Abstract
The superior orbital fissure syndrome (SOFS) is an uncommon complication rarely occurring in association with craniofacial trauma. Work-up of a patient injured by a traumatic right orbitozygomatic complex fracture and SOFS is presented. Accurate computed tomography scan and three-dimensional reconstruction showed a medial displacement of the lateral orbital wall, compressing the right superior orbital fissure (SOF), without intraorbital bone fragment displacement or hemorrhage. Imaging also revealed a frontosphenotemporal fracture, according to Pellerin et al, that is, frequently associated with visual impairment. Our primary choice of therapy was a corticosteroid treatment in association with an early surgical approach. It consisted in en bloc reduction and osteosynthesis of the fracture through a bicoronal approach, recovering SOF size. A prompt and almost complete recovery of the abducens movement, without diplopia, was achieved in 1 week. The authors discuss indications and management of SOFS. The presence of fractures should urgently lead to surgery. We deny waiting for a medical treatment result, while preferring the prompt reduction of the fractures and extrication of the soft tissues. The main focus of this study is on patient's anatomical feature and fracture patterns.
Keywords: superior orbital fissure syndrome, lateral orbital wall fracture, frontosphenotemporal fracture
Superior orbital fissure syndrome (SOFS) is a clinical syndrome first described by Hirschfield in 1858.1 It leads to a compression on the structures passing through the superior orbital fissure (SOF), especially on cranial nerves III, IV, VI, and V1 with its three branches: frontal, lacrimal, and nasociliary. This syndrome could be both complete and incomplete, depending on the number and the degree of distress of the structures involved. Clinical manifestation includes a variable combination of ophthalmoplegia, ptosis, proptosis, fixed and dilated pupil, hypo/anesthesia of the upper eyelid and forehead, and loss of corneal reflex.2 3 4 5 6 The optic nerve could be also involved, designating the orbital apex syndrome, as described by Kjaer in 1945.7 The SOFS etiology includes trauma, neoplasms, hemorrhage, vascular malformations, inflammation, or infectious process involving the retrobulbar space. A case of traumatic SOFS is described; interestingly, an accurate computed tomography (CT) scan analysis showed a particular fracture pattern frequently associated with visual impairment8 and excluded intraorbital lesions. The surgical treatment strategy has been oriented to re-establish the right SOF size without intraorbital intervention or bone disruption.
Case Report
A 75-year-old man came to our hospital following an injury to the right orbitozygomatic complex, reported as a domestic accident. He was confused about the incident and could not remember the event. At initial presentation, he complained about a sharp pain in the orbitozygomatic area without visual impairment and clinical exams reported a right periorbital hematoma. A basal craniomaxillofacial CT scan, without multiplanar reconstructions, revealed a right frontal–temporal skull fracture with ipsilateral involvement of the orbital roof, a fracture of the right orbital floor, a fracture of the posterior–lateral and anterior walls of the right maxillary sinus, and a fracture of the right zygomatic arch. All these fractures had subtle displacement. Consequently, according to both the minor clinical impact, the age of the patient and the minimal displacement of the fractures, surgical indications were not given while hospitalization and clinical observation were essential.
Unfortunately, after 12 hours, ocular signs and symptoms changed. While there was no proptosis, both pupils were equal in size and reactive to light, with intact accommodation and color vision; in contrast, a right ptosis became appreciable and ocular abduction was completely absent in the right eye, whereas there were normal movement functions in the left eye (Fig. 1).
Fig. 1.
