Abstract
OBJECTIVE
Intraoperative contrast-enhanced ultrasound (iCEUS) offers dynamic imaging and provides functional data in real time. However, no standardized protocols or validated quantitative data exist to guide its routine use in neurosurgery. The authors aimed to provide further clinical data on the versatile application of iCEUS through a technical note and illustrative case series.
METHODS
Five patients undergoing craniotomies for suspected tumors were included. iCEUS was performed using a contrast agent composed of lipid shell microspheres enclosing perflutren (octafluoropropane) gas. Perfusion data were acquired through a time-intensity curve analysis protocol obtained using iCEUS prior to biopsy and/or resection of all lesions.
RESULTS
Three primary tumors (gemistocytic astrocytoma, glioblastoma multiforme, and meningioma), 1 metastatic lesion (melanoma), and 1 tumefactive demyelinating lesion (multiple sclerosis) were assessed using real-time iCEUS. No intraoperative complications occurred following multiple administrations of contrast agent in all cases. In all neoplastic cases, iCEUS replicated enhancement patterns observed on preoperative Gd-enhanced MRI, facilitated safe tumor de-bulking by differentiating neoplastic tissue from normal brain parenchyma, and helped identify arterial feeders and draining veins in and around the surgical cavity. Intraoperative CEUS was also useful in guiding a successful intraoperative needle biopsy of a cerebellar tumefactive demyelinating lesion obtained during real-time perfusion analysis.
CONCLUSIONS
Intraoperative CEUS has potential for safe, real-time, dynamic contrast-based imaging for routine use in neurooncological surgery and image-guided biopsy. Intraoperative CEUS eliminates the effect of anatomical distortions associated with standard neuronavigation and provides quantitative perfusion data in real time, which may hold major implications for intraoperative diagnosis, tissue differentiation, and quantification of extent of resection. Further prospective studies will help standardize the role of iCEUS in neurosurgery.
Keywords: ultrasound, sonography, perfusion, microbubbles, contrast, dynamic imaging
Image-guided intraoperative navigation has become an integral component of modern cranial neurosurgery. Although preoperative MRI and CT scans can be reliably used for preoperative planning and intraoperative navigation, they are unable to account for intraoperative brain shifts due to patient positioning, CSF loss, tumor debulking, gravity, and cerebral edema.2,12,21 In contrast, intraoperative MRI (iMRI) and conventional intraoperative ultrasonography (iUS) provide an improved ability to account for real-time changes that occur during surgical intervention.23 While these modalities have shown promise for improving the extent of resection, they remain quite limited in their efficiency and ability to accurately guide neurooncological resection.
Conventional iUS provides an inexpensive method of visualizing lesions in real time. The quality of the images, however, is largely operator dependent, offers imaging sections and views that may not be in standard axial, coronal, or sagittal planes, and does not provide functional or perfusion data that may be beneficial for differentiating normal brain from surrounding pathology. As such, iUS as a stand-alone method has proved suboptimal in assessing the extent of brain tumor resection or detecting residual tumor.2,21 Though the development of iMRI obviated many limitations of the aforementioned imaging modalities, significant hurdles have limited its widespread use, including expense, availability, sterility and instrument concerns, poor resolution, artifact, and increased operative times.2,12,21 Perhaps most importantly, iMRI remains limited to providing static images, thereby potentially missing the dynamic enhancement patterns of lesions and their surrounding vascular networks.
Intraoperative contrast-enhanced US (iCEUS) offers an evolving tool for neuronavigation and intraoperative imaging, with the unique advantages of combining dynamic imaging and real-time perfusion data. Contrast agents used for this purpose are composed of microbubbles that remain within the vasculature, thereby allowing characterization of the arterial and venous network. This offers the potential for differentiating normal brain from neoplastic tissue, delineating feeding arteries and draining veins within the surgical bed, and providing information regarding hemodynamic alterations and real-time extent of resection.19,20 In addition, the safety profile of microbubbles allows multiple injections throughout the operation, highlighting changes in enhancement patterns at different stages of the procedure.14,24 Intraoperative CEUS has also been shown to detect lesions not visible on preoperative MRI and may even delineate higher-grade regions within areas of equivocal MRI enhancement, thereby more accurately guiding the selection of representative tissue for intraoperative biopsies.20
Although previous investigations have described the benefits of iCEUS in neurosurgery,18,20,26,27 the extent and potential of its application are still not well-defined, and no standardized protocol for guiding iCEUS and tumor resection exists in the United States. Furthermore, the technical nuances of extracting and analyzing perfusion data have been semiquantitative and varied.7,10,20,28 In the present article we aimed to provide further clinical evidence on the versatile application of iCEUS by offering a technical note on the quantitative extraction of perfusion parameters with a small, yet representative case series including iCEUS for real-time guidance and needle biopsy of intraaxial enhancing lesions.
Methods
Study Design and Patient Population
Five patients undergoing craniotomies for biopsy and/or resection of intraaxial lesions thought to be tumors on preoperative MRI were included (Table 1). Intraoperative CEUS was used in all cases as specified below. The University of Southern California Health Sciences Campus Institutional Review Board committee approved this study. All patients were informed about the surgical procedure and written consent was obtained.
TABLE 1.
Summary of included cases in which iCEUS was used
| Case No. | Pathology | Location | Ultrasound Device |
Transducer | Perfusion |
|---|---|---|---|---|---|
| 1 | Gemistocytic astrocytoma | Frontotemporal lobe | GE Logiq E9 | Linear (9L-D, 2–8 MHz) | Yes |
| 2 | Recurrent/residual glioblastoma | Frontal lobe | GE Logiq E9 | Linear (9L-D, 2–8 MHz) | – |
| 3 | Meningioma (meningothelial/ microcystic) |
Frontotemporal lobe | GE Logiq E9 | Linear (9L-D, 2–8 MHz) | – |
| 4 | Melanoma | 4th ventricle near the midbrain | Siemens Acuson | Transvaginal (8C4, 4–8 MHz) | – |
| 5 | Multiple sclerosis | Lt cerebellar peduncles & brainstem | Phillips Epiq 7 | Transvaginal (c10-3V, 3–10 MHz) | Yes |
– = not performed
Ultrasound Platforms
The following ultrasound units were used: A GE Logiq E9 (General Electric) with either linear (9 L-D, 2–8 MHz) or transvaginal (IC5–9D, 3–10 MHz) transducers, a Phillips EPIQ 7 (Philips Healthcare) with a transvaginal (c10–3 V, 3–10 MHz) transducer, or a Siemens Acuson (Siemens Healthcare) with transvaginal (8C4, 4–8 MHz) transducer to CEUS. In select circumstances, the transvaginal ultrasound probe is the ideal choice for iCEUS because of its small footprint and high frequency. The small footprint allows for insertion and imaging in a relatively small craniotomy, while the high-frequency imaging provides corresponding high near-field resolution. The ultrasound unit used in each case was based on availability, and transducer selection was ultimately determined by surgical exposure.
