Abstract
Vitamin D is essential for facilitating calcium absorption and preventing increases in parathyroid hormone (PTH), which can augment bone resorption. Our objectives were to examine serum levels of 25-hydroxyvitamin D [25(OH)D] and PTH, and factors related to longitudinal change in a population-based cohort. This is the first longitudinal population-based study looking at PTH and 25(OH)D levels. We analyzed 3896 blood samples from 1896 women and 829 men in the Canadian Multicentre Osteoporosis Study over a 10-year period starting in 1995 to 1997. We fit hierarchical models with all available data and adjusted for season. Over 10 years, vitamin D supplement intake increased by 317 (95% confidence interval [CI] 277 to 359) IU/day in women and by 193 (135 to 252) IU/day in men. Serum 25(OH)D (without adjustment) increased by 9.3 (7.3 to 11.4) nmol/L in women and by 3.5 (0.6 to 6.4) nmol/L in men but increased by 4.7 (2.4 to 7.0) nmol/L in women and by 2.7 (−0.6 to 6.2) nmol/L in men after adjustment for vitamin D supplements. The percentage of participants with 25(OH)D levels <50 nmol/L was 29.7% (26.2 to 33.2) at baseline and 19.8% (18.0 to 21.6) at year 10 follow-up. PTH decreased over 10 years by 7.9 (5.4 to 11.3) pg/mL in women and by 4.6 (0.2 to 9.0) pg/mL in men. Higher 25(OH)D levels were associated with summer, younger age, lower body mass index (BMI), regular physical activity, sun exposure, and higher total calcium intake. Lower PTH levels were associated with younger age and higher 25(OH)D levels in both women and men and with lower BMI and participation in regular physical activity in women only. We have observed concurrent increasing 25(OH)D levels and decreasing PTH levels over 10 years. Secular increases in supplemental vitamin D intake influenced both changes in serum 25(OH)D and PTH levels.
Keywords: 25(OH)D, PTH, POPULATION-BASED, LONGITUDINAL, VITAMIN D SUPPLEMENT
Introduction
Vitamin D is essential for facilitating calcium absorption and preventing increases in parathyroid hormone (PTH), which can augment bone resorption. Vitamin D may also have important extraskeletal health benefits, including decreasing the risk of chronic diseases such as multiple sclerosis, cancer, diabetes, and cardiovascular disease.(1,2) Nevertheless, what level of the major circulating metabolite of vitamin D, 25-hydroxyvitamin D [25(OH)D], might be required to attain such benefits(3) is unclear, and there are concerns about benefits and risks associated with higher levels of 25(OH)D.
The accepted functional indicator of vitamin D status is the serum level of 25(OH)D, which provides an integrated index of vitamin D derived from both skin synthesis and intestinal absorption and is used to assess vitamin D nutritional status. Recognizing differences in the definition of an optimal level of 25(OH)D, an assessment of the global status of vitamin D concluded that serum 25(OH)D levels below 50 or 75 nmol/L are a worldwide phenomenon and that hypovitaminosis D is reemerging as a major health problem globally.(4) A recent Institute of Medicine (IOM) report concluded, however, that a 25(OH)D level of 50 nmol/L would be sufficient to prevent 97.5% of healthy individuals from adverse bone health outcomes.(5) Population-based cross-sectional studies have shown that 25(OH)D levels vary by season,(6,7) are lower in the elderly,(8) are lower with deeper skin pigmentation,(9) are lower in obese individuals,(10) and vary by vitamin D intake.(7,10)
There are few population-based studies on temporal changes in 25(OH)D. The National Health and Nutrition Examination Surveys (NHANES) reported 25(OH)D values at different time points, although not on the same participants. Although they initially reported a decrease in 25(OH)D values,(11) most of the apparent reduction was subsequently ascribed to methodological differences.(12) The only longitudinal population-based cohort study tracked 25(OH)D over 14 years on the same participants,(13) finding that most subjects with low serum 25(OH)D levels are unlikely to have a substantial improvement in their vitamin D levels over time.
PTH is believed to be an important mediator of the skeletal effects of low vitamin D status; however, the only studies assessing the association of 25(OH)D and PTH are cross-sectional(8,14,15) or not population-based.(16–18) We therefore used the Canadian Multicentre Osteoporosis Study (CaMos) data set to examine, at the population level, longitudinal changes over time in serum levels of 25(OH)D and PTH, factors related to their change, and associations between the two.
Materials and Methods
Participants
CaMos is an ongoing, prospective cohort study of 9423 community-dwelling, randomly selected women (6539) and men (2884), aged 25 years and older at baseline, living within 50 km of nine Canadian cities (St John’s, Halifax, Quebec City, Kingston, Toronto, Hamilton, Saskatoon, Calgary, and Vancouver). Baseline cohort recruitment was between 1995 and 1997. CaMos objectives, methodology, and sampling framework are described in detail elsewhere.(19) Data collection at baseline included an extensive interviewer-administered questionnaire including sociodemographic information, medical history, family history, dietary intake, physical activity, and tobacco smoking. Clinical assessments included height and weight for all centers and blood collection in one center (Quebec City). Year 5 (2000 to 2002) and year 10 (2005 to 2007) follow-ups included an interviewer-administered questionnaire and clinical assessment of height and weight in all centers and blood collection in three centers at year 5 (Calgary, Hamilton, and Quebec City) and in seven centers at year 10 (Halifax, Quebec City, Kingston, Toronto, Saskatoon, Calgary, and Vancouver). Ethics approval was granted through McGill University and the appropriate research ethics board for each participating center. Signed informed consent was obtained from all study participants in accordance with the Helsinki Declaration.
