer. Despite of the lower intake of zinc by prostate cancer patients, our meta-analysis indicated that there is no evidence for an association between zinc intake and prostate cancer.
Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer death among men in United States. The American Cancer Society estimates that 180,890 new cases of prostate cancer will be diagnosed and about 26,120 men will die of prostate cancer in 2016, with African-American men having higher incidence and mortality [1]. Prostate cancer is a complex disease that results from genetic and environmental factors, and their interaction. The large variations in prostate cancer rates worldwide and within countries suggest diet may contribute to these variations; however, specific components of the diet have not been well-defined [2, 3].
A dietary factor that has been implicated in prostate cancer pathology is the essential micronutrient zinc. Zinc is widely distributed in the food supply. Major contributors to zinc intake include meats, fish and poultry, dairy, enriched and/or fortified foods, and dietary supplements [4]. The level of zinc in the body is maintained by regulating absorption of exogenous zinc and secretion and excretion of endogenous zinc [5]. In the US, the recommended dietary allowance (RDA) for men who are more than or equal to 19 years old is 11mg/day [6]; however, men consuming vegetarian diets have higher requirements (2 times) since the bioavailability of zinc to the body is reduced with phytate-rich foods (e.g., whole grains, fruits, vegetables). Based on data from the National Health and Nutrition Examination Survey (NHANES 2003–2004 & 2005–2006), most Americans meet the estimated average requirements for zinc [7]. However, non-Hispanic black, men had the lowest median daily dietary zinc intakes compared to non-Hispanic White, and Mexican American in the US population [8].
The levels of zinc in the body are tightly controlled as it is involved in many physiological processes such as enzyme activity, genomic stability, apoptosis, immunity, neurological function, response to oxidative stress, and cell signaling [9]. Normal prostate tissue contains one of the highest concentration of zinc in the body and several studies have shown that malignant prostate tissue lose the ability to accumulate Zinc leading to a decrease in zinc levels compared to normal or hyperplastic tissue (for reviews see [10, 11]). The decrease in prostatic zinc levels correlated with increased Gleason score [12], and level of the zinc uptake transporters (hZIP1, hZIP2 and ZIP3) [13, 14]. In addition, Rishi et al. reported decreased expression of hZIP1 and hZIP2 in prostatic tissue of black compared to white males [15]. The role of zinc in prostate cancer pathogenesis has not been elucidated, but there is evidence to suggest that zinc can inhibit energy production, growth and proliferation in normal prostate cells; and suppresses angiogenic and metastatic potentials of malignant prostate cells [11, 16].
Although experimental data supports the protective role of zinc in prostate cancer, epidemiological studies, including case-control, cohort and randomized clinical trials (RCT), have shown mixed results. There are some studies that showed zinc reduces the risk of developing prostate cancer [17–19] and prostate cancer mortality [20]. In addition, an ecologic study from South Carolina found an inverse relationship between soil zinc content and prostate cancer rate [21]. Other studies showed that advanced prostate cancer is associated with high intake of zinc [22–25]. However, many observed that dietary or supplemental zinc intake is not associated with prostate cancer risk or progression [26–34]. Reasons for these conflicting results could be due to different study design, measurements errors for zinc intake, or the lack of an accurate and reliable measurement for zinc status in humans.
The relationship between zinc and prostate cancer is indeed a “critical scientific, medical, and public interest issue” that has yet to be resolved [10]. Few studies assessed the role of zinc intake and prostate cancer among African American men, who are the most affected by prostate cancer [25, 31].
To address this issue, we assessed the association of dietary zinc intake among African Americans from a hospital based case-control study with demographic, lifestyle and clinical characteristics. We also conducted a systematic review of the literature and perform a meta-analysis to summarize the evidence regarding the association between zinc and prostate cancer.
Materials and Methods
Case-Control Study
Study population
Self-identified African American men aged between 40 and 85 were recruited from the Division of Urology at the Howard University Hospital and Washington D.C. area. Details of the study were previously published [35]. In brief, cases had histological confirmed prostate cancer with a PSA of > 3.5 ng/ml and a positive digital rectal examination (DRE). Controls were healthy, unaffected volunteers with no history of prostate cancer among first-degree relatives from the prostate cancer screening population of the Division of Urology. All participants provided written informed consent. The study and consent forms were approved by the Howard University Institutional Review Board [36, 37]. The present analysis is based on 248 men who provided demographic, lifestyle, dietary, and medical information.
Zinc assessment
Usual dietary intake was assessed at the time of recruitment (2000 to 2004) with the use of a block food frequency questionnaire (These self-administered FFQ were adapted from National Health and Nutrition Examination Survey 1999–2001 dietary recall data and validated for use in the African American population to estimate usual zinc intake during the year prior to recruitment into the study [38–40]. The FFQ consisted of 19 food items, 3 supplements questions, and questions to adjust for food fortification practices.
Statistical analysis
To compare sample characteristics, t-test for continuous variables and chi-square for categorical variables were used. Unconditional logistic regression was used to calculate the unadjusted and adjusted odds ratio (OR) and 95% confidence intervals (95% CI) associated with prostate cancer and zinc intake. The OR was adjusted for age, PSA, body mass index (BMI), family history of prostate cancer, education, income, fruit, vegetables, iron, calcium intake, smoking and alcohol intake. Zinc intake was analyzed as both continuous and tertiles with cut-points based on its distributions. We used the lowest tertile as the reference group. Linear trends across categories were tested in these models using ordinal variables. The SAS software (SAS Institute Inc., Cary, NC) was used for the analysis.
Systematic Review and Meta-Analysis
Literature search and selection
We conducted a literature search of PubMed, Embase and Cochrane databases from January 1st 1977 to March 31st 2016. The search strategy included a combination of free text and the Medical Subject Headings (MeSH) terms of the following terms: (zinc or ZN) AND (‘prostate cancer’ or ‘prostatic neoplasms’) AND (intake or status or diet or dietary or supplement or supplemental or hair or nail or toenail or plasma or serum or urine). No language restrictions were imposed. Titles and abstracts of identified studies were checked and the full text of relevant studies was assessed for eligibility against the predefined inclusion criteria. We also performed a manual search of references cited in the selected articles and published reviews to search for additional relevant studies and authors were contacted to request missing data. No additional eligible articles were identified in Embase or Cochrane Database of Reviews that we did not identify in PubMed. There was no Cochrane review on the topic. Studies were included in this meta-analysis if they fulfilled the following criteria: 1) presented original data from cohort, case–control, cross-sectional, or RCT studies, 2) the exposure of interest was zinc intake or status, 3) the outcome was prostate cancer occurrence, and 4) the studies reported effect estimates (OR, relative risk (RR), or hazard ratio (HR)) with 95% confidence intervals (95% CI).
