Abstract
Compounds were evaluated for antiviral activity in rhabdomyosarcoma (RD) cells against a recent 2014 clinical isolate of enterovirus D68 (EV-D68), a 1962 strain of EV-68D, rhinovirus 87 (RV-87, serologically the same as EV-D68), and enterovirus 71 (EV-71). Test substances included known-active antipicornavirus agents (enviroxime, guanidine HCl, pirodavir, pleconaril, and rupintrivir), nucleobase/nucleoside analogs (3-deazaguanine and ribavirin), and three novel epidithiodiketopiperazines (KCN-2,2’-epi-19, KCN-19, and KCN-21). Of these, rupintrivir was the most potent, with 50% inhibition of viral cytopathic effect (EC50) and 90% inhibition (EC90) of virus yield at 0.0022-0.0053 μM against EV-D68. Enviroxime, pleconaril and the KCN compounds showed efficacy at 0.01-0.3 μM; 3-deazaguanine and pirodavir inhibited EV-D68 at 7-13 μM, and guanidine HCl and ribavirin were inhibitory at 80-135 μM. Pirodavir was active against EV-71 (EC50 of 0.78 μM) but not against RV-87 or EV-D68, and all other compounds were less effective against EV-71 than against RV-87 and EV-D68. The most promising compound inhibiting both virus infections at low concentrations was rupintrivir. Antiviral activity was confirmed for the ten compounds in virus yield reduction (VYR) assays in RD cells, and for enviroxime, guanidine HCl, and pirodavir by cytopathic effect (CPE) assays in A549, HeLa-Ohio-1, and RD cells. These studies may serve as a basis for further pre-clinical discovery of anti-enterovirus inhibitors. Furthermore, the antiviral profiles and growth characteristics observed herein support the assertion that EV-D68 should be classified together with RV-87.
Keywords: Enterovirus D68, enterovirus 71, rhinovirus 87, picornavirus, antiviral
Enteroviruses, of which there are more than 100 known serotypes, are common causes of summer and fall illnesses, with up to 15 million infections annually in the United States. Enterovirus infections are associated with mild respiratory illness, febrile rash, and may also cause neurologic illness, such as aseptic meningitis and encephalitis (Solomon et al., 2010). According to the Centers for Disease Control and Prevention (CDC), non-polio enteroviruses are very common, and most people who get infected with these viruses do not get sick or have only a mild illness (Midgley et al., 2014). However, some people can have serious complications, especially infants and people with weakened immune systems.
Enterovirus D68 (EV-D68) is unique among non-polio enteroviruses in that it shares epidemiologic and biologic features with human rhinoviruses (Midgley et al., 2014; Oberste et al., 2004). It was first isolated in California in 1962 from 4 children with bronchiolitis and pneumonia. Since the original isolation of EV-D68, it has rarely been reported in the U.S. The National Enterovirus Surveillance System received only 79 EV-D68 reports during 2009-2013 (Schieble et al., 1967), but there was an increase in incidence in 2014 (Khan, 2015). EV-D68 is normally associated with mild to severe respiratory illness. However, infections with the 2014 virus strains caused more serious respiratory problems, especially in children who have asthma. The recent increase in the incidence of EV-D68 is also associated with an increase in unexplained polio-like cases in children, involving sudden onset of weakness in one or more limbs, referred to as acute flaccid myelitis in children (Greninger et al., 2015). Genetic sequencing of the virus found in respiratory secretions of children in California and Colorado who suffered from paralysis or muscle weakness in the fall of 2014 revealed that they were infected with a mutated strain of EV-D68 that is closer to polio than other strains common in previous years. This novel strain of EV-D68, called B1, appears to have emerged recently and has only 5 to 6 coding differences from previous strains commonly found in the United States. Of those six genetic polymorphisms in the B1 type of EV-D68 polyprotein, five are present in neuropathogenic poliovirus, enterovirus D70, or both (Greninger et al., 2015). Nucleotide sequencing, serology, and acid lability evidence suggest that EV-D68 is more similar to human rhinovirus 87 (RV-87) than to the enteroviruses (Blomqvist et al., 2002).
