Abstract
Background
Bone marrow cell-based treatment for critical limb ischemia (CLI) in diabetic patients yielded a modest therapeutic effect due to cell dysfunction. Therefore, approaches that improve diabetic stem/progenitor cell functions may provide therapeutic benefits. Here, we tested the hypotheses that restoration of hydrogen sulfide (H2S) production in diabetic bone marrow cells (BMCs) improves their reparative capacities.
Methods
Mouse BMCs were isolated by density-gradient centrifugation. Unilateral hind limb ischemia (HLI) was conducted in 12- to 14-week old db/+ and db/db mice by ligation of left femoral artery. H2S level was measured by either gas chromatography or staining with florescent dye sulfidefluor 7AM.
Results
Both H2S production and cystathionine γ-lyase (CSE), an H2S enzyme, levels were significantly decreased in BMCs from diabetic db/db mice. Administration of H2S donor diallyl trisulfide (DATS) or overexpression of CSE restored H2S production and enhanced cell survival and migratory capacity in high glucose (HG)-treated BMCs. Immediately after HLI surgery, the db/+ and db/db mice were administrated with DATS orally and/or local intramuscular injection of GFP-labeled BMCs or RFP-CSE-overexpressing BMCs (CSE-BMCs). Mice with HLI were divided into six groups: 1) db/+; 2) db/db; 3) db/db+BMCs; 4) db/db+DATS; 5) db/db+DATS+BMCs; 6) db/db+CSE-BMCs. DATS and CSE overexpression greatly enhanced diabetic BMCs retention in ischemic hind limbs (IHL) followed by improved blood perfusion, capillary/arteriole density, skeletal muscle architecture and cell survival, and decreased perivascular CD68+ cell infiltration in IHL of diabetic mice. Interestingly, DATS or CSE overexpression rescued HG-impaired migration, tube formation and survival of BMCs or mature human cardiac microvascular endothelial cells (HCMVECs). Mechanistically, DATS restored nitric oxide production and decreased eNOS-pT495 levels in HCMVECs, and improved BMC angiogenic activity under HG condition. Finally, silencing CSE by siRNA significantly increased eNOS-pT495 levels in HCMVECs.
Conclusions
Decreased CSE-mediated H2S bioavailability is an underlying source of BMC dysfunction in diabetes. Our data indicate that H2S and overexpression of CSE in diabetic BMCs may rescue their dysfunction and open novel avenues for cell-based therapeutics of CLI in diabetic patients.
Keywords: hydrogen sulfide, bone marrow cell, hind limb ischemia, diabetes, cell-based therapy
Journal Subject Terms: Angiogenesis, Basic Science Research, Cell Signaling, Cell Therapy, Diabetes, Vascular Disease
INTRODUCTION
Critical limb ischemia (CLI) imposes a major public health burden, resulting in high mortality and disability. Occurrence of CLI in patients with diabetes, the most prevalent metabolic problem in US adults, is very frequent. In the absence of effective treatments for increasing blood flow in ischemic limbs, cell-based therapies have rapidly emerged as a potential novel therapeutic approach for treatment of CLI. Especially after the initial characterization of putative bone marrow cells (BMCs),1 increasing evidence from animal models of experimental ischemic injuries suggest that BMCs participate in the process of angiogenesis/neovascularization and tissue injury repair. Our group and others have reported that transplantation of BMCs improves angiogenesis, neovascularization, and blood flow in ischemic hind limbs (IHL) and heart of mouse models.2–4 Available clinical trials provide sufficient evidence for safety and efficacy of BMC-based treatment for ischemic tissue injury.5, 6 However, similar trials have been disappointing in diabetic patients, presumably due to cell dysfunction.5 Therefore, enhancing survival and function of transplanted diabetic BMCs remains a major challenge and approaches which improve diabetic its function may provide better therapeutic benefits for CLI in diabetes.
Hydrogen sulfide (H2S) is an endogenously produced gasotransmitter that is critical for the regulation of cardiovascular homeostasis.7–9 In mammalian species, H2S is produced enzymatically by cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (MPST), of which CSE is the primary CSE enzyme expressed in the cardiovascular system, including in cardiomyocytes, in vascular endothelial cells (ECs), and smooth muscle cells.10 Cumulative evidence showed that both exogenous and endogenous H2S exert therapeutic effects on ischemic heart and limb injury.11–16 Recently, a role of H2S in the pathogenesis of diabetes has been suggested, where circulating levels of H2S were found to be inversely proportional to cardiovascular complications in diabetic animals.17, 18 Clinically, the negative association between diabetes and H2S is reinforced by decreased plasma H2S levels in diabetic patients.19, 20 To date, several studies have investigated the therapeutic effects of H2S in the ischemic tissue injury of diabetes.17, 18 However, regulation of biological functions of diabetic BMCs by H2S and signaling transduction pathways in diabetic ischemic limb repair remain to be elucidated.
It is well known that nitric oxide (NO) is an important signal molecule in cardiovascular homeostasis. NO is synthesized within ECs by endothelial NO synthase (eNOS) from L-arginine. Loss of NO bioavailability and eNOS activity has been implicated in several disease states such as coronary artery disease, hypertension, heart failure, and diabetes. Recent studies have demonstrated that NO and eNOS modulate angiogenesis in response to tissue ischemia in diabetes.21 Although H2S and NO are thought to modulate independent signaling pathways, recently cross-talk between these two molecules has been suggested.8 Exogenous H2S was shown to protect pressure overload- and myocardial infarction-induced heart failure via upregulation of eNOS and NO bioavailability.22, 23 In addition, lack of CSE was shown to diminish NO levels and exacerbate myocardial ischemia/reperfusion injury.8 However, the link between H2S and NO in the BMC-mediated angiogenesis in diabetes has not been studied.
The purpose of the present study was to evaluate the role and underlying mechanisms of H2S deficiency in diabetes-induced BMC dysfunction, especially, to examine the therapeutic effects of restoration of H2S production in diabetic BMCs on ischemic hind limb (IHL) injury in diabetic db/db mice. Mechanistically, we investigated the effects of H2S deficiency on eNOS/NO signaling pathways in under high glucose (HG) conditions.. We found that diallyl trisulfide (DATS), a stable H2S donor, and overexpression of CSE in diabetic BMCs restored their functional and reparative properties. Furthermore, our results demonstrate that the therapeutic actions of H2S are mediated by eNOS-pT495/NO signaling pathway. Therefore, our study supports the hypothesis that H2S deficiency plays a critical role in diabetes-induced BMC dysfunction.
METHODS
The detailed and expanded methodology is provided in the online-only Data Supplement.
Animal groups, hindlimb ischemia model, laser Doppler perfusion imaging of hind limb blood flow, and sample collection
Twelve- to 14-weeks-old, male, db/db mice lacking the gene encoding for leptin receptor and their control nondiabetic db/+ mice were obtained from Jackson Laboratories. All animal experiments were approved by the Institutional Animal Care and Use Committee of Temple University.
Six animal groups were used: 1) db/+; 2) db/db; 3) db/db +BMCs; 4) db/db+DATS; 5) db/db+DATS+ BMCs; 6) db/db+CSE-BMCs. DATS was administrated orally by gavage one day after the surgery (2 mg/mouse/day, for 3 weeks after surgery). BMCs were isolated from bone marrow of 12- to 14-week-old db/+ or db/db mice by density-gradient centrifugation as previously described3, 24 and labeled with GFP by transfection with GFP lentivirus (BMCs) or overexpressed CSE by transfection of RFP-tagged CSE lentivirus (CSE-BMCs). Induction of left hind limb ischemia was performed in db/+ and db/db mice as described previously.25 Immediately after femoral artery ligation, BMCs or CSE-BMCs were injected intramuscularly (5×105 cells).26 Blood flow measurements in each mouse hind limb were performed on pre- (day 0) and post-ligation days 7, 14 and 21 using a laser Doppler perfusion imager as previously described.25
Tube formation and cell migration
Tube formation was performed as previously described.3 Cell migration was determined by either transwells or scratch wound assay or scratch wound healing.3, 27
RNA interference
HCMVECs were transfected with CSE siRNA (60 nM, sc-78973, Santa Cruz) or a negative control RNA (NC-siRNA, SC-37007, Santa Cruz) was used at a final concentration of 60 nM according to the manufacturer’s instructions.
H2S production
Intracellular free H2S levels were determined in live BMCs and HCMVECs using a stable H2S fluorescent probe sulfidefluor 7AM (SF-7AM, Tocris, Cat. # 4943) as previously described.28 Free H2S levels were measured in mouse BMCs, serum, or medial tight muscles by gas chromatography chemiluminescence (Agilent 7890 GC gas chromatography system and G660XA Series chemiluminescence detector) as previously described.8
TUNEL staining
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) staining for apoptosis was determined on BMCs or ischemic skeletal muscle sections as manufacturer’s instructions (Cell death detection assay, Roche, Indianapolis, IN).
