Abstract
Memory T cells can often respond against pathogens that have evaded neutralizing antibodies and are thus key to vaccine-induced protection, yet the signals needed to optimize their responses are unclear. Here, we identify a dramatic and selective requirement for IL-6 to achieve optimal memory CD4 T cell recall following heterosubtypic influenza A virus (IAV) challenge of mice primed previously with wild-type or attenuated IAV strains. Through analysis of endogenous T cell responses and adoptive transfer of IAV-specific memory T cell populations, we find that without IL-6, CD4+, but not CD8+, secondary effector populations expand less and have blunted function and anti-viral impact. Early and direct IL-6 signals to memory CD4 T cells are required to program maximal secondary effector responses at the site of infection during heterosubtypic challenge indicating a novel role for a costimulatory cytokine in recall responses.
Introduction
Innate inflammatory responses are critical for protection against primary influenza A virus (IAV) infection. Triggering of innate immune recognition pathways with pathogen lysates or specific pathogen products to boost acute inflammation (1–5) dramatically improves the outcome of IAV challenge in unprimed mice. In contrast, mice deficient for toll-like receptor (TLR) adaptor proteins MyD88 and TRIF (6, 7), components of inflammasome pathways (8, 9), or treated with anti-inflammatory agents (10), are all far more susceptible to primary IAV challenge. A key function of innate immune recognition pathways is to induce production of costimulatory cytokines, but how individual components of the inflammatory response contribute to protection is not clear, at least in part because the impact of many cytokines and chemokines is multifactorial. For example, unprimed IL-6-deficient (Il6−/−) mice succumb to doses of IAV that are sublethal for WT mice, an outcome correlated in different studies with either impaired antibody (Ab) production, reduced neutrophil activity, or dysregulated immunopathology (11–13). Similarly, IL-1 (8, 14) and TNF (15–19) each have been shown to contribute to protection against primary IAV infection through multiple mechanisms.
Mice and humans primed with, or vaccinated against, one strain of IAV generate neutralizing Ab that prevents reinfection with the same strain providing long-lived ‘homotypic’ immunity. Priming also generates strong protection against IAV strains not recognized by neutralizing Ab. This ‘heterosubtypic’ immunity is largely mediated by memory T cells that recognize internal IAV protein epitopes that are shared between the priming and challenge virus (20). In contrast to the critical role of TLR pathways in protecting naïve animals against IAV, heterosubtypic protection does not require TLR signaling as IAV-primed WT and MyD88/TRIF-deficient mice are equally resistant to supralethal heterosubtypic challenge (6). This has been taken to indicate that innate inflammation plays only a minimal role in heterosubtypic immunity. However, memory CD4 T cells responding against IAV rapidly induce the production of high levels of several cytokines including IL-6, IL-1, and TNF from several innate immune cell subsets through a MyD88/TRIFF-independent mechanism (21). This T cell-driven inflammatory response correlates with viral control (22, 23) and suggests that production of some innate cytokines, triggered through pathways other than the traditional pattern recognition receptor pathways, may be critical for heterosubtypic protection (24).
Here, we analyze whether and how IL-6 expression impacts heterosubtypic immunity. Il6−/− mice primed with highly virulent or attenuated mouse-adapted IAV strains displayed impaired viral clearance and enhanced infection-associated morbidity following secondary challenge with heterosubtypic IAV. Analysis of endogenous polyclonal and adoptively transferred T cell receptor (TcR) transgenic CD4 and CD8 T cell responses reveal that the generation of IAV-specific T cell memory is not impacted by IL-6. However, the magnitude and functional potential of recall CD4 T cell responses is dramatically and selectively impaired. We confirm that protection against IAV mediated by adoptive transfer of memory CD4+, but not CD8+, T cells is severely compromised in Il6−/− hosts, as well as in WT hosts treated with IL-6-neutralizing Ab.
Mechanistically, the critical IL-6 signals required for optimal memory CD4 T cell-mediated protection are delivered only during the first few days post-infection (dpi). Early IL-6 drives maximum expansion of primed CD4 T cells and enhances production of the cytokines IL-2, TNF, and IFN-γ, especially in the cohort of cells responding in the lung. Finally, by analyzing IL-6 receptor deficient memory CD4 T cells responding in WT hosts, we show that direct IL-6 signals to memory CD4 T cells are responsible for promoting maximal secondary (2°) effector expansion and function.
These findings highlight striking differences in how IL-6 impacts the outcome of primary versus secondary IAV challenge, and in how IL-6 affects recall responses of CD4+ versus CD8+ T cells during acute viral infection. Our studies indicate a unique role for early IL-6 in promoting protective CD4 T cell memory responses and suggest that upregulated expression of IL-6 during this phase of the recall response might dramatically improve heterosubtypic protection against seasonal and pandemic IAV.
Materials and Methods
Mice
BALB/c, C57BL/6, C57BL/6.Thy1.1, JHD (BALB/c background), and Il6−/− (BALB/c and C57BL/6 background) mice were at least 8 weeks old at time of infection. Naïve CD4+ cells and CD8+ T cells were obtained from 5–8 week old HNT.Thy1.1 mice or Clone 4.Thy1.1 mice on a BALB/c background, respectively. Naïve CD4 T cells were also obtained from OT-II.Thy1.1.Thy1.2 mice or OT-II cells deficient for IL-6 receptor α (CD126) that were obtained by crossing C57BL/6 mice carrying a floxed IL-6 receptor α gene (Jackson) to OT-II mice expressing Cre driven by the CD4 promoter. HNT mice express a TcR recognizing aa 126–138 (HNTNGVTAACSHE) of A/Puerto Rico/8/34 (PR8, H1N1) hemagglutinin (HA), OT-II mice express a TcR recognizing aa 323–339 (ISQAVHAAHAEINEAGR) of chicken ovalbumin (OVA), and Clone 4 mice express a TCR recognizing aa 518–528 (IYSTVASSL) of PR8 HA. JHD and HNT TcR Tg mice were obtained from the animal breeding facility at Trudeau Institute or University of Massachusetts Medical School. Some mice were originally obtained from Jackson Laboratories and were bred at Trudeau Institute or UMASS Medical School. Experimental animal procedures were conducted in accordance with Animal Care and Use Committee guidelines.
Naïve cell isolation, memory T cell generation, cell transfer, and Ab treatments
Naïve T cells from TcR Tg mice were obtained from pooled spleen and lymph nodes as previously described (25). Resulting TcR Tg cells were routinely >97% TcR+ and expressed a characteristic naive phenotype (small size, CD62Lhi, CD44lo and CD25lo). TH1 or Tc1 effectors were generated from naïve TcR Tg T cells cultured as previously described (25–27). In vitro-generated memory cells were obtained from effector cultures that were washed several times and rested for at least 3 days in media free of antigen (Ag) or exogenous cytokine. In some experiments, cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes) prior to adoptive transfer. All cell populations were adoptively transferred to unprimed mice in 200 μl PBS by i.v. injection.
Mice were injected i.p. with IL-6-neutalizing Ab (MP5-20F3), or isotype control Ab (HRPN) (BioXcell) as indicated. In some experiments, mice were treated i.p. with 0.5 mg of anti Ly-6G Ab to deplete neutrophils (1A8) or isotype control (2A3) (BioXcell) on 0, 2, 4, and 6 dpi.