Another virus that has caused an increase in human infections is enterovirus 71 (EV-71). EV-71 is a major cause of hand, foot and mouth disease (HFMD) but also has the potential to induce severe neurological disease. EV-71 was first isolated from a two-month old patient with aseptic meningitis in California, USA in 1969 (Schmidt et al., 1974). Since that time, EV-71 has been identified as one of the causative agents for HFMD and is responsible for outbreaks in several countries including Taiwan, China, Australia, Singapore, Vietnam and Cambodia affecting up to millions of individuals at one time (Lin and Shih, 2014). These outbreaks often correlate with cohorts of children born that have not been previously exposed to the virus. While most cases of EV-71 present as a simple rash as seen in HFMD, approximately 10-20% of cases become neuropathogenic and cause severe neurological disease resulting in paralysis and death (McMinn, 2002; Ooi et al., 2010). According to the Global Disease Detection Program administered by the CDC, EV-71 is on the list of five infectious agents that are monitored worldwide for potential outbreaks (Christian et al., 2013). The CDC considers EV-71 a threat because of the potentially fatal nature of the disease, the ability to create large outbreaks through efficient spreading in daycare and school settings.
There is a need to identify inhibitors of enterovirus infections that may be developed for treatment. As we were in the process of preparing this manuscript for publication, two reports emerged describing antiviral activities of various anti-picornavirus agents against EV-D68 in vitro. Sun et al. reported efficacies of seven compounds against EV-D68 clusters A, B, and C viruses, with rupintrivir and SG85 being the most active compounds (Sun et al., 2015). Rhoden and colleagues evaluated 15 compounds from various classes against four EV-D68 strains (Rhoden et al., 2015). They also found the antipicornavirus agent rupintrivir to be highly active, but also similarly potent was the sialidase cleaving enzymatic compound DAS181. In this report we describe the antiviral activities of ten antiviral substances that have the potential to treat conditions caused by these viruses. Some of the compounds we investigated were recently reported (Rhoden et al., 2015; Sun et al., 2015), and other compounds are reported here for the first time, such as epidithiodiketopiperazine inhibitors. In addition to the information in the two recent publications, we show the cytotoxicity and selectivity indices of the compounds, their effects on virus yield, and comparative inhibitory activity of certain compounds in three human cell lines.
The following viruses were obtained from the American Type Culture Collection (ATCC, Manassas, VA) for evaluation: Enterovirus D68 (Fermon strain, VR-1076), Enterovirus 71 (Tainan/4643/98 strain, NR-471), and Rhinovirus 87 (F02-3607 Corn strain, VR-1197). Enterovirus D68 (US/KY/14-18953 strain, NR-49132) was from BEI Resources (Manassas, VA). Assays were performed in the following cell lines: A549 human lung carcinoma (CCL-185) and RD human rhabdomyosarcoma (CCL-136) cells from ATCC; and HeLa-Ohio-1 human cervical epithelioid carcinoma cells from Frederick Hayden (University of Virginia, Charlottesville, VA). Most of the studies were performed using RD cells.
Test compounds were obtained from various sources. Enviroxime was from the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), Ft. Detrick, Frederick, MD through the NIAID antiviral screening program. Guanidine HCl was purchased from Sigma Aldrich (St. Louis, MO). Pirodavir was purchased from AdooQ Bioscience, (Irvine, CA). Pleconaril and rupintrivir were obtained from the former Biota Pharmaceuticals (Notting Hill, Victoria, Australia). 3-deazaguanine and ribavirin were from the former ICN Pharmaceuticals (Costa Mesa, CA). Epidithiodiketopiperazine compounds KCN-2,2’-epi-19, KCN-19, and KCN-21 were prepared at The Scripps Research Institute (La Jolla, CA) as previously described (Nicolaou et al., 2012). Each compound was dissolved in DMSO or MEM then diluted in half-log10 increments, and added to cells immediately prior to addition of virus-containing medium. The compounds represented different types of inhibitors, such as those inhibiting viral uncoating (so-called virus canyon binders), viral protease, viral RNA polymerase, and other modes of action as discussed below.
In vitro antiviral studies were conducted by two well-established methods, the inhibition of virus-induced cytopathic effect (CPE) and virus yield reduction (VYR). CPE assays were conducted in triplicate wells of 96-well microplates of infected cells as described previously (Barnard et al., 1997; Barnard et al., 2004). EV-D68 and RV-87 viruses were evaluated in RD cells at 33°C with 25 mM MgCl2 and 2% FBS in minimal essential medium (MEM), typical conditions for evaluating rhinoviruses. EV-71 was tested in RD cells in MEM with 2% FBS but no MgCl2 and incubated at 37°C. Quantitation of percent virus destruction was made by neutral red dye uptake. The multiplicity of infection (MOI) used was the minimum inoculum that caused >80% CPE in 3-4 days of incubation for each virus (~16 cell culture infectious doses (CCID50) per microwell for EV-D68 (US/KY/14-18953), ~270 for EV-D68 (Fermon), ~430 for EV-71, and ~46 for RV-87). The MOI was varied to obtain equivalent CPE for comparisons. To verify that MOI differences did not confound the results, selected assays were also performed using equal MOIs; these yielded similar results as in Table 1 with the same comparisons and conclusions (data not shown).
