Abstract
We estimated peak bone mass (PBM) in 615 women and 527 men aged 16 to 40 years using longitudinal data from the Canadian Multicentre Osteoporosis Study (CaMos). Individual rates of change were averaged to find the mean rate of change for each baseline age. The age range for PBM was defined as the period during which bone mineral density (BMD) was stable. PBM was estimated via hierarchical models, weighted according to 2006 Canadian Census data. Lumbar spine PBM (1.046 ±0.123 g/cm2) occurred at ages 33 to 40 years in women and at 19 to 33 years in men (1.066 ± 0.129 g/cm2). Total hip PBM (0.981 ± 0.122 g/cm2) occurred at ages 16 to 19 years in women and 19 to 21 years in men (1.093 ± 0.169 g/cm2). Analysis of Canadian geographic variation revealed that the levels of PBM and of mean BMD in those over age 65 sometimes were discordant, suggesting that PBM and subsequent rates of bone loss may be subject to different genetic and/or environmental influences. Based on our longitudinally estimated PBM values, the estimated Canadian prevalences of osteoporosis (T-score <–2.5) were 12.0% (L1–L4) and 9.1% (total hip) in women aged 50 years and older and 2.9% (L1–L4) and 0.9% (total hip) in men aged 50 years and older. These were higher than prevalences using cross-sectional PBM data. In summary, we found that the age at which PBM is achieved varies by sex and skeletal site, and different reference values for PBM lead to different estimates of the prevalence of osteoporosis. Furthermore, lack of concordance of PBM and BMD over age 65 suggests different determinants of PBM and subsequent bone loss.
Keywords: PEAK BONE MASS, T-SCORES, OSTEOPOROSIS PREVALENCE, GEOGRAPHIC VARIATION, LONGITUDINAL
Introduction
The World Health Organization (WHO) expert panel has defined osteoporosis as a bone mineral density (BMD) 2.5 SD or more below the population-specific peak bone mass (PBM),(1) where PBM is the BMD during the stable period following growth and accrual of bone mass and prior to subsequent bone loss. Previously reported studies(2–6) have suggested that PBM at the hip occurs in the teens or twenties. Some studies have shown that PBM at the lumbar spine is achieved in the teens,(4,7) while others report reaching PBM in the twenties and the thirties.(2,8) Determination of time of PBM is essential for targeting interventions aimed at achieving optimal PBM. Many studies have estimated PBM from cross-sectional data,(3,6,9) and others have assessed the longitudinal change(4,8,10–15) but none has used longitudinal assessment in a population-based sample including teens and young adults.
There is evidence that BMD and fracture risk vary considerably among different populations.(16–18) We have previously reported geographic variation(17) of risk factors and fracture incidence across Canada. It is thought that both genetic variation and environmental determinants contribute to heterogeneity in the development of osteoporosis.(19) It is important to determine whether the geographic variation observed among the adult cohort is reflective of variation in PBM, hence related to genetics and early development,(20) or whether there are other determinants of BMD related to subsequent bone loss.
Our main objective was to determine PBM age range and levels in both sexes at the hip and lumbar spine using longitudinal BMD data from the Canadian Multicentre Osteoporosis Study (CaMos), a randomly selected community-dwelling population-based prospective cohort including teens and young adults. Our second objective was to determine the pattern of geographic variation in PBM and to assess whether this pattern is similar to that observed among older Canadian men and women. Our final objective was to determine the prevalence of osteoporosis based on the PBM determined from the present longitudinal analysis and compare this with estimates based on other PBM references values.
Methods
CaMos is an ongoing population-based prospective cohort study of 9423 randomly selected community-dwelling women (6539) and men (2884) aged 25 years and older at baseline (1995–1997) and living within 50 km of nine Canadian cities (St John’s, Halifax, Quebec City, Kingston, Toronto, Hamilton, Saskatoon, Calgary, and Vancouver) that we will refer to as centers. CaMos objectives, methodology, and sampling framework are described in detail elsewhere.(21) This cohort is referred to as the “original cohort.” In 2004, to better assess PBM and its determinants, the CaMos cohort was supplemented by a random sample of Canadians (n = 1001) between 16 and 24 years of age called the “youth cohort,” recruited using the same methodology. Signed informed consent was obtained from all CaMos participants. For participants under 18 years of age, we also obtained signed informed consent from one of the parents. Unlike those in the original cohort, after agreeing to participate but prior to the interview, the younger participants received a mailed form asking them to fill in information on their family history of osteoporosis, fractures, stooped posture, or hip fracture in grandparents. The selection criteria in both cohorts, however, were exactly the same. Participants in neither the youth cohort nor the original cohort were excluded if they had a family history of osteoporosis. In both the original and youth cohorts, data collection at baseline included an extensive interviewer-administered questionnaire and a clinical assessment. The questionnaire included sociodemographic information, medical and fracture history, family history, dietary intake, physical activity, tobacco smoking, and quality-of-life determinations. Clinical assessments included height, weight, and bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA). Year 2 follow-up in the youth cohort and 5-year follow-up in the original cohort included an interviewer-administered questionnaire and clinical assessment of height, weight, and BMD.
This study of PBM included 287 women and 235 men from the original cohort (25 to 40 years old) and 328 young women and 292 young men from the youth cohort (16 to 24 years old). All had two BMD measurements 2 to 5 years apart to estimate longitudinal BMD changes. The 16- to 40-year-old subgroup with at least one missing BMD will be referred to as the “excluded group” (286 women and 288 men). A further 4814 women and 1930 men aged 50 years or more from the original cohort were used to estimate osteoporosis prevalences in different age and sex subgroups.