Ultrasound Contrast Agent
For all cases, we used Definity (Lantheus Medical Imaging), a USCA composed of lipid shell microspheres enclosing perflutren (octafluoropropane) gas. The contrast was activated with 45 seconds of rapid agitation in an automated device (VialMix, Lantheus Medical Imaging). A bolus injection of 0.3 ml of USCA was given and followed by a 10-ml normal saline flush. Additional USCA injections were performed as clinically needed. Video clips of the CEUS examination were recorded, with images continuously acquired after contrast administration.
Time-Intensity Curve Analysis (Perfusion Parameters)
As this is a description of our preliminary experience with obtaining iCEUS perfusion data, two different systems were used. The GE Logiq E9 and Phillips EPIQ 7 systems were used to evaluate dynamic contrast enhancement patterns in separate patients (Cases 1 and 5). For both systems, a continuous cine clip of tumor enhancement was obtained after intravenous administration of the USCA. A time-intensity curve (TIC) was created for extraction of quantitative perfusion parameters.8 A region of interest (ROI) was placed on the tumor, and another ROI on normally enhancing adjacent tissue. A TIC was produced for each ROI. The mean echo of the ROI was then fit to a curve using a local density random walk model, from which multiple quantitative parameters were obtained. The peak intensity (PI) and time to peak (TTP) of the curves were identified, in decibels and seconds respectively, for each patient. The area under the curve was also calculated for tumor and adjacent normal parenchyma on both systems.
For the examination performed on the Phillips EPIQ 7 system (Case 5), additional parameters related to wash-in were obtained, including the rise time and wash-in-slope. By definition, the portion of the TIC before the peak was considered wash-in, and the portion after the peak was considered wash-out. The rise time was defined as the time needed to increase from 5% of PI to 95% of PI. The wash-in-slope was calculated by subtracting the intensity at 5% of PI from 95% of PI, then dividing by the rise time.
Case Reports
Case 1
A 57-year-old man taking immunosuppressant medications for rheumatoid arthritis presented with right-sided weakness of the face and extremities. MRI of the brain showed a large infiltrative left frontotemporal mass with significant midline shift and faint Gd enhancement, consistent with a diffuse glioma (Fig. 1). The patient underwent a left frontotemporal craniotomy for subtotal resection of a high-grade glioma and anterior temporal lobectomy. Prior to resection, iCEUS was used to localize the enhancing portions of the tumor and its relationship to surrounding brain tissue. Echogenic and heterogeneous tumor was noted within the left temporal lobe extending into the insular ribbon, uncus, and to the level of the corona radiata. Contrast-enhanced imaging evaluation of the tumor demonstrated robust enhancement of the majority of the tumor. The most pronounced enhancement (highest PI) and shortest TTP were noted within the uncus of the left temporal lobe. Of note, this portion of the tumor did not avidly enhance on preoperative MRI. Intraoperative CEUS was subsequently used to assess the extent of tumor debulking. A maximal safe tumor resection was achieved via an anterior temporal lobectomy. Pathology was consistent with gemistocytic Grade IV astrocytoma (glioblastoma multiforme). Postoperatively, the patient had some occasional word-finding difficulties but was otherwise back to his neurological baseline and was discharged home.
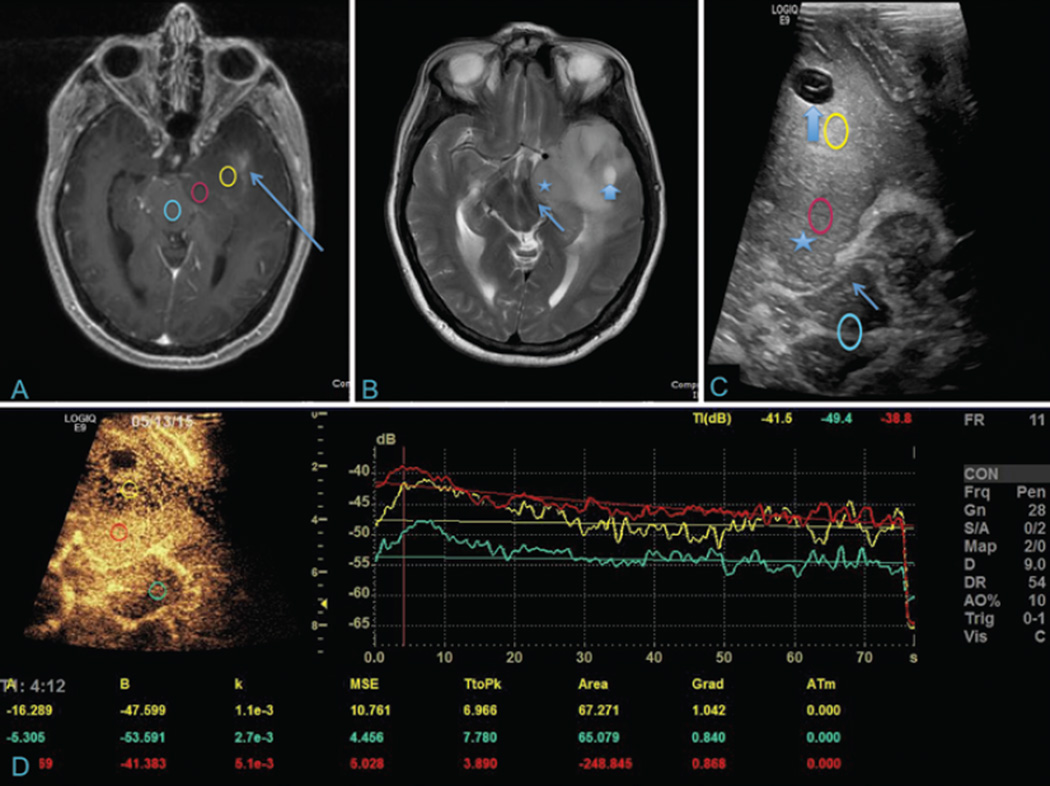
Fig. 1.
