Abstract
Summary
Canadian women over 50 years old were studied over a 10-year period to see if those who sustained a fracture (caused by minimal trauma) were receiving the recommended osteoporosis therapy. We found that approximately half of these women were not being treated, indicating a significant care gap in osteoporosis treatment.
Introduction
Prevalent fragility fracture strongly predicts future fracture. Previous studies have indicated that women with fragility fractures are not receiving the indicated treatment. We aimed to describe post fracture care in Canadian women using a large, population-based prospective cohort that began in 1995–1997.
Methods
We followed 5,566 women over 50 years of age from across Canada over a period of 10 years in the Canadian Multicentre Osteoporosis Study. Information on medication use and incident clinical fragility fractures was obtained during a yearly questionnaire or interview and fractures were confirmed by radiographic/medical reports.
Results
Over the 10-year study period, 42–56% of women with yearly incident clinical fragility fractures were not treated with an osteoporosis medication. During year 1 of the study, 22% of the women who had experienced a fragility fracture were on treatment with a bisphosphonate and 26% were on hormone therapy (HT). We were not able to differentiate HT use for menopause symptoms vs osteoporosis. Use of bisphosphonate therapy increased over time; odds ratio (OR) for use at year 10 compared to use at year 1 was 3.65 (95% confidence interval (CI) 1.83–7.26). In contrast, HT use declined, with an OR of 0.07 (95%CI 0.02–0.24) at year 10 compared to year 1 of the study.
Conclusion
In a large population-based cohort study, we found a therapeutic care gap in women with osteoporosis and fragility fractures. Although bisphosphonate therapy usage improved over time, a substantial gap remains.
Keywords: Bisphosphonates, Care gap, Fragility fracture, Osteoporosis, Postmenopausal women
Introduction
Having one osteoporotic fracture is a powerful predictor of future fractures. A history of any fragility fracture confers close to a twofold increased risk of future fracture [1]. Depending on the number and site of previous fractures, the risk of future fracture may be increased as high as 12-fold [2]. This increased risk is recognized in Canada’s clinical practice guidelines where prior fragility fracture is considered an indication for investigation and possible treatment of osteoporosis [3, 4]. Other guidelines also recommend testing and treatment of fragility fractures [5, 6].
Low trauma vertebral and hip fractures most commonly occur in post-menopausal women and are associated with significant excess morbidity and mortality [7–11]. Mortality further increases with increased numbers of vertebral fractures, whether symptomatic or incidentally discovered on radiograph [8, 11]. Osteoporosis affects up to one in three women, and fragility fractures are common [9]. A recent study from Quebec demonstrated that 81% of fractures in women over 50 years of age were fragility fractures [12]. Anti-osteoporosis therapies have been proven to be effective in preventing new fractures in women with existing osteoporotic fractures [13–17]. Despite the fact that therapy can decrease the risk of subsequent fracture by up to 40–60%, Ontario data have shown that less then 25% of patients presenting to hospital with a fragility fracture are investigated and treated for osteoporosis [18]. Hence, a care gap exists between best clinical practice for the diagnosis and treatment of osteoporosis and actual practice.
An osteoporosis care gap has been documented previously in Canada, but most studies have followed patients either post fracture clinic or in acute care, and most have been conducted over relatively short periods of time [19]. This has similarly been demonstrated internationally [20]. In 2008, the Canadian Multicentre Osteoporosis Study (CaMos) published the first prospective, population-based study showing an osteoporosis care gap in men with fragility fractures [21]. Based on these past studies, we predict that there is an osteoporosis care gap in women with fragility fractures in Canada. We also hypothesize that this care gap is improving over time. To test these hypotheses and better define the presumed care gap, we examined data collected over 10 years in the population-based Canadian Multicentre Osteoporosis Study to prospectively study the trends in osteoporosis treatment in Canadian women over time.
Methods
Study participants
We examined a population sample from participants in the ongoing population-based, community-dwelling longitudinal cohort study, the Canadian Multicentre Osteoporosis Study (CaMos). We included all women over the age of 50 years at baseline. Participant’s osteoporosis status was not known at the time of recruitment, as this was a population-based study to determine prevalence and incidence of osteoporosis. The cohort was followed for 10 years with baseline assessments taking place between 1995 and 1997.
The full methodological details of the CaMos study have been described previously [22]. Briefly, Canadians aged 25 years and older who live within a 50-km radius of one of nine study sites were randomly selected to participate in the Canadian Multicentre Osteoporosis Study. This prospective cohort study followed participants for 10 years at nine study centers across Canada (St. John’s, Halifax, Quebec City, Toronto, Hamilton, Kingston, Saskatoon, Calgary, and Vancouver). The study population consists of 9,423 community-dwelling individuals (6,539 women and 2,884 men) representing and age-stratified-, sex-, and region-specific samples. Recruitment occurred over an 18-month period from lists of random telephone numbers. Approval was received by the institutional review boards at each center and informed consent was obtained from each participant.