The current study included women and men who gave blood at baseline and at year 5 and/or year 10 follow-ups. We studied 1965 women and 850 men aged 25 years or older from eight of the CaMos centers, for a total of 4016 available blood samples drawn in 1995 to 1997, 2000 to 2002, and/or 2005 to 2007. Overall, 89.0% of the blood samples were collected within 90 days of their interview and 83.4% within the same season (as defined by summer and winter).
Serum 25(OH)D and PTH
All sera were analyzed at the same laboratory, using an identical technique, over a 3- to 4-month period with a single lot of quality controls and only three reagent lots. Fasting blood samples from participants had been aliquoted and stored at −80°C. Serum total 25(OH)D and PTH were measured using the Liaison (DiaSorin, Stillwater, MN, USA) autoimmunoanalyzer, which employs chemiluminescent immunoassay technology. Details on detection limit, assay variability, and quality controls are provided elsewhere.(10) The 95% PTH reference range is 22.2 to 108.9 pg/mL. The laboratory participates in the international Vitamin D External Quality Assessment Scheme (DEQAS).(20)
Calcium and vitamin D intake
Information on dietary calcium intake was obtained from an interviewer-administered, abbreviated semiquantitative food frequency questionnaire (FFQ). The semiquantitative FFQ included only the foods considered to be excellent sources of calcium. Dietary vitamin D intake was estimated from reported usual intake of vitamin D-fortified fluid milk, soya beverage, and yogurt. Fish consumption was not included in the CaMos FFQ, and therefore, vitamin D content from fish was not included in the dietary vitamin D intake. Vitamin D content of food was calculated using Canada’s Food and Drugs regulations on fortification standards and vitamin D quantity from food labels. The vitamin D content of medications and supplements was determined using Health Canada’s drug product database.(21) However, although almost all vitamin D supplements in Canada are vitamin D3 (in contrast to the United States, where most are vitamin D2), the type of vitamin D is not always included in the database and precluded us from obtaining the exact proportion of vitamin D2 versus vitamin D3 use. Details related to food and supplement data collection were previously reported.(22)
Statistical analyses
25(OH)D levels found to be “<10 nmol/L” were fixed to 10 nmol/L in the database. Percentage of participants with 25(OH)D levels <50 nmol/L [not meeting the 25(OH)D level consistent with the IOM’s Recommended Dietary Allowance(5)] and <75 nmol/L (not meeting the Endocrine Society’s recommended level(23)) were computed. Observed longitudinal change was computed using participants with two or more blood draws over time within the same season so that a positive difference implies an increase over time. Mean rate of change was converted to change over 10 years.
We used a hierarchical model to estimate the change in vitamin D supplement intake per year, adjusted for the season of interview (winter, summer). For this particular model, we used all available information on vitamin D supplement intake, even from a follow-up for which the participant did not give blood. Hierarchical models were also used to estimate the change over time in 25(OH)D levels and in PTH levels, adjusted for the season of blood draw (winter, summer). For 25(OH)D as outcome, we further adjusted for vitamin D supplement intake, when the latter was reported during the same season as the blood draw. Separate models were also fit for 25(OH)D levels as the outcome, based on the initial level of 25(OH)D: <50 nmol/L or ≥50 nmol/L. For PTH as an outcome, we further adjusted for polynomials of 25(OH)D levels. Models with polynomial terms of up to three degrees were considered. Mathematical minima(24) for this polynomial were computed using derivatives indicating the 25(OH)D level at which PTH levels stopped decreasing with increasing 25(OH)D. Separate models were also built for PTH levels by age groups (≤70 years or >70 years) and by season (summer or winter).
Hierarchical regression models were used to assess the association between change in 25(OH)D, change in PTH levels, and their possible respective determinants. These possible determinants included total calcium intake from dietary sources and supplements (mg/day), dietary vitamin D and vitamin D supplement intake (IU/day), sun exposure (≥30 minutes) in direct sunlight (never, seldom, regular/often), age group (25 to 50, 51 to 70, >70), body mass index (BMI; weight[kg]/height[m2]), season of blood draws (winter: November to April; summer: May to October), center, and participation in a regular physical program or activity (yes/no). Interactions of vitamin D supplement intake with season and sun exposure were considered. Our model, therefore, combined data from various levels of 25(OH)D and various times of blood collection in a complex Bayesian hierarchical model(25) that accounts for all levels of uncertainty. For the model with PTH over time as the outcome, we also considered polynomials of 25(OH)D levels and interaction of total calcium intake with 25(OH)D levels.
Separate models were run for men and women. The effects of season, vitamin D supplement, and all other possible determinants of 25(OH)D and PTH used were hypothesized not to vary with time or participants. Time was included in the models as an independent variable varying for each participant. The intercept, also varying for each participant, and the time variable were assumed to follow a normal distribution for each participant with mean mu and variance sigma. The means mu will follow their own regression lines, so that individual slopes will be allowed to vary. We used a Gibbs sampler algorithm implemented via WinBUGS software (Windows Bayesian inference Using Gibbs Sampling, Version 1.4.3 for Windows, MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK) to approximate the posterior densities of all mean and variance parameters. Between 20,000 and 50,000 iterations in WinBUGS were run to ensure convergence of the models and used for inferences. Noninformative prior distributions have been used so that all inferences will be driven by the data. Parameter estimates and their 95% credible intervals are provided as results from these models. Credible intervals are the equivalent to confidence intervals. Descriptive analyses were performed using SAS 9.1 (Cary, NC, USA).
Results
Characteristics of the studied sample
In our study, 1313 women and 576 men had one blood measurement, 454 women and 197 men had two measurements over time, and 198 women and 77 men had three measurements. The characteristics of the study participants at each time point are presented in Table 1. The percentage of women having their blood drawn during the summer was higher at year 10 than at baseline. Among the 4016 blood samples available, 44.6% were from Quebec City. At year 10, compared with baseline, the participants were older, had increased their sun exposure, and were more engaged in regular physical activity. Their total calcium and vitamin D intake had also increased during this period.
