Abstract
Context
Little is known about quality of care for occupational health disorders, although it may affect worker health and workers’ compensation costs. Carpal tunnel syndrome (CTS) is a common work-associated condition that causes substantial disability.
Objective
To describe the design of a study that is assessing quality of care for work-associated CTS and associations with clinical outcomes and costs.
Design
Prospective observational study of 477 individuals with new workers’ compensation claims for CTS without acute trauma who were treated at 30 occupational health clinics from 2011 to 2013 and followed for 18 months.
Main Outcome Measures
Timing of key clinical events, adherence to 45 quality measures, changes in scores on the Boston Carpal Tunnel Questionnaire and 12-item Short Form Health Survey Version 2 (SF-12v2), and costs associated with medical care and disability.
Results
Two hundred sixty-seven subjects (56%) received a diagnosis of CTS and had claims filed around the first visit to occupational health, 104 (22%) received a diagnosis before that visit and claim, and 98 (21%) received a diagnosis or had claims filed after that visit. One hundred seventy-eight (37%) subjects had time off work, which started around the time of surgery in 147 (83%) cases and lasted a median of 41 days (interquartile range = 42 days).
Conclusions
The timing of diagnosis varied, but time off work was generally short and related to surgery. If associations of quality of care with key medical, economic, and quality-of-life outcomes are identified for work-associated CTS, systematic efforts to evaluate and improve quality of medical care for this condition are warranted.
INTRODUCTION
Efforts to ensure that patients receive high-quality medical care have intensified in recent years, as the public has come to appreciate the pervasiveness of quality problems and their effects on clinical outcomes and costs.1–3 Because the entities paying for improvements in quality seldom reap the benefits,4 national programs designed to drive improvement now exist in most health care sectors.5–7 Workers’ compensation accounts for a relatively small percentage of US health care expenditures,8 but it has a unique characteristic9: Financial incentives for health care payers are intrinsically aligned with improving quality.
When workers return to health and function faster, employers may experience financial benefits because the employers are responsible for both medical and disability costs under workers’ compensation policies. Workers stand to gain not only clinically with a better recovery, but also financially because disability benefits cover only a portion of lost wages.10 Assuring the quality of care for occupational disorders may, therefore, present a unique opportunity to benefit both workers and their employers. A 2005 study from Spain demonstrated that improving care for musculoskeletal disorders reduced medical care and disability costs.11 Yet, little is known about the quality of health care provided in workers’ compensation systems in the US.
Carpal tunnel syndrome (CTS) is a common work-associated condition that can cause severe functional impairment and lead to sizable medical and disability costs.12,13 Working with policymakers, payers, and providers in the California workers’ compensation system, we sought to measure quality of care for CTS, to assess the value of higher quality of care to workers and employers, and to lay the groundwork for ongoing quality assessment and improvement programs in workers’ compensation settings. However, a major challenge to achieving this objective was conducting a rigorous evaluation of the relationship between quality of care for CTS and clinical and economic outcomes. A partnership between Kaiser Permanente Northern California Regional Occupational Health Department (KPNC-ROH), Kaiser Foundation Health Plan, and researchers at the RAND Corporation in Santa Monica, CA, made such a study possible.
In this article, we explain our study design and research approach, the unique characteristics of KPNC-ROH that were essential to conducting this analysis, and the implications that the study findings may have for the care of future patients with work-associated conditions treated at KPNC-Occupational Health Centers, elsewhere in California, and across the country. This approach can serve as a model for future studies designed to measure quality of care for patients in state workers’ compensation systems. If we identify associations of quality of care with key medical, economic, and quality-of-life outcomes, systematic efforts to measure and improve quality would be warranted.
Here, we describe 7 major steps that we have undertaken in our effort to evaluate the value of high-quality care for work-associated CTS: 1) developing quality-of-care measures for CTS, 2) selecting and recruiting the study population, 3) measuring quality of care, 4) assessing patient outcomes, 5) measuring medical care costs, 6) measuring disability benefit costs, and 7) measuring other costs to workers and employers. Steps 4 through 7 are still under way.
DEVELOPING QUALITY-OF-CARE MEASURES FOR CARPAL TUNNEL SYNDROME
To evaluate the quality of care for a condition such as CTS, specific measures were needed. Measure development often considers the framework of Donabedian,14 wherein quality can be assessed by examining the characteristics of the health care delivery system in which care is provided (its “structure”), the interactions between patients and physicians (“process”), and the changes in health that occur after receiving care (“outcome”). Care processes are widely studied because this focus gives specific information on what types of improvements are needed, and the quality measures are tailored to patient characteristics so case-mix adjustment is less a concern than when assessing outcomes.15 The National Committee for Quality Assurance (NCQA), for example, monitors how often patients in various health plans receive recommended vaccinations and cancer screenings, among other aspects of care.7 Before the current endeavor, no CTS-specific process measures existed.
We therefore developed a set of quality measures that could be applied to CTS, including work-associated CTS, meaning CTS that has been ascribed to occupational activities. We used a variation of the well-established RAND/UCLA (University of California, Los Angeles) Appropriateness Method, which incorporates a systematic review of the literature and a quantitative assessment reflecting the judgment of a group of experts.16,17 This method has good reliability and good content, construct, and predictive validity,18–21 and it has been used to develop measures of quality and surgical appropriateness for other musculoskeletal disorders as well as other conditions.2,22–24
Accordingly, we identified care processes for CTS that may be associated with improved outcomes and then asked content experts and a project advisory board, including the Director of KPNC-ROH, to refine, add, and delete draft measures. An 11-member multidisciplinary panel of experts in CTS reviewed a synopsis of the literature and rated the validity, feasibility, and importance of draft quality measures and the appropriateness of surgery in diverse clinical scenarios. Literature review methods and panel methods for selecting valid quality measures have been described previously.25–28
The full set of CTS measures addresses evaluation and monitoring; nonoperative management; electrodiagnostic testing; activity assessment and management; surgical appropriateness; and perioperative care. The current effort focuses on evaluation and monitoring (11 measures), nonoperative treatment (11 measures), activity assessment and management (10 measures), and appropriateness of surgery (13 measures), as shown in Table 1. During analysis, scores on individual measures will be aggregated into these categories. For these 45 measures, we developed a guidance document with detailed specifications for how each measure is to be scored, including eligibility criteria, definitions of terms, and adherence criteria. We also developed a paper data collection tool and pilot-tested it at a large workers’ compensation insurance company (California State Compensation Insurance Fund) and at KPNC-ROH. When issues potentially affecting feasibility or reliability were identified, the tool was refined accordingly.29 We later programmed the tool in Microsoft Access (Microsoft Corp, Redmond, WA) and conducted further pilot testing.
Table 1.