Data collection
An extensive interviewer-administered questionnaire (administered at years 3, 5, and 10), lateral lumbar and thoracic spine X-rays, and bone mineral density (BMD) testing were performed at baseline, and repeated after 5 and 10 years. BMD was also repeated at year 3, for women 40–60 years old at baseline. During other years, a two-page questionnaire was mailed to participants asking about hospitalizations and fractures within the past year and current use of prescription bone medications. All participants who experienced a fracture received a follow-up phone call to enquire about the circumstances surrounding the fracture including: date, fracture site, circumstances leading to fracture, and medical treatment. In addition, consent was obtained to allow contact with the treating physician or hospital and verification of the radiology report. Participants were told to bring the contents of their medicine cabinets with them to each in-person interview. All osteoporosis therapies, vitamin supplements, and other medications were documented in detail. The intervening year annual questionnaires displayed a list of bone-related medications and asked participants which they had taken during the previous year.
Bone mineral density
BMD of the lumbar spine (L1–L4) and hip were measured by DXA using Hologic QDR 1000, 2000, 4500, or Lunar DPX densitometers. Machines were calibrated daily, and quality assurance was performed following a standard daily and weekly schedule. Cross-calibration of the machines at the nine centers using a European Spine Phantom was performed initially, and subsequently the Bone Fide phantom was performed at baseline and in the year of every examination. Reports indicating bone density (g/cm2) and T-scores were sent to each participant, a physician named by the participant, or both depending on the center [23].
Fractures
Clinically recognized non-traumatic fractures were included. Minimal trauma fractures were defined as any clinical fragility fracture including fractures of the hip, ribs, spine, forearm, pelvis, and other, but excluding fractures of fingers, toes, or face.
At baseline, previous fractures were obtained by self-report only, but subsequent fractures that occurred after enrolment were reported by patients and confirmed by medical or radiographic reports.
Statistical analysis
The generalized estimating equations approach was performed to model differences in the use of osteoporosis medications over a 10-year period in individuals with osteoporotic fractures [24]. Osteoporosis medications were classified into three groups, bisphosphonates, hormone therapy (HT), and any bone-active therapy [including bisphosphonates, HT, selective estrogen receptor modulators (SERMS), or calcitonin] and analyzed separately. An auto-regressive correlation matrix was used for the analyses. All analyses were adjusted for age (years); combined BMD T-score at the lumbar spine, femoral neck, or trochanter (normal: T-score>−1, osteopenia: T-score<−1 and >−2.5, osteoporosis: T-score≤−2.5); smoking status (current/not current); and early menopausal status (<45 years). Adjusted odds ratios (OR) and corresponding 95% confidence intervals (CI) are reported. All statistical analyses were performed using the SAS/STAT (version 9.1; SAS Institute, Cary, NC, USA) software package running on Windows XP Professional. The criterion for statistical significance was set at α 0.05.
