Abstract
Activated neutrophils have been reported to affect peripheral resistance, for example, by plugging capillaries or adhering to the microvasculature. In vivo and ex vivo data indicate that activated neutrophils circulating in the blood also influence peripheral resistance. We used viscometry and microvascular mimics for in vitro corroboration. The rheological impact of differentiated neutrophil-like HL-60 promyelocytes (dHL60s) or human neutrophil suspensions stimulated with 10 nM fMet-Leu-Phe (fMLP) was quantified using a cone-plate rheometer (450 s−1 shear rate). To evaluate their impact on microscale flow resistance, we used 10-μm Isopore® membranes to model capillaries as well as single 200 × 50 μm microchannels and networks of twenty 20 × 50 μm microfluidic channels to mimic noncapillary microvasculature. Stimulation of dHL60 and neutrophil populations significantly altered their flow behavior as evidenced by their impact on suspension viscosity. Notably, hematocrit abrogated the impact of leukocyte activation on blood cell suspension viscosity. In micropore filters, activated cell suspensions enhanced flow resistance. This effect was further enhanced by the presence of erythrocytes. The resistance of our noncapillary microvascular mimics to flow of activated neutrophil suspensions was significantly increased only with hematocrit. Notably, it was elevated to a higher extent within the micronetwork chambers compared to the single-channel chambers. Collectively, our findings provide supportive evidence that activated neutrophils passing through the microcirculation may alter hemodynamic resistance due to their altered rheology in the noncapillary microvasculature. This effect is another way neutrophil activation due to chronic inflammation may, at least in part, contribute to the elevated hemodynamic resistance associated with cardiovascular diseases (e.g., hypertension and hypercholesterolemia).
Keywords: inflammation, viscosity, resistance, hematocrit, hemorrheology
1. Introduction
The transport of blood from the central circulation to the microvasculature drives the constant delivery of nutrients, gases, and signaling molecules to the peripheral tissues. Its regulation at various levels from the heart down to the microcirculation affirms the vital importance of the many redundant mechanisms that ensure efficient blood transport. The vascular wall endothelium is recognized as the primary modulator of blood flow via their control of arterial/arteriolar vasoactivity and their impact on leukocyte adhesion in the microcirculation [1]. However, the blood itself can also impact vascular flow.
The red blood cells (RBCs) have the dominant cellular effect on plasma viscosity due to their overwhelming numbers relative to the other cell types [2]. But, the neutrophils, which are the major leukocyte component, also have a rheological impact. This effect is related to the 4–500 μm diameter capillary and noncapillary vessels (i.e., arterioles, thoroughfare channels, and venules) that must accommodate the passage of neutrophils only 10–15 μm in diameter [3,4]. Under physiological (i.e., noninflamed) conditions, inactivated neutrophils are capable of efficiently “squeezing” through the capillaries and passing through the microvasculature as a result of their spherical, deformable, and nonadhesive state. However, upon activation, neutrophils undergo rapid (i.e., within seconds) changes in their shape, size, stiffness, and adhesivity while mounting an inflammatory response [5]. Notably, these changes in morphology and adhesivity impose a physical “side effect,” mainly on their flow in the microcirculation.
Inflammatory activation stimulates neutrophil adhesion to venular walls that, by effectively reducing cross-sectional area of the small vessels (i.e., venules) to flow, may raise microvascular resistance [6,7]. Interestingly, leukocytes from spontaneously hypertensive rats (SHRs) display features of an activated state (e.g., pseudopod projection), but they also exhibit reduced surface expression of CD18 integrins involved in capture by, and firm adhesion to, microvessel walls [8]. Despite this, SHRs display elevated microvascular resistance suggesting a way(s), other than cell adhesion to microvascular walls, by which chronically inflamed blood impairs tissue perfusion and contributes to organ injury [9].
To account for this, the influence of neutrophil pseudopod formation on blood rheology has been explored, particularly due to its reported effects on in vivo capillary flow [7]. When activated, neutrophils exhibit reduced capillary velocities due to their irregular shapes and increased stiffness [7,10]. This in turn causes an accumulation of erythrocytes upstream of the neutrophils leading to the disruption of a thin plasma lubrication layer that facilitates blood cell transport through the small capillaries and vessels [7,11]. In the extreme, activated neutrophils with pseudopod projections may plug capillaries. All of these likely raise microcirculatory resistance [6,10,12]. Along this line, previous studies using microporous filters or microfluidics-based capillary-like channels linked neutrophil activation to their reduced ability to pass through 4–6 μm diameter pores/channels, similar to those of the smallest capillaries [13–15]. The probability that these channels rapidly “plug up” during in vitro experiments, however, may have over-exaggerated the contribution of capillary plugging considering this phenomenon is typically not seen under physiological (i.e., healthy) conditions in vivo [16].
But there is another possible explanation for the association between activated neutrophils and elevated hemodynamic resistance in SHRs. Activated neutrophils in the blood may adversely impact microvascular resistance even without capillary plugging or microvascular adhesion. During flow, RBCs undergo “axial migration” to concentrate at the center of a flow field. This leaves a “cell-free” marginal zone adjacent to the vessel wall, resembling the plasma layer that lubricates blood cell passage through the capillaries [17,18]. As vessel diameters decrease, contributions from these low-viscosity peripheral zones become more prominent with the effect of reducing the apparent blood viscosity, i.e., the Fahraeus–Lindqvist effect [3,19,20]. The marginal layers measure only a few microns in thickness [21], so apparent viscosity is most affected in the smallest diameter vessels (i.e., <50 μm).
Interestingly, past ex vivo and in vivo evidence [22,23] points to a potential impact of cell activation on the rheological behavior of neutrophil suspensions flowing in the noncapillary microvasculature (e.g., venules, arterioles, etc.), which may lead to enhanced hemodynamic resistance. In contrast to inactivated cells that impose a minimal disturbance on red cell motion, it has been predicted, based on perfusion of neutrophil suspensions through skeletal muscle preparations, that activated pseudopod-projecting leukocytes with their irregular geometries likely tumble more in the bloodstream [23,24]. This, in turn, promotes their enhanced stochastic interactions with erythrocytes causing them to disturb the cell-free marginal zone and, thus, increase apparent blood viscosity and flow resistance in the microcirculation [7,23,24]. Surprisingly, there is little data, including in vitro evidence, to support that neutrophil pseudopod activity and its impact on blood rheology has a meaningful effect on microcirculatory resistance.
We conducted a series of in vitro experiments to further test the possibility that the altered rheological behavior of blood cell suspensions due to neutrophil activation significantly impacts microvascular-like flow. We used viscometry in conjunction with microfluidics-based microvascular mimics to relate neutrophil activation with their impact on the rheology of blood-like suspensions, which in turn affects microvessel flow resistance. We also explored the contribution of RBCs to the effects that neutrophil activation has on blood rheology and microvessel resistance. In this regard, we sought in vitro affirmation that, in the in vivo setting, sustained neutrophil activation in the blood may be one contributing factor that elevates peripheral hemodynamic resistance and contributes to microvascular pathobiology.
2. Experimental Methods
2.1. Cells.
HL-60 promyelocytes were purchased from the American Type Culture Collection (ATCC; cat. CCL-260), cultured under RPMI-1640 medium containing 10% fetal bovine serum (FBS; Hyclone) plus 1% penicillin/streptomycin/l-glutamine solution (Mediatech), and subcultured following the manufacturer's instruction. To induce a neutrophilic phenotype, HL-60 cells were differentiated by incubation with 1.25% dimethyl sulfoxide (DMSO; Sigma-Aldrich) for 5 days as reported [25,26]; we refer to these differentiated cells as dHL60 cells.
