Abstract
Osteoporosis Canada’s 2010 Clinical Practice Guidelines for the Diagnosis and Management of Osteoporosis in Canada focus on the clinical impact of fragility fractures, and on the assessment and management of women and men at high risk for fragility fracture. These guidelines now integrate a 10-year absolute fracture risk prediction into an overall management approach by using validated risk assessment tools. There currently is a large gap between optimal practices and those that are now being provided to Canadians with osteoporosis. These guidelines are part of a concerted effort to close this gap. Key changes from the 2002 guidelines of interest and relevance to radiologists are highlighted in this report.
Keywords: Osteoporosis, Fracture, Risk assessment, Bone mineral densitometry
The evaluation of persons suspected of having osteoporosis has evolved. Because the disease typically causes symptoms only when fractures result, the diagnosis tended, in the past, to be made late. The development of tools to measure bone mineral density (BMD), especially in the central skeleton, has allowed identification of individuals at high risk of fragility fracture before the first fracture.
A landmark was the publication of a diagnostic classification by a working group of the World Health Organization (WHO) in 1994 [1]. This defined osteoporosis in a post-menopausal woman as a BMD of 2.5 or more standard deviations (SD) below a young adult norm, that is, a T score of –2.5 or lower. This value roughly corresponded with the fraction of the population sustaining fragility fractures, although only a minority of fragility fractures occur in women with a T score this low. However, this became widely accepted as a BMD-derived definition of osteoporosis, was commonly used as a treatment threshold, and is now also applied to men older than age 50 years (Table 1) [2,3].
Table 1.
Recommended diagnostic categories for both men and women based on bone densitometry
| Age | Category | Criteriaa |
|---|---|---|
| <50 y | Below expected range for age | Z score ≤–2.0 |
| Within expected range for age | Z score >–2.0 | |
| ≥50 y | Severe (established) osteoporosis | T score ≤–2.5 with fragility fractures |
| Osteoporosis | T score ≤–2.5 | |
| Low bone mass | T score = –1.1 and –2.4 | |
| Normal | T score ≥–1.0 |
BMD = bone mineral density.
-
The T score is the number of standard deviations that BMD is above or below the mean normal peak BMD for young white women (the National Health and Nutrition Education Survey III for hip measurements).The Z score is the number of standard deviations that BMD above or below the mean normal BMD for sex, age, and (if reference data are available) race or ethnicity.
- Osteoporosis cannot be diagnosed by BMD alone below age 50 y.
- Based upon lowest value for lumbar spine (minimum 2 vertebral levels), total hip, and femoral neck. If either the lumbar spine or hip is invalid, then the forearm should be scanned and the distal third of the region reported.
- Fracture risk assessment under the Fracture Risk Assessment Tool / 2010 Canadian Association of Radiologists and Osteoporosis Canada system is based upon the femoral neck T score alone.
However, the WHO classification, despite its merits, proved to be limiting for a number of reasons, not the least of which was that it placed undue emphasis on the importance of BMD as a risk factor for osteoporotic fractures while obscuring the complexity of fracture risk, which is due to many factors.
The 2010 Osteoporosis Canada Clinical Practice Guidelines
The development of these guidelines followed the Appraisal of Guidelines, Research and Evaluation framework [4]. Primary care physicians, patients, osteoporosis specialists from different disciplines, radiologists, allied health professionals, and health policy makers were surveyed to identify priorities for the guidelines. Based on these, systematic reviews of the literature were conducted to update our knowledge in 2 key areas: (1) fracture risk assessment and (2) therapies for osteoporosis. Also, Canadian data are now available to inform many of these recommendations.