Results
A total of 5,566 women with mean age 67 (±9) years at baseline were followed over the 10 years. Table 1 displays the baseline characteristics of the cohort. During each year of the study, a median of 2.4 (1.9–2.9) percent of this cohort sustained a new minimal trauma fracture.
Table 1.
Baseline characteristics
| Characteristic | Women (n=5,566) number (percent) |
|---|---|
| Age (years) | |
| 50–59 | 1,351 (24.3) |
| 60–69 | 2,045 (36.7) |
| 70–79 | 1,647 (29.6) |
| 80+ | 523 (9.4) |
| Current smoker | 754 (13.6) |
| Regular activity program | 3,082 (55.4) |
| Diagnosis of osteoporosisa | 674 (12.4) |
| Clinical fragility fractureb | 1,551 (27.9) |
| Hip fracture | 71 (1.3) |
| Vertebral fracturec | 679 (12.2) |
| Current corticosteroid use | 95 (1.7) |
| Bisphosphonate use | 154 (2.8) |
| Hormone therapy use | 1,379 (24.8) |
| SERM use | 18 (0.3) |
| Calcitonin use | 1 (0.02) |
| BMD (n=4,826)d | |
| >−1.0 | 1,222 (25.3) |
| −1.0 to −2.5 | 2,601 (53.9) |
| <−2.5 | 1,003 (20.8) |
n=5433
Excludes fractures of fingers, toes, and face
Includes clinical and subclinical fractures
BMD scans were conducted in 4,826 women
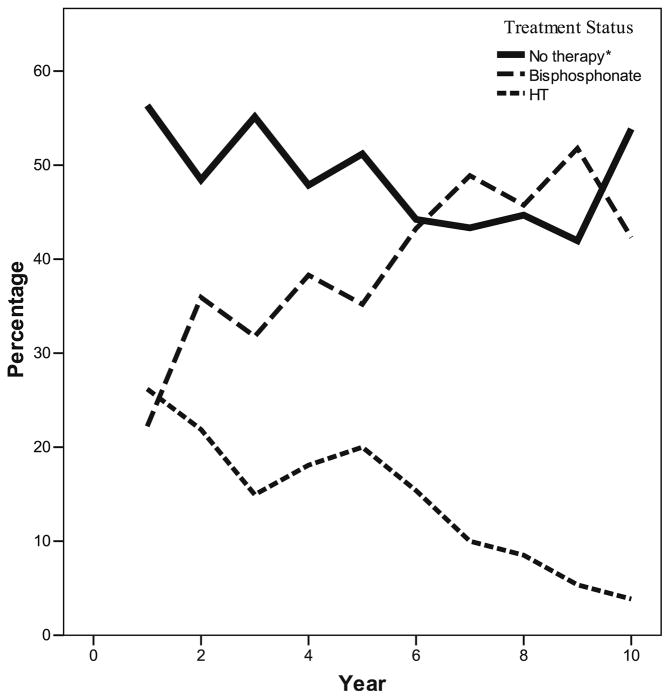
Treatment of these women with incident minimal trauma fractures over time is displayed in Fig. 1. During year 1 of the study, 22% of the women who had experienced a fragility fracture were on treatment with a bisphosphonate and 26% were on HT. The analysis did not differentiate the reason for HT use and, therefore, the proportion of this 26% that were using HT for menopausal symptoms, as opposed to bone protection, is unknown. Figure 1 shows the trend in treatment patterns over time, displaying decreasing HT use and increasing use of bisphosphonate therapy over the course of the 10-year study. Over the course of the 10-year study, up to 56% of women with incident fragility fractures per year were not treated with any osteoporosis therapy (Fig. 1).
Fig. 1.
Percentage of women with a new fragility fracture on no medical therapy for osteoporosis (solid line), or on treatment with a bisphosphonate (medium-sized dotted line), or on hormone therapy (small-sized dotted line)
Table 2 shows the OR for being on any osteoporosis treatment, bisphosphonate therapy, or HT during years 2–10 of the study compared to year 1 of the study, with results controlled for BMD, age, smoking, and early menopause status. Participants with incident fragility fractures were no more likely to be on treatment (of any type) for osteoporosis during the subsequent years of the study compared to year 1. The OR of HT use at year 7 compared to year 1 was 0.27 (95% CI 0.10–0.72) and by year 10 OR was 0.07 (95% CI 0.02–0.24). Bisphosphonate therapy use increased over time with an OR of 2.7 (95% CI 1.31–5.62) by year 4 of the study (Table 2).
Table 2.