Table 1.
Antiviral activities and cytotoxicities of test compounds against EV-D68, RV-87, and EV-71 by in vitro viral cytopathic effect (CPE) inhibition assays quantified by neutral red dye uptake.
| Enterovirus D68/US/KY/14-18593 | Enterovirus D68/Fermon | Rhinovirus 87 | Enterovirus 71 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | CC50 a | EC50 b | SI c | EC50 | SI | EC50 | SI | EC50 | SI |
| 3-Deazaguanine | 360±227 | 8.0±2.3 | 45 | 6.7±1.6 | 54 | 8.0±0.9 | 45 | 73±25 | 5 |
| Enviroxime | 95±27 | 0.31±0.22 | 306 | 0.27±0.19 | 352 | 0.19±0.11 | 500 | 1.0±1.1 | 92 |
| Guanidine HCl | 3670±1100 | 80±21 | 46 | 91±11 | 40 | 91±10 | 40 | 305±5 | 12 |
| KCN-2,2′-epi-19 | 1.9±1.0 | 0.130±0.003 | 15 | 0.084±0.030 | 23 | 0.13±0.06 | 14 | >1.9±0 | 0 |
| KCN-19 | 3.4±1.4 | 0.25±0.12 | 14 | 0.14±0.08 | 24 | 0.31±0.12 | 11 | 1.5±0.1 | 2 |
| KCN-21 | 2.4±1.5 | 0.017±0.005 | 141 | 0.014±0.009 | 171 | 0.031±0.026 | 77 | 0.93±0.57 | 3 |
| Pirodavir | 19±13 | 13±7.0 | 1.5 | 7.0±3.0 | 3 | >19±0 | 0 | 0.78±0.32 | 24 |
| Pleconaril | 11.3±6.3 | 0.13±0.03 | 87 | 0.13±0.09 | 87 | 1.6±1.0 | 7 | >11.3±0 | 0 |
| Ribavirin | 1100±450 | 135±74 | 8 | 147±74 | 7 | 106±26 | 10 | >1100±0 | 0 |
| Rupintrivir | 134±21 | 0.0046±0.0027 | 29130 | 0.0022±0.0120 | 60910 | 0.0053±0.0038 | 25280 | 0.018±0.015 | 7450 |
50% cytotoxic concentration (μM) ± SD (≥3 independent determinations), determined in confluent monolayers of RD cells.
50% effective (virus-inhibitory) concentration (μM) ± SD (≥3 independent determinations), determined in parallel with cytotoxicity.
Selectivity index (CC50 / EC50).
Compounds were evaluated at 8 half-log10 concentrations designed to bracket the 50% virus inhibitory concentration (EC50). The 50% cytotoxic concentration (CC50) of the compounds was determined in duplicate uninfected wells on the sample microtiter plate. Cells were in the confluent, stationary monolayer stage at the time of infection and treatment. VYR assays were conducted by first replicating the viruses in the presence of inhibitor, then after 3 days collecting the supernatant from each concentration of test compound and storing at −80°C. Later, virus yield was determined by endpoint dilution method (Reed and Muench, 1938). Briefly, supernatant virus was serially diluted in log10 increments then plated onto quadruplicate wells of 96-well plates seeded with RD cells. The presence or absence of CPE for determining a viral endpoint was evaluated by microscopic examination of cells 6 days after infection. From these data, 90% virus inhibitory concentrations (EC90) were determined by regression analysis.