Quality measures used in study by aspect of care, type of quality problem, and panelist-rated importance score, and description of provider task
| Measure | Aspect of care | Type of quality problem | Importance score | Description of provider task |
|---|---|---|---|---|
| Evaluation and monitoring: Obtain history, perform physical examination, order tests, and monitor symptoms | ||||
| 1 | New symptoms characteristic of CTS require detailed history | Underuse | 8 | Obtain history |
| 2 | New symptoms characteristic of CTS should lead to suspicion | Underuse | 7 | Obtain history |
| 3 | New hand or forearm pain requires evaluation for “red flags” | Underuse | 8 | Obtain history |
| 4 | New symptoms inconsistent with CTS require evaluation | Underuse | 8 | Obtain history |
| 5 | New CTS diagnosis requires assessment of medical risk factors | Underuse | 8 | Obtain history |
| 6 | New suspicion of CTS requires specific physical examination | Underuse | 8 | Perform physical examination |
| 7 | New suspicion of CTS requires evaluation for excessive weight | Underuse | 6 | Perform physical examination |
| 8 | Imaging should be used selectively for suspected CTS | Overuse | 7 | Order tests |
| 9 | Symptoms should be monitored after new diagnosis of CTS | Underuse | 7 | Monitor symptoms |
| 10 | Work-associated CTS symptoms require prompt follow-up | Underuse | 8 | Monitor symptoms |
| 11 | Preoperative electrodiagnostic testing is required for work-associated CTS | Underuse | 9 | Order tests |
| Nonoperative treatment: Prescribe splints, medications, and other treatments correctly | ||||
| 1 | Splints should be placed in neutral position | Underuse | 7 | Prescribe splints correctly |
| 2 | An attempt at splinting should last at least 6 weeks | Underuse | 7 | Prescribe splints correctly |
| 3 | NSAIDs should not be used for CTS | Overuse | 7 | Prescribe medications correctly |
| 4 | Muscle relaxants should not be used for CTS | Overuse | 7 | Prescribe medications correctly |
| 5 | Opioids should not be used for CTS | Overuse | 7 | Prescribe medications correctly |
| 6 | Diuretics should not be used for CTS | Overuse | 7 | Prescribe medications correctly |
| 7 | Corticosteroid treatment requires discussion of risks | Overuse | 6 | Prescribe medications correctly |
| 8 | Discuss benefits of surgery when offering steroids to patients with severe CTS | Underuse | 8 | Prescribe medications correctly |
| 9 | Steroids for work-associated symptoms require follow-up | Underuse | 7 | Prescribe medications correctly |
| 10 | Limit steroid injections to 4 | Overuse | 7 | Prescribe medications correctly |
| 11 | Laser therapy should not be used for CTS | Overuse | 7 | Order other treatment correctly |
| Activity assessment and management: Assess activity, assess causation, educate patients, recommend activity changes, and monitor activity | ||||
| 1 | New CTS diagnosis requires detailed occupational history | Underuse | 6 | Assess activity |
| 2 | New CTS diagnosis requires assessment of occupational factors: Vibration, force, and repetition | Underuse | 7 | Assess activity |
| 3 | New CTS diagnosis requires assessment of nonoccupational factors: Vibration, force, and repetition | Underuse | 7 | Assess activity |
| 4 | Exacerbating activities should be identified when symptoms limit functioning | Underuse | 7 | Assess activity |
| 5 | Rationale for work association should be documented | Underuse | 7 | Assess causation |
| 6 | Patients with a new diagnosis of CTS should be educated about the condition | Underuse | 7 | Educate patients |
| 7 | Exposures to vibration, force, and repetition should be minimized | Underuse | 7 | Recommend activity changes |
| 8 | Work status should be monitored when CTS appears work associated | Underuse | 7 | Monitor activity |
| 9 | Return to work after CTS-related disability requires follow-up assessment that includes functional limitations | Underuse | 6 | Monitor activity |
| 10 | Prolonged CTS-related disability should trigger evaluation | Underuse | 7 | Monitor activity |
| Surgical appropriatenessa: Assure potential benefits of surgery exceed risks | ||||
| 1 | Compelling indications for surgery when CTS is mild | Underuse | 9 | Perform necessary surgery |
| 2 | Compelling indications for surgery when CTS is moderate, Part 1 | Underuse | 9 | Perform necessary surgery |
| 3 | Compelling indications for surgery when CTS is moderate, Part 2 | Underuse | 9 | Perform necessary surgery |
| 4 | Compelling indications for surgery when CTS is severe, Part 1 | Underuse | 9 | Perform necessary surgery |
| 5 | Compelling indications for surgery when CTS is severe, Part 2 | Underuse | 9 | Perform necessary surgery |
| 6 | Compelling indications for surgery when CTS is severe, Part 3 | Underuse | 9 | Perform necessary surgery |
| 7 | Avoidance of carpal tunnel surgery during pregnancy | Overuse | 9 | Avoid inappropriate surgery |
| 8 | Compelling contraindications for surgery when CTS is mild, Part 1 | Overuse | 9 | Avoid inappropriate surgery |
| 9 | Compelling contraindications for surgery when CTS is mild, Part 2 | Overuse | 9 | Avoid inappropriate surgery |
| 10 | Compelling contraindications for surgery when CTS is moderate, Part 1 | Overuse | 9 | Avoid inappropriate surgery |
| 11 | Compelling contraindications for surgery when CTS is moderate, Part 2 | Overuse | 9 | Avoid inappropriate surgery |
| 12 | Compelling contraindications for surgery when CTS is moderate, Part 3 | Overuse | 9 | Avoid inappropriate surgery |
| 13 | Compelling contraindications for surgery when CTS is moderate, Part 4 | Overuse | 9 | Avoid inappropriate surgery |
Parts apply to different subpopulations within a given severity of carpal tunnel syndrome.
CTS = carpal tunnel syndrome; NSAID = nonsteroidal anti-inflammatory drug.
Pilot-testing also suggested that workers’ compensation claims for CTS may be filed at variable times in the clinical course of care. Some patients were referred to a KPNC-Occupational Health Center, received a diagnosis, and submitted a workers’ compensation claim within a short timeframe. However, CTS had been diagnosed in other patients years before they filed a claim.
Our study design accounted for this issue in two ways. First, the eligibility criteria for each measure included specific timeframes related to specific milestones in the clinical course of care. Physicians should perform certain tasks, for example, when evaluating symptoms that could represent CTS or when making a new diagnosis. Second, the data collection tool included the dates of major milestones related to measure eligibility, including making the first visit to a KPNC-Occupational Health Center for symptoms related to CTS, receiving a diagnosis of work-associated CTS, stopping work because of CTS, having surgery for CTS, and returning to work. Any diagnoses from before study enrollment required a positive electrodiagnostic test or an assessment by a specialist in musculoskeletal disorders.
