Abstract
Summary
It is not clear whether ankle fractures predict future osteoporotic fractures in women, and whether diabetes influences this relationship. We found that a prior ankle fracture does not predict subsequent osteoporotic fractures in women with or without diabetes.
Introduction
We aimed to determine: (1) whether a prior ankle fracture was a risk factor for a subsequent major osteoporotic fracture in older women; (2) whether this risk was modified by the presence of diabetes; (3) the risk factors for ankle fracture in older women.
Methods
We identified 3,054 women age 50 years and older with diabetes and 9,151 matched controls using the Manitoba Bone Density Program database. Multivariable regression models were used to examine factors associated with prior ankle fracture, and the importance of prior ankle fracture as a predictor of subsequent major osteoporotic fracture during a mean 4.8 years of observation.
Results
A prior ankle fracture was not a significant predictor of subsequent major osteoporotic fracture for women with diabetes (hazard ratio [HR] 1.13; 95% confidence interval [CI], 0.68–1.83; p=0.623) or women without diabetes (HR 1.16; 95% CI, 0.79–1.71; p=0.460), and there was no interaction between diabetes and ankle fracture after pooling all women in the cohort (p=0.971). The presence of diabetes was not independently associated with prior ankle fracture (adjusted odds ratio [OR] 1.14 [95% CI, 0.93–1.38], p=0.200), whereas higher body mass index (adjusted OR 1.04 per standard deviation increase [95% CI, 1.03–1.06], p<0.001), previous major osteoporotic fracture (adjusted OR 1.40 [95% CI, 1.13–1.75], p= 0.002), and multiple comorbidities (>6 ambulatory diagnostic groups) (adjusted OR 1.81 [95% CI, 1.40–2.36], p< 0.001) were related to prior ankle fracture.
Conclusions
Ankle fracture was not a significant predictor of major osteoporotic fracture in women, and a diagnosis of diabetes did not influence the relationship.
Keywords: Ankle fracture, Diabetes, Osteoporosis, Osteoporotic fracture, Risk factor
Introduction
Low-energy fragility fractures, or osteoporotic fractures, are often the first clinical sign of skeletal fragility [1]. Osteoporotic fractures have been defined as fractures of the hip, pelvis, spine, humerus, wrist, rib, clavicle, scapula, or sternum [2, 3]. Fractures of the hip and spine in particular are associated with severe disability, decreased health related quality of life, increased mortality and economic burden [4, 5]. Various risk factors have been identified that influence fracture risk including previous fracture, gender, body mass index (BMI), parental history of a hip fracture, smoking status, prolonged glucocorticoid exposure, rheumatoid arthritis, secondary causes of osteoporosis, and high alcohol intake [6, 7]. A previous osteoporotic fracture is considered to be one of the strongest risk factors for subsequent osteoporotic fracture, and this risk is largely independent of bone mineral density (BMD) [3]. Capturing osteoporotic fracture history provides valuable information for assessing future fracture risk and can be used with other key risk factors to initiate fracture prevention interventions [7, 8].
Ankle fractures are one of the most common fracture types that occur in the adult population, however it is unclear if these fractures should be considered osteoporotic fractures, or if they predict future fragility fractures [1, 9, 10]. Ankle fractures tend to occur more frequently in younger, obese individuals who are frequent fallers, and the incidence does not increase with age, excluding ankle fractures from the osteoporotic fracture class [1, 11, 12]. Current evidence suggests that adults with type 2 diabetes may be at increased risk of experiencing ankle fractures, in addition to the more typical osteoporotic fractures, despite having higher BMD [13–15]. Whether the occurrence of an ankle fracture signifies an increased risk of future fracture is unclear.
The purpose of the current study was to determine whether a prior ankle fracture was a risk factor for a subsequent major osteoporotic fracture in older women, and whether this risk was modified by the presence of diabetes.