Virus stocks and infections
A/Puerto Rico/8/34 (PR8), (H1N1) originating from stocks at St. Jude Children’s Hospital, A/PR8-OVAII (H1N1) (kindly provided by P. Doherty), A/Philippines/2/82/x-79 (A/Phil), (H3N2), and the cold-adapted attenuated strain A/Alaska/6/77 CR-29, (H3N2) (28), (both kindly provided by S. Epstein, NIH) were grown in the allantoic cavity of embryonated hen eggs at the Trudeau Institute and the egg infective dose (EID50) or tissue culture infective dose (TCID50) characterized. Mice were infected intranasally under light isoflurane anesthesia (Webster Veterinary Supply) with stated doses of virus in 50 μl PBS. Mice that received adoptively transferred T cells were infected on the same day as cell transfer. All infected mice were monitored daily for percent weight loss, hunched posture, ruffled fur, and lack of movement as previously described (29).
Tissue preparation
At different time points after virus infection, mice were euthanized by cervical dislocation followed by exsanguination by perforation of the abdominal aorta. Lungs were perfused by injecting 10 ml of PBS in the left ventricle of the heart. Lungs, spleen, and draining mediastinal lymph node (dLN) were prepared into single cell suspensions by mechanical disruption of organs and passage through a nylon membrane.
For assessment of immunopathology following IAV infection, lung lobes were isolated and immediately fixed in 10% neutral buffered formalin. Lung samples were subsequently processed, embedded in paraffin, sectioned, placed on L-lysine-coated slides, and stained with Hematoxylin and Eosin (H&E) using standard histological techniques. Sections were graded blindly from 0 to 4 by a board certified pathologist (S. Sell) on the basis of the extent of mononuclear cell infiltration and tissue damage.
Real Time-PCR
Viral titers were determined by quantitation of viral RNA. RNA was prepared from whole lung homogenates using TRIzol (Sigma-Aldrich), and 2.5 μg of RNA was reverse transcribed into cDNA using random hexamer primers and Superscript II Reverse Transcriptase (Invitrogen). Quantitative PCR was performed to amplify the polymerase (PA) gene of PR8 and A/Phil using an ABI Prism 7700 Sequence Detector (Applied Biosystems) with 50 ng of cDNA per reaction and the following primers and probe: forward primer, 5′-CGGTCCAAATTCCTGCTGA-3′; reverse primer, 5′CATTGGGTTCCTTCCATCCA-3′; probe, 5′-6-FAM-CCAAGTCATGAAGGAGAGGGAATACCGCT-3′. Data were analyzed with Sequence Detector v1.7a (Applied Biosystems). The copy number of the PA gene per 50 ng of cDNA was calculated using a PA-containing plasmid of known concentration as a standard.
Flow cytometry
Cell suspensions were washed, resuspended in FACS buffer (PBS plus 0.5% BSA and 0.02% sodium azide (NaN3); Sigma-Aldrich) and incubated on ice with 1 μg anti-FcR (2.4G2) followed by saturating concentrations of the following fluorochrome-labeled Abs for surface staining: anti-Thy1.1 (OX-7), anti-Thy1.2 (53-2.1), anti-CD4 (RM4.5), anti-CD8 (H35-17.2), anti-CD69 (H12F3), anti-CD25 (PC61.5), anti-CD127 (A7R34), anti-CD44 (IM7), anti-CD45.2 (104), anti-CD11b (M1/70), anti-Ly6-G (Gr-1, RB6-8C5). (Pharmingen, eBioscience, or BioLegend). To detect IAV-specific polyclonal CD4 T cells in IAV-primed mice, cells were stained for 1 hour (hr.) at 37°C with I-Ab/NP311–325-fluorochrome-labelled tetramer obtained from the NIH tetramer facility prior to surface marker staining.
For intracellular cytokine staining (ICCS), CD4 T cells were stimulated for 4 h with 10 ng/ml PMA and 50 ng/ml ionomycin (Sigma) or for 18 hrs. with 100 hemagglutination units (HAU) of IAV (PR8) lysate. After 2h, 10 μg/ml Brefeldin A (Sigma) was added. Cells were then surface stained and fixed for 20 min in 4% paraformaldehyde followed by permeablization for 10 min by incubation in 0.1% saponin buffer (PBS plus 1% FBS, 0.1% NaN3 and 0.1% saponin; Sigma-Aldrich) and staining for cytokine by the addition of anti-IFN-γ, -IL-2, and TNF fluorescently labeled Ab (Pharmingen, eBioscience, or BioLegend) for 20 minutes. Granzyme B and Foxp3 (ebioscience) expression was determined by intracellular staining as per manufactures instructions.
All FACS analysis was performed using LSRII and Canto flow cytometers (BD Biosciences) and FlowJo (Tree Star) analysis software.
ELISPOT Assay
ELISPOT analysis for detection of IFN-γ and IL-2 secreting T cells was performed as previously described (21). Briefly, 96-well plates (Millipore) were coated overnight with purified anti-mouse IFN-γ or IL-2 Ab. Plates were washed and blocked with complete T cell media prior to the addition of 105 lung cells or 106 spleen or lymph node cells and 106 syngeneic APC from spleen of unprimed mice. Wells were stimulated with 100 HAU of IAV lysate to elicit CD4 T cell responses or with 10 μg of PA224–233/Db, NP366–374/Db, or PB703–711/Kb peptides to elicit CD8 T cell responses. Plates were incubated overnight with biotinylated detection Ab against IFN-γ or IL-2 followed by streptavidin alkaline phosphate substrate. Plates were developed with 5-bromo-4-chloro-indolyl phosphate substrate and resulting spots were counted on an ImmunoSpot reader (CTL).
Detection of cytokines in culture supernatants
Luminex analysis with a multiplex kit (Millipore) was used to detect levels of cytokines present in culture supernatants. Purified memory OT-II cells were stimulated in vitro with 10 ng/ml PMA and 50 ng/ml ionomycin. After 8 hours, culture supernatants were harvested and analyzed using a Bio-Plex 200 System (Bio-Rad).
Statistical analysis
Unpaired, two-tailed, Students t-tests, ∞ = 0.05, were used to assess whether the means of two normally distributed groups differed significantly. The Welch-correction was applied when variances were found to differ. One-way ANOVA analysis with Bonferroni’s multiple comparison post-test was employed to compare multiple means. Significance is indicated as * P < 0.05, ** P < 0.005, *** P < 0.001, and **** P < 0.0001. The Log Rank test was used to test for significant differences in Kaplan-Meier survival curves. All error bars represent the standard deviation.