Odds of a woman with an incident fragility fracture being on medical treatment for osteoporosis in year 1 vs subsequent years prospectively documented in women aged 50 and older in the Canadian Multicentre Osteoporosis Study
| Year of CaMos study | Incident fracture rate n (%) | Any osteoporosis therapya
|
Bisphosphonate therapy
|
Hormone therapy
|
|||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | ||
| 2 | 128 (2.4) | 1.15 | 0.66–2.00 | 1.69 | 0.90–3.2 | 0.82 | 0.45–1.52 |
| 3 | 107 (2.1) | 0.95 | 0.53–1.73 | 1.74 | 0.88–3.42 | 0.60 | 0.35–1.04 |
| 4 | 94 (1.9) | 1.42 | 0.77–2.61 | 2.72 | 1.31–5.62 | 0.68 | 0.33–1.41 |
| 5 | 125 (2.6) | 1.09 | 0.64–1.84 | 1.90 | 0.97–3.70 | 0.60 | 0.32–1.11 |
| 6 | 104 (2.4) | 1.21 | 0.70–2.10 | 2.25 | 1.19–4.27 | 0.76 | 0.43–1.35 |
| 7 | 90 (2.1) | 1.51 | 0.85–2.69 | 3.57 | 1.79–7.15 | 0.27 | 0.10–0.72 |
| 8 | 94 (2.3) | 1.67 | 0.94–2.98 | 3.94 | 1.99–7.80 | 0.28 | 0.11–0.70 |
| 9 | 112 (2.9) | 1.50 | 0.88–2.55 | 4.14 | 2.21–7.74 | 0.15 | 0.06–0.36 |
| 10 | 104 (2.8) | 1.16 | 0.65–2.07 | 3.65 | 1.83–7.26 | 0.07 | 0.02–0.24 |
Hormone therapy, calcitonin, SERM, or bisphosphonate
OR controlled for BMD, age, smoking, and early menopause (younger than 45) status
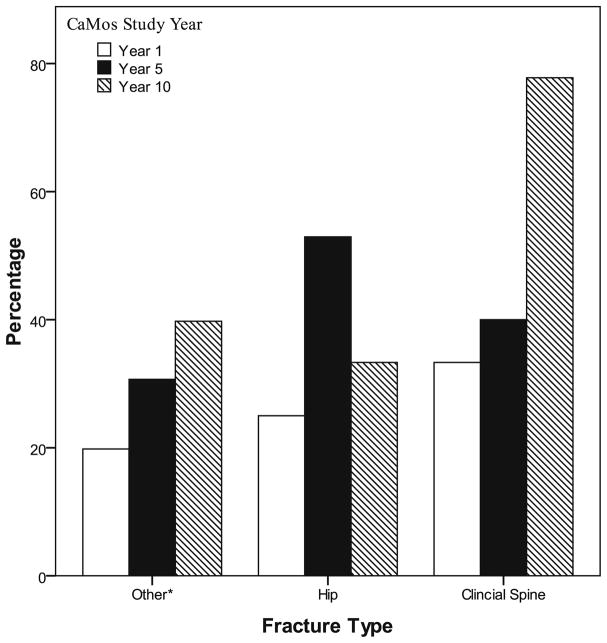
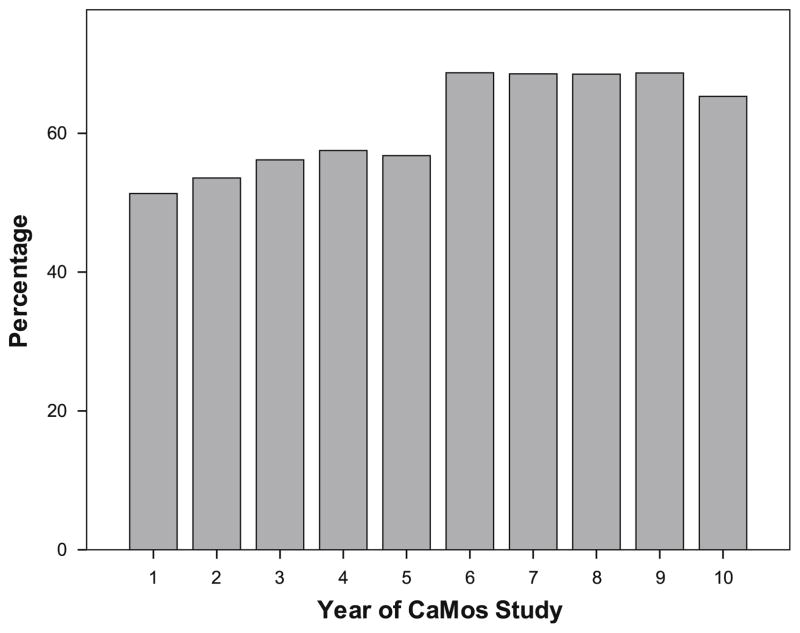
Figure 2 shows bisphosphonate treatment at years 1, 5, and 10 in women with different types of fragility fractures. In general, there was an increasing use of bisphosphonate therapy in all fracture types over time. The exception was a decline in bisphosphonate use for hip fractures (n=4) during year 10 (33%). Apart from year 10, the general trend in hip fracture treatment was one of increased bisphosphonate use over time, with more than half of hip fracture patients receiving treatment in years-8 and 9. When the study population was examined by BMD (instead of fractures), as shown in Fig. 3, treatment with an osteoporosis therapy (calcitonin, SERM, HT, bisphosphonate), in those with BMD≤−2.5, tended to increase over time. Fifty one percent of women with T-scores≤−2.5 were on therapy at year 1; when HT use is excluded, 36% were on therapy at year 1. However, by year 10, 65% were on bone-specific medications.
Fig. 2.
Percentage of women with incident fragility fracture on treatment with bisphosphonate, by fracture type
*Other: Clinical fragility fracture excluding fractures of the spine, hip, fingers, toes, and face
Fig. 3.
Percentage of women aged 50 and over with BMD≤−2.5 on therapy with any osteoporosis medication (calcitonin, SERM, HT, bisphosphonate) over each of the 10 years in the Canadian Multicentre Osteoporosis Study (CaMOS)
Discussion
In this prospective, population-based study of community-dwelling women aged 50 years and over, we found a therapeutic care gap in osteoporosis treatment. Only approximately half of the women in our study who sustained a fragility fracture were treated with an approved osteoporosis medication. Similarly, one-half to two-thirds of women meeting the WHO criteria for osteoporosis (T-score≤−2.5) were on medical therapy. This care gap demonstrated an improvement over the 10-year study period.