Fresh human blood was harvested from asymptomatic volunteers into K2-ethylenediaminetetraacetic acid (EDTA)-coated vacutainers (Becton-Dickinson, East Rutherford, NJ) by standard venipuncture following consent and blood collection procedures approved by the University of Kentucky Institutional Review Board (Lexington, KY). Neutrophils were isolated by two-step Histopaque–Percoll gradient centrifugation and subsequently resuspended in phosphate-buffered saline (PBS) as reported [27]. The resultant cell suspensions contained >90% neutrophils (data not shown). Finally, erythrocytes were harvested from whole blood by centrifugation at 150 × G for 5 min followed by three rinses in PBS.
2.2. Leukocyte Stimulation With fMLP.
Prior to morphometric and rheometric analyses, some dHL60 and human neutrophil populations were mildly stimulated by incubation in Hank's balanced salt solution (HBSS; 1:10 v/v; Invitrogen) containing 10 nM fMLP (Sigma Aldrich) for 5 min. Cell populations were then fixed in 1% p-formaldehyde in phosphate buffer for 30 min and subsequently rinsed in PBS in preparation for analyses.
2.3. Morphometric Analyses.
Aliquots of fixed dHL60 or human neutrophil suspensions were deposited on glass slides and the cells were imaged using an IX-70 inverted microscope (Olympus) under brightfield illumination at a 400× magnification. Three to seven random fields of cells were imaged and the shapes of the dHL60 or human neutrophils within these fields were manually traced using imagej software (National Institutes of Health). The circularity was determined for each traced cell as a morphological index of cell activation status.
2.4. Viscometric Analyses.
Suspensions of fixed dHL60s and primary neutrophils were subjected to a constant shear rate (450 s−1) in the absence or presence of autologous erythrocytes at 10% hematocrit for 10 min with a computer-interfaced DV-II+PRO digital cone-plate viscometer (Brookfield). Cone-plate viscometry was anticipated to provide a measure of neutrophil flow behavior since pseudopod extension due to cell activation is expected to enhance cell–cell interactions that, within the linear velocity gradient of the flow field, manifest as an increase in viscosity (Fig. 1(a)). The viscosities of cell suspensions during cone-plate rheometry were recorded at 30-s intervals for 5 min using wingather 32 software (Brookfield). To ensure data readings were stabilized, the viscometer was allowed to run for 30 s prior to data acquisition. During viscosity measurements, the cell suspensions were maintained at room temperature using a water-jacketed cooling system incorporated into the lower plate of the cone-plate system. For each experiment, the viscosities of the neutrophil suspensions were quantified by averaging measurements over the last 5 min of rheometry.
Fig. 1.
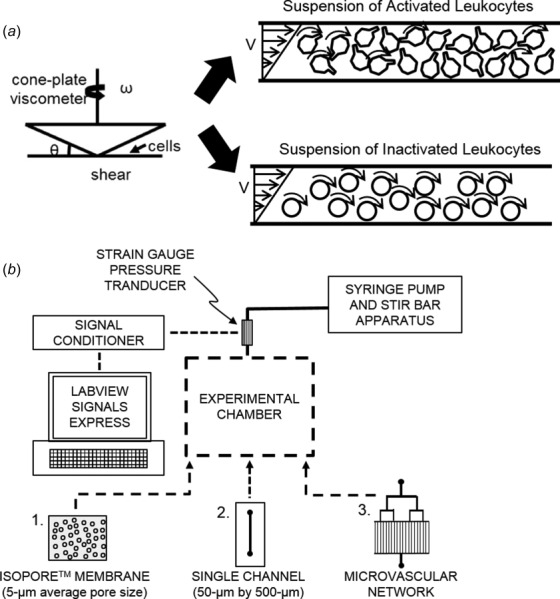
Schematics of the experimental flow setups used in the present study. (a) A cone-plate viscometer was used to measure the suspension viscosities of neutrophil populations under a uniform shear field. Activated neutrophils with extended pseudopods, in contrast to inactivated neutrophils, were anticipated to increase viscosity due to enhanced numbers of stochastic cell–cell interactions resulting from increased cell tumbling. (b) For flow studies, cells were perfused through three different experimental chambers designed to test the impact of neutrophil pseudopod activity on rheological flow properties. These chambers included (1) an isopore membrane with pore sizes of 10 μm to simulate microcapillary flow; (2) a single-channel microchamber (w: 500 μm; h: 50 μm; and l: 20 mm) to simulate flow through the large microvessels; and (3) a network of twenty 20 × 50 μm microfluidics channels to simulate flow through noncapillary microvasculature. Pressure changes were recorded with a strain gauge pressure transducer and analyzed in labview signals express.
2.5. Micropore Network Analyses.
To measure the impact of neutrophil pseudopod activity on capillary-like vessel flow resistance, we used a Harvard apparatus syringe pump (model 2000) to perfuse suspensions of neutrophilic cells through Isopore® membranes (Millipore) with 10 -μm diameter pores. These membranes were incorporated into a stainless steel filter holder connected to a mock flow setup (Fig. 1(b)). To measure the pressure gradient across the micropore network, the filter holder inlet was connected to a Statham pressure transducer while the outlet was left open to atmosphere. The pressure transducer was calibrated by manometry and interfaced to a carrier demodulator (Validyne) and a data acquisition device (NI USB-6008; National Instruments).
Fixed human neutrophils (1 × 106 cells/ml) that had been stimulated with either 0 or 10 nM fMLP for 5 min and suspended in HBSS were placed inside a 10 ml syringe containing a magnetic stir bar that, in conjunction with a stir plate, was used to gently agitate the cell suspensions and ensure the cells remained suspended. The cells were driven through the microporous membranes at a flow rate of 500 μl/min for 5 min. At this flow rate, the Reynolds number for flow through these porous membranes was extremely small (1) ensuring laminar flow conditions [28]. labview signalexpress (National Instruments) was used to record the infusing pressure (relative to the atmosphere; P t) for the duration of flow experiments. The pressure difference across the microporous network was calculated as ΔP = P t − Pt =0 with P t representing the average instantaneous pressure recorded over the last 2 min of constant flow (Q). For some experiments, autologous erythrocytes were re-added at a hematocrit of 10% to fixed neutrophil suspensions to assess the impact of neutrophil–erythrocyte interactions.
2.6. Microfluidics-Based Microvascular Mimics.
To measure the potential impact of neutrophil pseudopod activity on flow resistance in microfluidics mimics of noncapillary (i.e., arteriolar/venular) microvessels, two different configurations were used for testing. In one case, a polydimethylsiloxane (PDMS) chamber was made consisting of a single rectangular cross section microchannel (w: 500 μm; h: 50 μm; and l: 20 mm). We also constructed a PDMS chamber made to simulate a microvascular network with twenty 20 × 50 μm rectangular channels arranged in parallel. Both of these microvascular mimics were fabricated as previously reported [4] and used in conjunction with the flow setup (Fig. 1(b)) described in Sec. 2.5. Suspensions of fixed neutrophils (1 × 106 cells/ml) that had been stimulated with 0 or 10 nM fMLP for 5 min were perfused through the microfluidic chamber at flow rates of 1, 1.5, 2, and 5 ml/min in the absence or presence of autologous erythrocytes at 10% hematocrit for 3 min. We monitored the infusion pressure (relative to the atmosphere). For analyses, the baseline pressure reading (Pt =0) (obtained before the onset of flow) was subtracted from each instantaneous infusion pressure reading (Pt) and used as the instantaneous pressure gradient ΔP = P t − Pt =0. The pressure gradient values recorded over the last 2 min of constant flow (Q) at each flow rate were averaged and used to quantify the flow resistance (slope of a ΔP versus Q curve).
2.7. Statistics.
Statistical analyses were performed using microsoft excel. Paired t-tests with P < 0.05 were used to identify significant differences between quiescent and activated cell suspensions. For experiments involving the microvascular mimics, recorded pressure data were averaged and plotted in the software matlab (The MathWorks, Inc., Natick, MA) prior to statistical analyses.
