Summary
Background
In this study, we sought to assess whether a social network intervention was superior to HIV testing and counseling in impacting HIV incidence among PWID. Although this was not a primary study aim, it is associated with reducing drug and sex risk behaviors, which were primary aims.
Methods
PWID were recruited from street settings in Odessa, Donetsk, and Nikolayev, Ukraine for a clustered randomized clinical trial (RCT). “Index” or peer leaders, along with two of their network members, were randomly assigned to testing and counseling block (N=589) or testing and counseling plus a social network intervention block (N=611). Participants in the network intervention received 5-sessions to train their network members in risk reduction. Those assigned testing and counseling received no further intervention following counseling. Employing an intent to treat analyses, the primary outcome was HIV sero-conversion using Cox regression and incorporating a gamma frailty term to account for clustering. No stratification or minimization was utilized. The trail was registered with ClinicalTrial.gov, NCT01159704.
Findings
Between July 12, 2010 and November 23, 2012, 2,304 PWID were recruited, 1,200 of whom were HIV negative and included in the present study. At baseline, there were no significant differences between groups. Of the 1,200 HIV negative participants, 1,085 (90.4%) were retained at 12 months. Incidence density revealed 18.45 (95% CI 14.87 – 22.03, 102 events in 553.0 py) per 100 person years (py) for those in the intervention group and 31.78 (95% CI 26.83–36.74, 158 events in 497.1 py) per 100 py among control arm participants. This corresponded to a reduced hazard in the intervention group, HR= 0.53 (95% CI 0.38, 0.76, p =0.0003). There were no study-related adverse events.
Interpretation
These data provide strong support for integrating peer education into comprehensive HIV prevention programs for PWID and suggest the value in developing and testing peer-led interventions for improving access and adherence to PrEP and ART.
Funding
This study was funded by the National Institute on Drug Abuse (RO1 DA026739).
Introduction
Although worldwide the HIV epidemic is declining, in Eastern Europe and Central Asia it continues to increase. UNAIDS indicates that new HIV infections fell 38% since 2001, from 3.4 million [95% CI 3.3 – 3.6 million] in 2001 to 2.1 million in 2013 [95% CI 1.9 – 2.4 million] [1]. However, in Eastern Europe and Central Asia, new infections rose by 5% between 2005 and 2013 to 110,000 in 2013 [95% CI 86,000 – 130,000].
In 1995, the WHO characterized Ukraine as a country of low prevalence, [2] yet within two years all 27 regional capitals reported cases of HIV [3]. In 2011, the WHO and UNAIDS estimated that there were 230,000 [95% CI 180,000 – 310,000] people living with HIV in Ukraine [4]. At the end of 2014, Ukrainian officials reported a total of 264,489 HIV cases [5]. Similar to the United States and elsewhere, the HIV epidemic is unevenly distributed across Ukraine. Southern and eastern oblasts have approximately three times the HIV prevalence than the rest of Ukraine [6]. By 1997, people who inject drugs (PWID) accounted for nearly 85% of all infections [7]. Since 2008, sexual transmission of HIV has accounted for the majority of new infections [8,9], however, most of them are likely linked to PWID [10].
In 2010, we initiated an experimental randomized controlled trial comparing a social network intervention, combined with HIV testing and counseling, with testing and counseling alone targeting PWID in three south-eastern cities in Ukraine: Odessa, Donetsk, and Nikolayev. In a recent national survey, HIV prevalence among PWID in Ukraine was estimated to be 19.1%, including 30.2% in Odessa, 26.5% in Donetsk, and 31.8% in Nikolayev [11]. We selected a social network intervention approach since our prior experience suggested that PWIDs in Ukraine tended to have small, stable networks, and typically prepared drug solutions and injected together [12–14]. Network characteristics, including size and the length of time members have known one another, have been associated with drug and sex-related risk behaviors, as well as HIV serostatus [15,16]. Empowering active users to influence network members’ risk behaviors is a potentially low cost approach to promoting behavior change within difficult to reach populations. The intervention was based on theories of social norms, cognitive dissonance, social diffusion, social identity, and role theory [17–19]. The present investigation was designed to assess if peer educators could influence the injection and sex risk behaviors of members of their social network leading to reduced HIV incidence relative to those receiving the standard of care in Ukraine.
Methods
Study Design
We employed a randomized clinical trial (RCT) comparing experimental and control conditions with an enrollment ratio of 1:1. At each site, outreach workers employed by non-government organizations (NGOs) recruited peer educators or “index” participants. In addition to other eligibility criteria (see below), indexes were required to bring in two members of their injecting network to be eligible. When 16 eligible indexes were recruited, they were randomized along with their network members, to one of the two study arms in a 1:1 ratio using a blocked randomization scheme to ensure that balance between the two intervention conditions was maintained over the course of the entire trial and that the assignment sequence was not predictable In the final sample, 589 HIV negative individuals were included in the control block and 611HIV negative individuals in the intervention block. No stratification or minimization techniques were used. A total of 256 indexes at each site were assigned to one of the two study arms, half of whom, or 128, were trained to be peer leaders. For those randomized to the HIV testing and counseling-only arm, no further intervention was planned for indexes or their network members. Network members in the social network condition plus counseling and testing also received no further intervention, as the model was based on indexes in this arm providing interventions to members of their network after they were trained. Study procedures were approved by the Institutional Review Board (IRB) of the University of Colorado Denver and by the IRB of the Ukrainian Institute on Public Health Policy.