Bone mineral density
Lumbar spine (L1–L4), femoral neck, total hip, greater trochanter, and Ward’s triangle BMD values were measured by DXA using Hologic QDR (Marlborough, MA, USA) 1000, 2000, or 4500 or Lunar DPX (Piscataway, NJ, USA) densitometers. Machine calibration was done daily. Daily and weekly quality assurance tests were performed as recommended by the DXA manufacturers. Longitudinal stability was monitored using a spine phantom local to each site. In the original cohort, two of the nine centers in CaMos used GE Lunar machines and seven used Hologic machines. In the youth cohort, five centers used GE Lunar machines and four used Hologic machines. Lunar data were converted into equivalent Hologic values by standard methods.(22,23) All densitometers were calibrated at the start of the study and once each year thereafter using the Bona Fide Spine Phantom (BFP, Bio-Imaging Technologies, Newtown, PA, USA) to ensure site-to-site comparability. As an example, the precision, expressed as percent coefficient of variation (%CV), ranged between 0.17 and 0.39 in our 2008 monitoring period.
Of commercially available phantoms studied, the BFP exhibited the closest regression to human data for both the spine and the total hip.(24) The phantom was scanned annually 10 times without repositioning. As per the “CaMos Standardized Procedure Manual,” the same clinical technologist at each site was required to scan all subjects at all time points. In the original cohort, all Hologic measurements were redone by the same technologist and all Lunar measurements by two technologists. In the youth cohort, all BMD measurements were redone by the same two technologists. In both the original and youth cohorts, all subsequent BMD measurements were done on the same DXA machine as the baseline measurements. DXA measurements were performed at baseline and year 5 for the original cohort and at baseline and year 2 for the youth cohort. All the scan analyses were performed centrally to remove operator bias in the analytical phase.
Statistical analysis
Individual-level BMD slope estimates were computed for the lumbar spine, femoral neck, total hip, greater trochanter, and Ward’s triangle sites. The slope between the BMD values at baseline and year 5 in the original cohort and baseline and year 2 in the youth cohort were used as estimates of the rate of BMD change per year. The average of the slopes across subjects provides a longitudinal curve of BMD change per year across age (see Berger and colleagues(25) for methodologic details), where positive BMD changes imply an increase in BMD over time. A 5-degree polynomial was fit through the BMD change per year estimates to better illustrate the average change over time.
PBM was defined to have occurred when BMD change by age was no longer positive, that is, when there was no further gain in bone mass on average. After this peak, BMD remains stable or begins to decrease. The PBM age range was defined to be the age range of the BMD plateau or the age range 3 years following attainment of the highest BMD value if there was no stable period. When no peak was observed, the age of PBM was defined as the first 3 years of data acquisition, that is, ages 16 to 19 years. Stability was decided on exclusion of 0 from the 95% confidence interval of the BMD change estimate. However, priority was given to results from the 5-year age groups, as previously published.(25)
In order to estimate the mean PBM to best reflect the Canadian population, we used the same methodology as Tenenhouse and colleagues.(26) That is, we derived a weighted average of each center-specific BMD at baseline, and within each center, we assumed that the individual participant’s BMD values were normally distributed with a center-specific mean. For all skeletal sites, a single variance across all centers was employed, as indicated by the data. The nine center-specific means were defined in our study to be the PBM for each center, and a weight was attached to each value according to 2006 Canadian Census data for each of our nine centers. The nine PBM mean values were assumed to follow a normal distribution with an overall mean representing the overall PBM in Canada and with a variance parameter representing the spread of PBM between centers. Our model leads to estimates of the probability that one center has a higher PBM than any other center, which can be used to locate the centers with the highest or lowest PBM. A difference in PBM between two centers was said to be present if the estimated probability was higher than .95. We used the same methodology among those 65 years of age and older to provide inferences about geographic variation between the younger and older cohorts. This model can be described as a two-level Bayesian hierarchical model, and we used a Gibbs sampler algorithm implemented via BUGS software (Bayesian inference using Gibbs sampling, Version 0.603 for UNIX systems, MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK) to approximate the posterior densities of all mean and variance parameters. Between 4000 and 5000 iterations in BUGS were run to ensure convergence of the models and were used for inferences.
To obtain the age-specific percentage of PBM reached, the BMD was fixed within the PBM age range to the estimate of PBM. We then generated BMD values at each age outside the PBM age range by applying the estimated rates of BMD change. Finally, the BMD values derived in this fashion were divided by PBM.
The average percentage of change from baseline for bone mineral content (BMC), area, and BMD were computed for the youth cohort. Percentage change was given by the difference between both measurements (year 2 – baseline) within an individual divided by the measurement at baseline. This calculation was possible because both measurements for each individual were done on the same DXA machine, and since it was a unitless measure, it could be compared across centers. Graphs and mean comparisons by age allow comparisons of the evolution of BMC, area, and BMD over time.
Osteoporosis is defined as a BMD measurement 2.5 SD or more below PBM. For comparison purposes, four definitions of PBM were used to estimate the prevalence of osteoporosis. The first definition employed the CaMos Canadian PBM published by Tenenhouse and colleagues(26) using the original cohort and cross-sectional data only. The second definition employed PBM as determined earlier using longitudinal analysis with the addition of a younger cohort. Third, PBM was defined as the CaMos mean BMD at baseline in those 20 to 29 years old, as done by National Health and Nutrition Examination Survey (NHANES) of the United States.(5) Finally, the fourth definition used the published NHANES PBM.(5) All prevalences were obtained using weights from 2006 Canadian Census data.
