Abstract
Context
In the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly – Pivotal Fracture Trial (HORIZON-PFT), zoledronic acid (ZOL) 5 mg significantly reduced fracture risk.
Objective
The aim of the study was to identify factors associated with greater efficacy during ZOL 5 mg treatment.
Design, Setting, and Patients
We conducted a subgroup analysis (preplanned and post hoc) of a multicenter, double-blind, placebo-controlled, 36-month trial in 7765 women with postmenopausal osteoporosis.
Intervention
A single infusion of ZOL 5 mg or placebo was administered at baseline, 12, and 24 months.
Main Outcome Measures
Primary endpoints were new vertebral fracture and hip fracture. Secondary endpoints were nonvertebral fracture and change in femoral neck bone mineral density (BMD). Baseline risk factor subgroups were age, BMD T-score and vertebral fracture status, total hip BMD, race, weight, geographical region, smoking, height loss, history of falls, physical activity, prior bisphosphonates, creatinine clearance, body mass index, and concomitant osteoporosis medications.
Results
Greater ZOL induced effects on vertebral fracture risk were seen with younger age (treatment-by-subgroup interaction, P =0.05), normal creatinine clearance (P =0.04), and body mass index ≥ 25 kg/m2 (P = 0.02). There were no significant treatment–factor interactions for hip or nonvertebral fracture or for change in BMD.
Conclusions
ZOL appeared more effective in preventing vertebral fracture in younger women, overweight/obese women, and women with normal renal function. ZOL had similar effects irrespective of fracture risk factors or femoral neck BMD.
Zoledronic acid is a bisphosphonate that is administered iv for the treatment of postmenopausal osteoporosis; a single dose has been reported to decrease bone turnover and improve bone mineral density (BMD) for at least 12 months after infusion (1). Furthermore, recent data from the HORIZON-PFT (Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly – Pivotal Fracture Trial) showed that a once-yearly infusion of zoledronic acid 5 mg during a 3-yr period significantly reduced the risk of vertebral, hip, and other fractures (2).
A number of risk factors for fracture have been identified, and it is possible that the effect of zoledronic acid differs across categories of these risk factors; there may even be an interaction between these factors and the treatment. Age may affect the response to treatment because older people have faster hip bone loss than younger individuals (3), and the efficacies of risedronate, strontium, and PTH have been evaluated for elderly subjects (4–6). Data from the Hip Intervention Program (HIP) study have been used to suggest that risedronate does not reduce the risk of fracture in women over the age of 80 yr (7). A more likely interpretation, however, is that women were selected for the study based on risk factors for falls. Baseline BMD has also been claimed to be a determinant of response to treatment. The Fracture Intervention Trial 2 showed that in women who have a T-score above −2.5 at the hip without existing vertebral fractures, there is no effect of alendronate on fracture risk (8). Similarly, in the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe, there was no effect of ibandronate on fracture risk in women with a hip T-score above −3.0 and existing vertebral fractures (9). There are other patient characteristics that may have an effect on fracture risk; these effects could interact with treatment, but they have been insufficiently studied. These include race (10), although data from the Women’s Health Initiative hormone trials in U.S. women show no evidence of an interaction between hormone therapy, race (11), and body mass index (BMI), which is a very strong risk factor for fracture (12). In addition to effects of individual demographic parameters on fracture risk, there is also tremendous variability in hip fracture rates worldwide, with the highest rates observed in Northern European countries (13, 14). In contrast, the variability in vertebral fractures is much less. For example, Asian women have low rates of hip fracture but similar rates of vertebral fractures compared with white women (15). Other factors that are known to have an effect on fracture risk include smoking (smokers have an increased risk of fracture that is partially explained by their lower body weight) (16), height loss since age 25 yr (16), occurrence of a fall in the past 12 months (a marker of frailty) (17, 18), leisure-time activity (hours of walking) (19), and renal impairment (creatinine clearance <65 ml/min) (20). It is also possible that previous bisphosphonate use may influence response because prior therapy may have a persistent effect of reducing fracture risk, although the drug has been stopped (21, 22).
We analyzed the effect of zoledronic acid on vertebral fracture, hip fracture, nonvertebral fracture, and femoral neck BMD in patients from HORIZON-PFT divided into subgroups based on baseline risk factors to determine whether these factors were associated with greater treatment efficacy.
Subjects and Methods
Subjects
Full details of the HORIZON-PFT have been published previously (2). Postmenopausal women aged 65–89 yr were eligible for inclusion if they had a femoral neck T-score ≤−2.5 with or without evidence of an existing vertebral fracture, or a T-score ≤−1.5 with radiological evidence of at least two mild or one moderate vertebral fracture(s). Prior oral bisphosphonate use was allowed, with washout duration dependent on previous use (e.g. >48 wk of usage required a 2-yr washout). All subjects provided informed consent, and local institutional review board approval was obtained for the protocol. The study was conducted in accordance with good clinical practice and the Declaration of Helsinki.
Subjects were assigned to one of two strata on the basis of whether they were taking allowed osteoporosis medications at baseline. Stratum I was defined as women not taking any osteoporosis medications at the time of randomization, whereas stratum II was defined as those taking an allowed medication at the time of randomization.
Endpoints
The primary endpoints were new vertebral fractures (in stratum I) and hip fracture (in both strata). Secondary efficacy endpoints included any nonvertebral fracture, any clinical vertebral fracture, any clinical fracture (including hip fracture) and change in femoral neck BMD.
BMD
Dual-energy x-ray absorptiometry at the hip was performed at baseline and months 6, 12, 24, and 36. Total hip and femoral neck BMD were used to categorize patients into subgroups according to their BMD at baseline. Change in femoral neck BMD over 3 yr was also evaluated as an efficacy endpoint. Investigators and coordinators were blinded to comparative results from the individual follow-up scans. Bone loss was monitored centrally, and if it exceeded specified criteria, the site was notified and patient counseling occurred. All BMD measurements were corrected for variations related to machinery and site-related differences in BMD means.