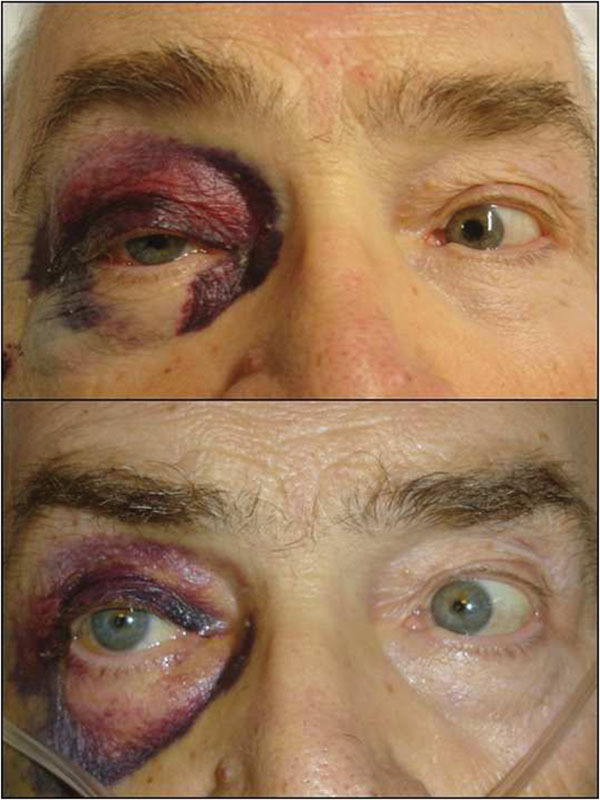
Up: Preoperatively, ptosis and ophthalmoplegia gazing to the right; down: 1 week postoperatively, almost complete recovery gazing to the right.
The patient was stable and there was no evidence of optic nerve involvement, thus identifying a provisional diagnosis of traumatic SOFS. According to the literature9 and to provide a better chance of neurologic recovery, the administration of intravenous (IV) steroids began (30 mg/kg IV loading dose of methylprednisolone followed by 15 mg/kg every 6 hours for 3 days). Pupillary reactions were checked throughout the night, to early identify an eventual ongoing relative afferent pupillary defect.
A new spiral craniomaxillofacial CT scan with coronal, sagittal, and three-dimensional (3D) reconstruction was performed the following morning. The imaging examination revealed a displacement of the lateral wall of the right SOF which was reduced in amplitude. The fracture was classified as a rare frontosphenotemporal fracture, according to Pellerin et al,8 that is frequently associated with visual impairment (Fig. 2). This pattern of fracture is characterized by the preservation of integrity of the lateral orbital wall and its whole dislocation. A thin layer of epidural blood of approximately 7 mm at the middle temporal fossa was also noticed, adjacent to the greater wing of the sphenoid bone.
Fig. 2.
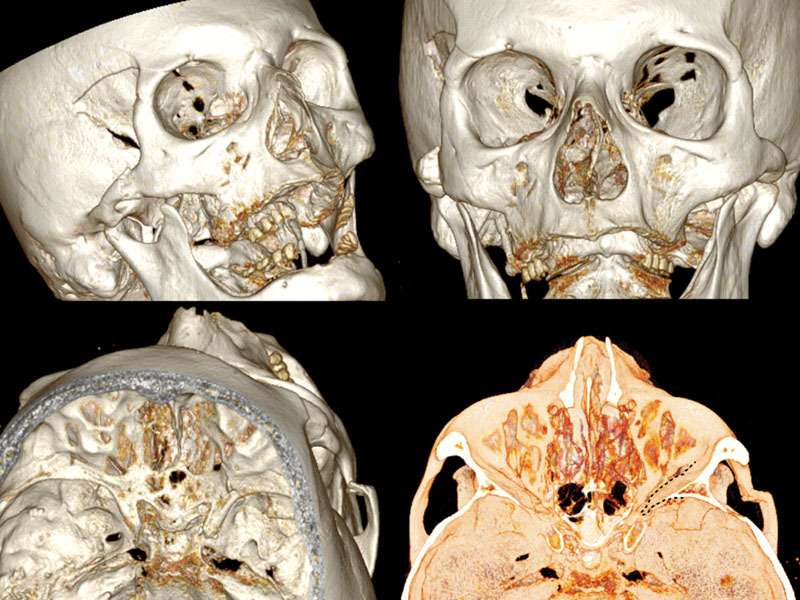
3D CT scan reconstruction showing four different projections of frontosphenotemporal Pellerin et al fracture pattern; note in frontal view (up right), SOF size reduction caused by medial displacement of the entire right lateral orbital wall; the black dashed line in the intracranial view (down right) shows the medial collapse of lateral orbital wall into the SOF. CT, computed tomography; SOF, superior orbital fissure; 3D, three-dimensional.
After 24 hours of steroid treatment, the ocular movements of the right eye were still absent, thus diagnosing a neuropraxia of the SOF nerves due to bone compression. In addition, neurosurgical evaluation of the epidural hematoma excluded the need for neurosurgical intervention.