Intracellular NO production in live HCMVECs
Intracellular NO production in live HCMVECs was determined by staining with 4-Amino-5-Methylamino-2′,7′-Difluorofluorescein (DAF-FM, Sigma) as previously described.29
Isolation of mouse lung microvascular ECs
Mouse lung microvascular endothelial cells (MMECs) were isolated using collagenase as previously described,30 and identified with staining of Von Willebrand factor (vWF, Santa Cruz SC-2780, 1:200) and CD31 (BD Biosciences; BD553370, 1:100).29
Statistics
In vitro studies were repeated at least 3 or more times with triplicates/group/experiment. Results are expressed as the mean ± SEM. For statistical comparison of single parameters, independent t test was used for two groups and one way ANOVA with Bonferr oni adjustment was performed for multiple groups. A probability value p <0.05 was considered to be significant.
RESULTS
H2S production is decreased in diabetic BMCs via downregulation of CSE
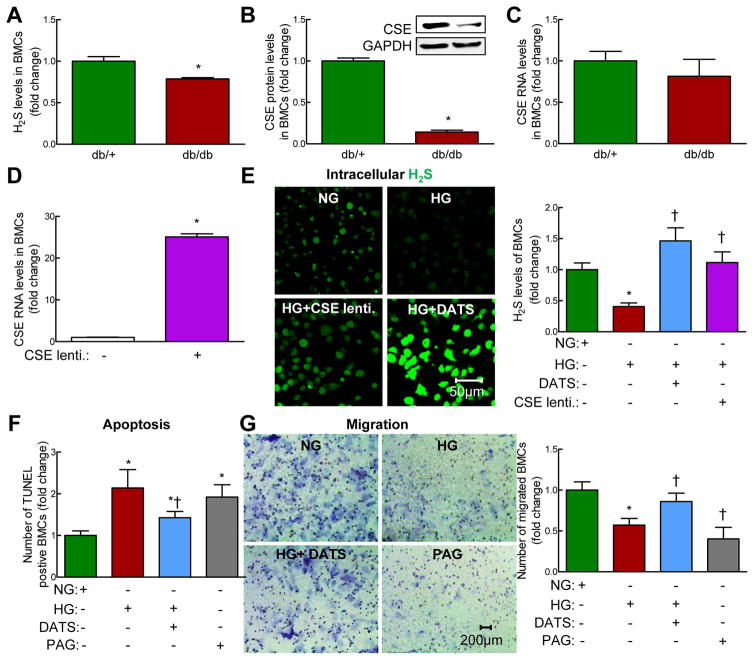
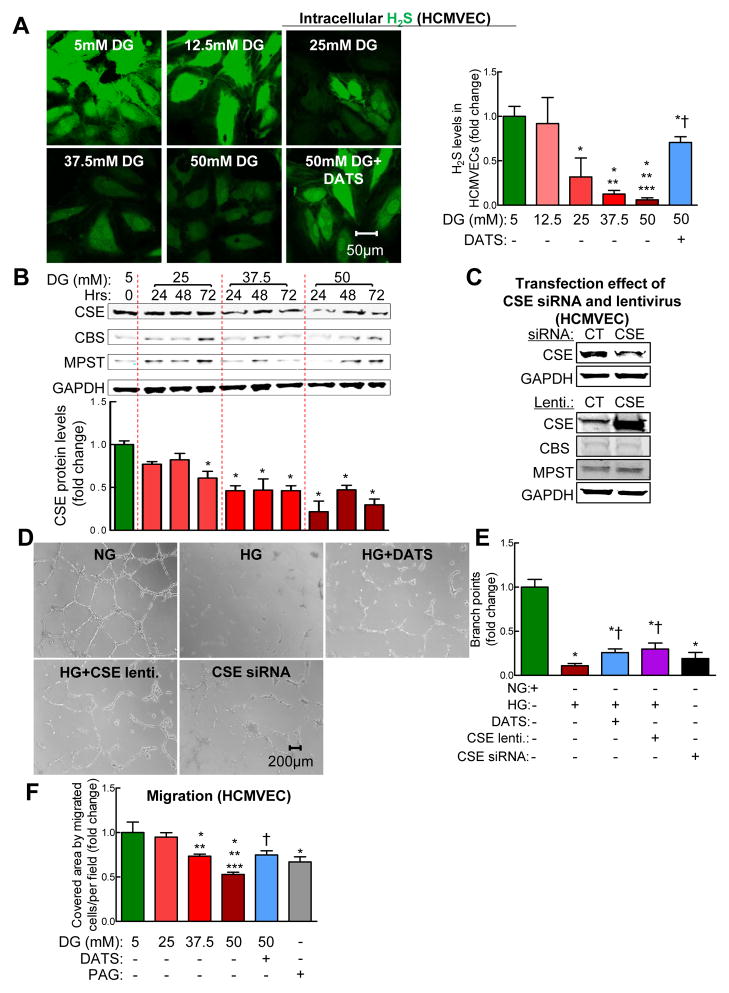
In diabetic db/db mice, H2S levels in plasma, medial thigh muscles and BMCs were significantly decreased compared to that of controls (non-diabetic db/+ mice). We next examined both protein and mRNA levels of H2S-synthesizing enzymes and found that mouse BMC expressed CSE. Protein level of CSE was significantly decreased in BMCs from diabetic db/db mice, whereas RNA levels of CSE was unchanged, indicating that downregulation of CSE in diabetic BMCs likely occurs at the post-transcriptional/translational level (Figure. 1B and C, p<0.05). CBS and MPST protein levels were virtually undetected (Supplementary Figure. 1D–G), indicating that CSE is a primary H2S-synthesizing enzyme in mouse BMAPC. In vitro, high glucose (HG), a key factor responsible for the pathogenesis of diabetes, significantly decreased H2S production in non-diabetic BMAPCs (Figure. 1D, p<0.05). Interestingly, CSE overexpression (Figure. 1D) or DATS treatment restored H2S levels in HG-treated BMCs (Figure. 1E, p<0.05). Moreover, to determine the translational value of our findings, we examined the effects of HG on CSE levels in human CD34+ cells (AllCell) which have been previously studied in phase III clinical trials. We found that CSE RNA, but not CBS and MPST RNA, level was significantly decreased in HG-treated human CD34+ cells (Supplementary Figure. 2A–C, p<0.05). Taken together, downregulation of CSE is responsible for H2S deficiency in diabetic and/or HG-treated BMAPC both in mice and humans.
Figure 1. H2S deficiency induced by CSE downregulation in diabetic BMCs enhanced BMC death and dysfunction.
BMCs were isolated from bone marrow of 12-week-old, male non-diabetic db/+ and diabetic db/db mice by gradient centrifugation. (A) Free H2S levels were measured by gas chromatography. Basal H2S levels were decreased in BMCs of db/db mice. (B) CSE protein levels were decreased in BMCs of db/db mice. (C) CSE RNA levels were not changed in BMCs of db/db mice. (D) CSE lentivirus transfection efficacy test. CSE overexpression using CSE lentivirus (MOI: 1 for 48 hrs) significantly increased CSE RNA levels in db/+ BMCs. BMCs isolated from db/+ mice were treated with normal D-glucose (DG) (NG, 5 mM) or high DG (HG, 50 mM) for 48 hrs. (E) Intracellular H2S content was measured with fluorescent probe sulfidefluor 7AM (SF-7AM, 25 μM, for 30 minutes) in BMCs from db/+ mice treated with NG for 48 hrs. Diallyl trisulfide (DATS, 10 μM, 48 hrs) and overexpression of CSE with CSE lentivirus significantly restored H2S production in high D-glucose (HG, 50 mM, 48 hrs)-treated BMCs. Left panel, representative photomicrographs; Right panel, relative quantification of H2S levels in live BMCs. (F) BMC death was measured using TUNEL Kit. DATS treatment inhibited HG-induced BMC death. Propargyl Glycine (PAG, 100 μM, 48hrs), a CSE-selective inhibitor, induced BMC death. (G) BMC migration was measured using Boyden transwell chamber. HG-impaired BMCs migration was restored by DATS, whereas PAG impaired BMC migration. Left panel, representative photographs; Right panel, relative quantification of BMC migration. n=3–5, *p<0.05 vs db/+ mice or NG-treated-BMCs; †p<0.05 vs HG-treated BMCs. BMCs, bone marrow cells; NG, normal glucose (5 mM): HG, High glucose (50 mM).
DATS rescued HG-induced BMC death
We next determined whether H2S deficiency is involved in HG-induced BMC death. We found that HG significantly increased TUNEL+ BMCs (Figure. 1F, Supplementary Figure. 3, p<0.05). DATS significantly reduced HG-induced BMC death. To study the role of CSE in BMC death, we examined the effect of DL-propargyl glycine (PAG), a selective CSE inhibitor, on BMC survival. We found that PAG significantly increased TUNEL+ BMCs (Figure. 1F, Supplementary Figure. 3, p<0.05), indicating that CSE-mediated H2S reduction plays a critical role in HG-induced BMC death.