Results
IL-6 is required for survival following high dose IAV priming
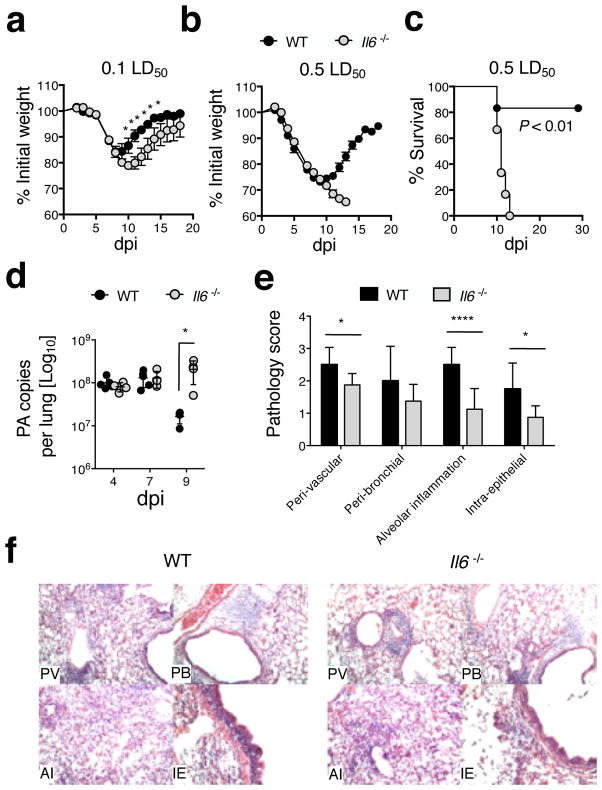
In order to study the role of IL-6 in heterosubtypic protection we first primed WT BALB/c or corresponding Il6−/− mice with a low dose (500 EID50 = 0.1 LD50 for WT mice) of the highly pathogenic mouse-adapted H1N1 IAV strain A/PR8 (30) and followed the course of the primary response. Only minimal differences in weight loss and recovery distinguished WT and Il6−/− mice (Fig 1a) and all mice survived. Because higher doses of IAV generate stronger T cell memory (31), we next challenged mice with 5 times more virus (2500 EID50, 0.5 LD50 for WT mice). Both strains initially lost equivalent weight (Fig 1b), but while virtually all WT mice recovered, none of the Il6−/− mice survived (Fig 1c). Identical results were observed in WT and Il6−/− C57Bl/6 mice (not shown). The reduced resistance of Il6−/− mice to a 2500 EID50 dose of IAV correlated with increased viral titers detected at 9 dpi but not at earlier time points (Fig 1d), consistent with observations of IL-6 deficiency leading to impaired viral clearance during primary infection (12, 13). Histological analysis at 7 dpi revealed greater inflammation in WT lungs based on perivascular, peribronchial, intraepithelial, and alveolar changes (Fig 1e and f). Together, these results confirm that IL-6 mediates pro-inflammatory and anti-viral effects that are required for the resolution of higher dose primary IAV infection (12).
Figure 1. High priming doses of IAV are lethal in the absence of IL-6.
WT (black symbols) or Il6−/− (grey symbols) mice on a BALB/c background were infected with a low dose (500 EID50 = 0.1 LD50 for WT mice) or high dose (2500 EID50 = 0.5 LD50) of A/PR8 (H1N1). Weight loss following (a) low and (b) high dose challenge (n = 5 mice/group; one of 5 experiments). On the indicated days following 2500 EID50 challenge, (c) survival, and (d) viral titers were assessed (n =3–5 mice/group/day). H&E stained lung sections were scored blindly for levels of histopathology on 7 dpi. Summary of analysis (n = 3–5 mice/group/day) (e), and representative H&E images at 10x (f) for categories of inflammation scored in (e) (PV = peri-vascular; PB = peri-bronchial; AI = alveolar inflammation; IE = Intra-epithelial).
IL-6 is required for optimal heterosubtypic immunity
We next tested the importance of IL-6 during heterosubtypic challenge of WT and Il6−/− mice primed with 500 EID50 of A/PR8 (H1N1), from which all mice recovered (Fig 1a). Primed mice were challenged after at least 45 days with a supralethal (150 LD50) dose of the H3N2 A/Phil IAV strain. Compared to WT mice Il6−/− mice lost significantly more body weight and their recovery was delayed several days (Fig 2a). Nevertheless, all primed Il6−/− mice survived the 150 LD50 challenge (Fig 2b). Enhanced weight loss in the Il6−/− mice correlated with higher viral titers and delayed viral clearance versus primed WT mice (Fig 2c), however an impressive degree of viral control was nonetheless evident in primed Il6−/− mice compared to unprimed WT mice challenged with the same dose of A/Phil (Fig 2c). These results demonstrate that IL-6 contributes significantly to optimal viral clearance and reduced morbidity following heterosubtypic IAV challenge. This could be due either to its actions during primary or secondary responses, or both.
Figure 2. IL-6 is required for optimal heterosubtypic protection.
WT or Il6−/− mice on a BALB/c background were primed with a low dose (500 EID50 = 0.1 LD50 for WT mice) of A/PR8 (H1N1). After 45 days mice were challenged with a 150 LD50 dose of A/Philippines (H3N2) virus and (a) morbidity and (b) survival monitored (n=5 mice/group; one of 2 experiments). Viral titers (c) from lungs of A/PR8-primed WT or Il6−/− mice (n=4 mice/group), or unprimed WT control mice (n=3) were assessed on the stated dpi after 150 A/Phil challenge. Data is representative of one of two experiments. The dashed line in (c) represents sensitivity cutoff of the assay.
Recall CD4 T cell responses are compromised in Il6−/− mice
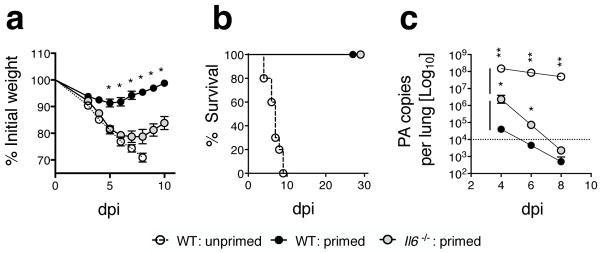
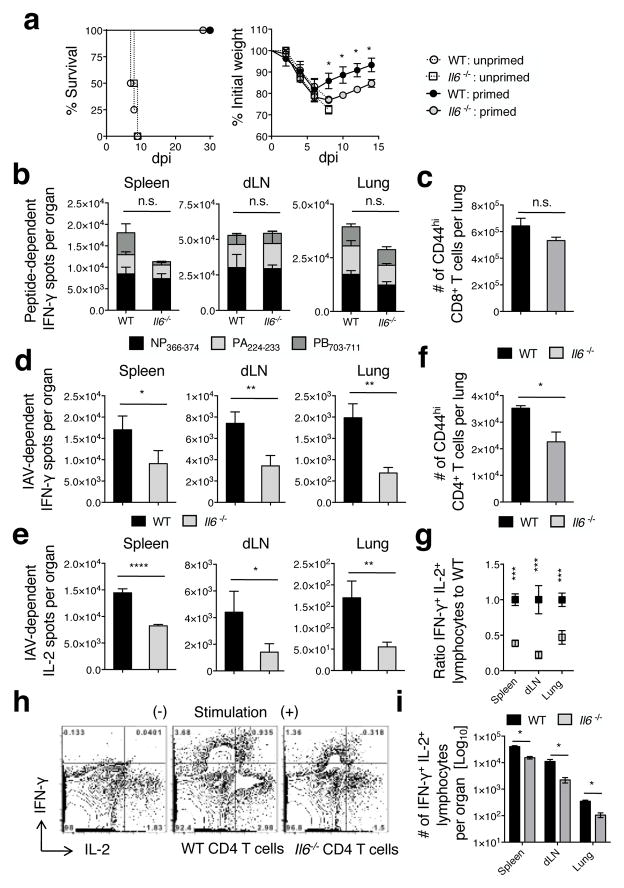
To test a role for IL-6 in a model more closely resembling vaccine-generated heterosubtypic immunity we used the attenuated cold-adapted H3N2 IAV strain A/Alaska/77 (21) as a priming virus. WT or Il6−/− C57BL/6 mice were primed with two doses of 2500 TCID50 A/Alaska/72 ten days apart, followed at least 45 days later by challenge with 20 LD50 A/PR8 as in our previous studies (21). All primed WT and Il6−/− mice survived the 20 LD50 A/PR8 challenge while unprimed mice succumbed by 10 dpi (Fig 3a, left panel), indicating induction of substantial heterosubtypic immunity by the attenuated IAV strain. However, primed Il6−/− mice lost significantly more weight and their recovery was delayed versus primed WT mice (Fig 3a, right panel), consistent with results observed in the live non-attenuated heterosubtypic model summarized in Figure 2.
