Abstract
Cytolytic T cells eliminate infected or cancer cells by recognizing peptides presented by MHC class I molecules on the cell surface. The antigenic peptides are derived primarily from newly synthesized proteins including those produced by cryptic translation mechanisms. Previous studies have shown that cryptic translation can be initiated by distinct mechanisms at non-AUG codons, in addition to conventional translation initiated at the canonical AUG start codon. Here, we show that presentation of endogenously translated cryptic peptides is enhanced by Toll-like receptor signaling pathways involved in pathogen recognition as well as by infection with different viruses. This enhancement of cryptic peptides was caused by pro-inflammatory cytokines, secreted in response to microbial infection. Furthermore, blocking these cytokines abrogated the enhancement of cryptic peptide presentation in response to infection. Thus presentation of cryptic peptides is selectively enhanced during inflammation and infection, which could allow the immune system to detect intracellular pathogens that might otherwise escape detection due to inhibition of conventional host translation mechanisms.
Introduction
On the cell surface, a diverse set of peptides is presented by Major Histocompatibility Complex (MHC) class I molecules (1–3). Cytotoxic CD8+ T cells of the immune system can bind these peptide-MHC complexes and trigger an immune response by recognizing non-self antigenic peptides. Peptides that are presented on MHC class I molecules to CD8+ T cells are derived mostly from endogenous sources - degradation of endogenously synthesized proteins or newly synthesized proteins. Apart from some viral proteins that are resistant to degradation, all endogenously synthesized proteins contribute to the antigenic peptide repertoire (3). This peptide repertoire is known to arise largely from newly synthesized proteins which allows early viral proteins to be detected regardless of their stability (4). Additionally, proteins undergoing turn-over would also contribute to the peptide display (5). The newly synthesized polypeptides that are targeted for degradation are known as defective ribosomal products or DRiPs, which are known to couple protein synthesis to the MHC class I presentation pathway (6). Some of the endogenously generated peptides in the MHC class I pathway can also originate from sources other than translation of the primary open reading frame. These sources are termed “cryptic” because their origin was unknown (7). The cryptic antigenic peptides contribute to the diversity of the peptide repertoire presented on the cell surface making the process of immune surveillance more effective.
Cryptically translated antigenic peptides can arise from several different sources. Some of these sources include alternative reading frames of an mRNA transcript (3, 8), read-through of stop codons into the 3′UTR (9) and ribosomal frameshifting (10, 11). Yet another source from which cryptic peptides arise is the use of a non-AUG initiation codon to initiate protein translation (12, 13).
Cryptic translation of either viral or endogenous mRNAs can give rise to cryptic pMHC (14). Cryptic peptides arising from the alternative reading frames (ARFs) of HIV and other retroviral ARFs and their role in protective immunity have been well characterized (15, 16). Some of these T-cell responses to cryptic peptides arising from HIV were shown to be necessary to control viral load in human HIV-infected patients (17). Furthermore, cryptic peptides arising from adenoviral vectors, used in a gene therapy trial, were also shown to elicit abnormal T-cell responses (18). In addition to virally induced cryptic peptides, there are several examples of cryptic peptides arising from endogenous sources. T-cell responses to cryptic epitopes arising from proteins AIM2 and NA17-A in melanoma patients were shown to be used for immune-monitoring (19). Furthermore, cryptic CD8+ T-cell epitopes from the VEGF gene were shown to arise through alternative initiation from a CUG codon (20). Given that all cell types are capable of presenting cryptic peptides (21), suggests that cryptic translation is a wide-spread phenomenon.
Since its initial discovery, the mechanism of CUG-initiated translation has been shown to be distinct from conventional AUG-initiated translation. A subset of ribosomes scans specifically for an alternate CUG initiation codon, which was unexpectedly found to be decoded as leucine rather than the canonical methionine residue (12, 13, 21). Furthermore, a novel initiator tRNA was found to be present at CUG initiation codons, which decoded CUG as a leucine (22). Perhaps due to the distinct mechanism, CUG-initiated translation was found to be resistant to several compounds that inhibited initiation at canonical AUG codons (23). This led to the question of, if other cellular stresses some of which inhibit conventional translation, could regulate presentation of cryptic peptides.
Here, we utilized in-vitro as well as ex-vivo model systems to study whether presentation of cryptic peptides was regulated by physiological stimuli. We found that T-cell responses to the cryptic CUG-initiated peptide were enhanced during viral infections and other inflammatory conditions. This enhancement in presentation of cryptic peptides was mediated by inflammatory cytokines.
Materials and Methods
Mice, cell lines and reagents
WI9.LYL8 transgenic mice have been described elsewhere (21), C57BL/6J and B10.D2 mice were purchased from Jackson Laboratory (Bar Harbor ME). All mouse work was done with the approval of the Animal Care and Use Committee of the University of California, Berkeley. Kb expressing L, Cos7, BCZ103 cell lines have been described before (24, 25), 11p9Z (26), 30NXZ (27), NIH3T3 and BALB 3T3 cells were obtained from American Tissue Culture Collection (ATCC, Manassas, VA.). C57BL/6J immortalized-macrophages were a gift from the laboratory of Prof. Greg Barton (University of California, Berkeley). MCSF-producing cells (3T3-MCSF) were a gift from the laboratory of Prof. Russell Vance (University of California, Berkeley). Toll-like receptor agonists were obtained from InVivogen.
Generation of primary WI9.LYL8 bone-marrow macrophages
Legs were dissected from WI9.LYL8 mice, and the femur and tibia were cleaned off from the surrounding muscle tissue and cleaned in 70 % ethanol. The bones were cut and the marrows were flushed using a 24 1/2 G needle into complete RPMI with 10% fetal bovine serum. The suspended marrows were then filtered through a 0.4 μm mesh filter. The bone marrow cells were re-suspended in 1mL red-blood cell lysis buffer for 1 minute and washed with complete RPMI media. Cells were then counted and plated into sterile petri dishes at 5–6 million cells per dish in special media with 20 % FBS and 20 % MCSF (macrophage colony stimulating factor).
mRNA transfection
mRNA was generated using the mMessage mMachine T7 Transcription kit (Ambion). Plasmid DNA was linearized with HpaI and used as templates for transcription by T7 RNA polymerase (mMessage mMachine T7; Ambion). Transcription reactions contained m7GTP or ARCA m7GTP cap analog (Ambion) to yield naturally capped mRNAs. The Poly(A) Tailing Kit (Ambion) was used to add poly(A) tails onto mRNAs. Transcribed mRNAs were purified using phenol: CHCl3 per the manufacturer’s recommendation followed by purification using illustra MicroSpin G-25 columns (GE Healthcare Life Sciences). In vitro transcribed mRNAs were stored in water at −80C. The TransMessenger Transfection Reagent (Qiagen) was used for transfection of mRNA into cells. For each μg of mRNA, a mix of 16.5 μL of Enhancer and up to 100μL Buffer EC was made and incubated for 5 minutes at room temperature. 33μL of TransMessenger Reagent was added and incubated for 10 minutes at room temperature. 900μL of serum-free media was added to the mix and added on to the cells. After 4 hours, media on cells was replaced with complete-RPMI with or without various Toll-like receptor agonists.
T-cell hybridoma assays
T-cell hybrids are LacZ inducible and LacZ activity is measured in the T-cell hybridoma assays. After co-culture of antigen-presenting cells and T-cell hybridoma cells for 18 hours in a 96-well flat bottom plate, 100μL of CPRG (Chlorophenol red-beta-D-galactopyranoside, purchased from Roche Diagnostics) solution is added to each well. Absorbance was measured at dual wavelengths, 595nm and 655 nm as reference.