DATS rescued HG-impaired BMC migration
The migratory function of BMC has been demonstrated to be one of the most important factors for neovascularization.31 We found that HG significantly decreased the number of migrated BMCs which was rescued by DATS (Figure. 1G and Supplementary Figure. 4,p<0.05). PAG impaired the migratory capacity of BMCs (Figure. 1G, p<0.05). Our findings suggest that CSE-mediated H2S deficiency impairs BMC migratory capacity under HG condition.
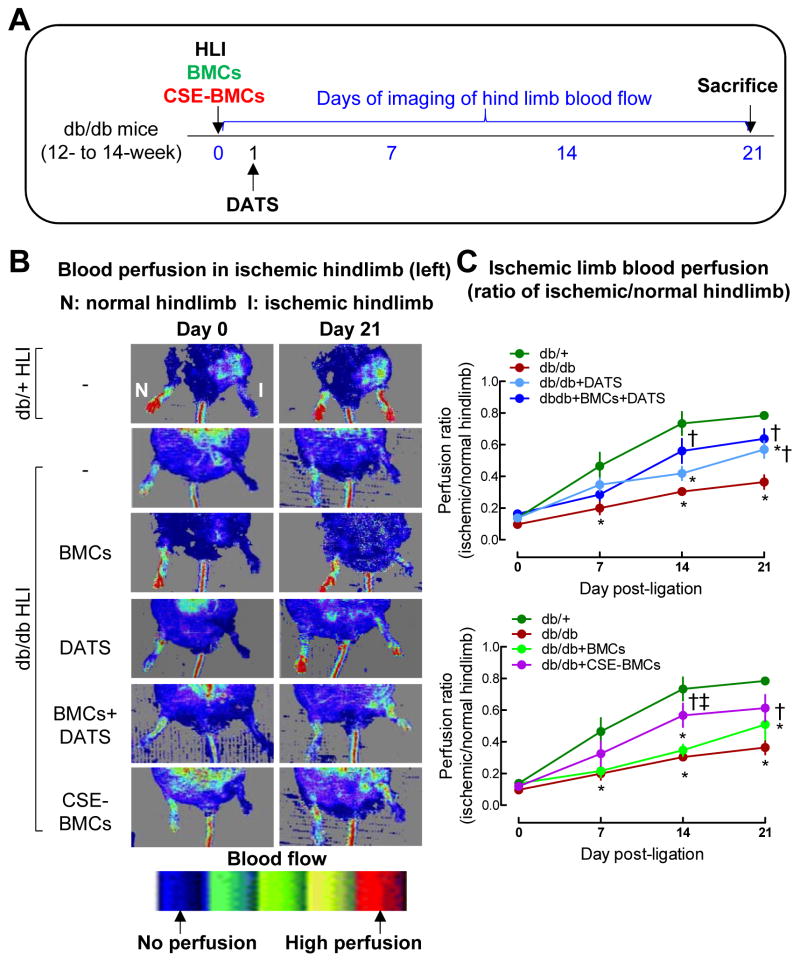
Administration of DATS or overexpression of CSE enhanced diabetic BMCs angiogenic potential and protected toe loss in IHL of diabetic mice
We next determined whether DATS or overexpression of CSE in BMCs rescues the defective phenotype and enhances the therapeutic effects of diabetic BMCs. For this purpose, the db/db mice with unilateral hind limb ischemia received DATS by gavage or local transplantation of BMCs alone, or received a combination of DATS and BMCs. To explore the role of CSE, the therapeutic effects of CSE-overexpressing diabetic BMCs were also examined. Blood perfusion in the ischemic limb was monitored by laser Doppler perfusion imaging (Figure. 2A). The ischemic/non-ischemic blood flow ratio was significantly decreased in diabetic mice by 38.6% 14 days after ligation and excision of femoral artery (Figure. 2B–C, p<0.05). Diabetic BMCs did not significantly increase the blood flow, whereas DATS significantly enhanced blood perfusion (Figure. 2B–C, p<0.05). Administration of DATS combined with diabetic BMCs dramatically increased blood perfusion. CSE-overexpressing diabetic BMCs also significantly increased blood flow (Figure. 2B–C, p<0.05).
Figure 2. Administration of DATS or overexpression of CSE potentiated diabetic BMC-mediated blood perfusion in IHL of db/db mice.
Diabetic BMCs were isolated from 12-week-old db/db mice by gradient centrifugation, then transfected with GFP (MOI: 1, BMCs) or RFP-labeled CSE lentivirus (MOI: 1, CSE-BMC) in the presence of polybrene (8 μg/ml) for 48 hrs. (A) db/db mice received diabetic BMCs or CSE-BMCs by local intramuscular injection (5×105) immediately following left femoral artery ligation (ischemic limb). DATS (2 mg/kg/day) was given by gavage starting on the first day post-ligation for 3 weeks. Doppler images for blood perfusion of IHL were taken at 0, 7, 14 and 21 days post-ligation. Ischemic limb blood perfusion was presented as ratio of blood flow in ischemic limbs divided by that in normal hind limb. Mice were euthanized at 21 days post-ligation. (B) Representative images showing blood flow recovery on days 0 and 21. (C) Quantification of perfusion ratio of blood flow recovery in ischemic limbs. n=5–7, *p<0.05 vs db/+ mice; †p<0.05 vs db/db mice; ‡p<0.05 vs db/db+BMCs; #p<0.05 vs db/db+DATS. BMCs, bone marrow cells; I, ischemic limb; HLI, hind limb ischemia; N, normal limb.
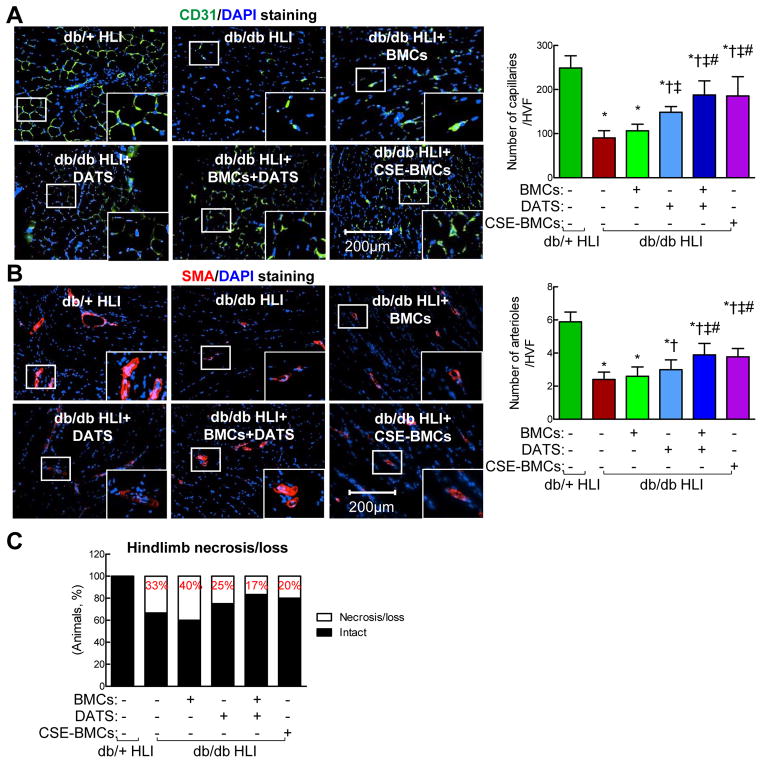
CD31 staining showed that the capillary density was significantly decreased in ischemic tissue of untreated diabetic mice compared to non-diabetic mice (Figure. 3A, p<0.05). Transplantation of diabetic BMCs slightly increased capillary density but not significantly, whereas DATS alone significantly increased capillary density in IHL of diabetic mice (Figure. 3A, p<0.05). A combination of DATS and diabetic BMC dramatically increased capillary formation. Transplantation of CSE-overexpressing diabetic BMCs to ischemic tissues significantly improved capillary density (Figure. 3A, p<0.05). Similarly, arteriole density (Smooth muscle actin+ vessels) was significantly decreased in IHL of diabetic mice compared to non-diabetic mice (Figure. 3B) which was not significantly improved by transplantation of diabetic BMC. DATS significantly increased the arteriole density (p<0.05), especially, potentiated BMC-mediated arteriole formation (Figure. 3B, p<0.05) in IHL of diabetes. Overexpression of CSE in BMCs significantly improved arteriole density (Figure. 3B, p<0.05). Taken together, these findings indicate that CSE downregulation-mediated H2S deficiency induces BMC dysfunction in diabetes and that restoration of endogenous H2S production by overexpression of CSE or exogenous supplementation of H2S donor DATS in diabetic BMCs rescues their dysfunction and improves blood perfusion and capillary/arteriole formation in IHL of diabetic mice which resulted in a lower rate of ischemic limb necrosis/loss (Figure. 3C,).
Figure 3. Administration of DATS or overexpression of CSE enhanced diabetic BMC-mediated angiogenesis and neovascularization in IHL of db/db mice.