3. Results
3.1. Morphological Changes Associated With Cell Activation Influences Viscosity of Neutrophilic Cell Suspensions.
Initial studies were conducted using populations of nonadherent dHL60 cells as a first test to confirm that altered cell morphologies (i.e., pseudopod extension) due to agonist stimulation impact cell suspension viscosity. We verified that while inactivated dHL60 cells remained rounded (Fig. 2(a)), they extended pseudopods and adopted a more irregular, nonuniform morphology after stimulation with 10 nM fMLP (Fig. 2(b)). This fMLP-induced morphological change was quantified as a significant decrease in circularity (Fig. 2(c)). Moreover, fMLP stimulation also affected the apparent viscosities of dHL60 suspensions (Fig. 2(d)). After agonist stimulation, the suspension viscosities of dHL60 cells were significantly enhanced relative to populations of untreated cells, and this effect was independent of cell number between 1 and 1.5 × 106 cell/ml densities (Fig. 2(e)).
Fig. 2.
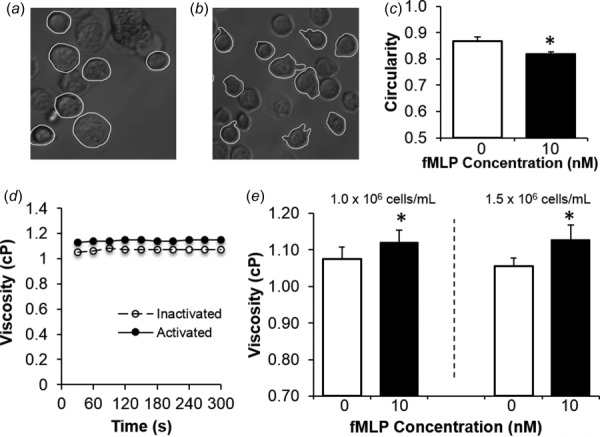
DHL60 pseudopod activity influences cell suspension viscosity. (a) and (b) Representative micrographs show the morphological changes associated with dHL60 stimulation. The dHL60 cells were either maintained in an inactivated state (a) or stimulated with 10 nM fMLP (b) for 5 min prior to fixation. Circularity was measured by manually tracing (white contour) the outlines of cells with imagej software. (c) Average circularities of inactivated (white bar) and activated (black bar) dHL60 neutrophil-like cells were assessed by tracing all cells present in 3–7 images per experimental treatment, with at least 50 cells analyzed. Bars are mean ± SEM for n = 3 independent experiments. (d) Viscosity measurements were obtained by cone-plate rheometry of inactivated (open circle) or activated (solid circle) dHL60 suspensions continuously recorded over a 5-min interval at 450 s−1. (e) Cone-plate rheometry was used to determine the average viscosities for suspensions of inactivated (white bar) or activated (black bar) dHL60 neutrophilic cells. Viscosity is expressed as mean ± SEM. *P < 0.05 compared to 0 nM fMLP stimulation (n = 3).
Activation of primary human neutrophils impacted suspension viscosity similarly to our dHL60 cultures. For these experiments, we confirmed that unstimulated neutrophils remained in a rounded, circular shape after harvest (Fig. 3(a)). Upon stimulation with 10 nM fMLP, most of these cells extended pseudopods and adopted a noncircular, elongated morphology (Fig. 3(b)) that resulted in significant reductions in their circularity compared to unstimulated cells (Fig. 3(c)). Moreover, fMLP-induced shape changes were associated with significant increases in the apparent viscosities of neutrophil suspensions (Figs. 3(d) and 3(e)).
Fig. 3.
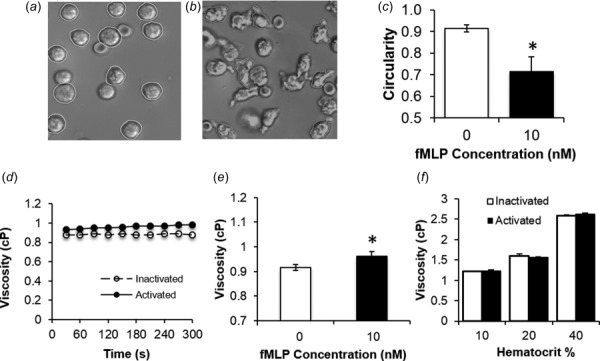
Extension of pseudopods by human neutrophils elevates suspension viscosity. (a) and (b) Representative micrographs show the morphological changes associated with human neutrophil stimulation. The human neutrophils were either maintained in an inactivated state (a) or stimulated with 10 nM fMLP (b) for 5 min prior to fixation. Circularity was measured by manually tracing (white contour) the outlines of cells with imagej software. (c) Average circularities of inactivated (white bar) and activated (black bar) neutrophils were assessed by tracing all cells present in 3–7 images per experimental treatment, with at least 50 cells analyzed. Bars are mean ± SEM for n = 3 independent experiments. (d) Viscosity measurements were obtained by cone-plate rheometry of inactivated (open circle) or activated (solid circle) human neutrophil suspensions continuously recorded over a 5-min interval at 450 s−1. (e) Cone-plate rheometry was used to determine the average viscosities for suspensions of inactivated (white bar) or activated (black bar) human neutrophils. Viscosity is expressed as mean ± SEM. (f) Average viscosity measurements were obtained for mixed populations of human neutrophils and erythrocytes exposed to a constant shear rate of 450 s−1. Erythrocytes were present at 10%, 20%, and 40% hematocrit, while human neutrophils were included as either inactivated (0 nM fMLP, white bars) or activated (10 nM fMLP, black bars) populations. Viscosity is expressed as mean ± SEM. n = 3. *P < 0.05 compared to 0 nM fMLP stimulation. n = 3.
3.2. Erythrocytes Abolish the Rheological Impact of Neutrophil Activation During Cone-Plate Viscometry.
The addition of erythrocytes to inactivated neutrophil suspensions resulted in an elevated suspension viscosity as the overall hematocrit concentration increased (Fig. 3(f)). The neutrophil suspension viscosity was found to double as hematocrit concentration increased from 10% to 40%. Activating the neutrophils, however, had no effect on suspension viscosity in the presence of erythrocytes at the hematocrits tested. For cone-plate flow, the presence of the erythrocytes appeared to mitigate the effects of neutrophil activation on suspension viscosity.
3.3. Erythrocytes Amplify the Effects of Neutrophil Perfusion on Flow Through Micropore Capillary Mimics.
Compared to populations of inactivated neutrophils, suspensions of activated cells generated significantly larger pressure gradients across Isopore® membranes while perfusing through them in the absence (Fig. 4(a)) or presence (Fig. 4(b)) of 10% hematocrit. The presence of hematocrit, however, generated a more dramatic change in pressure gradient than pure neutrophil suspensions alone. Compared to neutrophil suspensions without hematocrit, perfusion of those with 10% hematocrit elicited significantly greater pressure changes across the micropore membranes for the duration of the experiments (Fig. 4(c)). It should be noted that all cell suspensions tested were found to impose a gradual increase in the pressure gradient across the membrane during perfusion experiments, precluding us from determining a flow resistance for the micropore networks. Due to the presence of cells within the porosity of the Isopore® membranes after flow experiments, it is likely that the elevated pressure gradient arose from the entrapment of cells within pores (Fig. 4(d)). However, the degree or rate of clogging appeared to be similar for all perfusion conditions.
Fig. 4.