After 72 hours without any clinical improvement despite the steroid treatment, the patient underwent surgery. A bicoronal flap was harvested and only smooth exploration of the orbital roof and of the lateral orbital wall up to the SOF was performed, to exclude both undetected bone fragments displaced and subtle subperiosteal hematomas. The right temporalis muscle was completely dissected to achieve the complete exposure of the infratemporal fossa to master the frontotemporal skull portion of the fracture and an open standard Gillies elevation with Volkmann Bone Hook (Sklar Surgical Instruments, West Chester, PA) was performed, followed by internal rigid fixation at the right zygomatic arch and at the frontal–temporal buttresses (Fig. 3).
Fig. 3.
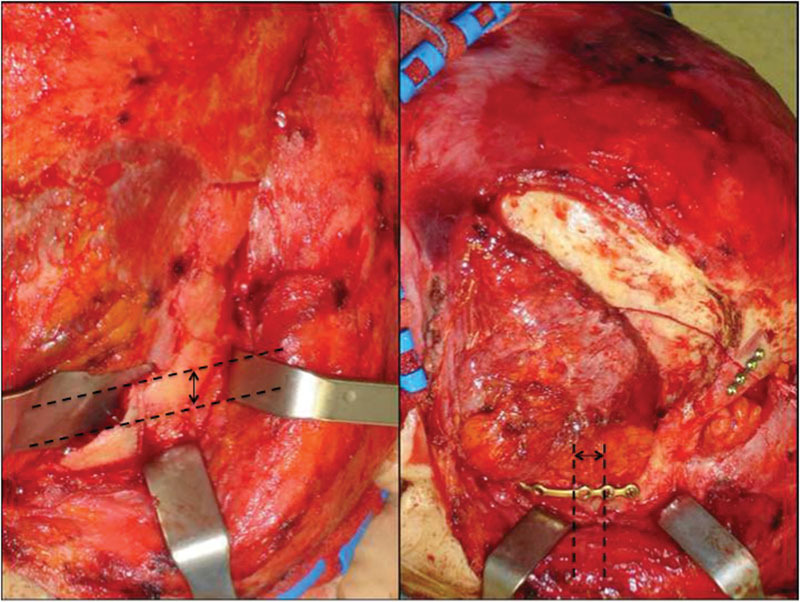
Left: Intraoperative view before reduction; right: Intraoperative view after reduction and rigid internal fixation. Black arrow shows displacement entity.
The postsurgical CT scan revealed a full recovery of the right SOF size (Fig. 4). One week postoperatively, physical examination showed a great improvement of all symptoms, a prompt and almost complete recovery of the abducens movement, without diplopia (Fig. 1). In 1 month, the patient completely healed.
Fig. 4.
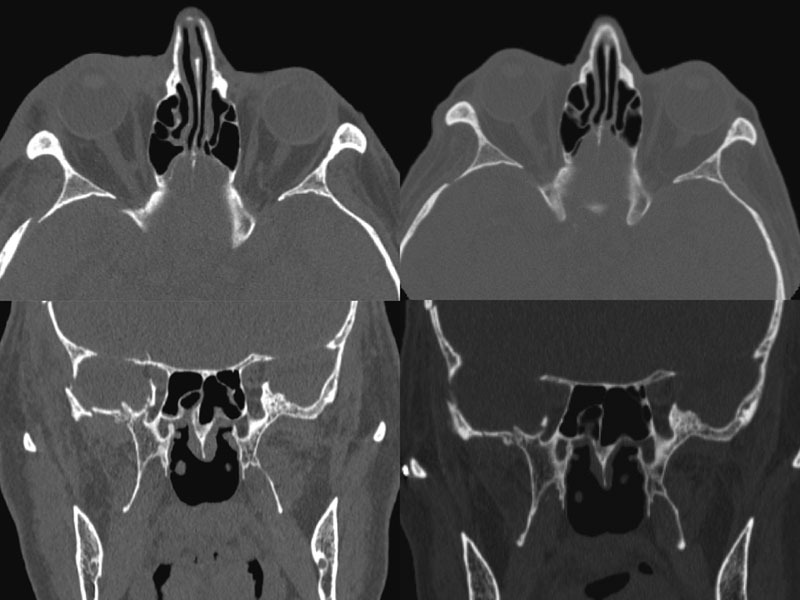
Left: Axial and coronal preoperative CT scan: note SOF size reduction; right: Axial and coronal postoperative CT scan: note SOF size recovery. CT, computed tomography; SOF, superior orbital fissure.