BMCs were isolated from 12-week-old diabetic db/db mice by gradient centrifugation, then transfected with GFP (MOI: 1, BMCs) or RFP-labeled CSE lentivirus (MOI: 1, CSE-BMC) in the presence of polybrene (8 μg/ml) for 48 hrs. Diabetic db/db mice received BMCs or CSE-BMCs by local intramuscular injection (5×105) immediately following left femoral artery ligation. DATS (2 mg/kg/day) was given by gavage starting on the first day post-ligation for 3 weeks. Ischemic skeletal muscle was collected at 21 days post-ligation and immunostained for CD31 or α-SMA. (A) Administration of DATS or overexpression of CSE potentiated diabetic BMC-mediated angiogenesis. Left panel, representative photomicrographs of CD31 staining (green) on ischemic skeletal muscle sections for examining capillaries density; Right panel, the average number of capillary/high-power visual field. (B) Administration of DATS or overexpression of CSE potentiated diabetic BMC-mediated neovascularization. Left panel, representative photomicrographs of α-SMA staining (red) on ischemic skeletal muscle sections for examining arteriole density; Right panel, the average number of arterioles/high-power visual field. Nuclei were stained with DAPI (blue). (C) Administration of DATS or overexpression of CSE in diabetic BMCs prevented ischemic hindlimb necrosis/loss. n=5–7, *p<0.05 vs db/+ mice; †p<0.05 vs db/db mice; ‡p<0.05 vs db/db+BMCs; #p<0.05 vs db/db+DATS. BMCs, bone marrow cells; HLI, hind limb ischemia; HVF, high-power visual field; α-SMA, α-smooth muscle actin.
Administration of DATS or overexpression of CSE improved diabetic BMC-restored ischemic skeletal muscle architecture in diabetic mice
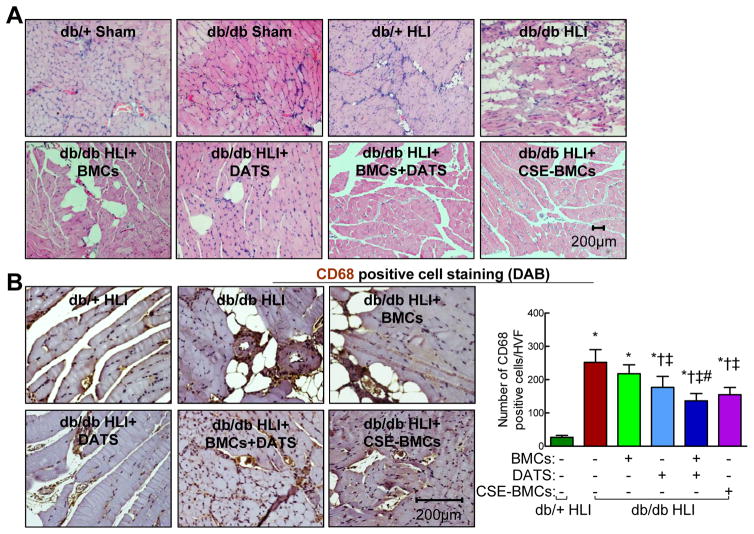
Non-diabetic mice showed slightly increased fat deposition in IHL compared to shams (Figure. 4A). There was massive muscle degeneration and scattered fat deposition in the ischemic regions of diabetic mice. DATS or diabetic BMC alone significantly rescued muscular degeneration and localized fat deposition, whereas a combination of DATS and diabetic BMCs or overexpression of CSE dramatically attenuated muscular degeneration and fat deposition (Figure. 4A), suggesting that sufficient H2S production is required for maintaining skeletal muscle architecture in IHL of diabetes.
Figure 4. Administration of DATS or overexpression of CSE potentiated BMC-mediated skeletal muscle architecture recovery and inhibition of CD68+ cell infiltration in perivascular space in IHL of db/db mice.
Diabetic db/db mice received BMCs or CSE-BMCs by local intramuscular injection (5×105) immediately following left femoral artery ligation. DATS (2 mg/kg/day) was given by gavage started on the first day post-ligation for 3 weeks. Ischemic skeletal muscle was collected at 21 days post-ligation for analysis. (A) Administration of DATS or overexpression of CSE potentiated BMC-mediated skeletal muscle architecture recovery. H&E staining of ischemic skeletal muscle sections. (B) Administration of DATS or overexpression of CSE potentiated BMC-mediated inhibition of CD68+ cell infiltration in perivascular space. Left panel, representative images of CD68+ cell staining (brown, DAB staining) on ischemic skeletal muscle sections; Right panel, the average number of CD68+ cells/high-power visual field. n=3–5, *p<0.05 vs db/+ mice; †p<0.05 vs db/db mice; ‡p<0.05 vs db/db+BMCs; #p<0.05 vs db/db+DATS. BMCs, bone marrow cells; HVF, high-power visual field.
Administration of DATS or overexpression of CSE in diabetic BMCs inhibited perivascular CD68+ cell infiltration in ischemic skeletal muscle of diabetic mice
Neovascularization is an integral process of inflammatory reactions and subsequent repair cascades in ischemic tissue injury. To evaluate therapeutic effects of administration of DATS and overexpression of CSE in diabetic BMCs on post-ischemic inflammatory responses, numbers of infiltrated CD68+ inflammatory cells were examined in ischemic muscle sections (Figure. 4B). We found that CD68+ cell infiltration was significantly increased in IHL of diabetic mice compared to non-diabetic mice (Figure. 4B, p<0.05). Transplantation of diabetic BMCs did not significantly alter the CD68+ cell infiltration. DATS alone significantly decreased CD68+ cell infiltration (p<0.05) and to a greater extent when combined with diabetic BMCs (p<0.05). CSE-overexpressing diabetic BMCs significantly reduced CD68+ cell infiltration (Figure. 4B, p<0.05), indicating that H2S deficiency caused by downregulation of CSE results in perivascular CD68+ cell infiltration of ischemic tissues in diabetes.
Administration of DATS or overexpression of CSE in diabetic BMCs attenuated apoptosis in IHL of diabetic mice
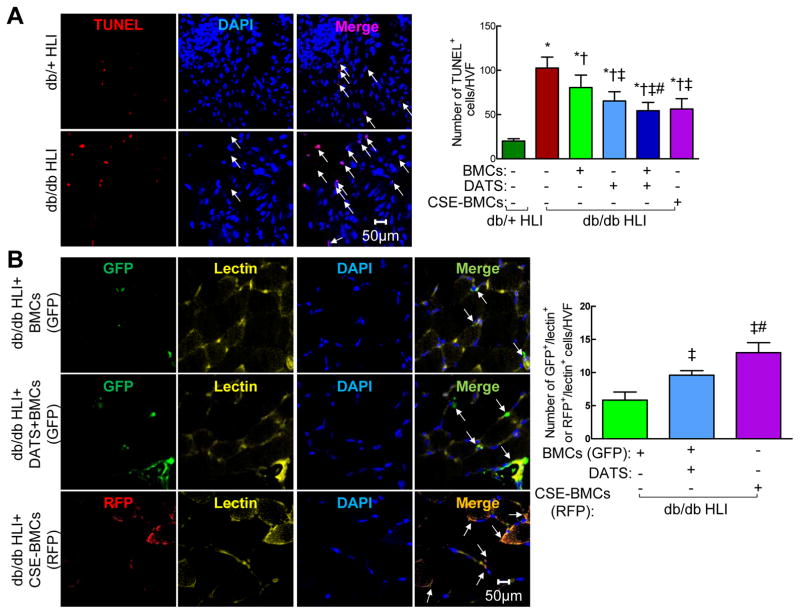
There was a significant increase of TUNEL+ apoptotic cells in IHL of diabetic mice compared to non-diabetic mice (Figure. 5A, p<0.05). We noted that the TUNEL+ cells were mainly expressed in the scar and scar zone. Diabetic BMCs and DATS alone significantly attenuated apoptosis, respectively (Figure. 5A, p<0.05). A combination of DATS and diabetic BMCs or overexpression of CSE in diabetic BMCs attenuated apoptosis to a greater extent (Figure. 5A, p<0.05), suggesting that CSE-mediated H2S is critically required for cell survival in ischemic tissues of diabetes.
Figure 5. DATS or overexpression of CSE potentiated BMC-mediated cell survival and engraftment of transplanted BMCs into vascular structure in IHL of db/db mice.
(A) DATS or overexpression of CSE potentiated BMC-mediated cell survival. Left panel, representative photomicrographs of TUNEL staining (red) of ischemic skeletal muscle; Right panel, the average number of TUNEL+ cells/high-power visual field. (B) CSE overexpression enhanced engraftment of transplanted BMCs into vascular structure. Representative photomicrographs of GFP (green)/lectin (yellow) and RFP (red)/lectin (yellow). Nuclei were stained with DAPI (blue). Ischemic skeletal muscle was collected at 21 day post-ligation. n=3–5, *p<0.05 vs db/+ mice; †p<0.05 vs db/db mice; ‡p<0.05 vs db/db+BMC; #p<0.05 vs db/db+DATS. BMCs, bone marrow cells; HVF, high-power visual field.