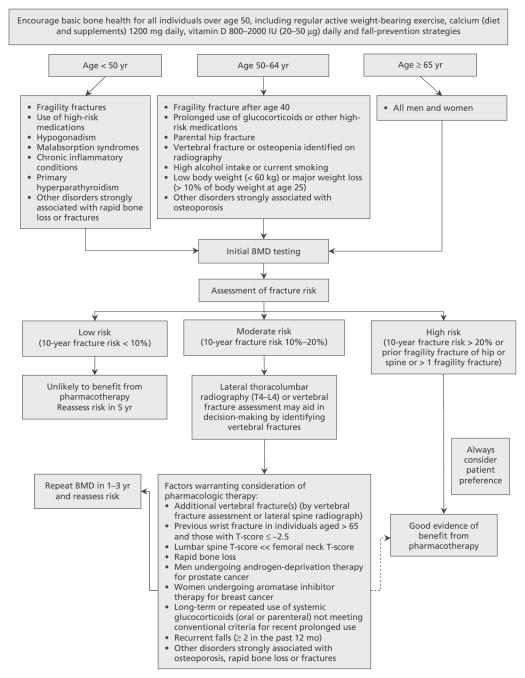
Appropriate interventions to prevent fractures need to accurately identify those at risk and, therefore, most likely to benefit from treatment [5]. Unfortunately, many of those who sustain a fragility fracture are neither appropriately assessed nor treated [6,7]. The 2010 guidelines focus on the assessment and management of women and men at high risk for such a fracture [7]. Low BMD is only one of several risk factors for fracture, and, in recognition of this, Osteoporosis Canada (OC) and the Canadian Association of Radiologists (CAR) adopted a system for 10-year absolute fracture risk assessment to be used in BMD reporting [8]. The 2010 guidelines have updated this absolute risk assessment model and developed recommendations for comprehensive care (Figure 1).
Figure 1.
An integrated approach to management of patients who are at risk for fracture. Dashed arrow indicates that evidence for benefit from pharmaco-therapy is not as strong in this instance as for other recommendations. BMD = bone mineral density. Reprinted from Papaioannou et al [7] with permission from CMAJ. © 2010 Canadian Medical Association.
Key Concepts
The 2010 guidelines use an integrated risk assessment (based on BMD and other risk factors) and treatment model to stratify women and men older than age 50 years into 3 fracture risk groups. These categories align with treatment implications: low risk (usually not requiring pharmacologic treatment), moderate risk (consider additional clinical risk factors to determine any need for pharmacologic treatment), and high risk (should be considered for pharmacologic treatment) [7]. Nonpharmacologic and lifestyle measures are applicable to all: optimizing calcium and vitamin D intake, regular weight-bearing activity, balance and strengthening exercises, and smoking cessation. In older patients, fall prevention should be considered, including a multifactorial assessment of causes contributing to risk.
These categories are defined as follows:
High risk for future fractures. Implied is a >20% probability of major osteoporotic fracture (ie, proximal femoral, clinical vertebral, forearm, or proximal humeral break) over the ensuing 10 years. Individuals older than age 50 years who have had a fragility fracture of the hip or vertebra and those who have had more than 1 fragility fracture are also considered to be high risk for future fractures, irrespective of BMD [9–12]. Pharmacologic therapy should be offered to these individuals.
Moderate risk for future fractures. Implied is 10%–20% probability for major osteoporotic fracture over 10 years. For those at moderate fracture risk with no other risk factors, treatment should be individualized and may include pharmacologic therapy, or just basic lifestyle measures with monitoring. There are more osteoporotic fractures in the moderate fracture risk group than in the high fracture risk group (because there are more individuals at moderate risk than at high risk), even though the individual fracture risk is higher in the high-risk group [13]. The moderate-risk group requires a careful evaluation to identify vertebral fractures (Table 2) or additional clinical risk factors (Figure 1), which may contribute to a decision to offer pharmacologic therapy.
Low risk for future fractures. Implied is <10% probability for major osteoporotic fracture over 10 years. These individuals usually do not require pharmacologic therapy. In general, the lifestyle measures referred to earlier are sufficient for those at low fracture risk who do not have additional risk factors for rapid BMD loss.
Table 2.
Key points on vertebral fracture recognition and radiologist reporting
|
Fracture Risk Assessment and the Importance of Radiologic Investigations
The term “osteopenia” has been replaced by “low bone mass” in the WHO classification. It is recommended that radiologists reclaim osteopenia as the term to describe low bone mass seen on plain radiographs or computed tomography recognizing the subjectivity of the observation and its dependance on technical factors in making the image.