Results
Baseline characteristics
Baseline characteristics of the participants included in the study sample were stratified by cohort (youth versus original) and are presented in Table 1. Both women and men in the youth cohort smoked less, weighed less, had lower body mass index (BMI). and were more engaged in regular physical activity than the original cohort. Younger men also reported fewer previous fractures and younger women were taller than the original cohort. Comparison of the same characteristics between the study sample and the excluded group (data not shown) revealed no clinically important differences except in 25- to 40-year-old men, in whom there was a higher percentage reporting current smoking (36.8%) in the excluded group. In particular, the average BMD at baseline in the excluded group was not different from that in the study sample. In the study sample, only 9 young women subjects were still premenarcheal at baseline, whereas 15 had their first period 1 or 2 years prior to baseline.
Table 1.
Baseline Characteristics by Gender and Cohort
| Women (n = 615)
|
Men (n = 527)
|
|||
|---|---|---|---|---|
| Youth cohort (16 to 24) | Original cohort (25 to 40) | Youth cohort (16 to 24) | Original cohort (25 to 40) | |
| N | 328 | 287 | 292 | 235 |
| White | 88.7% | 94.1% | 88.4% | 94.0% |
| Currently smoking | 7.0% | 20.6% | 12.3% | 23.4% |
| Regular activity | 65.2% | 54.0% | 69.5% | 57.9% |
| Previous fractures | 32.3% | 33.1% | 43.5% | 53.6% |
| Agea (years) | 19.6 (2.7) | 33.9 (4.3) | 19.4 (2.5) | 33.4 (4.2) |
| Menarchea (years) | 12.5 (1.3) | 12.7 (1.5) | — | — |
| Heighta (cm) | 164.7 (6.6) | 163.1 (6.1) | 178.0 (7.2) | 177.2 (7.2) |
| Weighta (kg) | 62.1 (11.9) | 68.2 (16.3) | 77.5 (15.7) | 82.6 (14.0) |
| BMIa (kg/m2) | 22.9 (4.2) | 25.6 (5.8) | 24.4 (4.7) | 26.2 (3.8) |
Reported as mean (SD).
Longitudinal BMD changes and age of PBM
BMD changes per year by sex and skeletal site are shown in Fig. 1. Longitudinal changes in BMD, BMC, and area occurred in parallel (data not shown).
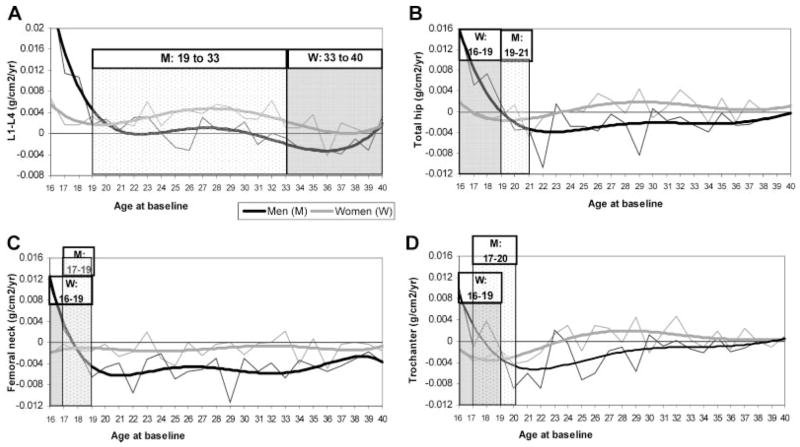
Fig. 1.
Longitudinal BMD change per year and PBM age range in women and men. Thin gray and black lines represent the average BMD change by age. The thicker gray and black lines represent the 5-degree polynomials that were fitted to the BMD change per year estimates: (A) L1–L4; (B) total hip; (C) femoral neck; (D) trochanter.
In younger women, L1–L4 BMD changes (Fig. 1A) were positive from 16 to 32 years of age but were not different from zero between ages 33 and 40 years. Therefore, PBM was defined to occur between the ages 33 and 40 years in women. For total hip (Fig. 1B) and femoral neck (Fig. 1C) in women, BMD changes were not different from zero at all ages from 16 to 24 years (except for a 1-year decline at age 21 in the total hip). PBM was thought to have been achieved prior to 16 years of age at the total hip and femoral neck in women; for practical purposes, the age of PBM in both total hip and femoral neck was assigned to have occurred between 16 and 19 years of age. In the trochanter (Fig. 1D) and also in Ward’s triangle (data not shown), a decrease in BMD was already observed at age 16; therefore, PBM also was defined to occur between 16 and 19 years of age.
In young men, lumbar spine BMD changes (Fig. 1A) were positive from ages 16 to 18 years, were not different from zero between 19 and 33 years of age, and decreased from 34 to 40 years of age. Therefore, L1–L4 PBM was defined to occur between 19 and 33 years of age in men. Total-hip BMD change (Fig. 1B) was positive in young men from ages 16 to 18 years and decreased after age 22. Therefore, PBM was defined to have occurred between 19 and 21 years of age. At the femoral neck (Fig. 1C), PBM was positive at age 16 and decreased after age 19. The age of PBM therefore was defined to occur between 17 and 19 years. In the trochanter (Fig. 1D) and Ward’s triangle (data not shown), the age of PBM was defined as 17 to 20 years and 16 to 19 years, respectively.
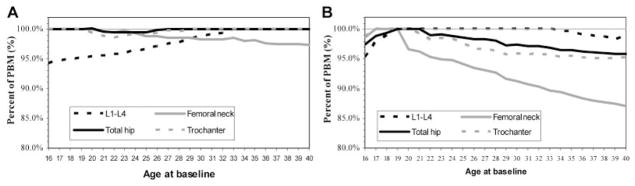
The percentages of BMD relative to PBM at different ages are depicted in Fig. 2. These figures show that at 16 years of age in both women and men, more than 94% of BMD is already acquired, even though some continued gain in BMD may be observed after age 16 depending on the skeletal site. The most rapid loss of BMD after attaining PBM is seen in both women and men in Ward‘s triangle. In men, there is also rapid bone loss after PBM at the femoral neck.
Fig. 2.