Participants
NGOs in each of the three cities, Odessa, Donetsk, and Nikolayev, were located in areas with high concentrations of drug injectors, with staff familiar recruiting, interviewing, and intervening with PWID. Although recruitment was extended throughout all districts in each city, specific areas were targeted based on the NGO staff’s knowledge of where PWID congregated. Indexes were recruited by recovering drug users serving as outreach workers, an approach shown to be effective in recruiting PWID [20,21]. Potential indexes were initially screened for eligibility on the street by outreach workers and referred to NGO offices where eligibility was finalized by interviewers. Eligibility requirements included: 16 years of age or older; self-reported drug injection in the past 30 days; willingness to be interviewed for approximately 1-hour and tested for HIV; not too impaired to comprehend and provide informed consent; and, for this paper, testing HIV negative at baseline. Comprehension of informed consent was assessed using an 8-item questionnaire covering key items from the Consent Form. Indexes were also required to bring two members of their injecting network, who also met eligibility criteria, for study participation. Drug injection was verified through visual signs of recent injection. Although urinalysis was conducted, a positive sample was not required due to the frequent low quality of drugs in Ukraine, particularly opiates [12]. Participants were compensated the equivalent of $6.00 U.S. for their baseline interview, $7.00 for the 6-month interview, and $8.00 for the 12-month interview. In addition, indexes received the equivalent of $5.00 U.S. for each eligible network member they brought to the project, $2.00 for completing the Network Inventory, $4.00 for each of the five training sessions attended, and a $7.00 bonus if they attended all sessions.
Interviews were conducted using an audio computer-administered self-interview (ACASI) to minimize social desirability. The interview schedule was adapted from the Risk Behavior Assessment (RBA) developed during the Cooperative Agreement sponsored by the National Institute on Drug Abuse (NIDA). It assessed demographics, health history, criminal justice involvement, drug use, and injection- and sex-related risk behaviors. Reliability and validity assessments of the RBA support its use with PWIDs for this purpose [22,23]. We modified the instrument based on information from focus groups with drug users and dealers and a review by NGO staff. For example, a common method of obtaining drugs in Ukraine is by purchasing pre-loaded syringes. Russian translation was conducted for both the questionnaire and consent form by an IRB-certified translator and verified. Following the interview, participants were provided HIV testing, using the HIV I + II One-Step Test finger-stick rapid test (Orgenics Ltd., Yavne, Israel) registered by the Ministry of Health.
There were 16 phases of recruitment, each lasting 8 weeks, with four weeks dedicated to recruitment of indexes and their two network members, one week of randomization and informing indexes in the experimental condition of the training schedule, two weeks of intervention training, and a week planning for the next phase. Overall, recruitment took 64 weeks, beginning July 12, 2010 and extending to November 23, 2012. Participants were re-interviewed and tested for HIV at 6 and 12 months.
Randomization and masking
Randomization was conducted by the study statistician, a member of the U.S. team, who e-mailed subject numbers to the Ukraine data coordinator allocating participants to the two conditions. Randomization occurred after participants received their baseline interview and after HIV testing and counseling, hence masking was not necessary.
Procedures
At the beginning of the project, a one-week centralized training was held in Yalta for all staff from the three NGOs, including outreach workers, interviewers, HIV testers/counselors and Directors. The training was conducted by the U.S. team and included a detailed presentation of the research protocol and of Good Research Practices (GRP) for all staff. Following this, separate trainings were held for interviewers, outreach workers, and HIV counselors.
HIV Testing and Counseling
The testing and counseling intervention, Ukraine’s standard of care, was an updated version of the Counseling and Education (C & E) model developed during NIDA’s Cooperative Agreement [24]. The manual was updated based on the HIV rapid test. In the pre-test counseling session, a series of cue cards describing basic information about HIV/AIDS and how to reduce HIV transmission were discussed with the participant. The content of the cue cards was modified from the original C & E model based on the injection practices of Ukraine drug users. Participants rehearsed how to clean injection equipment and how to use a condom with an anatomical model. HIV test results were then provided and additional cue cards presented based on the test results. Those testing positive were provided a list of HIV service agencies and referred to the AIDS Centre in their city for confirmation of test results.
The C & E intervention was selected for the comparison condition primarily because in an earlier study we conducted in Ukraine, findings reveled that participants in the C & E-only arm reduced their HIV-related drug and sex risk behaviors as much as those receiving an intensive individually-focused intervention plus the C & E model [25]. This lack of significant differences between more intensive and sophisticated interventions and the C & E alone was similar to that found during NIDA’s Cooperative Agreement across multiple sites [26,27].
Peer Leader Intervention
This intervention was developed by Latkin and colleagues [17,19]. Intervention training consisted of 5 sessions delivered in small groups over a two-week period designed to motivate peer leaders to become educators within their injection network and provide them with skills training in how to effectively teach their network members HIV risk reduction behaviors. Peer leaders were encouraged to model safe behaviors with their network members. Training sessions consisted of role-playing and other interactive learning techniques. The initial sessions included discussions of how HIV was affecting their community and the role they could play in reducing transmission. The sessions included exercises on how and when to talk with network members about HIV risk reduction. Role-plays focused on problem solving scenarios, especially overcoming barriers to risk reduction. At the end of each session (lasting approximately 90 minutes) peer leaders were provided outreach assignments to conduct with their network members. In the subsequent session they were asked to discuss their experiences, with the group helping to address issues that may have arisen. The final session included a graduation ceremony. Outreach workers from the NGOs were expected to conduct similar five-session trainings with peer leaders once the recruitment began. No intervention sessions were to occur with leaders after the final training session and none with network members.