Table 1.
Characteristics of the Participants by Follow-up Year
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Year | Baseline | 5 | 10 | Baseline | 5 | 10 |
| No. | 465 | 1013 | 1337 | 185 | 439 | 577 |
| Percentage by sex per year | ||||||
| Summer | 51.4% | 53.7% | 56.8% | 51.9% | 50.8% | 51.3% |
| Quebec | 100.0% | 47.0% | 24.0% | 100.0% | 48.3% | 23.2% |
| Age (years) | ||||||
| 25–50 | 17.6% | 9.5% | 6.6% | 26.5% | 14.1% | 10.6% |
| 51–70 | 61.3% | 51.9% | 51.0% | 48.7% | 49.4% | 49.0% |
| >70 | 21.1% | 38.6% | 42.4% | 24.8% | 36.5% | 40.4% |
| Sun exposure | ||||||
| Never | 86.2% | 53.0% | 45.2% | 76.8% | 36.9% | 35.4% |
| Seldom | 11.8% | 31.6% | 29.4% | 20.0% | 44.2% | 31.9% |
| Regular/often | 2.0% | 15.4% | 25.4% | 3.2% | 18.9% | 32.6% |
| Regular activity | 48.8% | 42.1% | 54.8% | 46.0% | 42.6% | 53.5% |
| Antiresorptive use | 30.5% | 48.5% | 35.4% | 0.0% | 5.2% | 7.3% |
| Mean (SD) | ||||||
| PTH (pg/mL) | 70.8 (35.6) | 72.6 (34.6) | 62.0 (27.9) | 67.7 (28.2) | 72.7 (32.5) | 63.7 (34.5) |
| 25(OH)D (nmol/L) | 59.5 (20.7) | 64.4 (23.2) | 70.7 (24.7) | 64.7 (23.2) | 67.0 (23.7) | 69.9 (25.0) |
| Vitamin D supplement (IU/day) | 118 (688) | 243 (330) | 522 (934) | 38 (134) | 96 (206) | 273 (742) |
| Vitamin D from milk +beverages (IU/day) | 103 (128) | 109 (111) | 112 (115) | 108 (123) | 126 (155) | 114 (128) |
| Calcium supplement (mg/day) | 180 (314) | 424 (463) | 516 (505) | 48 (206) | 117 (250) | 217 (363) |
| Dietary calcium (mg/day) | 762 (463) | 828 (473) | 866 (518) | 721 (403) | 855 (609) | 825 (513) |
| BMI (kg/m2) | 26.2 (4.9) | 27.2 (5.4) | 27.2 (5.5) | 26.4 (3.7) | 27.3 (3.9) | 27.6 (4.9) |
Calcium and vitamin D supplement intakes
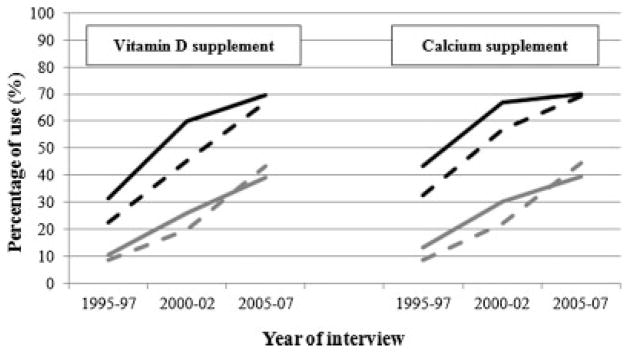
The calcium and vitamin D intake from the diet stayed stable over time, and the change in total calcium and vitamin D intakes were due to an increase in supplement intakes. At baseline, 36.0% of women and 10.3% of men were taking calcium supplements for a mean (SE) calcium supplement intake in users of 487 (24) mg/day in women and 466 (110) mg/day in men. At year 10, these percentages had increased to 69.5% in women and 42.3% in men for a mean intake in users of 744 (15) mg/day in women and 494 (26) mg/day in men. At baseline, 25.6% of women and 9.2% of men were taking vitamin D supplements for a mean (SE) vitamin D supplement intake in users of 463 (122) IU/day in women and 416 (49) IU/day in men. At year 10, these percentages had increased to 68.1% in women and 41.4% in men for a mean intake in users of 755 (34) IU/day in women and 650 (69) IU/day in men. Of those using vitamin D supplements, 94.0% of women and 76.5% of men at baseline and 94.7% of women and 92.7% of men at year 10 were also taking calcium supplements. The use of calcium and vitamin D supplement by season is depicted in Fig. 1 and shows a slightly greater use in winter compared with summer.
Fig. 1.
Calcium and vitamin D supplement use by sex (women: black; men: gray) and season (winter: solid line; summer: dotted line).
We estimated that vitamin D supplement intake increased annually by 32 (27 to 36) IU/day in women and 19 (14 to 25) IU/day in men. Summer was associated with an estimate decrease in vitamin D supplement intake of 27 (−10 to 64) IU/day in women and 6 (−33 to 45) IU/day in men.
25(OH)D levels and change in 25(OH)D levels over time
The percentages of participants by sex, season, and year of follow-up with 25(OH)D levels <50 nmol/L and <75nmol/L are presented in Table 2 along with their 95% confidence intervals. Overall, the percentage of participants with 25(OH)D levels <50 nmol/L and <75 nmol/L had decreased by 9.9% and by 16.2%, respectively, over 10 years, with a bigger decrease in the winter compared with the summer.
Table 2.