Percent of PBM achieved between 16 and 40 years of age in women and men: (A) women; (B) men.
When examining changes in total-hip BMD relative to the number of years since menarche, we estimated an increase (0.007g/cm2 per year with a 95% confidence interval of 0.002–0.013) until 3 years after menarche, after which BMD remained stable.
PBM reference values using age of PBM from longitudinal versus cross-sectional analyses
Using the age ranges for PBM as defined earlier, we computed the PBM values (g/cm2) for each geographic center and for the overall CaMos PBM. The overall CaMos PBM values are presented in Table 2. The CaMos PBM reference value was compared with the CaMos PBM value obtained previously by cross-sectional analysis(26) and with cross-sectional PBM values reported by NHANES.(5) When data at corresponding skeletal sites were available to perform comparisons, the current CaMos PBM estimates, obtained through longitudinal analysis using a younger cohort, were consistently higher than both the previous CaMos PBM estimates and the NHANES PBM, both of which relied on cross-sectional data.
Table 2.
Comparison of Canadian PBM and NHANES PBM
| Previous CaMos PBM
|
Current CaMos PBM
|
NHANES PBM
|
||||
|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | ||
| L1–L4 | Women | 1.042 (0.121) | (1.024, 1.059) | 1.046 (0.123) | (1.025, 1.067) | |
| Men | 1.058 (0.127) | (1.040, 1.077) | 1.066 (0.129) | (1.046, 1.082) | — | |
| Femoral neck | Women | 0.857 (0.125) | (0.816, 0.904) | 0.877 (0.114) | (0.860, 0.894) | 0.858 (0.120) |
| Men | 0.910 (0.125) | (0.875, 0.945) | 0.971 (0.151) | (0.945, 0.998) | 0.934 (0.137) | |
| Total hip | Women | — | 0.981 (0.122) | (0.963, 0.999) | 0.942 (0.122) | |
| Men | — | 1.093 (0.169) | (1.059, 1.126) | 1.041 (0.144) | ||
| Trochanter | Women | — | 0.720 (0.111) | (0.703, 0.736) | 0.708 (0.099) | |
| Men | — | 0.817 (0.140) | (0.793, 0.841) | 0.778 (0.118) | ||
Geographic variation and center differences between men and women
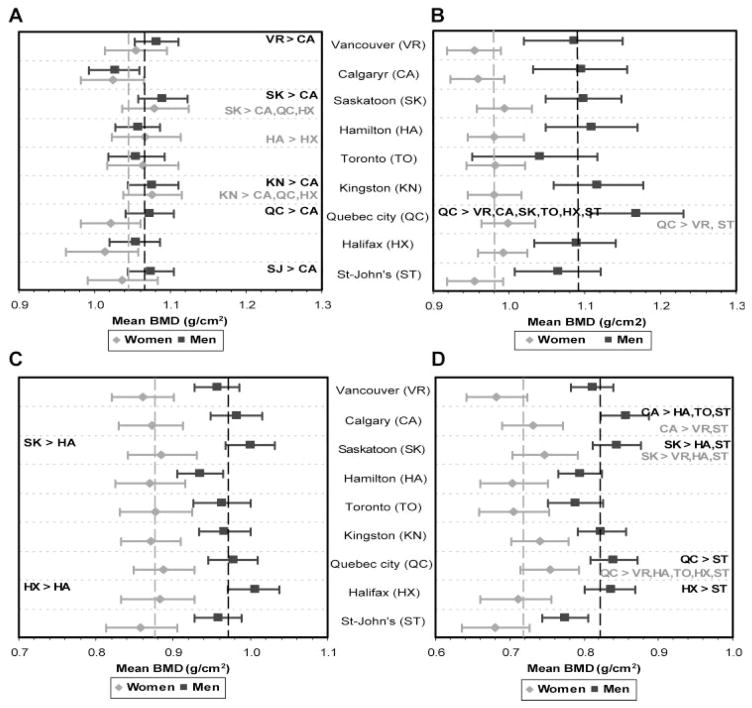
The PBM values by center are presented in Fig. 3. The dotted vertical line indicates the sex-specific overall Canadian PBM values. When centers were compared with each other, among the statistically different centers (with probability ≥95%), the PBM differences ranged from 0.042 to 0.084 g/cm2 in women and from 0.046 to 0.127 g/cm2 in men. Quebec City, Saskatoon, and Kingston were among the centers with the highest PBM values in women, whereas Quebec City was the center with the highest PBM values in men. In contrast, Vancouver and St-John’s for women and St-John’s and Calgary for men were among the centers with the lowest PBM values.
Fig. 3.
PBM (95% confidence interval) by center in women and men and geographic comparisons. The dotted vertical line indicates the sex-specific overall Canadian PBM (black for men; gray for women). Statistically different centers (with probability ≥95) are noted (black for men; gray for women): (A) L1–L4; (B) total hip; (C) femoral neck; (D) trochanter.
In terms of differences between sexes in the different centers, there were no differences between women and men in spine PBM estimates in any center except for Quebec City, where men, on average, possessed a PBM that was 0.052 g/cm2 (95% confidence interval 0.002–0.102) higher than women. At the hip (total hip, femoral neck, trochanter, and Ward’s triangle; data not shown), men had consistently higher PBM estimates except in the Toronto center, where total hip values were not different.
Geographic variation in PBM relative to Mean BMD over age 65
The pattern of geographic variations in mean BMD in those 65 years of age and older is different in the lumbar spine in men and at the total hip in both women and men in comparison with the geographic variation in PBM. In particular, in those age 65 years and older, participants in Quebec City, Halifax, and St-John’s (eastern Canada) had the lowest mean BMD, whereas Kingston and Toronto (Ontario) had the highest mean BMD. However, intercenter variations in PBM often were the opposite.