Methods
Design overview
A matched cohort study was carried out using the anonymized Population Health Information System (POPULIS) data repository at the Manitoba Centre for Health Policy (MCHP), University of Manitoba [16]. The population based data repository contains information regarding: patient demographics; date and type of service received; in-patient, out-patient, and office-based diagnoses and procedures performed; and prescription medications dispensed to out-patients. Medications are classified according to the Anatomic Therapeutic Chemical (ATC) of the WHO Collaboration Centre for Drugs Statistics Methodology. The accuracy and validity of the POPULIS databases has been previously published [17]. For this analysis we used repository data from April 1, 1987 to March 31, 2007 (pharmacy dispensation data were only available after April 1, 1995).
The MHCP data repository is linked to the Manitoba Bone Density Program database through an anonymized (scrambled) personal health identification number. Details on the construction and validation of the Manitoba Bone Density Program database have been described elsewhere [18]. Briefly, the database contains all clinical BMD measurements for individuals tested in Manitoba since January 1, 1990. In the province of Manitoba’s publicly funded medical care system, BMD testing is provided for all women who are older than 65 years, or for younger women with a fragility fracture, who use systemic glucocorticoids for more than 3 months per year, have osteopenia diagnosed by X-ray, have experienced menopause prior to age 45 years, or who present with other risk factors for osteoporosis as determined by their referring physician.
Study population
This study used the Manitoba Bone Density Program database to identify women who (as of March 31, 2007) were over the age of 50 years at the time of BMD measurement, had a valid recorded height and weight, and had a BMD measurement recorded for the femoral neck (FN) site. For those who had more than one DXA assessment, the first BMD measurement was used for analysis. Diabetes was identified by the presence of either two separate physician claims for diabetes within 2 years, or a single hospitalization with a diagnosis of diabetes (International Classification of Diseases 9th revision Clinical Modification [ICD-9-CM] code 250). This method of identifying individuals with diabetes has been well validated [19]. Each diabetes subject was matched to three controls in the database without diabetes. Subjects with diabetes and controls were matched based on: BMD testing at the same facility; BMD testing performed within 12 months; and age within 2.5 years. The research ethics board for the University of Manitoba approved this study, and data access was granted by the Manitoba Health Information Privacy Committee.
Fracture ascertainment
Prior ankle fractures were identified according to the ICD-9-CM 824 code. This code includes medial malleolus, lateral malleolus, bimalleolar, and trimalleolar fractures. The proportion of individuals with prevalent fractures was calculated by dividing the number of fractures that occurred prior to the date of BMD testing by the number of individuals in each group. The following ICD-9-CM codes were used to identify major osteoporotic fractures: ICD-9-CM 820–821 with site specific fracture fixation code (hip); ICD-9-CM 813 with site specific fracture fixation or casting code (forearm); ICD-9-CM 805 (clinical spine); ICD-9-CM 812 (proximal humerus). Fracture definitions required the presence of two or more relevant hospital or physician ICD-9-CM fracture codes [20]. All fractures that were associated with trauma codes were excluded from the analysis. Incident fractures were identified as fractures that occurred between the date of BMD testing and March 31, 2007. To minimize the potential for counting health care interactions related to the same injury, fracture codes for the same site that occurred within 180 days were ignored.
Covariates
Femoral neck (FN) BMD measurements were assessed using either pencil-beam dual X-ray absorptiometry (DXA) (Lunar DPX; GE Lunar, Madison, WI, USA) if the measurement was taken before the year 2000, or fan-beam DXA (Lunar Prodigy; GE Lunar) after the year 2000. There were no clinically significant differences between the analyses using the different instruments (T-score differences <0.2). All systems showed constant long-term performance, and adequate in vivo system precision [21]. The DXA scans were analyzed by technicians according to the manufacturer’s guidelines. The T-scores and Z-scores were calculated based on reference data for U.S. white females from the NHANES III survey [22]. Additional variables collected at the time of BMD measurement were weight and height (by self-report before year 2000, measured after year 2000). The provincial pharmacy database was used to identify use of osteoporosis-related medication (90 days or more of pharmacy dispensations for hormone therapy, bisphosphonate, selective estrogen receptor modulator, parathyroid hormone or recombinant human parathyroid hormone analog [1–34], calcitonin during the year prior to the BMD test). A global comorbidity score was constructed based upon the Johns Hopkins Case-Mix System [23]. Briefly, the number of ambulatory diagnostic groups (ADGs) is reflective of the number and severity of comorbid conditions. The number of ADGs was categorized as 0–2 (reference), 3–5, or 6 or more ADGs. Other covariates associated with skeletal fragility were defined from the POPULIS data repository: chronic obstructive pulmonary disease (a proxy for smoking, as smoking cannot be defined from administrative data) [24], alcohol and substance abuse diagnosis, rheumatoid arthritis, hyperthyroidism, dementia, and prolonged glucocorticoid use (at least 90 days of pharmacy dispensations in the year before BMD testing at a mean daily dose of prednisone-equivalent 7.5 mg or greater) [25, 26].