The ten compounds were evaluated against EV-D68, RV-87, and EV-71 strains in CPE assays to determine viral inhibition (Table 1). Compounds that were potent inhibitors of EV-D68 and RV-87 included rupintrivir, pleconaril, enviroxime, and three KCN compounds. However, these same compounds were less active or ineffective against EV-71. Of the compounds tested, rupintrivir exhibited the most potent effects against EV-D68, RV-87, and EV-71 viruses. Guanidine HCl, 3-deazaguanine, pirodavir, and ribavirin also exhibited slight antiviral activity against EV-D68, RV-87 and/or EV-71. Pirodavir was the only compound that was more active against EV-71 than it was against EV-D68. Importantly, the antiviral sensitivity profile of EV-D68 more closely resembled that of RV-87 than of EV-71, supporting the assertions by Bloomqvist et.al. (2002) that RV-87 and EV-68 are highly similar and should be reclassified as a single serotype, perhaps as rhinoviruses rather than enteroviruses.
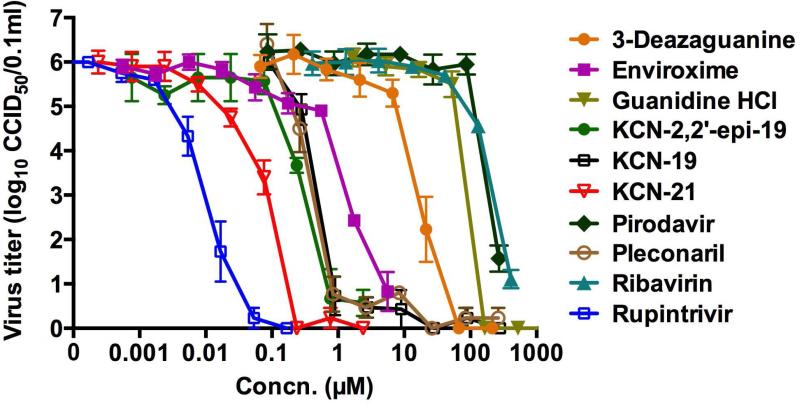
Dose-responsive effects of compounds on virus yield are shown graphically in Figure 1. Rupintrivir and KCN-21 were the most potent of the compounds against EV-D68, whereas pirodavir and ribavirin were the least active. From these data, concentrations inhibiting virus yield by 90% (EC90) were calculated by regression analysis (Table 2). A one-log10 reduction in viral titer (EC90) largely correlated to a 50% inhibition in viral CPE (EC50). The one exception was for pirodavir, which inhibited CPE at a much lower concentration than it did virus yield. For this reason we deem pirodavir to be poorly effective against EV-D68 virus.
Figure 1.
Dose-response curves for the inhibition of EV-D68 (US/KY/14-18953 strain) yield by ten antiviral compounds. Mean values ± SD were derived from 3 independent assays, determined using supernatant fluids from RD cells at 3 days after infection.
Table 2.
Activities of test compounds against EV-D68 (US/KY/14-18953 strain) by virus yield reduction assays in RD cells.
| Compound | EC90a (μM) ± SD | SI b |
|---|---|---|
| 3-Deazaguanine | 8.0 ± 1.1 | 45 |
| Enviroxime | 0.61 ± 0.08 | 156 |
| Guanidine HCl | 60 ± 1.3 | 64 |
| KCN-2,2’-epi-19 | 0.14 ± 0.03 | 14 |
| KCN-19 | 0.27 ± 0.03 | 13 |
| KCN-21 | 0.024 ± 0.002 | 100 |
| Pirodavir | 106 ± 12 | <1 |
| Pleconaril | 0.21 ± 0.05 | 54 |
| Ribavirin | 93 ± 23 | 12 |
| Rupintrivir | 0.004 ± 0.002 | 33,500 |
90% (1 log10) reduction of virus yield; mean ± SD calculated from three independent assays.
Selectivity index (CC50 [from Table 1] / EC90).
We also evaluated the antiviral activities of selected inhibitors against EV-D68 in three different human cell lines: A549, Hela-Ohio-1, and RD (Table 3). There was a general consistency in EC50 and CC50 values for each inhibitor across the three cell lines. Pirodavir was less cytotoxic to A549 cells than it was to the other two cell lines, which resulted in a higher selectivity index (SI). Enviroxime was more active in RD cells, although the variability between assays for enviroxime was high depending on subtle differences in cell health and confluence.
Table 3.