Overexpression of CSE in diabetic BMCs improved their engraftment and angiogenic activity in IHL of diabetic mice
To track the distribution of injected BMCs, control and CSE-overexpressing diabetic BMCs were tagged with GFP and RFP-labeled CSE lentivirus, respectively (Supplementary Figure. 5A and B). The CSE mRNA level was increased by 25-fold in transfected BMCs compared to the control (Figure. 1D, p<0.05).
Double staining of ischemic tissues with lectin (yellow) and GFP (green, for diabetic BMCs) or RFP (red, for CSE-overexpressing diabetic BMCs, Figure. 5B) showed that lectin+ capillaries or arterioles contained either GFP+ (Figure. 5B, upper panel) or RFP+ cells (Figure. 5B, lower panel), confirming the presence of hybrid blood vessels composed of transplanted BMCs. It is noteworthy that lectin+/GFP+ cells in capillaries and arterioles were increased by 1.64-fold in the mice treated with DATS+BMCs compared with that treated with BMCs alone (Figure. 5B, p<0.05). Lectin+/RFP+ cells in capillaries and arterioles were 2.22- and 1.58-fold more than lectin+/GFP+ cells in mice treated with BMCs and DATS+BMCs, respectively (Figure. 5B, p<0.05), suggesting that DATS or overexpression of CSE in diabetic BMCs increased their cooperative and differentiation properties for angiogenesis and neovascularization. Overexpression of CSE in diabetic BMCs increased their angiogenic capability to a greater extent than administration of DATS plus BMCs. Moreover, there were more transplanted BMCs detectable in ischemic tissues of mice with DATS+BMCs or CSE-BMCs compared to that with BMCs alone (Figure. 5B), suggesting that overexpression of CSE increases diabetic BMC retention in ischemic tissues of diabetic mice. Taken together, these results suggest that endogenous H2S production resulting from overexpression of CSE increases diabetic BMC survival rate, retention and engraftment into ischemic tissue, and subsequently induces vascular network formation to a greater extent than exogenous H2S production in the ischemic muscles after transplantation.
Hyperglycemic culture conditions reduced H2S production and impaired HCMVEC function via downregulation of CSE
Human cardiac microvascular endothelial cell (HCMVEC) is a cardiovascular-relevant cellular model in which to study the role of H2S in endothelial cell regulatory mechanisms of angiogenesis and neovascularization under high glucose (HG) condition in human. We found that H2S production and CSE protein levels were reduced in HCMVECs dose-dependently (Figure. 6A and B, p<0.05). DATS significantly increased H2S levels in HCMVECs (Figure. 6A and B, p<0.05). CBS or MPST protein levels were not affected by HG (Figure. 6B), suggesting that CSE plays a major role for H2S production in HCMVECs.
Figure 6. DATS or overexpression of CSE rescued HG-impaired HCMVECs tube formation and migration.
Human cardiac microvascular endothelial cells (HCMVECs) were treated with normal concentration of D-glucose (NG, 5 mM) or indicated concentrations of D-glucose (HG) in the presence or absence of DATS (10 μM) for 48 hrs. Intracellular H2S was detected with 25 μM SF-7AM stain for 30 minutes. (A) High concentrations of D-glucose (HG) decreased H2S production in HCMVECs dose-dependently. DATS restored HG-induced H2S. Left panel, representative photomicrographs of intracellular H2S production in live HCMVECs; Right panel, relative quantification of H2S production. (B) HG decreased CSE levels in HCMVECs in a dose-dependent manner. Upper panel, representative CSE, MPST and CBS protein levels in HCMVECs; lower panel, relative quantification of CSE protein levels. HCMVECs were treated with NG or indicated concentrations of HG for indicated times. (C) CSE siRNA knocked down CSE levels, whereas CSE lentivirus significantly increased in HCMVECs. (D); and (E) administration of DATS (10 μM, 48 hrs) and overexpression of CSE prevented HG (50 mM, 48 hrs)-impaired HCMVECs tube formation. Silencing CSE by CSE siRNA significantly impaired HCMVECs tube formation. (F) DATS (10 μM, 48 hrs) rescued HG (50 mM, 48 hrs)-impaired HCMVECs migration. CSE inhibitor DL-Propargyl Glycine (PAG, 100 μM, 48 hrs) significantly impaired HCMVECs migration. Relative quantification of number of migrated HCMVECs by scratch wound assay (Supplementary Figure 6). Cell migration was quantified by covered area of migrated cells/per field. For silencing of CSE, HCMVECs were treated with CSE siRNA (60 nM) for 72 hrs; for overexpressing CSE gene, HCMVECs were treated with CSE lentivirus (MOI; 1) plus polybrene (8 μg/ml) for 48 hrs. n=3–5, *p<0.05 vs NG-treated HCMVECs (CT); **p<0.05 vs 25 mM DG-treated HCMVECs; ***<0.05 vs 37.5 mM DG-treated HCMVECs; †p<0.05 vs 50 mM DG-treated HCMVECs. DG, D-glucose; HCMVECs, human cardiac microvascular endothelial cells; HVF, high-power visual field; lenti, lentivirus.
We used a matrigel model to examine the role of H2S in HG-impaired tube formation, a standard in vitro experimental model for studying angiogenic properties of HCMVECs. We found that HG dramatically impaired tube formation, which was significantly improved either by DATS or overexpression of CSE (Figure. 6D–F, p<0.05). Silencing CSE by siRNA significantly impaired tube formation of HCMVECs.
HCMVEC migration was evaluated using the scratch wound assay. HG significantly reduced HCMVEC migration dose-dependently (Figure. 6F and Supplementary Figure. 6, p<0.05). DATS rescued HG-impaired HCMVEC migration while PAG impaired HCMVEC migration similarly to HG exposure (p<0.05). Taken together, our results indicate that CSE-mediated H2S deficiency plays a critical role in HG-impaired HCMVEC tube formation and migration.
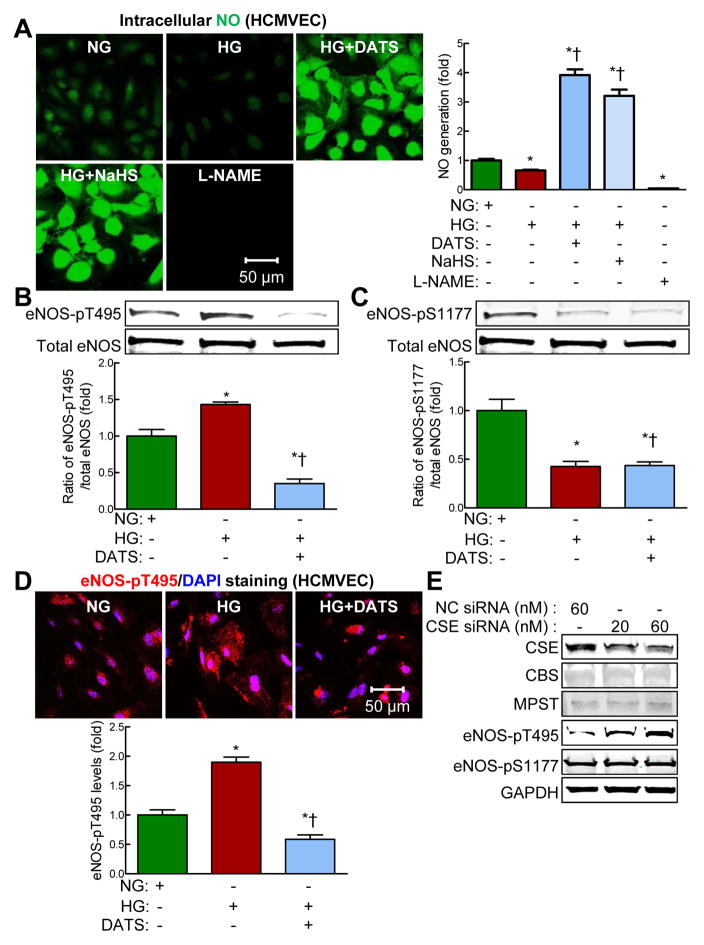
DATS reversed HG-induced eNOS phosphorylation at threonine 495 and NO reduction in HCMVECs
Evidence suggests that H2S requires NO to exerts positive effects.32 Next, we examined the effects of H2S deficiency on NO production. We found that HG inhibited NO production in HCMVECs (Figure. 7A, p<0.05) which was rescued by either DATS or sodium hydrosulfide, suggesting that H2S deficiency mediates HG-induced NO reduction in HCMVECs (Figure. 7A, p<0.05).
Figure 7. DATS rescued HG-reduced nitric oxide production via downregulation of eNOS-pT495 in HCMVECs.