Statistical analysis
Baseline cohort characteristics as of the index date (time of BMD measurement) are presented as mean (SD) or frequencies. Differences in baseline characteristics between individuals with and without diabetes were evaluated using unpaired Student’s t-tests (continuous variables) and chi-square tests (categorical variables). Multivariable logistic regression analysis was performed to model the relationship between prior ankle fracture and diabetes adjusted for: age, BMI, FN BMD, previous major osteoporotic fracture, number of ADGs, chronic obstructive pulmonary disease, alcohol and substance abuse diagnosis, rheumatoid arthritis, hyperthyroidism, dementia, prolonged glucocorticoid use and osteoporotic medication use [25–27]. A multivariable Cox proportional hazards regression model was used to determine the predictors of time to major osteoporotic fracture as a function of the same variables. In addition to survival models stratified for the presence/absence of diabetes, a combined model was constructed that included an interaction term for diabetes and prior ankle fracture. Odds ratios (OR) and hazard ratios (HR) were generated from the regression models with corresponding 95% confidence intervals (CI). All statistical analyses were performed using Statistica (Version 8.0, StatSoft, Inc., Tulsa, OK, USA). The criterion for statistical significance was set at a p value of 0.05.
Results
The characteristics of the 3054 women with a diagnosis of diabetes and 9151 women without a diagnosis of diabetes are shown in Table 1. The cohorts were well matched for age: mean for women with diabetes 68.0±9.3 years, mean for controls without diabetes 67.9±9.2 years (p=0.773). On average, the women with diabetes had a greater BMI compared to the non-diabetic control cohort (29.9±6.3 versus 26.6±5.0 kg/m2, p<0.001). Women with diabetes had a greater number of ADGs than women without diabetes, and also higher average FN bone density T-scores and Z-scores (both p<0.001) (Table 1).
Table 1.
Descriptive characteristics of the study cohort
| Women with diabetes (n=3,054) | Women without diabetes (n=9,151) | p Value | |
|---|---|---|---|
| Age | |||
| 50–64 years; n (%) | 1,166 (38.2) | 3,496 (38.2) | 0.940 |
| 65–79 years; n (%) | 1,583 (51.8) | 4,722 (51.6) | |
| ≥80 years; n (%) | 305 (10.0) | 933 (10.2) | |
| BMI, kg/m2; mean (SD) | 29.9 (6.3) | 26.6 (5.0) | <0.001 |
| Number of ADGs for year prior to BMD testing | |||
| 0–2; n (%) | 460 (15.1) | 2,131 (23.3) | <0.001 |
| 3–5; n (%) | 1,376 (45.1) | 4,294 (46.9) | |
| 6 or more; n (%) | 1,218 (39.9) | 2,726 (29.8) | |
| Use of osteoporosis medicationa; n (%) | 607 (19.9) | 2,323 (23.4) | <0.001 |
| Bone densitometry | |||
| Femoral neck T-score; mean (SD) | −1.3 (1.1) | −1.6 (0.9) | <0.001 |
| Femoral neck Z-score; mean (SD) | 0.4 (1.1) | 0.0 (1.0) | <0.001 |
| Prior fractures | |||
| Ankle; n (%) | 173 (5.7) | 386 (4.2) | <0.001 |
| Clinical vertebral; n (%) | 98 (3.2) | 274 (3.0) | 0.550 |
| Any major non-vertebral fractureb; n (%) | 203 (6.7) | 442 (4.8) | <0.001 |
BMI body mass index, ADGs ambulatory diagnostic groups
Osteoporosis medication use in the preceding year (hormone therapy; bisphosphonate; selective estrogen receptor modulator; parathyroid hormone or recombinant human parathyroid hormone analog [1–34]; or calcitonin)
Major non-vertebral fracture includes hip, distal forearm, proximal humerus
Despite having higher FN bone density measurements compared to controls, women with diabetes had experienced a greater number of prior ankle fractures (5.7% versus 4.2%, p<0.001) (Table 1). In the overall study cohort, greater BMI, previous major osteoporotic fracture, and six or more ADGs were associated with a prior ankle fracture (Table 2). There was no relationship between a diagnosis of diabetes and prior ankle fracture after adjustment for other factors (OR 1.13; 95% CI, 0.93–1.38; p=0.200).