Data extraction
The following data were extracted from each study: first author’s last name, publication year, study name, study location, ethnic origin of participants, age (mean or range), sample size, zinc intake categories, dietary assessment method, covariates adjusted for in the multivariable analysis, and effect estimates with their 95% CI for each category of zinc. Data extraction was conducted independently by 2 investigators using a standardized data extraction excel sheet, and disagreements resolved by consensus. The reporting protocol for the meta-analysis is shown in S1 Checklist.
Statistical analysis
We calculated the standard error for each study using the reported 95% CIs and computed summary RR and 95% CIs from the RR, OR and HR for the highest versus the lowest zinc category using the inverse variance weighted method. Both fixed effects [41] and random effects [42] models were estimated. Chi-square and I2 statistics were used to evaluate the statistical heterogeneity among the studies.I2 statistic values on the order of 25%, 50%, and 75% are considered as low, moderate, and high, respectively [43].Sources of heterogeneity were assessed by using stratified analyses and meta-regression with source of zinc (dietary, supplemental, total, serum/hair/nail), study design (case-control, cohort, nested case-control and RCT), disease status (early stages (cancer is clinically confined to the prostate gland), late stages (cancer is clinically disseminated outside the prostate gland) [44], mixed/unspecified (all stages combined/not specified by authors), country (USA& Canada, Europe, other), total sample size (<100, 100–500, >500 subjects), publication year (before 1995, 1995–2006, after 2006) and year the recruitment started (before 1984, 1984–1993, after 1993) as potential explanatory factors. We also tested the influence of individual study on the results in sensitivity analyses and performed a cumulative meta-analysis. Cumulative meta-analysis shows the evolution of the estimate of the pooled effect in a meta-analysis with publication year. We examined the publication bias through visual inspection of the Begg’s funnel plots and formally tested using Egger’s regression asymmetry method with results considered to indicate potential small-study bias when P <0.10.
To model the relationship between zinc intake and prostate cancer, we applied a 2-stage random-effects dose-response meta-analysis developed by Orisini et al [42] using generalized least squares for trend (GLST) analysis and restricted cubic splines with three knots at percentiles 10%, 50%, and 90% of the distribution. We computed study-specific RR and 95% CI from the natural logarithms of the effect estimates across categories of zinc intake [45]. The dose–response results are presented for a 100 mg/d increment of zinc intake. The numbers of cases and the denominators in the cohort studies and the median or mean intake for each category were required [42]. Several papers had missing data needed to estimate the dose-response associations. We contacted the authors for the missing information. If we were unable to obtain the data, we derived the missing values using information reported in the papers instead of excluding potentially important studies using recommendations described by Bekkering et al [46]. When the median or mean intake per category was not provided, we assigned the midpoint of the upper and lower boundaries in each category as the average intake. If the lower or upper boundary for the lowest and highest category respectively was not reported, we assigned half the dose to the lower boundary and multiplied the highest level by 1.5. The dose of zinc was not collected by the authors in two studies [18, 25]. For Kristal et al, 1999 [18] we used the dose of zinc from a subsequent paper from the same author (Kristal et al. 2010) [28]; and used the data from Gonzalez et al 2009 [17] for Zhang et al 2009 [25], as the later measured both dose and frequency. All analyses were performed with Stata 11.0 (StataCorp), with a 2-tailed α of 0.05 using a combination of published macros, including metan, metareg, metafunnel, metabias, GLST and MKSPLINE.
Results
Case-Control Study
Table 1 summarizes the descriptive characteristics of the study participants in the case-control study. The cases had lower educational level and income and less likely to eat red meat and vegetables or be current drinker. The daily zinc intake was lower in the case than control (p = 0.06). However, prostate cancer cases did not differ significantly from the healthy controls for BMI, family history of prostate cancer, daily dietary intake of total iron, total calcium, total fat and mean energy intake. We examined the association between total zinc intake and prostate cancer as a continuous variable or divided into tertiles (<6.5, 6.5–12.5, >12.5mg/day). In Table 2, we report crude and adjusted prostate risk estimates in relation to total zinc intake. We did not find an association between zinc intake (treated as continuous variable) and prostate cancer after adjusting for age, food energy, body mass index, education, income, smoking history, alcohol, total fat, family history of prostate cancer, PSA levels, vegetables and fruit servings per day, total calcium and iron levels (OR = 0.97, 95% CI: 0.46, 2.1). When we examined tertiles of zinc intake, we observed a non-linear increase in the odds of prostate cancer with increasing zinc intake (p-trend of adjusted model = 0.6).
Table 1. Demographic and health-related characteristics of the Washington D.C. prostate cancer study by outcome.