HPLC fractionation assay
Cos7 cells (150,000–250,000 cells per well of 6 well-plate) were transfected with the different [ATG]-YL8 or [CTG]-YL8, Toll-like receptor 2 and Kb constructs and thereafter stimulated with Pam3CSK4. Transfected cells were re-suspended in 10% acetic acid and boiled at 100 deg. C for 10 minutes. The boiled suspension of cells was then spun down at 10,000 rpm for 15 minutes. The supernatant was transferred to a 10kDa Millipore filter (Ambion) and spun down at 13,000 rpm for 45 minutes. The flow-through was injected into a C18 column and separated by reverse-phase HPLC. A program with a ratio of 80 % Buffer A (0.1% Tri-fluoro acetic acid in water) and 20 % Buffer B (0.1 % Tri-fluoro acetic acid in acetonitrile) was used. 3-drop fractions were collected in a flat-bottom 96-well plate. The plates were dried by spinning overnight in a vacuum-trap based plate dryer. The following day, 50,000 Kb-L cells and 100,000 BCZ103 hybridoma were added to each well of the 96 well plate. The antigenic peptide activity was detected and quantified by the T cell activation assay described above.
Virus infection
Mouse Cytomegalovirus (MCMV) (Smith strain) and influenza virus (Strains WSN33 and PR8) were obtained from the laboratory of Prof. Laurent Coscoy (University of California, Berkeley). For both infections, 1 million bone marrow macrophages were plated into each well of a 6 well plate. The next day, the media on the cells was removed and stored in a 50mL tube as conditioned media. 1 mL of viral supernatant (at MOI of 0.5–1.0) or complete RPMI media was added to the cells for 2 hours. This was then removed and replaced with 1 mL of fresh media and 1mL of the conditioned media. After 6 hours, the macrophages were harvested with cold PBS with 1mM EDTA, and scraped off the plate using cell-lifters. Cells were re-suspended in complete RPMI and a T-cell assay was set up.
Virus preparation
1 million NIH3T3 cells were plated into a T75 flask on the previous night in 10 mLs of complete DMEM media. Media on the cells was replaced with DMEM complete media + MCMV virus at an MOI of 0.1, in a total volume of 4 mLs, for 2 hours. 6 mLs of complete DMEM was added thereafter, and the cells were observed for 4 days. Once a beaded formation of cells began to form, the supernatant from the cells was harvested the next day. To harvest the virus, supernatant from the cells was spun down at 1200 rpm for 5 minutes. Supernatant was then filtered through a 0.45μm filter and stored at −80 deg. C.
Plaque assay
150,000 BALB 3T3 cells were plated into each well of a 6-well plate. Serial dilutions of virus were made in complete DMEM media. Serial dilutions of 1:10, 1:100, 1:1000, 1:10,000 were made by adding 300μL of virus into 2.7 mLs of complete DMEM. 1mL of the dilution was added into each well, and was incubated for 2 hours. After 2 hours, the supernatant was aspirated, and a mixture of Low-melting agarose, MEM + serum, antibiotic, and 40% glucose was added to the cells. On the fifth day, plaques were fixed by adding 10% formaldehyde and visualized by removing agarose and adding crystal violet.
TNF intracellular staining assay
Brefeldin-A (Golgi Plug) was added to the treatment condition after 2 hours of treatment initiation, and left in solution for 4–6 hours. Cells were then harvested in FACS buffer and stained for surface MHC Class I expression. Primary macrophages were treated with FcBlock at a 1:200 concentration in a final volume of 50μL for 30 minutes. Cells were then fixed and permeabilized using CytoFix/CytoPerm solution (100μL per well) for 20 minutes. Cells were then washed with 1X Perm/wash buffer and incubated with anti-TNF antibody (anti-mouse TNF-alpha-PE, Clone: MP6-XT22, eBiosciences) or an Isotype Control antibody (Isotype-PE conjugated, Rat IgG1, Clone: eBRG1, eBiosciences) at a concentration of 1:100 in a final volume of 50μL for 30 minutes – 1hour. Cells were then analyzed on the FC-500.
Co-culture assays with B10.D2 cells
Primary macrophages were prepared from B10.D2 mice, which have the MHC-H2D haplotype. B10.D2 macrophages were first infected with MCMV for 6 hours. Cells were then harvested, washed, and titrated into a 96-well plate or split into a 96-well plate. B6WT primary macrophages were then added to these cells for 6 hours. The supernatant was removed and 100,000 BCZ103 cells were added to the mixture of B10.D2 cells and BCZ103 cells, and a T-cell assay was performed.
UV-inactivated virus
MCMV-GFP (obtained from the laboratory of Dr. Laurent Coscoy) was exposed to UV light inside a sterile hood for 30 minutes. Cells were infected with this supernatant and GFP expression was then tested in these cells by flow cytometry. Supernatant that was exposed to UV did not exhibit any GFP expression in the cells, whilst wild-type MCMV-GFP exhibited GFP production in the cells. Inactivation was also tested by a plaque assay. UV-inactivated MCMV did not produce any plaques in the diluted samples.
TNF and IFNAR-blocking experiments
Primary macrophages were infected with MCMV or left untreated and treated with anti-TNFα antibody (Mouse TNFα Antibody, Monoclonal Rat IgG1, Clone # MP6-XT22, R&D Systems) or an Isotype control antibody (Rat IgG1 Isotype Control, Monoclonal Rat IgG1, Clone # 43414, R&D Systems) for 6 hours. For IFNAR blocking, an anti-IFNAR-1 antibody (LEAF Purified anti-mouse IFNAR-1, Clone MAR1-5A3, BioLegend) or Isotype control (LEAF Purified Mouse IgG1κ Isotype Control, Clone MG1-45, BioLegend) was used. Cells were harvested and T-cell assay was performed. The antibodies (Blocking or Isotype control) were included during the incubation with T-cells. During the incubation with T-cells, anti-TNFα or Isotype antibody was included.
Luciferase assays with L929-ISRE cells
Supernatant from uninfected and infected cells were added to L929-ISRE cells (obtained from Dr. Astar Winoto’s laboratory, UC Berkeley). These cells were then lysed using the reagents of the Promega Luciferase assay. Luciferin reagent was then added to the lysed cells and the absorbance was measured.
Results
Model system to study cryptic translation of antigenic peptides
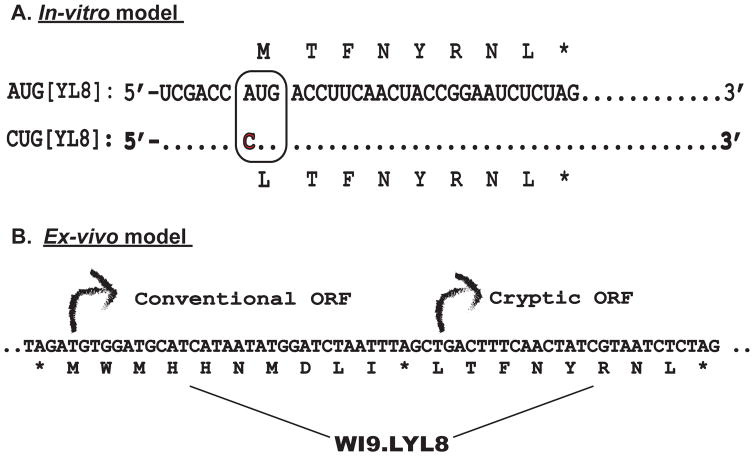
We examined the presentation of cryptic pMHC I using cDNA constructs encoding either the LYL8 (LTFNYRNL) peptide or its analog MYL8 (MTFNYRNL) as a model (Figure 1 top panel). These two constructs differ only in the first amino acid encoded by either the CUG (cryptic) or AUG (conventional) initiation codon. Cells transfected with these constructs decode their CUG or AUG initiation codons as Leu and Met respectively by either cryptic or canonical translation (Schwab et al, 2004). An important feature of these constructs is that a CUG start codon can be decoded into a Methionine amino acid by wobble base pairing. Therefore, the construct starting with a CUG gives rise to a combination of MYL8 and LYL8 peptides (Schwab et al, 2004) (Starck et al, 2008). To obviate transcription and posttranscriptional regulation, mRNA generated from these cDNAs encoding LYL8 or MYL8 were transfected into macrophages, followed by TLR agonist stimulation. The presentation of translated peptides was measured with the BCZ103 T cell hybridoma, which responds to either the LYL8, or the MYL8 peptides bound to the Kb MHC Class I molecule (Malarkannan et al, 1999). Upon activation, the BCZ103 hybridoma produces the reporter lacZ that is measured by the conversion of the CPRG substrate to a red product by its absorbance at 595nm. Therefore, antigen presentation and T-cell activation is used as a read out for the translation levels of these peptides in the cell.