Table 2.
Risk factors associated with prior ankle fractures in entire cohort of women (N=12,205)
| OR (95% CI) | p Value | |
|---|---|---|
| Diabetes | 1.14 (0.93–1.38) | 0.200 |
| Age, per 10 years increase | 1.00 (0.99–1.01) | 0.439 |
| BMI, per SD increase | 1.04 (1.03–1.06) | <0.001 |
| FN BMD, per SD decrease | 0.91 (0.82–1.01) | 0.078 |
| Previous major fracture | 1.40 (1.13–1.75) | 0.002 |
| Number of ADGs | ||
| 0–2 | 1.00 reference | |
| 3–5 | 1.27 (0.99–1.65) | 0.063 |
| 6 or more | 1.81 (1.40–2.36) | <0.001 |
Adjusted odds ratios (OR) and 95% confidence intervals (CI) predicted with logistic regression model. The model was also adjusted for chronic obstructive pulmonary disease, alcohol and substance abuse diagnosis, rheumatoid arthritis, hyperthyroidism, dementia, prolonged corticosteroid use and prior osteoporotic medication use. These factors were not significantly related with prior ankle fractures
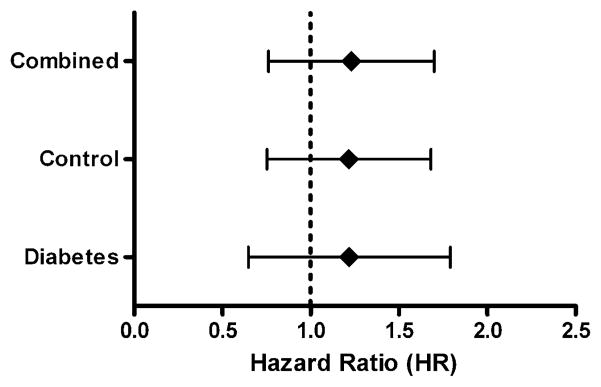
No difference was found in the number of incident ankle fractures during mean 4.8 years of follow-up: 17/3054 (0.6%) for women with diabetes versus 49/9151 (0.5%) for women without diabetes (p=0.892). A prior ankle fracture did not show a significant association with subsequent major osteoporotic fracture in women with diabetes (adjusted HR 1.13; 95% CI, 0.70–1.83; p=0.623) or in women without diabetes (adjusted HR 1.16; 95% CI, 0.79–1.71; p=0.460). When the two groups were combined, the relationship was also not significant (adjusted HR 1.17; 95% CI, 0.79–1.73; p=0.316) and there was no evidence an interaction between diabetes and ankle fracture (p=0.971) (Fig. 1).
Fig. 1.
Forest plot indicating the risk associated with a subsequent major osteoporotic fracture in women with a previous ankle fracture
Discussion
In this large matched-cohort study, we found that a prior ankle fracture did not predict a subsequent major osteoporotic fracture in older women with or without diabetes. Elevated BMI, previous major osteoporotic fracture, and six or more ADGs were significant risk factors for ankle fracture.