Figure 3. CD4 but not CD8 T cell cytokine responses are reduced during heterosubtypic challenge of mice primed with attenuated IAV.
WT or Il6−/− C57BL/6 mice were primed with two doses of 2500 TCID50 A/Alaska ten days apart. At day 45 post-priming mice were challenged with 20 LD50 A/PR8. Survival and weight loss (a) of primed or unprimed control WT and Il6−/− mice (n=4 mice/group; one of two experiments). IFN-γ responses elicited by immunodominant MHC I-restricted peptides were enumerated by ELISPOT from cells isolated from stated organs 5 days after A/PR8 challenge primed WT or Il6−/− mice (b) and numbers of CD44hi CD8 T cells in the lung determined by FACS (c) (n=3 mice/group; one of two experiments). Similarly, IFN-γ (d) and IL-2 (e) responses were enumerated in stated organs by ELISPOT using IAV lysate to stimulate CD4 T cells (n=3 mice/group; one of two experiments) and the number of CD44hi CD4 T cells in the lung determined by FACS (f). Dual IFN-γ and IL-2 production by CD4 T cells in the stated organs in response to IAV lysate was determined by ICCS and the frequency expressed as a ratio to the WT response (g), representative staining of spleen cells (h), and the enumeration of dual cytokine producing cells (i) is shown.
Heterosubtypic immunity is largely dependent on memory T cell responses directed against epitopes of internal proteins that are shared between the priming and challenge virus (32). Therefore, we used ELISPOT analysis at 5 dpi, the peak of secondary T cell responses in this model (21), to enumerate virus-specific IL-2- and IFN-γ-producing T cells. Immunodominant MHC I-restricted peptides derived from the nucleoprotein (NP366–374) or RNA polymerase proteins (PA224–233 and PB1703–711) that are highly conserved between A/Alaska and A/PR8 (21) were used to detect CD8 T cell responses. IAV (PR8) lysate (33) was used to stimulate a broad spectrum of IAV-reactive CD4 T cells.
The number of IFN-γ-secreting T cells elicited by the immunodominant CD8 T cell epitopes was similar in WT and Il6−/− mice (Fig 3b) as was the total number of activated CD44hi CD8+ T cells present in the lung (Fig 3c). There was an indication that PB1703–711 responses in the spleen may be somewhat dependent on IL-6, but the differences did not reach significance and were not present in the dLN or lung. Very few peptide-dependent IL-2 spots were detected from CD8 T cells (not shown). Thus, the generation of 2° IAV-specific CD8 T cell responses did not depend on the presence of IL-6 either during the primary response or following secondary challenge.
In contrast at 5 dpi, both IL-2 and IFN-γ spots generated in response to IAV lysate in the spleen, dLN, and lung (Fig 3d and e), and the total number of activated CD44hi CD4+ T cells detected in the lung (Fig 3f) were significantly reduced in Il6−/− mice indicating by inference an impaired recall CD4 T cell response. To verify that the CD4 T cell response is impaired, the frequency and number of IAV-specific CD4+ T cells capable of dual IFN-γ/IL-2 production at 5 dpi was assessed in the spleen, dLN, and lung by intracellular cytokine staining. When compared to WT hosts, a significant reduction in the frequency (Fig 3g and h), expressed as a ratio of the WT response, and number of dual IFN-γ/IL-2 cytokine producers (Fig 3i) was observed in Il6−/− mice. Virtually no IAV lysate- or MHC I peptide-dependent spots were detected in unprimed mice challenged with A/Phil at this time point consistent with previous studies (21). In total, these results suggest that compromised heterosubtypic protection observed in Il6−/− mice is likely to be largely due to the reduced efficacy of recall CD4+ T cell responses.
Memory CD4 T cell-generation is not impaired in Il6−/− mice
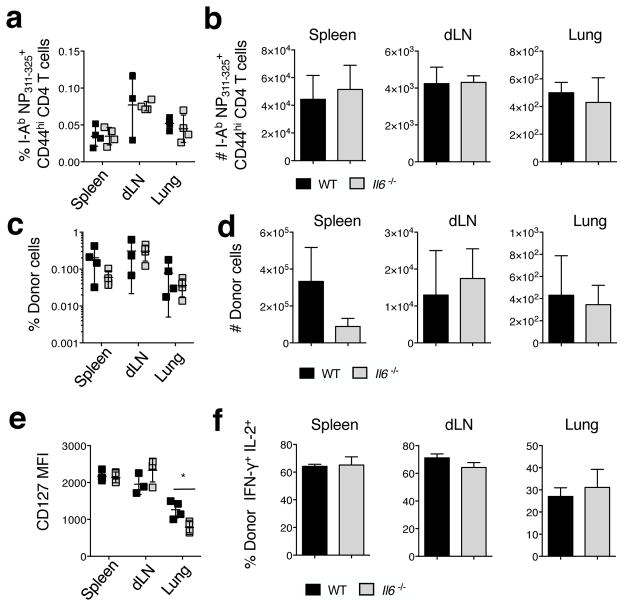
The decreased recall CD4 T cell responses in Il6−/− mice could reflect impaired memory cell generation after initial priming, reduced CD4 T cell memory maintenance, or a reduced secondary CD4 T cell response following rechallenge. To test these possibilities, we first enumerated memory CD4 T cells recognizing NP311–325 in A/Alaska-primed WT and Il6−/− mice prior to heterosubtypic challenge using an MHC II tetramer (34). The frequency and absolute number of CD44high tetramer+ cells detected in all organs tested at 45 dpi was equivalent in WT and Il6−/− mice (Fig 4a and b). We also observed that the efficiency of memory formation from naïve OT-II TcR transgenic CD4 T cell donors transferred to WT or Il6−/− hosts that were subsequently challenged with A/PR8-OVAII (Fig 4c and d) was similar at 45 dpi. Furthermore, the IAV-primed OT-II cells in the spleen and dLN expressed similar levels of the IL-7 receptor alpha chain (CD127) (Fig 4e), levels of which strongly correlate with the survival fitness of memory CD4 T cells (34). Interestingly, donor cells in the lung expressed reduced CD127 in Il6−/− hosts (Fig 4e). Finally, cytokine production from restimulated memory OT-II cells isolated from WT or Il6−/− hosts, including the frequency of dual IFN-γ+IL-2+ producing cells that are often correlated with superior protective function (35) was similar (Fig 4f). Together, these results suggest that the absence of IL-6 expression does not significantly impact the generation or maintenance of functional memory CD4 T cells following IAV priming.
Figure 4. Memory CD4 T cell formation following IAV priming is not impaired in the absence of IL-6.