Vertebral fractures are strongly associated with future osteoporotic fractures independent of prior clinical fracture history [10,12,14]. The 2010 OC Guidelines emphasize the potential for a change in height measurement to detect vertebral fractures. When accurately measured, height loss that exceeds 2 cm in less than 3 years may indicate the presence of such a fracture and when observed should be investigated by means of a lateral thoracic and lumbar spine radiograph [15]. Vertebral fracture is the most common manifestation of osteoporosis. However, about two-thirds of these fractures are asymptomatic or present with symptoms that do not lead to a diagnosis [14]. In the Canadian Multi-centre Osteoporosis Study (CaMos), radiographic fractures were associated with an increased risk of future fractures independent of clinical fracture and have been found to affect quality of life [6].
Osteoporotic vertebral fractures are best defined on lateral radiographs as 25% or greater height loss of the anterior, mid, or posterior vertical dimensions of an individual vertebra, especially if there is end-plate disruption [16]. Mild spinal deformities (<25% height loss without definite end-plate fracture) are not strong predictors of future osteoporotic fractures or low bone density [10]. Vertebral fractures should be assessed visually and graded by the semiquantitative method of Genant and should include T4-L4 vertebrae (Table 2) [17].
What Is the Role of the Radiologist in Reporting Vertebral Fractures?
Vertebral fracture recognition and reporting by radiologists was the subject of a review by OC and the CAR [14,18]. Recognizing and reporting vertebral fractures as near-certain signs of osteoporosis are important roles that radiologists can play in guiding physicians to reduce the risk of future osteoporotic fractures and close the associated care gap (Table 2) [18,19]. Lateral radiographs or vertebral fracture assessment (VFA) of the thoracolumbar spine to diagnose unrecognized vertebral compression fractures assist in further stratifying risk and clinical decision making. In a Canadian study, 1 in 6 elderly patients who had a chest radiograph was found to have a vertebral fracture [20]. Only 60% of vertebral fractures were mentioned in the radiology report [20]. The care gap in identifying vertebral fractures on chest radiographs has been found to be as low as 20%–50% in both national and international studies [21].
VFA with BMD Measurement
VFA is an option in which bone densitometers use fan-beam imaging to examine the spine [2]. To date, Canadian centres have been slow to adopt VFA technology, despite its potential clinical value in identifying patients with previously unrecognized vertebral fractures, such as radiographic fractures. VFA findings predict future osteoporotic and hip fractures independently of age, weight, and BMD [18,22].
Tools to Measure 10-Year Fracture Risk
The 2010 OC Guidelines include 2 closely related tools for estimating the 10-year risk of a major osteoporotic fracture as defined above: an updated version of the Canadian Association of Radiologists and Osteoporosis Canada (CAROC) [23] and the Fracture Risk Assessment Tool (FRAX) of the WHO, specific for Canada [24]. Both have been calibrated by using the same Canadian fracture data and have been directly validated in Canadian databases (Table 3) [5,25,26].
Table 3.
Comparison of the 2010 CAROC and Canadian FRAX risk assessment tools
| 2010 CAROC | Canadian FRAX | |
|---|---|---|
| Model | Semiquantitative (low, moderate, high) | Quantitative (fracture probability) |
| BMDa | Femoral neck (required) | Femoral neck (optional) |
| Clinical | Fragility fracture; prolonged steroids | Fragility fracture; prolonged steroids; body mass index; parental hip fracture; current smoking; high alcohol use; rheumatoid arthritis; secondary causes |
| Output | Major fracture risk | Major fracture risk; hip fracture risk |
| High risk | >20% major fracture | >20% major fracture |
| Validation | Level 1 evidence | Level 1 evidence |
BMD = bone mineral density; CAROC = Canadian Association of Radiologists and Osteoporosis Canada; FRAX = Fracture Risk Assessment Tool.
The T score for the femoral neck is derived from the National Health and Nutrition Education Survey III reference database for white women.