Percentage (95% CI) of Participants With 25(OH)D Levels <50 nmol/L [Not Meeting the 25(OH)D Level Consistent With the IOM’s Recommendation] or <75 nmol/L (Not Meeting the Endocrine Society’s Recommended Level) per Follow-up Year
| Baseline, % (95% CI)
|
Year 5, % (95% CI)
|
Year 10, % (95% CI)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | <50 nmol/L | <75 nmol/L | n | <50 nmol/L | <75 nmol/L | n | <50 nmol/L | <75 nmol/L | |
| Women | |||||||||
| Summer | 239 | 20.9 (15.7–26.1) | 74.1 (68.5–79.7) | 544 | 21.7 (18.2–25.3) | 65.8 (61.8–69.8) | 760 | 16.2 (13.6–18.8) | 56.5 (53.0–60.0) |
| Winter | 226 | 42.5 (36.1–48.9) | 82.7 (77.8–87.6) | 469 | 32.6 (28.4–36.8) | 77.4 (73.6–81.2) | 577 | 22.9 (19.5–26.3) | 65.7 (61.8–69.6) |
| Summer and winter | 465 | 31.4 (27.2–35.6) | 78.3 (74.6–82.0) | 1013 | 26.8 (24.1–29.5) | 71.2 (68.4–74.0) | 1337 | 19.1 (17.0–21.2) | 60.4 (57.8–63.0) |
| Men | |||||||||
| Summer | 96 | 19.8 (11.8–27.8) | 62.5 (52.8–72.2) | 223 | 13.5 (9.0–18.0) | 59.2 (52.7–65.7) | 296 | 18.2 (13.8–22.6) | 54.4 (48.7–60.1) |
| Winter | 89 | 31.5 (21.8–41.2) | 80.9 (72.7–89.1) | 216 | 30.1 (24.0–36.2) | 74.5 (68.9–80.3) | 281 | 24.9 (19.8–30.0) | 64.8 (59.2–70.4) |
| Summer and winter | 185 | 25.4 (19.1–31.7) | 71.4 (64.9–77.9) | 439 | 21.6 (17.8–25.4) | 66.7 (62.3–71.1) | 577 | 21.5 (18.1–24.9) | 59.5 (55.5–63.5) |
| Women and men | |||||||||
| Summer | 335 | 20.6 (16.3–24.9) | 70.8 (65.9–75.7) | 767 | 19.3 (16.5–22.1) | 63.9 (60.5–67.3) | 1056 | 16.8 (14.5–19.1) | 55.9 (52.9–58.9) |
| Winter | 315 | 39.4 (34.0–44.8) | 82.2 (78.0–85.4) | 685 | 31.8 (28.3–35.3) | 76.5 (73.3–79.7) | 858 | 23.5 (20.7–26.3) | 65.4 (62.2–68.6) |
| Summer and winter | 650 | 29.7 (26.2–33.2) | 76.3 (73.0–79.6) | 1452 | 25.2 (23.0–27.4) | 69.8 (67.4–72.2) | 1914 | 19.8 (18.0–21.6) | 60.1 (57.9–62.3) |
Based on participants with repeated blood draws in the same season as the initial blood draw, we found that 30.5% of women and men had a 25(OH)D increase >20 nmol/L over 10 years, whereas 20.2% had a 25(OH)D decrease >20 nmol/L. These results translated into an estimated 10-year increase of 25(OH)D levels by 9.3 (7.3 to 11.4) nmol/L in women and by 3.5 (0.6 to 6.4) nmol/L in men (Table 3). Vitamin D intake from supplements accounted for 44% of the serum 25(OH)D increase in women and 25% of the increase in men. Serum 25(OH)D was higher in samples collected in summer in both women (by 6.9 [4.9 to 8.8] nmol/L) and men (by 8.4 [5.4 to 11.5] nmol/L). Individuals with initial 25(OH)D levels <50 nmol/L showed a larger increase in 25(OH)D levels over the 10 years (Table 3). Overall, the use of 400 IU/day of vitamin D supplement increased 25(OH)D levels by an estimated 5.3 (4.8 to 5.8) nmol/L in women and 4.5 (3.4 to 5.5) nmol/L in men. When the initial serum 25(OH)D value was <50 nmol/L, these increases were 10.9 (8.6 to 13.1) nmol/L in women and 7.9 (−2.0 to 17.8) nmol/L in men.
Table 3.
Estimated 10-Year Change in 25(OH)D (nmol/L per Year)
| Covariables in model | Models with initial 25(OH)D <50 nmol/L | Models with initial 25(OH)D ≥50 nmol/L | Models with all 25(OH)D values | |
|---|---|---|---|---|
| Women | Season | 20.4 (15.9 to 24.9) | 0.9 (−1.8 to 3.6) | 9.3 (7.3 to 11.4) |
| Season and vitamin D supplement | 13.5 (8.6 to 18.3) | −1.81 (−5.1 to 1.5) | 4.7 (2.4 to 7.0) | |
| Men | Season | 14.7 (7.2 to 22.4) | 13.0 (8.5 to 17.1) | 3.5 (0.6 to 6.4) |
| Season and vitamin D supplement | 12.4 (4.2 to 20.7) | −0.9 (−5.6 to 3.8) | 2.7 (−0.6 to 6.2) |
Factors associated with longitudinal 25(OH)D levels
Factors associated with increased 25(OH)D levels included summer season, higher intake of vitamin D supplements, younger age, lower BMI, participation in regular physical activity, sun exposure, and higher total calcium intake (Table 4). We found different patterns of association between 25(OH)D levels and vitamin D supplement intake depending on the season and/or the sun exposure (interactions). In these multivariate models, the 10-year increases in serum 25(OH)D levels were estimated to be 4.6 (2.3 to 7.3) nmol/L in women and 3.0 (−0.7 to 6.9) nmol/L in men.
Table 4.