The evidence on ankle fracture as a risk factor for a subsequent major osteoporotic fracture is limited, as smaller cohort and questionnaire-based studies have produced conflicting results. In a retrospective study, ankle fractures were associated with hip fractures in women 70 years of age or younger (OR 1.81; 95% CI, 1.02–3.19) and spine fractures in women 71 years of age or older (OR 1.49; 95% CI, 1.01–2.19) [28]. However, in a 4-year prospective cohort study of 451 postmenopausal women found no association between radiograph-confirmed prior ankle fracture and subsequent hip fracture in women between 60 and 99 years (RR 1.3; 95% CI, 0.6–2.7) [29]. In a recent report from the Dubbo Osteoporosis Epidemiology Study, ankle fractures were not associated with subsequent fractures in women (RR 0.84; 95% CI, 0.40–1.78) [30]. Our study provides additional evidence that prior ankle fractures in women over age 50 years, with or without diabetes, are not predictive of osteoporotic fractures, and as suggested by others, should not be considered an osteoporotic fracture [2].
We demonstrated that a prior major osteoporotic fracture, elevated BMI, and the presence of multiple comorbidities (6 or more ADGs) were significantly related to prior ankle fracture. These data complement the findings from a cohort study of perimenopausal women from Finland which revealed that elevated BMI, prior osteoporotic fracture, and three or more prescription drugs independently increased the risk of ankle fracture [31]. Though not assessed in this study, the duration of diabetes and type 2 diabetes-related complications such as retinopathy may also contribute to ankle fracture risk, as these factors are known to influence the risk of other fractures in patients with diabetes [32]. The relationship between obesity and ankle fracture may be driven by the higher-weight-associated increase in energy transmitted through the ankle joint during a fall. Additional risk factors that have been associated with ankle fractures include a history of falls, vigorous physical activity, family history of hip fracture, and younger age [11, 31, 33, 34]. The pathology of ankle fracture is complex, and specific covariates like obesity, treatment with exogenous insulin, the presence of comorbid conditions, and having an osteoporotic fracture history appear to be more important contributors than BMD and the presence of diabetes alone.
There were several study limitations. We were unable to differentiate between diagnoses of type 1 and type 2 diabetes; however, it is likely that most of the cases had type 2 diabetes given the mean age of our cohort [35, 36]. We were unable to evaluate the use of insulin or other anti-hyperglycemic medications. Various reports have indicated that the use of thiazolidinediones is associated with an increased fracture risk [37–39].
In conclusion, we found that prior ankle fracture was not a risk factor for subsequent major osteoporotic fracture in women with and without diabetes. Therefore, the presence of an ankle fracture alone does not indicate a need for further osteoporosis assessment in women greater than 50 years of age. Although having a diagnosis of diabetes does not appear to increase the risk of ankle fractures, other clinical risk factors that may be more prevalent in individuals with diabetes, such as obesity and multiple comorbidities, are associated with increased ankle fracture risk. Further study is warranted to confirm clinical risk factors for osteoporotic fracture risk that may be specific to individuals with diabetes.
Acknowledgments
We are indebted to Manitoba Health for providing data (HIPC File No. 2008/2009–08). The results and conclusions are those of the authors, and no official endorsement by Manitoba Health is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
Footnotes
Conflicts of interest W.D. Leslie has served on the advisory board for Amgen, Genzyme and Novartis; has received unrestricted research grants from Amgen, Genzyme, Merck Frosst, Proctor & Gamble and Sanofi-Aventis; has received speaker fees from Amgen and Merck Frosst; has received travel funds from Genzyme.
J.D. Adachi has served as a consultant and speaker for Amgen, Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, GSK, Merck Frosst, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Servier and Wyeth; has received clinical trials grants from Amgen, Bristol-Myers Squibb, Eli Lilly, GSK, Merck Frosst; Novartis, Pfizer, Procter & Gamble, Sanofi Aventis, Roche and Wyeth.
A. Papaioannou has served on the advisory board for Amgen, Eli Lilly, Merck Frosst, Proctor & Gamble and Novartis; has consulted for Amgen, Aventis Pharma, Eli Lilly, Lundbeck Canada Inc., Merck Frosst, Novartis, Proctor & Gamble, Servier, Warner Chillcott and Wyeth-Ayerst; has received unrestricted research grants from Amgen, Eli Lilly, Merck Frosst, Proctor & Gamble and Sanofi-Aventis; has received clinical trial grants from Novartis and Phizer, has received a research grant from the Ontario Ministry of Health and Long-Term Care; has served as a member of the Continuing Medical Education Steering Committee of the Ontario College of Family Physicians.