Discussion
The SOF is a cleft between the greater and the lesser wing of the sphenoid bone, representing an important area of connection between the middle cranial fossa and the orbit.
Numerous structures pass through the SOF: the oculomotor (III) and trochlear nerves (IV), the ophthalmic division of the trigeminal nerve (VI) with its frontal, lacrimal, and nasociliary branch, the abducens nerve (VI), and both the ophthalmic veins, superior and inferior.
Some authors10 recognize two compartments, as a topographical division: a superior or superolateral part, which includes the trochlear, lacrimal, and frontal nerves and the superior ophthalmic vein, and an inferior or inferomedial part, which includes the superior and inferior branches of the oculomotor nerve, the nasociliary nerve, the abducens nerve, and both the sensory and the sympathetic root of the ciliary ganglion. The inferior ophthalmic vein localization is variable, occasionally passing through the tendinous annulus of Zinn.
Other authors11 observe three separate compartments, identifying a lateral, medial, and inferior portion. The lateral one is composed of the narrowest part of the superolateral fissure which contains the trochlear nerve, the frontal nerve, the lacrimal nerve, and the superior ophthalmic vein. The medial portion is constituted by the tendinous annulus of Zinn, and it also contains the superior and inferior branches of the oculomotor nerve as well as the nasociliary nerve, the abducens nerve, and the roots of the ganglion. The inferior portion lies below the tendinous annulus and is mainly filled with adipose tissue; the inferior ophthalmic vein is found here, when it is present.10 11 12
The key point is that the location of the contents within the fissure is fairly constant, despite the descriptive anatomical subdivision. The superior branch of the oculomotor nerve is the structure closest to the medial rim of the fissure; the trochlear nerve is the closest to the superior rim, and the abducens nerve is the closest to the inferior rim13 (Fig. 5). Moreover, the VI nerve passes through the common tendinous ring and has a long intracranial course. All these aforementioned aspects usually lead to a major severity of damage to abducens nerve.14
Fig. 5.
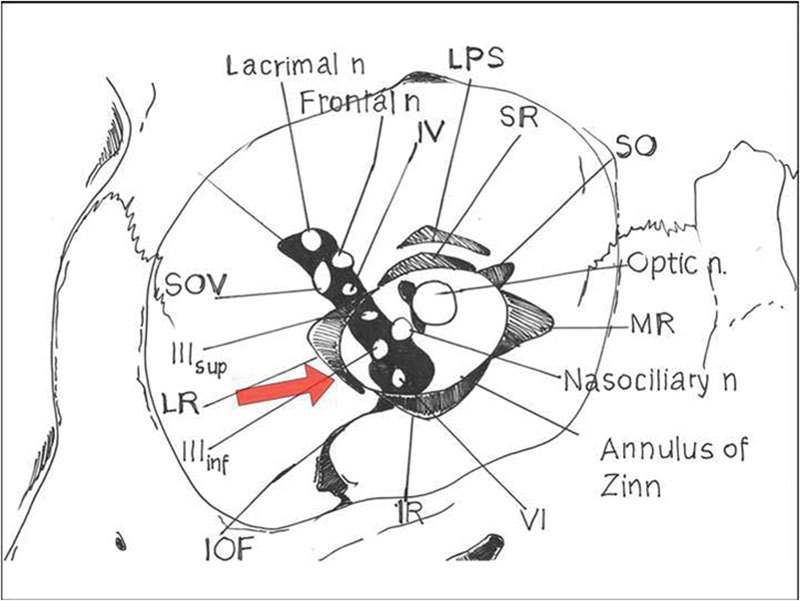
SOF and topographic positioning of its content. The red arrow indicates the direction of displacement of the lateral orbital wall, with consequent distortion of annulus of Zinn insertion and impingement on abducens nerve. IOF, inferior orbital fissure; IIIinf, oculomotor inferior branch; IIIsup, oculomotor superior branch; IR, inferior rectus; LPS, levator palpebrae superioris; LR, lateral rectus; MR, medial rectus; n, nerve; SO, superior oblique; SOF, superior orbital fissure; SOV, superior ophthalmic vein; SR, superior rectus; VI, abducens.