Figure 1. Model systems to study Cryptic Translation.
A. mRNA sequences encoding the MYL8 or [AUG]-YL8 and the LYL8 or [CUG]-YL8 peptides are shown. The sequences differ only at a single nucleotide (A>C) within the boxed initiation codon. B. Bicistronic transgene encoding the WI9 and LYL8 peptides. The conventional [AUG] initiated WI9 is followed by a stop codon and the cryptic [CUG] initiated LYL8 peptide.
In addition to this in-vitro assay, we also used primary cells from a transgenic mouse model, which expresses a bicistronic transgene WI9.LYL8, were obtained (Figure 1 bottom panel) (Schwab et al, 2003). This transgene enables identification of naturally processed peptides that are generated in the conventional versus cryptic context. The transgene encodes two peptides; the first one is a WI9 peptide derived from the Y-chromosome gene Uty, placed in a conventional AUG initiated translational context. The second peptide, LYL8 was placed in a “cryptic” translational context, downstream of the stop codon with CUG as the initiation codon. In this model system, however, the CUG start codon is decoded only as a leucine and gives rise to the LYL8 peptide exclusively (Schwab et al, 2003). Therefore, this transgenic model allows study of conventional and cryptic translation from the same gene. The WI9 peptide is presented on the Db MHC Class I molecule and is detected by the lac Z inducible, 11p9Z T-cell hybridoma, whilst the LYL8 peptide, as described above is presented on the Kb MHC Class I molecule and detected by the BCZ103 T-cell hybridoma.
TLR agonists enhance presentation of cryptic pMHC I
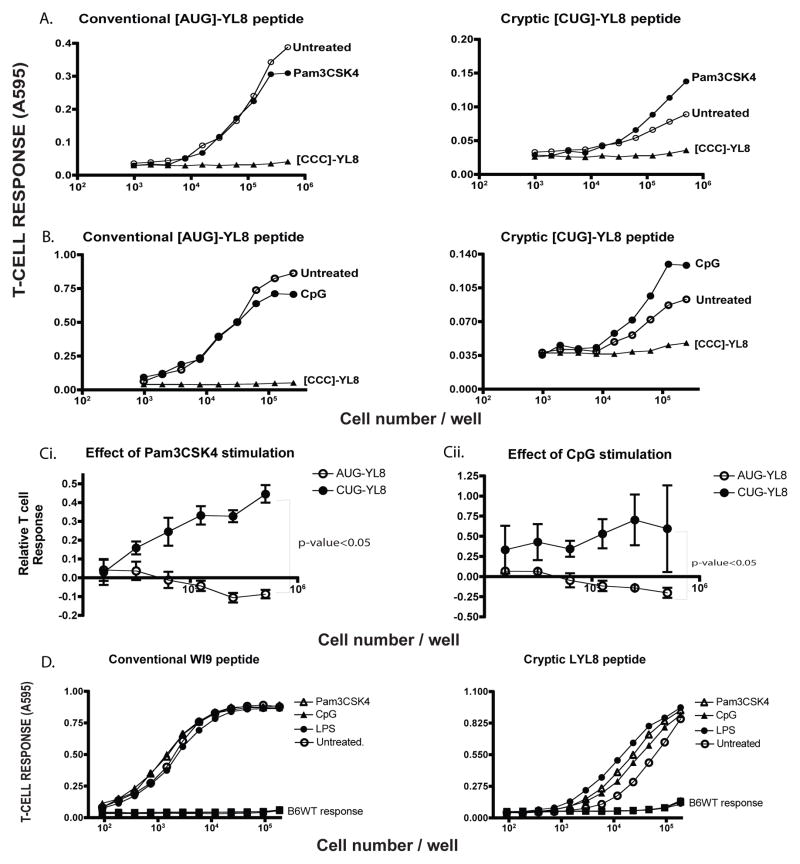
When the mRNA generated from these constructs were transfected into macrophages, followed by treatment with Pam3CSK4 (a bacterial lipoprotein) a TLR2 agonist, the T-cell response to the CUG-initiated peptide, LYL8, was enhanced, compared to the untreated sample (Figure 2A). In contrast, the T cell response to cells transfected with the AUG-initiated peptide MYL8 was not altered by the Pam3CSK treatment. Note that cells transfected with mRNAs of the control construct [CCC]-YL8 did not yield detectable pMHC I because [CCC] is not an effective initiation codon. A similar enhancement in antigen presentation was observed when transfected cells were treated with CpG (unmethylated CpG nucleotides), a TLR9 agonist was used (Figure 2B). To establish statistical significance, T-cell responses to the AUG-YL8 and CUG-YL8 peptide, upon Pam3CSK4 and CpG stimulation, were normalized to that of the untreated cells, averaged across 3 distinct experiments and plotted as a line graph. The trend of each line indicates the effect of the condition (Figure 2C). T-cell responses to the cryptic peptide are significantly enhanced compared to that of the conventional peptide, for which the T-cell responses tend to diminish upon TLR ligand stimulation.
Figure 2. Toll-Like Receptor (TLR) agonists enhance cryptic peptide presentation.
A. The mRNA encoding MYL8 ([AUG]-YL8), LYL8 ([CUG]-YL8) peptides along with the negative control ([CCC]-YL8) were transfected into wild-type immortalized macrophages. After 3 hours, half of the transfected samples were treated with TLR2 agonist - Pam3CSK4 (1μg/mL) for 4 hours. The macrophages were then harvested and cultured with the lacZ inducible, BCZ103 T-cell hybridoma. The response of BCZ103 T-cells, shown on the Y-axis is a measure of the β-galactosidase activity determined by the conversion of the CPRG substrate to a colored product with an absorbance at 595 nm. The cell numbers in each well is indicated on the X-axis. Data is representative of 11 experiments. B. Identical experimental setup as in (A) except TLR9 agonist - CpG was used as the agonist. Y-axis indicates the BCZ103 T-cell response and X-axis indicates the cell number per well. Data is representative of 8 experiments. C. T-cell responses to the AUG-YL8 and CUG-YL8 peptides, upon Pam3CSK4 (i) stimulation or CpG stimulation (ii), were normalized to that of the untreated samples for 3 distinct experiments. An unpaired t-test (with Welch’s correction) was performed, comparing the 2 variables - p-value<0.05. D. Primary macrophages were isolated from the WI9.LYL8 transgenic mice and stimulated with indicated TLR agonists (1 μg/mL) for 6 hours and then cultured with either the T-cell hybridoma 11p9Z specific for the WI9 peptide or the LYL8 specific BCZ103 hybridoma. Macrophages from wild-type mice were used as a negative control. Y-axis indicates the T-cell response (11p9Z specific hybridoma response for conventional peptide and BCZ103 hybridoma response for the cryptic peptide) and the X-axis indicates the cell number per well.
In addition to using immortalized cell-lines in the in-vitro model system, we used primary cells isolated from the WI9.LYL8 transgenic mice to extend these observations to normal cells. Bone-marrow derived macrophages were generated from these transgenic mice and the macrophages were stimulated with these various agonists such as Pam3CSK4, CpG, and LPS. Cells treated with each agonist enhanced the cryptic peptide specific T-cell response robustly, compared to the untreated cells. In contrast, the 11p9Z T-cell response against the AUG-initiated WI9 peptide remained unchanged with or without agonist treatment (Figure 2D). To establish statistical significance, T-cell responses to the WI9 and LYL8 peptide, upon Pam3CSK4, CpG, LPS stimulation, were normalized to that of the untreated cells, averaged across 3 distinct experiments and plotted as a line graph. T-cell responses to TLR-ligand stimulation were significantly enhanced for the LYL8 peptide. T-cell responses to the WI9 peptide were not affected in a significant manner, upon TLR-ligand stimulation (Supplementary Figure S1A). Statistical significance of the enhancement was calculated in a similar way as described for Figure 2C.