Parameter Estimates of Predictors of Longitudinal 25(OH)D and PTH Levels
| Parameter estimate and 95% CI
|
||||
|---|---|---|---|---|
| 25(OH)D (nmol/L)
|
PTH (pg/mL)
|
|||
| Women | Men | Women | Men | |
| Change per year | 0.5 (0.2 to 0.7) | 0.3 (−0.1 to 0.7) | −0.7 (−1.0 to −0.4) | −0.7 (−1.2 to −0.2) |
| Age (years) | ||||
| 25–50 | 6.5 (3.1 to 10.0) | 5.1 (0.3 to 9.9) | −10.8 (−15.3 to −6.2) | −15.3 (−21.5 to −9.1) |
| 51–70 | 0.7 (−0.3 to 1.6) | −0.9 (−2.4 to 0.6) | −2.7 (−3.9 to −1.4) | −5.4 (−7.4 to −3.4) |
| 71+ | Reference category | Reference category | ||
| BMI (kg/m2) | −0.7 (−0.91 to −0.56) | −0.6 (−1.0 to −0.2) | 0.48 (0.3 to 0.7) | 0.3 (−0.2 to 0.7) |
| Center | ||||
| Vancouver | 3.4 (−4.2 to 11.1) | −9.7 (−27.2 to 8.0) | 2.1 (−6.0 to 10.0) | −3.2 (−21.6 to 15.2) |
| Calgary | −0.7 (−2.0 to 0.6) | −1.3 (−3.5 to 0.8) | −3.0 (−4.8 to −1.2) | −1.5 (−4.4 to 1.3) |
| Saskatoon | −1.6 (−2.8 to 0.4) | −1.3 (−3.2 to 0.8) | −1.9 (−3.4 to −0.2) | 0.2 (−2.5 to 2.9) |
| Hamilton | 0.7 (−0.4 to 1.8) | 1.7 (−0.1 to 3.5) | −4.5 (−5.8 to −3.1) | −4.1 (−6.2 to −1.9) |
| Toronto | −0.3 (−1.1 to 0.4) | −1.0 (−2.2 to 0.2) | −1.5 (−2.6 to −0.4) | −0.2 (−1.9 to 1.5) |
| Kingston | −0.2 (−0.8 to 0.5) | 0.2 (−1.0 to 1.4) | −0.6 (−1.5 to 0.3) | −0.4 (−2.0 to 1.2) |
| Halifax | 0.3 (−0.5 to 1.1) | 0.4 (−0.8 to 1.6) | −1.0 (−2.1 to 0.2) | −1.4 (−3.1 to 0.4) |
| Quebec city | Reference category | Reference category | ||
| Participation in a regular physical activity | 1.9 (0.2 to 3.6) | 4.1 (1.4 to 6.8) | −3.4 (−5.6 to −1.1) | −0.7 (−4.2 to 2.8) |
| Season of blood draw | ||||
| Summer | 8.2 (6.2 to 10.2) | 9.0 (5.9 to 12.2) | −0.4 (−2.7 to 2.0) | 1.1 (−2.5 to 4.7) |
| Winter | Reference category | Reference category | ||
| Sun exposure | ||||
| Never | Reference category | Reference category | ||
| Seldom | 2.8 (1.8 to 3.9) | 2.2 (0.7 to 3.6) | 1.0 (−0.4 to 2.4) | 1.4 (−0.6 to 3.3) |
| Regular/often | 2.6 (1.6 to 3.5) | 2.9 (1.7 to 4.1) | 0.6 (−0.6 to 1.7) | 0.5 (−1.2 to 2.1) |
| Total calcium intake (per 300 mg/day) | 1.2 (0.8 to 1.6) | 1.3 (0.7 to 2.0) | −0.4 (−0.9 to 0.1) | 0.1 (−0.8 to 1.0) |
| Vitamin D supplement intake (per 400 IU/day) | 7.2 (6.2 to 8.1) | 5.6 (4.1 to 7.1) | — | — |
| Summer by vitamin D supplement (per 400 IU/day) | −2.5 (−3.5 to −1.4) | −2.9 (−4.9 to −0.9) | — | — |
| Sun exposure by vitamin D supplement (per 400 IU/day) | ||||
| Never | Reference category | Reference category | ||
| Seldom | −2.1 (−3.1 to −1.0) | — | — | — |
| Regular/often | −1.4 (−3.1 to 0.3) | — | — | — |
| 25(OH)D (per 10 nmol/L) | ||||
| 25(OH)D | — | — | −14.2 (−18.3 to −10.1) | −6.9 (−9.5 to −4.2) |
| [25(OH)D]2 | — | — | 1.1 (0.6 to 1.6) | 0.2 (−0.1 to 0.4) |
| [25(OH)D]3 | — | — | −0.03 (−0.04 to −0.01) | — |
Results with 95% CI excluding zero are shown in bold.
PTH levels and change in PTH levels over time
Based on participants with repeated blood draws in the same season as the initial draw, we found that 31.5% of women and men had a PTH decrease >25 pg/mL over 10 years, whereas 20.7% had a PTH increase >25 pg/ml. When stratifying the participants according to their initial 25(OH)D levels (<50 nmol/L or ≥50 nmol/L), we found that those who had initial serum 25(OH)D values <50 nmol/L that later increased to ≥50 nmol/L had an average decrease in PTH over 10 years of 20.7 pg/mL.