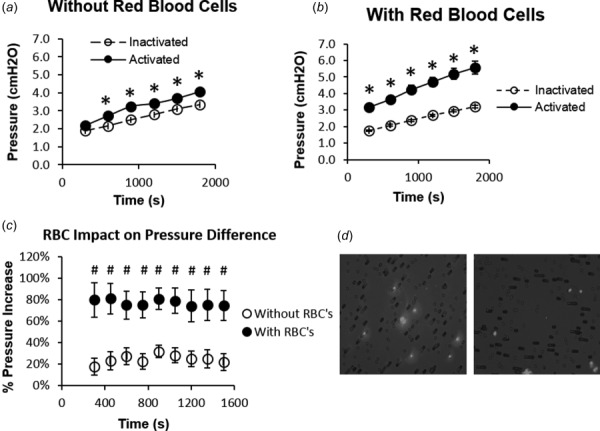
RBCs exacerbate neutrophil activation-related elevations in perfusion pressure associated with flow of blood-like cell suspensions through micropore capillary mimics. (a) and (b) Plots of perfusion pressure versus time were generated to quantify the effects of cell activation (in the form of pseudopod extension) on the flow of neutrophil suspensions through micropore filter-based capillary mimics. Neutrophil populations that had either been maintained in an inactivated state (open circle) or activated with 10 nM fMLP for 5 min (solid circle) were perfused across micropore filters (10 μm pore diameters) in the absence (a) or presence (b) of 10% hematocrit. Average perfusion pressure determined from n = 3 experiments was expressed as mean ± SEM. (c) The percent increase in perfusion pressure due to cell activation was quantified by normalizing the perfusion pressures for activated neutrophil suspensions with that for inactivated cell suspensions. This normalization was carried out and plotted for suspensions lacking (open circle) or containing (solid circle) 10% hematocrit. Each data point is expressed as mean ± SEM. *P < 0.05 compared to inactivated controls. # P < 0.05 compared to populations without RBCs. n = 3. (d) Perfusion of purified suspensions of inactivated (right image) and activated (left image) neutrophils (stained with nuclear label 4', 6-diamidino-2-phenylindole (DAPI); black staining) through micropore filters (gray color) was associated with cell plugging.
3.4. Erythrocytes Enhance the Rheological Impact of Neutrophil Activation in Microscale Flows.
A single-channel microchamber (w: 500 μm; h: 50 μm; and l: 20 mm) was used to simulate flow without plugging through microvessels considered to be the largest within the microcirculation. The perfusion pressure readings obtained from flowing activated neutrophil suspensions through this chamber were similar to those obtained for inactivated neutrophil suspensions (Fig. 5(a)). We also utilized a microvascular network consisting of twenty 20 × 50 μm channels arranged in parallel to simulate microscale flow at dimensions smaller than the single-channel microchamber. Similar to the single-channel microchamber, the perfusion pressure readings obtained by flowing activated neutrophil suspensions were similar to those obtained for inactivated neutrophils (Fig. 5(b)). Based on pressure versus flow curves generated for each chamber (Fig. 5(c)), the impacts of activated and inactivated cell suspensions on microchannel and micronetwork flow resistance were determined to be similar between inactivated and activated cell populations (Table 1).
Fig. 5.
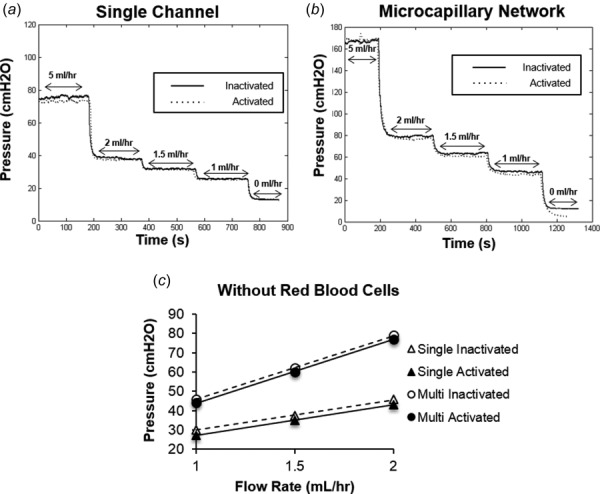
In the absence of RBCs, neutrophil activation does not significantly impact flow resistance of microvascular mimics. (a) and (b) Plots of perfusion pressure versus time for microfluidics chambers comprised of either a single 500 × 50 μm channel (a) or a network of twenty 20 × 50 μm microchannels (b) were generated at various flow rates (0, 1, 1.5, 2, and 5 ml/hr) for purified neutrophil suspensions that had either been maintained in an inactivated state (solid-black line) or activated with 10 nM fMLP (dotted-gray line). (c) Based on these raw data, pressure versus flow curves were generated to determine effects of neutrophil activation on microchannel flow resistance. For each experiment, pressure values were obtained by averaging the measured pressure gradient over the last 2 min at each flow rate. From these curves, resistance values corresponding to single channel (inactivated—open triangles and activated—solid triangle) or microvascular network (inactivated—open circles and activated—solid circles) were found by computing the slope of each line. Points are mean ± SEM. n = 3.
Table 1.
Effects of cell activation on microchannel flow resistance for purified neutrophil suspensions in the absence of hematocrit.
|
Resistance (cm H2O/(ml hr)) |
|||
|---|---|---|---|
| Inactivated | Activated | P value | |
| Single channel | 18.92 ± 6.26 | 18.65 ± 5.54 | P > 0.05 |
| Multinetwork | 27.89 ± 3.76 | 27.9 ± 3.28 | P > 0.05 |
Resistance values (expressed as average ± SEM) calculated for each microfluidic chamber in the absence of RBCs. P values are listed to the right.
Similar experiments were repeated, but in the presence of 10% hematocrit, in order to evaluate the contributions of the erythrocytes. For both the single-channel microchamber (Fig. 6(a)) and the micronetwork chamber (Fig. 6(b)), the perfusion pressure readings obtained from the flow of activated neutrophil suspensions appeared to be elevated in comparison to that of inactivated neutrophil populations. In line with these observations, neutrophil activation in the presence of erythrocytes appeared to increase the slope of the pressure flow curves for both chambers (Fig. 6(c)). Quantitatively, while addition of 10% hematocrit alone appeared to increase baseline resistances of both chambers to the flow of neutrophils, fMLP stimulation, in combination with the red cells, significantly (p < 0.05) elevated the flow resistance exhibited by both of these chambers (Table 2). Notably, the fold change/increase in the flow resistance of the micronetwork chamber due to neutrophil activation in the presence of hematocrit was significantly (p < 0.05) higher than that of the single-channel microchamber (Table 3).
Fig. 6.
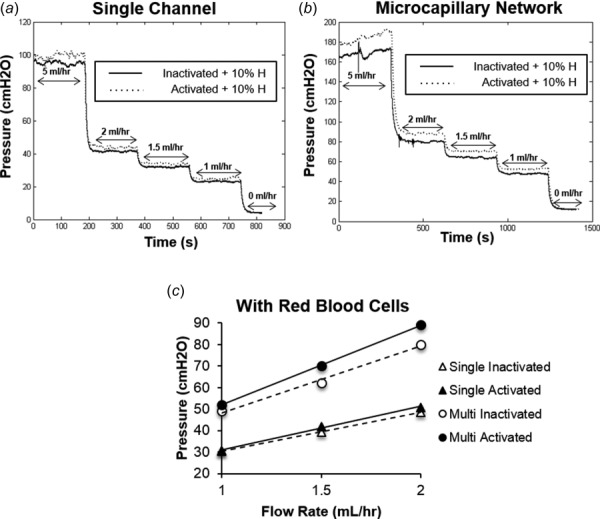
In the presence of RBCs, neutrophil activation and pseudopod extension significantly elevate flow resistance of microvascular mimics. (a) and (b) Perfusion pressure versus time plots for microfluidics chambers comprised of either a single 500 × 50 μm channel (a) or a network of twenty 20 × 50 μm microchannels (b) were generated at various flow rates (0, 1, 1.5, 2, and 5 ml/hr) for purified neutrophil suspensions that had either been maintained in an inactivated state (solid-black line) or activated with 10 nM fMLP (dotted-gray line), fixed, and subsequently supplemented with 10% hematocrit. (c) Based on these raw data, pressure versus flow curves were generated to determine effects of neutrophil activation on microchannel flow resistance in the presence of hematocrit. For each experiment, pressure values were obtained by averaging the measured pressure gradient over the last 2 min at each flow rate. From these curves, resistance values corresponding to single channel (inactivated—open triangles and activated—solid triangle) or microvascular network (inactivated—open circles and activated—solid circles) were found by computing the slope of each line. Points are mean ± SEM. n = 3.