Fractures
Lateral radiographs of the spine were obtained at baseline and at 12, 24, and 36 months (or early termination) for stratum I, and at baseline and 36 months (or early termination) for stratum II. All vertebrae from T4 to L4 were evaluated by quantitative morphometry by an expert reader at a central imaging laboratory (Synarc, Inc., San Francisco, CA) using standard methods (23). Incident morphometric vertebral fractures were defined by any vertebral height reduction from baseline of at least 20% and 4 mm by quantitative morphometry, confirmed by one severity grade or greater increase from baseline by semiquantitative reading (23). Baseline prevalent fractures were defined as those with height ratios at least 3 SD values below the mean height ratio for that specific vertebral level on the quantitative reading.
The present analysis of vertebral fracture focuses only on incidence of vertebral fracture at 3 yr. Patient reports of clinical fractures (including hip fracture) were obtained at each patient contact. Each report of a nonvertebral fracture required central confirmation (University of California, San Francisco Coordinating Center) based on a radiological or surgical procedure report or a copy of the radiograph. Fractures to toes, facial bones, and fingers and those resulting from excessive trauma [as assessed by a priori criteria (2)] were excluded. For clinical vertebral fractures, the community-obtained radiograph was compared with the baseline study radiograph by a central reader (Synarc). Evidence of an incident vertebral fracture by semi quantitative criteria was required to confirm a clinical vertebral fracture.
Adverse events
Safety was assessed by the recording of all adverse events and serious adverse events and by physical examination, regular measurement of vital signs, and regular monitoring of hematological, blood chemical, and urinary values. A more detailed description of adverse event reporting has been published previously (2).
Statistical analysis
Analysis of the percentage of subjects with at least one new morphometric vertebral fracture was performed for the modified intent-to-treat population (defined as all randomized subjects who were evaluable for at least one vertebra at baseline and at least one follow-up radiograph). Women in stratum I who had incident vertebral fractures at month 12 and month 24 had their fracture results carried forward to subsequent time points. Logistic regression adjusting for the effects of treatment, number of prevalent baseline vertebral fractures, subgroup, and treatment-by-subgroup interaction was used to report odds ratios and P values. For clinical fracture endpoints, Cox proportional hazard models adjusting for the effects of treatment, subgroup, and treatment-by-subgroup interaction were used to report relative hazard ratios and P values. Percentage change from baseline in femoral neck BMD was analyzed using an ANOVA model with treatment, geographic region, subgroup, and treatment-by-subgroup interactions as factors in the models. Treatment-by-subgroup interaction was considered statistically and clinically significant at P <0.05, and relevant trends were noted with 0.05 < P < 0.2. Categorical subgroup variables were used for all analyses. Because the study was not generally designed to detect difference within and/or across levels of subgroups, the information provided should be interpreted as being descriptive, with the P values being used to guide in the interpretation of individual results. All efficacy analyses, other than vertebral fractures, were performed on the complete intent-to-treat population.
In the present subgroup analysis, the preplanned determinants of response considered were: age (<70, 70–74, ≥75 yr), femoral neck BMD T-score (above or below −2.5) and prevalent vertebral fracture status at baseline (present or absent), race (Caucasian or other), weight (tertiles), region (Europe, Asia, the Americas), past use of bisphosphonates (yes or no), stratum (with or without concomitant osteoporosis medication, including hormone replacement therapy), and creatinine clearance calculated using the Cockcroft-Gault equation (<60 ml/min, ≥60 ml/min). Post hoc determinants of response considered were: total hip BMD by tertile, smoking (current smoker or not), height loss since age 25 yr (by tertile), fall in past 12 months (yes or no), walking duration (by tertile), and BMI (<18, 18–24.9, 25–29.9, and ≥30 kg/m2).
Results
Results of the primary and secondary analyses of the HORIZON-PFT have been reported previously (2). Baseline characteristics for women in strata I and II are shown in Table 1, and for stratum I alone in Table 2. Baseline characteristics in the combined strata I and II population were comparable between zoledronic acid and placebo groups, with the exception of BMI and weight. In stratum I, baseline characteristics were comparable between the treatment groups, except for mean BMI and mean weight. A total of 286 patients in the zoledronic acid group and 289 patients in the placebo group were included, although they had a baseline femoral neck T-score above −2.5 and no vertebral fracture and thus deviated from the entry criteria for the study.
TABLE 1.