Statistical analysis
Using an intent-to-treat design, the primary outcome measure was differences in HIV incidence between intervention arms (referred to in the following as C & E only and C & E plus). HIV incidence was not a primary outcome in the original study design that sought to examine changes in HIV risk behaviors resulting from the intervention. However, given the high prevalence of HIV among PWID in this region, and a large population of HIV negative individuals recruited at baseline, we were able to utilize an opportunistic approach to track HIV incidence and how it may be associated with the intervention and HIV risks. Sample size estimates were based on an earlier 5-year grant we had in Ukraine, as well as a pilot study, targeting the same HIV-related risk behaviors. Allowing for an estimated 10% loss to follow-up at 6-months and 15% at 12-months, 125 index participants in each arm at each location provided adequate power (80–90%) to detect differences of 20% or more in rates of endorsing key behaviors between interventions. To assess possible differences due to lost to follow-up between groups we developed a Generalized Estimating Equation (GEE) logistic regression model. This model utilized an exchangeable error structure to account for correlations within peer networks. Baseline predictors of attrition were explored and tested for differences between the two groups. Interactions by intervention arm were also explored to examine behaviors related to attrition that may have been unique to each intervention. Participation at 12-months was the binomial outcome of interest, with a saturated model of covariates utilized along with backwards selection to arrive at a parsimonious model. Additionally, the success of randomization was evaluated between the intervention and comparison conditions using t-tests or chi-square, as appropriate.
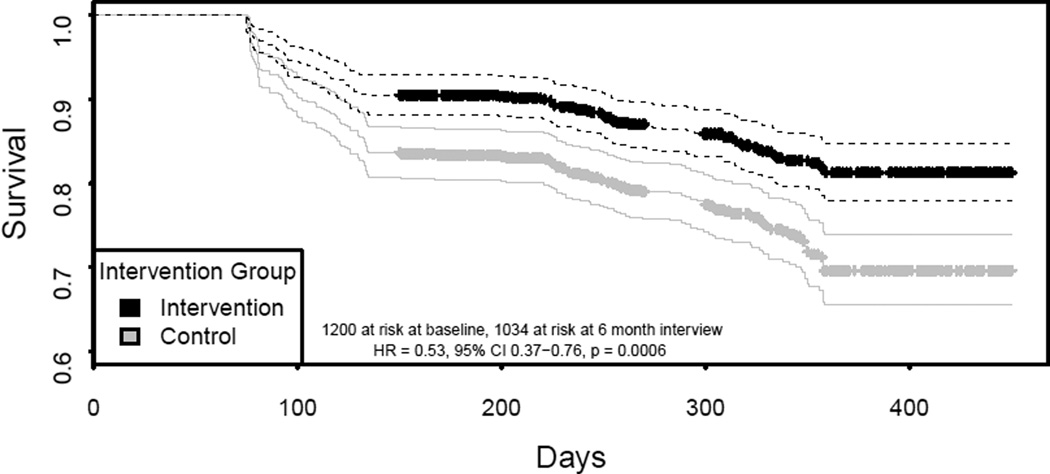
The date of HIV seroconversion was estimated to be the midpoint between an individual’s last negative and first positive test. Overall incidence density (ID), and ID stratified by intervention group, are reported and 95% confidence intervals (CI) calculated using normal approximation given the frequent events (Table 2). Figure 1 presents Kaplan Meier survival curves of groups and a log rank test to test differences between groups. At baseline, 1200 were at risk and, at the 6 month interview, 1034 were at risk. Intra-class correlation (ICC) of networks was estimated utilizing a linear random effects for peer networks model with person-time as the offset and HIV event as the outcome.
Table 2.
| a: Time invariant characteristics |
Overall (baseline) |
HIV + | Remained HIV− | Unadjusted HR (95%CI) | |
|---|---|---|---|---|---|
| Control | 589 (49.1%) | 150 | 385 | Ref | |
| Intervention | 611 (51.0%) | 94 | 456 | 0.53 (0.37,0.76) p=0.0006 | |
| City | |||||
| Odessa | 421 (35.1%) | 98 | 299 | 0.96 (0.62, 1.51) p=0.87 | |
| Donetsk | 363 (30.3%) | 93 | 214 | Ref | |
| Nikolayev | 416 (34.7%) | 53 | 328 | 0.36 (0.23, 0.56) p<0.0001 | |
| Sex | |||||
| Male | 899 (74.9%) | 179 | 630 | Ref | |
| Female | 301 (25.1%) | 65 | 211 | 1.28 (0.92, 1.80) p=0.15 | |
| b: Time Varying Exposures |
Overall (baseline) |
HIV + | Remained HIV− | Unadjusted HR (95%CI) | |
|---|---|---|---|---|---|
| Mean Age (sd) | 31.81 (8.36) | 35.02 (7.72) | 31.73 (8.48) | 1.05 (1.03, 1.07) p =0.0016 | |
| Daily Injector | |||||
| yes | 520 (43.3%) | 120 | 310 | 1.50 (1.11, 2.02) p=0.007 | |
| no | 680 (56.7%) | 128 | 523 | Ref | |
| Always Inject with others | |||||
| yes | 584 (48.7%) | 99 | 326 | 1.00 (0.75, 1.30) p=0.92 | |
| no | 616 (51.3%) | 149 | 507 | Ref | |
| Mean Years of injecting (sd) |
11.65 (8.97) | 14.88 (8.34) | 11.54 (9.16) | 1.05 (1.03, 1.07) p<0.0001 | |
| Log Injection frequency mean (sd) |
3.04 (0.89) | 3.11 (1.20) | 2.92 (1.01) | 1.10 (1.04, 1.16) p=0.0004* | |
| Common Container (N=1199) |
|||||
| Yes | 464 (38.7%) | 94 | 332 | 1.06 (0.77, 1.45) p=0.74 | |
| no | 735 (61.3%) | 154 | 500 | Ref | |
| Front and Back Loading with others |
|||||
| Yes | 863 (71.9%) | 198 | 605 | 1.59 (1.11, 2.28) p=0.012 | |
| No | 337 (28.1%) | 50 | 227 | Ref | |
| Front back loading with dealer and/ or others |
|||||
| Yes | 1162 (96.8%) | 242 | 799 | 6.20 (1.45, 26.5.9) p=0.014 | |
| No | 38 (3.2%) | 6 | 34 | Ref | |
| Shared Works (N=1199) | |||||
| Yes | 341 (28.4%) | 88 | 274 | 1.42 (1.02, 2.00) p=0.04 | |
| No | 858 (71.6%) | 160 | 559 | Ref | |
| Mean overall drug risk score (sd) |
1.59 (0.90) | 1.57 (0.94) | 1.52 (0.89) | 1.13 (0.95, 1.33) p=0.18 | |
| More than one sexual partner (N=1194) |
|||||
| Yes | 267 (22.3%) | 157 | 673 | 0.61 (0.45, 0.84) p=0.003 | |
| No | 927 (77.6%) | 91 | 160 | Ref | |
| Unprotected Sex (N=1180) |
|||||
| Yes | 481(41.0%) | 87 | 292 | 1.07 (0.80, 1.43) p=0.63 | |
| No | 699 (59.3%) | 161 | 536 | Ref | |
| Sex with an injection drug user (N=1185) |
|||||
| Yes | 493 (41.6%) | 105 | 399 | 0.92 (0.67, 1.25) p=0.59 | |
| No | 692 (58.4%) | 141 | 424 | ref | |
| Sex with HIV positive | |||||
| Yes | 31 (2.6%) | 11 | 22 | 1.05 (0.43, 2.54) p=0.92 | |
| No | 1169 (97.4%) | 237 | 811 | ref | |
| Sex for trade (N=1197) | |||||
| Yes | 25 (2.1%) | 3 | 20 | 0.88 (0.32, 2.43) p=0.81 | |
| No | 1172 (97.9%) | 245 | 813 | ref | |
a) Time invariant characteristics are presented for final visit. Totals will not sum to total baseline numbers due to follow up. Total denominator is 1200 unless otherwise reported.