Temporal glioblastoma with corresponding iCEUS and perfusion data. A: Axial T1-weighted contrast-enhanced MR image showing a left temporal glioblastoma (yellow) with the uncus (red) compressing the ipsilateral midbrain (blue). Faint Gd enhancement is noted in the anterior temporal lobe (arrow). B: Axial T2-weighted MR image demonstrating extensive hyperintensity surrounding the lesion, including within the uncus (star). There is mass effect on the midbrain and ipsilateral cerebral peduncle (thin arrow). A cystic component is seen in the anterior temporal lobe (thick arrow). C: Corresponding B-mode ultrasound image showing the cystic lesion (thick arrow), uncus (star), and compressed cerebral peduncle (thin arrow). Colored circles correspond to those in A. D: Intraoperative CEUS with perfusion graph showing peak contrast enhancement, overall blood flow, and blood volume for the three respective lesions. The left uncus demonstrates avid contrast enhancement with the greatest degree of early arterial enhancement, which is highly suggestive of a higher grade of tumor. Of note, this region did not significantly enhance on the preoperative T1-weighted contrast-enhanced MRI.
Case 2
A 52-year-old man with a medical history of recurrent glioblastoma multiforme, who was previously treated with two resections and adjuvant chemoradiation, was admitted with altered mental status. On neurological examination, he demonstrated new left upper-extremity weakness (Grade 4+/5). MRI showed interval expansion of T2-weighted FLAIR signal in the right frontal lobe with surrounding edema and midline shift (Fig. 2). The patient underwent a redo frontoparietal craniotomy. Intraoperative CEUS was used to localize the tumor and define its relationship to surrounding brain tissue. A heterogeneous pattern of enhancement was observed, with rapid arterial phase at 6 seconds, PI at 14 seconds, and venous washout at 34 seconds. These findings were consistent with the parameters for high-grade glioma described by Prada et al.,19 and the final pathology showed recurrent/residual glioblastoma. Postoperatively, the patient’s clinical examination status remained the same and he was discharged to home.
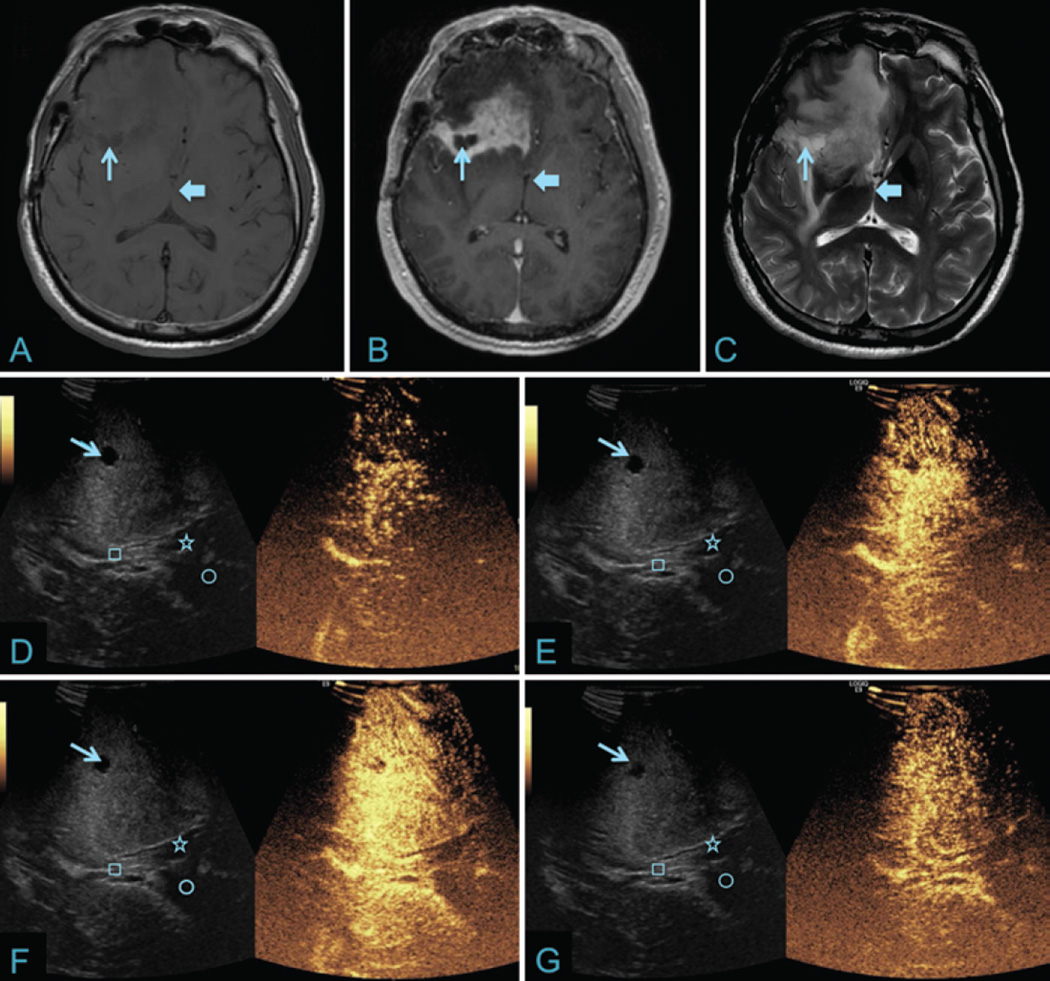
Fig. 2.
Recurrent right frontal glioblastoma with corresponding iCEUS images. A: Axial T1-weighted noncontrast axial MR image of the right frontal glioblastoma showing an iso- to hypointense lesion with a T1 signal hypointense cystic component (thin arrow). The third ventricle is shown for anatomical reference to iCEUS images (thick arrow). B: Axial T1-weighted contrast-enhanced MR image showing avid enhancement within the lesion as well as a nonenhancing cystic cavity (thin arrow). C: Axial T2-weighted MR image showing infiltrative edema surrounding the lesion and hyperintensity within the cystic cavity (thin arrow). D–G: Corresponding B-mode (left) and iCEUS (right) images showing contrast perfusion at four different time points (7, 10, 14, and 34 seconds). Note the rapid arterial phase (D), a peak phase around 14 seconds (F), and a delayed venous washout phase (G). Corresponding B-mode ultrasound image better displays the corresponding anatomy including the cystic component of the lesion (thin arrow), roof of the third ventricle/fornix (square), and right (star) and left (circle) lateral ventricles.