Table 2.
Effects of cell activation on microchannel flow resistance for purified neutrophil suspensions in the presence of hematocrit.
|
Resistance (cm H2O/(ml hr)) |
|||
|---|---|---|---|
| Inactivated | Activated | P value | |
| Single channel | 21.91 ± 6.87 | 22.7 ± 7.41 | P < 0.05 |
| Multinetwork | 31.98 ± 2.21 | 34.91 ± 3.44 | P < 0.05 |
Resistance values (expressed as average ± SEM) calculated for each microfluidic chamber in the presence of RBCs. P values are listed to the right.
Table 3.
Summary of the effects of cell activation in the presence of hematocrit on microscale channel resistance elevation.
P < 0.05 compared to 0% difference (one-sample t-test).
P < 0.05 compared to percent difference in single channel (Student's t-test).
Percent difference between inactivated and activated neutrophil suspensions calculated for each microfluidic chamber in the absence and presence of RBCs.
4. Discussion
The results from the present study provided further support that cell activation, along with the accompanying morphological and surface compositional changes, influences the flow of leukocyte suspensions through microvessels with sizes in the range of those that make up the noncapillary microvasculature. Initial testing utilized dHL60 cells, which are useful in modeling the behavior of human neutrophils [29,30], to reveal first relationships between the leukocyte activation state and the rheological impact of blood cell suspensions on microvascular flow. To carry out these studies, we stimulated cells with the bacteria-derived agonist fMLP, resulting in the extension of pseudopods and adoption of irregular, noncircular (i.e., nonspherical) morphologies as previously reported for both dHL60 neutrophilic leukocytes and primary human neutrophils [31,32]. It should be noted that, although neutrophils and dHL60s may form aggregates due to agonist stimulation, we did not anticipate this to be a problem during our cone-plate experiments. Previously published data indicated that neutrophil homotypic aggregate formation is not significant during cone-plate shear [33], while stimulation with 10 nM fMLP merely resulted in minimal formation of 2–3 cell neutrophil aggregates [34]. Additionally, the use of fixative after fMLP stimulation is reportedly capable of arresting aggregate formation [35]. For these reasons, results from our viscometry studies were likely attributed to cell morphological effects, rather than aggregate formation.
Rheometric analyses confirmed cell activation to be capable of altering the rheology of dHL60 cell suspensions through cell morphological changes related to fMLP stimulation. The biological activity of the cells had no effect on our viscometric measurements, considering the cells were fixed after fMLP treatment and prior to analyses. It was necessary to fix cells prior to analyses, since neutrophil-like cells are sensitive to shear stress and exposure to shear would result in pseudopod retraction over the duration of the experiment [36,37]. Fixation prevents this response by ensuring cells maintain extended pseudopods, allowing us to draw conclusions based on cell morphology. Thus, the effect on apparent viscosity primarily depended on the noncircular morphology that the cells adopted upon activation. Conceivably, the shape changes caused the activated dHL60s to undergo a greater degree of tumbling in the linear velocity gradient of the Couette flow field generated by the cone-plate viscometer. Indeed, primary neutrophils themselves are prone to increased numbers of cell collisions once activated [38], suggesting that dHL60 cells behave similarly. While further analyses on activated cells are required to verify this effect, it may be possible that the enhanced stochastic interactions between activated dHL60s resulted in reduced transport efficiency in the direction of flow and/or disturbance of a putative cell-free marginal zone near the cone surface [39]. Either way these effects of cell activation manifested into a change in suspension viscosity.
A similar relationship between fMLP stimulation and cell suspension viscosity was observed for purified populations of primary human neutrophils. Importantly, while the cell activation state impacted the viscosity of pure neutrophil suspensions, this effect was lost in the presence of 10% hematocrit. In all likelihood, the relative scale of the blood cells with respect to the overall flow field geometry governed the flow behavior of these cell suspensions. In this regard, the rheological properties of our blood cell suspensions for cone-plate flow with dimensions on the order of many hundreds of microns to millimeters may reflect the behavior of blood in macroscale flows. Reportedly, the viscosities of mixed leukocyte–erythrocyte suspensions at physiological (i.e., healthy) proportions reflect the dominant impact of hematocrit (i.e., 10–40% [40,41]) on apparent blood viscosity [42].
Based on this, it is likely that, for the macrocirculation, changes in the rheological behavior of flowing neutrophils upon agonist stimulation will be obscured by the influence of the RBCs that far outnumber the neutrophils within the circulation. This would result in changes to neutrophil flow and dynamics, although additional testing beyond the scope of this paper would be required to determine how RBCs are involved in this process. It may be possible that the dominant RBC presence in our test samples minimized the number of homotypic collisions between neutrophils and increased stochastic interactions between neutrophils and RBCs similar to those reported by Goldsmith et al. [43,44]. In turn, the high deformability of the RBCs probably minimizes the impact of homotypic interactions as well as heterotypic collisions with neutrophils by dissipating the energy associated with these interactions. In these ways, the presence of large numbers of RBCs likely dilute and effectively mask the impact of neutrophil activation in macroscale flow fields. Furthermore, any disturbance to the cell-free marginal zone that may occur due to neutrophil activation is likely negligible in these flow geometries. Thus, the erythrocytes appear to prevent neutrophil activation from impacting macroscale flows, including cylindrical flows found within the macrocirculation.
Reportedly, suspensions of agonist-stimulated neutrophils perfused through skeletal muscle microvasculature significantly raised the hemodynamic flow resistance in these ex vivo preparations [45]. Such resistance elevations were linked to a morphological impact that required the presence of physiological hematocrits [23,24]. These results indicated that RBC contributions to the leukocyte impact on blood flow depend on the size of the vasculature. Although this influence appears lost at the macroscale, the role of leukocyte morphology may become more relevant as the scale diminishes.
One way activated neutrophils may influence microvascular flow resistance is via an impact on flow through the tiniest vessels, the capillaries. To mimic this and verify our experimental approach, we measured how neutrophil morphology can affect cell passage through micropores. While the filters we used had pore sizes that were larger than those predicted for the smallest capillaries, we chose to use membranes with 10 μm-diameter pores to minimize the impact of cell clogging [46]. With these pores, we intended to measure how neutrophils impact flow rheology based on pseudopod activity alone. Furthermore, we performed our microscale flow studies with 10% hematocrit characteristic of the in vivo microvasculature, as opposed to the 40% hematocrit normally found at the macroscale [47]. The goal of these studies was to provide a basis of comparison with our later studies, as well as other published data [45], using microfluidics-based mimics of noncapillary microvasculature.
As expected, the perfusion pressures across our micropore networks were elevated after our neutrophil suspensions were stimulated with fMLP and flowed through the Isopore® membranes, either with or without hematocrit. These results suggest that the neutrophil activation state can, at least partially, impact microvascular resistance due to extended pseudopods. We selected 10 μm pores in our experiment to enable cells to freely pass through the membrane, thereby limiting changes in flow resistance to pseudopod activity alone. However, we still observed a degree of plugging for all samples tested. Consequently, the entrapment of activated neutrophils within the pores also contributed to elevated flow resistance, similar to plugging within the microcapillaries [22,48]. However, after accounting for plugging, we observed that neutrophil morphological changes in the presence of hematocrit altered flow resistance the most among the conditions tested. Since neutrophils, in comparison to RBCs, travel at reduced velocities through the capillaries, it is likely that the presence of hematocrit resulted in RBCs accumulating upstream of neutrophils and disrupting the peripheral marginal layer during flow through the micropores as described previously [11].