b) Time varying exposures are presented for 6 month visit to parallel the lagged cox regression analysis. Injection frequency is right tailed and log transformed.
per 10 injections
Figure 1.
A Cox proportional hazards model with a gamma frailty term was utilized to identify predictors of HIV seroconversion and to test HIV hazard differences between groups. The gamma frailty term was used to fit a random intercept for peer networks. The proportional hazard assumption was assessed by visual inspection, chi-square analysis of Schoenfeld residual trends, and interactions with time. Time varying exposures, such as injection frequency, were lagged; for example, behaviors reported at baseline were assumed to be the exposure for an HIV seroconversion event occurring between the baseline and 6 month visit.
The primary test of interest in the multivariate Cox regression was HIV hazard ratio differences between intervention arms. Covariates were explored manually using forward selection, including age and gender, as well as HIV drug and sex risk behaviors. Variables examined at both baseline and follow-up, which assessed the 30 day period before the interview, included: always injecting with others; frequency of injecting; years injecting; sharing injection paraphernalia; using needles/syringes known to have been used by another injector; more than one sex partner; unprotected sex; sex with another PWID; sex with someone known to have been HIV positive or whose HIV status was unknown; and, sex for trade. Sex between men was reported too infrequently to be included. All covariates met the proportional hazard assumption and were significant at an alpha of less than 0.05. No corrections for multiple comparisons were made. R version 3.0.1, the survival package, and the geepack package were utilized for analyses [28]. There was no data monitoring committee. The study was registered at ClinicalTrial.gov, a service of the U.S. National Institutes of Health (NCT01159704).
Role of the funding source
The sponsor of the study had no role in the study’s design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between July 12, 2010 and November 23, 2012, 2,304 PWID were recruited into the study, 1,200 of whom were HIV negative and included in these analyses. Six month follow-ups occurred from 07/14/2010 to 11/07/2012 and 12-month follow-ups from 07/13/2010 to 11/23/2012. Of the 1,200 negative participants, 1085 (90.4%) were retained at 12 months. Overall, 40.4% of those interviewed at baseline were HIV positive (931 of 2131 where 173 were not followed and missing HIV status). PWID who tested positive were referred to the local AIDS Centre for further testing and, if confirmed positive, treatment. At baseline, the cohort was 75.0% (N = 899) male, with an average age of 31.8 years and an average age of 20.2 at first injection. There were no statistically significant differences between intervention and comparison groups. The ICC of peer networks was estimated to be 0.22, suggesting a level of correlation of HIV events within individuals in shared peer networks.
At the 12 month follow-up, there were 260 HIV seroconversions among 1,050.1person years. Overall density during the study was 24.8 (95% CI 21.8 – 27.8) events per 100 person-years (py). In the C & E only arm, HIV incidence density was 31.9 (95% CI 26.8 – 36.7, 158 events in 497.1 py) per 100 py, while in the C & E plus arm it was 18.4 (95% CI 14.8 – 22.0, 102 events in 553.0 py) per 100 py. Figure 1 shows a Kaplan Meyer survival curve, which reflects a 42.1% reduction in HIV incidence graphically depicted in the C & E plus intervention arm relative to the C&E only arm (p<0.001 log rank test).
Results of the univariate hazard ratio (HR) analyses of HIV drug and sex risk behaviors are shown on Table 2. This was a randomized clinical trial that investigated the effects of a behavioral intervention on HIV incidence in 1200 HIV negative PWID over 12 months. Importantly, C & E plus had a marked 47% reduced HR compared to the C & E only arm.
Table 3 displays multivariate Cox proportional hazards regression results. Notably, after adjustment for significant HIV drug and sex risk factors, a significant 47% reduction in HIV hazard was retained in the C & E plus arm. Increased injection frequency and increased age were associated with increased hazard of HIV.. Meanwhile important differences in HIV hazard were found among the cities where, relative to Donetsk, both Odessa and Nikolayev had reduced HIV hazard.
Table 3.