Case 3
A 48-year-old woman presented with a 6-month history of headaches that worsened when lying down. There were no focal neurological findings on examination. MRI demonstrated a large right frontotemporal extraaxial mass compatible with a meningioma (Fig. 3). On MRI, heterogeneous enhancement and T2 signal hyperintensity along the peripheral margin raised the possibility of a microcystic component. Intraoperative CEUS was used to assess the tumor enhancement and relationship to surrounding brain tissue and subsequently the extent of tumor resection. The dural component and core of the meningioma were nonenhancing on iCEUS, likely due to sacrifice of the middle meningeal arterial supply during exposure. A thick enhancing rim of tissue was noted at the periphery of the tumor that appeared to fill from a central vessel. This may have been due to parasitization of the middle cerebral artery circulation by the meningioma (Video 1).
Video 1. Meningioma with corresponding iCEUS. Cine clip with side-by-side B-mode (left) and iCEUS (right) demonstrated avid arterial enhancement in the peripheral rim of the meningioma, which appeared to arise from the cerebral (middle cerebral artery) circulation. The core and peripheral cystic component did not display significant contrast enhancement due to its low vascularity. Copyright University of Southern California. Published with permission. Click here to view.
Nonenhancing cystic areas in the tumor were also noted on iCEUS images, which corresponded to the preoperative MRI findings. During resection, certain areas within the deeper margins of the lesion appeared grossly invasive and the cyst capsule was densely adherent to and invading the underlying parenchyma. Pathological examination confirmed a benign meningioma with mixed meningothelial and microcystic subtypes. The patient maintained baseline neurological function postoperatively and was discharged to home.
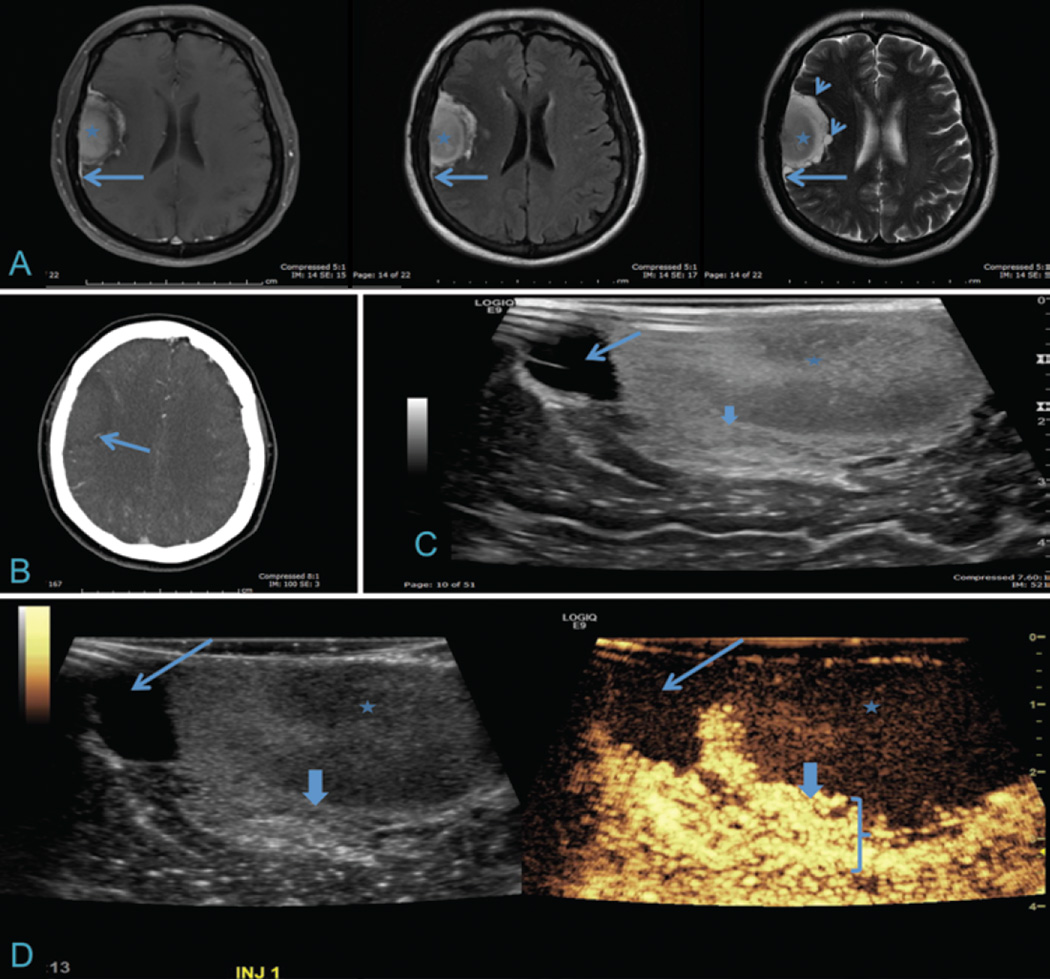
Fig. 3.
Meningioma with corresponding iCEUS images. A: Axial T1-weighted contrast-enhanced, FLAIR, and T2-weighted MR images of a right frontotemporal, dura-based, extraaxial lesion. The core of the lesion is mildly heterogeneous (asterisk) with a dural tail (long arrows) and cystic components seen on T2-weighted images (short arrows). B: Axial CT angiogram demonstrating a feeding vessel from the middle cerebral artery distribution (arrow). C: Intraoperative ultrasound image demonstrates a cystic component (long arrow) and heterogeneous echotexture within the core of the lesion (asterisk) and a thick heterogeneous peripheral component (thick arrow). D: Corresponding B-mode (left) and iCEUS (right) images demonstrating a central tumor with low vascularity (asterisk), nonenhancing cystic component (long arrow), and thick, avidly enhancing peripheral rim (thick arrow and bracket). Differentiation can be appreciated between enhancing tumor and normal, adjacent brain parenchyma.
Case 4
An 81-year-old woman with a medical history of melanoma presented with altered mental status and gait instability. The neurological examination was significant for dysmetria in the left upper extremity. A large enhancing mass in the superior aspect of the fourth ventricle near the midbrain and noncommunicating hydrocephalus were documented on MRI (Fig. 4). The patient underwent a suboccipital craniotomy, with modified telovelar approach, and iCEUS was used for tumor localization and assessment of the extent of tumor resection. The tumor demonstrated avid arterial enhancement, prolonged uptake of the contrast material followed by delayed washout, which was inconsistent with prior reported enhancement patterns of glial tumors. At this point, intraoperative diagnosis of a metastatic melanoma lesion was favored given the findings of iCEUS and frozen section. Postresection CEUS identified residual tumor in the superior aspect of the fourth ventricle and quadrigeminal plate that was thought to be unsafe for additional resection based on its location and relationship with the midbrain. In this case, iCEUS facilitated real-time dynamic evaluation for guiding maximal tumor resection with no intraoperative complications. The final pathological findings were consistent with metastatic melanoma. The patient exhibited no changes in neurological status on postoperative examination.