Antiviral activities and toxicities of three inhibitors against EV-D68 in different cell lines by neutral red assay.
| Enviroxime | Guanidine HCl | Pirodavir | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell Line | EC50a | CC50b | SIc | EC50 | CC50 | SI | EC50 | CC50 | SI |
| A549 | 0.77 ± 1.1 | 68 ± 13 | 89 | 32 ± 4.8 | 2990 ± 500 | 93 | 11 ± 7.2 | 140 ± 33 | 13 |
| HeLa-Ohio-1 | 1.7 ± 0.72 | 78 ± 24 | 45 | 66 ± 22 | 3040 ± 79 | 46 | 6.1 ± 2.8 | 33 ± 6.1 | 5.3 |
| RD | 0.27 ± 0.23 | 66 ± 28 | 240 | 76 ± 28 | 3960 ± 510 | 52 | 8.1 ± 3.9 | 32 ± 18 | 3.9 |
50% effective concentration (μM) ± SD (≥3 independent determinations) against EV-D68/US/KY/14-18953.
50% cytotoxic concentration (μM) ± SD (≥3 independent determinations) in stationary cell monolayers.
Selectivity index (CC50 / EC50).
The inhibitors tested in these assays have diverse modes of action, some of which are known versus picornaviruses, and others that can be inferred from their effects against other viruses. Rupintrivir, the most active substance, was chemically designed to inhibit picornavirus proteases (Dragovich et al., 1999; Patick et al., 1999) and represents the most potent compound tested. Our antiviral results with rupintrivir agree with those recently published (Rhoden et al., 2015; Sun et al., 2015). Rupintrivir was found to be effective in treating an EV-71 infection in suckling mice by reducing mortality and clinical scores (Zhang et al., 2013). In the same experiment ribavirin was found to be ineffective. Rupintrivir is considered a potential agent to treat EV-71 infections (Zhang et al., 2010), and the same could be said for treating infections caused by EV-D68, based upon its activity. Patick et al. (Patick et al., 1999) reported that rupintrivir also inhibits rhinovirus 87, which is reported to be the same virus serotype as EV-D68 (Blomqvist et al., 2002).
Pirodavir and pleconaril are picornavirus capsid binding inhibitors that prevent attachment and/or virus uncoating (Barnard et al., 2004; Kaiser et al., 2000; Liu et al., 2015). Activities of these two compounds against EV-D68 and EV-71 were previously reported against the Fermon 1962 strain (Liu et al., 2015), and recent publications evaluated one or both of these inhibitors with data similar to ours (Rhoden et al., 2015; Sun et al., 2015). Enviroxime has been implicated to inhibit multiple picornavirus targets, but the viral nonstructural protein 3A(B) may be the predominant one (Brown-Augsburger et al., 1999; Heinz and Vance, 1995). The modes of action of ribavirin and 3-deazaguanine have not been specifically identified against picornaviruses. Both compounds (once metabolized to 5’-monophosphate nucleotides) inhibit cellular inosine monophosphate dehydrogenase, resulting in decreases in intracellular guanosine triphosphate levels (Allen et al., 1977; Streeter et al., 1973). This affects the rates of viral and cellular RNA synthesis. Whether these compounds in their intracellular nucleoside triphosphate forms inhibit picornavirus RNA polymerases has not been determined, although ribavirin-resistant EV-71 viruses have mutations in viral 3D polymerase (Sadeghipour et al., 2013). Guanidine HCl inhibits picornavirus RNA synthesis by interaction with viral non-structural protein 2C (Sadeghipour et al., 2012). The present report is the first to document the antiviral activity of this compound against EV-D68. Guanidine HCl has been used to treat picornavirus infections in mice (Eggers, 1976; Herrmann et al., 1982). KCN-2,2’-epi-19, KCN-19, and KCN-21 are closely related compounds that we first reported as inhibitors of poliovirus replication (Nicolaou et al., 2012). Here we report their activities against EV-D68 and EV-71 for the first time. Their mode(s) of action against EV-D68, EV-71, and poliovirus are not known.
Compounds other that those discussed above that are under investigation as anti-picornavirus agents include itraconazole and vapendavir (Gao et al., 2015; Tijsma et al., 2014). Itraconazole inhibits picornavirus nonstructural protein 3A (similar to enviroxime), but viruses resistant to enviroxime-like compounds are not cross resistant to itraconazole. Vapendavir is a virus capsid binding inhibitor that was reported to inhibit EV-71 (Tijsma et al., 2014). We have tested vapendavir against EV-D68, and found it to be inactive (D.F. Smee et al., unpublished). This agrees with Rhoden et al. (2015) in RD cells, but not with Sun et al. (2015) who indicated that vapendavir is active against ten strains of EV-D68 in HeLa cells. Differences in activity of vapendavir between labs may relate to the type of cells used for assay.