Baseline characteristics of subjects in overall population (strata I and II combined)
| Zoledronic acid | Placebo | |
|---|---|---|
| n | 3875 | 3861 |
| Age (yr) | 73.1 ± 5.3 | 73.0 ± 5.4 |
| Age group | ||
| <70 yr | 1140 (29.4) | 1174 (30.4) |
| 70–74 yr | 1238 (31.9) | 1235 (32.0) |
| ≥75 yr | 1497 (38.6) | 1452 (37.6) |
| Age at menopause (yr) | 48.0 ± 5.5 | 47.9 ± 5.5 |
| Stratum | ||
| I | 3045 (78.6) | 3039 (78.7) |
| II | 830 (21.4) | 822 (21.3) |
| Race | ||
| Caucasian | 3054 (78.8) | 3055 (79.1) |
| Asian | 562 (14.5) | 559 (14.5) |
| Other | 259 (6.7) | 247 (6.4) |
| Region | ||
| Americas | 1391 (35.9) | 1387 (35.9) |
| Asia | 550 (14.2) | 540 (14.0) |
| Europe | 1934 (49.9) | 1934 (50.1) |
| BMI (kg/m2)a | 25.1 ± 4.3 | 25.4 ± 4.3 |
| BMIa | ||
| <18 kg/m2 | 98 (2.5) | 91 (2.4) |
| 18–24.9 kg/m2 | 1969 (50.9) | 1845 (47.8) |
| 25–29.9 kg/m2 | 1333 (34.5) | 1420 (36.8) |
| ≥30 kg/m2 | 468 (12.1) | 500 (13.0) |
| Weight (kg)a | 59.9 ± 11.1 | 60.6 ± 11.3 |
| Height loss | ||
| <−5.65 cm | 1243 (32.9) | 1276 (33.8) |
| −5.65 to −3 cm | 1283 (34.0) | 1242 (32.9) |
| >−3 cm | 1253 (33.2) | 1262 (33.4) |
| Baseline VFx | ||
| Yes | 2416 (62.4) | 2477 (64.2) |
| No | 1457 (37.6) | 1383 (35.8) |
| Current smoker | ||
| Yes | 344 (8.9) | 316 (8.2) |
| No | 3531 (91.1) | 3544 (91.8) |
| History of falls | ||
| Yes | 1139 (29.5) | 1130 (29.3) |
| No | 2706 (70.0) | 2711 (70.3) |
| Unknown | 20 (0.5) | 15 (0.4) |
| Physical activity (hours walking/wk) | 19.6 ± 18.0 | 19.8 ± 17.9 |
| Prior bisphosphonate usage | ||
| Yes | 565 (14.6) | 557 (14.4) |
| No | 3293 (85.1) | 3282 (85.1) |
| Total hip BMD (g/cm2) | 0.65 ± 0.09 | 0.65 ± 0.09 |
| Standardized total hip BMD (g/cm2) | ||
| 0.23–0.61 | 1290 (33.6) | 1262 (32.9) |
| >0.61–0.68 | 1301 (33.8) | 1276 (33.2) |
| >0.68–1.32 | 1253 (32.6) | 1301 (33.9) |
| Femoral neck BMD (g/cm2) | 0.53 ± 0.06 | 0.53 ± 0.06 |
| Femoral neck T-score | −2.8 ± 0.5 | −2.7 ± 0.6 |
| Femoral neck T-score | ||
| ≤−2.5 | 2814 (73.1) | 2734 (71.1) |
| >−2.5 | 1037 (26.9) | 1111 (28.9) |
| Femoral neck T-score by baseline VFx status | ||
| ≤−2.5 + VFx | 1649 (42.8) | 1645 (42.8) |
| ≤−2.5 − VFx | 1163 (30.2) | 1088 (28.3) |
| >−2.5 + VFx | 751 (19.5) | 822 (21.4) |
| >−2.5 − VFx | 286 (7.4) | 289 (7.5) |
| Creatinine clearance | ||
| <60 ml/min | 1786 (46.1) | 1728 (44.8) |
| ≥60 ml/min | 2089 (53.9) | 2133 (55.2) |
Data are presented as mean ± SD or number (%). VFx, Vertebral fracture.
Significant difference (P < 0.05) between treatment groups (t test for continuous variables; χ2 test for categorical variables).
TABLE 2.
Baseline characteristics for patients in stratum I
| Zoledronic acid | Placebo | |
|---|---|---|
| n | 2822 | 2853 |
| Age (yr) | 73.0 ± 5.2 | 3.1 ± 5.4 |
| Age group | ||
| <70 yr | 832 (29.5) | 852 (29.9) |
| 70–74 yr | 907 (32.1) | 923 (32.4) |
| ≥75 yr | 1083 (38.4) | 1078 (37.8) |
| Age at menopause (yr) | 48.2 ± 5.3 | 48.0 ± 5.4 |
| Race | ||
| Caucasian | 2135 (75.7) | 2179 (76.4) |
| Asian | 482 (17.1) | 477 (16.7) |
| Other | 205 (7.3) | 197 (6.9) |
| Region | ||
| Americas | 917 (32.5) | 931 (32.6) |
| Asia | 473 (16.8) | 461 (16.2) |
| Europe | 1432 (50.7) | 1461 (51.2) |
| BMI (kg/m2)a | 25.2 ± 4.2 | 25.5 ± 4.3 |
| BMI | ||
| <18 kg/m2 | 67 (2.4) | 63 (2.2) |
| 18–24.9 kg/m2 | 1400 (49.7) | 1348 (47.3) |
| 25–29.9 kg/m2 | 1014 (36.0) | 1059 (37.2) |
| ≥30 kg/m2 | 334 (11.9) | 379 (13.3) |
| Weight (kg)a | 59.9 ± 11.0 | 60.6 ± 11.4 |
| Height loss | ||
| <−5.65 cm | 966 (32.7) | 981 (33.0) |
| −5.65 to −3 cm | 1005 (34.0) | 969 (32.6) |
| >−3 cm | 985 (33.3) | 1021 (34.4) |
| Baseline VFx | ||
| Yes | 1752 (62.1) | 1815 (63.6) |
| No | 1070 (37.9) | 1038 (36.4) |
| Current smoker | ||
| Yes | 235 (8.3) | 218 (7.6) |
| No | 2587 (91.7) | 2635 (92.4) |
| History of falls | ||
| Yes | 811 (28.7) | 837 (29.3) |
| No | 1998 (70.8) | 2006 (70.3) |
| Unknown | 13 (0.5) | 10 (0.4) |
| Physical activity (hours walking/wk) | 19.8 ± 17.9 | 20.0 ± 18.0 |
| Prior bisphosphonate usage | ||
| Yes | 335 (11.9) | 339 (11.9) |
| No | 2479 (87.8) | 2502 (87.7) |
| Total hip BMD (g/cm2) | 0.65 ± 0.09 | 0.65 ± 0.09 |
| Standardized total hip BMD (g/cm2) | ||
| 0.23–0.61 | 931 (33.2) | 951 (33.5) |
| >0.61–0.68 | 939 (33.5) | 939 (33.0) |
| >0.68–1.32 | 933 (33.3) | 952 (33.5) |
| Femoral neck BMD (g/cm2) | 0.53 ± 0.06 | 0.53 ± 0.06 |
| Femoral neck T-score | −2.7 ± 0.5 | −2.8 ± 0.5 |
| Femoral neck T-score | ||
| ≤−2.5 | 2037 (72.7) | 2054 (72.3) |
| >−2.5 | 766 (27.3) | 788 (27.7) |
| Femoral neck T-score by baseline VFx status | ||
| ≤−2.5 + VFx | 1185 (42.3) | 1238 (43.6) |
| ≤−2.5 − VFx | 852 (30.4) | 816 (28.7) |
| >−2.5 + VFx | 556 (19.8) | 571 (20.1) |
| >−2.5 − VFx | 210 (7.5) | 217 (7.6) |
| Creatinine clearance | ||
| <60 ml/min | 1289 (45.7) | 1274 (44.7) |
| ≥60 ml/min | 1533 (54.3) | 1579 (55.3) |
Data are presented as mean ± SD or number (%). VFx, Vertebral fracture.
Significant difference (P < 0.05) between treatment groups (t test for continuous variables; χ2 test for categorical variables).
Adverse events were not analyzed separately in all the subgroups reported here. However, adverse events in the overall population have been reported previously, where zoledronic acid (5 mg) treatment was generally safe and well tolerated (2).