| Cases (n = 127) | Controls (81) | P-Value | |
|---|---|---|---|
| Mean (SD) Age at diagnosis, years | 66.5 (9) | 66 (10.7) | 0.75 |
| Income, % | |||
| $0- $29,999 | 57 | 44 | 0.09 |
| $30,000-$59,999 | 26 | 28 | |
| over $60,000 | 17 | 28 | |
| Education, % | |||
| less than high school | 63 | 46 | 0.01* |
| High school or more | 37 | 54 | |
| Body Mass Index at diagnosis (kg/m2), % | |||
| Normal weight (<25) | 33 | 29 | 0.8 |
| Over weight (25–30) | 39 | 42 | |
| Obese (>30) | 28 | 29 | |
| Smoking status (%) | |||
| Current smoker | 20 | 25 | 0.6 |
| Former smoker | 47 | 40 | |
| Never smoker | 33 | 35 | |
| Alcohol use (%) | |||
| Current Drinkers | 37 | 52 | 0.08 |
| Former Drinker | 35 | 23 | |
| Non-drinkers | 28 | 25 | |
| Family history of Prostate cancer (%) | |||
| Yes | 19 | 14 | 0.42 |
| PSA ng/mL at the evaluation or diagnosis (%) | |||
| 0–2.5 | 19 | 51 | <0.0001* |
| 2.6–9.9 | 44 | 43 | |
| 10–19.9 | 15 | 2 | |
| >20 | 22 | 4 | |
| Zinc, Daily intake (±SD) | |||
| Total Zinc (mg) | 10.8 (7.4) | 14.3 (15.4) | 0.06 |
| Zinc from supplements (mg) | 5.2 (11.9) | 6.7 (12.5) | 0.4 |
| Potential modifiers of zinc intake, Daily intake (±SD) | |||
| Red meat (servings) | 2.1 (1.4) | 3.1 (2.9) | 0.0008* |
| Vegetables (servings) | 3.3 (2.3) | 4.2 (3.5) | 0.03* |
| Fruits (servings) | 1.8 (1.2) | 1.8 (1.4) | 0.8 |
| Total Iron (mg) | 14.9 (9.2) | 17.2 (14.4) | 0.19 |
| Total Calcium (mg) | 730.6 (470.1) | 736.8 (611.8) | 0.94 |
| Energy intake (kcal) | 2029.2 (1225.7) | 2387.9 (1890.1) | 0.13 |
| Dietary total fat (g) | 81.6 (56.3) | 96.8 (80.5) | 0.14 |
| Saturated fat | 23.1 (16.2) | 26.4 (23.4) | 0.26 |
| Polyunsaturated fat | 21.2 (15.51) | 25.9 (20.8) | 0.08 |
| Monounsaturated fat | 30.9 (22.3) | 36.6 (31.7) | 0.16 |
* p<0.05
Table 2. Crude and adjusted prostate cancer estimates.
| Total zinc intake | Unadjusted OR (95% CI) | Adjusted OR (95%CI)a |
|---|---|---|
| ≤6.50 (mg/day) | Reference | Reference |
| 6.5–12.50 | 1.51 (0.57, 2.3) | 1.8 (0.6, 5.6) |
| >12.5 | 0.81 (0.41, 1.61) | 1.3 (0.2, 6.5) |
| bp-trend = 0.5 | p-trend = 0.6 | |
| Continuous (10mg/day) | 0.75 (0.57, 0.99) | 0.97 (0.46, 2.1) |
a) OR generated from logistic regression model adjusting for age, food energy, meat consumption. body mass index, education, income, smoking history, alcohol, total fat, family history of prostate cancer, PSA levels, vegetables and fruit servings per day, total calcium and iron levels.
b) p value for trend
Meta-Analysis
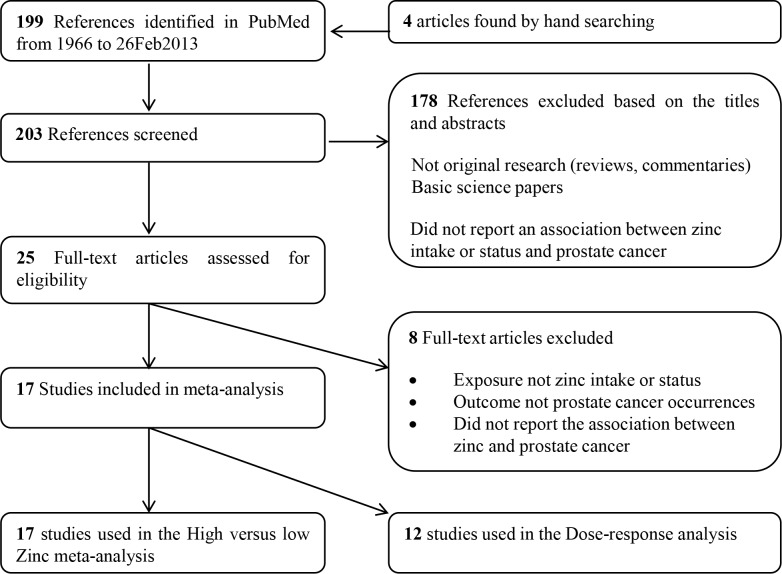
We also conducted a systematic review of the literature and performed a meta-analysis to summarize and pool the findings from multiple studies that measured the association between zinc and prostate cancer risk in the general population. We identified a total of 199 articles from our systematic review and 4 from hand-searching. We scanned the titles and abstract of the 203 articles to remove commentaries, reviews, basic science papers resulting in 25 articles. The full text-articles of the 25 articles that assessed the association between zinc and prostate cancer were reviewed. Eight articles were excluded as they did not report the association between zinc and risk of prostate cancer [17, 20, 21, 47–52] and we included the remaining 17 articles in our meta-analysis [17–19, 22–34, 53] (Fig 1).
Fig 1. Flow diagram of systematic literature search on zinc and the risk of prostate cancer.
Characteristics of the Included Studies
The studies that met our inclusion criteria included 3 cohort, 2 nested case-control, 11 case-control, and 1 randomized clinical trial with a total of 111,199 participants and 11,689 cases of prostate cancer, were used for the meta-analysis. Details of each of these studies are summarized in Table 3. The numbers of participants in each study ranged from 143 to 46,974. The studies were generally from the United States (n = 10) or European countries (n = 4); 2 studies was based in China and 1 from Malaysia. Four studies assessed zinc status from nail, hair and plasma, and the rest used FFQ to estimate zinc intake. Dose of zinc in most of the studies was mg/day. All of the studies adjusted for age, as age is an established risk factor for prostate cancer.
Table 3. Characteristics of papers included in the meta-analysis.