Cytopathic effect inhibition studies performed here were followed up by virus yield reduction assays, indicating the compounds were inhibitors of virus replication and not just of viral cytopathic effect. Except for the pirodavir data, it was shown that a 90% reduction in virus yield reduction (EC90) correlated approximately to a 50% reduction in viral cytopathic effect (EC50). This is an observation we have made repeatedly with multiple viruses (such as influenza), but have never published.
We found that EV-D68 did not replicate well in cell culture unless we lowered incubation temperature to 33°C and added magnesium chloride to the medium, conditions commonly used for rhinoviruses, not enteroviruses. Using these conditions, we investigated a number of cell types from human and monkey origin, and developed CPE assays in three human cell lines: A549, Hela-Ohio-1, and RD. African green monkey kidney (Vero 76) and other monkey kidney cell lines were not suitable for EV-D68 antiviral assays, whereas EV-71 replicated well and induced strong CPE in Vero 76 cells, and we routinely perform antiviral studies using Vero 76 cells. This highlights yet another way in which EV-D68 differs from classical enteroviruses.
Mouse models have been developed for evaluation of inhibitors of EV-71 infections, such as the use of newborn/suckling mice (Chen et al., 2004; Yu et al., 2000) and adult AG129 mice that lack the interferon α/β receptor (Caine et al., 2013; Khong et al., 2012). Presently there is no mouse model for EV-D68 infections. Until an EV-D68 model is developed, new broad-spectrum anti-picornavirus agents must be evaluated in other picornavirus models of infection, such as in EV-71-infected mice.
Broad-spectrum anti-picornavirus agents, i.e., compounds that inhibit EV-D68, EV-71, and other picornaviruses such as rhinoviruses, coxsackieviruses, and polioviruses, are more likely to be successful as drug candidates than those inhibiting only a limited number of these viruses. Recently it was determined that rupintrivir inhibits noroviruses (Rocha-Pereira et al., 2014), which are not in the picornaviridae family. Perhaps this broad-spectrum activity will revitalize interest in rupintrivir as a treatment for picornaviruses, or else will serve to inspire the synthesis and development of other related compounds that may have a broader antiviral spectrum. The work presented here has identified novel diketopiperazines (KCN-2,2’-epi-19, KCN-19, and KCN-21) that inhibit EV-D68. They have previously been reported by us to inhibit poliovirus (Nicolaou et al., 2012), thus these compounds deserve further investigation as anti-picornavirus agents. Many of the compounds studied here are not being pursued for further development but they should be. Because of the medical need for treating enterovirus infections, these compounds could serve as a basis for further pre-clinical discovery.
Highlights.
Ten compounds were evaluated for inhibition of EV-D68, EV-71 and RV-87.
Nine compounds inhibited EV-D68 and RV-87, with rupintrivir being most active.
Three novel epidithiodiketopiperazines were among the active inhibitors of EV-D68.
Inhibition of EV-D68 was confirmed in three cell lines and by virus yield assays.
Antiviral profiles support classifying EV-D68 with RV-87.
Acknowledgements
This project has been funded in part with Federal funds from the Virology Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Numbers HHSN272201100019I/HHSN27200009/B16 and HHSN272201100019I/HHSN27200011/B18. The opinions expressed herein are those of the authors and not of the Federal Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen LB, Huffman JH, Dan Cook P, Meyer RB, Jr., Robins RK, Sidwell RW. Antiviral activity of 3-deazaguanine, 3-deazaguanosine, and 3-deazaguanylic acid. Antimicrob. Agents Chemother. 1977;12:114–119. doi: 10.1128/aac.12.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard DL, Bischofberger N, Kim CU, Huffman JH, Sidwell RW, Dougherty JP, Lew W, Williams MA, Yang W. Acyclic phosphonomethylether nucleoside inhibitors of respiratory viruses. Antivir. Chem. Chemother. 1997;8:223–233. [Google Scholar]