Changes in fracture risk
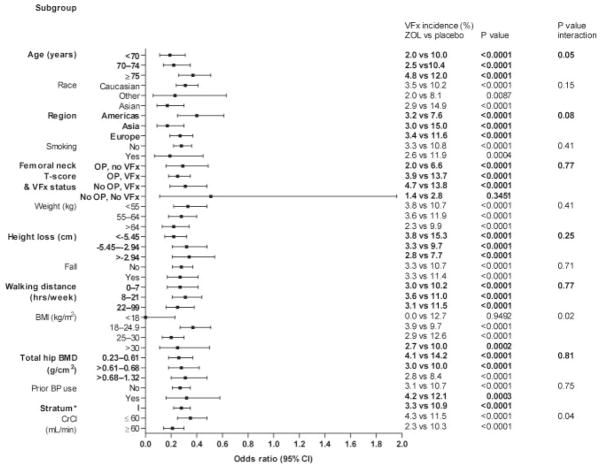
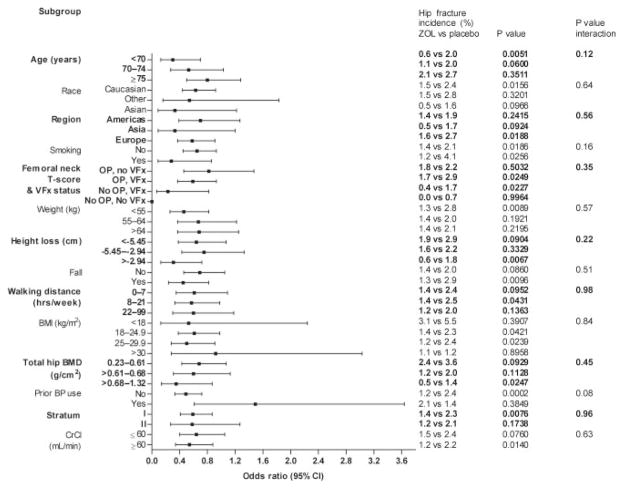
Zoledronic acid treatment was associated with significant reductions in the risk of vertebral fractures at 3 yr in all categories (Fig. 1). Zoledronic acid treatment reduced the risk of hip fracture and nonvertebral fracture over 3 yr across all subgroups (hazard ratio <1) except for those treated previously with bisphosphonates (Figs. 2 and 3).
FIG. 1.
Effect of baseline risk factors on reduction in vertebral fracture with zoledronic acid 5 mg at 3 yr in stratum I. OP, Osteoporosis (femoral neck T-score ≤−2.5); VFx, vertebral fracture; BP, bisphosphonate; CrCl, creatinine clearance; ZOL, zoledronic acid; CI, confidence interval. *, Vertebral fracture risk was assessed in the stratum I modified intent-to-treat population.
FIG. 2.
Effect of baseline risk factors on reduction in hip fracture with zoledronic acid 5 mg at 3 yr (strata I and II). OP, Osteoporosis (femoral neck T-score ≤−2.5); VFx, vertebral fracture; BP, bisphosphonate; CrCl, creatinine clearance; ZOL, zoledronic acid; CI, confidence interval.
FIG. 3.
Effect of baseline risk factors on reduction in nonvertebral fracture with zoledronic acid 5 mg at 3 yr (strata I and II). OP, Osteoporosis (femoral neck T-score ≤−2.5); VFx, vertebral fracture; BP, bisphosphonate; CrCl, creatinine clearance; ZOL, zoledronic acid; CI, confidence interval.
With vertebral fracture risk, significant treatment–factor interactions were observed for age (P = 0.05), BMI (P = 0.02), and creatinine clearance (P = 0.04), with greater effects of zoledronic acid in younger women (<70 yr), women who were overweight or obese (BMI >30 g/cm2), and women with creatinine clearance above 60 ml/min (Fig. 1). There were no significant treatment–factor interactions (P < 0.05) for hip fractures (Fig. 2) or nonvertebral fractures (Fig. 3).
Changes in femoral neck BMD
There was a significant beneficial effect of zoledronic acid treatment on femoral neck BMD in all subgroups compared with placebo (all P < 0.0001) (Table 3). The following subgroup categories had significant treatment-by-subgroup interactions (P < 0.05) and were associated with different magnitudes of greater increase in BMD by zoledronic acid relative to placebo: smaller absolute total hip BMD (0.23–0.61 g/cm2) and BMD T-score below −2.5 with and without prevalent vertebral fractures. However, these results relate to percentage changes in femoral neck BMD. When we calculated the absolute changes in femoral neck BMD, we found that the interaction with smaller absolute total hip BMD (0.23–0.61 g/cm2) was not significant (P = 0.06), and BMD T-score below −2.5 with and without prevalent vertebral fractures was not significant either (P = 0.5).
TABLE 3.