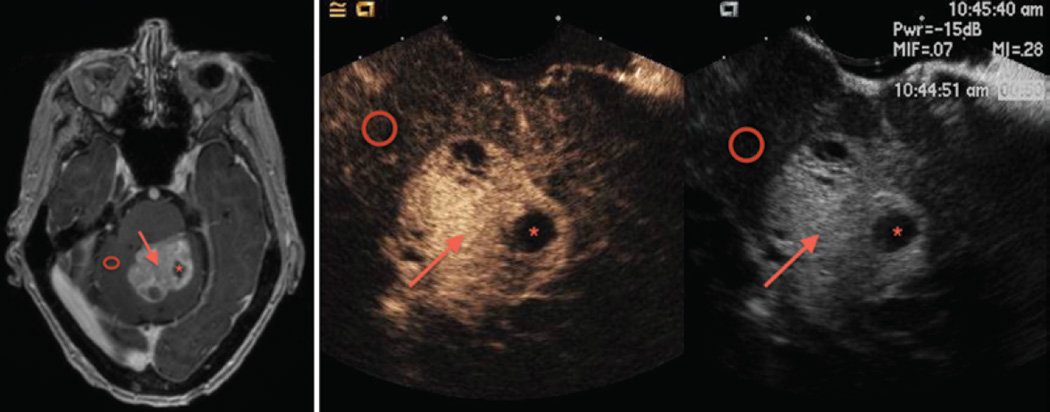
Fig. 4.
Metastatic melanoma with corresponding iCEUS images. Left: Axial T1-weighted contrast-enhanced MR image demonstrating heterogeneous enhancement of the brainstem lesion (arrow) with two distinct intralesional cystic/necrotic areas (asterisk). Adjacent normal cerebellar parenchyma is noted (circle). Right: Corresponding side-by-side iCEUS (left) and B-mode (right) images demonstrating the same lesion (arrow) and cystic/necrotic areas (asterisk). Solid components of the tumor display avid early arterial enhancement with delayed washout (not pictured). No significant abnormal enhancement is noted in the adjacent normal cerebellar parenchyma (circle).
Case 5
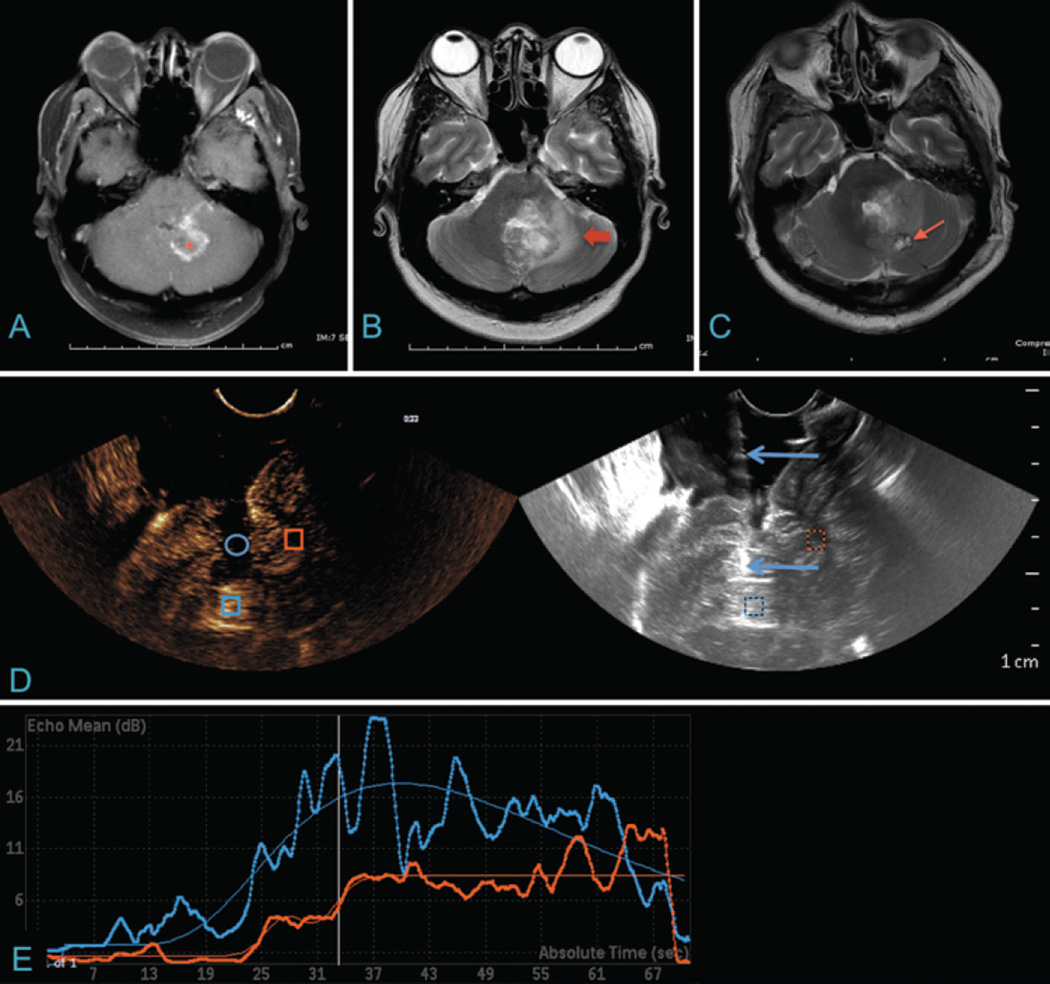
A 45-year-old woman presented with worsening ataxia, vertigo, and left hand paresthesias. Neurological examination was significant for a left abducens nerve palsy, decreased sensation in the left cranial nerve V2 and V3 distributions, left-sided dysdiadochokinesia, and dysmetria. MRI demonstrated progressive T2-weighted/FLAIR hyperintensity and Gd enhancement within the left inferior cerebellar peduncle, middle cerebellar peduncle, superior cerebellar peduncle, left cerebellar hemisphere, and vermis (Fig. 5). Involvement of the left dorsal midbrain, pons, and medulla was also demonstrated. The preoperative diagnosis was an infiltrative high-grade glioma. A left suboccipital craniotomy was performed to biopsy the cerebellar/brainstem mass using conventional intraoperative neuronavigation, which resulted in equivocal findings on pathological examination due to patient positioning and brain shift in the prone position. Subsequent MRI showed that the biopsied region was outside the area of enhancement. The patient underwent a second suboccipital craniotomy to biopsy the cerebellar lesion and iCEUS was used. An area of avid enhancement with prolonged TTP and delayed contrast washout was identified intraoperatively and was biopsied using real-time guidance of an ultrasound-compatible biopsy needle. The observed perfusion enhancement pattern was not typical for glial tumor or metastatic disease.20 Final pathological features were consistent with a demyelinating process, most likely multiple sclerosis. Postoperatively, the patient’s neurological function improved following treatment for multiple sclerosis.