2010 CAROC System
The 2010 OC Guidelines provided an opportunity to update CAROC [7,23] and to calibrate and validate it by using Canadian data. The 2010 CAROC risk assessment tool uses the patient’s age and sex, together with the femoral neck T score to derive a person’s initial fracture risk, and this may then be adjusted upward as determined by 2 major clinical risk factors, namely fragility fractures after age 40 years (especially vertebral compression fractures) and recent prolonged systemic glucocorticoid use (ie, at least 3 months cumulative during the preceding year at a prednisone equivalent dose of ≥7.5 mg daily) [27]. When both of these clinical risk factors are present, the 10-year absolute fracture risk is considered to be high, irrespective of the BMD result.
The method of determining absolute fracture risk (see Table 4):
Table 4.
2010 CAROC zones of fracture risk for women and men by using femoral neck T scorea
| Women
|
|||
|---|---|---|---|
| Age, y | Low risk | Moderate risk | High risk |
| 50 | Above −2.5 | −2.5 to −3.8 | Below −3.8 |
| 55 | Above −2.5 | −2.5 to −3.8 | Below −3.8 |
| 60 | Above −2.3 | −2.3 to −3.7 | Below −3.7 |
| 65 | Above −1.9 | −1.9 to −3.5 | Below −3.5 |
| 70 | Above −1.7 | −1.7 to −3.2 | Below −3.2 |
| 75 | Above −1.2 | −1.2 to −2.9 | Below −2.9 |
| 80 | Above −0.5 | −0.5 to −2.6 | Below −2.6 |
| 85 | Above +0.1 | +0.1 to −2.2 | Below −2.2 |
| Men | |||
|---|---|---|---|
|
| |||
| Age, y | Low risk | Moderate risk | High risk |
| 50 | Above −2.5 | −2.5 to −3.9 | Below −3.9 |
| 55 | Above −2.5 | −2.5 to −3.9 | Below −3.9 |
| 60 | Above −2.5 | −2.5 to −3.7 | Below −3.7 |
| 65 | Above −2.4 | −2.4 to −3.7 | Below −3.7 |
| 70 | Above −2.3 | −2.3 to −3.7 | Below −3.7 |
| 75 | Above −2.3 | −2.3 to −3.8 | Below −3.8 |
| 80 | Above −2.1 | −2.1 to −3.8 | Below −3.8 |
| 85 | Above −2.0 | −2.0 to −3.8 | Below −3.8 |
CAROC = Canadian Association of Radiologists and Osteoporosis Canada.
The T score for the femoral neck is derived from the National Health and Nutrition Education Survey III reference database for white women.
Begin with the table appropriate for the patient’s sex.
Identify the row that is closest to the patient’s age.
Determine the individual’s fracture risk category by using the femoral neck T score (risk assessment by using the updated CAROC system is based upon the femoral neck T score only). However, when determining the risk category, a patient with a T score of the spine, total hip, or femoral neck in the osteoporotic range (ie, ≤ 2.5, at any age) should be classified as having at least moderate risk.
Evaluate clinical factors (ie, fragility fractures after age 40 years and recent prolonged systemic glucocorticoid use) that may move the patient into a higher fracture risk category. When both factors are present (ie, fragility fractures and prolonged systemic glucocorticoid use), the patient is considered to be at high fracture risk regardless of the BMD result.
Canadian FRAX System
In parallel with the development of the CAROC tool, OC has worked with the WHO to develop a Canadian version of the FRAX tool [24]. It encompasses some additional factors (Table 3) and is country specific. Some clinical practitioners may prefer the versatility of FRAX, which allows for risk assessment in the absence of a BMD measurement and is more quantitatively accurate for those patients with one of more of the additional risk factors listed above. As well, anyone may readily access the tool.
Some limitations to the use of the Canadian FRAX tool are of note. First, it is computer based and requires access to the FRAX Web site, an iPhone (Apple Inc., Cupertino, CA) application, or specialized software on DXA (dual-emission x-ray absorptiometry) machines (not now widely available in Canada). Second, radiologists who use FRAX would need to make a more complete assessment of clinical risk factors that necessitates more detailed history taking. The clinical history provided to radiologists by referring physicians is rarely adequate, and, if the only patient contact involves the technologists performing DXA, then additional technologist training must be provided to ensure accurate documentation of the relevant clinical risk factors.