Effect of factors on mean percentage change from baseline in femoral neck BMD at 3 yr (strata I and II)
| Category | n | Mean % change in FN BMD
|
Least squares mean between-treatment difference (95% CI) | P value within category | P value interaction | |
|---|---|---|---|---|---|---|
| Zoledronic acid | Placebo | |||||
| Age (yr) | ||||||
| <70 | 959 | 4.33 | −0.76 | 5.09 (4.55, 5.62) | <0.0001 | 0.99 |
| 70–74 | 1015 | 4.09 | −0.96 | 5.05 (4.52, 5.57) | <0.0001 | |
| ≥75 | 1093 | 3.90 | −1.14 | 5.04 (4.53, 5.55) | <0.0001 | |
| Race | ||||||
| Asian | 469 | 4.37 | −0.49 | 4.85 (4.08, 5.63) | <0.0001 | 0.51 |
| Caucasian | 2390 | 4.08 | −0.96 | 5.04 (4.70, 5.38) | <0.0001 | |
| Other | 208 | 3.71 | −1.97 | 5.68 (4.50, 6.85) | <0.0001 | |
| Region | ||||||
| Americas | 1074 | 3.5 | −1.47 | 4.98 (4.47, 5.48) | <0.0001 | 0.70 |
| Asia | 459 | 4.35 | −0.48 | 4.82 (4.04, 5.61) | <0.0001 | |
| Europe | 1534 | 4.44 | −0.73 | 5.17 (4.74, 5.60) | <0.0001 | |
| Smoking | ||||||
| No | 2810 | 4.17 | −0.92 | 5.08 (4.77, 5.40) | <0.0001 | 0.58 |
| Yes | 257 | 3.35 | −1.42 | 4.77 (3.70, 5.84) | <0.0001 | |
| Femoral neck T-score and VFx status | ||||||
| OP, no VFx | 953 | 4.81 | −0.56 | 5.37 (4.82, 5.92) | <0.0001 | 0.004 |
| OP, VFx | 1258 | 4.44 | −0.92 | 5.36 (4.89, 5.83) | <0.0001 | |
| No OP, no VFx | 238 | 3.21 | −1.38 | 4.59 (3.53, 5.65) | <0.0001 | |
| No OP, VFx | 617 | 2.65 | −1.37 | 4.02 (3.36, 4.68) | <0.0001 | |
| Weight (kg) | ||||||
| <55 | 1040 | 3.92 | −1.29 | 5.21 (4.68, 5.74) | <0.0001 | 0.77 |
| 55–64 | 1067 | 3.97 | −1.10 | 5.07 (4.56, 5.58) | <0.0001 | |
| >64 | 957 | 4.43 | −0.50 | 4.93 (4.40, 5.46) | <0.0001 | |
| Height loss (cm) | ||||||
| <−5.65 | 1015 | 4.05 | −1.48 | 5.53 (5.00, 6.05) | <0.0001 | 0.10 |
| −5.65 to −3 | 1009 | 4.02 | −0.86 | 4.89 (4.37, 5.41) | <0.0001 | |
| >−3 | 972 | 4.26 | −0.53 | 4.79 (4.26, 5.31) | <0.0001 | |
| BMI (kg/m2) | ||||||
| <18 | 74 | 2.66 | −2.42 | 5.07 (3.07, 7.07) | <0.0001 | 0.27 |
| 18–24.9 | 1574 | 3.95 | −1.15 | 5.10 (4.67, 5.53) | <0.0001 | |
| 25–29.9 | 1064 | 4.39 | −0.90 | 5.29 (4.79, 5.79) | <0.0001 | |
| >30 | 350 | 4.22 | −0.07 | 4.28 (3.41, 5.16) | <0.0001 | |
| Total hip BMD (g/cm2) | ||||||
| 0.23–0.61 | 1035 | 4.72 | −1.27 | 5.98 (5.46, 6.51) | <0.0001 | <0.0001 |
| >0.61–0.68 | 1019 | 3.99 | −1.06 | 5.05 (4.52, 5.57) | <0.0001 | |
| >0.68–1.32 | 1006 | 3.56 | −0.55 | 4.10 (3.58, 4.63) | <0.0001 | |
| Prior BP use | ||||||
| No | 2604 | 4.3 | −0.86 | 5.16 (4.84, 5.49) | <0.0001 | 0.26 |
| Yes | 455 | 2.86 | −1.60 | 4.46 (3.67, 5.26) | <0.0001 | |
| Stratum | ||||||
| I | 2394 | 4.26 | −0.87 | 5.13 (4.79, 5.47) | <0.0001 | 0.40 |
| II | 673 | 3.53 | −1.29 | 4.81 (4.16, 5.47) | <0.0001 | |
| CrCl (ml/min) | ||||||
| <60 | 1361 | 3.88 | −1.31 | 5.18 (4.73, 5.64) | <0.0001 | 0.48 |
| ≥60 | 1706 | 4.27 | −0.69 | 4.96 (4.56, 5.37) | <0.0001 | |
Falls and walking distance had no effect on BMD change by category and so are not listed. CI, Confidence interval; BP, bisphosphonate; FN, femoral neck; OP, osteoporosis (femoral neck T-score ≤−2.5); VFx, vertebral fracture; CrCl, creatinine clearance.
Discussion
In general, this analysis showed that zoledronic acid 5 mg had similar effects on femoral neck BMD, hip and nonvertebral fracture independent of patient demographics, and baseline risk factors for fracture. However, although zoledronic acid showed significant reductions in vertebral fractures across all subgroups, there appeared to be larger reductions in vertebral fractures with zoledronic acid in younger women, women with higher BMI, and women with normal renal function. BMI and renal function have not previously been reported as factors that increase or decrease the efficacy of an anticatabolic treatment for postmenopausal osteoporosis. These interactions would be more compelling had they been consistently significant among fracture types and BMD changes. However, there was little evidence that these factors modified the effect of zoledronic acid for the other endpoints evaluated.
In interpreting the subgroup analyses presented, it is important to consider the well-known statistical limitations of subgroup analyses including the lack of power and violation of model assumptions that occur by including such interaction terms in the statistical model and confound the interpretation of effects within and across levels of subgroups (24). As recommended (24), interpretation of results has primarily been based on tests of interaction, recognizing that a nonsignificant interaction may reflect lack of power and may not demonstrate homogeneity of effect. Also, when interpreting these analyses, we took into account the results from other studies and the consistency of results within ours.
Fracture risk, particularly for hip and vertebral fractures, increases exponentially with age, and it is therefore of paramount importance to establish the efficacy of treatments among those over 75 and over 80 yr. The importance of age as a determinant of responsiveness to treatments for postmenopausal osteoporosis has been considered for alendronate, risedronate, strontium ranelate, and teriparatide, as well as for hormone replacement therapy (4–6, 11, 25, 26) for vertebral and for nonvertebral fractures. Nonvertebral fracture risk reduction as a result of treatment with bisphosphonates has not been demonstrated in patients over 80. However, the numbers of patients over 80 yr (or over 75 yr) in these studies were limited. In addition, the studies were not specifically designed (or powered) to assess fracture risk reduction in old age. As a result, the number of nonvertebral fracture events in elderly subsets of the trial populations was relatively small, and the confidence limits were relatively wide. This was demonstrated by the effects of risedronate on fracture risk in 8680 women from the HIP and Vertebral Efficacy with Risedronate Therapy (VERT) studies (4). The effect of risedronate on vertebral fracture risk was similar in subjects above and below the age of 80 yr (4). However, the effect of risedronate on nonvertebral fracture risk was only significant in subjects below the age of 80 yr (4). Similarly, the Fracture Intervention Trial (FIT) showed significant reductions in vertebral fracture in the subgroup over age 75 with alendronate treatment. Although the reductions in clinical fractures overall were not statistically significant in those over 75, the treatment-by-age interaction was not significant, supporting a homogeneity of effect across age groups (25). HORIZON-PFT is the first study to show a significant reduction in nonvertebral fractures with bisphosphonates among those over age 75.