Our findings of a therapeutic care gap are in keeping with what has been reported previously in the literature. In Canada, treatment rates after fragility fracture have been reported in 5.2–38% of individuals [19]. Although this “care gap” has been previously reported, most studies have been performed retrospectively using large databases or chart review [20, 25, 26]. Such studies risk underestimating the number of patients on therapy, as databases may not record patients who use out-of-plan pharmacy services. Our prospective cohort study does not face this limitation. Another population-based cohort study from the province of Manitoba, Canada, conducted between 1997 and 2002 showed that, in 1997, only 4.9% of women with a fragility fracture went on to be treated with an osteoporosis medication during the 12 months following the fracture [27]. This study documented an improvement in treatment rates over time, with 17.6% of patients being treated in 2001. This improvement over time has been noted in other studies as well [28]. Our study also showed improved treatment rates, with respect to bisphosphonate use in women with fragility fractures, over the 10-year study period. Bisphosphonate use in these women increased from 22% in year 1 to 42% in year 10. The rate of osteoporosis treatment using any medication (HT, SERM, bisphosphonate, or calcitonin) also showed a trend towards increase, from 44% at year 1 to 46% at year 10. Our reported treatment rates, while not ideal, are far higher than previously reported rates in Canada [27]. This may be the result of increased participant and physician awareness that resulted from being involved in the CaMos study. Results of all BMD tests and X-rays were reported by the CaMos researchers directly to participants, to their physicians, or to both. This reporting introduces a bias towards increased awareness of osteoporosis within our study and may have led to higher treatment rates than found in the general population. Similarly, the Osteoporosis Canada guidelines for diagnosis and treatment of osteoporosis came out in 2002, and a concentrated effort was made to circulate the guidelines to physicians [3]. The year 2002 correlates with years 5–6 of our study and, therefore, introduction of these guidelines may help explain the sharp rise in bisphosphonate use seen at this time as demonstrated in Fig. 1.
Alternatively, HT use fell from 26% in year 1 to 4% in year 10. The Women’s Health Initiative combined hormone therapy arm (WHI) published in 2002 raised awareness to the risks associated with long-term HT use and resulted in HT no longer being considered a first-line therapy for osteoporosis [29]. The WHI was published during year 5–6 of our study and the steep trend towards decreased HT use after this time is evident in Fig. 1. The trend towards no improvement in treatment (of any type) in participants with incident fragility fractures (as seen in Table 2) was likely influenced by this decline in HT use as well.
Despite these improvements in bisphosphonate use in women with fragility fractures, we still found that approximately half of fracturing participants were not receiving bisphosphonate therapy. Many studies have shown that a history of fracture is a significant risk factor for future fractures [1, 2, 30–33]. Medical therapy for osteoporosis, in particular, therapy with bisphosphonates, has been proven to be effective at preventing future fractures. In the 2004 report by Roux et al., when postmenopausal women with clinical vertebral fractures were treated with risedronate (vs placebo), subsequent fracture risk was decreased within 6 months, with risk decreased by 69% after 1 year of therapy [34]. Morphometric vertebral fractures were also decreased by 71%. Similarly, risedronate has been shown to decrease nonvertebral fractures in women with and without previous vertebral fractures, within 6 months, with fracture risk decreased as low as 74% at 1 year [35]. Other bisphosphonates have also shown significant efficacy at decreasing fracture risk [13, 36]. Hence, there is strong evidence to support immediate treatment with bisphosphonate therapy in all patients with fragility fracture, regardless of life expectancy or other circumstances. Despite the strength of the evidence, previous reports have found that doctors are more likely to initiate treatment based on BMD results than on the history of a fragility fracture [12]. The evidence for treatment is less strong using BMD alone as a predictor of fracture [37]; however, most guidelines do recommend treating postmenopausal women with a BMD T-score of ≤−2.5 [3, 38]. Despite this, we found only 51–69% of the women in our study with a BMD T-score of ≤−2.5 were receiving treatment with an osteoporosis medication.
Fracture type affects the likelihood of treatment. Previous reports have found that vertebral fractures are more likely to be treated than other types of fracture [20]. We also found this generally to be true in our study, although at year 5 (as seen in Fig. 2), women with an incident hip fracture were more likely to receive treatment than women with clinical vertebral fractures. We found a decrease in treatment rates of hip fracture at year 10; this discrepancy was likely secondary to the low number of hip fractures (n=4) that occurred that year. All fractures in postmenopausal women require attention. Even if a clinician is uncertain as to whether a patient has had a “fragility” fracture or if the fracture was trauma-related, an osteoporosis work-up is still warranted as decreased BMD scores and increased risk of osteoporosis have been identified in women over 50 with “traumatic” fractures [39].
Reasons for the existing care gap are not entirely clear. Previous reports have cited reasons such as the cost of therapies and cost of resources for diagnosis, concerns about medication side effects, and lack of knowledge about osteoporosis, BMD measurements, and osteoporosis treatments [25, 40]. Poor communication among health care providers about who is responsible for initiating and following therapy has been cited to be a significant issue. Although orthopedic surgeons are responsible for the acute care of fracture patients, they often consider preventative therapies such as osteoporosis treatment to be the realm of primary care providers [25]. In Canada, where universal healthcare is available, the cost of resources to diagnose osteoporosis should not be an issue. Similarly, oral bisphosphonate therapy is covered either by restricted or open access by most provincial drug benefit plans [41]. Therefore, the presence of the existing care gap in Canada, as demonstrated by our study, points to causes beyond financial ones as areas in need of attention to address this issue.