Another way that activated neutrophils may influence peripheral resistance is by their impact on blood flow in the noncapillary vasculature, including the arterioles, venules, and thoroughfare channels (arteriovenous shunts). We modeled this using two different microfluidics-based models: a single channel and a microvascular network, both with rectangular cross-sectional areas. We selected rectangular geometries for experimentation since fabricating smooth, circular geometries with PDMS chambers can be difficult to accomplish [49]. While not a perfect approximation of in vivo microvascular flow, rectangular microchambers have been observed to exhibit flow behavior similar to microvessels [50,51]. For both our single channel and microvascular network, the flow resistance of these microfluidic chambers was affected by neutrophil activation, but only in the presence of 10% hematocrit. This indicates that neutrophil morphological changes, in conjunction with their stochastic interactions between RBCs, are capable of affecting flow resistance in these small vessels. Based on this, it is likely that the cell-free marginal zones within these microfluidic channels played a role. While cell-free peripheral zones have been described for circular vessels, similar regions are also present in rectangular microchannels like ours [52]. Accordingly, we were able to make inferences on in vivo microvessels based on our microfluidic analyses. Thus, the impact that stochastic interactions between neutrophils and RBCs have on the cell-free marginal zone likely explains the observed effects of the neutrophil activation on the microchannel flow resistance when hematocrit is present.
It is important to note that our microfluidic experiments modeled two extremes of the microscale, both of which gave results pointing to a dependence of microvessel resistance on neutrophil pseudopod activity. Although there may have been neutrophil aggregate formation which may have affected flow resistance, we expected this contribution to be small considering we (1) stimulated the cells with fMLP at levels shown to induce minimal aggregation [34] and (2) fixed these cells after activation to prevent further aggregate formation [35]. Thus, in comparison to our macroscale results, our microchannel data suggest that leukocyte morphology becomes more relevant as the scale diminishes. However, additional testing is needed to further reveal the extent that cell morphology affects flow resistance.
Considering that the rheological impact of the marginal zone is enhanced as microfluidic channels decrease in size [52], it is reasonable that the blood-like suspensions with activated neutrophils had a greater effect on flow resistance in the microvessel network chamber compared to the single-channel microchamber. Classically, the total resistance of a parallel network is inversely proportional to the sum of the reciprocals of each individual vessel resistance value. Small resistance elevations within individual vessels contribute to large elevations across the entire network [53]. In terms of microscale flows, we expect that this relationship derives from the stochastic interactions between neutrophils and RBCs [23]. Conceivably, these collisions, in turn, perturb the cell-free marginal zone, leading to a significant elevation in flow resistance across the entire network chamber due to the combined effects occurring within each individual channel. A similar relationship likely holds in the microvasculature.
In summary, the evidence reported in this study is consistent with the possibility that neutrophil activity, and its impact on blood rheology, is another factor that may contribute toward elevated peripheral resistance, among others that have been reported (e.g., blood vessel contractility, capillary plugging, leukocyte adhesion, etc.) [12,23,45]. In this regard, it is not surprising that the circulation has multiple mechanisms charged with maintaining neutrophils in an inactivated state until they are required to respond. Otherwise, the resulting elevations in flow resistance due to sustained leukocyte activity could have negative effects on tissue perfusion. In fact, there is a growing body of evidence [7,54,55] that chronic neutrophil activation and its impact on microvascular function contributes to the connection between a chronic inflammatory blood state and many human disease states associated with organ/tissue injury arising from impaired tissue blood flow (e.g., peripheral arterial disease, Alzheimer's, diabetes, stroke, vasculitis, etc.). Consequently, it is essential to consider all of the contributions that activated neutrophils have on microvascular flow.
Acknowledgment
This work was supported by an American Heart Association Beginning Grant-in-Aid (No. 09BGIA2250309) and a National Science Foundation (NSF)-Kentucky Experimental Program on Stimulating Competitive Research (No. 0814194) Bioengineering Initiative grant. We also acknowledge the support from an NSF-Integrated Graduate Education and Research Training Program on Bioactive Interfaces (No. 0653710) and a Kentucky Opportunity Fellowship, both of which were awarded to M.L.A. Finally, we would like to thank Ms. Xiaoyan Zhang Ph.D. for her assistance in establishing experimental protocols.
Contributor Information
Michael L. Akenhead, Department of Biomedical Engineering, , University of Kentucky, , Lexington, KY 40506-0070.
Nolan M. Horrall, Department of Biomedical Engineering, , University of Kentucky, , Lexington, KY 40506-0070.
Dylan Rowe, Math, Science and Technology Center, , Paul L. Dunbar High School, , Lexington, KY 40513.
Palaniappan Sethu, Division of Cardiovascular Disease, , University of Alabama-Birmingham, , Birmingham, AL 35294-0006.
Hainsworth Y. Shin, Department of Biomedical Engineering, , University of Kentucky, , Lexington, KY 40506-0070 , e-mail: hy.shin@uky.edu.
References
- [1]. Granger, D. N. , and Senchenkova, E. , 2010, “Leukocyte-Endothelial Cell Adhesion,” Inflammation and the Microcirculation (Integrated Systems Physiology—From Cell to Function), Morgan & Claypool Life Sciences, San Rafael, CA. [PubMed] [Google Scholar]
- [2]. Baskurt, O. K. , and Meiselman, H. J. , 2003, “Blood Rheology and Hemodynamics,” Semin. Thromb. Hemostasis, 29(5), pp. 435–450. [DOI] [PubMed] [Google Scholar]