Prevalence of osteoporosis
The estimated Canadian prevalence of osteoporosis, from CaMos data for women and men 50 years of age and older, are presented in Table 3 for different skeletal sites. Men were found to have a lower prevalence of osteoporosis than women regardless of skeletal site. The last line of the table shows the estimated Canadian prevalence of osteoporosis as defined by the International Society for Clinical Densitometry (ISCD),(27) that is, using the lowest T-score of lumbar spine, femoral neck, or total hip. However, this last osteoporosis prevalence estimate is the same as one using the lowest T-score of lumbar spine or femoral neck. Moreover, adding trochanter to the list of sites does not change the prevalence. Thus, when the ISCD method is employed, the prevalence estimates are driven by BMD from only two skeletal sites—lumbar spine and femoral neck. When using female-specific PBM to generate men’s T-scores, the prevalence of osteoporosis, using the ISCD method, is 4.2% in men aged 50 to 64 years, 6.6% in men 65 years of age or older, and 5.0% in men 50 years of age or older.
Table 3.
Estimates of Canadian Prevalence of Osteoporosisa (%) (95% CI) Analyses Using Updated CaMos PBM Values Determined by Longitudinal Analysis
| Women
|
Men
|
|||||
|---|---|---|---|---|---|---|
| Site used for prevalence (T-score) | 50–64 years | 65+ years | 50+ years | 50–64 years | 65+ years | 50+ years |
| Lumbar spine | 7.5 (6.0, 9.0) | 19.7 (16.8, 22.6) | 12.0 (10.6, 13.5) | 2.9 (2.0, 3.9) | 2.8 (1.5, 4.2) | 2.9 (2.1, 3.7) |
| Femoral neck | 8.1 (6.5, 9.6) | 29.8 (26.5, 33.2) | 16.1 (14.4, 17.7) | 2.4 (1.5, 3.3) | 5.1 (3.3, 6.9) | 3.3 (2.5, 4.2) |
| Total hip | 4.1 (3.0, 5.2) | 17.6 (14.8, 20.4) | 9.1 (7.8, 10.4) | 0.5 (0.1, 0.9) | 1.8 (0.7, 3.0) | 0.9 (0.5, 1.4) |
| Trochanter | 1.4 (0.7, 2.1) | 5.8 (4.0, 7.5) | 3.0 (2.3, 3.8) | 0.2 (0.0, 0.4) | 1.3 (0.3, 2.2) | 0.5 (0.2, 0.9) |
| Lowest T-score in L1–L4, femoral neck, total hip | 12.0 (10.2, 13.9) | 37.1 (33.6, 40.7) | 21.3 (19.5, 23.1) | 4.7 (3.5, 5.9) | 6.9 (4.8, 9.0) | 5.5 (4.4, 6.5) |
Osteoporosis is defined as a T-score ≤–2.5.
Table 4 compares four estimates of Canadian prevalences of osteoporosis based on femoral neck or total hip. In both men and women, prevalence estimates were lower when total-hip values were used rather than femoral neck values irrespective of the method used to determine PBM. In men, within the same skeletal site, estimates of prevalence in CaMos were not appreciably different regardless of the methods and population used to determine PBM. However, in women, using PBM reference values obtained from longitudinal analysis and a younger cohort resulted in higher estimates of prevalence than with any of the other methods.
Table 4.
Estimates of Canadian Prevalence of Osteoporosisa (%) (95% CI) in Women and Men 50 Years Old or Older by Different Definitions of PBM
| Women
|
Men
|
|||
|---|---|---|---|---|
| PBM use to generate T-score in CaMos | Femoral neck | Total hip | Femoral neck | Total hip |
| Previous CaMos PBM | 7.6 | — | 3.7 | — |
| (6.4, 8.7) | — | (2.8, 4.6) | — | |
| Current CaMos PBM | 16.1 | 9.1 | 3.3 | 0.9 |
| (14.4, 17.7) | (7.8. 10.4) | (2.5, 4.2) | (0.5, 1.4) | |
| CaMos 20- to 29-year mean BMD as PBM | 11.4 | 7.6 | 3.0 | 1.7 |
| (10.0, 12.8) | (6.4, 8.8) | (2.2, 3.8) | (1.0, 2.3) | |
| NHANES PBM | 9.5 | 5.2 | 3.2 | 1.2 |
| (8.2, 10.8) | (4.2, 6.2) | (2.3, 4.0) | (0.7, 1.7) | |
Osteoporosis is defined as a T-score ≤–2.5.
Discussion
We report here the first population-based estimates of PBM that used longitudinal areal BMD data from young men and women aged 16 years and older and that included rural and urban regions. The highest value of areal BMD, BMC, and volumetric BMD (vBMD) have been reported by various investigators to be achieved at different chronologic ages. A recent longitudinal study in a US population performed by quantitative compute tomography (QCT) reported substantial trabecular bone loss at multiple sites (particularly at the lumbar spine) beginning in the third decade in both sexes.(28) The term PBM therefore needs to be defined not only by whether it is cross-sectional or longitudinal but also by the method used to assess it. In our studies, longitudinal changes in BMD, BMC, and area occurred in parallel, likely because most participants were postpubertal. The estimated age of PBM attainment therefore also may differ in relation to the technique (ie, DXA versus QCT) employed because DXA based measurements do not clearly distinguish cortical and trabecular bone. Nevertheless, for purposes of calculation of T-scores from DXA scans for determination of the presence of osteoporosis, as defined by the WHO,(1) PBM has been defined as the BMD during the stable period following growth and accrual of bone mass and prior to subsequent bone loss. Thus our definition of PBM corresponds to the highest areal bone mineral density (aBMD) that is the basis for T-scores.