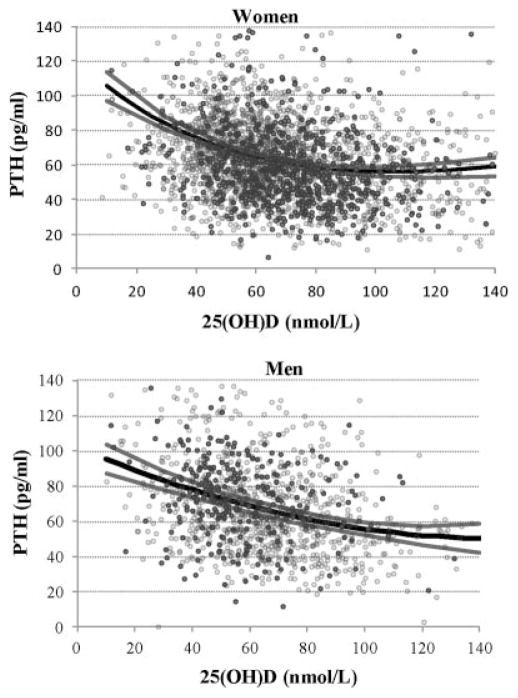
We estimated that PTH decreased over 10 years by 7.9 (5.4 to 11.3) pg/mL in women and by 4.6 (0.2 to 9.0) pg/mL in men. Models with a polynomial of degree three (cubic) and of second degree (quadratic) in 25(OH)D were used to explain PTH in women and men, respectively. Fig. 2 shows the relation obtained from PTH and 25(OH)D in the summer. In the winter, the curves are offset by −0.27 (−2.56 to 2.06) pg/mL in women and by −1.08 (−4.58 to 2.49) pg/mL in men. We found that the 25(OH)D levels above which PTH is no longer decreasing with increases in serum 25(OH)D were 104.2 nmol/L in women and 147.8 nmol/L in men. The 10-year declines in PTH were 5.7 (3.2 to 8.4) pg/mL in women and 3.3 (−0.7 to 7.5) pg/mL in men. Further adjusting for total calcium intake or separate models by season or by age group (≤70 or >70 years) did not change the decrease in PTH over time. The relationship between serum 25(OH)D and PTH did not change when the analysis was stratified by season. Models by age group showed a mathematical minima higher in women >70 years (118.6 nmol/L) compared with the overall model, whereas in men both models were linear and therefore not showing any stable period for PTH.
Fig. 2.
Estimated summer 2005 to 2007 PTH by 25(OH)D levels (black line) with 95% CI (gray lines) with observed PTH by 25(OH)D levels in the corresponding summer 2005 to 2007 period (dark gray dots) and all other observed PTH by 25(OH)D levels, ie, in 1995 to 1997, 2000 to 2002, and the winter 2005 to 2007 (pale gray circles).
Factors associated with longitudinal PTH levels
Factors associated with lower PTH levels were higher 25(OH)D levels, younger age, and center in both women and men (Table 4). In women, lower PTH levels were further associated with lower BMI and participation in a regular physical activity. PTH levels were not associated with total calcium intake. In these multivariate models, the 10-year decreases in PTH levels were estimated to be 7.4 (3.9 to 10.4) pg/mL in women and 6.7 (1.5 to 11.9) pg/mL in men.
Discussion
In this population-based Canadian cohort, we found that 25(OH)D levels increased over 10 years in all age groups and in both sexes but especially in women. In contrast, NHANES had concluded that there was no change over time in the US population.(12) In the only other population-based study examining 25(OH)D over time in the same participants, Jorde and colleagues,(13) using the Tromso cohort, followed women and men aged 25 to 84 years over 14 years. They found that 43.0% of participants had 25(OH)D levels <50 nmol/L in 1994 and 40.6% in 2008, a decrease of 2.4% over 14 years. In contrast, we found in the CaMos cohort that 29.7% of participants had 25(OH)D levels <50 nmol/L in 1995 to 1997, and this had fallen to 19.8% of the population by 2005 to 2007, a decrease of 9.9% over 10 years. Those not meeting that vitamin D threshold decreased more markedly during winter but also during summer, and more markedly in women but also in men. The Canadian Health Measures Survey (CHMS) has recently reported, from a cross-sectional study, prevalences of 25(OH)D levels <50 nmol/L that are similar to the CaMos year 10 results.(26) In view of the stability of the serum 25(OH)D levels in the Tromso population, Jorde and colleagues concluded that their results provided some support for the use of one single measurement of 25(OH)D to predict future health outcomes. Our results suggest that at least in our population, this might not be the case. When only those whose blood samples were drawn during the same season were used, the Tromso study also found that 20.7% of their participants had a change (increase or decrease) of more than 20 nmol/L over 14 years. In comparison, we found that 50.6% sustained a change in 25(OH)D but in 10 years, confirming a much greater rate of change in the CaMos population.
Concomitant with the increase in 25(OH)D levels in our study, we observed a progressive increase in intake of vitamin D supplements over the 10 years, which was more marked in women than men and somewhat more marked in winter than summer. The higher vitamin D supplement use in winter therefore suggests that an appropriate risk mitigation strategy was being used by some participants, and this increased over the course of the study. The increase in vitamin D supplement intake might be the result of increased awareness and education of the general public about the need to consume calcium and vitamin D, but it might also be the result of increased awareness and education of participants in CaMos, a study of osteoporosis. In either case, it suggests that educational strategies directed toward increasing calcium and vitamin D intake can have a positive impact on an important approach to ameliorating osteoporosis. We also found clear increasing trends over a 10-year period in regular physical activity (a proxy for outdoor activity), sun exposure, and longitudinal trends in these factors contributing to increases in serum 25(OH)D levels. We concur with the Tromso study that season, vitamin D supplementation, BMI, and physical activity are important determinants of 25(OH)D changes over time.