| First Author, year | Study design | Population (Study period) | Cases # | Total # | Age (years) range/mean | Source of Zinc | Highest category | Stratification/Adjustment variables |
|---|---|---|---|---|---|---|---|---|
| Kolonel, 1988 | case-control | Hawaii, USA: 37% Japanese, 31% white (1977–1983) | 452 | 1351 | >70 (43%) & ≤70 (57%) | Dietary & supplements | >100 (mg/day) | Stratified by Age (<70 & ≥70); zinc intake (dietary & total); Adjusted for ethnicity |
| West, 1991 | case-control | Utah, USA: LDS members (1984–1985) | 358 | 1037 | 45–74 | Dietary | >16 (mg/day) | Matched by age and residence; stratified by age (45–67; 68–74) and pathology (all tumors, aggressive tumor; adjustment for energy and age |
| Andersson, 1996 | case-control | Sweden (1989–1994) | 526 | 1062 | 45–74 | Dietary | >13.5 (mg/day) | Stratified by pathology (all stages & advanced); adjusted for age, energy |
| Key, 1997 | case-control | UK (1990–1994) | 328 | 656 | 68.1 | Dietary | ≥ 11 (mg/day) | Matched for age; adjusted for social class |
| Vlajinac, 1997 | case-control | Serbia (1990–1994) | 101 | 303 | 70.5 cases & 71.5 control | Dietary | NR | Matched for age, hospital admittance and place of residence; adjusted for energy, protein, total fat, saturated fatty acids, carbohydrates, total sugar, fiber, retinol equivalent, alpha-tocopherol, folic acid, vitamin B12, potassium, calcium, phosphorous, magnesium and iron |
| Lee, 1998 | case-control | China (1989–1992) | 133 | 398 | 50–80 | Dietary | NR | Adjusted for region, fat, carotenoids and selenium |
| Kristal,1999 | case-control | Washington, USA: 95–98% white (1993–1996) | 697 | 1363 | 40–64 | Supplements | ≥ 7 (frequency/week) | Stratified by stage and grade; adjusted for age, energy, fat, race, family history, BMI, PSA testing, education |
| Platz, 2002 | nested case-control | CLUEII cohort, USA (1989–1996) | 115 | 342 | 59–74 | Toe nail | 259.1 (ppm) | Matched on age, race, date of blood collection and size of toenail clipping; adjusted for education, adult height, current BMI, BMI at age 21, father or brother with prostate cancer, cigarette smoking, and multivitamin use |
| Litzmann, 2003 | cohort | Health professionals cohort, USA (1986–2000) | 2901 | 46974 | 44–66 | Supplements | 101 mg | Stratified by stage; adjusted for age, energy, BMI, height, smoking, family history, physical activity, aspirin use, dietary calcium, supplemental calcium, fructose, supplemental vitamin E, tomato-based foods, fish, red meat, and alpha -linolenic acid. |
| Meyer, 2005 | RCT | SU.VI.MAX trial, Canada (1994–2004) | 101 | 4830 | 45–60 | Serum | ≥ 13.4 mmol/L | Matched for age, PSA, smoking, BMI, serumβ-carotene, α-tocopherol, vitamin C, Selenium |
| Gallus, 2007 | case-control | multicenter hospital study from Italy (1991–2002) | 1294 | 2745 | 46–74 | Dietary | >15.65 (mg/day) | Adjusted for age, study center, education, physical activity, family history, BMI and total energy intake |
| Zhang, 2009 | case-control | Case-Control Surveillance Study: 77–84% White, USA (1976–2006) | 1706 | 4110 | 40–79 | Supplements | ≥10 years | Adjusted for matching variables age, study center, year of interview and race, and for education, BMI, alcohol, current smoking, family history, use of other vitamins & mineral supplements. |
| Gonzalez, 2009 | cohort | Vitamin and Lifestyle cohort: 93–94% white, USA (2000–2004) | 832 | 35244 | 50–76 | Dietary & supplements | 152 mg/day | Stratified by zinc category, stage, grade &vegetable and fruit intake; adjusted for education, race, family history, PSA-test within the 2 years prior to baseline, & current multivitamin use. |
| Kristal, 2010 | cohort | Prostate cancer prevention trial: 93–94% white, USA& Canada (1994–2003) | 1703 | 9559 | cases: 63.6 & controls: 62.6 | Dietary & supplements | >22 mg/day | Adjusted for age, race, family history, treatment arm, BMI and pathology |
| Karimi, 2012 | case-control | Hospital-based study from Malaysia: 47% Malays, 33% Chinese and 20% Indians (2010–2012) | 50 | 50 | 50–86 | Hair and Nails | hair: >3.75 mg/g & Nails: >3.32 mg/g | Matched for age and ethnicity |
| Park, 2013 | Nested case-control | Multiethnic cohort study from Hawaii and California USA: 46% AA, 20% Japanese (1993–2006) | 392 | 1175 | 45–75 | Serum | >102.5 μg/dl | Matched for geographic location, race, birth year, date of blood draw, time of blood draw, and fasting hours prior to blood draw, family history, BMI, and education |
Abbreviations: AA: African American, RCT: Randomized control-trial, BMI: body mass index
Highest versus Lowest Zinc Category Meta-Analysis
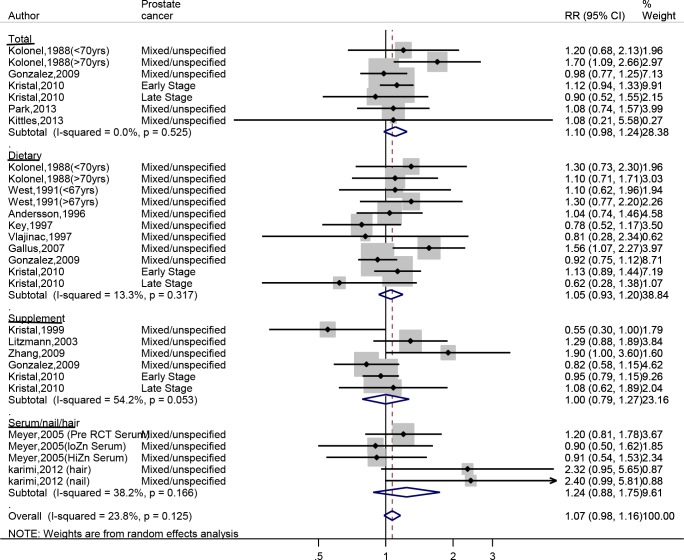
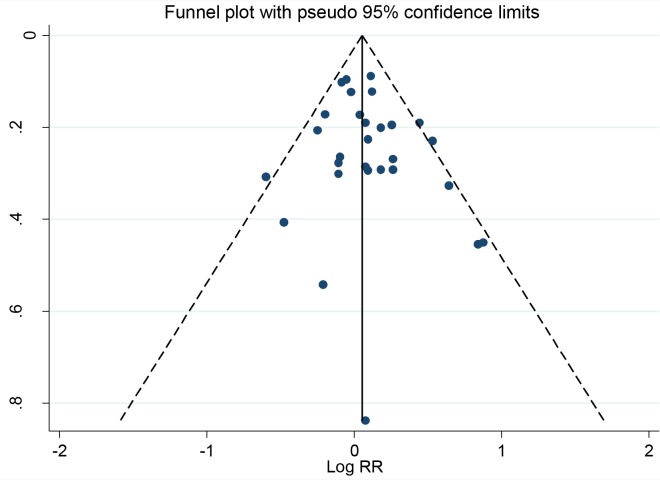
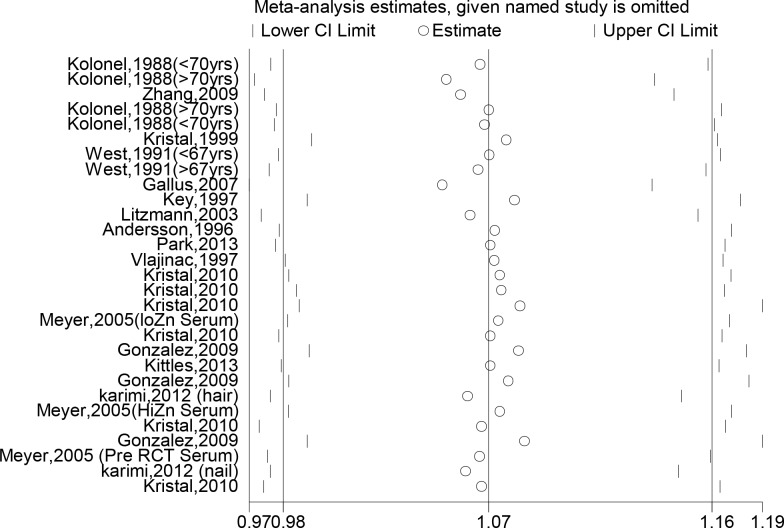