The clinical risk factors used in the CAROC model are fewer than those used in FRAX but do capture the major risks for fracture, and risk categorization with this tool accurately reflects national fracture data. The updated 2010 CAROC system shows a high overall degree of concordance in risk categorization with the Canadian FRAX tool (approximately 90% agreement). Differences, when they occur, usually relate to the presence of one or more risk factors that contribute to FRAX but that are not considered in the CAROC tool (namely, parental history of hip fracture, smoking, excess alcohol intake, rheumatoid arthritis).
The updated version of the CAROC fracture risk assessment tool is easy to use and, therefore, is recommended instead of FRAX for BMD reporting in Canada. Family physicians, osteoporosis specialists, and those communicating DXA findings are already familiar both with risk assessment in general [28] and with the 2005 CAROC model in particular; this should allow for a more seamless integration of the 2010 CAROC system into reporting. This situation may change as FRAX becomes more widely used.
Addressing Other Issues
Why Is the Lumbar Spine Not Included in the Fracture Risk Assessment? How Do I Evaluate Risk When the Lumbar Spine BMD Is Much Lower Than That at the Hip?
The FRAX and related 2010 CAROC risk assessment systems were calibrated for use of femoral neck BMD based upon: (a) the strength of the association of BMD with subsequent fractures (particularly hip fractures), (b) representation among the FRAX derivation cohorts, and (c) availability of a reference standard database for BMD normalization (NHANES III [National Health and Nutrition Examination Survey III] white female). These risk assessment models do not include lumbar spine BMD, which is known to be strongly associated with vertebral fracture risk [29]. Given the modest correlation between lumbar spine and femoral neck BMD [29–33], the T scores from these 2 sites are not uncommonly “discordant” [34]. Although there is no accepted definition of discordance, it usually is described as an absolute difference in T scores higher than 1 or 2 SDs. The idea of using the minimum T score for major osteoporotic fracture prediction is not supported by evidence from multiple cohorts [30–33]. Substitution of the minimum T score in the FRAX paradigm overestimates fracture probability. Simple procedures that integrate the femoral neck and lumbar spine T scores in the assessment of major osteoporotic fracture risk within the FRAX and 2010 CAROC systems are currently in development but require further validation before they can be recommended [34]. Meanwhile, a lumbar spine T score that is significantly worse than the femoral neck T score is considered an additional factor that may warrant pharmacologic treatment in those at moderate fracture risk (Figure 1).
Why Is It Recommended That Male T Scores Be Generated by Using a Female Reference Database?
In 1994, the WHO expert panel set the operational definition of osteoporosis in postmenopausal white women as a BMD T score of 2.5 or more SDs below the normal BMD for young healthy white women [1]. The WHO Collaborating Centre has recently provided guidance on the diagnosis of osteoporosis in older white and nonwhite women and men, designating BMD measurement made at the femoral neck with DXA as the reference standard [35]. The recommended reference range is the NHANES III reference database for femoral neck measurements in white women aged 20–29 years by using a similar cutoff value for both men and women (BMD T score 2.5 SD or more below the average for young adult women). The WHO position remains controversial, and other groups advocate sex-matched reference data [36,37]. A recent report from CaMos supports the WHO position, and, therefore, this is now the recommendation for BMD reporting in Canada [38]. Using T scores derived from male reference data (currently the default on DXA machines) will slightly overestimate the fracture risk in men.
What Recommendations Apply to Other Groups?
For premenopausal women, children, and younger men, the diagnosis of osteoporosis should not be made on the basis of BMD score alone (Table 1). In these age groups, OC and the International Society for Clinical Densitometry recommend using a Z score above or below –2.0 to categorize BMD as “within the expected range for age” or “below the expected range for age” [33].
What Is a Fragility Fracture?