An inverse relationship has been reported between PTH levels and 25(OH)D levels,(27) and we found a similar relationship in our studies. Previous studies from cross-sectional, population-based data or from intervention studies have generally shown curvilinear relationships between 25(OH)D and PTH, with a plateau reached at various serum levels of 25(OH)D.(15,28) One study using a convenience sample(29) and another using a population-based study(8) found an inverse relationship with no plateau. In our study, the relationship appeared similar in summer and winter for both women and men but was different between the sexes. The relationship appeared to be similar in women ≤70 and those >70 years old but with a PTH plateau somewhat later in the older age group. The relationship by age groups in men was similar. Thus a plateau of PTH levels appeared to occur in women at about 104 nmol/L of 25(OH)D, but in men there appeared to be a progressive decline with no leveling off if at all until at least 147 nmol/L of 25(OH)D. Differences in the results of the relationships reported in the literature have been ascribed to the use of different assays for PTH and 25(OH)D measurement as well as the age, calcium intake, ethnicity, mobility, and BMI of the participant.(28–30) In our study, PTH levels appeared to vary inversely with BMI levels and with regular physical activity but not with sun exposure. At least part of the complexity of the relationship between PTH and 25(OH)D, however, lies in the fact that serum 25(OH)D is only an indirect modulator of circulating PTH levels. Thus, the direct mediators of PTH secretion are the ambient serum calcium and the levels of the active metabolite of vitamin D, 1,25dihydroxyvitamin D, which is present in target tissues. Both of these are closely homeostatically regulated. Thus, although each of these direct mediators are influenced by serum 25(OH)D, measurement of this inactive metabolite provides only an indirect assessment of the control of PTH secretion. Nevertheless, elevated serum PTH level is a well-recognized biomarker of low serum 25(OH)D levels, which we therefore used in our longitudinal analysis of vitamin D changes. To date no longitudinal analysis of PTH as a biomarker of changes in 25(OH)D has been reported. More than half (52.2%) of the population showed a change (decrease or increase) of 25 pg/mL or greater over a 10-year period, and as 25(OH)D rose from baseline to year 10, PTH levels progressively fell in all age groups and in both sexes. Consequently, the longitudinal changes in serum PTH support the longitudinal changes in serum 25(OH)D as relevant to skeletal health outcomes. Elevated PTH levels have been associated with increased markers of bone resorption in osteoporotic women,(31) and decreases in hip fracture rates have been reported in Canada over the past two decades.(32) Whether the lower PTH levels observed in our studies over time are relevant to the decline in hip fractures or to other aspects of bone health will, however, require further study.
Undoubtedly, strengths of this study are that CaMos is a population-based longitudinal cohort comprising a recent, large sample of adults, which has collected data on an array of biological, behavioural, and environmental correlates. As well, the method of blood sample analysis ensured quality and high comparability between the different years of blood collection. Questions on sun exposure, although limited, were included to provide a relative estimate of sun-exposure rank. Household nonresponse bias related to bone health was slight, although some is still possible.(33) Another limitation is that our vitamin D intake was derived only from milk products, soya beverages, and supplements and did not include, for instance, fatty fish. However, using data from the 2004 Canadian Community Health Survey Cycle 2.2, Vatanparast and colleagues(34) reported that meat and meat alternatives (including fish) represented only 31.1% of the dietary vitamin D intake. Thus, we found that the most likely explanation for the secular increase in 25(OH)D was the increased consumption of vitamin D supplements, but we cannot preclude vitamin D contributions from nondairy sources, including fatty fish. Finally, a little less than half of the blood samples were from Quebec city; thus our results might not be representative of all centers, especially for PTH, because the center variable is associated with PTH but not with 25(OH)D in our multivariate models. Our comparisons with the CHMS in terms of 25(OH)D levels tend to show that CaMos was not biased toward having healthier people because of their long-term participation in an osteoporosis study.
In summary, these results indicate a trend toward increasing 25(OH)D levels and decreasing PTH levels over 10 years. Secular increases in supplemental vitamin D have impacted both serum 25(OH)D and PTH but do not explain all of the variation in these measures.
Acknowledgments
CaMos is funded by the Canadian Institutes of Health Research (CIHR), Amgen, Eli Lilly Canada Inc., Merck Frosst Canada Ltd., Novartis Pharmaceuticals Inc., and The Dairy Farmers of Canada.
The authors thank Steve Brooks from the Bureau of Nutritional Sciences, Health Canada, for his valuable review of the manuscript. We also thank all the participants in the Canadian Multi-centre Osteoporosis Study who made this study possible.
The members of the CaMos Research Group are: David Goltzman (co-principal investigator, McGill University, Montreal), Nancy Kreiger (co-principal investigator, University of Toronto, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto). CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (former national coordinator), Suzanne Godmaire (research assistant), Silvia Dumont (research assistant), Claudie Berger (statistician), Wei Zhou (statistician). Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator). Dal-housie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator). Laval University, Quebec City, Quebec: Jacques P Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator). Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator). University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator). McMaster University, Hamilton, Ontario: Jonathan D Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator). University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P Olszynski (director), K Shawn Davison (co-director), Jola Thingvold (coordinator). University of Calgary, Calgary, Alberta: David A Hanley (director), Jane Allan (coordinator). University of British Columbia, Vancouver, British Columbia: Jerilynn C Prior (director), Millan Patel (co-director), Brian Lentle (radiologist), Yvette Vigna (coordinator).
Authors’ roles: Data acquisition: LG-F, NK, JBR, CSK, NH, KS, KSD, JDA, JPB, DAH, JCP, and DG. Study design and data analysis: CB, LJ, LL, and DG. Drafting manuscript: CB and DG. All authors have read, revised, and approved the final manuscript. CB takes responsibility for the integrity of the data analysis.