Furthermore, to ensure that this was a direct effect of the TLR agonist stimulation and subsequent activation of signaling pathways in the macrophage, TNF-α production was measured by intra-cellular cytokine staining and analysis by flow cytometry. All of the TLR agonist stimulations, led to production in macrophages, showing that this was an effect of macrophage activation (Supplementary Fig S1B).
Therefore, in both the in-vitro model system and the ex-vivo model systems, we found that different TLR agonists enhanced cryptic peptide presentation substantially more than conventional peptide presentation.
TLR agonist enhanced amounts of naturally processed cryptic peptide
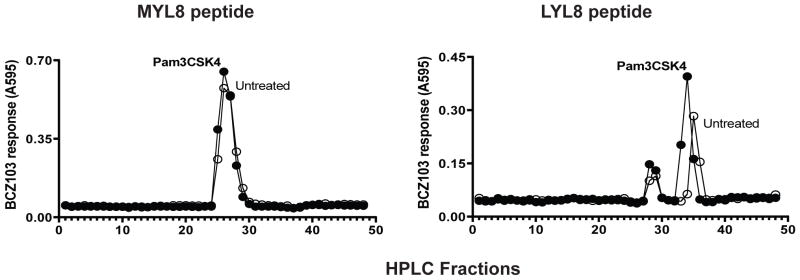
We analyzed the translated peptides directly by fractionating lysates of transfected cells by reverse-phase High Performance Liquid Chromatography (HPLC). The cDNA constructs transfected into COS 7 cells and antigenic peptides were detected using Kb-expressing L fibroblasts (K89s) as APC and activation of the BCZ103 hybridoma. The T-cell response shown on the Y-axis to each fraction reflects the relative amount of peptide in each fraction (Figure 3). In addition, the elution profile of the antigenic activity revealed the structural features of the peptide as established by comparison with synthetic analogs. For both the [AUG]-YL8 and the [CUG]-YL8 constructs, the antigenic activities were found in essentially the same fractions indicating that the same peptides were generated from these constructs. Note that the MYL8 peptide elutes in fractions 25–28 and comparable amounts were generated with or without Pam3CSK treatment of cells transfected with the [AUG]-YL8 construct (Figure. 3, left panel). In contrast, cells transfected with the [CUG]-YL8 construct produced two distinct antigenic activities. The first peak in fractions 25–28 matches the MYL8 peptide, produced by conventional translation and the second peak at fraction 34–36 corresponds to the LYL8 peptide that is produced by a cryptic translation mechanism (Figure. 3, right panel). Notably, Pam3CSK treatment enhanced the recovery of only the LYL8 peptide but did not change the recovered MYL8 peptide. Thus, the observed increase of antigen presentation activity by TLR agonists can be attributed to an increase in cryptic rather than conventional translation. Moreover, TLR agonist stimulation does not modify the peptide in any manner and therefore, as evidenced by the peptides eluting at the same fraction with or without TLR agonist treatment.
Figure 3. TLR agonist enhances amount of cryptic peptide.
cDNA constructs encoding [ATG]-YL8 or [CTG]-YL8, along with the Kb MHC Class I molecule and TLR2 were transfected into Cos7 cells. These cells were then treated with Pam3CSK4. Peptides were extracted by acid and fractionated by RP-HPLC. K89 antigen presenting cells and BCZ103 hybridoma cells were added to the fractionated peptide samples to measure the amount of peptide through the T-cell assay. Y-axis indicates the BCZ103 T-cell response and the X-axis indicates the fraction numbers after running the samples through the RP-HPLC. Data is representative of 3 experiments.
Pathogen infection enhances presentation of cryptic peptides
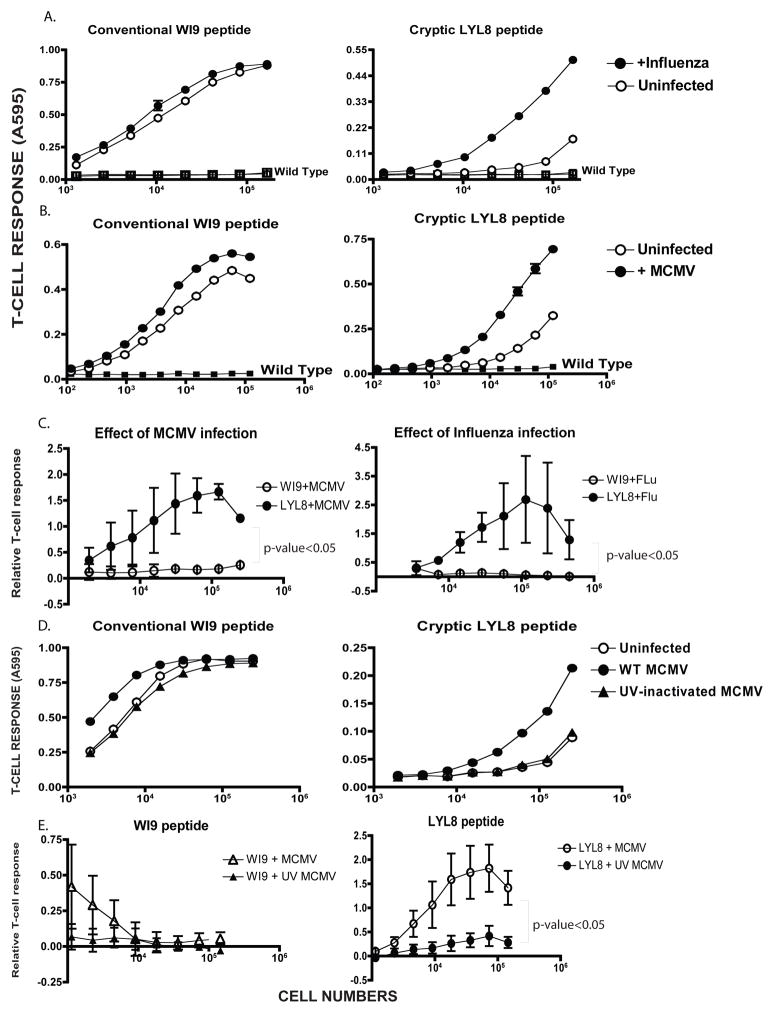
TLR agonists are merely simulators of pathogenic infection. In order to obtain a more physiological assessment, we tested whether virus infection would affect the presentation of cryptic peptides. First we used the Influenza virus because it is known to inhibit host protein synthesis (28) The WI9.LYL8 macrophages were infected with Influenza (PR8 strain) for a duration of 6 hours and 24 hours. Conventional peptide presentation was mildly enhanced at both of those time points, however cryptic peptide presentation was significantly enhanced (Figure 4A). Influenza infection of cells was confirmed using an IFN-dependent luciferase reporter assay.
Figure 4. Cryptic peptide presentation is enhanced by virus infection.
A. Primary macrophages from the WI9.LYL8 transgenic mice were infected with Influenza virus at an MOI of 1.0 for 6 hours, after which the cells were harvested and assayed for antigen presentation activity using T-cell hybridomas as described in Figure 2D. The Y-axis indicates the T-cell response (11p9Z hybridoma response for the conventional WI9 peptide and the BCZ103 hybridoma response for the cryptic LYL8 peptide) and X-axis shows the the cell numbers per well. Data is representative of 3 experiments. B. WI9.LYL8 primary macrophages were infected with Mouse-cytomegalovirus (MCMV) at an MOI of 1.0 for 6 hours. Cells were then harvested and a T-cell hybridoma assay was set up as described in Figure 2D. The Y-axis indicates the T-cell response (11p9Z specific hybridoma response for the conventional peptide and the BCZ103 specific hybridoma response for the cryptic peptide) and X-axis indicates the cell numbers per well. Data is representative of 4 experimental repeats C. T-cell responses to the WI9 and LYL8 peptides, upon MCMV infection (left panel), Influenza infection (right panel), were normalized to that of the uninfected samples for 3 distinct experiments. An unpaired t-test (with Welch’s correction) was performed, comparing the conventional and cryptic peptide responses - p-value<0.05 D. WI9.LYL8 macrophages were infected with wild-type MCMV or UV-inactivated MCMV for 6 hours. Cells were then harvested and a T-cell hybridoma assay was set up as described in Figure 2. The Y-axis indicates the T-cell response (11p9Z specific hybridoma response for the conventional peptide and the BCZ103 specific hybridoma response for the cryptic peptide) and X-axis indicates the cell numbers plated. Data is representative of 3 experiments. E. T-cell responses to the WI9 (left panel) and LYL8 (right panel) peptides upon wild-type and UV-inactivated MCMV infection, were normalized to that of the uninfected samples for 3 distinct experiments. An unpaired t-test (with Welch’s correction) was performed, comparing the wild-type and UV-inactivated T-cell responses.