In the teriparatide study, which included 1085 women from the fracture prevention study, the effect of teriparatide on vertebral fractures was similar in subjects above and below the age of 75 yr (5). However, the effect on nonvertebral fractures was only significant below the age of 75 yr. Again, the subset of elderly patients was relatively small, as was the number of nonvertebral fractures, limiting the statistical power to address nonvertebral fracture efficacy. The strontium ranelate study prospectively assessed 1488 women over age 80 yr and showed a significant reduction in vertebral and nonvertebral fractures (6). The Women’s Health Initiative hormone trials did not find a difference in the effects of hormone replacement therapy on fracture risk according to age (11, 26).
Whether reductions in hip fracture risk with therapy decrease with age has been the subject of some controversy. We found no treatment by age interaction for hip fracture and so cannot claim less efficacy of zoledronic acid in hip fracture risk in the elderly. In the HIP study of risedronate, in the subgroup of women over age 80, the reduction in hip fracture risk was 20% (7) but was not statistically significant. This oldest group of women was recruited based on their age plus an additional risk factor for hip fracture but did not necessarily have low BMD. Whether this lack of reduction was due to the presence of other risk factors (e.g. high risk of falling), absence of low BMD, or other factors such as low compliance cannot be determined.
We cannot be certain whether or not there is an attenuation of effect of osteoporosis treatments on fracture risk reduction with increasing age, and this merits further study. It is likely that there is some attenuation because there is a lesser dependency of fracture risk on BMD with age (12).
There have been no analyses of any treatment interaction with renal function, although poor renal function has been associated with greater fracture risk (20). The impact of renal function was attenuated after adjustment for age. We defined our threshold for decreased renal function based on the definition of stage 3 chronic kidney disease, i.e. estimated creatinine clearance between 30 and 59 ml/min, and we limited inclusion of patients to those with creatinine clearance above 30 ml/min measured at two separate occasions before randomization. This level of chronic kidney disease can be associated with the early stages of renal osteodystrophy, such as secondary hyperparathyroidism, and this may attenuate the benefit of the drug (27). However, this is not as likely because there was no treatment-by-BMD interaction in the current study. There is an association between fall risk and renal impairment (20), and so falls may have a more important role in fracture risk in such subjects. More studies are needed to examine for an interaction between renal function and treatment on fracture risk.
The least expected interaction was between BMI and treatment for vertebral fracture in which those with highest BMI had the strongest reduction. There is a similar (nonsignificant) trend for nonvertebral fractures. Low BMI is a strong indicator of risk for all fractures, but the relationship is most evident for hip fractures (13). This effect is mainly mediated through low BMD, and so it might have been expected that women with low BMI would be more responsive to zoledronic acid than overweight women (with high BMI). Falls are considered to be an important risk factor for vertebral fracture (18, 28); it is possible that they are particularly important for slender women because there is insufficient “padding” to protect against a fall. If the trauma of a fall was more important in slender women, then zoledronic acid would not be expected to be as protective as when low BMD is a more important factor. More studies are needed to examine for an interaction between BMI and treatment on fracture risk.
The interactions between risk factors and treatment were not significant for nonvertebral fracture or hip fracture. For both, there was a weak relationship with prior use of bisphosphonates. We appreciate that bisphosphonates such as alendronate may continue to suppress bone turnover and possibly fracture risk for several years after stopping them (21, 22), and this might mean that the effect of a newly introduced treatment might be less effective. This is further supported by the smaller increase in BMD noted with zoledronic acid after prior bisphosphonate use in the current study, although there was no significant treatment-by-prior-use interaction with femoral neck BMD. Several previous studies have noted an interaction between baseline BMD and nonvertebral fracture reduction for bisphosphonates wherein nonvertebral fracture reductions were great among those with lower BMD (7, 9, 29). However, greater efficacy for nonvertebral or hip fractures was not observed with zoledronic acid for those with lower BMD. However, it should be noted that in the current study, women were selected by baseline BMD T-score and vertebral fracture status in such a way that no women with high BMD (T-score >−2.5) without a vertebral fracture were included (2). Therefore, the effect of baseline BMD on reduction of fracture or BMD could not be independently evaluated.
The only significant interaction with treatment and hip BMD was for baseline total hip BMD; there was a larger BMD increase among those with lower BMD. This may relate to the higher bone turnover rate found in women with low BMD. Therefore, higher bone turnover might be expected to be associated with bigger increases in BMD in response to zoledronic acid, because the level of mineralization would be lower at baseline and the remodeling space higher. However, this could be a common variable effect; when we examined absolute change in BMD, we found that there was no longer a significant interaction with low BMD.
In conclusion, we found that zoledronic acid had similar effects on hip and nonvertebral fracture and change in femoral neck BMD independent of demographics and baseline risk factors for fracture. However, zoledronic acid appeared more effective in preventing vertebral fracture in younger women, women with higher BMI, and women with normal renal function. Overall, in general, even when treatment-by-factor interactions were present, the efficacy of zoledronic acid was positive across the different levels of subgroups, indicative of the robust efficacy profile of zoledronic acid as a once-a-year therapy for the treatment of postmenopausal osteoporosis.
Acknowledgments
Special thanks are due Zeb Horowitz (now at Savient Pharmaceuticals), John Orloff (Novartis), and the following: Steering Committee members—Dennis Black, Steven Cummings, Pierre Delmas, Richard Eastell, Ian Reid, Steven Boonen, Jane Cauley, Felicia Cosman, Péter Lakatos, Ping C. Leung, Zulema Man, Erik Fink Eriksen (Novartis), Peter Mesenbrink (Novartis); Past Steering Committee members—Edith Lau, Saloman Jasqui, Carlos Mautalen, Theresa Rosario-Jansen (Novartis), John Caminis (Novartis); Data Safety Monitoring Board—Lawrence Raisz (chairman), Peter Bauer, Juliet Compston, David DeMets, Raimund Hirschberg, Olof Johnell, Stuart Ralston, Robert Wallace; DSMB Consultants—Michael Farkough; Novartis—Mary Flood; University of California, San Francisco (UCSF) Coordinating Center—Douglas Bauer, Lisa Palermo; UCSF Radiology (QCT analysis)—Thomas Lang.