By using a younger cohort, it was possible to determine that the period of PBM is specific for gender and skeletal site. In particular, for young women, PBM at the hip is established before age of entry into the cohort (16 years). For young men, there is a longer time of bone mass accrual at the hip compared with women. In young men, the increase at the spine occurred over the same time period as the hip, but the stable period after accrual is longer in the spine than in the hip, where it decreases shortly after a stable level is reached. Even with the later bone accrual in the spine in both women and men and in the hip in young men, most of the bone accrual in both young men and young women occurs before age 16, with more than 94% of PBM already achieved by age 16. These results indicate that early intervention before and during puberty is necessary for the attainment of optimal PBM. The different timing and duration of puberty in young women and men have been shown to result in sexual dimorphism in bone structure after puberty, even though the rate of growth in both sexes is thought to be similar both before and during puberty.(29)
Only one other longitudinal and population-based study(30) of PBM was identified. Thus, in a small cohort, Sundberg and colleagues(30) studied 45 girls and 48 boys aged 12 and 13 years for 4 years in a rural village in southern Sweden (Sösdala, population 2900). They concluded that growth in bone size precedes the accrual of mass in both genders and that growth in bone size and accrual of mass in girls precede the growth in bone size and accrual of mass in boys. In our study, beginning at age 16, there was no discordance between bone size and bone mass in either young women or young men, likely because the vast majority of participants were postpubertal.
A recent non-population-based study by Boot and colleagues(15) that combined participants from a longitudinal and a cross-sectional study found that PBM in the spine occurred at 17.8 years in women and 20.6 years in men. Walsh and colleagues(13) concluded that skeletal maturation and bone mineral accrual at the lumbar spine continue into the third decade. Mein and colleagues(8) studied 9-year changes in BMD in young women (mean age 18 years) and found similar results to those reported here: an increase at the lumbar spine but a decrease at the femoral neck that was already observed at age 18. The results in men are also consistent with results of an Australian cross-sectional but population-based study in men aged 20 to 97 years.(6) These authors found small increases in spine BMD in their cohort, but mean BMD decreases in hip sites were already evident at 20 years of age. In contrast, Bachrach and colleagues,(4) using a convenience sample, described a plateau in spine BMD in women at 15.7 years of age, and Lloyd and colleagues(12) concluded that there was no change in BMD of the femoral neck in girls 17 to 22 years of age. We did not see large differences in the magnitude of the lumbar spine PBM between the two sexes. Thus differences in BMD between men and women after the age at which PBM is reached may reflect either or both of the following: a lesser rate of bone loss in men than in women or earlier and more extensive development of artifacts such as degenerative changes in the spine or both.
Geographic variation in PBM was observed in this study, with some clinically and statistically important differences between centers. PBM also varied by sex and skeletal site. Although our sample size was larger than most, we still did not have the power to assess the possibility of geographic variation in the age of PBM. Geographic differences among those 65 years of age and older were similar to those noted in the overall adult cohort by Langsetmo and colleagues,(17) with a higher BMD in Ontario (particularly Toronto and Kingston) and a lower BMD in Atlantic Canada. However, the pattern of variation in PBM in our study sometimes was discordant with the mean BMD in the adult cohort or those aged 65 years of age and older. In sites where concordance was observed (ie, a higher PBM was associated with a higher mean BMD in older age and vice versa), the level of PBM may well be the major factor determining later life BMD, with relatively similar rates of decline after PBM is reached. In contrast, for those with lower BMD levels in old age despite equivalent or higher PBM values, rate of BMD loss after skeletal maturity is reached may be the determining factor. Both genetic and environmental factors may influence PBM and the rate of bone loss after maturity, but the specific factors and/or their contributions may not be the same. The identification of population groups that manifest these different patterns therefore may aid in identifying the precise nature of these determinants.
The longitudinal analysis of lumbar spine BMD change showed small changes in BMD among both men and women not noted in our previous cross-sectional analyses.(26) These changes and the inclusion of a younger cohort had an impact on the defined age range of PBM but minimal effect on the estimate of PBM. Lumbar spine BMD, for example, was very stable over the whole age range. For the femoral neck region, however, there were clinically important differences in the PBM in women and clinically and statistically important differences in men. Thus the updated femoral neck PBM in men was 0.971 g/cm2 compared with the previously published value of 0.910 g/cm2, which did not translate into a major change in prevalence of osteoporosis in men because the SD also was increased. In contrast, however, in women, the difference in PBM determined by our current approach, combined with a decrease in the SD, doubled the prevalence of osteoporosis. Therefore, including the younger cohort had a direct impact on the estimates of the prevalence of osteoporosis because estimates of prevalence are sensitive to even modest changes in estimates of PBM.
These results using different PBM ages, different PBM values, and different skeletal sites to compute prevalence of osteoporosis demonstrate that the use of different definitions or references will result in different osteoporosis prevalences. Other recent articles(9,31,32) similarly have demonstrated that different PBM definitions (in particular, PBM age and skeletal site) may lead to different prevalences of osteoporosis. In addition to the impact of PBM definitions on assessing prevalence, the determination of BMD T-scores remains a critically important tool for clinical decision making, and therefore it is important that clinicians understand the spectrum of factors defining T-scores. A recent article by Rochmis and colleagues(33) has noted that the Hologic NHANES III femoral neck scores are disproportionately low in comparison with the total-hip and trochanter regions, which may result in misclassification of patients as osteoporotic. Our studies reaffirm how variable the thresholds for T-scores may be and that calculation of the reference values is strongly dependent on the population and skeletal site used. There are critical clinical consequences of this fact: T-score calculations have an obvious impact on the diagnosis of osteoporosis and on tools used for the estimation of absolute 10-year fracture risk (eg, FRAX: www.shef.ac.uk/FRAX) when these calculations include T-score values. The definition of the reference values for T-score calculations therefore ultimately may significantly affect the clinical decision to treat or not to treat. Consequently, until alternative criteria are developed,(34) care is necessary in the definition and use of PBM reference standards.