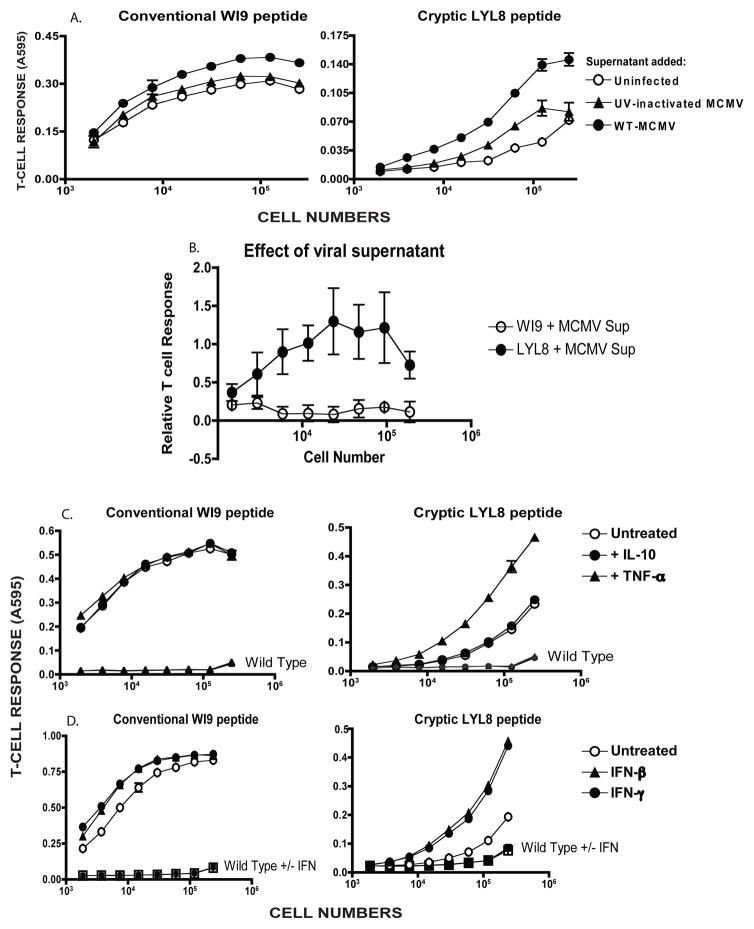
Next, we used the mouse cytomegalovirus (MCMV) a member of the herpesvirus family. Primary bone marrow derived macrophages were generated from the WI9.LYL8 transgenic mice. These cells were then infected with MCMV at an MOI of 1.0 for a duration of 6 hours. Cells were then harvested and a T-cell assay was performed by culturing the cells with either the BCZ103 or the 11p9Z hybridoma. In the presence of the virus, cryptic peptide presentation was considerably enhanced as indicated by the increased BCZ103 response of the infected samples compared to the uninfected samples (Figure 4B). Additionally, we established that T-cell responses to LYL8 peptide were enhanced in a statistically significant manner, in response to MCMV and Influenza infection, compared to the T-cell responses to the WI9 peptide (Figure 4C).
In order to determine if the enhanced presentation activity was caused by the virus in particular or by a cell-signaling event, mutant MCMV that were lacking multiple different open-reading frames (ORFs) (obtained from the laboratory of Dr. Laurent Coscoy) were used to infect the WI9.LYL8 transgenic macrophages. All of the mutants were shown to enhance cryptic peptide presentation and the conventional peptide presentation remained unchanged. Only the mutant that was lacking MHC Class I inhibitors, showed increased peptide presentation for both the conventional and cryptic peptides (Supplementary Fig S2A). This showed that no single viral gene was responsible for this phenomenon in particular. In this analysis, we also incorporated the T-cell responses to an endogenous peptide (Supplementary Fig S2A iii), outside of the transgene, as a control, to show that the cryptic peptides are indeed differentially enhanced compared to other conventional or endogenous peptides.
Next, we asked whether virus gene expression was required to cause increase in presentation of cryptic peptides. We tested live versus UV inactivated MCMV-GFP (MCMV that also had a GFP coding sequence in its genome) for this experiment. The viral supernatant was inactivated by exposure to ultra-violet (UV) light. The enhancement of cryptic peptide presentation, in this experiment, was 3000-fold in the presence of wild-type MCMV and there was no enhancement of cryptic peptide presentation in the presence of UV-inactivated MCMV. For conventional peptide presentation, there was only a 4-fold enhancement in the relative T-cell response in the presence of wild-type MCMV, whilst relative T-cell response was decreased by 30% in the presence of UV-inactivated MCMV (Figure 4D). This enhancement was calculated by obtaining the number of cells required to establish half-maximal T-cell response. This number of cells was then normalized to that of the uninfected condition. This normalized number was compared across wild-type MCMV treated and UV-inactivated MCMV treated conditions. This suggested that the live virus was triggering a signaling activity that UV inactivated virus could not. Moreover, it was expected that UV inactivated virus would behave like a TLR agonist, but that was not the case. The inactivation of the virus was measured by flow cytometry by looking for GFP positive cells, which were completely absent when UV inactivated virus was used to infect cells. Furthermore, UV inactivated virus failed to induce any TNF-α production in an intracellular cytokine-staining assay (Supplementary Fig S2B). UV-inactivated MCMV has been shown to exhibit reduced inflammatory cytokine production (29). Therefore, it is consistent that we observe decreased TNF-α production when macrophages are infected with UV-inactivated virus. Furthermore, T-cell responses to the LYL8 peptide, upon wild-type MCMV virus infection were enhanced in a statistically significant manner in comparison to UV-inactivated virus infection (Figure 4E). T-cell responses to the WI9 peptide are unchanged upon wild-type MCMV or UV-inactivated virus infection and display an overall decreasing trend.
From these results, we can infer that live pathogen infection enhances presentation of cryptic peptides to T-cells, substantially more than presentation of conventional peptides. Moreover, this effect is also greater than what was seen with TLR agonists. Furthermore, UV-inactivated virus was unable to cause the enhancement of cryptic peptides due to the inability of stimulating cytokine production in macrophages.
Enhancement of cryptic peptide presentation does not require direct virus infection
To determine if the enhanced cryptic peptide presentation was caused directly by live virus infection, a co-culture assay was performed. The WI9.LYL8 macrophages are derived from the C57BL/6 strain, which expresses the H-2b haplotype and the Kb and Db MHC Class I molecules. In this experiment, we used primary macrophages of a different haplotype- H-2d – which express distinct MHC Class I molecules, Kd, Dd and Ld, which do not present the WI9 or the LYL8 peptides. We infected the H-2d macrophages with either wild-type MCMV or UV inactivated MCMV for 6 hours. These H-2d macrophages were then co-cultured with WI9.LYL8 macrophages for a period of 3–6 hours. Next, the supernatant from the H-2d macrophages was filtered through a 0.2μM filter to remove any cells, and added to the WI9.LYL8 macrophages. After a duration of 3–6 hours, T-cell hybridomas were added to the co-cultured cells (Figure 5A). The WI9.LYL8 macrophages that were co-cultured with the WT MCMV infected H-2d macrophages showed enhanced cryptic peptide presentation, whilst those co-cultured with the UV-MCMV infected H-2d macrophages showed reduced increase in cryptic peptide presentation. Interestingly, when the supernatants were added to the WI9.LYL8 macrophages, the uninfected and UV-inactivated supernatant did not induce measurable enhancement in cryptic peptide presentation, but the WT MCMV supernatant showed enhanced cryptic peptide presentation. These results suggest that the antigen-presenting cells do not have to be infected with the virus in order to show enhanced peptide presentation. Moreover, T-cell responses to the LYL8 peptide were significantly enhanced upon WT MCMV supernatant stimulation compared to the WI9 peptide (Figure 5B), which did not show any signs of enhancement. These results suggest that co-culture with infected cells was sufficient to induce enhanced cryptic peptide presentation. Moreover, co-culture with just the supernatant from infected cells was also sufficient to induce enhanced cryptic peptide presentation. This result implicates soluble factors secreted from infected cells as potential mediators of enhanced antigen presentation activity. This also suggests that the UV-inactivated supernatant might induce very little soluble factors to be secreted into the supernatant, which is why it was unable to induce enhanced cryptic peptide presentation.