Reduced multivariate cox proportional hazard model
| HR | 95% CI | p-value | |
|---|---|---|---|
| Intervention | 0.53 | (0.38, 0.75) | 0.0003 |
| Injection frequency | 1.06 | (1.007, 1.12 | 0.026 |
| Age | 1.06 | (1.03, 1.06) | <0.0001 |
| Odessa* | 0.62 | (0.41, 0.94) | 0.030 |
| Nikolayev* | 0.30 | (0.20, 0.48) | <0.0001 |
Verses Donetsk
Overall, including those with incident HIV during the study, attrition was similar between groups 10.1% (N = 62 of 611) C & E plus, and 9.2% (N = 53 of 589) C & E only, p=0.63). Similarly 53 (8.7%) HIV negative individuals dropped from the C & E plus group and 46 (7.8%) from the C & E only group before the final HIV assay was conducted at the 12 month interview. Direct causes for individual dropouts are unknown however baseline predictors were identified. Two significant covariates were found related to attrition: city, where participants from Nikolayev (OR = 0.51, 95% CI 0.30 – 0.88, p = 0.02) and Odessa (OR = 0.39, 95% CI 0.21 – 0.70 p = 0.002) had lower odds of attrition than Donetsk; and, those who always injected with others had lower attrition (OR = 0.72, 95% CI 0.52 – 0.995, p = 0.047). No other predictors, including interaction terms by intervention arm, were significant (p> 0.15).
Discussion
We found that the peer leader network intervention condition (C & E plus) was associated with markedly reduced HIV incidence relative to the control condition (18.4 vs. 31.8 per 100 person years), which was associated with a 47% reduced HIV hazard in the C & E plus group. This is a stigmatized and marginalized population engaging in high drug and sex risk behaviors [29–31].,. These results replicate and expand on a similar intervention applied in St Petersburg Russia, where HIV incidence density was lowered from 19.6 to 7.8 per 100 person years [32], and further supports the utilization of peer-led interventions as a viable and economical mechanism for reducing HIV incidence among exceptionally risky and difficult to reach populations. A number of previous studies have supported this approach through observed changes in behavior. Specifically, the SHIELD and STEP studies in Baltimore [33–35] and the HIV Prevention Trials Network 037 study in Philadelphia [36], as well as studies in other areas of Ukraine [13,14], demonstrated the effectiveness of peer-led interventions in reducing HIV risk behaviors among PWID. Unlike these previous investigations, which focused on self-reported risk behaviors, this study demonstrated a strong statistically significant reduction in HIV incidence associated with assignment to the experimental condition.
We also observed a number of risk factors associated with HIV incidence. Increased injection frequency and increased age were associated with increased HIV risk in the multivariate analysis. However, the extent to which peer educators influenced injection and sex risks leading to reduced HIV incidence is unclear. Further planned analyses will address this topic and, hopefully, elucidate the effect of peer-led interventions on risk behaviors. Additionally, we observed reduced hazard of HIV in Odessa and Nikolayev versus Donetsk, warranting additional investigation. Reports of behavioral differences between other Ukraine cities, however, suggest that HIV risk behaviors among PWID, including the types of drugs injected as well as injection and sex behaviors, may differ across cities in Ukraine [37–39].
There are several limitations to consider when drawing inferences from the study. Primarily, other than HIV testing data, findings were based on self-report. While extensive research in PWID populations have demonstrated the utility of self-report [40,41], errors in self-report could have contributed to the lack of findings of specific behavioral changes regarding injection practices, such as injection frequency, that could help explain the HIV incidence reduction. Recall error should have been minimized, however, by the brief 30-day period participants were asked to remember. Importantly, there is no indication that the recall of behaviors was differential between arms, suggesting the differences detected are valid. Social desirability could also have influenced participants’ self-report, although this was the same in both conditions. Additionally, the recruitment strategy called for recruiting leaders in each city based on outreach workers’ knowledge or recommendations by members of the network. It is not possible to know how representative the samples were of PWID leaders or their standing within their network. Because of the street-recruitment approach utilized, the sample likely over-represents those willing to spend the time required to participate and motivated by the modest stipend. Another possible limitation is the difference we observed in HIV incidence and prevalence and that of other investigators who, generally, rely on “official” figures from government agencies in Ukraine for their HIV estimates [42]. Our samples were recruited on the street by NGO outreach workers who were former drug injectors. They had knowledge of where PWID congregated and the rapport necessary to establish trust and recruit index participants who were active drug injectors. They in turn recruited other active drug injectors. We verified drug use through inspection for recent venipuncture and urinalysis. Our experience over the past 15 years working in Ukraine is that most street users will not go to AIDS Centers or military recruitment facilities (for example), to be tested, as there is little benefit to be gained and much to lose (e.g., stigma, discrimination, etc.). Moreover, a recent study by J-I Cakalo, et al. found that misclassification of PWID occurred frequently [43]. The authors estimated that as many as 34.5% of men reported as heterosexual from 2005 to 2011 could have actually been PWID. Moreover, our samples were recruited from Odessa, Donetsk, and Nikolayev, located in the southern and eastern regions of Ukraine where, historically and today, rates of HIV are the highest in the country. Conversely, this study has notable strengths, including the outcome measure of HIV seroconversion and the randomized longitudinal clinical trial design with a large sample size. Finally, there were no study-related adverse events.
Despite the strong intervention effect observed in the peer network arm, HIV incidence was still unacceptably high, indicating that the intervention should be implemented alongside other HIV prevention interventions found to be effective. Further work leveraging the peer-led intervention’s ability to reach marginalized populations, combined with expanded access to needle-exchange programs (NEPs) and opioid agonist therapies (e.g., methadone and buprenorphine), HIV anti-retroviral treatment and pre-exposure prophylaxis, could lead to further reductions in HIV incidence and improve access and retention in HIV medical care. In light of the current conflict in eastern Ukraine, especially Donetsk, it is imperative that WHO and other international health organizations continue to assess the health of drug users and implement feasible and effective interventions.