SELECTING AND RECRUITING A STUDY POPULATION
Identifying a suitable population was the most substantial challenge this study faced. Given that our objectives included informing health care policy, we sought workers with CTS from diverse industries. Also, we needed access to high-quality databases that included diagnosis and treatment codes, medical care utilization, time off work, and disability ratings, among other variables. Many large studies of musculoskeletal disorders have been conducted in countries with national databases,30,31 offering access to populations of adequate sample size and reducing the time and expense involved in recruiting subjects. Few national databases are available in the US, but some large integrated health care systems have both large patient populations and advanced databases.
Although KPNC is best known for its large regional integrated health care systems and prepaid (ie, capitated) care, KPNC-ROH is a major provider of fee-for-service occupational health care in Northern California. It has been selected by numerous large and small employers, workers’ compensation insurance carriers, and third-party administrators of workers’ compensation claims. Occupational conditions are referred to KPNC-Occupational Health Center specialists based in 30 clinics. In addition to an electronic medical record system, KPNC-ROH maintains a comprehensive database of workers’ compensation claims that includes information on employer, payer, patient characteristics and diagnoses, recommended worksite accommodations, recommended and actual work status, health care utilization and claims (by diagnosis), and prescriptions and pharmacy claims (by diagnosis). Copies of California workers’ compensation forms, including Doctor’s First Reports, Progress Reports, and Permanent and Stationary Reports, are also included. About 70% of patients treated by KPNC-Occupational Health Centers also have general health insurance through KPNC.
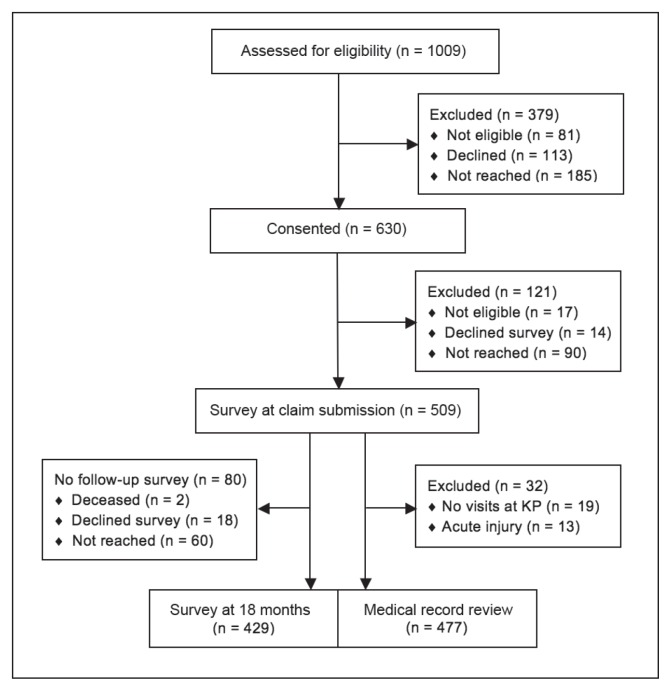
The KPNC-ROH partners in this study used these internal workers’ compensation databases to prospectively identify 1009 adults aged 18 years and older who had a primary or secondary diagnosis of CTS that was linked to a workers’ compensation claim (July 2011 to February 2013). We included secondary diagnoses of CTS because some patients with claims related to other upper extremity conditions are later found to have CTS. After KPNC-ROH contacted potential subjects, 630 (67.9%) consented to participate; another 81 were found to be ineligible (ineligible subjects included KPNC employees, subjects who did not speak English or Spanish, or subjects who were unable to provide consent), 113 declined to participate, and 185 could not be reached (Figure 1). Given the challenges inherent in making a diagnosis of CTS, subjects remained eligible if the diagnosis was later changed or the workers’ compensation claim was dropped or denied, enabling us to evaluate the quality of the initial evaluation and its effects on outcomes.
Figure 1.
Enrollment of Study Subjects, Survey Responses, and Medical Record Review.
KP = Kaiser Permanente.
MEASURING QUALITY OF CARE
In addition to recruiting an appropriate and sizable population, we needed to assess the quality of care by multiple physicians in different locations, including ancillary services such as physical therapy or imaging. A systemwide electronic medical record system eliminated the problem of legibility and made data from multiple sites and physicians accessible.
Specially trained medical-record abstractors at the KPNC Division of Research with experience collecting data for quality measurement reviewed each subject’s electronic medical record and identified visits related to the CTS claim. Abstractors collected data needed to determine eligibility and adherence for each quality measure. After excluding patients with no visits to KPNC-Occupational Health Center physicians (n = 19) or CTS related to an acute injury (acute injuries were excluded from the study; n = 13), abstractors reviewed records for 477 patients. Abstractors also obtained additional variables, including work status at each visit, clinical symptoms and signs, results of electrodiagnostic tests, and the dates of major milestones in the clinical course of care (see section Developing Quality-of-Care Measures for Carpal Tunnel Syndrome).
To ensure that assessments of quality were valid and reliable, abstractors underwent a 2-day training session, including applying the guidance document and Microsoft Access data collection tool (described earlier) to practice cases. Abstractors initially reviewed medical records in pairs, discussed findings, and resolved any discrepancies together and, when questions arose, with the research team. Next, to ensure proficiency, we compared abstractors’ reviews for 3 cases against those of an occupational medicine physician. Subsequently, abstractors scored cases independently, except for reliability assessments. Through site visits and phone meetings, an experienced nurse researcher (CR) monitored data validity, clarified definitions of terms and variables, and maintained a log of questions and answers. A total of 58 cases underwent duplicate review, including 35 used to estimate reliability.
ASSESSING PATIENT OUTCOMES
We used a prospective, observational study design to examine the relationship between quality of care for CTS and patient outcomes. Telephone surveys measured changes in each patient’s symptoms and functional status between the time of claim submission and 18 months later. Of the 630 subjects initially recruited, 17 were found to be ineligible; 509 (83.0%) of the remaining 613 subjects completed the baseline survey, 14 declined, and 90 could not be reached. For the follow-up survey, 429 (84.3% of the 509 subjects who filled out the baseline survey) responded, 18 declined, 2 were deceased, and 60 could not be reached.
To assess outcomes, we used the Boston Carpal Tunnel Questionnaire and the 12-item Short-Form Health Survey Version 2 because condition-specific instruments tend to be more responsive and general instruments facilitate comparisons across conditions. The Boston Carpal Tunnel Questionnaire is specifically tailored for CTS and has demonstrated validity, reliability, and responsiveness.32–39 It includes 2 subscales: symptom severity and functional status. Overall scores are obtained by calculating the mean response across component questions; weighting each question according to the importance to the patient increases responsiveness.32 The widely used 12-item Short Form Health Survey Version 2 includes physical and mental health component scores, with the former being more responsive in CTS.33 Finally, we obtained information on any permanent disability via the medical record and Permanent and Stationary Reports completed by the treating physicians.