The adult size of the SOF is roughly 22 mm in length, 2 to 3 mm in width at the apex and 7 to 8 mm at the base.12 Concerning the shape, generally, there are only two morphologically different types of SOF (Fig. 6). Type A has a noticeable narrowing, mainly caused by a bulge on the inferior rim; type B has a smooth outline without any noticeable narrowing. Moreover, type A has a significant greater length, while the maximum width of the fissure has small and irrelevant differences between the two types. As a consequence, the area of type B fissure is significantly smaller than the area of the type A fissure.12 In addition, being more crowded, type B is probably more inclined to SOFS in the case of size reduction, with minor chances of spontaneous healing, by spatial rearrangement of the structures involved and/or by edema reabsorption. As a general rule, the shape of the fissure remains the same on both sides of the skull.12
Fig. 6.
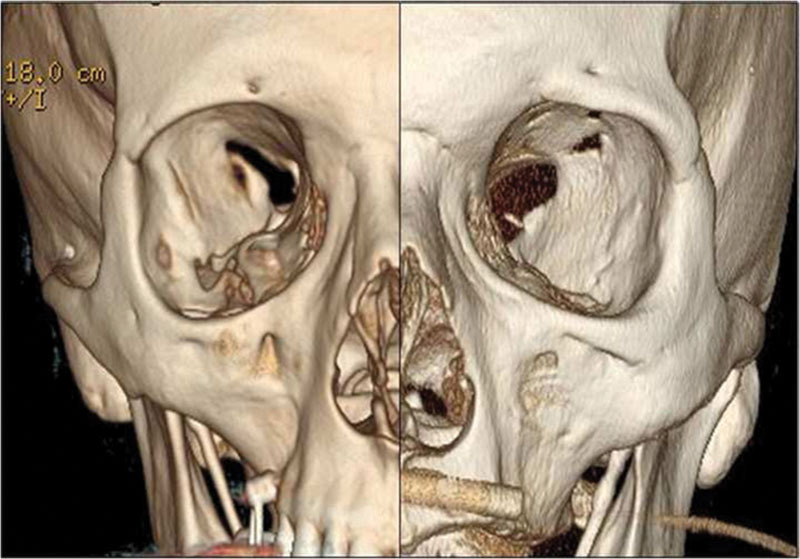
3D CT scan reconstruction showing a nonfractured type B SOF on the left and a type A SOF on the right. CT, computed tomography; SOF, superior orbital fissure; 3D, three-dimensional.
In the setting of traumas, a CT scan of brain and orbits is helpful either to identify bone dislocation, with SOF and orbital apex fractures, or to evaluate eventual nerve impingement due to hematoma or hemorrhage. Spiral CT scans reduce imaging time and permit coronal and sagittal reconstructions, as well as those in 3D, being helpful in identifying the cause of a SOFS or an orbital apex syndrome clinically diagnosticated and being, therefore, essential for surgical planning.15 16 17 A recent study also confirms the accuracy of CT scan on the width measurement, comparing values obtained on the horizontal plane including the optic canal to the real width on cadavers.18 Consequently, we recommend the use of spiral CT scan to correctly valuate the whole orbital structure in each plan.
The incidence of SOFS has historically been less than 1%, as confirmed by a recent review at 0.33%.9 The causes of SOFS could be classified as traumatic (craniofacial fractures, intracranial hypertension caused by an epidural or subdural hematoma, carotid-cavernous sinus fistula, or retrobulbar and subperiosteal hematomas) and nontraumatic (syphilis, paranasal sinus infections, or tumors), as described by Llorente Pendás and Albertos Castro.19 Furthermore, in the case of traumatic SOFS, the lesion mechanism can be direct (nerves traversing the fissure are interrupted or compressed by displaced bone fragments) or indirect (the orbital walls behave like a nonexpandable box, determining that every increase in internal pressure, caused either by posterior displacement of the eyeball at the moment of the injury or later by edema and bleeding, may compress the nerves against the rims of the fissure). Consequently, in traumatic SOFS, the limitation of ocular motility may be related to either bone impingement on extraocular muscles, causing an actual mechanical restriction of movement, or to a cranial nerve palsy.
In our case, the spiral CT scan permitted to identify a size reduction of the ipsilateral SOF and a contralateral type B fissure. The associated clinical presentation of right ptosis and complete absence of ocular movements in the right eye led to the diagnosis of right SOFS, with a traumatic physiopathological mechanism. The VI nerve was the most severely injured.