Supplementary Material
Table 1.
Baseline unadjusted comparisons between intervention arms
| C & E plus | C & E only | |
|---|---|---|
| City | ||
| Odessa | 216 | 205 |
| Dontesk | 176 | 187 |
| Nikolayev | 219 | 197 |
| Gender | ||
| Male | 446 | 453 |
| Female | 165 | 136 |
| Age, Mean (sd) | 31.66 (7.96) | 31.96 (8.77) |
| Daily Injector | ||
| Yes | 274 | 246 |
| No | 337 | 343 |
| Always Inject with others | ||
| Yes | 294 | 290 |
| No | 317 | 299 |
| Years of Injecting, mean (sd) | 11.35 (8.79) | 11.96 (9.14) |
| Log 30 day Injection Frequency |
3.05 (0.90 | 3.03 (0.89) |
| *Common Container | ||
| Yes | 228 | 236 |
| No | 382 | 353 |
|
*Front and back loading with others |
||
| Yes | 433 | 430 |
| No | 178 | 159 |
| *Shared Works | ||
| Yes | 170 | 170 |
| No | 441 | 417 |
| Mean overall drug risk score | 1.56 (0.91) | 1.62 (0.88) |
| More than one sexual partner | ||
| Yes | 122 | 117 |
| No | 487 | 468 |
| Unprotected sex | ||
| Yes | 241 | 240 |
| No | 359 | 340 |
| Sex with an injection drug user |
||
| Yes | 255 | 238 |
| No | 350 | 342 |
| Sex with HIV positive partner | ||
| Yes | 15 | 16 |
| No | 595 | 574 |
Common container refers to groups of PWID each drawing the drug solution from the same jar or cup; front or back loading occurs when the needle or plunger, respectively, is removed from the syringe and the drug solution squirted in; shared works refers to sharing drug paraphernalia, such as cotton, cooker or water.
Panel: Research in context.
Systematic Review
Using electronic databases (International Social Network Analysis website, MEDLINE, PubMed, PsycINFO, Social Science Citation Index, and Web of Science), we conducted a literature search from January 1980 to January 2016 with terms of ‘substance use’, ‘drug use’, ‘injecting drug use’, ‘people who inject drugs’ ‘HIV’, ‘AIDS’ combined with the terms ‘social network’ and similar terms (‘sociometric’, ‘sociograms’, and ‘respondent driven sampling’). We identified 15 HIV prevention interventions targeting PWID; 11 studies included control groups, and 6 were randomized clinical trials. The prior evidence from these studies suggest that social network interventions could alter HIV risk behaviors among PWID. However, there was a dearth of evidence as to whether this approach would lead to a significant reduction in HIV incidence, as reductions in risk behaviors have not always translated into reductions in HIV incidence.
Added value of this study
This study suggests that social network interventions are a viable method to reduce new HIV infections among PWID and that active drug users can serve as effective change agents within their social networks to promote behavior changes that lead to reductions in HIV incidence.
Interpretation
These results, in combination with findings from other social network interventions, suggest that in addition to syringe exchange and opioid substitution programs, practitioners and policy makers should involve drug users in HIV prevention activities and train them to promote risk reduction and health promotion behaviors with their sex and injection partners. Future research should examine the feasibility and utility of involving active drug users and other key populations in HIV care and medication adherence programs as well.
Acknowledgments
This study was supported by the National Institute on Drug Abuse (grant RO1 DA026739). S. Strathdee was supported by a NIDA MERIT award (R37DA019829). We acknowledge the dedicated staff and directors who participated in this project, including Dimitry Kryzhko with Health of Nation in Makeyevka/Donetsk; Olga Kostyuk and Tatiana Semikop with Faith, Hope and Love in Odessa; and Elena Goryacheva with the Charity Foundation Vykhod in Nikolayev. Their commitment to preventing the further spread of HIV in their country is inspiring. We are also indebted to the drug users who agreed to participate and gave their time, without which we could not have conducted this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
R.E. Booth, C.A. Latkin, S. Dvoryak and J.T. Brewster were responsible for the proposal, securing funding, and drafting the protocol. R.E. Booth, Principal Investigator, supervised all aspects of the study. J.M. Davis was responsible for data cleaning, writing the statistical analysis plan, and conducting the statistical analyses. C.A. Latkin conceptualized the social network intervention and provided support for intervention development and implementation. S. Strathdee assisted with the analyses and data interpretation. S. Dvoryak and J.T. Brewster conducted intervention training and monitoring. O. Lisovska oversaw data collection at each NGO through quarterly site visits and monitored the trial during the course of intervention delivery. R.E. Booth, C.A. Latkin, J.M. Davis, S. Strathdee, and J.T. Brewster wrote the initial draft of the manuscript. All authors contributed to, and approved, the final manuscript.
Declaration of interests
We declare that we have no conflicts of interests.
Human Participant Protection
The study, including procedures for informed consent, was approved by the Colorado Multiple Institutional Review Board at the University of Colorado Denver and by the Ukrainian Institute on Public Health Policy.
Contributor Information
Robert E. Booth, Department of Psychiatry, University of Colorado Denver, Denver, Colorado, U.S.A. 1557 Ogden Street, Denver, CO, 80218.
Jonathan M. Davis, Department of Psychiatry, University of Colorado Denver, Denver, Colorado, U.S.A.
Sergey Dvoryak, Ukrainian Institute on Public Health Policy, Kiev, Ukraine.
John T. Brewster, Department of Psychiatry, University of Colorado Denver, Denver, Colorado, U.S.A.
Oksana Lisovska, Ukrainian Institute on Public Health Policy, Kiev, Ukraine.
Steffanie A. Strathdee, Global Health Institute, University of California San Diego Department of Medicine, San Diego, California, U.S.A..