WT or Il6−/− C57Bl/6 mice were primed with A/Alaska as described in Figure 3. The (a) percentage and (b) absolute number of NP311–325 tetramer+ CD44high CD4 T cells was enumerated at 45 dpi (n=4 mice/group; one of 2 similar experiments). In separate experiments, WT or Il6−/− mice receiving 1×106 Thy-disparate naïve OT-II donor cells were primed with 0.1 LD50 A/PR8-OVAII. At 45 dpi, the (c) percentage and (d) absolute number of donor cells detected in stated organs was determined (n=4 mice/group; one of 2 experiments). The mean fluorescence intensity of CD127 on donor cells was determined by FACS (e). The percentage of donor cells co-producing IFN-γ and IL-2 was determined by intracellular cytokine staining following ex vivo stimulation (f).
Memory CD4 T cell-mediated protection is compromised in the absence of IL-6
We next directly tested if IL-6 expression impacts the protective potential of memory T cell responding against IAV. We employed an adoptive transfer model using well-characterized memory CD8+ or CD4+ T cell populations generated in vitro from naïve TcR transgenic precursors that we have shown protect against IAV and are equivalent to polyclonal in situ-generated IAV-specific memory cells in their response (22, 27, 36, 37). This experimental design allows us to focus on the protective capacity of the donor memory cells and eliminates contributions from host IAV-primed memory T and B cells, Ab, and IAV-primed lung environment that can all independently contribute to protection in IAV-primed animals (20).
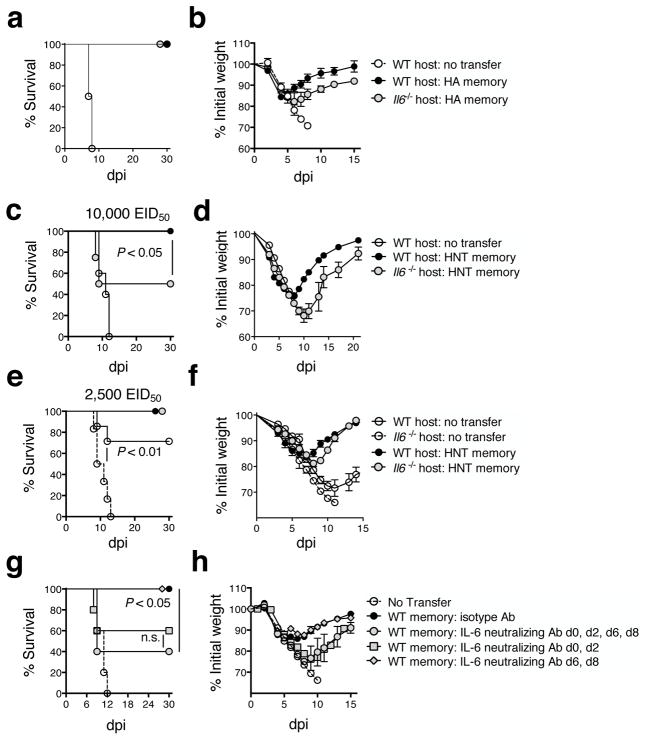
We transferred 5×106 Tc1-polarized memory CD8 T cells, generated from HA (clone 4) TcR transgenic cells, or Th1-polarized memory CD4 T cells, generated from HNT TcR transgenic cells, to unprimed WT or Il6−/− hosts. This number of HA CD8+ or HNT CD4+ memory cells provides robust protection to unprimed mice against 10,000 EID50 (2 LD50) A/PR8 challenge (27, 36). Memory CD8 T cells fully protected WT and Il6−/− hosts, with only slightly more weight loss observed in Il6−/− mice (Fig 5a and b). In contrast, while memory CD4 T cells fully protected WT recipients, only about half of the Il6−/− recipients survived (Fig 5c), with more weight loss and delayed time to recovery versus that observed in WT recipients (Fig 5d). Transfer of 5×106 memory CD4 T cells did protect Il6−/− mice against a 4-fold lower 2500 EID50 A/PR8 challenge (still lethal to unprimed Il6−/− mice, the open circle with dashed line and as shown in Figure 1). However, delayed recovery in Il6−/− versus WT hosts was still evident (Fig 5e and f). These results confirm that memory CD4+, but not CD8+, T cell-mediated protection against IAV is dramatically reduced in the absence IL-6.
Figure 5. Protection mediated by memory CD4+, but not CD8+, T cells is compromised in the absence of IL-6.
Memory CD8 T cells, 5×106, generated from HA TcR transgenic cells were transferred to naïve WT or Il6−/− BALB/c mice. Mice were subsequently infected with 10,000 EID50 A/PR8 and their (a) survival and (b) weight loss monitored (n=10 mice/group) and compared to mice not receiving donor memory cells (n=3 mice). WT or Il6−/− BALB/c mice that received 5×106 memory CD4 T cells generated from HNT TcR transgenic cells were challenged with 10,000 EID50 A/PR8, and (c) survival and (d) weight loss monitored. Analysis was also done on mice challenged with 2500 EID50 A/PR8 (e and f) (n=10 mice/group). In separate experiments, WT mice receiving 5×106 memory HNT CD4 T cells were treated with 0.5 mg of IL-6 neutralizing Ab on the stated days post 10,000 EID50 A/PR8 challenge. Control mice were treated with isotype control Ab on 0, 2, 4, 6, and 8 dpi. Survival (g) and weight loss (h) is depicted for 5 mice/group (one of three experiments).
Early host-derived IL-6 is required for optimal memory CD4 T cell-mediated protection
To confirm the importance of IL-6 removed acutely rather than IL-6 absent throughout host development, we treated WT recipients of memory CD4 T cells with IL-6-neutralizing or isotype control Ab from 0–8 dpi. The IL-6 neutralizing Ab treatment reduced the protective efficacy of transferred memory CD4 T cells (Fig 5g, black vs. grey filled circles) to an extent similar to that observed in Il6−/− hosts. This result also indicates that host derived IL-6 plays a key role in maximizing memory CD4 T cell-mediated protection as similar impaired protection was seen following transfer of WT memory CD4+ T cells to Il6−/− mice, where donor cell-derived IL-6 could potentially impact the outcome, and in WT hosts treated with IL-6 neutralizing Ab, where all IL-6 (donor and host-derived) is subject to blocking.
To ask when IL-6 signals are needed, we treated recipients with IL-6 neutralizing Ab from 0–8 dpi, or only at early (0 and 2 dpi), or later time points (6 and 8 dpi). Early blockade of IL-6 impaired survival (Fig 5g) and increased morbidity (Fig 5h) as dramatically as did treatment throughout 0–8 dpi. In contrast, late administration of IL-6 neutralizing Ab had no impact (Fig 5g and h). This finding indicates that IL-6 is needed only during the first few days post-challenge to support optimal protective memory CD4 T cell responses.
Impaired B cell and neutrophil responses do not underlie reduced CD4 T cell protection
The reduced protective efficacy of memory CD4 T cells in the absence of IL-6 could be due to impaired responses of memory CD4 T cells themselves and/or to IL-6-dependent effects on other cell types known to contribute to IAV clearance. Diminished B cell Ab production has been implicated in the enhanced susceptibility of Il6−/− mice to primary IAV challenge (11). To test if IL-6 is needed for memory CD4 T cell protection through a B cell-dependent mechanism, we utilized JHD mice that lack B cells as hosts. JHD recipients of memory CD4 T cells were treated with IL-6 neutralizing or control Ab following a lethal A/PR8 challenge for this strain (2500 EID50) (36). As expected from our earlier studies, memory CD4 T cells were able to transfer protection to the B cell-deficient JHD hosts (36), but this protection was abrogated by IL-6 neutralizing Ab treatment (Supp Fig 1a and b), strongly suggesting that the requirement for IL-6 in memory CD4 T cell-mediated protection is independent of any action, direct or indirect, on B cells.