We performed a meta-analysis including our case-control study comparing the highest to the lowest zinc intake category (Fig 2). The random effect summary risk estimates from the 17 studies indicated that the combined high zinc consumption increased the risk of prostate cancer by 7% (OR high vs. low = 1.07, 95% CI: 0.98, 1.64). Visual inspection of the funnel plot did not identify any substantial asymmetry (Fig 3) and the Egger linear regression test also indicated no evidence of significant publication or small-study bias (p = 0.679). Sensitivity analysis showed that no specific study has a strong influence on the summary estimate as the overall OR after exclusion of any individual study was between 0.97 and 1.19 (Fig 4).
Fig 2. Forest plot of included studies for the highest versus lowest meta-analysis, stratified by zinc intake (dietary, supplement, and total) or zinc status (serum, nail, and hair).
Fig 3. Funnel plot of studies examining the association between zinc and prostate cancer incidence as a test for publication bias.
Fig 4. Sensitivity analysis investigating the influence of each individual study on the overall meta-analysis of zinc and risk of prostate cancer.
The meta-analysis of all studies except the “omitted” study named on the left margin is presented as a horizontal confidence interval. The full, “combined” results are shown as the solid vertical lines.
Subgroup analyses were conducted to further examine the relationship between zinc and prostate cancer (Table 4). There was a 10% increase in the risk of prostate cancer when total zinc intake was examined (RR = 1.10, 95% CI: 0.98, 1.24) that was borderline statistically significant (p = 0.107) and low heterogeneity (I2 = 0%) among the all studies. The increased risk was absent for the relationship between supplemental zinc intake and prostate cancer (RR = 1, 95% CI: 0.79, 1.27) with strong evidence for heterogeneity among these studies (I2 = 54.4). We observed greater extent of homogeneity among studies assessing dietary (I2 = 22%) or total zinc intake (I2 = 0%) compared to supplemental intake (I2 = 54%) or zinc status (I2 = 38%). There was 21% increase in the risk of prostate cancer among case-control studies compared with cohort studies (p = 0.04) with medium heterogeneity (I2 = 38%). The source of this heterogeneity seems to be the studies where supplemental zinc was assessed.
Table 4. Summary of the meta-analyses for the association of zinc and prostate cancer.
| n | RR | (95%CI) | p-value | I2 | p-value* | |
|---|---|---|---|---|---|---|
| Highest versus lowest meta-analysis | ||||||
| All studies | 29 | 1.07 | (0.98,1.64) | 0.127 | 23.8% | 0.125 |
| By Zinc category | ||||||
| Zinc intake (dietary, supplemental, total) | 24 | 1.05 | (0.97,1.15) | 0.232 | 21.6% | 0.169 |
| Dietary | 11 | 1.05 | (0.93,1.20) | 0.416 | 13.3% | 0.317 |
| Supplemental | 6 | 1.00 | (0.79,1.27) | 0.999 | 54.4% | 0.053 |
| Total | 7 | 1.10 | (0.98,1.245) | 0.107 | 0% | 0.525 |
| Zinc status (serum/toes /hair) | 5 | 1.24 | (0.88,1.75) | 0.166 | 38.2% | 0.166 |
| By Pathology | ||||||
| Early | 3 | 1.06 | (0.95,1.12) | 0.329 | 0% | 0.374 |
| Late | 3 | 0.90 | (0.63,1.28) | 0.553 | 0% | 0.536 |
| Mixed/unspecified | 23 | 1.10 | (0.98,1.23) | 0.113 | 32.7% | 0.067 |
| By Study design | ||||||
| Cohort and nested case-control | 14 | 1.01 | (0.94,1.10) | 0.735 | 0% | 0.745 |
| Case-Control | 15 | 1.21 | (1.01,1.46) | 0.041 | 39% | 0.059 |
| Total Zinc only | ||||||
| By Pathology | ||||||
| Early | 1 | 1.12 | (0.94,1.33) | 0.201 | - | - |
| Late | 1 | 0.90 | (0.52,1.55) | 0.704 | - | - |
| Mixed/unspecified | 5 | 1.13 | (0.93,1.38) | 0.228 | 12.5% | 0.334 |
| By Study design | ||||||
| Cohort and nested case-control | 4 | 1.06 | (0.93,1.21) | 0.364 | 0% | 0.765 |
| Case-Control | 3 | 1.47 | (1.04,2.07) | 0.029 | 0% | 0.6 |
| Dietary Zinc only | ||||||
| By Pathology | ||||||
| Early | 1 | 1.13 | (0.89,1.44) | 0.32 | - | - |
| Late | 1 | 0.62 | (0.28,1.38) | 0.24 | - | - |
| Mixed/unspecified | 9 | 1.06 | (0.91,1.22) | 0.47 | 15.5% | 0.304 |
| By Study design | ||||||
| Cohort and nested case-control | 3 | 0.98 | (0.80,1.21) | 0.841 | 33.1% | 0.224 |
| Case-Control | 8 | 1.123 | (0.95,1.33) | 0.173 | 2.7% | 0.409 |
| Supplementary Zinc only | ||||||
| By Pathology | ||||||
| Early | 1 | 0.95 | (0.79,1.15) | 0.592 | - | - |
| Late | 1 | 1.08 | (0.62,1.90) | 0.788 | - | - |
| Mixed/unspecified | 4 | 1.02 | (0.65,1.58) | 0.944 | 71.90% | 0.014 |
| By Study design | ||||||
| Cohort and nested case-control | 4 | 0.98 | (0.84,1.15) | 0.788 | 8.30% | 0.352 |
| Case-Control | 2 | 1.02 | (0.30,3.43) | 0.978 | 86.90% | 0.006 |
n = number of observations; RR = relative risk; CI = confidence interval; I2 = Heterogeneity,