To enable adjustment for covariates, surveys included questions on prior workers’ compensation claims in the study hand (the hand with work-associated CTS included in the study); duration, location, and timing of CTS symptoms; involvement of an attorney; demographic covariates; medical conditions associated with CTS; whether the patient received care for those symptoms before or in addition to being seen at KPNC-Occupational Health Center; and smoking status, alcohol or substance abuse, and anxiety.
MEASURING MEDICAL CARE COSTS
To evaluate the association between quality and costs, detailed information was needed on the costs of medical care related to CTS, including physician visits, physical and occupational therapy visits, medications, diagnostic tests, and surgery. Accordingly, KPNC-ROH provided RAND partners with de-identified datasets containing procedure codes, diagnosis codes, dates of service, and billed amounts for all services billed to workers’ compensation. Because many workers’ compensation claims involved additional diagnoses, we included only services involving diagnosis codes potentially related to CTS. We adjusted the billed amounts to match California’s fee schedule and summed the spending associated with each CTS claim from the time of submission until 18 months later. Of the 477 patients for whom quality of care was assessed, 3 lacked data on medical care expenditures because of having had only a single visit to a KPNC-Occupational Health Center.
MEASURING DISABILITY BENEFIT COSTS
Because of the importance of disability costs when considering the value of high-quality care for work-associated CTS, we needed to estimate the costs associated with temporary and permanent disability benefits. Actual payments were not available to us, so we needed to estimate them based on other information. Physicians at KPNC-Occupational Health Centers document activity restrictions and work status in detail at each clinic visit, and the physicians’ Permanent and Stationary Reports with permanent disability ratings are part of the medical record. “Work status” is documented by the physicians as “full duty,” “off work,” or “work with restriction of activities.”
Using work status documented at each visit with a primary treating physician as well as data from the KPNC-ROH workers’ compensation database, we constructed event histories that tracked each patient’s daily work status throughout the 18-month follow-up period. We estimated statutory temporary total disability benefits using each patient’s self-reported monthly personal income before filing the claim, and statutory permanent disability benefits based on patients’ permanent disability ratings and statewide data on earnings losses for patients with CTS.
MEASURING OTHER COSTS TO WORKERS AND EMPLOYERS
CTS imposes a variety of costs on workers and employers beyond the financial cost of medical and disability benefits paid by the workers’ compensation system during the first 18 months of a claim.40–43 Employers are responsible for the lifetime cost of medical treatment for occupational injury and illness, and most spending on the average workers’ compensation claim occurs well after our follow-up period.44
To estimate uncompensated economic losses for workers during the 18-month follow-up period, we used self-reported data from our baseline and follow-up surveys on employment, monthly wages, annual household and personal income, job changes, out-of-pocket medical spending, and out-of-pocket personal care expenditures. We used these data to estimate the effect of higher-quality care on labor income, employment, return to work at the at-injury employer, and out-of-pocket medical costs.
In addition to the costs of reduced employment and earnings that are borne by workers, employers may also bear the cost of absenteeism (time off work) and “presenteeism” (reduced productivity while at work) because of residual disability following recovery from CTS. We estimated costs associated with absenteeism and presenteeism at baseline and follow-up using standardized instruments and monetized the employer costs using data on the daily wage rate and multipliers estimated by Nicholson et al41 for absenteeism and by Pauly et al42 for presenteeism.
ANALYSIS
First, we have undertaken descriptive analyses. Table 2 presents key characteristics of the study population obtained from the baseline survey at claim submission. The age and sex of the study population is typical for CTS, including disproportionately affecting women.45 The personal income in the study population was relatively high, consistent with the geographic area.
Table 2.
Characteristics of study population at submission of workers’ compensation claim for CTS (N = 509)
| Characteristic category | Value |
|---|---|
| Demographic characteristics | |
| Age, years, mean (SD) | 47.6 (10.4) |
| Female sex, % | 73.3 |
| Spanish speaking, % | 4 |
| Clinical characteristics | |
| Right hand is study hand (affected hand, or dominant hand if bilateral), % | 81.1 |
| Katz hand diagram rating, % | |
| Classic | 5.1 |
| Probable | 34.6 |
| Possible | 55.4 |
| Unlikely | 4.9 |
| Median nerve digit score, % | |
| 2 (most likely) | 86.8 |
| 1 (intermediate) | 8.3 |
| 0 (least likely) | 4.9 |
| Self-efficacy (1–4, confidence in ability to manage CTS), mean (SD) | 2.3 (0.8) |
| Health status measures | |
| Boston Carpal Tunnel Questionnaire | |
| Symptom severity score (1–5, 5 = worst), mean (SD) | 2.9 (0.8) |
| Functional status score (1–5, 5 = worst), mean (SD) | 2.5 (0.9) |
| Short Form Health Survey (12 Item, Version 2) | |
| Physical component score (1–100, 100 = best), mean (SD) | 40.1 (9.7) |
| Mental component score (1–100, 100 = best), mean (SD) | 50.4 (11.5) |
| Economic characteristics | |
| Work status, % | |
| Working full time | 63.5 |
| Working part time | 11.0 |
| Not working | 25.0 |
| Personal income (US$) in last 4 weeks, % | |
| ≤ 1600 | 24.2 |
| 1601–2500 | 14.9 |
| 2501–3750 | 15.9 |
| 3751–5000 | 19.3 |
| 5001–6250 | 9.2 |
| > 6251 | 8.6 |
| Data missing | 7.9 |
CTS = carpal tunnel syndrome; SD = standard deviation.