Management of traumatic SOFS is still controversial. Restriction of movement due to edema or hematoma could spontaneously improve, thus conservative management and close observation without surgical intervention has historically been proposed.1 4 20 21 22 23 Literature review suggests that megadoses corticosteroid treatment may be helpful and successful when there is no evidence of bone dislocation22 24 25 and that patients treated with steroids have a better chance of neurologic recovery than those with observation alone.9 Medical management followed is 30 mg/kg IV loading dose of methylprednisolone followed by 15 mg/kg every 6 hours for 3 days.9 On the contrary, the restriction of movements caused by tethering of either the extraocular muscles or their associated network of fat and fibrous septa by fracture fragments necessitates the reduction of the fractures, the extrication of the soft tissues, and the restoration of the pretraumatic SOF size.24 26
Additional randomized clinical trials are necessary to further understand the role of steroids in the treatment of SOFS in case of slight or nondisplaced fractures. Nonetheless, previous data seem to support that surgical decompression may be beneficial to those patients with evident compression of the SOF.24
Through our extensive experience of more than 20 years in a major trauma hospital, despite the rarity of this syndrome, a prompt surgical intervention seems to provide a better and early chance of functional recovery. As a matter of fact, in case of fractures, the limitation of ocular motility is most likely a result of both mechanical (restrictive) and neurologic (paralytic) causes. Prompt surgical anatomical reduction should solve mechanical hindrance and symptoms immediately improve. Subsequently, the full recovery may be obtained in a few days or weeks, leading to the whole neurological restoration. Corticosteroid treatment should speed up the healing process, lasting few weeks instead of months, hastening the return of nerve function. On the contrary, mechanical ratio might partially persist with a sole conservative approach.
In this paradigmatic case, the early treatment consisted in a simplified surgical approach, to achieve the full recovery of the right SOF size, by fracture reduction and internal rigid fixation, without intraorbital intervention or bone disruption. A brisk and almost complete recovery of the abducens movement, without diplopia, was shown in a week and full healing in a month.
As a consequence, megadose corticosteroid treatment in association with a prompt surgical approach reduces hospitalization and gives a more rapid clinical resolution. Moreover, this kind of surgery requires a simple and quick access, thus representing a primary choice of therapy. We deny waiting for months a recovery that is likely obtained in a more rapid way and with a better chance of functional resolution.
In case of impacted lateral orbital wall fractures, Stanley et al have previously classified those into four different types according to the pattern of its involvement.27 Furthermore, in case of dislocation of the sphenotemporal buttress, as in the Pellerin et al fracture pattern,8 there is a distortion of the muscular insertions on the tendinous annulus of Zinn and a SOF size reduction, with a multiple cranial nerve palsy. In addition, all these aforementioned fracture patterns have been noted to have a high incidence of associated ophthalmologic injuries, ranging from ocular dysmotility, which improve with fracture reduction, to globe rupture, requiring ocular enucleation.27
As for impacted lateral wall fractures,27 in our case, the surgical management has provided a rapid return to excellent movement function by eliminating mechanical restrictions and hastening the return of nerve function.
In this perspective, the Pellerin et al pattern fracture appears to be legitimately classified as an impacted lateral orbital wall fracture. Hence, the authors propose to introduce a fifth type in Stanley et al's impacted lateral orbital wall fracture classification (Fig. 7). This new classification could assist surgeons in both recalling and recognizing this rare pattern of fracture, to provide a prompt surgical treatment.
Fig. 7.