* = p-value for heterogeneity
Dose-Response Meta-Analysis
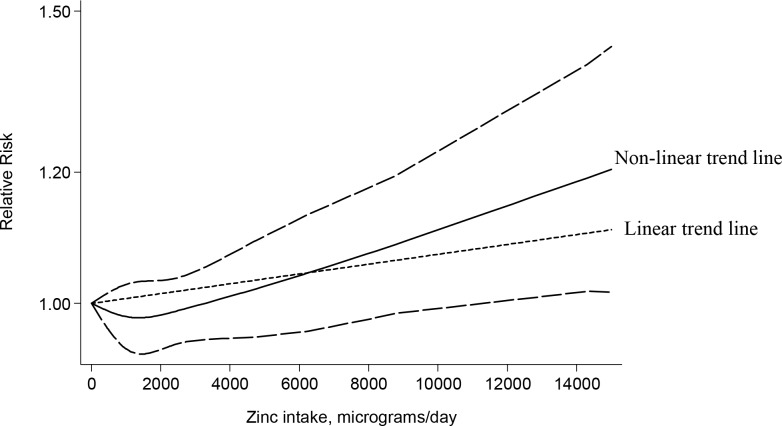
Twelve studies were included in the dose–response analysis [17, 18, 22–28, 33, 34]. Publication bias was not evident with Egger’s test (p = 0.84). The result of the dose-response meta-analysis indicates that there is no association between zinc intake and prostate cancer (RR 100mg/day increase = 1.07, 95% CI: 0.90, 1.28). If we assume that the relationship between zinc and prostate cancer is linear, an intake of 100 mg/day of zinc would increase the risk of prostate cancer by 7%. However, the relationship between zinc intake and prostate cancer is not linear (non-linearity test p = 0.0022) (Fig 5).
Fig 5. Dose-response relations between zinc intake and RR of prostate cancer (P for nonlinearity = 0.0022).
The fitted nonlinear trend is represented by the solid line with the 95% confidence intervals line in long dashes. Lines with short dashes represent the linear trend.
Discussion
Prostate cancer disproportionately affects men of African descent. Protective role of zinc in prostate cancer has been observed in animal and in vitro studies but epidemiological studies, including case-control, cohort and RCT, have shown mixed results. To address this issue, we assessed the association of dietary zinc intake among African Americans from a hospital based case-control study with demographic, lifestyle and clinical characteristics. We also conducted a systematic review of the literature and perform a meta-analysis to summarize the evidence regarding the association between zinc and prostate cancer.
The results of our case-control study suggest that there is a non-linear increase in risk of prostate cancer with increasing intake of zinc which is not statistically significant. Although this is the first study to examine the relationship between zinc intake and prostate cancer in African American, this is not an unusual finding as an increase in risk of prostate cancer has been previously reported in response to other vitamins and minerals [22–25]. For instance, vitamin E supplementation was shown to be associated with increased prostate cancer among the participants of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) [54] depending on the basal status or interactions with other nutrients [55]. Kolenko et al [56] in a recent report explains the increased risk of prostate cancer with increasing intake of zinc as a result of the mechanisms regulating zinc hemostasis and bioavailability.
We conducted a meta-analysis to systematically summarize the results from multiple studies to generate a pooled estimate of the effect of zinc and prostate cancer among the general population. We performed an overall and dose-response meta-analyses to examine the shape of the relationship between zinc intake and risk of prostate cancer. The high versus low meta-analysis showed a statistically non-significant positive association between zinc intake and prostate cancer (summary estimate OR = 1.07, 95% CI: 0.98, 1.16). Subgroup analysis showed when looking at dietary (RR = 1.05 (0.93, 1.2)), supplemental (RR = 1.0 (0.79, 1.27)) or total zinc (RR = 1.10 (0.98, 1.245), there was no apparent association with prostate cancer risk. However, there was a 24% increase risk of prostate cancer associated with increased zinc intake measured in serum [30] and hair/nail [53]. The observed increased association between levels of zinc status (serum and nail) and prostate cancer could be a result of a better estimation of zinc exposure, eliminating recall bias. On the other hand, it could be due to the lack of correlation between zinc biomarkers and zinc status [57].
Other limitations of our study should also be acknowledged. Our case-control study has small sample size and the recall bias associated with case-control studies and FFQ assessments. In the meta-analyses errors in measurement of zinc intake could have attenuated individual study results and led to the null association between zinc intake and risk of prostate cancer. All the studies in our analysis (except for two) assessed zinc intake using FFQ, several of which have been validated with reasonable reproducibility and validity [38–40]. Misreporting of intake was still inevitable. Although we did not observe publication bias, it could still be present as the published results might not be representative of the conducted studies. The presence of high heterogeneity among the studies that assessed supplemental zinc is very evident (I2>71%) but using random effect models and performing sensitivity analysis showed that this high level of heterogeneity among the studies did not influence the summary estimate.