Carl A. Latkin, Department of Health, Behavior and Society, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, U.S.A..
References
- 1.UNAIDS Fact Sheet. [Accessed from April 5, 2015];2014 http://search.unaids.org/search.asp?lg=en&search=hiv%20statistics%20in%20the%20world. [Google Scholar]
- 2.World Health Organization. The current global situation of the HIV/AIDS pandemic. Wkly Epidemiol Rec. 1995;70:355. [Google Scholar]
- 3.Khodakevich L, Dehne K. Inaugural Meeting of the Global Research Network on HIV Prevention in Drug-Using Populations. Geneva: 1998. Jun, HIV epidemics in drug using populations and increasing drug use in central and Eastern Europe. [Google Scholar]
- 4.WHO, UNAIDS, UNICEF. Monitoring and reporting on the health sector response to HIV/AIDS; Ukraine country report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 5.HIV infection in Ukraine Informational Bulletin. Issue 43. Kyiv: Ministry of Health of Ukraine, Ukrainian Center for Socially Dangerous Diseases Control, Gromashevsky Institute of Epidemiology and Infectious Diseases. 2015 [Google Scholar]
- 6.Ministry of Health Ukraine. HIV-infection in Ukraine: Information Bulletin no. 27. Ministry of Health Ukraine, Ukrainian AIDS Centre, L.V. Gromashevskogo; 2007. [Google Scholar]
- 7.UNAIDS/WHO. Joint United Nations Programme on HIV/AIDS: AIDS epidemic update. Geneva, Switzerland: 2002. Dec, [Google Scholar]
- 8.Kruglov YV, Kobyshcha YV, Salyuk T, Varetska O, Shakarishvili A, Saldanha VP. The most severe HIV epidemic in Europe: Ukrain’s national prevalence for 2007. Sex Transm. Infect. 2008;84(Suppl 1):i37–i41. doi: 10.1136/sti.2008.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. AIDS Epidemic Update: November 2009. New York: UNAIDS; 2010. [Google Scholar]
- 10.Vitek CR, Cakalo JI, Kruglov YV, Dumchev KV, Salyuk TO, Bozicevic I, et al. Slowing of the HIV epidemic in Ukraine: Evidence from case reporting and Key Population Surveys, 2005–2012. PloS one. 2014;9(9):e103657. doi: 10.1371/journal.pone.0103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International HIV/AIDS Alliance in Ukraine. Summary of the Analytical Report: Monitoring the Behavior and HIV-Infection Prevalence among People Who Inject Drugs as a Component of the HIV Second Generation Surveillance. 2014 [Google Scholar]
- 12.Booth RE, Kennedy JK, Brewster JT, Semerik O. Drug injectors and dealers in Odessa, Ukraine. Journal of Psychoactive Drugs. 2003;35:419–426. doi: 10.1080/02791072.2003.10400488. [DOI] [PubMed] [Google Scholar]
- 13.Booth RE, Lehman WE, Latkin CA, Brewster JT, Sinitsyna, Dvoryak S. Use of a peer leader intervention model to reduce needle-related risk behaviors among drug injectors in Ukraine. Journal of Drug Issues. 2009 Spring;:1945–1962. [Google Scholar]
- 14.Booth RE, Lehman WE, Latkin CS, Dvoryak S, Brewster JT, Royer MS, Sinitsyna L. Individual and network interventions with injection drug users in five Ukraine cities. American Journal of Public Health. 2011;101:336–343. doi: 10.2105/AJPH.2009.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latkin CA, Mandell W, Oziemkowska M, Vlahov D, Celentano DA. The relationship between sexual behavior, alcohol use, and personal network characteristics among injection drug users in Baltimore, MD. JAIDS. 1994;13:273–280. doi: 10.1097/00007435-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population group saturation. American Journal of Epidemiology. 2000;152:913–922. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 17.Latkin CA. Outreach in natural setting: the use of peer leaders for HIV prevention among injecting drug users’ networks. Public Health Reports. 1998;113:151–159. [PMC free article] [PubMed] [Google Scholar]
- 18.Latkin CA, Knowlton AR. New directions in HIV prevention among drug users - settings, norms, and pproaches to AIDS prevention (SNNAAP): a social influence approach. Advances in Medical Sociology. 2000;7:261–287. [Google Scholar]
- 19.Latkin CA, Mandell W, Vlahov D, Oziemkowska M, Celentano DD. The long term outcomes of a personal network-oriented intervention for injecting drug users: the SAFE Study. American Journal of Community Psychology. 1996;24:341–365. doi: 10.1007/BF02512026. [DOI] [PubMed] [Google Scholar]
- 20.Carlson R, Wang J, Siegal H, Falck R, Guo J. An Ethnographic Approach to Targeted Sampling: Problems and Solutions in AIDS Prevention Research among Injection Drug and Crack-Cocaine Users. Hum. Organ. 1994;53:279–286. [Google Scholar]
- 21.Wiebel W. Combining ethnographic and epidemiologic methods in targeted AIDS interventions: the Chicago model. In: Battjes RJ, Pick RW, editors. Needle Shar. Intraven. Drug Users Natl. Int. Perspect. Washington, D.C: US Printing Office; 1998. pp. 137–150. [PubMed] [Google Scholar]
- 22.Dowling-Guyer S, Others A. Reliability of drug users’ self-reported HIV risk behaviors and validity of self-reported recent drug use. Assessment. 1994;1:383–392. [Google Scholar]
- 23.Weatherby N, Needle R, Cesar H, Booth R, McCoy C, Watters J, et al. Validity of self-reported drug use among injection drug users and crack smokers recruited through street outreach. 1994:347–355. [Google Scholar]