- [3]. Popel, A. S. , and Johnson, P. C. , 2005, “Microcirculation and Hemorheology,” Annu. Rev. Fluid Mech., 37, pp. 43–69. 10.1146/annurev.fluid.37.042604.133933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Sethu, P. , Moldawer, L. L. , Mindrinos, M. N. , Scumpia, P. O. , Tannahill, C. L. , Wilhelmy, J. , Efron, P. A. , Brownstein, B. H. , Tompkins, R. G. , and Toner, M. , 2006, “Microfluidic Isolation of Leukocytes From Whole Blood for Phenotype and Gene Expression Analysis,” Anal. Chem., 78(15), pp. 5453–5461. 10.1021/ac060140c [DOI] [PubMed] [Google Scholar]
- [5]. Schmid-Schonbein, G. W. , 2006, “Analysis of Inflammation,” Annu. Rev. Biomed. Eng., 8, pp. 93–131. 10.1146/annurev.bioeng.8.061505.095708 [DOI] [PubMed] [Google Scholar]
- [6]. Lipowsky, H. H. , 2005, “Microvascular Rheology and Hemodynamics,” Microcirculation, 12(1), pp. 5–15. 10.1080/10739680590894966 [DOI] [PubMed] [Google Scholar]
- [7]. Mazzoni, M. C. , and Schmid-Schonbein, G. W. , 1996, “Mechanisms and Consequences of Cell Activation in the Microcirculation,” Cardiovas. Res., 32(4), pp. 709–719. 10.1016/0008-6363(96)00146-0 [DOI] [PubMed] [Google Scholar]
- [8]. Arndt, H. , Smith, C. W. , and Granger, D. N. , 1993, “Leukocyte-Endothelial Cell Adhesion in Spontaneously Hypertensive and Normotensive Rats,” Hypertension, 21(5), pp. 667–673. 10.1161/01.HYP.21.5.667 [DOI] [PubMed] [Google Scholar]
- [9]. Williams, S. A. , and Tooke, J. E. , 1992, “Noninvasive Estimation of Increased Structurally-Based Resistance to Blood Flow in the Skin of Subjects With Essential Hypertension,” Int. J. Microcirc., Clin. Exp., 11(1), pp. 109–116. [PubMed] [Google Scholar]
- [10]. Worthen, G. S. , Schwab, B., 3rd , Elson, E. L. , and Downey, G. P. , 1989, “Mechanics of Stimulated Neutrophils: Cell Stiffening Induces Retention in Capillaries,” Science, 245(4914), pp. 183–186. 10.1126/science.2749255 [DOI] [PubMed] [Google Scholar]
- [11]. Schmid-Schonbein, G. W. , Usami, S. , Skalak, R. , and Chien, S. , 1980, “The Interaction of Leukocytes and Erythrocytes in Capillary and Postcapillary Vessels,” Microvas. Res., 19(1), pp. 45–70. 10.1016/0026-2862(80)90083-7 [DOI] [PubMed] [Google Scholar]
- [12]. Eppihimer, M. J. , and Lipowsky, H. H. , 1996, “Effects of Leukocyte-Capillary Plugging on the Resistance to Flow in the Microvasculature of Cremaster Muscle for Normal and Activated Leukocytes,” Microvas. Res., 51(2), pp. 187–201. 10.1006/mvre.1996.0020 [DOI] [PubMed] [Google Scholar]
- [13]. Adams, R. A. , Evans, S. A. , and Jones, J. G. , 1994, “Characterization of Leukocytes by Filtration of Diluted Blood,” Biorheology, 31(6), pp. 603–615. [DOI] [PubMed] [Google Scholar]
- [14]. Kikuchi, Y. , 1995, “Effect of Leukocytes and Platelets on Blood Flow Through a Parallel Array of Microchannels: Micro- and Macroflow Relation and Rheological Measures of Leukocyte and Platelet Activities,” Microvas. Res., 50(2), pp. 288–300. 10.1006/mvre.1995.1059 [DOI] [PubMed] [Google Scholar]
- [15]. Kikuchi, Y. , Sato, K. , and Mizuguchi, Y. , 1994, “Modified Cell-Flow Microchannels in a Single-Crystal Silicon Substrate and Flow Behavior of Blood Cells,” Microvas. Res., 47(1), pp. 126–139. 10.1006/mvre.1994.1008 [DOI] [PubMed] [Google Scholar]
- [16]. Schmid-Schonbein, G. W. , 1987, “Capillary Plugging by Granulocytes and the No-Reflow Phenomenon in the Microcirculation,” Fed. Proc., 46(7), pp. 2397–2401. [PubMed] [Google Scholar]
- [17]. Karnis, A. , Goldsmith, H. L. , and Mason, S. G. , 1963, “Axial Migration of Particles in Poiseuille Flow,” Nature, 200(4902), pp. 159–160. 10.1038/200159a0 [DOI] [Google Scholar]
- [18]. Pries, A. R. , Secomb, T. W. , and Gaehtgens, P. , 1996, “Biophysical Aspects of Blood Flow in the Microvasculature,” Cardiovas. Res., 32(4), pp. 654–667. 10.1016/0008-6363(96)00065-X [DOI] [PubMed] [Google Scholar]
- [19]. Fåhræus, R. , and Lindqvist, T. , 1931, “The Viscosity of the Blood in Narrow Capillary Tubes,” 96(3), pp. 562–568. [Google Scholar]
- [20]. Kim, S. , Ong, P. K. , Yalcin, O. , Intaglietta, M. , and Johnson, P. C. , 2009, “The Cell-Free Layer in Microvascular Blood Flow,” Biorheology, 46(3), pp. 181–189. 10.3233/BIR-2009-0530 [DOI] [PubMed] [Google Scholar]
- [21]. Freund, J. B. , and Orescanin, M. M. , 2011, “Cellular Flow in a Small Blood Vessel,” J. Fluid Mech., 671, pp. 466–490. 10.1017/S0022112010005835 [DOI] [Google Scholar]
- [22]. Harris, A. G. , and Skalak, T. C. , 1993, “Effects of Leukocyte Activation on Capillary Hemodynamics in Skeletal Muscle,” Am. J. Physiol., 264(3 Pt 2), pp. H909–916. [DOI] [PubMed] [Google Scholar]
- [23]. Helmke, B. P. , Bremner, S. N. , Zweifach, B. W. , Skalak, R. , and Schmid-Schonbein, G. W. , 1997, “Mechanisms for Increased Blood Flow Resistance Due to Leukocytes,” Am. J. Physiol., 273(6 Pt 2), pp. H2884–2890. [DOI] [PubMed] [Google Scholar]
- [24]. Helmke, B. P. , Sugihara-Seki, M. , Skalak, R. , and Schmid-Schonbein, G. W. , 1998, “A Mechanism for Erythrocyte-Mediated Elevation of Apparent Viscosity by Leukocytes In Vivo Without Adhesion to the Endothelium,” Biorheology, 35(6), pp. 437–448. 10.1016/S0006-355X(99)80021-3 [DOI] [PubMed] [Google Scholar]
- [25]. Makino, A. , Glogauer, M. , Bokoch, G. M. , Chien, S. , and Schmid-Schonbein, G. W. , 2005, “Control of Neutrophil Pseudopods by Fluid Shear: Role of Rho Family GTPases,” Am. J. Physiol. Cell Physiol., 288(4), pp. C863–871. [DOI] [PubMed] [Google Scholar]
- [26]. Makino, A. , Prossnitz, E. R. , Bunemann, M. , Wang, J. M. , Yao, W. , and Schmid-Schonbein, G. W. , 2006, “G Protein-Coupled Receptors Serve as Mechanosensors for Fluid Shear Stress in Neutrophils,” Am. J. Physiol. Cell Physiol., 290(6), pp. C1633–1639. 10.1152/ajpcell.00576.2005 [DOI] [PubMed] [Google Scholar]
- [27]. Rainger, G. E. , Buckley, C. D. , Simmons, D. L. , and Nash, G. B. , 1999, “Neutrophils Sense Flow-Generated Stress and Direct Their Migration Through AlphaVbeta3-Integrin,” Am. J. Physiol., 276(3 Pt 2), pp. H858–864. [DOI] [PubMed] [Google Scholar]
- [28]. Usami, S. , Chen, H. H. , Zhao, Y. , Chien, S. , and Skalak, R. , 1993, “Design and Construction of a Linear Shear Stress Flow Chamber,” Ann. Biomed. Eng., 21(1), pp. 77–83. 10.1007/BF02368167 [DOI] [PubMed] [Google Scholar]
- [29]. Carrigan, S. O. , Weppler, A. L. , Issekutz, A. C. , and Stadnyk, A. W. , 2005, “Neutrophil Differentiated HL-60 Cells Model Mac-1 (CD11b/CD18)-Independent Neutrophil Transepithelial Migration,” Immunology, 115(1), pp. 108–117. 10.1111/j.1365-2567.2005.02131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Hauert, A. B. , Martinelli, S. , Marone, C. , and Niggli, V. , 2002, “Differentiated HL-60 Cells Are a Valid Model System for the Analysis of Human Neutrophil Migration and Chemotaxis,” Int. J. Biochem. Cell Biol., 34(7), pp. 838–854. 10.1016/S1357-2725(02)00010-9 [DOI] [PubMed] [Google Scholar]