In summary, we found that low dietary intake of zinc in our case-control study among African Americans showed an increase in prostate cancer risk albeit statistically non-significant. However, the pooled estimates from published studies indicated that zinc intake was not associated with prostate cancer risk. This inconsistency could be explained by the study limitations we mentioned above including small sample size and the recall bias however, it is likely that the major explanation resides within the population heterogeneity in the studies we included in the meta-analysis. There is a lack in studies that examined the association between zinc intake and prostate cancer exclusively in African American. All the studies we included in the meta-analysis examined zinc intake in the general population with no racial/ethnic stratification. An important factor that has not been considered in these epidemiological studies is the heterogeneity of zinc uptake by the prostate gland among different populations. This is supported by previous studies reporting a significant downregulation of the two major zinc transporters, hZIP1 and hZIP2, in normal prostate tissues from African American men when compared with age-matched white men [15]. The reason behind this phenotype is thought to be evolutionary. Since Africa is a mineral-rich continent, Africans may have genetically downregulated their zinc absorption capacity to avoid high toxic levels of zinc that might result in various serious neurodegenerative and biochemical disorders [58]. It has been also shown that the expression of zinc transporters correlates significantly with zinc levels in prostate gland tissues [59]. These findings indicate that African American men need to have higher zinc intake than their White counterparts and might also explain the association we encountered between low zinc intake and prostate cancer in African American while no association was found in the meta-analysis that comprised heterogeneous populations. Thus, the current study indicates the imminent need to conduct further studies that represent racial/ethnic minorities, especially African American who are more susceptible to have zinc deficiency and unintentionally underrepresented in the current literature, to examine the association between zinc intake and the risk of prostate cancer. Also, large epidemiologic studies based on prospective zinc data, preferably using biologic samples (eg, toenails) with repeated collection over time to better reflect long-term exposures, could give a better insight into this critical question.
Supporting Information
(DOCX)
Acknowledgments
We are grateful to all individuals who participated as research subjects in this study. The authors would also like to thank the urologists and clinic staff at all the participating sites.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the following funding sources: National Institutes of Health (5U54CA91431-01) (RAK) and Egyptian Ministry of Higher Education GM842 (AMM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016 Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Marshall JR. Diet and prostate cancer prevention. World journal of urology. 2012;30(2):157–65. 10.1007/s00345-011-0810-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. The Proceedings of the Nutrition Society. 2008;67(3):253–6. 10.1017/S002966510800712X . [DOI] [PubMed] [Google Scholar]
- 4.Otten JJ, Hellwig JP, Meyers LD, Board FN, Medicine I. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements: National Academies Press; 2006. [Google Scholar]
- 5.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. The Journal of nutrition. 2000;130(5S Suppl):1374S–7S. . [DOI] [PubMed] [Google Scholar]
- 6.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Journal of the American Dietetic Association. 2001;101(3):294–301. 10.1016/S0002-8223(01)00078-5 . [DOI] [PubMed] [Google Scholar]
- 7.Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? The Journal of nutrition. 2011;141(10):1847–54. 10.3945/jn.111.142257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. The Journal of nutrition. 2000;130(5S Suppl):1367S–73S. . [DOI] [PubMed] [Google Scholar]
- 9.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Archives of toxicology. 2012;86(4):521–34. 10.1007/s00204-011-0775-1 . [DOI] [PubMed] [Google Scholar]
- 10.Costello LC, Franklin RB, Feng P, Tan M, Bagasra O. Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States). Cancer causes & control: CCC. 2005;16(8):901–15. 10.1007/s10552-005-2367-y . [DOI] [PubMed] [Google Scholar]
- 11.Ho E, Song Y. Zinc and prostatic cancer. Current opinion in clinical nutrition and metabolic care. 2009;12(6):640–5. 10.1097/MCO.0b013e32833106ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortesi M, Fridman E, Volkov A, Shilstein S, Chechik R, Breskin A, et al. Clinical assessment of the cancer diagnostic value of prostatic zinc: a comprehensive needle-biopsy study. The Prostate. 2008;68(9):994–1006. 10.1002/pros.20766 . [DOI] [PubMed] [Google Scholar]
- 13.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Molecular cancer. 2007;6:37 10.1186/1476-4598-6-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Molecular cancer. 2005;4:32 10.1186/1476-4598-4-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rishi I, Baidouri H, Abbasi JA, Bullard-Dillard R, Kajdacsy-Balla A, Pestaner JP, et al. Prostate cancer in African American men is associated with downregulation of zinc transporters. Applied immunohistochemistry & molecular morphology: AIMM / official publication of the Society for Applied Immunohistochemistry. 2003;11(3):253–60. . [DOI] [PubMed] [Google Scholar]
- 16.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Molecular cancer. 2006;5:17 10.1186/1476-4598-5-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez A, Peters U, Lampe JW, White E. Zinc intake from supplements and diet and prostate cancer. Nutrition and cancer. 2009;61(2):206–15. 10.1080/01635580802419749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1999;8(10):887–92. . [PubMed] [Google Scholar]
- 19.Li XM, Zhang L, Li J, Li Y, Wang HL, Ji GY, et al. Measurement of serum zinc improves prostate cancer detection efficiency in patients with PSA levels between 4 ng/mL and 10 ng/mL. Asian journal of andrology. 2005;7(3):323–8. 10.1111/j.1745-7262.2005.00044.x . [DOI] [PubMed] [Google Scholar]
- 20.Epstein MM, Kasperzyk JL, Andren O, Giovannucci EL, Wolk A, Hakansson N, et al. Dietary zinc and prostate cancer survival in a Swedish cohort. The American journal of clinical nutrition. 2011;93(3):586–93. 10.3945/ajcn.110.004804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner SE, Burch JB, Hussey J, Temples T, Bolick-Aldrich S, Mosley-Broughton C, et al. Soil zinc content, groundwater usage, and prostate cancer incidence in South Carolina. Cancer causes & control: CCC. 2009;20(3):345–53. 10.1007/s10552-008-9248-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. European urology. 2007;52(4):1052–6. 10.1016/j.eururo.2007.01.094 . [DOI] [PubMed] [Google Scholar]
