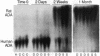
Abstract
Genetically engineered fibroblasts have been successfully used to produce therapeutic proteins in animals, but sustained production of the proteins has not been achieved. This limits the potential of fibroblast-mediated gene therapy in humans. We have studied the phenomenon of decreased production in rats by using retroviral vectors carrying genes encoding human adenosine deaminase and neomycin phosphotransferase. While transplanted skin fibroblasts containing vector sequences persisted at constant levels for at least 8.5 mo, vector expression decreased by greater than 1500-fold after 1 mo. Cellular or antibody-mediated immune responses were not detected in transplanted animals, and expression could not be restored in fibroblasts recultivated from the grafts. This phenomenon is reminiscent of sequence-specific gene inactivation observed in other cell types. Because genetic manipulation and expression of foreign proteins did not affect survival of the transplanted cells, effective long-term therapy may be possible with the use of alternative gene regulatory elements.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Franz T., Hilberg F., Seliger B., Stocking C., Ostertag W. Retroviral mutants efficiently expressed in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3292–3296. doi: 10.1073/pnas.83.10.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver R. I., Jr, Chytil A., Courtney M., Crystal R. G. Clonal gene therapy: transplanted mouse fibroblast clones express human alpha 1-antitrypsin gene in vivo. Science. 1987 Aug 14;237(4816):762–764. doi: 10.1126/science.3497452. [DOI] [PubMed] [Google Scholar]
- Ham R. G. Dermal fibroblasts. Methods Cell Biol. 1980;21A:255–276. [PubMed] [Google Scholar]
- Hilberg F., Stocking C., Ostertag W., Grez M. Functional analysis of a retroviral host-range mutant: altered long terminal repeat sequences allow expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5232–5236. doi: 10.1073/pnas.84.15.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D., Osborne W. R. Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood. 1989 Aug 1;74(2):876–881. [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Stewart C. L., Harbers K., Löhler J., Simon I., Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Garcia J. V., Osborne W. R., Miller A. D. Expression of human adenosine deaminase in mice after transplantation of genetically-modified bone marrow. Blood. 1990 Apr 15;75(8):1733–1741. [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Progress toward human gene therapy. Blood. 1990 Jul 15;76(2):271–278. [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Osborne W. R., Hock R. A., Kaleko M., Miller A. D. Long-term expression of human adenosine deaminase in mice after transplantation of bone marrow infected with amphotropic retroviral vectors. Hum Gene Ther. 1990 Spring;1(1):31–41. doi: 10.1089/hum.1990.1.1-31. [DOI] [PubMed] [Google Scholar]
- Osborne W. R. Human red cell purine nucleoside phosphorylase. Purification by biospecific affinity chromatography and physical properties. J Biol Chem. 1980 Aug 10;255(15):7089–7092. [PubMed] [Google Scholar]
- Osborne W. R., Miller A. D. Design of vectors for efficient expression of human purine nucleoside phosphorylase in skin fibroblasts from enzyme-deficient humans. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6851–6855. doi: 10.1073/pnas.85.18.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Spencer N. Partial purification and properties of the common inherited forms of adenosine deaminase from human erythrocytes. Biochem J. 1973 May;133(1):117–123. doi: 10.1042/bj1330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. D., Hock R. A., Osborne W. R., Miller A. D. Efficient retrovirus-mediated transfer and expression of a human adenosine deaminase gene in diploid skin fibroblasts from an adenosine deaminase-deficient human. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1055–1059. doi: 10.1073/pnas.84.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. D., Thompson A. R., Miller A. D. Production of human factor IX in animals by genetically modified skin fibroblasts: potential therapy for hemophilia B. Blood. 1989 Feb;73(2):438–445. [PubMed] [Google Scholar]
- Rosenberg M. B., Friedmann T., Robertson R. C., Tuszynski M., Wolff J. A., Breakefield X. O., Gage F. H. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1988 Dec 16;242(4885):1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- Selden R. F., Skośkiewicz M. J., Russell P. S., Goodman H. M. Regulation of insulin-gene expression. Implications for gene therapy. N Engl J Med. 1987 Oct 22;317(17):1067–1076. doi: 10.1056/NEJM198710223171706. [DOI] [PubMed] [Google Scholar]
- St Louis D., Verma I. M. An alternative approach to somatic cell gene therapy. Proc Natl Acad Sci U S A. 1988 May;85(9):3150–3154. doi: 10.1073/pnas.85.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., Barklis E., Ostertag W., Jaenisch R. Two distinct sequence elements mediate retroviral gene expression in embryonal carcinoma cells. J Virol. 1987 Sep;61(9):2742–2746. doi: 10.1128/jvi.61.9.2742-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Fisher L. J., Xu L., Jinnah H. A., Langlais P. J., Iuvone P. M., O'Malley K. L., Rosenberg M. B., Shimohama S., Friedmann T. Grafting fibroblasts genetically modified to produce L-dopa in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9011–9014. doi: 10.1073/pnas.86.22.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannas I. V., Burke J. F., Orgill D. P., Skrabut E. M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982 Jan 8;215(4529):174–176. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- ZIEVERINK W. D., MOLONEY W. C. USE OF Y CHROMOSOME IN THE WISTAR/FURTH RAT AS A CELLULAR MARKER. Proc Soc Exp Biol Med. 1965 Jun;119:370–373. doi: 10.3181/00379727-119-30184. [DOI] [PubMed] [Google Scholar]