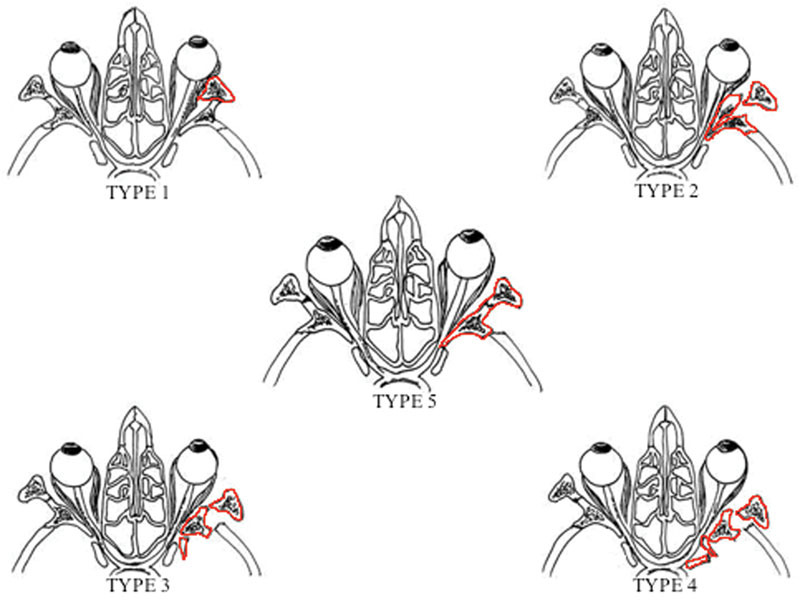
Modified Stanley et al's lateral orbital wall fracture classification introducing a fifth type of fracture pattern according to Pellerin et al. Bony fragments are shown in red.
Conclusion
Management of traumatic SOFS is still controversial. Our proposal is to adopt conservative management with megadoses of corticosteroids in the absence of displaced fractures, steroid treatment with 30 mg/kg IV loading dose of methylprednisolone, followed by 15 mg/kg every 6 hours for 3 days and gradual reduction.
The presence of fractures, instead, should urgently lead to surgery. We deny waiting for a medical treatment result, while preferring the prompt reduction of the fractures and extrication of the soft tissues. Steroid treatment should be mastered both at the same time of surgery and following it, with a dose of 15 mg/kg every 6 hours for 3 days and a gradual reduction.
Spiral CT scans have to be considered the standard approach on the recognition of SOFS, due to the possibility of coronal, sagittal, and 3D reconstruction. As a matter of fact, this multiplanar approach could recognize contralateral type B SOF, suggesting a prompt surgical approach.
Impacted lateral orbital wall fractures have a high incidence of associated ophthalmologic injuries, and in these fracture patterns, from type I to type V as here proposed, prompt surgical intervention may have a positive influence in selected patients on the recovery of visual function.
Acknowledgments
The authors declare that they have not received any source of financial support for the work being published.
References
- 1.Hirschfield L. Épanchement de sang dans le sinus caverneux du côté gauche diagnostiqué pendant la vie. CR Soc Biol. 1858;10:138–139. [Google Scholar]
- 2.Bowerman J E. The superior orbital fissure syndrome complicating fractures of the facial skeleton. Br J Oral Surg. 1969;7(1):1–6. doi: 10.1016/s0007-117x(69)80053-3. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson J W. An unusual skull fracture. Br J Oral Surg. 1974;12(2):244–245. doi: 10.1016/0007-117x(74)90133-4. [DOI] [PubMed] [Google Scholar]
- 4.Pogrel M A. The superior orbital fissure syndrome: report of case. J Oral Surg. 1980;38(3):215–217. [PubMed] [Google Scholar]
- 5.Ghobrial W, Amstutz S, Mathog R H. Fractures of the sphenoid bone. Head Neck Surg. 1986;8(6):447–455. doi: 10.1002/hed.2890080609. [DOI] [PubMed] [Google Scholar]
- 6.Hedstrom J, Parsons J, Maloney P L, Doku H C. Superior orbital fissure syndrome: report of case. J Oral Surg. 1974;32(3):198–201. [PubMed] [Google Scholar]
- 7.Kjaer I. A case of orbital apex syndrome in collateral pansinusitis. Acta Ophthalmol (Copenh) 1945;23:357–366. [Google Scholar]
- 8.Pellerin P, Dhellemmes P, Jomin M, Laine E, Donazzan M. [Frontosphenotemporal fractures : a separate anatomical and clinical entity (author's transl)] Rev Stomatol Chir Maxillofac. 1980;81(4):246–252. [PubMed] [Google Scholar]
- 9.Chen C T, Chen Y R. Traumatic superior orbital fissure syndrome: current management. Craniomaxillofac Trauma Reconstr. 2010;3(1):9–16. doi: 10.1055/s-0030-1249369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergin D J. New York: Raven Press; 1987. Anatomy of the eyelids, lacrimal system and orbit; pp. 46–47. [Google Scholar]