Fig. 5.
Multiple sclerosis with corresponding iCEUS and perfusion data. A: Axial T1-weighted contrast-enhanced MR image shows an incompletely ring-enhancing multiple sclerosis lesion in the cerebellar white matter (asterisk). B: Axial T2-weighted MR image of the same lesion shows surrounding edema (thick arrow). C: Axial T2-weighted MR image more inferiorly shows the first biopsy site (arrow), which missed the lesion of interest when using neuronavigation without iCEUS. D: iCEUS (left) and corresponding B-mode US (right) images demonstrating the previous failed biopsy site (circle) and a biopsy needle (arrows) en route to contrast-enhancing lesion of interest (blue square). Second area of interest is demarcated over an area of normal cerebellar parenchyma (orange square). E: TIC demonstrated delayed USCA uptake in the enhancing lesion (blue curve) with prolonged retention of contrast and a delayed washout phase. The TIC for the adjacent normal cerebellar parenchyma (orange curve) displays gradual contrast enhancement without washout.
Discussion
This case series reinforces the concept that iCEUS is a safe, feasible, and efficient adjuvant imaging modality that can be used to guide the neurosurgical management of both neoplastic and nonneoplastic lesions. Compared with conventional neuronavigation, iCEUS’ greatest assets are its ability to account for brain shift during surgical manipulation, to evaluate dynamic contrast enhancement of lesions, and to identify the location of feeding arteries and draining veins associated with brain tumors. Although its accuracy and efficacy remain unknown compared with intraoperative MRI, iCEUS offers the clear relative benefits of real-time, dynamic imaging with a capacity to quantitatively assess contrast perfusion of neoplastic and nonneoplastic tissue, the option for repeated contrast infusions and assessments, and markedly improved surgical workflow. Although limited to two patients in this series, we demonstrated a quantitative method for extracting perfusion data in the operating room when using iCEUS. Another benefit to iCEUS is that it provided real-time guidance of a needle tissue biopsy in a deep, eloquent location that had been previously unsuccessful when using standard neuronavigation, by locating a focus of enhancement via real-time perfusion analysis.
Evolution of Quantitative Perfusion in iCEUS
Intraoperative CEUS has been used to evaluate brain perfusion and response to stroke therapy in patients with cerebrovascular disorders for more than a decade, and experience with its use in brain tumor imaging is continuously growing.3 The earliest studies on brain tumor perfusion were performed at the bedside, and the quality was limited by the thickness of the cranial vault. Nonetheless, Vicenzini et al. used a standardized software system to evaluate several hemorrhagic and neoplastic lesions in the preoperative setting and generated TICs, from which important perfusion parameters were derived: PI, TTP, mean transit time, cerebral blood volume, and cerebral blood flow.28 Engelhardt et al. then used a similar methodology in the intraoperative setting and analyzed the perfusion characteristics of 6 patients obtained using a second generation USCA (SonoVue). Due to the limited number of patients, however, they were unable to generate any significance or recommendations from their findings.7 More recently, Prada et al. used iCEUS with a semiquantitative offline interobserver analysis to assess the timing, degree of contrast enhancement, and contrast distribution.20 They reported the largest patient series to date, but their perfusion parameters were reported in ranges (compared to discrete means with confidence intervals) since quantitative abstraction from TICs were not performed. In Cases 1 and 5, we used iCEUS to acquire quantitative perfusion parameters by employing a protocol similar to that of Vicenzini et al. (Fig. 1).28 By analyzing the values in the TIC, we were able to extract specific values of PI, TTP, and area under the curve to assign an objective, quantifiable value corresponding to the visual degree of enhancement. To our knowledge, this is the first confirmation on Vicenzini and colleagues’ preliminary findings.
Prior cranial ultrasonography techniques had many limitations. Kanno et al. reported the first intraoperative application of CEUS by evaluating 30 brain lesions (13 gliomas, 9 metastastic lesions, and 6 meningiomas) using a first-generation contrast agent (Levovist).10 By recording changes in the echo signals with power Doppler ultrasound, the authors were able to delineate the tumor borders after administration of contrast. However, the reported data were crude, since peak contrast intensity was measured by pausing videocassette recordings whenever the contrast intensity was deemed to have reached its peak. He et al. also evaluated 22 gliomas and 7 meningiomas using the second-generation CE SonoVue.9 However, their ultrasound spatial resolution and definition were limited since they used a phased-array probe with low frequency and a wide viewing window (as in transcranial Doppler ultrasonography).
Use of iCEUS for Neoplastic Lesions
Prior studies have shown that the extent of primary intraaxial tumor resection is associated with improved progression- free survival.1,22,25 The inherent challenge associated with this axiom is the adverse neurological outcomes that can result from resecting adjacent eloquent brain tissue. Differentiating infiltrative tumors from brain matter intraoperatively can be difficult, and many intraoperative contrast and optical agents are currently under evaluation for this purpose. Although intraoperative MRI ameliorates many of these issues by providing a snapshot view of the extent of resection, its widespread use has remained limited based on resources, sterility concerns, operative times, and lack of Class I data to support its use. As presented in Cases 1–4, the utility of iCEUS to help differentiate high-grade gliomas, cystic meningiomas, and metastatic lesions from normal brain parenchyma, predict tumor vascularity, and navigate around vascular structures holds great potential.
To date, Prada et al. have the most extensive experience with iCEUS in neurosurgery.15–17,19,20 Through multiple studies, they analyzed 53 gliomas, 15 meningiomas (including 10 skull base lesions), 6 metastases, and various other neoplasms including ependymomas, pituitary adenomas, and craniopharyngiomas. Given the large number of lesions analyzed, they were able to delineate specific iCEUS patterns related to each different lesion. For example, glioblastoma showed a heterogeneous iCEUS pattern with rapid arterial and venous phases and high overall enhancement, as compared with brain parenchyma. This was consistent with our observed iCEUS findings in our recurrent/residual glioblastoma case (Case 2).