We are indebted to the HORIZON-PFT Clinical Site Investigators—Argentina: Eduardo Kerzberg, Zulema Man, Carlos Mautalen, Maria Ridruejo, Guillermo Tate, Jorge Velasco; Australia: Michael Hooper, Mark Kotowicz, Peter Nash, Richard Prince, Anthony Roberts, Philip Sambrook; Austria: Harald Dobnig, Gerd Finkenstedt, Guenter Hoefle, Klaus Klaushofer, Martin Pecherstorfer, Peter Peichl; Belgium: Jean Body, Steven Boonen, Jean-Pierre Devogelaer, Piet Geusens, Jean Kaufman; Brazil: João Brenol, Jussara Kochen, Rubem Lederman, Sebastiao Radominski, Vera Szejnfeld, Cristiano Zerbini; Canada: Jonathan Adachi, Jacques Brown, Denis Choquette, David Hanley, Robert Josse, David Kendler, Richard Kremer, Frederic Morin, Wojciech Olszynski, Alexandra Papaioannou, Chiu Kin Yuen; China: Baoying Chen, Shouqing Lin; Colombia: Nohemi Casas, Monique Chalem, Juan Jaller, Jose Molina; Finland: Hannu Aro, Jorma Heikkinen, Heikki Kröger, Lasse Mäkinen, Juha Saltevo, Jorma Salmi, Matti Välimäki; France: Claude-Laurent Benhamou, Pierre Delmas, Patrice Fardellone, Georges Werhya; Germany: Bruno Allolio, Dieter Felsenberg, Joachim Happ, Manfred Hartard, Johannes Hensen, Peter Kaps, Joern Kekow, Ruediger Moericke, Bernd Ortloff, Peter Schneider, Siegfried Wassenberg; Hong Kong: Ping Chung Leung; Hungary: Adam Balogh, Bela Gomor, Tibor Hidvégi, Laszlo Koranyi, Péter Lakatos, Gyula Poór, Zsolt Tulassay; Israel: Rivka Dresner Pollak, Varda Eshed, A. Joseph Foldes, Sophia Ish-Shalom, Iris Vered, Mordechai Weiss; Italy: Silvano Adami, Antonella Barone, Gerolamo Bianchi, Sandro Giannini, Giovanni Carlo Isaia, Giovanni Luisetto, Salvatore Minisola, Nicola Molea, Ranuccio Nuti, Sergio Ortolani, Mario Passeri, Alessandro Rubinacci, Bruno Seriolo, Luigi Sinigaglia; Korea (Republic of): Woong-Hwan Choi, Moo-II Kang, Ghi-Su Kim, Hye-Soon Kim, Yong-Ki Kim, Sung-Kil Lim, Ho-Young Son, Hyun-Koo Yoon; Mexico: Carlos Abud, Pedro Garcia, Salomon Jasqui, Luis Ochoa, Javier Orozco, Javier Santos; New Zealand: Ian Reid; Norway: Sigbjørn Elle, Johan Halse, Arne Høiseth, Hans Olav, Høivik Ingun Røed, Arne Skag, Jacob Stakkestad, Unni Syversen; Poland: Janusz Badurski, Edward Czerwinski, Roman Lorenc, EwaMarcinowska-Suchowierska, Andrzej Sawicki, Jerzy Supronik; Russia: Eduard Ailamazyan, Lidiya Benevolenskaya, Alexander Dreval, Leonid Dvoretsky, Raisa Dyomina, Vadim Mazurov, Galina Melnichenko, Ashot Mkrtoumyan, Alexander Orlov-Morozov, Olga Ostroumova, Eduard Pikhlak, Tatiana Shemerovskaya, Nadezhda Shostak, Irina Skripnikova, Vera Smetnik, Evgenia Tsyrlina, Galina Usova, Alsu Zalevskaya, Irina Zazerskaya, Eugeny Zotkin; Sweden: Osten Ljunggren, Johan Lofgren, Mats Palmér, Maria Saaf, Martin Stenström; Switzerland: Paul Hasler, Olivier Lamy, Kurt Lippuner, Claude Merlin, René Rizzoli, Robert Theiler, Alan Tyndall, Daniel Uebelhart; Taiwan: Jung-Fu Chen, Po-Quang Chen, Lin-show Chin, Jawl-Shan Hwang, Tzay-Shing Yang, Mayuree Jirapinyo; Thailand: Mayuree Jirapinyo, Rojanasthien Sattaya, Sutin Sriussadaporn, Soontrapa Supasin, Nimit Taechakraichana, Kittisak Wilawan; United Kingdom: Hugh Donnachie, Richard Eastell, William Fraser, Alistair McLellan, David Reid; United States: John Abruzzo, Ronald Ackerman, Robert Adler, John Aloia, Charles Birbara, Barbara Bode, Henry Bone, Donald Brandon, Jane Cauley, Felicia Cosman, Daniel Dionne, Robert Downs Jr., James Dreyfus, Victor Elinoff. Ronald Emkey, Joseph Fanciullo, Darrell Fiske, Palmieri Genaro, M. Gollapudi, Richard Gordon, James Hennessey, Paul Howard, Karen Johnson, Conrad Johnston, Risa Kagan, Shelly Kafka, Jeffrey Kaine, Terry Klein, William Koltun, Meryl Leboff, Bruce Levine, E. Michael Lewiecki, Cora Elizabeth Lewis, Angelo Licata, Michael Lillestol, Barry Lubin, Raymond Malamet, Antoinette Mangione, Velimir Matkovic, Daksha Mehta, Paul Miller, Sam Miller, Frederik T. Murphy, Susan Nattrass, David Podlecki, Christopher Recknor, Clifford Rosen, Daniel Rowe, Robert Rude, Thomas Schnitzer, Yvonne Sherrer, Stuart Silverman, Kenna Stephenson, Barbara Troupin, Joseph Tucci, Reina Villareal, Nelson Watts, Richard Weinstein, Robert Weinstein, Michael Weitz, and Richard White.
This work was supported by Novartis Pharma.
Abbreviations
- BMD
Bone mineral density
- BMI
body mass index
Footnotes
Clinical Trials.gov no. NCT00049829.