Reduced neutrophil responses have also been suggested to underlie the enhanced susceptibility of Il6−/− mice to primary IAV infection (12). To test the importance of neutrophils in contributing to memory CD4 T cell-mediated protection we thoroughly depleted neutrophils in recipients of memory CD4 T cells prior to lethal A/PR8 challenge. Protection was robust in both WT and JHD hosts whether neutrophils were present or not (Supp Fig 1c–f), indicating that any defects in neutrophil responses are not responsible the reduced protective efficacy of memory CD4 T cells responding against IAV in the absence of IL-6.
Early memory CD4 T cell responses are not impacted by IL-6
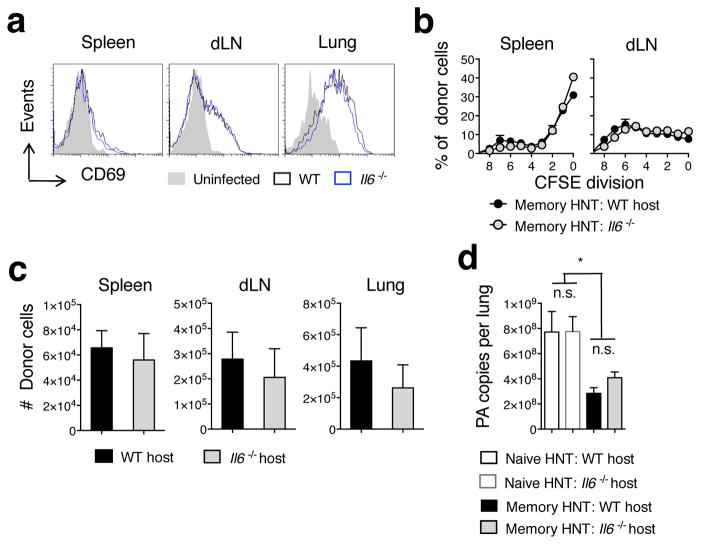
Since IL-6 is required only within the first few dpi for optimal memory CD4 T cell-mediated protection, we asked if memory CD4 T cell activation is compromised in Il6−/− hosts following IAV challenge, as has been suggested previously (38). Donor memory CD4 T cells were CFSE-labeled and transferred to WT or Il6−/− mice followed by challenge with 10,000 EID50 A/PR8. No differences in CD69 expression (Fig 6a), division profile based on analysis of CFSE dilution (Fig 6b), or donor cell number were detected between WT and Il6−/− hosts at 4 dpi in the spleen or dLN (Fig 6c).
Figure 6. Initial activation of memory CD4 T cells and early viral control are not impacted by IL-6.
Unprimed thy-disparate WT or Il6−/− mice received 1×106 CFSE-labeled HNT memory CD4 and were challenge with 10,000 EID50 A/PR8. Representative (a) donor CD69 expression, (b) proliferation profile determined by loss of CFSE label, and (c) cell number at 4 dpi were assessed in stated organs (n=3 mice/group). Results from 1 of 3 similar experiments. In separate experiments, (d) pulmonary viral titers were determined on 4 dpi from lungs of WT BALB/c or Il6−/− mice receiving 5×106 memory HNT cells or not and challenged with 10,000 EID50 A/PR8 (n = 4/group). Results representative of 2 separate experiments.
Memory CD4 T cells recognizing IAV Ag on MHC II+ APC in the lung drive potent innate immune activation resulting in reduced viral titers by 4 dpi (22, 39). We tested a role for IL-6 in orchestrating this early memory CD4 T cell-mediated viral control by assessing A/PR8 titers in WT or Il6−/− recipients of memory CD4 T cells at 4 dpi. Both WT and Il6−/− recipients displayed comparable and significant viral control at 4 dpi compared to control mice not receiving donor cells (Fig 6d). These results indicate that neither the initial activation of, nor initial viral control mediated by memory CD4 T cells is compromised in the absence of IL-6.
2° CD4 T cell effector number and function are reduced in the absence of IL-6
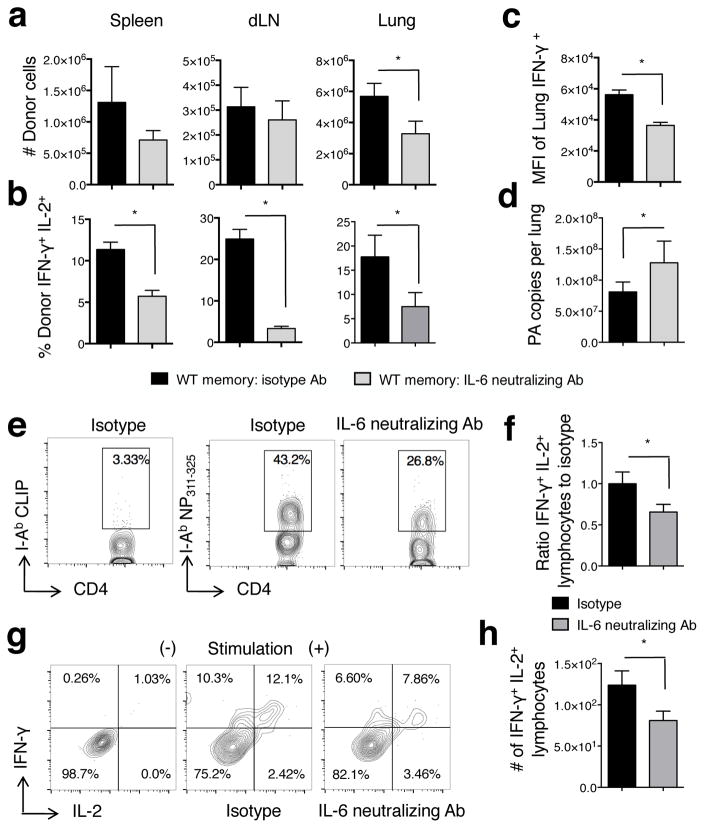
We next asked if IL-6 impacts the magnitude and/or function of 2° CD4 T cell effectors present at 7 dpi, as this late phase of the recall response is ultimately critical for memory CD4 T cell-mediated protection (40). We transferred donor memory cells to WT mice, challenged with A/PR8, and treated recipients with IL-6 neutralizing or control Ab from 0–6 dpi. We used 2,500 EID50 rather than 10,000 EID50 as the onset of death of some mice challenged with the higher dose when treated with IL-6 neutralizing Ab could complicate analysis. At 7dpi, the peak of 2° effector responses in this model (37), the number of donor cells detected in the spleen and dLN of mice treated with IL-6 neutralizing or isotype Ab was similar (Fig 7a). However, the number of donor cells in the lung was significantly reduced by IL-6 neutralizing Ab treatment (Fig 7a), indicating that IL-6 is critical for maximal 2° effector accumulation at the site of infection where many of the key effector functions of CD4 T cells are thought to be mediated (41).