HCMVECs were treated with normal concentration of D-glucose (NG, 5 mM) or HG (50 mM) in the presence or absence of DATS (10 μM), and NaHS (100 μM) for 48 hrs. HCMVECs treated with L-NAME (100 μM) for 0.5 hr were used as negative controls. For silencing of CSE, HCMVECs were treated with CSE siRNA (20 and 60 nM) for 72 hrs. (A) DATS and NaHS rescued high glucose (HG)-induced nitric oxide (NO) reduction in HCMVECs. Left panel, representative photomicrographs of intracellular NO production in live HCMVECs; Right panel, relative quantification of NO. NO levels were assayed by staining with fluorescence probe DAF-FM (10 μM) for 30 minutes. (B) DATS significantly reduced HG (50 mM, 48 hrs)-induced eNOS-pT495. Representative eNOS-pT495 and total eNOS levels and relative quantification of eNOS-pT495/total eNOS. (C) DATS did not rescue HG (50 mM, 48 hrs)-reduced eNOS-pS1177. Representative eNOS-pS1177 and total eNOS levels and relative quantification of eNOS-pS1177/total eNOS. (D) HG significantly increased eNOS-pT495 expression in the cytoplasm of HCMVECs which was rescued by DATS. Upper panel, representative photomicrographs of eNOS-pT495 (red); lower panel, relative quantification of eNOS-pT495 levels. (E) Silencing of CSE by CSE siRNA in HCMVECs increased eNOS-pT495 level dose-dependently, but eNOS-pS1177 level was not affected. Representative CSE, CBS, MPST, eNOS-pT495, eNOS-pS1177 and GAPDH protein levels in HCMVECs treated with indicated concentrations of CSE siRNA. A negative control (NC-siRNA) was used at a final concentration of 60 nM according to the manufacturer’s instructions. n=3–5, *p<0.05 vs NG-treated HCMVECs (CT); †p<0.05 vs 50 mM DG-treated HCMVECs. DAF-FM, 4-Amino-5-Methylamino-2′,7′-Difluorofluorescein; eNOS, endothelial nitric oxide synthase; eNOS-pS1177, eNOS phosphorylation at serine 1177; eNOS-pT495, eNOS phosphorylation at threonine 495; HCMVECs, human cardiac microvascular endothelial cells; L-NAME, L-NG-nitroarginine methyl ester; NO, nitric oxide.
Recently, we reported that HG decreases eNOS activity and NO production via eNOS phosphorylation at threonine 495 (eNOS-pT495) — a negative regulator of eNOS activity.29 To investigate whether H2S induces eNOS-pT495, we examined the effect of DATS on eNOS-pT495 in HG-treated HCMVECs. HG increased eNOS-pT495 levels in a dose- and time-dependent manner (Figure. 7B and Supplementary Figure. 7A and B, p < 0.05) and this effect was reduced by DATS (p < 0.05). HG significantly decreased eNOS phosphorylation at serine 1177 (eNOS-pS1177) — a positive regulator of eNOS activity. Interestingly DATS did not significantly affect eNOS-pS1177 levels (Figure. 7C, p<0.05). By immunohistochemistry, we also found that HG significantly increased eNOS-pT495 expression in the cytoplasm of HCMVECs which was also rescued by DATS (Figure. 7D, p<0.05). Interestingly, knockdown of CSE expression dose-dependently increased protein levels of eNOS-pT495, but not eNOS-pS1177, in HCMVECs (Figure. 7E). Taken together, our findings suggest that H2S deficiency mediated HG-induced NO reduction via eNOS-pT495 in HCMVECs.
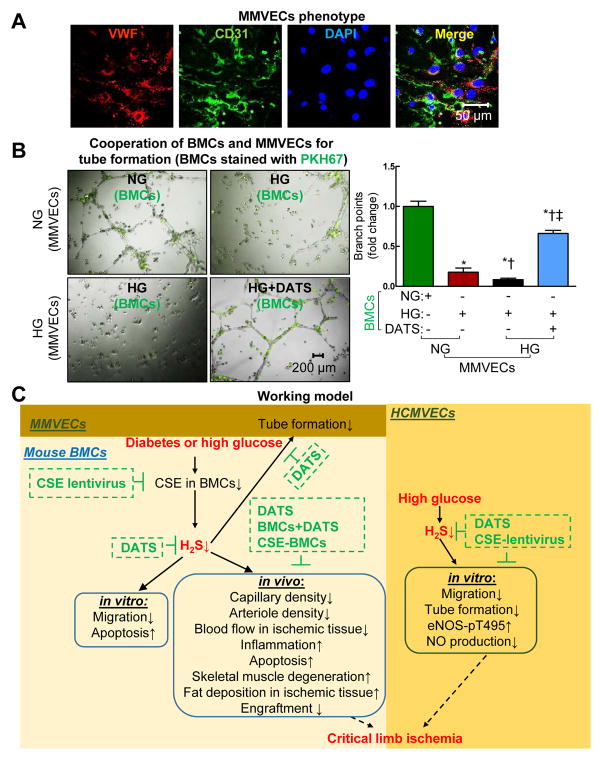
DATS rescued HG-impaired tube formation by incorporation of BMCs and ECs
Tube formation by incorporation of BMCs and ECs has been suggested for studying the role of BMC in promoting tube formation in vivo.33 We found that BMCs became incorporated with mouse microvascular endothelial cells (MMECs) for tube formation, and HG-treated BMCs significantly decreased tube number of length, respectively (Figure. 8A and B, p<0.05). DATS significantly increased the number of BMCs integrated into tubular structures and improved tube formation of MMECs (Figure. 8B, p<0.05), suggesting that H2S deficiency plays a critical role in impaired tube formation of diabetic BMCs and ECs.
Figure 8. HG-impaired tube formation by incorporation of HG-treated BMCs and ECs was rescued by DATS.
(A) Identification of MMVECs. MMVECs isolated from lung of non-diabetic db/+ mice (p3) were identified by endothelial marker vWF (red, Von Willebrand factor) and CD31 (green). Nuclei were stained with DAPI (blue, 4′,6-Diamidino-2-Phenylindole). MMVECs were isolated from 12–14-week non-diabetic db/+ mice using collagenase. (B) HG-treated BMCs exacerbated HG-impaired MMVECs tube formation, which was rescued by DATS. For exploring the BMC migratory phenotype, a novel tube formation assay assessing the ability of BMCs to incorporate into tube formation of MMVECs was examined. BMCs were isolated from 12–14-week non-diabetic db/+ mice by gradient centrifugation. BMCs and MMVECs were treated with NG (5 mM) and/or HG (50 mM) in the presence or absence of DATS (10 μM) for 48 hrs. Then the BMCs were labeled with PKH67 (green) and mixed with MMVECs for tube formation. (C) Schematic representation of mechanisms of diabetes-mediated BMC dysfunction and impaired blood perfusion in IHL. n=3, *p<0.05 vs NG-treated BMCs and MMVECs; †p<0.05 vs HG-treated BMCs+NG-treated MMVECs; ‡p<0.05 vs HG-treated BMCs+HG-treated MMVECs. BMCs, bone marrow cells; MMVECs, mouse microvascular endothelial cells.
DISCUSSION
CLI is one of the major vascular complications in diabetes, which is estimated to affect 400 million or 4.4% of the world population in the next 20 years.34 CLI is defined as advanced stage of peripheral arterial disease with arterial occlusion in lower extremity and results in limb loss, disability and mortality. The first objective in treating CLI is to increase blood flow to the affected limb. Previously, surgical vascular reconstruction and adjunctive pharmacotherapy with antithrombotic treatment have been extensively applied in clinical practice. However, the results have failed to meeting expectations, partially due to relapse.35 In the last decades, cellular therapy has emerged as a potential strategy for treatment of CLI in patients. Since the discovery of BMCs in peripheral circulation,1 the regenerative property of BMCs has been studied extensively. We and others reported that transplantation of BMCs improved angiogenesis/neovascularization and blood flow in ischemic heart and limbs.3, 4 Moreover, the beneficial effects of BMC-based therapy for CLI in diabetic patients have been investigated.5 However, its functional benefits in diabetic patients are modest due to diabetes-induced cell dysfunction.5 Therefore, functional improvement of diabetic BMCs would be a promising approach for treatment of CLI in diabetic patients.
In this study, we aimed to reveal the role and underlying mechanisms of H2S deficiency in the regulation of BMC function, and its effect on ischemic tissue repair in diabetic mice. Here we have demonstrated that; 1) CSE is the dominant H2S-synthesizing enzyme in BMCs. 2) CSE level is downregulated in diabetic BMCs or HG-treated mature HMVECs and thus results in H2S reduction. 3) H2S donor DATS or overexpression of CSE improves reparative capacity of diabetic-BMC in vitro and in vivo. And 4) DATS restored H2S deficiency-induced eNOS-pT495 and NO reduction under HG condition. We demonstrate that our findings may have translational value which opens new avenues for treatment of CLI in diabetes. A summary of our findings are shown in Figure. 8C.