Disclosure Summary: R.E. serves as a consultant, has received honoraria for speaking, and has received grant funding from Novartis, Amgen, Sanofi-Aventis, Lilly, Organon, Pfizer, and Procter & Gamble Pharmaceuticals. D.M.B. reports holding research contracts with Novartis and Roche, receiving speaker fees from NPS Pharmaceuticals, and consulting for GlaxoSmithKline (GSK). S.B. reports receiving consulting fees and research funding from Novartis and having received research funding from Amgen, GlaxoSmithKline, Merck, Novartis, Procter & Gamble, Roche, and Sanofi-Aventis. S.A. serves on advisory committees for Roche-GSK, Merck Sharp & Dohme (MSD), Novartis, and Lilly. D.F. has received research grants from GSK, General Electric, MSD, Novartis, Procter & Gamble, Lilly, Nycomed, Servier, Wyeth, and Chugai and has served on advisory boards for Novartis, MSD, Procter & Gamble, Lilly, Roche, Amgen, TEVA Pharmaceuticals, and Bayer. K.L. has received research grants from Amgen, MSD, Novartis, and Roche-GSK and serves on advisory boards for Amgen, Eli Lilly, MSD, Novartis, and Roche-GSK. S.R.C. reports receiving consulting/speaker fees from Novartis, Lilly, Amgen, Zelos, and Procter & Gamble. P.D.D. received research grants from Amgen, Eli Lilly, and Procter & Gamble, and consulting and/or speaker fees from Acceleron, Amgen, Eli Lilly, GSK, MSD, Novartis, Nycomed, Organon, Pfizer, Procter & Gamble, Roche, Sanofi-Aventis, Servier, Wyeth, and Zelos. L.P. received consulting/data analysis fees from NPS Pharmaceuticals. P.M. is an employee of Novartis and owns stock in the company. J.A.C. has received research grants from Novartis, Pfizer, Merck, Eli Lilly, and Procter & Gamble and honoraria from Novartis.
References
- 1.Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, Widmer A, Devogelaer JP, Kaufman JM, Jaeger P, Body JJ, Brandi ML, Broell J, Di Micco R, Genazzani AR, Felsenberg D, Happ J, Hooper MJ, Ittner J, Leb G, Mallmin H, Murray T, Ortolani S, Rubinacci A, Saaf M, Samsioe G, Verbruggen L, Meunier PJ. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–661. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 3.Jones G, Nguyen T, Sambrook P, Kelly PJ, Eisman JA. Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo osteoporosis epidemiology study. BMJ. 1994;309:691–695. [PMC free article] [PubMed] [Google Scholar]
- 4.Boonen S, McClung MR, Eastell R, El-Hajj Fuleihan G, Barton IP, Delmas P. Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc. 2004;52:1832–1839. doi: 10.1111/j.1532-5415.2004.52506.x. [DOI] [PubMed] [Google Scholar]
- 5.Boonen S, Marin F, Mellstrom D, Xie L, Desaiah D, Krege JH, Rosen CJ. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54:782–789. doi: 10.1111/j.1532-5415.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 6.Seeman E, Vellas B, Benhamou C, Aquino JP, Semler J, Kaufman JM, Hoszowski K, Varela AR, Fiore C, Brixen K, Reginster JY, Boonen S. Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res. 2006;21:1113–1120. doi: 10.1359/jbmr.060404. [DOI] [PubMed] [Google Scholar]
- 7.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 8.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 9.Chesnut CH, III, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 11.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 12.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 13.De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 15.Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl):57S–63S. doi: 10.1016/8756-3282(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 17.Tobias JH, Hutchinson AP, Hunt LP, McCloskey EV, Stone MD, Martin JC, Thompson PW, Palferman TG, Bhalla AK. Use of clinical risk factors to identify postmenopausal women with vertebral fractures. Osteoporos Int. 2007;18:35–43. doi: 10.1007/s00198-006-0209-8. [DOI] [PubMed] [Google Scholar]
- 18.Freitas SS, Barrett-Connor E, Ensrud KE, Fink HA, Bauer DC, Cawthon PM, Lambert LC, Orwoll ES. Rate and circumstances of clinical vertebral fractures in older men. Osteoporos Int. 2008;19:615–623. doi: 10.1007/s00198-007-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;288:2300–2306. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- 20.Dukas L, Schacht E, Stähelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int. 2005;16:1683–1690. doi: 10.1007/s00198-005-1903-7. [DOI] [PubMed] [Google Scholar]
- 21.Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19:1259–1269. doi: 10.1359/JBMR.040326. [DOI] [PubMed] [Google Scholar]
- 22.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 23.Black DM, Palermo L, Nevitt MC, Genant HK, Christensen L, Cummings SR. Defining incident vertebral deformity: a prospective comparison of several approaches. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:90–101. doi: 10.1359/jbmr.1999.14.1.90. [DOI] [PubMed] [Google Scholar]
- 24.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine – reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 25.Ensrud KE, Black DM, Palermo L, Bauer DC, Barrett-Connor E, Quandt SA, Thompson DE, Karpf DB. Treatment with alendronate prevents fractures in women at highest risk: results from the Fracture Intervention Trial. Arch Intern Med. 1997;157:2617–2624. [PubMed] [Google Scholar]
- 26.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA, Ko MG, Naughton MJ, Satterfield S, Bassford T. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women’s Health Initiative Randomized Trial. J Bone Miner Res. 2006;21:817–828. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 27.Coen G, Manni M, Addari O, Ballanti P, Pasquali M, Chicca S, Mazzaferro S, Napoletano I, Sardella D, Bonucci E. Metabolic acidosis and osteodystrophic bone disease in predialysis chronic renal failure: effect of calcitriol treatment. Miner Electrolyte Metab. 1995;21:375–382. [PubMed] [Google Scholar]
- 28.Nevitt MC, Cummings SR, Stone KL, Palermo L, Black DM, Bauer DC, Genant HK, Hochberg MC, Ensrud KE, Hillier TA, Cauley JA. Risk factors for a first-incident radiographic vertebral fracture in women >or =65 years of age: the study of osteoporotic fractures. J Bone Miner Res. 2005;20:131–140. doi: 10.1359/JBMR.041003. [DOI] [PubMed] [Google Scholar]
- 29.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]