The current study has several limitations. It is likely that women (and the care providers for these women) involved in this 10-year osteoporosis study had increased awareness of osteoporosis and fragility fractures. Similarly, participation in CaMos was voluntary, and therefore, the study is subject to volunteer bias that may make the cohort healthier and more motivated than individuals in general society. Both of these issues, however, should lead to a more conservative estimate of a care gap and, if anything, an underestimate of the actual problem. Similarly, patient adherence with osteoporosis medications was not measured; therefore, patients with osteoporosis medications in their medicine cabinet (and therefore recorded during their CaMos interview) may not have been taking them or not taking them appropriately, leading to an overestimate of the number of women on therapy. The current study focuses on treatment using approved osteoporosis medications. It does not report the use of calcium and vitamin D supplements or lifestyle modification as a means to fracture prevention [42]. However, although calcium and vitamin D are important components in the treatment of osteoporosis, the superiority of combining adequate calcium plus vitamin D with an osteoporosis medication has been proven in multiple large randomized controlled trials [43, 44].
In summary, we found an osteoporosis treatment care gap in Canadian women aged 50 and over who suffer a fragility fracture or who have a BMD T-score≤−2.5. Although our results show an improvement over the course of 10 years with more women with fragility fractures receiving treatment (particularly treatment with bisphosphonates), approximately half of these women are still not receiving treatment despite strong evidence to support its efficacy and guidelines recommending its use. This care gap therefore remains an important barrier to the effective treatment of osteoporosis and public health officials, patient societies, and health care providers need not be complacent, but continue to target educational and other interventions aimed at addressing this problem.
Acknowledgments
The authors thank all the participants in the CaMos study, who’s participation made this research possible. The CaMos Research Group: David Goltzman (co-principal investigator, McGill University), Nancy Kreiger (co-principal investigator, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto). CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant), Silvia Dumont (administrative assistant), Claudie Berger (study statistician), Wei Zhou (statistician). Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator). Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator). Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator). Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator). University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator). McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator). University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director), Jola Thingvold (coordinator). University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator). University British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Millan Patel (co-director), Yvette Vigna (coordinator), Brian Lentle (radiologist).
The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research (CIHR); Merck Frosst Canada Ltd.; Eli Lilly Canada Inc.; Novartis Pharmaceuticals Inc.; The Alliance: sanofiaventis & Procter and Gamble Pharmaceuticals Canada Inc.; Servier Canada Inc.; Amgen Canada Inc.; The Dairy Farmers of Canada; and The Arthritis Society.
This work was supported by the Osteoporosis Canada and CaMos joint Fellowship Research Award, www.osteoporosis.ca. Dr. Fraser was also supported by the University of Western Ontario Resident Research Career Development Program.
Footnotes
Conflicts of interest Dr. Fraser, Dr. Ioannidis, Ms. Pickard, Dr. Prior, Dr. Olszynski, Dr. Anastassiades, Dr. Jamal, Dr. Josse, and Dr. Goltzman have no conflicts of interest to declare for this manuscript.
Dr. Adachi has been a consultant/speaker for: Amgen, Astra Zeneca, Eli Lilly, GSK, Merck, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Servier, Wyeth and Bristol-Myers Squibb; and has conducted clinical trials for: Amgen, Eli Lilly, GSK, Merck, Novartis, Pfizer, Procter & Gamble, Sanofi Aventis, Roche, Wyeth, and Bristol-Myers Squibb.
Dr. Kaiser has been on the advisory board for: Amgen, Novartis, Eli Lilly, Bristol-Myers Squibb, and Astra Zeneca; and speakers bureau for: Amgen, Novartis, Procter & Gamble/Sanofi Aventis, Merck Frosst, and Eli Lilly.
Dr. Jacques Brown has received consulting fees or other remuneration from: Abbott, Amgen, Eli Lilly, Novartis, Merck, and Warner Chilcott; received research grants from: Abbott, Amgen, Bristol-Myers-Squibb, Eli Lilly, Pfizer, and Roche; and been on the speakers bureau for: Eli Lilly, Amgen, Novartis, Merck, and Warner Chilcott.
Dr. Hanley has been on the advisory board for: Amgen, Merck Frosst, Warner-Chilcott, Novartis, and Eli Lilly; performed clinical trials for: Amgen, Merck Frosst, Procter & Gamble/Sanofi Aventis, Novartis, NPS Pharmaceuticals, Eli Lilly, Pfizer, and Wyeth-Ayerst; and received speaking honoraria from: Amgen, Merck Frosst, Procter & Gamble/Sanofi Aventis, Novartis, NPS Pharmaceuticals, Eli Lilly, Pfizer, and Wyeth-Ayerst.