- 11.Natori Y, Rhoton A L Jr. Microsurgical anatomy of the superior orbital fissure. Neurosurgery. 1995;36(4):762–775. doi: 10.1227/00006123-199504000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Reymond J, Kwiatkowski J, Wysocki J. Clinical anatomy of the superior orbital fissure and the orbital apex. J Craniomaxillofac Surg. 2008;36(6):346–353. doi: 10.1016/j.jcms.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Govsa F, Kayalioglu G, Erturk M, Ozgur T. The superior orbital fissure and its contents. Surg Radiol Anat. 1999;21(3):181–185. doi: 10.1007/BF01630898. [DOI] [PubMed] [Google Scholar]
- 14.Chen C T, Wang T Y, Tsay P K, Huang F, Lai J P, Chen Y R. Traumatic superior orbital fissure syndrome: assessment of cranial nerve recovery in 33 cases. Plast Reconstr Surg. 2010;126(1):205–212. doi: 10.1097/PRS.0b013e3181dab658. [DOI] [PubMed] [Google Scholar]
- 15.Suojanen J N, Mukherji S K, Dupuy D E, Takahashi J H, Costello P. Spiral CT in evaluation of head and neck lesions: work in progress. Radiology. 1992;183(1):281–283. doi: 10.1148/radiology.183.1.1549687. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman R A, Gusnard D A, Bilaniuk L T. Pediatric craniocervical spiral CT. Neuroradiology. 1992;34(2):112–116. doi: 10.1007/BF00588155. [DOI] [PubMed] [Google Scholar]
- 17.Seiff S R, Berger M S, Guyon J, Pitts L H. Computed tomographic evaluation of the optic canal in sudden traumatic blindness. Am J Ophthalmol. 1984;98(6):751–755. doi: 10.1016/0002-9394(84)90693-7. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara T, Matsuda K, Kubo T, Tomita K, Yano K, Hosokawa K. Superior orbital fissure syndrome after repair of maxillary and naso-orbito-ethmoid fractures: a case study. J Plast Reconstr Aesthet Surg. 2009;62(12):e565–e569. doi: 10.1016/j.bjps.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 19.Llorente Pendás S, Albertos Castro J M. Traumatic superior orbital fissure syndrome: report of case. J Oral Maxillofac Surg. 1995;53(8):934–936. doi: 10.1016/0278-2391(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 20.Sieverink N P, van der Wal K G. Superior orbital fissure syndrome in a 7-year-old boy. Int J Oral Surg. 1980;9(3):216–220. doi: 10.1016/s0300-9785(80)80021-4. [DOI] [PubMed] [Google Scholar]
- 21.Zachariades N. The superior orbital fissure syndrome. Review of the literature and report of a case. Oral Surg Oral Med Oral Pathol. 1982;53(3):237–240. doi: 10.1016/0030-4220(82)90296-1. [DOI] [PubMed] [Google Scholar]
- 22.Rowe N L. Edinburg: Churchill Livingstone; 1985. Fractures of the zygomatic complex and orbit; pp. 530–531. [Google Scholar]
- 23.Menard P, Foussadier F, Ricbourg B. Syndrome de la fente sphénoïdale au cours des fractures de l'orbite. Présentation d'un cas et revue de la littérature. Rev Stomatol Chir Maxillofac. 1991;92(3):193–198. [PubMed] [Google Scholar]
- 24.Postma M P, Seldomridge G W, Vines F S. Superior orbital fissure syndrome and bilateral internal carotid pseudoaneurysms. J Oral Maxillofac Surg. 1990;48(5):503–508. doi: 10.1016/0278-2391(90)90241-s. [DOI] [PubMed] [Google Scholar]
- 25.Acartürk S, Seküçoğlu T, Kesiktäs E. Mega dose corticosteroid treatment for traumatic superior orbital fissure and orbital apex syndromes. Ann Plast Surg. 2004;53(1):60–64. doi: 10.1097/01.sap.0000106424.54415.dc. [DOI] [PubMed] [Google Scholar]
- 26.Koornneef L. Current concepts on the management of orbital blow-out fractures. Ann Plast Surg. 1982;9(3):185–200. doi: 10.1097/00000637-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Stanley R B Jr, Sires B S, Funk G F, Nerad J A. Management of displaced lateral orbital wall fractures associated with visual and ocular motility disturbances. Plast Reconstr Surg. 1998;102(4):972–979. doi: 10.1097/00006534-199809040-00006. [DOI] [PubMed] [Google Scholar]