Figure 7. 2° CD4 T cell effector number and function are reduced in the absence of IL-6.
HNT memory cells, 1×106, were transferred to naïve WT BALB/c subsequently infected with 2,500 EID50 A/PR8 and treated with 0.5mg of isotype or IL-6 neutralizing Ab on days 0, 2, 4, and 6 dpi. Donor cells were enumerated at 7 dpi (a). Dual IFN-γ+/IL-2+ cytokine producing cells (b) and mean fluorescence intensity of IFN-γ signal (c) was assessed at 7 dpi by ICCS (n=4 mice/group; representative of 3 similar experiments). Viral titers (d) were determined on 7 dpi in mice receiving donor HNT memory cells followed by 2,500 EID50 A/PR8 challenge (n=4 mice/group; one of two experiments). WT C57Bl/6 mice were primed with A/Alaska, challenged with A/PR8 as described in Figure 3, and mice treated with isotype or IL-6 neutralizing Ab as described above. On 5dpi, NP311–325 tetramer+ CD4 T cells detected in the lung (e) were assessed for dual IFN-γ+/IL-2+ cytokine producing cells. The frequency (f and g) and number (h) is shown (n=4 mice/group; one of 2 similar experiments).
We analyzed 2° effector function to look for additional effects of IL-6. Strikingly, neutralization of IL-6 reduced the frequency of cells producing IFN-γ together with IL-2 in all organs tested (Fig 7b) and the MFI of IFN-γ+ cells was also reduced (Fig 7c). Only very low numbers of IL-10+ or IL-17+ donor cells were detected, consistent with previous studies (37) and these were not impacted by Ab treatment (not shown). Most importantly, impaired viral clearance by adoptively transferred memory CD4 T cells at 7 dpi was observed in hosts treated with IL-6 neutralizing Ab (Fig 7d).
To confirm the absence of IL-6 signals only during heterosubtypic challenge also reduces dual IFN-γ+/IL-2+ 2° effector responses from polyclonal CD4 T cells, we challenged WT A/Alaska/6/77-primed mice with PR8 as in Figure 3, treated mice with IL-6-neutralizing or isotype control antibodies on day 0, 2, and 4 post-PR8 challenge, and assessed the cytokine response of NP311–325 tetramer+ CD4 T cells (Fig 7e). In comparison to isotype treated mice, reduced frequencies and total numbers of dual IFN-γ+/IL-2+ NP311–325+ cells were detected at 5 dpi in mice treated with IL-6-neutralizing antibodies (Fig 7f–h). These findings are consistent with the impaired cytokine production of Il6−/− versus WT mice detected in response to IAV lysate stimulation of polyclonal CD4 T cells responding to heterosubtypic challenge summarized in Figure 3. In total, these results strongly support the hypothesis that early IL-6 signals to memory CD4 T cells play a key role in maximizing the anti-viral capacity of late-acting 2° CD4 T cell effectors.
In contrast to the dramatic impact on 2° CD4 T cell effectors, IL-6 blockade did not impact the magnitude of, nor IFN-γ or granzyme B expression by 2° CD8 T cell effectors derived memory TcR transgenic HA cells (Supp Fig 2). This is in agreement with similar CD8 T cell responses in WT and Il6−/− mice during heterosubtypic challenge (Fig 3). IL-6 blockade also did not alter 1° CD4 T cell effector responses generated from naïve HNT TcR transgenic donor cells, consistent with previous studies (12) (not shown). Together, these results thus support a unique role for IL-6 in selectively programming the maximal efficacy of memory CD4 T cell responses against IAV.
Direct IL-6 signals to memory CD4 T cells maximize 2° effector responses
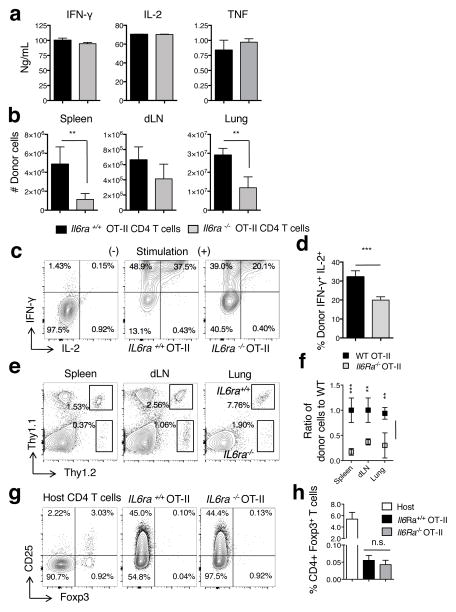
IL-6 could signal memory CD4 T cells directly or it could exert indirect IL-6-dependent positive effects on the 2° CD4 T cell effector response by signaling other adaptive or innate immune populations, including APC or Foxp3+ Tregs (42). To determine if direct IL-6 signals to IAV-specific memory CD4 T cells are necessary to enhance 2° effector responses, we generated memory CD4 T cells from OT-II cells lacking expression of the IL-6 receptor alpha chain (Il6ra−/−) and from OT-II cells obtained from Il6ra+/+ littermates. The spectrum and level of cytokines detected upon restimulation of the in vitro-generated Il6ra−/− and Il6ra+/+ memory CD4 T cells was similar (Fig 8a), as was the yield of cells recovered (not shown). This indicates that direct IL-6 signals to unprimed CD4 T cells are not required for the generation of functional Th1-like memory populations in vitro, which agrees with the in vivo studies summarized in Figure 4. However, when transferred to WT C57BL/6 hosts then challenged with A/PR8-OVAII, the 2° CD4 T cell effector response in the lung generated from Il6ra−/− donor cells was severely impaired in terms of absolute number and cytokine production compared to 2° effectors generated from Il6ra+/+ donor cells (Fig 8b, c, and d).
Figure 8. Direct IL-6 signals to memory CD4 T cells maximize2° effector responses.
Memory populations were generated in vitro from IL6ra+/+ and Il6ra−/− OT-II cells. Cytokine production (a) from an equal number of IL6ra+/+ and Il6ra−/− memory cells was determined by luminex analysis of supernatants from triplicate wells 8 hours post-stimulation. Results representative of 3 separate experiments. IL6ra+/+ or Il6ra−/− OT-II memory cells, 1×106, were transferred to different Thy-disparate hosts followed by infection with 0.1 LD50 A/PR8-OVAII. At 7 dpi, the total number of donor cells (b) and IFN-γ+ and IL-2+ cytokine production in the lung were determined from 4 mice/group (one of 2 separate experiments). Representative ICCS staining of lung cells in the absence and presence of stimulation is shown in (c) and the percentage of dual IFN-γ+ IL-2+ donor cells in (d). In separate experiments, WT.Thy1.2/Thy1.1 or Il6ra−/−.Thy1.2 OT-II memory cells, 1×106, were co-transferred to the same WT C57BL/6.Thy1.1 host followed by infection with 0.1 LD50 A/PR8-OVAII. At 7 dpi, the total frequency of donor cells (e), the ratio of Il6ra−/− to WT OT-II memory cells (f), and the frequency of CD25+ Foxp3+ CD4+ T cells lymphocytes from the host or donor populations (g and h) was determined from 4 mice/group (one of 2 separate experiments).