Intraoperative CEUS can also provide a more accurate measure of tumor grade. Prada et al. found 5 lesions that were initially diagnosed as low-grade on the basis of preoperative MRI, which were later confirmed to be anaplastic gliomas.20 For each of these cases, iCEUS showed foci of dense vascular network with higher contrast enhancement compared with other histologically proven low-grade lesions. They also found iCEUS to be a more sensitive modality for the detection of gliomas.19 This information offered with iCEUS was particularly pertinent when diagnosing our glioblastoma case (Case 1). In this case, the most marked region of iCEUS enhancement was located in the left uncus. However, this area did not demonstrate significant enhancement on preoperative MRI. Failure to sample and resect this area may have led to inappropriate downgrading of the lesion and suboptimal resection. This is further evidence that iCEUS can potentially help guide more accurate biopsy of neoplastic lesions, minimize sampling and diagnostic error, and guide the extent of resection during surgery.13 For meningiomas and other lesions with complex vasculature, iCEUS can help identify the location of arterial and venous vessels relative to the tumor mass.
Nonneoplastic Lesions
Image-guided biopsy techniques can provide a high diagnostic yield with a low rate of morbidity. It has previously been shown that 18% (28 of 158) of presumed tumor lesions were found to have other etiologies including demyelination and infectious and inflammatory reactions after stereotactic biopsy.30 Although rare, acute tumefactive demyelinating lesions in multiple sclerosis can appear as a ring-enhancing lesion on MRI.6 This can present a diagnostic challenge as described in Case 5, and may need a brain biopsy for confirmation prior to treatment.11 In Case 5, the utilization of iCEUS with perfusion allowed successful biopsy after neuronavigation failed to locate the region of interest due to brain shift in the posterior fossa (Fig. 5). Prada et al. also noted that they did not observe perfusion of avascular regions (abscess and radiation necrosis), which offers additional support that iCEUS can aid the differential diagnosis of rim enhancing lesions that may warrant biopsy.20
Advantages of iCEUS
Intraoperative CEUS provides several advantages over other imaging modalities. USCAs consist of gas microbubbles enclosed in protein or lipid shells. The gases used in USCAs are eliminated via the lungs and are not metabolized. The shells, on the other hand, are composed of substances that are easily metabolized, such as phospholipid or albumin. USCA microbubbles (mean diameter range 1.1–3.3 µm) are slightly smaller than red blood cells and are therefore considered pure intravascular contrast agents (blood pool agents). Unlike CT and MRI contrast agents, USCAs remain completely within the vasculature and do not cross into the interstitial space. As an intravascular contrast agent, iCEUS maps the vascular network of tumor and brain parenchyma down to the capillary level. CT and MR perfusion, conversely, both demonstrate parenchymal contamination as a result of permeability of the smaller contrast agents used in those modalities. Intraoperative CEUS also provides high temporal resolution in real time by using a contrast-specific mode with a low mechanical index (< 0.1). Additional advantages of USCAs with CEUS include a low incidence of side effects and lack of nephrotoxicity. This allows the application of iCEUS in patients with iodine allergies and impaired renal function.5 The only relative contraindication is that iCEUS is not recommended for patients with right-to-left, bidirectional, or transient right-to-left cardiovascular shunts.18
There are three so-called second-generation USCAs available in the US, all of which contain a fluorinated gas core. The second-generation agents demonstrate increased stability in the blood when compared with the first-generation agents.4 Definity is a second-generation agent, and it is frequently used at our institution for noncardiac CEUS applications. There are two other second-generation USCAs available in the US, Optison and Lumason, and they have very similar pharmacokinetics. The safety of Definity has previously been reported,29 and the agent is approved by the Food and Drug Administration (FDA) for echocardiography. While the FDA has not approved Definity for iCEUS, this did not influence our research. Many noncardiac applications remain off-label in the US, and USCAs are extensively used in other countries for a variety of applications.
Limitations
Both the iCEUS technique and this retrospective study are not without limitations. The technique employed is limited in that no standardized data are yet available to accurately guide intraoperative management decisions pertaining to diagnosis and differentiation from normal tissue. Furthermore, the intraoperative ultrasound hard-ware employed with contrast capabilities has not yet been designed specifically to accommodate surgical exposures during craniotomy or with minimally invasive neurosurgical techniques. The imaging views and orientations acquired during iCEUS may not be standard in the sense of corresponding to traditional axial, coronal, and sagittal planes, thereby often adding an element of unfamiliarity to the surgeon. In this retrospective analysis of a very small and heterogeneous cohort, perfusion data were not available for all patients. Rather, this case series demonstrated the feasibility of off-label use of an FDA-approved microbubble contrast agent for the purpose of iCEUS, and there were numerous benefits that are potentially provided by this adjunctive technique, including dynamic contrast-based imaging, differentiation of tissue and assessment of extent of resection, quantitative perfusion analysis, and selective guidance of tissue biopsy in real time. Future investigations are needed to validate the time-intensity perfusion curves used in this study, and how they may augment intraoperative decision-making pertaining to diagnosis and maximizing the extent of tumor resection.
Conclusions
Intraoperative CEUS holds tremendous potential for safe, real-time, dynamic contrast-based imaging for routine use in neurooncological surgery and image-guided biopsy. Intraoperative CEUS eliminates the effect of intraoperative anatomical distortions associated with standard neuronavigation and provides quantitative perfusion data in real-time, which may hold major implications for intraoperative diagnosis, tissue differentiation, and quantification of extent of resection. Further prospective studies will help standardize the role of iCEUS in neurosurgery.
Acknowledgments
Dr. Shiroishi is supported in part by a grant from the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR000131) and by a grant from Toshiba American Medical Systems. Dr. Grant reports receiving grant support from General Electric.
Definity (Lantheus Medical Imaging) is not approved by the Food and Drug Administration for intraoperative contrast-enhanced ultrasound imaging.
ABBREVIATIONS
- FDA
Food and Drug Administration
- iCEUS
intraoperative contrast-enhanced ultrasound
- iMRI
intraoperative MRI
- iUS
intraoperative ultrasound
- PI
peak intensity
- ROI
region of interest
- TIC
time-intensity curve
- TTP
time to peak
- USCA
ultrasound contrast agent
Footnotes
Disclosures
Dr. Shiroishi reports being a consultant for Guerbet.
Author Contributions
Conception and design: Zada, Lekht. Acquisition of data: Zada, Lekht, Brauner, Bakhsheshian, Chang, Gulati, Christian. Analysis and interpretation of data: Zada, Lekht, Brauner, Bakhsheshian, Chang, Gulati, Shiroishi. Drafting the article: Lekht, Brauner, Bakhsheshian, Chang, Gulati. Critically revising the article: Lekht, Brauner, Bakhsheshian, Chang, Gulati, Shiroishi, Grant, Christian. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Zada. Study supervision: Zada, Lekht, Grant.