The most serious manifestation of osteoporosis is a fragility fracture, defined as a fracture that occurs spontaneously or after minor trauma such as a fall from standing height or less [39]. Fragility fractures (which exclude craniofacial, hand, ankle, and foot fractures) represent 80% of all fractures that occur in postmenopausal women aged 50 years and older. A fracture remains one of the most-significant risk factors for predicting future fractures [12]. Forty percent of women who experience a fracture have a history of prior fracture [40]. The risk of experiencing another fracture in the year after a hip fracture is 5%–10% [6] and 20% after a vertebral fracture [10].
What Do I Do When Someone Has Had More Than One Fragility Fracture?
Refining the history of fracture is important in risk stratification. In the CAROC and FRAX systems, fractures are only captured as a dichotomous (yes/no) variable. However, multiple fractures confer greater risk than a single fracture. Individuals with more than 1 low-trauma fracture should be regarded as at particularly high risk for future fracture [12]. Multiple vertebral fractures also confer a stronger risk for future fractures than a single vertebral fracture.
DXA in Practice
Reporting
CAR recently published a standard for BMD reporting [41]. To generate a meaningful report, the referring physician will need to provide important clinical data, including indication for BMD testing, factors relevant to scan assessment (such as joint replacement, bone surgery, or bone disease in scan regions), osteoporosis medication history, factors crucial to fracture-risk determination (such as fragility fracture history and glucocorticoid use) and other pertinent medication information. In turn, there is a physician preference for absolute fracture reporting [42].
All first-time BMD reports should include demographic data, diagnostic category, fracture-risk category (if the patient is 50 years old or older), BMD data (including BMD in g/cm2, BMD T score, and the reference database used), and limitations to the assessment, if any [8]. There should follow the reporting physician’s interpretation and suggestions for any follow-up, if appropriate. When reporting BMD data, left femoral neck, total hip, and lumbar spine should be reported. If disease or artifacts affect the interpretation of the scan at the lumbar spine, then a forearm scan of the nondominant arm should be done. Similarly, if the left hip examination is compromised, then the right side can be used. Scan date, report date, referring physician, reporting physician, facility name and location, and machine identification (brand, model, and serial number) are other important components of the report.
On follow-up, large changes in height and weight should be reported. A measured prospective height loss of 2 cm or more may suggest vertebral fracturing (most of these are silent) and warrants further radiographic investigations (lateral spine radiographs or VFA). Weight changes of 10% also are prone to introduce artifact in monitoring change. In addition, absolute (not percentage) changes in bone density should be examined for statistical significance (when the machine, positioning, and region of interest assignment are consistent). This should be based upon practice and site and DXA-machine-specific determinations of precision (g/cm2). These data are then used for the specific determination of the least significant change (g/cm2) and the 95% confidence levels for statistically significant change at the different scan sites.
Although fracture-risk prediction is valid when using either the FRAX or 2010 CAROC models, it is recommended that the reports should clearly identify the tool used to the referring physician. With respect to therapy, neither the FRAX nor CAROC tools reflect the risk reduction associated with pharmacologic therapy. These tools reflect the theoretical risk for a hypothetical patient who is treatment naive, that is, on treatment or having ceased a course of treatment.
Quality Control and Assurance
For a meaningful BMD result, care must be taken in performing DXA. Quality control starts with scanning a spine phantom daily to exclude machine drift. Preventive maintenance of the machine should be done at least annually. Ideally, a limited number of dedicated and educated technologists should operate each scanner to ensure consistent results. Precision tests should be repeated whenever there are hardware or personnel changes. It should involve subjects representative of the patient population by using protocols that are published and available on the CAR Web site.
When scanning a patient, it is important to adhere to manufacturer protocols regarding proper positioning, determination of regions of interest, bone mapping, and subregion assignments. For follow-up scans, it is important that the machine, positioning, regions of interest, bone mapping, and subregion assignments are the same. The reproducibility of the area in each region of interest is a guide to the consistency of patient positioning between examinations. The total proximal femur and L1-L4 lumbar spine segments combined should be used for the determination of total lumbar spine T score, unless there are reasons, degenerative disease or artifacts, to exclude up to 2 vertebrae (a T score at least 1 SD different from the supra- or subadjacent vertebrae should prompt but not mandate examining the image and other evidence to decide if a segment needs to be excluded).