- Barnard DL, Hubbard VD, Smee DF, Sidwell RW, Watson KG, Tucker SP, Reece PA. In vitro activity of expanded-spectrum pyridazinyl oxime ethers related to pirodavir: novel capsid-binding inhibitors with potent antipicornavirus activity. Antimicrob. Agents Chemother. 2004;48:1766–1772. doi: 10.1128/AAC.48.5.1766-1772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Microbiol. 2002;40:4218–4223. doi: 10.1128/JCM.40.11.4218-4223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Augsburger P, Vance LM, Malcolm SK, Hsiung H, Smith DP, Heinz BA. Evidence that enviroxime targets multiple components of the rhinovirus 14 replication complex. Arch. Virol. 1999;144:1569–1585. doi: 10.1007/s007050050611. [DOI] [PubMed] [Google Scholar]
- Caine EA, Partidos CD, Santangelo JD, Osorio JE. Adaptation of enterovirus 71 to adult interferon deficient mice. PLoS One. 2013;8:e59501. doi: 10.1371/journal.pone.0059501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Yu CK, Wang YF, Liu CC, Su IJ, Lei HY. A murine oral enterovirus 71 infection model with central nervous system involvement. J. Gen. Virol. 2004;85:69–77. doi: 10.1099/vir.0.19423-0. [DOI] [PubMed] [Google Scholar]
- Christian KA, Ijaz K, Dowell SF, Chow CC, Chitale RA, Bresee JS, Mintz E, Pallansch MA, Wassilak S, McCray E, Arthur RR. What we are watching--five top global infectious disease threats, 2012: a perspective from CDC's Global Disease Detection Operations Center. Emerg. Health Threats J. 2013;6:20632. doi: 10.3402/ehtj.v6i0.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, Patick AK, Matthews DA, Lee CA, Ford CE, Burke BJ, Rejto PA, Hendrickson TF, Tuntland T, Brown EL, Meador JW, 3rd, Ferre RA, Harr JE, Kosa MB, Worland ST. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements. J. Med. Chem. 1999;42:1213–1224. doi: 10.1021/jm9805384. [DOI] [PubMed] [Google Scholar]
- Eggers HJ. Successful treatment of enterovirus-infected mice by 2-(alpha-hydroxybenzyl)-benzimidazole and guanidine. J. Exp. Med. 1976;143:1367–1381. doi: 10.1084/jem.143.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Yuan S, Zhang C, Wang Y, Wang Y, He G, Zhang S, Altmeyer R, Zou G. Discovery of itraconazole with broad-spectrum in vitro antienterovirus activity that targets nonstructural protein 3A. Antimicrob. Agents Chemother. 2015;59:2654–2665. doi: 10.1128/AAC.05108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greninger AL, Naccache SN, Messacar K, Clayton A, Yu G, Somasekar S, Federman S, Stryke D, Anderson C, Yagi S, Messenger S, Wadford D, Xia D, Watt JP, Van Haren K, Dominguez SR, Glaser C, Aldrovandi G, Chiu CY. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012-14): a retrospective cohort study. Lancet Infect. Dis. 2015;15:671–682. doi: 10.1016/S1473-3099(15)70093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz BA, Vance LM. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J. Virol. 1995;69:4189–4197. doi: 10.1128/jvi.69.7.4189-4197.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann EC, Jr., Herrmann JA, Delong DC. Prevention of death in mice infected with coxsackievirus A16 using guanidine HCl mixed with substituted benzimidazoles. Antiviral Res. 1982;2:339–346. doi: 10.1016/0166-3542(82)90003-1. [DOI] [PubMed] [Google Scholar]
- Kaiser L, Crump CE, Hayden FG. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antiviral Res. 2000;47:215–220. doi: 10.1016/s0166-3542(00)00106-6. [DOI] [PubMed] [Google Scholar]
- Khan F. Enterovirus D68: acute respiratory illness and the 2014 outbreak. Emerg. Med. Clin. North Am. 2015;33:e19–32. doi: 10.1016/j.emc.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Khong WX, Yan B, Yeo H, Tan EL, Lee JJ, Ng JK, Chow VT, Alonso S. A non-mouse-adapted enterovirus 71 (EV71) strain exhibits neurotropism, causing neurological manifestations in a novel mouse model of EV71 infection. J. Virol. 2012;86:2121–2131. doi: 10.1128/JVI.06103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Shih SR. Cell and tissue tropism of enterovirus 71 and other enteroviruses infections. J. Biomed. Sci. 2014;21:18. doi: 10.1186/1423-0127-21-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sheng J, Fokine A, Meng G, Shin WH, Long F, Kuhn RJ, Kihara D, Rossmann MG. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science. 2015;347:71–74. doi: 10.1126/science.1261962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Midgley CM, Jackson MA, Selvarangan R, Turabelidze G, Obringer E, Johnson D, Giles BL, Patel A, Echols F, Oberste MS, Nix WA, Watson JT, Gerber SI. Severe respiratory illness associated with enterovirus D68 - Missouri and Illinois, 2014. MMWR Morb. Mortal. Wkly. Rep. 2014;63:798–799. [PMC free article] [PubMed] [Google Scholar]