Supplemental Information
- Video 1. https://vimeo.com/151025212.
References
- 1.Aizer AA, Bi WL, Kandola MS, Lee EQ, Nayak L, Rinne ML, et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer. 2015;121:4376–4381. doi: 10.1002/cncr.29639. [DOI] [PubMed] [Google Scholar]
- 2.Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2015;15:517. doi: 10.1007/s11910-014-0517-x. [DOI] [PubMed] [Google Scholar]
- 3.Bartels E, Henning S, Wellmer A, Giraldo-Velásquez M, Kermer P. Evaluation of cerebral perfusion deficit in stroke patients using new transcranial contrast imaging CPS technology—preliminary results. Ultraschall Med. 2005;26:478–486. doi: 10.1055/s-2005-858765. [DOI] [PubMed] [Google Scholar]
- 4.Borden MA, Qin S, Ferrara KW. Ultrasound contrast agents. In: Weissleder R, editor. Molecular Imaging: Principles and Practice. Shelton, CT: People’s Medical Publishing House; 2010. pp. 425–444. [Google Scholar]
- 5.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 6.Dagher AP, Smirniotopoulos J. Tumefactive demyelinating lesions. Neuroradiology. 1996;38:560–565. doi: 10.1007/BF00626098. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt M, Hansen C, Eyding J, Wilkening W, Brenke C, Krogias C, et al. Feasibility of contrast-enhanced sonography during resection of cerebral tumours: initial results of a prospective study. Ultrasound Med Biol. 2007;33:571–575. doi: 10.1016/j.ultrasmedbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2011;49:137–149. doi: 10.3233/CH-2011-1464. [DOI] [PubMed] [Google Scholar]
- 9.He W, Jiang XQ, Wang S, Zhang MZ, Zhao JZ, Liu HZ, et al. Intraoperative contrast-enhanced ultrasound for brain tumors. Clin Imaging. 2008;32:419–424. doi: 10.1016/j.clinimag.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Kanno H, Ozawa Y, Sakata K, Sato H, Tanabe Y, Shimizu N, et al. Intraoperative power Doppler ultrasonography with a contrast-enhancing agent for intracranial tumors. J Neurosurg. 2005;102:295–301. doi: 10.3171/jns.2005.102.2.0295. [DOI] [PubMed] [Google Scholar]
- 11.Maarouf M, Kuchta J, Miletic H, Ebel H, Hesselmann V, Hilker R, et al. Acute demyelination: diagnostic difficulties and the need for brain biopsy. Acta Neurochir (Wien) 2003;145:961–969. doi: 10.1007/s00701-003-0113-3. [DOI] [PubMed] [Google Scholar]
- 12.Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices. 2012;9:491–500. doi: 10.1586/erd.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Prada F, Del Bene M, Casali C, Saladino A, Legnani FG, Perin A, et al. Intraoperative navigated angiosonography for skull base tumor surgery. World Neurosurg. 2015;84:1699–1707. doi: 10.1016/j.wneu.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Prada F, Del Bene M, Mattei L, Casali C, Filippini A, Legnani F, et al. Fusion imaging for intra-operative ultrasound-based navigation in neurosurgery. J Ultrasound. 2014;17:243–251. doi: 10.1007/s40477-014-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prada F, Del Bene M, Moiraghi A, Casali C, Legnani FG, Saladino A, et al. From grey scale B-mode to elastosonography: multimodal ultrasound imaging in meningioma surgery-pictorial essay and literature review. Bio Med Res Int. 2015;2015:925729. doi: 10.1155/2015/925729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prada F, Del Bene M, Saini M, Ferroli P, DiMeco F. Intraoperative cerebral angiosonography with ultrasound contrast agents: how I do it. Acta Neurochir (Wien) 2015;157:1025–1029. doi: 10.1007/s00701-015-2412-x. [DOI] [PubMed] [Google Scholar]
- 19.Prada F, Mattei L, Del Bene M, Aiani L, Saini M, Casali C, et al. Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. BioMed Res Int. 2014;2014:484261. doi: 10.1155/2014/484261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prada F, Perin A, Martegani A, Aiani L, Solbiati L, Lamperti M, et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery. 2014;74:542–552. doi: 10.1227/NEU.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 21.Reinges MH, Nguyen HH, Krings T, Hütter BO, Rohde V, Gilsbach JM. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien) 2004;146:369–377. doi: 10.1007/s00701-003-0204-1. [DOI] [PubMed] [Google Scholar]
- 22.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. 264–266. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 23.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 24.Sidhu PS, Choi BI, Nielsen MB. The EFSUMB Guidelines on the Non-hepatic Clinical Applications of Contrast Enhanced Ultrasound (CEUS): a new dawn for the escalating use of this ubiquitous technique. Ultraschall Med. 2012;33:5–7. doi: 10.1055/s-0031-1299141. [DOI] [PubMed] [Google Scholar]
- 25.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vetrano IG, Prada F, Erbetta A, DiMeco F. Intraoperative ultrasound and contrast-enhanced ultrasound (CEUS) features in a case of intradural extramedullary dorsal schwannoma mimicking an intramedullary lesion. Ultraschall Med. 2015;36:307–310. doi: 10.1055/s-0034-1399669. [DOI] [PubMed] [Google Scholar]
- 27.Vetrano IG, Prada F, Nataloni IF, Bene MD, Dimeco F, Valentini LG. Discrete or diffuse intramedullary tumor? Contrast-enhanced intraoperative ultrasound in a case of intramedullary cervicothoracic hemangioblastomas mimicking a diffuse infiltrative glioma: technical note and case report. Neurosurg Focus. 2015;39(2):E17. doi: 10.3171/2015.5.FOCUS15162. [DOI] [PubMed] [Google Scholar]
- 28.Vicenzini E, Delfini R, Magri F, Puccinelli F, Altieri M, Santoro A, et al. Semiquantitative human cerebral perfusion assessment with ultrasound in brain space-occupying lesions: preliminary data. J Ultrasound Med. 2008;27:685–692. doi: 10.7863/jum.2008.27.5.685. [DOI] [PubMed] [Google Scholar]
- 29.Wei K, Mulvagh SL, Carson L, Davidoff R, Gabriel R, Grimm RA, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008;21:1202–1206. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Whiting DM, Barnett GH, Estes ML, Sila CA, Rudick RA, Hassenbusch SJ, et al. Stereotactic biopsy of non-neoplastic lesions in adults. Cleve Clin J Med. 1992;59:48–55. doi: 10.3949/ccjm.59.1.48. [DOI] [PubMed] [Google Scholar]