- 24.Coyle SL. The NIDA HIV Counseling and Education Intervention Manual. Rockville, MD: National Institute on Drug Abuse; 1993. [Google Scholar]
- 25.Booth RE, Lehman WE, Dvoryak S, Brewster JT, Sinitsyna L. Interventions with injection drug users in Ukraine. Addiction. 2009;104:1864–1873. doi: 10.1111/j.1360-0443.2009.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booth RE, Kwiatkowski CF, Stephens RC. Effectiveness of HIV/AIDS interventions for out-of-treatment injection drug users. Journal of Psychoactive Drugs. 1998b;30:269–278. doi: 10.1080/02791072.1998.10399702. PMID:9798793. [DOI] [PubMed] [Google Scholar]
- 27.Stephens RC, Kwiatkowski CF, Booth RE. The impact of the NIDA Cooperative Agreement programs among crack and injection drug users. In: Levy JA, Stephens RC, McBride DC, editors. Advances in Medical Sociology. Stamford, CT: JAI Press; 2000. pp. 241–259. [Google Scholar]
- 28. https://cran.r-project/org/ [Google Scholar]
- 29.Booth RE, Lehman WE, Kwiatkowski CF, Brewster JT, Sinitsyna, Dvoryak S. Stimulant injectors in Ukraine: the next wave of the epidemic? AIDS and Behav. 2008;12:652–661. doi: 10.1007/s10461-008-9359-3. [DOI] [PubMed] [Google Scholar]
- 30.Booth RE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Predictors of HIV serostatus among drug injectors at three Ukraine sites. AIDS. 2006;20:1–7. doi: 10.1097/QAD.0b013e328010e019. [DOI] [PubMed] [Google Scholar]
- 31.Booth RE, Kwiatkowski CF, Mikulich-Gilbertson, Brewster JT, Salomsonsen-Sautel S, Corsi KF, Sinitsyna L. Predictors of risky needle use following interventions with drug injectors in Ukraine. Drug and Alcohol Dependence. 2006:S49–S57. doi: 10.1016/s0376-8716(06)80009-8. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman IF, Latkin CA, Kukhareva PV, Malov SV, Batluk JV, Shaboltas AV, Skochilov RV, Sokolov NV, Verevochkin SV, Hudgens MG, Kozlov AP. A peer-educator network HIV prevention intervention among injection drug users: results of a randomized controlled trial in St. Petersburg, Russia. AIDS Behav. 2013 Sep;17(7):2510–2520. doi: 10.1007/s10461-013-0563-4. PubMed PMID: 23881187; PubMed Central PMCID: PMC3950300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihailovic A, Tobin K, Latkin C. The Influence of a Peer-Based HIV Prevention intervention on conversation about HIV prevention among people who inject drugs in Baltimore, Maryland. AIDS Behav. 2015 Apr 7; doi: 10.1007/s10461-015-1048-4. [Epub ahead of print] PubMed PMID: 25845530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobin KE, Kuramoto SJ, Davey-Rothwell MA, Latkin CA. The STEP into Action study: a peer-based, personal risk network-focused HIV prevention intervention with injection drug users in Baltimore, Maryland. Addiction. 2011 Feb;106(2):366–375. doi: 10.1111/j.1360-0443.2010.03146.x. Epub 2010 Nov 4. PubMed PMID: 21054614; PubMed Central PMCID: PMC3049994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol. 2003 Jul;22(4):332–339. doi: 10.1037/0278-6133.22.4.332. PubMed PMID: 12940388. [DOI] [PubMed] [Google Scholar]
- 36.Latkin CA, Donnell D, Metzger D, Sherman S, Aramrattna A, Davis-Vogel A, Quan VM, Gandham S, Vongchak T, Perdue T, Celentano DD. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Soc Sci Med. 2009 Feb;68(4):740–748. doi: 10.1016/j.socscimed.2008.11.019. Epub 2008 Dec 13. PubMed PMID: 19070413; PubMed Central PMCID: PMC2724962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth RE, Mikulich-Gilbertson SK, Brewster JT, Salomonson-Sautel S, Semerik O. Predictors of self-reported HIV infection among drug injectors in Ukraine. JAIDS. 2004;35:82–88. doi: 10.1097/00126334-200401010-00012. [DOI] [PubMed] [Google Scholar]
- 38.Booth RE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Predictors of HIV serostatus among drug injectors at three Ukraine sites. AIDS. 2006;20:1–7. doi: 10.1097/QAD.0b013e328010e019. [DOI] [PubMed] [Google Scholar]
- 39.Booth RE, Davis JM, Brewster JT, Lisovska O, Dvoryak S. Krokodile injectors in Ukraine: Fueling the epidemic? AIDS and Behav. 2016;20:369–376. doi: 10.1007/s10461-015-1008-z. [DOI] [PubMed] [Google Scholar]
- 40.Maisto S, McKay J, Conners G. Self-reported issues in substance abuse: state of the art and future directions. Behav Assess. 1990;121:117–134. [Google Scholar]
- 41.Booth RE, Crowley TJ, Zhang Y. Substance abuse treatment entry, retention, and effectiveness: out-of-treatment opiate injection drug users. Drug Alcohol Depend. 1996;42:11–20. doi: 10.1016/0376-8716(96)01257-4. [DOI] [PubMed] [Google Scholar]
- 42.Vitek CR, Cakalo J-I, Kruglov YV, Dumchev KV, Salyuk TO, Bozicevic I, et al. Slowing of the HIV epidemic in Ukraine: Evidence from case reporting and key population surveys, 2005 – 2012. PLOS ONE. 2014 Sep;9(9):e103657. doi: 10.1371/journal.pone.0103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cakalo J-I, Bozicevic I, Vitek C, Mandel JS, Salyuk T, Rutherford GW. Misclassification of men with reported HIV infection in Ukraine. AIDS Behav. doi: 10.1007/s10461-015-1112-0. Published online DOI 10.1007/s10461-015-112-0: June 13, 2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.