Dr. Papaioannou has been a consultant/speaker for: Amgen, Aventis, Eli Lilly, Merck Frosst, Novartis, Procter & Gamble, Servier, and Wyeth-Ayerst; conducted clinical trials for: Eli Lilly, Merck Frosst, Novartis, Procter & Gamble, and Sanofi-Aventis; and received unrestricted grants from: Amgen, Eli Lilly, Merck Frosst, Procter & Gamble, and Sanofi-Aventis.
Contributor Information
L.-A. Fraser, Departments of Epidemiology and Medicine, Hamilton Health Sciences—Chedoke Site, McMaster University, 1200 Main Street West, Hamilton, ON L8N 3Z5, Canada
G. Ioannidis, Departments of Epidemiology and Medicine, Hamilton Health Sciences—Chedoke Site, McMaster University, 1200 Main Street West, Hamilton, ON L8N 3Z5, Canada
J. D. Adachi, Departments of Epidemiology and Medicine, Hamilton Health Sciences—Chedoke Site, McMaster University, 1200 Main Street West, Hamilton, ON L8N 3Z5, Canada
L. Pickard, Departments of Epidemiology and Medicine, Hamilton Health Sciences—Chedoke Site, McMaster University, 1200 Main Street West, Hamilton, ON L8N 3Z5, Canada
S. M. Kaiser, Department of Medicine, Dalhousie University, Halifax, NS, Canada
J. Prior, Department of Medicine, University of British Columbia, Vancouver, BC, Canada
J. P. Brown, Department of Medicine, Laval University, Quebec City, QC, Canada
D. A. Hanley, Department of Medicine, University of Calgary, Calgary, AB, Canada
W. P. Olszynski, Department of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
T. Anastassiades, Department of Medicine, Queen’s University, Kingston, ON, Canada
S. Jamal, Department of Medicine, University of Toronto, Toronto, ON, Canada
R. Josse, Department of Medicine, University of Toronto, Toronto, ON, Canada
D. Goltzman, Department of Medicine, McGill University, Montreal, QC, Canada
A. Papaioannou, Departments of Epidemiology and Medicine, Hamilton Health Sciences—Chedoke Site, McMaster University, 1200 Main Street West, Hamilton, ON L8N 3Z5, Canada
References
- 1.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 3.Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167:S1–S34. [PMC free article] [PubMed] [Google Scholar]
- 4.Siminoski K, Leslie WD, Frame H, Hodsman A, Josse RG, Khan A, Lentle BC, Levesque J, Lyons DJ, Tarulli G, Brown JP. Recommendations for bone mineral density reporting in Canada: a shift to absolute fracture risk assessment. J Clin Densitom. 2007;10:120–123. doi: 10.1016/j.jocd.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 5.The National Osteoporosis Foundation (NOF) Clinician’s Guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation; Washington, DC: 2008. [Google Scholar]
- 6.AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract. 2003;9:544–564. doi: 10.4158/EP.9.6.544. [DOI] [PubMed] [Google Scholar]
- 7.Papaioannou A, Kennedy CC, Ioannidis G, Sawka A, Hopman WM, Pickard L, Brown JP, et al. The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2009;20:703–714. doi: 10.1007/s00198-008-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kado KM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporostic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 9.Keen RW. Burden of osteoporosis and fractures. Curr Osteoporos Rep. 2003;1:66–70. doi: 10.1007/s11914-003-0011-x. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181:265–271. doi: 10.1503/cmaj.081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 12.Bessette L, Ste-Marie LG, Jean S, et al. The care gap in diagnosis and treatment of women with a fragility fracture. Osteoporos Int. 2008;19:79–86. doi: 10.1007/s00198-007-0426-9. [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 14.Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi J, Shea B, Tugwell P, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–516. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2002;23:CD001155. doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Cranney A, Welch V, Adachi JD, Guyatt G, Krolicki N, Griffith L, Shea B, Tugwell P, Wells G. Etidronate for treating and preventing postmenopausal osteoporosis. Cochrane Database Syst Rev. 2001;4:CD003376. doi: 10.1002/14651858.CD003376. [DOI] [PubMed] [Google Scholar]
- 17.Cranney A, Waldegger L, Zytaruk N, Shea B, Weaver B, Papaioannou A, Robinson V, Wells G, Tugwell P, Adachi JD, Guyatt G. Risedronate for the prevention and treatment of postmenopausal osteoporosis. 2003;4:CD004523. doi: 10.1002/14651858.CD004523. [DOI] [PubMed] [Google Scholar]
- 18.Hajcsar EE, Hawker G, Bogoch ER. Investigation and treatment of osteoporosis in patients with fragility fracture. Can Med Assoc J. 2000;163:819–822. [PMC free article] [PubMed] [Google Scholar]