Recent evidence suggests that H2S plays an important role in the maintenance of cardiovascular homeostasis and proliferation, differentiation, anti-inflammatory and anti-apoptosis properties of stem/progenitor cells.11–14, 36 The role and mechanism of H2S in biological functions of diabetic BMC remains unclear. In concordance with recent studies that H2S production was decreased and related to the pathogenesis of CLI and diabetes mellitus,19, 20, 37 we observed H2S production was reduced in plasma, hind limb skeletal muscles and BMCs from diabetic mice. Here, we provide the first evidence showing that H2S production was significantly reduced in BMCs of diabetic mice, and in HG-treated BMCs and mature HMVECs. Importantly we show that restoration of H2S by administration of DATS or overexpression of CSE improved diabetic BMC and HG-treated mature HCMVEC function in vivo or in vitro. Our findings indicate that insufficient H2S plays a critical role in diabetes-induced BMC and mature EC dysfunction.
Although H2S reduction in diabetic patients and animals has been reported, regulation of H2S synthesizing enzymes in diabetes is controversial. CSE levels were either increased in the pancreas, kidney, and liver38 or not altered in the aorta, liver, kidney and heart,39, 40 or decreased in the skin41 which may depend on the disease stage, species, tissues and organs. CSE has been suggested as a dominant enzyme responsible for H2S generation in the cardiovascular system,2 and largely expressed in cardiomyocytes, vascular endothelial cells (ECs), and smooth muscle cells.10 Genetic deletion of the CSE enzyme in mice markedly reduces H2S levels in the serum, heart, aorta, and other tissues.10, 42 The role of CSE and CSE-dependent H2S production in diabetic BMCs is unclear. Our data suggest that BMCs predominantly express CSE. Interestingly, diabetic/hyperglycemic BMCs showed reduced CSE and H2S levels and overexpression of CSE restored H2S production in HG-treated BMCs. Further studies to investigate signaling pathways mediating CSE levels in diabetic BMCs are warranted.
In this study, we examined the effects of H2S on IHL repair in a HLI model of diabetic db/db mice with either systemic administration of DATS or overexpression of CSE in BMCs isolated from db/db mice. We observed that DATS potentiated diabetic BMC-mediated blood perfusion, cell survival, angiogenesis/neovascularization and skeletal muscle architecture recovery. Importantly, we observed that overexpression of CSE in diabetic BMCs significantly improved their reparative capacity. Our studies provide strong evidence for downregulation of CSE as a key factor in diabetes-induced BMC dysfunction. These data suggest that improving diabetic BMC function by overexpression of CSE may present a novel treatment option for autologous cell-based treatment of CLI in diabetic patients.
Prolonged inflammation has been implicated with reduced BMC mobilization, cell death, and functional impairment.3, 24, 43 We have reported that co-administration of interlukin-10 (IL-10) and BMCs enhanced cell survival and angiogenesis/neovascularization after myocardial infarction in mice via suppression of miR-375.3, 24, 43 Moreover, IL-10 KO mice display functional impairment and decreased survival of BMCs when BMCS are transplanted in ischemic myocardium.24 In this study, we observed that DATS or overexpression of CSE in BMCs reduced perivascular CD68 (monocytes/macrophage maker) infiltration and enhanced angiogenesis/neovascularization in ischemic limbs. This is in line with recent reports showing that H2S reduces neutrophil recruitment, stimulates ischemic vascular remodeling and promotes angiogenesis in HLI animal models.15, 16 Our study suggests a significant recruitment of inflammatory cells that was associated with CSE-mediated H2S reduction in diabetic BMCs.
NO is an important signal molecule in cardiovascular homeostasis. Loss of NO bioavailability has been implicated in several disease states such as coronary artery disease, heart failure, and diabetes. Several lines of evidence suggest that H2S requires NO to exert its protective effects in the cardiovascular system.8 H2S and NO cooperatively regulate angiogenesis/neovascularization under physiological and ischemic conditions.32 Pharmacological inhibition or genetic ablation of eNOS has been shown to diminish the beneficial effects of H2S in angiogenesis and wound healing.8, 44, 45 Additionally, H2S-mediated eNOS phosphorylation is important for NO production.8, 46 Furthermore, CSE KO mice exhibited lower eNOS phosphorylation at serine-1177 (eNOS-pS1177, active site) than at threonine-495 (eNOS-pT495, inhibitory site) in heart.8 Acute in vivo administration of DATS restored eNOS-pS1177 but not eNOS-pT495 levels in the heart of CSE KO mice.8 Recently, we have shown that HG induced eNOS-pT495 in aortic endothelial cells.29 Consistent with these findings, DATS significantly inhibited hyperglycemia-induced eNOS-pT495. Our results suggest H2S deficiency plays an important role in HG-impaired BMCl function. Importantly, we observed that HG-decreased eNOS-pS1177 in HCMVECs and was not rescued by DATS. Moreover, we also found that silencing of CSE by siRNA dose-dependently increased eNOS-pT495 but did not affect eNOS-pS1177 levels in HCMVECs. The contradictory findings on effects of DATS and the role of CSE on the regulation of eNOS-pT495 and eNOS-pT1177 may be due to the differences in doses and routes of DATS administration, as well as experimental species, conditions, organs and cell types. Our data indicate that downregulation of CSE-mediated H2S deficiency plays an important role in eNOS-pT495/NO reduction/EC dysfunction cascade under HG condition. We are the first to demonstrate that the beneficial effects induced by administration of DATS or overexpression of CSE in diabetic BMCs may be mediated via eNOS-pT495/NO signaling pathways.
Several other mechanisms were also reported to be involved in H2S-regulated angiogenesis and stem/progenitor cell function and biology. For instance, H2S improved endothelial progenitor cell function and wound healing in db/db mice via activation of angiopoietin-1.41 H2S maintains mesenchymal stem cell (MSC) function via sulfhydration of calcium channel.14 CSE/H2S protects MSC from hypoxia and serum-deprivation induced cell death via attenuation of the mitochondrial injury pathway, inhibition of endoplasmic reticulum stress and activation of the PI3K/Akt signaling pathway.12 NaHS improved human adipose tissue-derived stem cells survival via increased antioxidant defense, enhanced ERK-phosphorylation and decreased AKT-phosphorylation.47 Taken together, our data along with published literature suggests that H2S pathway may incorporate multiple molecular and signaling modules to exert its effect in cell and disease specific manner.
Despite evidence supporting a cell-protective role of H2S in animal models, translation of preclinical findings of cell protection from the bench to the bedside is still challenging due to uncertainty of dosing, timing, route and stability of H2S donors.48 In addition, H2S donor-induced side effects, such as hypothermia, anti-inflammatory and pro-inflammatory properties, have not been defined clearly.48 Therefore, it is imperative to test the safety, tolerability, efficacy and stability, as well as define the side effects, of novel H2S donors in large animal models which are clinically relevant and express cardiovascular similarities with humans before translating H2S therapy to patients with cardiovascular diseases.49 Therefore it is still challenging to treat CLI in diabetic patients using H2S donors. Increasing endogenous H2S in diabetic BMCs may emerge as a potential therapeutic strategy for CLI in diabetic patients.
DATS act as H2S donor when they react with biological thiols including glutathione (GSH), or by human red blood cells via glucose-supported reaction. DATS exerts various beneficial biological effects in different organs, including anti-hyperlipidemia, -hypoglycemia, -cancer, –thrombosis, –inflammatory responses, and –oxidative stress in appropriate doses (see review 50). In good accordance, here we provide strong evidence that systemic administration of DATS improved diabetic BMC-mediated angiogenesis/neovascularization in ischemic hind limbs of diabetic db/db mice, suggesting that H2S deficiency plays important role in regulation of diabetic BMC biology and function.
Although a single study reported that systemic administration of H2S donor NaHS and hydroxythiobenzamide (HTB) restored diabetic BMC-mediated wound healing in db/db mice,41 the biological function of H2S deficiency in BMC-mediated repair of ischemic limb injury in diabetes has never been studied. Consistent with the above study, we observed that systemic administration of DATS potentiates diabetic BMC functional and biological properties in vivo and in vitro. To extend these findings, here we provide evidence that increasing endogenous H2S production by overexpression of CSE in diabetic BMCs rescues diabetes-induced BMC dysfunctional and reparative properties. We demonstrate that increasing endogenous H2S production by overexpressing CSE in diabetic BMCs may serve as a therapeutic modality to treat CLI in diabetic patients in the future.
CONCLUSIONS
Downregulation of CSE plays a major role in diabetes-induced H2S deficiency in BMCs. Systemic administration of H2S donor DATS or overexpression of CSE in BMCs restores diabetic BMC reparative capacity for ischemic hind limb ischemia in diabetic db/db mice. Our findings suggest that overexpression of CSE in diabetic BMCs may boost the efficacy of autologous cell-based treatment of CLI in diabetic patients.
Supplementary Material
CLINICAL PERSPECTIVE.
What Is New?
We provide evidence that diminished H2S levels in diabetic bone marrow cells impair their angiogenic reparative activity. Particularly:
Diabetes or hyperglycemia (HG) leads to the reduction of H2S levels
CSE is the dominant H2S-synthesizing enzyme in BMCs and is downregulated in diabetic BMCs or HG-treated mature HMVECs which results in H2S deficiency.