Summary
The 2010 OC Guidelines integrate 10-year absolute fracture risk prediction into an overall management approach by using validated risk assessment tools. There currently is a large gap between the optimal practices and treatments that are currently being provided to Canadians with osteoporosis. These guidelines are part of a concerted effort to close this.
Acknowledgments
The authors are indebted to Ms Donna Spafford and the staff at OC for logistical support.
References
- 1.World Health Organization. Assessment of osteoporotic fracture risk and its role in screening for postmenopausal osteoporosis. Geneva: WHO; 1994. p. 5. Technical Report Series, no. 843. [Google Scholar]
- 2.Kanis JA, Oden A, Johnell O, et al. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12:417–27. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 3.Tenenhouse A, Joseph L, Kreiger N, et al. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:897–904. doi: 10.1007/s001980070050. [DOI] [PubMed] [Google Scholar]
- 4.AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P, Krege JH, Adachi JD, et al. Vertebral fracture status and the World Health Organization risk factors for predicting osteoporotic fracture risk. J Bone Miner Res. 2009;24:495–502. doi: 10.1359/jbmr.081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaioannou A, Wiktorowicz ME, Adachi JD, et al. Mortality, independence in living, and re-fracture, one year following hip fracture in Canadians. J SOGC. 2000;22:591–7. [Google Scholar]
- 7.Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–73. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siminoski K, Leslie WD, Frame H, et al. Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J. 2005;56:178–88. [PubMed] [Google Scholar]
- 9.Colon-Emeric C, Kuchibhatia M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporos Int. 2003;14:879–93. doi: 10.1007/s00198-003-1460-x. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–3. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 11.Hodsman AB, Leslie WD, Tsang JF, et al. 10-year probability of recurrent fractures following wrist and other osteoporotic fractures in a large clinical cohort: an analysis from the Manitoba Bone Density Program. Arch Intern Med. 2008;168:2261–7. doi: 10.1001/archinte.168.20.2261. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–82. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lentle BC, Brown JP, Khan A, et al. Recognizing and reporting vertebral fractures: reducing the risk of future osteoporotic fractures. Can Assoc Radiol J. 2007;58:27–36. [PubMed] [Google Scholar]
- 15.Siminoski K, Adachi JG, Hanley DA, et al. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporosis Int. 2005;16:403–10. doi: 10.1007/s00198-004-1709-z. [DOI] [PubMed] [Google Scholar]
- 16.Ferrar L, Jiang G, Adams J, et al. Identification of vertebral fractures: an update. Osteoporos Int. 2005;16:717–28. doi: 10.1007/s00198-005-1880-x. [DOI] [PubMed] [Google Scholar]
- 17.Genant HK, Wu C, van Kujik C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 18.Guglielmi G, Diacinti D. Vertebral morphometry. In: Grampp S, editor. Radiology of Osteoporosis. 2. Berlin Heidelberg, Germany: Springer-Verlag; 2008. pp. 125–36. [Google Scholar]
- 19.Bessette L, Ste-Marie LG, Jean S, et al. The care gap in diagnosis and treatment of women with a fragility fracture. Osteoporos Int. 2008;19:79–86. doi: 10.1007/s00198-007-0426-9. [DOI] [PubMed] [Google Scholar]
- 20.Colman I, Chahal AM, Raymond G, et al. Incidental vertebral fractures discovered with chest radiography in the emergency department: prevalence, recognition, and osteoporosis management in a cohort of elderly patients. Arch Intern Med. 2009;165:905–9. doi: 10.1001/archinte.165.8.905. [DOI] [PubMed] [Google Scholar]
- 21.Gehlbach SH, Begelow C, Heimisdottir M, et al. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11:577–82. doi: 10.1007/s001980070078. [DOI] [PubMed] [Google Scholar]
- 22.McCloskey EV, Vasireddy S, Threlkeld J, et al. Vertebral fracture assessment (VFA) with a densitometer predicts future fractures in elderly women unselected for osteoporosis. J Bone Miner Res. 2008;23:1561–8. doi: 10.1359/jbmr.080515. [DOI] [PubMed] [Google Scholar]
- 23.Osteoporosis Canada. [Accessed February 8, 2011]; Available at: http://www.osteoporosis.ca.