Figure 5. Enhancement of cryptic peptide presentation does not require direct virus infection.
A. Primary macrophages of the H-2d haplotype were infected with wild-type MCMV-GFP or UV-inactivated MCMV-GFP for 6 hours. Supernatant from the infected H-2d macrophages were filtered with a 0.2μM filter and added to the WI9.LYL8 macrophages (H-2b haplotype). The WI9.LYL8 macrophages were then harvested and a T-cell hybridoma assay was set up as described in Figure 2. The Y-axis indicates the T-cell response (11p9Z specific hybridoma response for the conventional peptide and the BCZ103 specific hybridoma response for the cryptic peptide) and X-axis indicates the cell numbers plated. Data is representative of 3 experiments. B. T-cell responses to the WI9 and LYL8 peptides were normalized to that of the uninfected samples for 3 distinct experiments. An unpaired t-test (with Welch’s correction) was performed, comparing the conventional and cryptic peptide responses – p-value<0.05 C. WI9.LYL8 primary macrophages were stimulated with TNFα (0.1ug/mL) and IL-10 (0.1ug/mL). Cells were then harvested and a T-cell hybridoma assay was set up as described in Figure 2. The Y-axis indicates the T-cell response (11p9Z specific hybridoma response for the conventional peptide and the BCZ103 specific hybridoma response for the cryptic peptide) and X-axis indicates the cell numbers plated. Data is representative of 2 experiments. D. WI9.LYL8 macrophages were stimulated with Type I or Type II IFN. Cells were then harvested and a T-cell hybridoma assay was set up as described in Figure 2. The Y-axis indicates the T-cell response (11p9Z specific hybridoma response for the conventional peptide and the BCZ103 specific hybridoma response for the cryptic peptide) and X-axis indicates the cell numbers plated. Data is representative of 2 experiments.
To determine if there was any reinfection of the WI9.LYL8 macrophages, the macrophages were stained with MHC Class I surface antibodies. The H2-D macrophages stained positively for both Dd antibody and MCMV-GFP (Supplementary Fig S3A). However, the WI9.LYL8 macrophages stained positively only for the Db antibody and not for MCMV-GFP at all (Supplementary Fig S3B) ruling out the possibility that the WI9.LYL8 macrophages were infected with residual virus.
To test if soluble factors, secreted upon viral infection, were responsible for the enhancement of cryptic peptide presentation, we decided to test if cytokines were able to direct regulate cryptic peptide presentation. We added TNF-α to WI9.LYL8 primary macrophages for 6 hours. Cells were than harvested and a T-cell assay was performed. The cells were also treated with the non-inflammatory cytokine – IL-10. Notably, TNF-α enhanced cryptic peptide presentation by 3-fold whilst IL-10 did not cause any measurable change in cryptic peptide presentation compared to untreated cells (Figure 5C). Moreover, conventional peptide presentation was actually decreased by 20% in the presence of these two cytokines, as measured by the relative T-cell response. Furthermore, T-cell responses to LYL8 peptide were enhanced in a statistically significant manner upon TNF-α stimulation, compared to the IL-10 stimulation, whereas the T-cell responses to the WI9 peptide were not significantly affected by either cytokine (Supplementary Fig S4A and S4B). T-cell responses to the LYL8 peptide were also significantly enhanced upon TNF-α stimulation compared to the T-cell responses to the WI9 peptide (Supplementary Fig S4C). Activated macrophages are also known to secrete Interferons upon TLR stimulation. So, we treated primary WI9.LYL8 macrophages with Type I and Type II Interferon. Cryptic, but not conventional peptide presentation was enhanced in the presence of both the Type I (IFNβ) and Type II Interferon (IFNγ) by 8-fold and 5-fold respectively, whilst conventional peptide presentation was increased only by 2-fold (Figure 5D). Furthermore, T-cell responses to the LYL8 peptide were significantly enhanced upon Type I and Type II interferon stimulation compared to the WI9 peptide (Supplementary Fig S4D and S4E). Therefore, we have further shown that enhancement of cryptic peptide presentation does not require direct infection with virus; the enhancement can be caused by inflammatory cytokines.
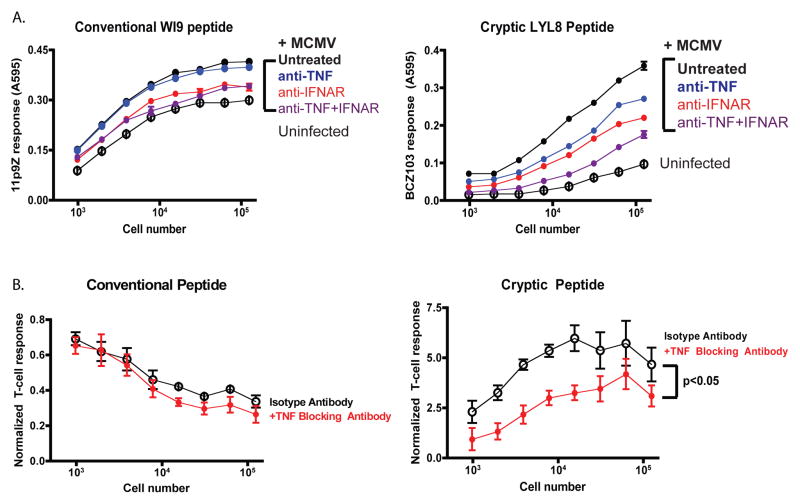
To determine if similar cytokines enhanced cryptic peptide presentation during MCMV infection, we used anti-TNF and anti-interferon receptor (IFNAR) to block the effect of these cytokines. A variety of different concentrations of this blocking antibody were tested. Primary WI9.LYL8 macrophages were infected with MCMV virus and cultured with TNF-α blocking antibody or IgG Isotype control antibody. When the TNF-α blocking antibodies were used, enhancement of cryptic peptide presentation was inhibited. As measured by the relative T-cell response, the enhancement with the TNF Isotype antibody was 3-fold, whilst the enhancement with the TNF blocking antibody was just 2-fold. This suggested that there might be other inflammatory cytokines that could be acting to enhance cryptic peptide presentation. To determine if removal of Interferon can diminish the enhanced cryptic peptide presentation phenomenon, IFNAR I blocking antibodies were used at a concentration of 1.0ug/mL. WI9.LYL8 macrophages were infected with MCMV and cultured with Isotype control (IgG) antibodies or IFNAR I blocking antibodies. As an additional control, infected cells were cultured with both TNF-α and IFNAR I blocking antibodies (Figure 6A). In the presence of IFNAR I blocking antibodies, enhancement of cryptic peptide presentation was comparable to that with the Isotype control antibodies. Furthermore, in the presence of both TNF-α and IFNAR I blocking antibodies, both cryptic and conventional peptide presentation were significantly diminished. This showed that both inflammatory cytokines play a role in causing enhanced cryptic peptide presentation. However, when the effect of TNF-α alone was examined, we found that TNF-α affected cryptic peptide presentation more than conventional peptide presentation. This was shown by quantifying the effect of the TNF-α blocking antibodies. In order to quantify and combine different experiments, all the points on each curve, for the infected conditions, were normalized to its corresponding point on the uninfected curve. These values were then averaged across all the different experiments and plotted as a line graph (Figure 6B). The trend of each line indicates the effect of the condition. MCMV infection clearly enhances cryptic peptide presentation compared to uninfected cells but conventional peptide presentation is diminished in comparison to uninfected cells. With the TNF-α blocking antibody treatment, there is a more significant inhibition of cryptic peptide presentation, compared to conventional peptide presentation.