- 23.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case-control study in Hawaii. American journal of epidemiology. 1988;127(5):999–1012. . [DOI] [PubMed] [Google Scholar]
- 24.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. Journal of the National Cancer Institute. 2003;95(13):1004–7. . [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Coogan P, Palmer JR, Strom BL, Rosenberg L. Vitamin and mineral use and risk of prostate cancer: the case-control surveillance study. Cancer causes & control: CCC. 2009;20(5):691–8. 10.1007/s10552-008-9282-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson SO, Wolk A, Bergstrom R, Giovannucci E, Lindgren C, Baron J, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. International journal of cancer. 1996;68(6):716–22. . [DOI] [PubMed] [Google Scholar]
- 27.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. British journal of cancer. 1997;76(5):678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA, Albanes D, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. American journal of epidemiology. 2010;172(5):566–77. 10.1093/aje/kwq148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MM, Wang RT, Hsing AW, Gu FL, Wang T, Spitz M. Case-control study of diet and prostate cancer in China. Cancer causes & control: CCC. 1998;9(6):545–52. . [DOI] [PubMed] [Google Scholar]
- 30.Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. International journal of cancer. 2005;116(2):182–6. 10.1002/ijc.21058 . [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Wilkens LR, Morris JS, Henderson BE, Kolonel LN. Serum zinc and prostate cancer risk in a nested case-control study: The multiethnic cohort. The Prostate. 2013;73(3):261–6. 10.1002/pros.22565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platz EA, Helzlsouer KJ, Hoffman SC, Morris JS, Baskett CK, Comstock GW. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. The Prostate. 2002;52(4):288–96. 10.1002/pros.10115 . [DOI] [PubMed] [Google Scholar]
- 33.Vlajinac HD, Marinkovic JM, Ilic MD, Kocev NI. Diet and prostate cancer: a case-control study. European journal of cancer. 1997;33(1):101–7. . [DOI] [PubMed] [Google Scholar]
- 34.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer causes & control: CCC. 1991;2(2):85–94. . [DOI] [PubMed] [Google Scholar]
- 35.Hernandez W, Grenade C, Santos ER, Bonilla C, Ahaghotu C, Kittles RA. IGF-1 and IGFBP-3 gene variants influence on serum levels and prostate cancer risk in African-Americans. Carcinogenesis. 2007;28(10):2154–9. 10.1093/carcin/bgm190 . [DOI] [PubMed] [Google Scholar]
- 36.Robbins CM, Hooker S, Kittles RA, Carpten JD. EphB2 SNPs and sporadic prostate cancer risk in African American men. PLoS One. 2011;6(5):e19494 10.1371/journal.pone.0019494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonilla C, Hooker S, Mason T, Bock CH, Kittles RA. Prostate cancer susceptibility Loci identified on chromosome 12 in African Americans. PLoS One. 2011;6(2):e16044 10.1371/journal.pone.0016044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. Journal of clinical epidemiology. 1990;43(12):1327–35. . [DOI] [PubMed] [Google Scholar]
- 39.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. American journal of epidemiology. 1986;124(3):453–69. . [DOI] [PubMed] [Google Scholar]
- 40.Coates RJ, Eley JW, Block G, Gunter EW, Sowell AL, Grossman C, et al. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. American journal of epidemiology. 1991;134(6):658–71. . [DOI] [PubMed] [Google Scholar]
- 41.Leonard T, Duffy JC. A Bayesian fixed effects analysis of the Mantel-Haenszel model applied to meta-analysis. Stat Med. 2002;21(16):2295–312. 10.1002/sim.1048 . [DOI] [PubMed] [Google Scholar]
- 42.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. American journal of epidemiology. 2012;175(1):66–73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tempany CM, Zhou X, Zerhouni EA, Rifkin MD, Quint LE, Piccoli CW, et al. Staging of prostate cancer: results of Radiology Diagnostic Oncology Group project comparison of three MR imaging techniques. Radiology. 1994;192(1):47–54. 10.1148/radiology.192.1.8208963 . [DOI] [PubMed] [Google Scholar]
- 45.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. American journal of epidemiology. 1992;135(11):1301–9. . [DOI] [PubMed] [Google Scholar]
- 46.Bekkering GE, Harris RJ, Thomas S, Mayer AM, Beynon R, Ness AR, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? American journal of epidemiology. 2008;167(9):1017–26. 10.1093/aje/kwn005 . [DOI] [PubMed] [Google Scholar]
- 47.Greenlee H, White E, Patterson RE, Kristal AR, Vitamins, Lifestyle Study C. Supplement use among cancer survivors in the Vitamins and Lifestyle (VITAL) study cohort. Journal of alternative and complementary medicine. 2004;10(4):660–6. 10.1089/acm.2004.10.660 . [DOI] [PubMed] [Google Scholar]
- 48.Heshmat MY, Kaul L, Kovi J, Jackson MA, Jackson AG, Jones GW, et al. Nutrition and prostate cancer: a case-control study. The Prostate. 1985;6(1):7–17. . [DOI] [PubMed] [Google Scholar]
- 49.Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, Penson DF, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. American journal of epidemiology. 2008;167(8):925–34. 10.1093/aje/kwm389 . [DOI] [PubMed] [Google Scholar]
- 50.Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. Journal of the National Cancer Institute. 2007;99(10):754–64. 10.1093/jnci/djk177 . [DOI] [PubMed] [Google Scholar]
- 51.Lin YS, Caffrey JL, Lin JW, Bayliss D, Faramawi MF, Bateson TF, et al. Increased risk of cancer mortality associated with cadmium exposures in older Americans with low zinc intake. Journal of toxicology and environmental health Part A. 2013;76(1):1–15. 10.1080/15287394.2012.722185 . [DOI] [PubMed] [Google Scholar]
- 52.Neuhouser ML, Kristal AR, Patterson RE, Goodman PJ, Thompson IM. Dietary supplement use in the Prostate Cancer Prevention Trial: implications for prevention trials. Nutrition and cancer. 2001;39(1):12–8. 10.1207/S15327914nc391_2 . [DOI] [PubMed] [Google Scholar]
- 53.Karimi G, Shahar S, Homayouni N, Rajikan R, Abu Bakar NF, Othman MS. Association between trace element and heavy metal levels in hair and nail with prostate cancer. Asian Pacific journal of cancer prevention: APJCP. 2012;13(9):4249–53. . [DOI] [PubMed] [Google Scholar]
- 54.Klein EA, Thompson IM Jr., Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama. 2011;306(14):1549–56. 10.1001/jama.2011.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, et al. Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(8):1494–504. 10.1158/1055-9965.EPI-14-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nature reviews Urology. 2013;10(4):219–26. 10.1038/nrurol.2013.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. The American journal of clinical nutrition. 2010;91(5):1478S–83S. 10.3945/ajcn.2010.28674I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7(2):111–7. 10.1038/sj.pcan.4500712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52(4):316–21. 10.1016/j.ymeth.2010.08.004 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.