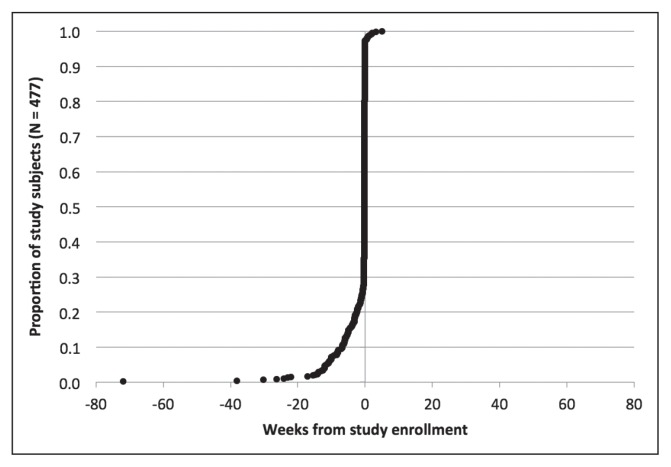
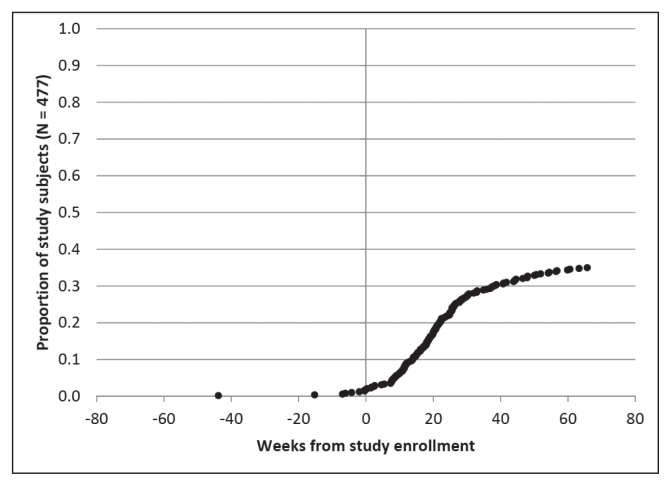
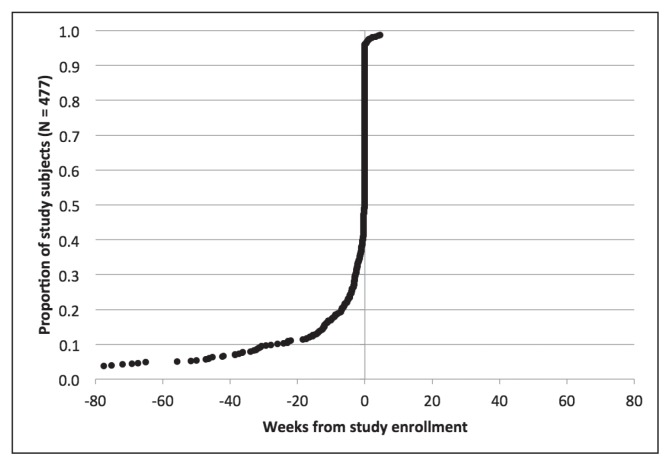
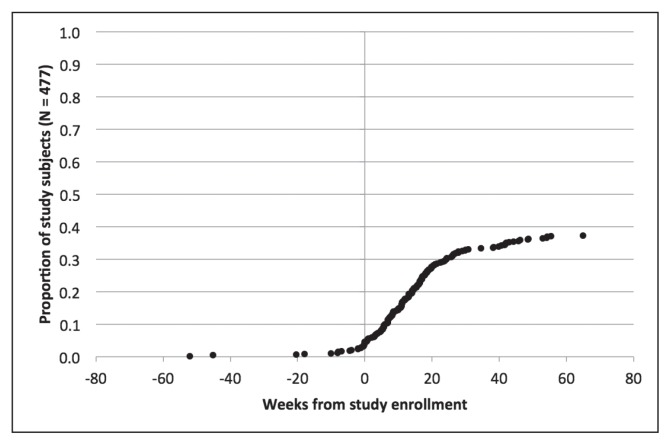
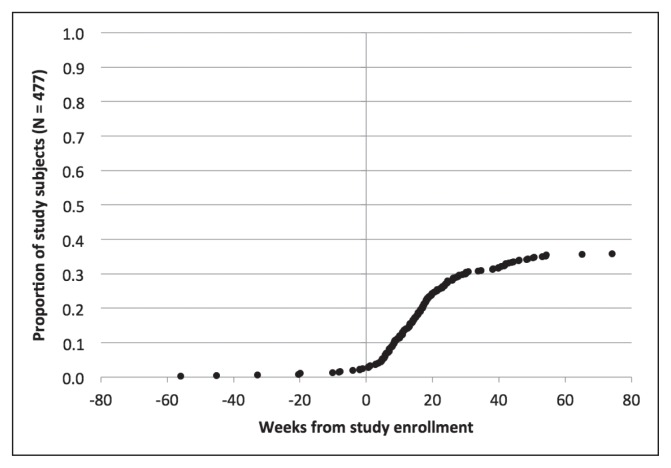
Figures 2 through 6 show the timing of major milestones in relation to study enrollment for the 477 patients for whom quality of care was assessed. These milestones include making the first visit to a KPNC-Occupational Health Center for CTS-related symptoms, receiving a diagnosis of work-associated CTS, stopping work because of CTS, having surgery for CTS, and returning to work.
Figure 2.
Clinical milestones: Percentage of study subjects achieving each milestone by weeks from study enrollment (time = 0): Making first visit to KPNC-ROH because of symptoms of carpal tunnel syndrome.
KPNC-ROH = Kaiser Permanente Northern California-Regional Occupational Health Department.
Figure 6.
Clinical milestones: Percentage of study subjects achieving each milestone by weeks from study enrollment (time = 0): Returning to work.
Looking at the timing for when these 477 subjects met the first 2 milestones, most subjects fell into 1 of 4 groups. The most common situation was for both the first visit and the diagnosis to occur within 1 week of study enrollment (267 subjects, 56.1%). For 104 subjects (21.8%), the diagnosis preceded the first visit and enrollment by at least a week, and sometimes by several years: This could reflect later referral to a KPNC-Occupational Health Center, transfer of care, or recurrent symptoms. The first visit preceded both diagnosis and enrollment by a week or more in 48 subjects (10.0%): This could indicate that physicians did not suspect CTS initially or sought additional evidence before making a diagnosis. For 50 subjects (10.5%), the first visit and the diagnosis occurred within a week of each other but more than a week before enrollment. In these cases, the occupational medicine physician may have documented a provisional diagnosis of CTS at the first visit but delayed linking it to a workers’ compensation claim pending greater insight into work association. These findings highlight the importance of tailoring eligibility for each measure to the specific clinical circumstances at a given time point. For example, some measures related to the initial evaluation would not apply to patients who already have well-established diagnoses of work-associated CTS at the time of presentation to a KPNC-Occupational Health Center.
Regarding the other 3 major milestones, 178 subjects (37.3%) stopped work because of CTS. The median interval from study enrollment to stopping work was 91 days (interquartile range = 103 days). Most patients who stopped work did so to have surgery, consistent with practices at KPNC-ROH. Of patients who stopped work because of CTS, 147 (82.6%) stopped working within a week of surgery. A few patients stopped work and underwent surgery before presenting to a KPNC-Occupational Health Center. The median duration off work was 41 days (interquartile range = 42 days) among the 163 patients for which this could be calculated.