- 24.World Health Organization. [Accessed August 3, 2011];WHO facture risk assessment tool. Available at: http://www.shef.ac.uk./FRAX/
- 25.Leslie WD, Tsang JF, Lix L. Simplified system for absolute fracture risk assessment: clinical validation in Canadian women. J Bone Miner Res. 2009;24:353–60. doi: 10.1359/jbmr.081012. [DOI] [PubMed] [Google Scholar]
- 26.Leslie WD, Berger C, Langsetmo L, et al. Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: results from the CaMos and Manitoba cohorts. Osteoporos Int. 2011;22:1873–83. doi: 10.1007/s00198-010-1445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–9. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 28.McPherson R, Frohlich J, Fodor G, et al. Canadian Cardiovascular Society position statement: Recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22:913–27. doi: 10.1016/s0828-282x(06)70310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie WD, Tsang JT, Caetano PA, et al. Number of osteoporotic sites and fracture risk assessment: a cohort study from the Manitoba Bone Density Program. J Bone Miner Res. 2007;22:476–83. doi: 10.1359/jbmr.061112. [DOI] [PubMed] [Google Scholar]
- 30.Blake GM, Knapp KM, Spector TD, et al. Predicting the risk of fracture at any site in the skeleton: are all bone mineral density measurement sites equally effective? Calcif Tissue Int. 2006;78:9–17. doi: 10.1007/s00223-005-0127-3. [DOI] [PubMed] [Google Scholar]
- 31.Leslie WD, Lix LM, Tsang JF, et al. Single-site vs multisite bone density measurement for fracture prediction. Arch Intern Med. 2007;167:1641–7. doi: 10.1001/archinte.167.15.1641. [DOI] [PubMed] [Google Scholar]
- 32.Kanis JA, Johnell O, Oden A, et al. The use of multiple sites for the diagnosis of osteoporosis. Osteoporos Int. 2006;17:527–34. doi: 10.1007/s00198-005-0014-9. [DOI] [PubMed] [Google Scholar]
- 33.Lewiecki EM, Watts NB, McClung MR, et al. Official positions of the International Society for Clinical Densitometry. J Clin Endocrinol Metab. 2004;89:3651–5. doi: 10.1210/jc.2004-0124. [DOI] [PubMed] [Google Scholar]
- 34.Leslie WD, Lix LM, Johansson H, et al. Spine-hip discordance and fracture risk assessment: a physician-friendly FRAX enhancement. Osteoporos Int. 2011;22:839–47. doi: 10.1007/s00198-010-1461-5. [DOI] [PubMed] [Google Scholar]
- 35.Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008;42:467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Leslie WD, Adler RA, El-Hajj Fuleihan G, et al. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:22–30. doi: 10.1016/j.jocd.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Binkley NC, Schmeer P, Wasnich RD, et al. What are the criteria by which a densitometric diagnosis of osteoporosis can be made in males and non-Caucasians? J Clin Densitom. 2002;5(suppl):S19–27. doi: 10.1385/jcd:5:3s:s19. [DOI] [PubMed] [Google Scholar]
- 38.Langsetmo L, Leslie WD, Zhou W, et al. Using the same bone density reference database for men and women provides a simpler estimation of fracture risk. J Bone Miner Res. 2010;25:2108–14. doi: 10.1002/jbmr.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleerekoper M. The evaluation of patients with osteoporosis. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA: Academic Press; 1966. pp. 1011–8. [Google Scholar]
- 40.Kanis JA, Johansson H, Oden A, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029–37. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Siminoski K. Canadian Association of Radiologists technical standards for bone mineral densitometry reporting. Can Assoc Radiol J. 2011;62:166–75. doi: 10.1016/j.carj.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Leslie WD. Absolute fracture risk reporting in clinical practice: a physician-centered survey. Osteoporosis Int. 2008;19:459–63. doi: 10.1007/s00198-008-0565-7. [DOI] [PubMed] [Google Scholar]