J.M. Pritchard, L.M. Giangregorio and G. Ioannidis have no conflict of interest to declare.
Contributor Information
J. M. Pritchard, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada. Hamilton Health Sciences, St. Peter’s Hospital, Room 159, 1200 Main Street West, Juravinski Research Centre, Hamilton, ON, Canada L8N 3Z5
L. M. Giangregorio, Department of Kinesiology, University of Waterloo, Waterloo, Ontario, Canada
G. Ioannidis, Department of Medicine, McMaster University, Hamilton, Ontario, Canada
A. Papaioannou, Department of Medicine, McMaster University, Hamilton, Ontario, Canada
J. D. Adachi, Department of Medicine, McMaster University, Hamilton, Ontario, Canada
W. D. Leslie, Department of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada
References
- 1.Kanis JA, Oden A, Johnell O, Jonsson B, De Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12:417–427. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl 2):S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. A meta-analysis of previous fracture and sub-sequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Wiktorowicz MEGR, Papaioannou A, Adachi JD, Papadimitropoulos E. Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int. 2001;12:271–278. doi: 10.1007/s001980170116. [DOI] [PubMed] [Google Scholar]
- 5.Papaioannou A, Kennedy C, Ioannidis G, Sawka A, Hopman WM, Pickard L, Brown JP, Josse RG, Kaiser S, Anastassiades T, Goltzman D, Papadimitropoulous M, Tenenhouse A, Prior JC, Olszynski WP, Adachi JD for the CaMos Study Group. The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2009;20:703–714. doi: 10.1007/s00198-008-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA, Kaiser SM, Kvern B, Siminoski K, Leslie WD. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamdy RC, Baim S, Broy SB, Lewiecki EM, Morgan SL, Tanner SB, Williamson HF. Algorithm for the management of osteoporosis. South Med J. 2010;103(10):1009–1015. doi: 10.1097/SMJ.0b013e3181f0e8d6. [DOI] [PubMed] [Google Scholar]
- 9.Ho PY, Tang N, Law SW, Tsui HF, Lam TP, Leung KS. A prospective case-control study of ankle fracture in postmenopausal women. Hong Kong Med J. 2006;12(3):208–211. [PubMed] [Google Scholar]
- 10.Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus. J Bone Jt Surg Br. 2005;87(4):489–495. doi: 10.1302/0301-620X.87B4.15724. [DOI] [PubMed] [Google Scholar]
- 11.Hasselman CT, Vogt MT, Stone KL, Cauley JA, Conti SF. Foot and ankle fractures in elderly white women. Incidence and risk factors. J Bone Jt Surg Am. 2003;85-A(5):820–824. doi: 10.2106/00004623-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Eastell R, Reid DM, Compston J, Cooper C, Fogelman I, Francis RM, Hay SM, Hosking DJ, Purdie DW, Ralston SH, Reeve J, Russell RG, Stevenson JC. Secondary prevention of osteoporosis: when should a non-vertebral fracture be a trigger for action? Q J Med. 2001;94(11):575–597. doi: 10.1093/qjmed/94.11.575. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res. 2008;23(8):1334–1342. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91(9):3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 16.Lix LM, Yohendran MS, Leslie WD, Shaw SY, Baumgartner R, Bowman C, Metge C, Gumel A, Hux J, James RC. Using multiple data features improved the validity of osteoporosis case ascertainment from administrative databases. J Clin Epidemiol. 2008;61:1250–1260. doi: 10.1016/j.jclinepi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Roos LL, Mustard CA, Nicol JP, McLerran DF, Malenka DJ, Young TK, Cohen MM. Registeries and administrative data: organization and accuracy. Med Care. 1993;31:201–212. doi: 10.1097/00005650-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Leslie WD, Caetano PA, MacWilliam LR, Finlayson GS. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8(1):25–30. doi: 10.1385/jcd:8:1:025. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard JF, Ludwig S, Wajda A, Dean H, Anderson K, Kendall O. Incidence and prevalence of diabetes in Manitoba. 1986–1991. Diabetes Care. 1996;27:1047–1053. doi: 10.2337/diacare.19.8.807. [DOI] [PubMed] [Google Scholar]