After performing these descriptive analyses, we plan to undertake 2 basic types of multivariate regression analyses. The first will involve examining rates of adherence to the measures individually and by aspect of care, and then using multivariate regression models to identify predictors of receiving recommended care. Such predictors will include demographic characteristics, clinical features of the CTS (eg, symptom duration, pattern, timing, and severity; neurologic signs; results of electrodiagnostic testing), characteristics of workers’ compensation claims (eg, having a prior claim, involvement of an attorney), clinic characteristics (specifically, volume of patients with CTS treated per year in each of the 30 clinics).
We plan to use a patient-measure-level dataset in this regression analysis. Each patient will contribute 1 observation (ie, row) to this dataset per quality measure for which they are eligible (eg, 10 observations if eligible for 10 measures). Patient-level characteristics (eg, sex and age) will be copied to each corresponding observation, a categorical variable will represent the quality measure for the observation, and a binary variable will indicate whether care for the patient adhered to the recommendation in the quality measure. We plan to use logistic regression with patient-level random effects to control for patient-level heterogeneity and will use measure-level fixed effects to control for variability in the pass rates across the measures.
The second type of analysis will involve examining whether patients who receive higher-quality care, specifically those who have less evidence of underuse and overuse of care, have better clinical outcomes, less disability, and lower costs. Clinical outcomes include changes in scores on the Boston Carpal Tunnel Questionnaire and the 12-item Short Form Health Survey Version 2 from baseline to 18 months. Disability outcomes will include days on temporary disability and the presence of permanent disability under the workers’ compensation claim. Cost outcomes will include health care expenditures related to the workers’ compensation claim for CTS as well as disability-related costs.
We will also use multivariate regression to assess the relationship between quality of care and clinical and economic outcomes. These analyses must be performed at the patient level, instead of the patient-measure level, because changes in health and costs occur at the patient level. Hence, we will need to develop patient-level indexes that aggregate data on adherence to measure recommendations. For instance, we will create indexes reflecting underuse of care and overuse of care, and indexes for each aspect of care (ie, diagnostic evaluation, nonoperative treatment, activity management, and appropriateness of surgery). Creating each index will involve dividing the number of times care adhered to the recommendations within a group of related measures (eg, related to underuse), by the number of times each patient was eligible for those measures. We will weight the indexes by the measures’ importance scores. We will tailor the modeling approach (eg, linear, Poisson) to each outcome of interest. For example, we assume Poisson regression will be needed to determine how days on temporary disability vary with quality of care. All analyses will control for relevant patient-level characteristics, including demographic characteristics, CTS symptoms, clinical signs, electrodiagnostic test results, and characteristics that have been found to influence clinical and cost outcomes for work-associated CTS.
CONCLUSIONS
We have undertaken a comprehensive effort to evaluate the quality of medical care provided to individuals with work-associated CTS, and to assess the associations between quality and both clinical outcomes and diverse costs. However, to achieve our goals, we needed to overcome multiple challenges. These included developing a set of quality measures de novo, recruiting a sizable and diverse population, assessing quality by reviewing medical records from multiple treating physicians over a 12-month period, comparing patients’ self-reported outcomes at claim submission and 18 months later, and using diverse sources of data to estimate health care, disability payment, and other costs. Necessary data sources included surveys, medical records, administrative data sets on health care utilization, detailed information on work status, and Permanent and Stationary Reports. In partnership with KPNC-ROH, which provides a high volume of care for occupational disorders, we were able to recruit and survey 509 adults shortly after submission of workers’ compensation claims in 2011 to 2013 and obtain the requisite data. The final stages of data collection and analysis are under way. We hope that this study can eventually provide a model for future efforts to test the association between quality of care and outcomes in workers’ compensation for other conditions or in other settings.
Evidence that higher-quality care for work-associated CTS is associated with improved outcomes or lower costs would have important implications for patients treated at a KPNC-Occupational Health Center, elsewhere in California, and in workers’ compensation systems across the country. Several prior studies have demonstrated that greater adherence to recommended care processes is associated with improved patient outcomes.18,19,24 Workers’ compensation is unique in the US health care system in that the parties responsible for health care expenditures, employers and workers’ compensation payers, are also responsible for disability costs. This creates a natural alignment of incentives such that employers and payers may experience returns on investments in quality. More importantly, individuals with occupational disorders stand to benefit from better health, a more rapid return to work, and reduced economic losses.
If we find that higher-quality care is associated with improved outcomes and lower costs, as hypothesized, systematic efforts to monitor and improve quality would be warranted. To date little has been done to improve quality of care for patients with occupational disorders, in contrast with the myriad efforts in place in other health care settings. For example, the Centers for Medicare and Medicaid Services has instituted various public reporting policies and financial penalties for hospitals, targeting deficient care processes and worse-than-expected outcomes, including readmissions, health care-associated infections, and other problems. For commercial health plans, the National Committee for Quality Assurance has long overseen the Healthcare Effectiveness Data and Information Set, which issues report cards that highlight preventive care and the treatment of chronic diseases. Best practices for incentivizing and improving quality could be adapted from other health care sectors to workers’ compensation settings in California and elsewhere.
Our descriptive analyses reveal some potential challenges. In our population, time off work was relatively short and largely limited to patients undergoing surgery. This may make it harder for us to detect associations between quality and time on temporary disability.46 At least one in five patients were diagnosed with work-associated CTS before presenting to a KPNC-Occupational Health Center—and some even underwent surgery. This could hamper future efforts to improve care for this population. Finally, we detected some delays between presentation to a KPNC-Occupational Health Center and diagnosis of CTS, and between diagnosis of CTS and the filing of a claim. This can be warranted in some patients, such as those with atypical symptoms. However, such delays also can contribute to worse outcomes.47–49
This research effort has several limitations. Some patients in our sample who had an initial diagnosis of CTS were found to have other conditions. However, our quality measures were designed to include the challenges in making an accurate diagnosis of CTS. We have so far been unable to include quality measures related to electrodiagnostic studies and perioperative care, which may reduce our ability to detect an association between quality, outcomes, and costs. Finally, because of the unique environment at KPNC-ROH, potential findings may not be fully reflective of conditions in workers’ compensation settings nationwide. The quality of care for CTS may also be higher at KPNC-ROH, making it harder to detect an effect at KPNC-ROH on clinical outcomes and costs.
Demonstrating an association between higher-quality care for work-associated CTS and both clinical outcomes and costs has important implications for policy. If associations are detected as a result of the work we have undertaken, systematic efforts to monitor and improve quality of care for patients with CTS will be warranted at KPNC-ROH, across California, and nationwide.
Figure 3.
Clinical milestones: Percentage of study subjects achieving each milestone by weeks from study enrollment (time = 0): Receiving a diagnosis of work-associated carpal tunnel syndrome.
Figure 4.
Clinical milestones: Percentage of study subjects achieving each milestone by weeks from study enrollment (time = 0): Stopping work because of carpal tunnel syndrome.
Figure 5.