Strengths of this investigation include the use of a randomly selected population-based cohort that was followed prospectively, the quality control that was performed routinely to ensure longitudinal reliability of all the BMD measures, and the inclusion of both men and women. Those lost to follow-up were similar at baseline in terms of age, menarche age, BMD, BMI, history of fracture, regular activity, and race. Although there is no obvious source or direction of bias in measured variables, as in all longitudinal observational cohort studies, limitations include possible selection bias and loss to follow-up. The fact that we have used two measurements in a short time can be a limitation by generating low-slope precision estimates. However, we combined data across large numbers of subjects and used average slopes to determine PBM. Therefore, any uncertainty associated with each individual slope is greatly reduced in our reported average estimates. Finally, DXA is a very useful clinical tool, but it has significant limitations, particulary in growing bones, where change in bone size may result in disproportionately increased aBMD and also could affect the analysis of longitudinal changes in real (volumetric) BMD in the same.(35) Although most of our participants were postpubertal and over 94% of BMD had already been acquired, nevertheless, further studies are needed to compare our results with similar analyses using other techniques such as QCT.
In summary, using the longitudinal rate of change in BMD, it was concluded that the age at which PBM is achieved varies by sex and skeletal site, with hip PBM occurring at an earlier age than lumbar spine PBM and with men achieving lumbar spine PBM earlier than women but achieving hip PBM later. Geographic variation in PBM, which was sometimes discordant with mean BMD in those over age 65, may indicate that PBM and subsequent rates of bone loss may be subject to different genetic and/or environmental influences. Using a younger cohort and longitudinal assessment leads to different PBM values in the femoral neck than our earlier PBM estimates,(26) resulting in an apparent increased prevalence of osteoporosis in women and a small decrease in men. Different reference values for PBM lead to different prevalences of osteoporosis, thereby demonstrating the importance of optimal determination of such values.
Acknowledgments
We wish to thank all the participants in the Canadian Multicentre Osteoporosis Study. The CaMos Research Group includes David Goltzman (co-principal investigator, McGill University, Montreal), Nancy Kreiger (co-principal investigator, University of Toronto, Toronto), and Alan Tenenhouse (principal investigator emeritus, Toronto). CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant) and Claudie Berger (study statistician). Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), and Emma Sheppard (coordinator). Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), and Barbara Stanfield (coordinator). Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), and Marc Gendreau (coordinator). Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), and Barbara Matthews (coordinator). University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie Jamal (co-director), Tim Murray (past director), and Barbara Gardner-Bray (coordinator). McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), and Laura Pickard (coordinator). University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K Shawn Davison (co-director), and Jola Thingvold (coordinator). University of Calgary, Calgary, Alberta: David A. Hanley (director) and Jane Allan (coordinator). University of British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Millan Patel (co-director), Brian Lentle (radiologist), and Yvette Vigna (coordinator).
CaMos was funded by the Canadian Institutes of Health Research (CIHR), Merck Frosst Canada, Ltd., Eli Lilly Canada, Inc., Novartis Pharmaceuticals, Inc., The Alliance for Better Bone Health (Sanofi-Aventis and Procter & Gamble Pharmaceuticals Canada, Inc.), Amgen, The Dairy Farmers of Canada, and The Arthritis Society.
Footnotes
Disclosures
DAH and KSD have received honoraria from Amgen, Proctor & Gamble, Sanofi-Aventis, Merck Frosst, and Servier. DG and RGJ have received honoraria from and served on the advisory boards of Amgen, Eli Lilly, Proctor & Gamble, Merck Frosst, Novartis, and Servier. CB, LL, LJ, JCP, NK, and AT state that they have no conflicts of interest.
All authors were involved in the conception and design of the study. LJ, DG, AT, LL, and CB were involved in the analyses and interpretation of data. CB, DG, and LL were involved in drafting the manuscript, whereas all the authors revised critically and approved the final version of the manuscript.
References
- 1.Report of a WHO Study Group. World Health Organ Techical Report Series 843. Geneva: WHO; 1994. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis; pp. 1–129. [PubMed] [Google Scholar]
- 2.Pedrazzoni M, Girasole G, Bertoldo F, et al. Definition of a population-specific DXA reference standard in Italian women: the Densitometric Italian Normative Study (DINS) Osteoporos Int. 2003;14:978–982. doi: 10.1007/s00198-003-1521-1. [DOI] [PubMed] [Google Scholar]
- 3.Hoiberg M, Nielsen TL, Wraae K, et al. Population-based reference values for bone mineral density in young men. Osteoporos Int. 2007;18:1507–1514. doi: 10.1007/s00198-007-0399-8. [DOI] [PubMed] [Google Scholar]
- 4.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 6.Henry MJ, Pasco JA, Korn S, Gibson JE, Kotowicz MA, Nicholson GC. Bone mineral density reference ranges for Australian men: Geelong Osteoporosis Study. Osteoporos Int. 2010;21:909–917. doi: 10.1007/s00198-009-1042-7. [DOI] [PubMed] [Google Scholar]