- 20.Giangregorio LM, Leslie WD. Time since prior fracture is a risk modifier for ten year osteoporotic fractures. J Bone Miner Res. 2010;25(6):1400–1405. doi: 10.1002/jbmr.35. [DOI] [PubMed] [Google Scholar]
- 21.Leslie WD. The importance of spectrum bias on bone density monitoring in clinical practice. Bone. 2006;39:361–368. doi: 10.1016/j.bone.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 23.Leslie WD, Derksen S, Prior HJ, Lix LM, Metge C, O’Neil J. The interaction of ethnicity and chronic disease as risk factors for osteoporotic fractures: a comparison in Canadian Aboriginals and non-Aboriginals. Osteoporos Int. 2006;17(9):1358–1368. doi: 10.1007/s00198-006-0111-4. [DOI] [PubMed] [Google Scholar]
- 24.Leslie WDBC, Langsetmo L, Adachi JD, Hanley DA, Ioannidis G, Goltzman D, Papaioannou A, Josse R, Kovacs CS, Olszynski WP, Towheed T, Kaiser SM, Prior J, Jamal S, Kreiger N, Brown JP the CaMos Research Group. Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: Results from the CaMos and Manitoba BMD cohorts. Osteoporos Int. 2011 doi: 10.1007/s00198-010-1445-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): What they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11(4):473–477. doi: 10.1016/j.jocd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Fraser M, Lovell F, Reece A, McLellan AR. Characteristics of males over 50 years who present with a fracture. J Bone Jt Surg (Br) 2008;90B(1):72–77. doi: 10.1302/0301-620X.90B1.18773. [DOI] [PubMed] [Google Scholar]
- 27.Langsetmo L, Hanley DA, Kreiger N, Jamal SA, Prior J, Adachi JD, Davison KS, Kovacs C, Anastassiades T, Tenenhouse A, Goltzman D. Geographic variation of bone mineral density and selected risk factors for prediction of incident fracture among Canadians 50 and older. Bone. 2008;43(4):672–678. doi: 10.1016/j.bone.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunnes M, Mellstrom D, Johnell O. How well can a previous fracture indicate a new fracture? A questionnaire study of 29,802 postmenopausal women. Acta Orthop Scand. 1998;69(5):508–512. doi: 10.3109/17453679808997788. [DOI] [PubMed] [Google Scholar]
- 29.Lauritzen JB, Lund B. Risk of hip fracture after osteoporosis fractures. 451 women with fracture of lumbar spine, olecranon, knee or ankle. Acta Orthop Scand. 1993;64(3):297–300. doi: 10.3109/17453679308993629. [DOI] [PubMed] [Google Scholar]
- 30.Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297(4):387–394. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 31.Valtola A, Honkanen R, Kroger H, Tuppurainen M, Saarikoski S, Alhava E. Lifestyle and other factors predict ankle fractures in perimenopausal women: a population-based prospective cohort study. Bone. 2002;30(1):238–242. doi: 10.1016/s8756-3282(01)00649-4. [DOI] [PubMed] [Google Scholar]
- 32.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care. 2001;24(7):1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 33.Honkanen R, Tuppurainen M, Kroger H, Alhava E, Saarikoski S. Relationships between risk factors and fractures differ by type of fracture: a population-based study of 12192 perimenopausal women. Osteoporos Int. 1998;8:25–31. doi: 10.1007/s001980050044. [DOI] [PubMed] [Google Scholar]
- 34.Seeley DG, Kelsey J, Jergas M, Nevitt MC. Predictors of ankle and foot fractures in older women. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11(9):1347–1355. doi: 10.1002/jbmr.5650110920. [DOI] [PubMed] [Google Scholar]
- 35.Evans JMM, MacDonald TM, Leese GP, Ruta DA, Morris AD. Impact of type 1 and type 2 diabetes on patterns and costs of drug prescribing. Diabetes Care. 2000;23:770–774. doi: 10.2337/diacare.23.6.770. [DOI] [PubMed] [Google Scholar]
- 36.Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24(7):1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 37.Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168(8):820–825. doi: 10.1001/archinte.168.8.820. [DOI] [PubMed] [Google Scholar]
- 38.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180(1):32–39. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91(9):3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]