Clinical milestones: Percentage of study subjects achieving each milestone by weeks from study enrollment (time = 0): Having surgery for carpal tunnel syndrome.
Acknowledgments
This work was funded by a grant from the Agency for Healthcare Research and Quality (5R01HS018982-03). Prior stages in the work (quality measure development) was supported with funding from the California Commission on Health and Safety and Workers’ Compensation (CHSWC) and with an unrestricted gift from Zenith Insurance. CHSWC is a state-sponsored joint labor-management body charged with examining the health and safety and workers’ compensation systems in California and recommending administrative or legislative modifications to improve their operation. The funders played no role in the design, conduct, or reporting of this work.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The RAND Corporation has received funding from Insurance and Care NSW, Australia, for the evaluation of workers’ compensation treatment guidelines (Teryl Nuckols), and from the Collaborative Spine Research Foundation (Teryl Nuckols). The author(s) have no other potential conflicts of interest to report.
References
- 1.Institute of Medicine of the National Academies. Workshop summary. Washington, DC: The National Academies Press; 2009. Dec 16, Value in health care: accounting for cost, quality, safety, outcomes, and innovation. [PubMed] [Google Scholar]
- 2.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003 Jun 26;348(26):2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 3.Key features of the Affordable Care Act by year [Internet] Washington, DC: US Department of Health and Human Services; 2015. Aug 13, [cited 2016 Apr 25]. Available from: www.hhs.gov/healthcare/facts/timeline/timeline-text.html. [Google Scholar]
- 4.Leatherman S, Berwick D, Iles D, et al. The business case for quality: case studies and an analysis. Health Aff (Millwood) 2003 Mar-Apr;22(2):17–30. doi: 10.1377/hlthaff.22.2.17. [DOI] [PubMed] [Google Scholar]
- 5.ACS National Surgical Quality Improvement Program (ACS NSQIP) [Internet] Chicago, IL: American College of Surgeons; c2016. [cited 2016 Apr 8]. Available from: www.facs.org/quality-programs/acs-nsqip. [Google Scholar]
- 6.Roadmap for quality measurement in the traditional medicare fee-for-service program [Internet] Baltimore, MD: Centers for Medicare and Medicaid Services; 2009. [cited 2016 Apr 25]. Available from: www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/QualityMeasurementRoadmap_OEA1-16_508.pdf. [Google Scholar]
- 7.HEDIS & performance measurement [Internet] Washington, DC: National Committee for Quality Assurance; c2016. [cited 2016 Apr 25] Available from: www.ncqa.org/HEDISQualityMeasurement.aspx. [Google Scholar]
- 8.Centers for Medicare & Medicaid Services, Office of the Actuary National Health Statistics Group. National health expenditure accounts [Internet] Baltimore, MD: Centers for Medicare and Medicaid Services; 2013. [cited 2016 Apr 25]. Available from: www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nationalhealthaccountshistorical.html. [Google Scholar]
- 9.Porter ME. What is value in health care? N Engl J Med. 2010 Dec 23;363(26):2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 10.Uncompensated wage loss for injured workers with permanent disabilities [Internet] San Francisco, CA: State of California Department of Industrial Relations; 2007. May, [cited 2016 Apr 25]. Available from: www.dir.ca.gov/dwc/UncompensatedWageLossforInjuredWorkerswithPD/UncompensatedWageLossforInjuredWorkerswithPD.html. [Google Scholar]
- 11.Abásolo L, Blanco M, Bachiller J, et al. A health system program to reduce work disability related to musculoskeletal disorders. Ann Intern Med. 2005 Sep 20;143(6):404–14. doi: 10.7326/0003-4819-143-6-200509200-00005. doi: 10.7326/0003-4819-143-6-200509200-00005. [DOI] [PubMed] [Google Scholar]
- 12.Dale AM, Harris-Adamson C, Rempel D, et al. Prevalence and incidence of carpal tunnel syndrome in US working populations: pooled analysis of six prospective studies. Scand J Work Environ Health. 2013 Sep 1;39(5):495–505. doi: 10.5271/sjweh.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spector JT, Turner JA, Fulton-Kehoe D, Franklin G. Pre-surgery disability compensation predicts long-term disability among workers with carpal tunnel syndrome. Am J Ind Med. 2012 Sep;55(9):816–32. doi: 10.1002/ajim.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966 Jul;44(3) Suppl:166–206. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 15.Brook RH, McGlynn EA, Cleary PD. Quality of health care. Part 2: measuring quality of care. N Engl J Med. 1996 Sep 26;335(13):966–70. doi: 10.1056/NEJM199609263351311. [DOI] [PubMed] [Google Scholar]
- 16.Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation; 2001. [Google Scholar]
- 17.Shekelle P. The appropriateness method. Med Decis Making. 2004 Mar-Apr;24(2):228–31. doi: 10.1177/0272989X04264212. [DOI] [PubMed] [Google Scholar]
- 18.Hepner KA, Rowe M, Rost K, et al. The effect of adherence to practice guidelines on depression outcomes. Ann Intern Med. 2007 Sep 4;147(5):320–9. doi: 10.7326/0003-4819-147-5-200709040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Higashi T, Shekelle PG, Adams JL, et al. Quality of care is associated with survival in vulnerable older patients. Ann Intern Med. 2005 Aug 16;143(4):274–81. doi: 10.7326/0003-4819-143-4-200508160-00008. [DOI] [PubMed] [Google Scholar]
- 20.Shekelle PG, Chassin MR, Park RE. Assessing the predictive validity of the RAND/UCLA appropriateness method criteria for performing carotid endarterectomy. Int J Technol Assess Health Care. 1998 Fall;14(4):707–27. doi: 10.1017/s0266462300012022. [DOI] [PubMed] [Google Scholar]
- 21.Shekelle PG, Kahan JP, Bernstein SJ, Leape LL, Kamberg CJ, Park RE. The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med. 1998 Jun 25;338(26):1888–95. doi: 10.1056/nejm199806253382607. [DOI] [PubMed] [Google Scholar]
- 22.Ganz DA, Chang JT, Roth CP, et al. Quality of osteoarthritis care for community-dwelling older adults. Arthritis Rheum. 2006 Apr 15;55(2):241–7. doi: 10.1002/art.21844. [DOI] [PubMed] [Google Scholar]