- 19.Papaioannou A, Giangregorio L, Kvern B, et al. The osteoporosis care gap in Canada. BMC Musculoskelet Disord. 2004;5:11. doi: 10.1186/1471-2474-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giangregorio L, Papaioanou A, Cranney A, Zytaruk N, Adachi JD. Fragility Fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35:293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Papaioannou A, Kennedy CC, Ioannidis G, et al. The osteoporosis care gap in men with fragility fractures: the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2008;19:581–587. doi: 10.1007/s00198-007-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreiger N, Tenenhouse A, Joseph L, Mackenzie T, Poliquin S, Brown JP, Prior JC, Rittmaster RS. The Canadian Multi-centre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging. 1999;18:376–387. [Google Scholar]
- 23.Kingwell E, Prior JC, Ratner PA, Kennedy SM. Direct-to-participant feedback and awareness of bone mineral density testing results in a population-based sample of mid-aged Canadians. Osteoporos Int. 2010;21:307–319. doi: 10.1007/s00198-009-0966-2. [DOI] [PubMed] [Google Scholar]
- 24.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linearmodels. Biometrika. 1986;73:13–22. [Google Scholar]
- 25.Feldstein AC, Nichols GA, Elmer PJ, Smith DH, Aickin M, Herson M. Older women with fractures: patients falling through the cracks of guideline-recommended osteoporosis screening and treatment. J Bone Joint Surg Am. 2003;85:2294–2302. [PubMed] [Google Scholar]
- 26.Feldstein A, Elmer PJ, Orwoll E, Herson M, Hillier T. Bone mineral density measurement and treatment for osteoporosis in older individuals with fractures. A gap in evidence-based practice guideline implementation. Arch Intern Med. 2003;163:2165–2172. doi: 10.1001/archinte.163.18.2165. [DOI] [PubMed] [Google Scholar]
- 27.Metge CJ, Leslie WD, Manness LJ, Yogendran M, Yuen CK, Kvern B the Maximizing Osteoporosis Management in Manitoba Steering Committee. Postfracture care for older women. Gaps between optimal care and actual care. Can Fam Physician. 2008;54:1270–1276. [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner MJ, Flik KR, Mooar P, Lane JM. Improvement in the undertreatment of osteoporosis following hip fracture. J Bone Jt Surg. 2002;84A:1342–1348. doi: 10.2106/00004623-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 30.Klotzbuecher CM, Ross PD, Landsmen PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 31.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 32.Nevitt MC, Ross PD, Palermo L, Musliner T, Genant HK, Thompson DE. Association of prevalent vertebral fractures, bone density, and alendronate treatment with incident vertebral fractures: effect of number and spinal location of fractures. The Fracture Intervention Trial Research Group. Bone. 1999;25:613–619. doi: 10.1016/s8756-3282(99)00202-1. [DOI] [PubMed] [Google Scholar]
- 33.Chen P, Krege JH, Adachi JD, Prior JC, Tenenhouse A, Brown JP, Papadimitropoulos M, Kreiger N, Olszynski WP, Josse RG, Goltzman D. Vertebral fracture status and the World Health Organization (WHO) risk factors for predicting osteoporotic fracture risk. J Bone Miner Res. 2009;24:495–502. doi: 10.1359/jbmr.081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roux C, Seeman E, Eastell R, Adachi J, Jackson RD, Felsenberg D, Songcharoen S, Rizzoli R, Di Munno O, Horlait S, Valent D, Watts NB. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin. 2004;20:433–493. doi: 10.1185/030079903125003125. [DOI] [PubMed] [Google Scholar]
- 35.Harrington JT, Ste-Marie LG, Brandi ML, Civitelli R, Fardellone P, Grauer A, Barton I, Boonen S. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–135. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- 36.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 37.Cranney A, Jamal SA, Tsang TF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575–580. doi: 10.1503/cmaj.070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 39.Sanders KM, Pasco JA, Ugoni AM, Nicholson GC, Seeman E, Martin TJ, Skoric B, Panahi S, Kotowicz MA. The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: the Geelong Osteoporosis Study. J Bone Miner Res. 1998;13:1337–1342. doi: 10.1359/jbmr.1998.13.8.1337. [DOI] [PubMed] [Google Scholar]
- 40.Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int. 2004;15:767–778. doi: 10.1007/s00198-004-1675-5. [DOI] [PubMed] [Google Scholar]
- 41.Osteoporosis Canada. Provincial access to etidronate, alendronate, risedronate, calcitonin, raloxifene and teriparatide. 2007 Retrieved November 22, 2009 from http://www.osteoporosis.ca/index.php/ci_id/5521/la_id/1.htm.
- 42.Poliquin S, Joseph L, Gray-Donald K. Calcium and vitamin D intakes in an adult Canadian population. Can J Diet Pract Res. 2009;70(1):21–27. doi: 10.3148/70.1.2009.21. [DOI] [PubMed] [Google Scholar]
- 43.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 44.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–4124. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]