Footnotes
Disclosures
The following authors declared potential conflicts of interest as follows: DG (Amgen, Eli Lilly, Merck Frosst, and Novartis), DAH (Amgen, Eli Lilly, Novartis, Procter & Gamble, Sanofi Aventis, Merck Frosst, Servier, Warner Chilcott, and NPS Pharmaceuticals), CSK (Amgen, Eli Lilly, and Novartis), JDA (Amgen, Eli Lilly, GSK, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott, and Bristol-Myers Squibb), JPB (Abbott, Amgen, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofi Aventis, Servier, and Warner Chilcott). CB, LL, LJ, LG-F, JBR, KS, KSD, JCP, and NK did not have any conflicts of interest.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006 Jul;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academy Press; 2011. [Google Scholar]
- 4.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj FG, Josse RG, Lips P, Morales-Torres J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009 Nov;20(11):1807–20. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 5.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011 Jan;96(1):53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woitge HW, Scheidt-Nave C, Kissling C, Leidig-Bruckner G, Meyer K, Grauer A, Scharla SH, Ziegler R, Seibel MJ. Seasonal variation of biochemical indexes of bone turnover: results of a population-based study. J Clin Endocrinol Metab. 1998 Jan;83(1):68–75. doi: 10.1210/jcem.83.1.4522. [DOI] [PubMed] [Google Scholar]
- 7.Barake R, Weiler H, Payette H, Gray-Donald K. Vitamin D supplement consumption is required to achieve a minimal target 25-hydroxyvitamin D concentration of >or=75 nmol/L in older people. J Nutr. 2010 Mar;140(3):551–6. doi: 10.3945/jn.109.115626. [DOI] [PubMed] [Google Scholar]
- 8.Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009 Apr;94(4):1244–50. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011 Jun;22(6):1745–53. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene-Finestone LS, Berger C, de Groh M, Hanley DA, Hidiroglou N, Sarafin K, Poliquin S, Krieger J, Richards JB, Goltzman D. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011 May;22(5):1389–99. doi: 10.1007/s00198-010-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009 Mar;169(6):626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008 Dec;88(6):1519–27. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010 Apr;171(8):903–8. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Molero I, Morcillo S, Valdes S, Perez-Valero V, Botas P, Delgado E, Hernandez D, Olveira G, Rojo G, Gutierrez-Repiso C, Rubio-Martin E, Menendez E, Soriquer F. Vitamin D deficiency in Spain: a population-based cohort study. Eur J Clin Nutr. 2011 Mar;65(3):321–8. doi: 10.1038/ejcn.2010.265. [DOI] [PubMed] [Google Scholar]
- 15.Ginde AA, Wolfe P, Camargo CA, Jr, Schwartz RS. Defining vitamin D status by secondary hyperparathyroidism in the U.S. population. J Endocrinol Invest. 2011 May 23; doi: 10.3275/7742. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Bacon CJ, Woo J, Lau EM, Lam CW, Gamble GD, Reid IR. Effects of 25-hydroxyvitamin D level and its change on parathyroid hormone in premenopausal Chinese women. Osteoporos Int. 2010 Nov;21(11):1935–41. doi: 10.1007/s00198-009-1163-z. [DOI] [PubMed] [Google Scholar]
- 17.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001 Mar;86(3):1212–21. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 18.Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr. 2006 Sep;84(3):602–9. doi: 10.1093/ajcn/84.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiger N, Tenenhouse A, Joseph L, MacKenzie T, Poliquin S, Brown J, Prior JC, Rittmaster RS. The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging. 1999;18:376–87. [Google Scholar]
- 20.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004 Nov;50(11):2195–7. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 21.Health Canada. Drug product database online query. Health Canada; Canada: 2009. http://web-prod3.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp. [Google Scholar]
- 22.Poliquin S, Joseph L, Gray-Donald K. Calcium and vitamin D intakes in an adult Canadian population. Can J Diet Pract Res. 2009;70(1):21–7. doi: 10.3148/70.1.2009.21. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 24.Spivak M. Calculus. 2. Houston, TX: Publish or Perish, Inc; 1980. [Google Scholar]
- 25.Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge (UK): Cambridge University Press; 2006. [Google Scholar]
- 26.Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr. 2011 Jul;94(1):128–35. doi: 10.3945/ajcn.111.013268. [DOI] [PubMed] [Google Scholar]
- 27.Pasco JA, Henry MJ, Kotowicz MA, Sanders KM, Seeman E, Pasco JR, Schneider HG, Nicholson GC. Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Gee-long Osteoporosis Study. J Bone Miner Res. 2004 May;19(5):752–8. doi: 10.1359/JBMR.040125. [DOI] [PubMed] [Google Scholar]
- 28.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurds-son G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005 Nov;294(18):2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 29.Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003 Jan;88(1):185–91. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkman M, Sorva A, Tilvis R. Responses of parathyroid hormone to vitamin D supplementation: a systematic review of clinical trials. Arch Gerontol Geriatr. 2009 Mar;48(2):160–6. doi: 10.1016/j.archger.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, Stein EM, Fleischer J, Rosen CJ, Rogers H, Staron RB, Lemaster J, Shane E. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int. 2012 Jan;23(1):171–82. doi: 10.1007/s00198-011-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leslie WD, O’Donnell S, Jean S, Lagace C, Walsh P, Bancej C, Morin S, Hanley DA, Papaioannou A. Trends in hip fracture rates in Canada. JAMA. 2009 Aug;302(8):883–9. doi: 10.1001/jama.2009.1231. [DOI] [PubMed] [Google Scholar]
- 33.Kmetic A, Joseph L, Berger C, Tenenhouse A. Multiple imputation to account for missing data in a survey: estimating the prevalence of osteoporosis. Epidemiology. 2002 Jul;13(4):437–44. doi: 10.1097/00001648-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Vatanparast H, Calvo MS, Green TJ, Whiting SJ. Despite mandatory fortification of staple foods, vitamin D intakes of Canadian children and adults are inadequate. J Steroid Biochem Mol Biol. 2010 Jul;121(1–2):301–3. doi: 10.1016/j.jsbmb.2010.03.079. [DOI] [PubMed] [Google Scholar]