H2S donor DATS or overexpression of CSE improves reparative capacity of diabetic-BMC in vitro and in vivo.
Finally, DATS restored H2S deficiency-induced eNOS-pT495 and NO reduction under HG condition.
What Are the Clinical Implications?
These findings strongly support the emerging concept that restoration of H2S levels may be of clinical importance and may potentially be a therapeutic target in diabetic patients with cardiovascular diseases.
Particularly, our findings have important translational value and open new avenues for modulating H2S levels in diabetic BMCs before treatment of CLI.
Acknowledgments
FUNDING SOURCE
This work was supported in part by the NIH grants HL091983, HL053354 and HL126186 (R.K.) and American Heart Association grant SDG16390004 (Z.C.).
Footnotes
DISCLOSURES
None
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants & redox signaling. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 3.Garikipati VN, Krishnamurthy P, Verma SK, Khan M, Abramova T, Mackie AR, Qin G, Benedict C, Nickoloff E, Johnson J, Gao E, Losordo DW, Houser SR, Koch WJ, Kishore R. Negative regulation of mir-375 by interleukin-10 enhances bone marrow-derived progenitor cell-mediated myocardial repair and function after myocardial infarction. Stem Cells. 2015;33:3519–3529. doi: 10.1002/stem.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circulation research. 2011;109:1280–1289. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasala GP, Minguell JJ. Vascular disease and stem cell therapies. British medical bulletin. 2011;98:187–197. doi: 10.1093/bmb/ldr017. [DOI] [PubMed] [Google Scholar]
- 6.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circulation research. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through nrf2 signaling. Circulation research. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circulation research. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2s as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Y, Liu D, Liu Y, Zhang C, Wang J, Wang S. Physiologic levels of endogenous hydrogen sulfide maintain the proliferation and differentiation capacity of periodontal ligament stem cells. Journal of periodontology. 2015;86:1276–1286. doi: 10.1902/jop.2015.150240. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Li CS, Wang CM, Xie YJ, Wang AL. Cse/h2s system protects mesenchymal stem cells from hypoxia and serum deprivationinduced apoptosis via mitochondrial injury, endoplasmic reticulum stress and pi3k/akt activation pathways. Molecular medicine reports. 2015;12:2128–2134. doi: 10.3892/mmr.2015.3651. [DOI] [PubMed] [Google Scholar]
- 13.Fox B, Schantz JT, Haigh R, Wood ME, Moore PK, Viner N, Spencer JP, Winyard PG, Whiteman M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: Is h2s a novel cytoprotective mediator in the inflamed joint? Journal of cellular and molecular medicine. 2012;16:896–910. doi: 10.1111/j.1582-4934.2011.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, Shi S. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of ca(2+) channel sulfhydration. Cell Stem Cell. 2014;15:66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. Journal of the American Heart Association. 2012;1:1–17. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor nahs promotes angiogenesis in a rat model of hind limb ischemia. Antioxidants & redox signaling. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 17.Lambert JP, Nicholson CK, Amin H, Amin S, Calvert JW. Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the risk pathway. Medical gas research. 2014;4:1–11. doi: 10.1186/s13618-014-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Bucci M, Cirino G. Biosynthesis of h2s is impaired in non-obese diabetic (nod) mice. British journal of pharmacology. 2008;155:673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 20.Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxidants & redox signaling. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bir SC, Pattillo CB, Pardue S, Kolluru GK, Shen X, Giordano T, Kevil CG. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a no/vegf-dependent manner. Diabetes. 2014;63:270–281. doi: 10.2337/db13-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Sr, Gojon G, Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H(2)s protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. American journal of physiology Heart and circulatory physiology. 2012;302:H2410–2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. Il-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of stat3 and suppression of hur. Circulation research. 2009;104:e9–18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nature protocols. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW, Zhang HZ, Kim CE, An HS, Kim JM, Kim MH. Amniotic mesenchymal stem cells have robust angiogenic properties and are effective in treating hindlimb ischaemia. Cardiovascular research. 2012;93:525–534. doi: 10.1093/cvr/cvr328. [DOI] [PubMed] [Google Scholar]
- 27.Kishore R, Qin G, Luedemann C, Bord E, Hanley A, Silver M, Gavin M, Yoon YS, Goukassian D, Losordo DW. The cytoskeletal protein ezrin regulates ec proliferation and angiogenesis via tnf-alpha-induced transcriptional repression of cyclin a. The Journal of clinical investigation. 2005;115:1785–1796. doi: 10.1172/JCI22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mier PD, van den Hurk JJ. Lysosomal hydrolases of the epidermis. I. Glycosidases. The British journal of dermatology. 1975;93:1–10. doi: 10.1111/j.1365-2133.1975.tb06468.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Z, Jiang X, Pansuria M, Fang P, Mai J, Mallilankaraman K, Gandhirajan RK, Eguchi S, Scalia R, Madesh M, Yang X, Wang H. Hyperhomocysteinemia and hyperglycemia induce and potentiate endothelial dysfunction via mu-calpain activation. Diabetes. 2015;64:947–959. doi: 10.2337/db14-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Z, Jiang X, Kruger WD, Pratico D, Gupta S, Mallilankaraman K, Madesh M, Schafer AI, Durante W, Yang X, Wang H. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118:1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, Tran-Son-Tay R, Grant MB. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 32.Yuan S, Kevil CG. Nitric oxide and hydrogen sulfide regulation of ischemic vascular remodeling. Microcirculation. 2015;23:134–145. doi: 10.1111/micc.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi Y, Okazaki Y, Seta N, Satoh T, Takahashi K, Ikezawa Z, Kuwana M. Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis research & therapy. 2010;12:1–11. doi: 10.1186/ar3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the united states, 1990–2010. The New England journal of medicine. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 35.Borkosky SL, Roukis TS. Incidence of re-amputation following partial first ray amputation associated with diabetes mellitus and peripheral sensory neuropathy: A systematic review. Diabetic foot & ankle. 2012;3:1–5. doi: 10.3402/dfa.v3i0.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie X, Sun A, Zhu W, Huang Z, Hu X, Jia J, Zou Y, Ge J. Transplantation of mesenchymal stem cells preconditioned with hydrogen sulfide enhances repair of myocardial infarction in rats. The Tohoku journal of experimental medicine. 2012;226:29–36. doi: 10.1620/tjem.226.29. [DOI] [PubMed] [Google Scholar]
- 37.Islam KN, Polhemus DJ, Donnarumma E, Brewster LP, Lefer DJ. Hydrogen sulfide levels and nuclear factor-erythroid 2-related factor 2 (nrf2) activity are attenuated in the setting of critical limb ischemia (cli) Journal of the American Heart Association. 2015;4:1–10. doi: 10.1161/JAHA.115.001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, Ni J, Yu C, Yao T, Huang Y, Wang R, Lu L. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:2119–2126. doi: 10.1093/ndt/gfq749. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denizalti M, Bozkurt TE, Akpulat U, Sahin-Erdemli I, Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn-Schmiedeberg’s archives of pharmacology. 2011;383:509–517. doi: 10.1007/s00210-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 41.Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, Chen AF. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014;63:1763–1778. doi: 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voskuil M, Hoefer IE, van Royen N, Hua J, de Graaf S, Bode C, Buschmann IR, Piek JJ. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9:287–292. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 43.Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, Thal M, Mackie A, Hoxha E, Ramirez V, Qin G, Sadayappan S, Ghosh AK, Kishore R. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappab. Circulation. 2012;126:418–429. doi: 10.1161/CIRCULATIONAHA.112.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Candiloros H, Muller S, Zeghari N, Donner M, Drouin P, Ziegler O. Decreased erythrocyte membrane fluidity in poorly controlled iddm. Influence of ketone bodies. Diabetes care. 1995;18:549–551. doi: 10.2337/diacare.18.4.549. [DOI] [PubMed] [Google Scholar]
- 45.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. Journal of cellular and molecular medicine. 2013;17:879–888. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dongo E, Benko Z, Csizmazia A, Marosi G, Grottke A, Jucker M, Schumacher U, Kiss L. H2s preconditioning of human adipose tissue-derived stem cells increases their efficacy in an in vitro model of cell therapy for simulated ischemia. Life sciences. 2014;113:14–21. doi: 10.1016/j.lfs.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 48.Wagner F, Asfar P, Calzia E, Radermacher P, Szabo C. Bench-to-bedside review: Hydrogen sulfide--the third gaseous transmitter: Applications for critical care. Crit Care. 2009;13:213. doi: 10.1186/cc7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salloum FN. Hydrogen sulfide and cardioprotection--mechanistic insights and clinical translatability. Pharmacology & therapeutics. 2015;152:11–17. doi: 10.1016/j.pharmthera.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Ji X, Ji K, Wang B. Hydrogen sulfide prodrugs-a review. Acta pharmaceutica Sinica B. 2015;5:367–377. doi: 10.1016/j.apsb.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.