- [31]. Sham, R. L. , Packman, C. H. , Abboud, C. N. , and Lichtman, M. A. , 1991, “Signal Transduction and the Regulation of Actin Conformation During Myeloid Maturation: Studies in HL60 Cells,” Blood, 77(2), pp. 363–370. [PubMed] [Google Scholar]
- [32]. Zhelev, D. V. , Alteraifi, A. M. , and Chodniewicz, D. , 2004, “Controlled Pseudopod Extension of Human Neutrophils Stimulated With Different Chemoattractants,” Biophys. J., 87(1), pp. 688–695. 10.1529/biophysj.103.036699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Neelamegham, S. , Taylor, A. D. , Burns, A. R. , Smith, C. W. , and Simon, S. I. , 1998, “Hydrodynamic Shear Shows Distinct Roles for LFA-1 and Mac-1 in Neutrophil Adhesion to Intercellular Adhesion Molecule-1,” Blood, 92(5), pp. 1626–1638. [PubMed] [Google Scholar]
- [34]. Russo, R. G. , Liotta, L. A. , Thorgeirsson, U. , Brundage, R. , and Schiffmann, E. , 1981, “Polymorphonuclear Leukocyte Migration Through Human Amnion Membrane,” J. Cell Biol., 91(2 Pt 1), pp. 459–467. 10.1083/jcb.91.2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Rochon, Y. P. , and Frojmovic, M. M. , 1991, “Dynamics of Human Neutrophil Aggregation Evaluated by Flow Cytometry,” J. Leukocyte Biol., 50(5), pp. 434–443. [DOI] [PubMed] [Google Scholar]
- [36]. Fukuda, S. , Yasu, T. , Predescu, D. N. , and Schmid-Schonbein, G. W. , 2000, “Mechanisms for Regulation of Fluid Shear Stress Response in Circulating Leukocytes,” Circ. Res., 86(1), pp. E13–E18. 10.1161/01.RES.86.1.e13 [DOI] [PubMed] [Google Scholar]
- [37]. Moazzam, F. , DeLano, F. A. , Zweifach, B. W. , and Schmid-Schonbein, G. W. , 1997, “The Leukocyte Response to Fluid Stress,” Proc. Natl. Acad. Sci. U. S. A., 94(10), pp. 5338–5343. 10.1073/pnas.94.10.5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Taylor, A. D. , Neelamegham, S. , Hellums, J. D. , Smith, C. W. , and Simon, S. I. , 1996, “Molecular Dynamics of the Transition From L-Selectin- to Beta 2-Integrin-Dependent Neutrophil Adhesion Under Defined Hydrodynamic Shear,” Biophys. J., 71(6), pp. 3488–3500. 10.1016/S0006-3495(96)79544-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Merrill, E. W. , 1969, “Rheology of Blood,” Physiol. Rev., 49(4), pp. 863–888. [DOI] [PubMed] [Google Scholar]
- [40]. Besarab, A. , Bolton, W. K. , Browne, J. K. , Egrie, J. C. , Nissenson, A. R. , Okamoto, D. M. , Schwab, S. J. , and Goodkin, D. A. , 1998, “The Effects of Normal as Compared With Low Hematocrit Values in Patients With Cardiac Disease Who Are Receiving Hemodialysis and Epoetin,” N. Engl. J. Med., 339(9), pp. 584–590. 10.1056/NEJM199808273390903 [DOI] [PubMed] [Google Scholar]
- [41]. Fang, W. C. , Helm, R. E. , Krieger, K. H. , Rosengart, T. K. , DuBois, W. J. , Sason, C. , Lesser, M. L. , Isom, O. W. , and Gold, J. P. , 1997, “Impact of Minimum Hematocrit During Cardiopulmonary Bypass on Mortality in Patients Undergoing Coronary Artery Surgery,” Circulation, 96(9 Suppl), pp. II-194–199. [PubMed] [Google Scholar]
- [42]. Miller, G. E. , 2010, Fundamentals of Biomedical Transport Processes, Morgan & Claypool, San Rafael, CA. [Google Scholar]
- [43]. Goldsmith, H. L. , Lichtarge, O. , Tessier-Lavigne, M. , and Spain, S. , 1981, “Some Model Experiments in Hemodynamics: VI. Two-Body Collisions Between Blood Cells,” Biorheology, 18(3–6), pp. 531–555. [DOI] [PubMed] [Google Scholar]
- [44]. Goldsmith, H. L. , Quinn, T. A. , Drury, G. , Spanos, C. , McIntosh, F. A. , and Simon, S. I. , 2001, “Dynamics of Neutrophil Aggregation in Couette Flow Revealed by Videomicroscopy: Effect of Shear Rate on Two-Body Collision Efficiency and Doublet Lifetime,” Biophys. J., 81(4), pp. 2020–2034. 10.1016/S0006-3495(01)75852-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Sutton, D. W. , and Schmid-Schonbein, G. W. , 1992, “Elevation of Organ Resistance Due to Leukocyte Perfusion,” Am. J. Physiol., 262(6 Pt 2), pp. H1646–H1650. [DOI] [PubMed] [Google Scholar]
- [46]. Bagge, U. , and Braide, M. , 1982, “Leukocyte Plugging of Capillaries In Vivo,” White Blood Cells (Microcirculation Reviews), Vol. 1, Bagge U., Born G. V. R., and Gaehtgens P., eds., Springer, Amsterdam, The Netherlands, pp. 89–98. [Google Scholar]
- [47]. Klitzman, B. , and Duling, B. R. , 1979, “Microvascular Hematocrit and Red Cell Flow in Resting and Contracting Striated Muscle,” Am. J. Physiol., 237(4), pp. H481–H490. [DOI] [PubMed] [Google Scholar]
- [48]. Meisel, S. R. , Shapiro, H. , Radnay, J. , Neuman, Y. , Khaskia, A. R. , Gruener, N. , Pauzner, H. , and David, D. , 1998, “Increased Expression of Neutrophil and Monocyte Adhesion Molecules LFA-1 and Mac-1 and Their Ligand ICAM-1 and VLA-4 Throughout the Acute Phase of Myocardial Infarction: Possible Implications for Leukocyte Aggregation and Microvascular Plugging,” J. Am. Coll. Cardiol., 31(1), pp. 120–125. 10.1016/S0735-1097(97)00424-5 [DOI] [PubMed] [Google Scholar]
- [49]. De Ville, M. , Coquet, P. , Brunet, P. , and Boukherroub, R. , 2012, “Simple and Low-Cost Fabrication of PDMS Microfluidic Round Channels by Surface-Wetting Parameters Optimization,” Microfluid. Nanofluid., 12(6), pp. 953–961. 10.1007/s10404-011-0929-8 [DOI] [Google Scholar]
- [50]. Faivre, M. , Abkarian, M. , Bickraj, K. , and Stone, H. A. , 2006, “Geometrical Focusing of Cells in a Microfluidic Device: An Approach to Separate Blood Plasma,” Biorheology, 43(2), pp. 147–159. [PubMed] [Google Scholar]
- [51]. Shevkoplyas, S. S. , Gifford, S. C. , Yoshida, T. , and Bitensky, M. W. , 2003, “Prototype of an In Vitro Model of the Microcirculation,” Microvas. Res., 65(2), pp. 132–136. 10.1016/S0026-2862(02)00034-1 [DOI] [PubMed] [Google Scholar]
- [52]. Lima, R. , Wada, S. , Tanaka, S. , Takeda, M. , Ishikawa, T. , Tsubota, K. , Imai, Y. , and Yamaguchi, T. , 2008, “In Vitro Blood Flow in a Rectangular PDMS Microchannel: Experimental Observations Using a Confocal Micro-System,” Biomed. Microdevices, 10(2), pp. 153–167. 10.1007/s10544-007-9121-z [DOI] [PubMed] [Google Scholar]
- [53]. Goyal, M. R. , 2013, Biofluid Dynamics of Human Body Systems, Apple Academic Press, Toronto, Canada. [Google Scholar]
- [54]. Doring, Y. , Drechsler, M. , Soehnlein, O. , and Weber, C. , 2014, “Neutrophils in Atherosclerosis: From Mice to Man,” Arterioscler., Thromb., Vasc. Biol., 35, pp. 485–491. 10.1161/ATVBAHA.114.303564 [DOI] [PubMed] [Google Scholar]
- [55]. Hansen, P. R. , 1995, “Role of Neutrophils in Myocardial Ischemia and Reperfusion,” Circulation, 91(6), pp. 1872–1885. 10.1161/01.CIR.91.6.1872 [DOI] [PubMed] [Google Scholar]