- 7.Sabatier JP, Guaydier-Souquieres G, Laroche D, et al. Bone mineral acquisition during adolescence and early adulthood: A study in 574 healthy females 10–24 years of age. Ospeoporos Int. 1996;6:141–148. doi: 10.1007/BF01623938. [DOI] [PubMed] [Google Scholar]
- 8.Mein AL, Briffa NK, Dhaliwal SS, Price RI. Lifestyle influences on 9-year changes in BMD in young women. J Bone Miner Res. 2004;19:1092–1098. doi: 10.1359/JBMR.040310. [DOI] [PubMed] [Google Scholar]
- 9.Ribom EL, Ljunggren O, Mallmin H. Use of a Swedish T-score reference population for women causes a two-fold increase in the amount of postmenopausal Swedish patients that fulfill the WHO criteria for osteoporosis. J Clin Densitom. 2008;11:404–411. doi: 10.1016/j.jocd.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 11.Slosman DO, Rizzoli R, Pichard C, Donath A, Bonjour JP. Longitudinal measurement of regional and whole body bone mass in young healthy adults. Osteoporos Int. 1994;4:185–190. doi: 10.1007/BF01623238. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd T, Petit MA, Lin HM, Beck TJ. Lifestyle factors and the development of bone mass and bone strength in young women. J Pediatr. 2004;144:776–782. doi: 10.1016/j.jpeds.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 13.Walsh JS, Henry YM, Fatayerji D, Eastell R. Lumbar spine peak bone mass and bone turnover in men and women: a longitudinal study. Osteoporos Int. 2009;20:355–362. doi: 10.1007/s00198-008-0672-5. [DOI] [PubMed] [Google Scholar]
- 14.Baxter-Jones AD, McKay H, Burrows M, et al. International longitudinal paediatric reference standards for bone mineral content. Bone. 2010;46:208–216. doi: 10.1016/j.bone.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boot AM, de Ridder MA, van dS I, van SI, Krenning EP, de Muinck Keizer-Schrama SM. Peak bone mineral density, lean body mass and fractures. Bone. 2010;46:336–341. doi: 10.1016/j.bone.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Kaptoge S, da Silva JA, Brixen K, et al. Geographical variation in DXA bone mineral density in young European men and women. Results from the Network in Europe on Male Osteoporosis (NEMO) study. Bone. 2008;43:332–339. doi: 10.1016/j.bone.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Langsetmo L, Hanley DA, Kreiger N, et al. Geographic variation of bone mineral density and selected risk factors for prediction of incident fracture among Canadians 50 and older. Bone. 2008;43:672–678. doi: 10.1016/j.bone.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petley GW, Cotton AM, Murrills AJ, et al. Reference ranges of bone mineral density for women in southern England: the impact of local data on the diagnosis of osteoporosis. Br J Radiol. 1996;69:655–660. doi: 10.1259/0007-1285-69-823-655. [DOI] [PubMed] [Google Scholar]
- 19.Richards JB, Kavvoura FK, Rivadeneira F, et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151:528–537. doi: 10.7326/0003-4819-151-8-200910200-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javaid MK, Cooper C. Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab. 2002;16:349–367. doi: 10.1053/beem.2002.0199. [DOI] [PubMed] [Google Scholar]
- 21.Kreiger N, Tenenhouse A, Joseph L, et al. Research notes: the Canadian Multicentre Osteoporosis Study (CaMos) - background, rationale, methods. Can J Aging. 1999;18:376–387. [Google Scholar]
- 22.Genant HK, Grampp S, Gluer CC, et al. Universal standardization for dual x-ray absorptiometry: Patient and phantom cross-calibration results. J Bone Miner Res. 1994;9:1503–1514. doi: 10.1002/jbmr.5650091002. [DOI] [PubMed] [Google Scholar]
- 23.Hanson J. Standardization of proximal femur BMD measurements. International Committee for Standards in Bone Measurement. Osteoporos Int. 1997;7:500–501. doi: 10.1007/s001980050039. [DOI] [PubMed] [Google Scholar]
- 24.Jackson SA, Miller CG. Choice of Cross-Calibration Phantom for DXA of the Lumbar Spine and Total Hip. ASBMR. 2003 [Google Scholar]
- 25.Berger C, Langsetmo L, Joseph L, et al. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. CMAJ. 2008;178:1660–1668. doi: 10.1503/cmaj.071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenenhouse A, Joseph L, Kreiger N, et al. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: The Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:897–904. doi: 10.1007/s001980070050. [DOI] [PubMed] [Google Scholar]
- 27.ISCD. Official Position of the ISCD. 2007 http://www.iscd.org.
- 28.Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iuliano-Burns S, Hopper J, Seeman E. The age of puberty determines sexual dimorphism in bone structure: a male/female co-twin control study. J Clin Endocrinol Metab. 2009;94:1638–1643. doi: 10.1210/jc.2008-1522. [DOI] [PubMed] [Google Scholar]
- 30.Sundberg M, Gardsell P, Johnell O, Ornstein E, Karlsson MK, Sernbo I. Pubertal bone growth in the femoral neck is predominantly characterized by increased bone size and not by increased bone density–a 4-year longitudinal study. Osteoporos Int. 2003;14:548–558. doi: 10.1007/s00198-003-1406-3. [DOI] [PubMed] [Google Scholar]
- 31.Leslie WD, Caetano PA, Roe EB. The impact of hip subregion reference data on osteoporosis diagnosis. Osteoporos Int. 2005;16:1669–1674. doi: 10.1007/s00198-005-1901-9. [DOI] [PubMed] [Google Scholar]
- 32.Lofman O, Larsson L, Toss G. Bone mineral density in diagnosis of osteoporosis: reference population, definition of peak bone mass, and measured site determine prevalence. J Clin Densitom. 2000;3:177–186. doi: 10.1385/jcd:3:2:177. [DOI] [PubMed] [Google Scholar]
- 33.Rochmis PG, Sheridan MJ, Perry L. Is the NHANES III femoral neck database discordant with the total hip and trochanteric region databases? J Clin Densitom. 2009;12:224–228. doi: 10.1016/j.jocd.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Melton LJ., III The prevalence of osteoporosis: gender and racial comparison. Calcif Tissue Int. 2001;69:179–181. doi: 10.1007/s00223-001-1043-9. [DOI] [PubMed] [Google Scholar]
- 35.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]