- 23.MacLean CH, Louie R, Leake B, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000 Aug;284(8):23–30. 984–92. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 24.Quintana JM, Escobar A, Arostegui I, et al. Health-related quality of life and appropriateness of knee or hip joint replacement. Arch Intern Med. 2006 Jan 23;166(2):220–6. doi: 10.1001/archinte.166.2.220. [DOI] [PubMed] [Google Scholar]
- 25.Maggard MA, Harness NG, Chang WT, Parikh JA, Asch SM, Nuckols TK Carpal Tunnel Quality Group. Indications for performing carpal tunnel surgery: clinical quality measures. Plast Reconstr Surg. 2010 Jul;126(1):169–79. doi: 10.1097/prs.0b013e3181da8685. doi: 10.1097/prs.0b013e3181da8685. [DOI] [PubMed] [Google Scholar]
- 26.Nuckols T, Harber P, Sandin K, et al. Quality measures for the diagnosis and non-operative management of carpal tunnel syndrome in occupational settings. J Occup Rehabil. 2011 Mar;21(1):100–9. doi: 10.1007/s10926-010-9260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuckols TK, Maggard Gibbons M, Harness NG, Chang WT, Chung KC, Asch SM Carpal Tunnel Quality Group. Clinical quality measures for intraoperative and perioperative management in carpal tunnel surgery. Hand (N Y) 2011 Jun;6(2):119–31. doi: 10.1007/s11552-011-9325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandin KJ, Asch SM, Jablecki CK, Kilmer DD, Nuckols TK Carpal Tunnel Quality Group. Clinical quality measures for electrodiagnosis in suspected carpal tunnel syndrome. Muscle Nerve. 2010 Apr;41(4):444–52. doi: 10.1002/mus.21617. [DOI] [PubMed] [Google Scholar]
- 29.Nuckols TK, Griffin A, Asch SM, et al. RAND/UCLA quality-of-care measures for carpal tunnel syndrome: tools for assessing quality of care and appropriateness of surgery [Internet] Santa Monica, CA: RAND Corporation; 2011. [cited 2016 Apr 25] Available from: www.rand.org/pubs/technical_reports/TR809.html. [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen JH, Thomsen JF, Overgaard E, et al. Computer use and carpal tunnel syndrome: a 1-year follow-up study. JAMA. 2003 Jun 11;289(22):2963–9. doi: 10.1001/jama.289.22.2963. [DOI] [PubMed] [Google Scholar]
- 31.Atroshi I, Gurnmesson C, Ornstein E, Johnsson R, Ranstam J. Carpal tunnel syndrome and keyboard use at work: a population-based study. Arthritis Rheum. 2007 Nov;56(11):3620–5. doi: 10.1002/art.22956. [DOI] [PubMed] [Google Scholar]
- 32.Amadio PC, Silverstein MD, Ilstrup DM, Schleck CD, Jensen LM. Outcome assessment for carpal tunnel surgery: the relative responsiveness of generic, arthritis-specific, disease-specific, and physical examination measures. J Hand Surg Am. 1996 May;21(3):338–46. doi: 10.1016/S0363-5023(96)80340-6. . [DOI] [PubMed] [Google Scholar]
- 33.Bessette L, Sangha O, Kuntz KM, et al. Comparative responsiveness of generic versus disease-specific and weighted versus unweighted health status measures in carpal tunnel syndrome. Med Care. 1998 Apr;36(4):491–502. doi: 10.1097/00005650-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 35.Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the disabilities of the arm, shoulder, and hand, the carpal tunnel questionnaire, and the SF-36 to clinical change after carpal tunnel release. J Hand Surg Am. 2003 Mar;28(2):250–4. doi: 10.1097/00005650-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Katz JN, Gelberman RH, Wright EA, Lew RA, Liang MH. Responsiveness of self-reported and objective measures of disease severity in carpal tunnel syndrome. Med Care. 1994 Nov;32(11):1127–33. doi: 10.1097/00005650-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Leite JC, Jerosch-Herold C, Song F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskelet Disord. 2006 Oct 20;7:78. doi: 10.1097/00005650-199411000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993 Nov;75(11):1585–92. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Sambandam SN, Priyanka P, Gul A, Ilango B. Critical analysis of outcome measures used in the assessment of carpal tunnel syndrome. Int Orthop. 2008 Aug;32(4):497–504. doi: 10.1007/s00264-007-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boden LI, Galizzi M. Economic consequences of workplace injuries and illnesses: lost earnings and benefit adequacy. Am J Ind Med. 1999 Nov;36(5):487–503. doi: 10.1002/(sici)1097-0274(199911)36:5<487::aid-ajim1>3.0.co;2-2. . [DOI] [PubMed] [Google Scholar]
- 41.Nicholson S, Pauly MV, Polsky D, Sharda C, Szrek H, Berger ML. Measuring the effects of work loss on productivity with team production. Health Econ. 2006 Feb;15(2):111–23. doi: 10.1002/hec.1052. [DOI] [PubMed] [Google Scholar]
- 42.Pauly MV, Nicholson S, Polsky D, Berger ML, Sharda C. Valuing reductions in on-the-job illness: ‘presenteeism’ from managerial and economic perspectives. Health Econ. 2008 Apr;17(4):469–85. doi: 10.1002/Hec.1266. [DOI] [PubMed] [Google Scholar]
- 43.Reville RT, Polich S, Seabury SA, Giddens E. Permanent disability at private, self-insured firms A study of earnings loss, replacement, and return to work for workers’ compensation claimants. Santa Monica, CA: RAND; 2001. [Google Scholar]
- 44.Bellusci D. California workers’ compensation at 100 years part II: how do we rate? Part 2. Proceedings of the WCIRB Annual Workers’ Compensation Conference; 2015 Jun 11; San Francisco, CA. Oakland, CA: WCIRB California; 2015. Jun 17, [Google Scholar]
- 45.Kleopa KA. In the clinic. Carpal tunnel syndrome. Ann Intern Med. 2015 Sep 1;163(5):ITC1. doi: 10.7326/AITC201509010. [DOI] [PubMed] [Google Scholar]
- 46.Cheadle A, Franklin G, Wolfhagen C, et al. Factors influencing the duration of work-related disability: a population-based study of Washington State workers’ compensation. Am J Public Health. 1994 Feb;84(2):190–6. doi: 10.2105/ajph.84.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniell WE, Fulton-Kehoe D, Chiou LA, Franklin GM. Work-related carpal tunnel syndrome in Washington State workers’ compensation: temporal trends, clinical practices, and disability. Am J Ind Med. 2005 Oct;48(4):259–69. doi: 10.1002/ajim.20203. [DOI] [PubMed] [Google Scholar]
- 48.Steyers CM. Recurrent carpal tunnel syndrome. Hand Clin. 2002 May;18(2):339–45. doi: 10.1016/s0749-0712(01)00005-1. . [DOI] [PubMed] [Google Scholar]
- 49.Wickizer TM, Franklin G, Fulton-Kehoe D, et al. Improving quality, preventing disability and reducing costs in workers’ compensation healthcare: a population-based intervention study. Med Care. 2011 Dec;49(12):1105–11. doi: 10.1097/MLR.0b013e31823670e3. [DOI] [PubMed] [Google Scholar]