Figure 6. Blocking TNF- and Type I Interferon signaling further inhibits the enhancement of cryptic peptide presentation upon MCMV infection.
A. WI9.LYL8 primary macrophages were infected with MCMV and then cultured with either TNF blocking antibody (MP6XT22), IFNAR 1 blocking antibody along with the respective isotype control antibodies. Data is representative of 2 independent experiments with each experiment having a triplicate of samples for each condition. Cells were then harvested and a T-cell hybridoma assay was set up as described in Figure 2. The Y-axis indicates the T-cell response (11p9Z specific hybridoma response for the conventional peptide and the BCZ103 specific hybridoma response for the cryptic peptide) and X-axis indicates the cell numbers plated. B. T-cell response to infection and blocking antibody, from different experiments, were normalized to that of uninfected samples. The reciprocal of this value was taken and plotted as a % of the uninfected. Mann-Whitney test showed a statistically significant effect of TNF blocking antibody on cryptic peptide presentation.
Therefore, we have further shown that enhancement of cryptic peptide presentation does not require direct infection with virus; the enhancement can be caused by inflammatory cytokines. Using blocking antibodies against inflammatory cytokines such as Type I IFN and TNF-α can diminish the enhancement of cryptic peptide presentation. However, TNF-α has a more significant effect on cryptic peptide presentation than the conventional peptide.
Discussion
Presentation of peptides generated by cryptic translation has emerged as an important source of antigenic precursors. Here, we show that cryptic peptide presentation can be enhanced by physiological stimuli typically found in inflammatory milieu. Presentation of cryptic peptide was selectively enhanced when cells were stimulated with various TLR agonists or cells were infected with viruses such as MCMV or Influenza. Remarkably, the enhanced cryptic peptide presentation could also be accomplished without direct virus infection, by treating the cells with the inflammatory cytokines TNF or type I interferon. These findings clearly show that presentation of cryptic peptides is not simply a constitutive process, but can also be regulated.
TLR stimulation induces a wide variety of transcriptional responses, relating to innate immunity, within the cell (30). Therefore, it is expected that TLRs would enhance global protein translation to induce immune response (31). This is indeed corroborated by our results with respect to TLR stimulation enhancing cryptic and conventional peptide presentation. Viral infection and cytokine treatments also enhanced presentation of cryptic peptides substantially more than conventionally translated peptides in antigen presenting cells. This selective effect on cryptic versus conventional peptides is consistent with the distinct translational mechanisms that produce their antigenic precursors. Previous studies showed that initiation at the cryptic CUG codon was decoded as a leucine rather than the canonical methionine residue (12). Furthermore, the translational start at the CUG versus the canonical AUG codons was shown to be resistant to inhibitors of conventional translation (13, 21). Finally, a novel initiator tRNA was found that allowed translation initiation at CUG start codons (22). Because later stages of the antigen presentation pathway such as cytoplasmic proteolysis and TAP transport are unlikely to distinguish peptides arising from cryptic versus conventional translation, it is likely that selective regulation of cryptic peptide presentation occurs at an early stage, perhaps during polypeptide synthesis itself. This would suggest that a distinct factor(s) regulates the translation of peptides from CUG versus AUG start codons.
One possibility is a factor that is involved in cellular stress responses. TLRs have been implicated in regulating different cellular stress responses relating to endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) (31). Furthermore, MCMV has also been shown to prevent the inhibition of global protein translation by regulating the UPR (32). Studying factors involved in the UPR and identifying if they are linked to the differential CUG-initiated translation mechanism would help establish a mechanism for TLRs and MCMV enhancing cryptic peptide presentation.
Another possibility is to identify factors that are downstream of the TNF and IFN signaling pathways. One such factor is the p38 MAP kinase pathway, which can also be induced by different kinds of cellular stresses, osmotic shock, heat, and proinflammatory cytokines (33). Moreover, p38 MAP kinase is an important downstream effector of all Toll-like receptor signaling pathways (34, 35) and inhibition of p38 MAPK signaling has been shown to cause distinct inflammatory responses (36, 37). Therefore, studying the p38 MAPK pathway and its downstream effectors could help identify a potential mechanism for regulation of cryptic peptide presentation.
One possible downstream effector could be one that is involved with the translation initiation pathway. For example, previous studies on the regulation of Met-initiator tRNA (Met-tRNAiMet) binding to the eIF2 complex have shown that this binding determines the efficiency of translation initiation (38). The AIMP3/p18 protein is anchored to the methionyl tRNA synthetase complex. AIMP3 knockdown resulted in reduced delivery of Met-initiator tRNA to eIF2 and inhibition of protein synthesis. The AIMP3 is found in a complex with AIMP1 and AIMP2 proteins and is known to mediate TNF signaling and activate macrophages through the p38 MAPK pathway (39). The AIMP2/p38 complex is also important for the assembly of the tRNA synthetase complex and has been shown to be an important regulator in response to genotoxic stresses (40). Given the localization of these AIMP proteins within the tRNA synthetase complex and their diverse functionality, it would be of interest to test the expression of these AIMP proteins in the context of MCMV infection and treatment with inflammatory cytokines, to determine if they can also regulate cryptic translation of antigenic precursors.
Regardless of the underlying mechanism, our findings imply that it may be possible to exploit enhanced cryptic peptide presentation for vaccines against viruses and even cancer cells. Viruses have been known to inhibit conventional antigen presentation and therefore it would be interesting to determine if cryptic peptides, which are clearly enhanced upon viral infection, could be used to develop more effective vaccines. Likewise, enhanced cryptic peptide presentation could also be applicable to the cancer microenvironment that is rife with inflammatory cytokines like TNF and Interferons. If cryptic peptides are also enhanced in tumor cells, they could be used to develop effective peptide-based vaccines against cancer cells as well.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Federico Gonzalez for support and David King for peptide synthesis.
Footnotes
This work was supported by grants from the NIH to N.S. S.R.S was supported in part by an NIH training grant, a postdoctoral fellowship from the Cancer Research Institute and the NRSA fellowship from the National Institutes of Health.
Author Contributions
S.P. and N.S. designed the study. S.P. and S.R.S. carried out the experiments and analyzed the data. S.P. and N.S. wrote the manuscript.