- Nicolaou KC, Lu M, Totokotsopoulos S, Heretsch P, Giguere D, Sun YP, Sarlah D, Nguyen TH, Wolf IC, Smee DF, Day CW, Bopp S, Winzeler EA. Synthesis and biological evaluation of epidithio-, epitetrathio-, and bis-(methylthio)diketopiperazines: synthetic methodology, enantioselective total synthesis of epicoccin G, 8,8′-epi-ent-rostratin B, gliotoxin, gliotoxin G, emethallicin E, and haematocin and discovery of new antiviral and antimalarial agents. J. Am. Chem. Soc. 2012;134:17320–17332. doi: 10.1021/ja308429f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, Sessions W, Kirk C, Chatterjee N, Fuller S, Hanauer JM, Pallansch MA. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J. Gen. Virol. 2004;85:2577–2584. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9:1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- Patick AK, Binford SL, Brothers MA, Jackson RL, Ford CE, Diem MD, Maldonado F, Dragovich PS, Zhou R, Prins TJ, Fuhrman SA, Meador JW, Zalman LS, Matthews DA, Worland ST. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 1999;43:2444–2450. doi: 10.1128/aac.43.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–498. [Google Scholar]
- Rhoden E, Zhang M, Nix WA, Oberste MS. In vitro efficacy of antiviral compounds against enterovirus D68. Antimicrob. Agents Chemother. 2015 doi: 10.1128/AAC.00766-15. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Pereira J, Nascimento MS, Ma Q, Hilgenfeld R, Neyts J, Jochmans D. The enterovirus protease inhibitor rupintrivir exerts cross-genotypic anti-norovirus activity and clears cells from the norovirus replicon. Antimicrob. Agents Chemother. 2014;58:4675–4681. doi: 10.1128/AAC.02546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghipour S, Bek EJ, McMinn PC. Selection and characterisation of guanidine-resistant mutants of human enterovirus 71. Virus Res. 2012;169:72–79. doi: 10.1016/j.virusres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Sadeghipour S, Bek EJ, McMinn PC. Ribavirin-resistant mutants of human enterovirus 71 express a high replication fidelity phenotype during growth in cell culture. J. Virol. 2013;87:1759–1769. doi: 10.1128/JVI.02139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am. J. Epidemiol. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. Journal Infect. Dis. 1974;129:304–309. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- Streeter DG, Witkowski JT, Khare GP, Sidwell RW, Bauer RJ, Robins RK, Simon LN. Mechanism of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. USA. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Meijer A, Froeyen M, Zhang L, Thibaut HJ, Baggen J, George S, Vernachio J, van Kuppeveld FJ, Leyssen P, Hilgenfeld R, Neyts J, Delang L. Antiviral activity of broad-spectrum and enterovirus-specific inhibitors against clinical isolates of enterovirus D68. Antimicrob. Agents Chemother. 2015 doi: 10.1128/AAC.01375-15. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsma A, Franco D, Tucker S, Hilgenfeld R, Froeyen M, Leyssen P, Neyts J. The capsid binder Vapendavir and the novel protease inhibitor SG85 inhibit enterovirus 71 replication. Antimicrob. Agents Chemother. 2014;58:6990–6992. doi: 10.1128/AAC.03328-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CK, Chen CC, Chen CL, Wang JR, Liu CC, Yan JJ, Su IJ. Neutralizing antibody provided protection against enterovirus type 71 lethal challenge in neonatal mice. J. Biomed. Sci. 2000;7:523–528. doi: 10.1007/BF02253368. [DOI] [PubMed] [Google Scholar]
- Zhang X, Song Z, Qin B, Zhang X, Chen L, Hu Y, Yuan Z. Rupintrivir is a promising candidate for treating severe cases of enterovirus-71 infection: evaluation of antiviral efficacy in a murine infection model. Antiviral Res. 2013;97:264–269. doi: 10.1016/j.antiviral.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Zhang XN, Song ZG, Jiang T, Shi BS, Hu YW, Yuan ZH. Rupintrivir is a promising candidate for treating severe cases of Enterovirus-71 infection. World J. Gastroenterol. 2010;16:201–209. doi: 10.3748/wjg.v16.i2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]