References
- 1.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 3.Shastri N, Schwab S, Serwold T. Producing nature’s gene-chips. The generation of peptides for display by MHC class I molecules. Annu Rev Immunol. 2002;20:463–493. doi: 10.1146/annurev.immunol.20.100301.064819. [DOI] [PubMed] [Google Scholar]
- 4.Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol. 2006;27:368–373. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Farfan-Arribas DJ, Stern LJ, Rock KL. Using intein catalysis to probe the origin of major histocompatibility complex class I-presented peptides. Proc Nat Acad Sci USA. 2012;109:16998–17003. doi: 10.1073/pnas.1210271109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yewdell JW. DRiPs solidify: progress in understanding endogenous MHC class I antigen processing. Trends in immunology. 2011;32:548–558. doi: 10.1016/j.it.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malarkannan S, Afkarian M, Shastri N. A rare cryptic translation product is presented by Kb major histocompatibility complex class I molecule to alloreactive T cells. J Exp Med. 1995;182:1739–1750. doi: 10.1084/jem.182.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinaud S, Moris A, Fevrier M, Rohrlich PS, Weiss L, Langlade-Demoyen P, Lemonnier FA, Schwartz O, Habel A. Identification of Cryptic MHC I-restricted Epitopes Encoded by HIV-1 Alternative Reading Frames. J Exp Med. 2004;199:1053–1063. doi: 10.1084/jem.20031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodenough E, Robinson TM, Zook MB, Flanigan KM, Atkins JF, Howard MT, Eisenlohr LC. Cryptic MHC class I-binding peptides are revealed by aminoglycoside-induced stop codon read-through into the 3′ UTR. Proc Nat Acad Sci USA. 2014;111:5670–5675. doi: 10.1073/pnas.1402670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garbe Y, Maletzki C, Linnebacher M. An MSI Tumor Specific Frameshift Mutation in a Coding Microsatellite of MSH3 Encodes for HLA-A0201-Restricted CD8(+) Cytotoxic T Cell Epitopes. PLoS ONE. 2011;6:e26517. doi: 10.1371/journal.pone.0026517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zook MB, Howard MT, Sinnathamby G, Atkins JF, Eisenlohr LC. Epitopes derived by incidental translational frameshifting give rise to a protective CTL response. J Immunol. 2006;176:6928–6934. doi: 10.4049/jimmunol.176.11.6928. [DOI] [PubMed] [Google Scholar]
- 12.Malarkannan S, Horng T, Shih PP, Schwab S, Shastri N. Presentation of out-of-frame peptide/MHC class I complexes by a novel translation initiation mechanism. Immunity. 1999;10:681–690. doi: 10.1016/s1074-7613(00)80067-9. [DOI] [PubMed] [Google Scholar]
- 13.Schwab SR, Shugart JA, Horng T, Malarkannan S, Shastri N. Unanticipated antigens: Translation initiation at CUG with leucine. Plos Biology. 2004;2:1774–1784. doi: 10.1371/journal.pbio.0020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starck SR, Shastri N. Non-conventional sources of peptides presented by MHC class I. Cell Mol Life Sci. 2011;68:1471–1479. doi: 10.1007/s00018-011-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinaud S, Consiglieri G, Bouziat R, Urrutia A, Graff-Dubois S, Fourati S, Malet I, Guergnon J, Guihot A, Katlama C, Autran B, van Endert P, Lemonnier FA, Appay V, Schwartz O, Kloetzel PM, Moris A. CTL escape mediated by proteasomal destruction of an HIV-1 cryptic epitope. PLoS Pathogens. 2011;7:e1002049. doi: 10.1371/journal.ppat.1002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutkowski MR, Ho O, Green WR. Defining the mechanism(s) of protection by cytolytic CD8 T cells against a cryptic epitope derived from a retroviral alternative reading frame. Virology. 2009;390:228–238. doi: 10.1016/j.virol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrison KE, Champiat S, York VA, Agrawal AT, Kallas EG, Martin JN, Hecht FM, Deeks SG, Nixon DF. Transcriptional Errors in Human Immunodeficiency Virus Type 1 Generate Targets for T-Cell Responses. Clinical and Vaccine Immunology. 2009;16:1369–1371. doi: 10.1128/CVI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Goudy K, Hirsch M, Asokan A, Fan Y, Alexander J, Sun J, Monahan P, Seiber D, Sidney J, Sette A, Tisch R, Frelinger J, Samulski RJ. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc Natl Acad Sci U S A. 2009;106:10770–10774. doi: 10.1073/pnas.0902269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen RS, Andersen SR, Hjortsø MD, Lyngaa R, Idorn M, Køllgård TM, Met Ö, thor Straten P, Hadrup SR. High frequency of T cells specific for cryptic epitopes in melanoma patients. Oncoimmunology. 2013;2:e25374. doi: 10.4161/onci.25374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinzierl AO, Maurer D, Altenberend F, Schneiderhan-Marra N, Klingel K, Schoor O, Wernet D, Joos T, Rammensee HG, Stevanovic S. A cryptic vascular endothelial growth factor T-cell epitope: identification and characterization by mass spectrometry and T-cell assays. Cancer Res. 2008;68:2447–2454. doi: 10.1158/0008-5472.CAN-07-2540. [DOI] [PubMed] [Google Scholar]
- 21.Schwab SR, Li KC, Kang C, Shastri N. Constitutive display of cryptic translation products by MHC class I molecules. Science. 2003;301:1367–1371. doi: 10.1126/science.1085650. [DOI] [PubMed] [Google Scholar]
- 22.Starck SR, Jiang V, Pavon-Eternod M, Prasad S, McCarthy B, Pan T, Shastri N. Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science. 2012;336:1719–1723. doi: 10.1126/science.1220270. [DOI] [PubMed] [Google Scholar]
- 23.Starck SR, Ow Y, Jiang V, Tokuyama M, Rivera M, Qi X, Roberts RW, Shastri N. A distinct translation initiation mechanism generates cryptic peptides for immune surveillance. PLoS ONE. 2008;3:e3460. doi: 10.1371/journal.pone.0003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malarkannan S, Shih PP, Eden PA, Horng T, Zuberi AR, Christianson G, Roopenian D, Shastri N. The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol. 1998;161:3501–3509. [PubMed] [Google Scholar]
- 26.Serwold T, Gaw S, Shastri N. ER aminopeptidases generate a unique pool of peptides for MHC class I molecules. Nat Immunol. 2001;2:644–651. doi: 10.1038/89800. [DOI] [PubMed] [Google Scholar]
- 27.Mendoza LM, Paz P, Zuberi A, Christianson G, Roopenian D, Shastri N. Minors held by majors: the H13 minor histocompatibility locus defined as a peptide/MHC class I complex. Immunity. 1997;7:461–472. doi: 10.1016/s1074-7613(00)80368-4. [DOI] [PubMed] [Google Scholar]
- 28.Aragon T, de la Luna S, Novoa I, Carrasco L, Ortin J, Nieto A. Eukaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus. Mol Cell Biol. 2000;20:6259–6268. doi: 10.1128/mcb.20.17.6259-6268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Chen DG. Recombinant murine cytomegalovirus vector activates human monocyte-derived dendritic cells in a NF-kappaB dependent pathway. Mol Immunol. 2009;46:3462–3465. doi: 10.1016/j.molimm.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 31.Claudio N, Dalet A, Gatti E, Pierre P. Mapping the crossroads of immune activation and cellular stress response pathways. EMBO J. 2013;32:1214–1224. doi: 10.1038/emboj.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian Z, Xuan B, Chapa TJ, Gualberto N, Yu D. Murine cytomegalovirus targets transcription factor ATF4 to exploit the unfolded-protein response. J Virol. 2012;86:6712–6723. doi: 10.1128/JVI.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsoulidis E, LY, Mers H, Platanias LC. The p38 Mitogen-Activated Protein Kinase Pathway in Interferon Signal Transduction. Journal of Interferon & Cytokine Research. 2005;25:749–756. doi: 10.1089/jir.2005.25.749. [DOI] [PubMed] [Google Scholar]
- 34.Dunne A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 35.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007;109:4313–4319. doi: 10.1182/blood-2006-10-048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarnicki AG, Conroy H, Brereton C, Donnelly G, Toomey D, Walsh K, Sweeney C, Leavy O, Fletcher J, Lavelle EC, Dunne P, Mills KH. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180:3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 38.Kang T, Kwon NH, Lee JY, Park MC, Kang E, Kim HH, Kang TJ, Kim S. AIMP3/p18 Controls Translational Initiation by Mediating the Delivery of Charged Initiator tRNA to Initiation Complex. Journal of Molecular Biology. 2012;423:475–481. doi: 10.1016/j.jmb.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Park SG, Choi EC, Kim S. Aminoacyl-tRNA synthetase–interacting multifunctional proteins (AIMPs): A triad for cellular homeostasis. IUBMB Life. 2010;62:296–302. doi: 10.1002/iub.324. [DOI] [PubMed] [Google Scholar]
- 40.Han JM, Park BJ, Park SG, Oh YS, Choi SJ, Lee SW, Hwang SK, Chang SH, Cho MH, Kim S. AIMP2/p38, the scaffold for the multi-tRNA synthetase complex, responds to genotoxic stresses via p53. Proc Natl Acad Sci U S A. 2008;105:11206–11211. doi: 10.1073/pnas.0800297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.