To confirm that IL-6 has a direct role on memory CD4 T cells responding to IAV, we co-transferred WT (Thy1.1/Thy1.2) and Il6ra−/− (Thy1.2) OT-II memory cells to WT C57BL/6.Thy1.1 hosts. The memory cells from Il6ra−/− donors displayed impaired 2° effector accumulation (Fig 8e and f) and cytokine production (not shown). As both donor populations are responding in the presence of the same endogenous Foxp3+ Treg population, and the Il6ra−/− and Il6ra+/+ 2° effectors contain minimal but comparable frequencies of CD25+ Foxp3+ cells (Fig g and h), these results further reveal the importance of IL-6 signals directly received by memory CD4 T cells that program maximal protective 2° CD4 T cell effector responses during heterosubtypic IAV challenge.
Discussion
Optimal clearance of heterosubtypic IAV infection requires a complex interplay of multiple elements of the innate and adaptive arms of the immune system that is incompletely understood. Memory CD4 T cells provide strong protection against experimental IAV infection (36, 43–46), and increased numbers of multifunctional IAV-specific memory CD4 T cells correlate with improved outcomes in human studies (47, 48). We have reported that late-acting 2° effectors, which peak around 7 dpi, are critical for complete memory CD4 T cell-mediated protection against IAV (21). Uncovering signals that maximize this 2° effector response could lead to strategies to improve vaccine-induced T cell protection against IAV and other pathogens against which neutralizing Ab alone cannot confer long-term immunity. We recently found that autocrine IL-2 maximizes the magnitude of 1° but not 2° CD4 T cell effector populations in the lung (34), suggesting that unique cytokine signals other than IL-2 might act to specifically regulate 2° effectors accumulation at the site of infection. Here, we show that direct IL-6 signals to memory CD4 T cells delivered early after IAV challenge, support maximal 2° effector responses in terms of magnitude, function, and protective capacity.
Through analysis of endogenous T cells in IAV-primed animals and adoptive transfer models, we find that IL-6 impacts memory CD4 T cell recall more than secondary CD8 T cell responses. This suggests that the amount of IL-6 produced early on during heterosubtypic challenge may be key in determining the relative contribution of IAV-specific memory CD4+ versus CD8+ T cells to protection. Such a mechanism could, at least in part, underlie observations of multiple seemingly distinct forms of heterosubtypic immunity observed in different experimental systems that rely more or less on either memory CD4+ or CD8+ T responses (20). The level of IL-6 produced following heterosubtypic challenge may be impacted by several variables including the dose and strain of viruses used, the duration between priming and recall challenge, and the age, strain, or sex of the animals analyzed. We speculate that other innate cytokines produced upon IAV infection may also have a selective regulatory role on the 2° CD8 T cell effector response during heterosubtypic challenge. In support of this hypothesis, memory CD8 T cells deficient for expression of type I IFN receptor have been shown to be less efficient at promoting IAV clearance than WT memory CD8 T cells of the same specificity (49). The ability of early innate inflammatory signals to selectively impact the protective capacity of recall CD4+ versus CD8+ T cell responses suggests there is an important initial therapeutic window during which delivery of targeted mediators may significantly improve vaccine-induced, or adoptively transferred memory T cell responses.
The increased magnitude of 2° effector responses following early IL-6 signaling to memory CD4 T cells is consistent with the known pro-survival activity of the cytokine (50–52). Previously in vitro, we found that IL-6 produced during cognate interactions between CD4 T cells and dendritic cells enhanced expression of the anti-apoptotic molecule Bcl2 in the responding T cells (53). We did not observe changes in Bcl2 expression by 2° effectors responding against IAV in the presence or absence of IL-6 (not shown), but this does not rule out that regulation by IL-6 of other pro- or anti-apoptotic molecules plays a role in maximizing the recall CD4 T cell response. Alternatively, or in addition to an anti-apoptotic effect, IL-6 may optimize 2° effector infiltration and/or retention in the lung either by impacting chemokine gradients or chemokine receptor expression on the 2° effector cells (54). Recent work also suggests that IL-6 can impact mitochondrial respiration in effector T cells thereby enhancing cytokine production (55). Finally, IL-6 may regulate elements of the autophagy pathway (56), which has recently been shown to play an important role in effector CD4 T cell survival in vivo (57). Detailed further studies are needed to discriminate the importance of each of these potential mechanisms in contributing to IL-6’s role in maximizing protective 2° CD4 T cell effector responses against IAV.
While not required for memory CD4 T cell generation, we show herein that IL-6 signals are needed for optimal recall of memory CD4 T cells. This is in agreement with recent observations of Nish et al. (58), however, in contrast to their findings we did not observe impaired 1° effector function in the absence of IL-6 in our studies. This may be due to differences in the experimental system employed as IAV, a rapidly replicating pathogen, likely generates a more robust and sustained inflammatory response than the immunizations and models of autoimmunity utilized by Nish et al. One or more IAV-induced inflammatory factors may compensate in the absence of IL-6 to allow for adequate primary effector responses in the studies summarized here. Interestingly, impaired recall CD4 T cell responses against IAV in Il6−/− mice have also been attributed to IL-6 being required to reduce Treg-mediated suppression (38). Our results clearly implicate direct IL-6 signals to memory CD4 T cells as critical for maximizing 2° effector responses, but do not formally rule out an impact of IL-6 on Treg-mediated suppression in addition to its direct impact on memory CD4 T cells. However, we note that recall CD8 T cell responses were not impaired in the absence of IL-6 in our studies while a recent report found dramatic regulation of the magnitude of secondary CD8 T cell responses by Tregs in IAV-primed mice (59).
The importance of adjuvants in stimulating innate inflammatory responses to improve vaccine-induced generation of memory T and B cell subsets is well known. The role innate signals play in recall responses of primed lymphocytes is much less clear. The therapeutic manipulation of IL-6, and likely other inflammatory mediators, may offer a means to maximize or to tailor, vaccine-induced T cell responses against pathogens and cancers. Our findings also highlight broad differences in how IL-6 affects the outcome of primary versus secondary IAV challenge. Il6−/− mice primed with cold-adapted IAV survive doses of heterosubtypic virus at least an order or magnitude greater than is lethal to unprimed mice, albeit with more morbidity and delayed viral clearance compared to IAV-primed WT mice. It is most likely that heterosubtypic immunity remains strong in primed Il6−/− mice due to IAV-specific memory CD8 T cell responses that are largely unaffected by the absence of IL-6, and are able to potently clear IAV through multiple distinct mechanisms (27). These studies further support the overall concept that vaccines able to generate strong IAV-specific memory CD4+ (47), as well as CD8+ (60) T cells and neutralizing Ab should together provide the strongest protection against IAV infection, including in situations where innate immune responses may be compromised (61).
Supplementary Material
Acknowledgments
We thank Drs. R.W. Dutton and H. Tsukamoto for helpful discussions and the University of Massachusetts Medical School DERC Morphology Core for assistance with histology preparation. We also acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of I-Ab/NP311–325 tetramers.
References
- 1.Tan AC, Mifsud EJ, Zeng W, Edenborough K, McVernon J, Brown LE, Jackson DC. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm. 2012;9:2710–2718. doi: 10.1021/mp300257x. [DOI] [PubMed] [Google Scholar]
- 2.Lau YF, Tang LH, Ooi EE, Subbarao K. Activation of the innate immune system provides broad-spectrum protection against influenza A viruses with pandemic potential in mice. Virology. 2010;406:80–87. doi: 10.1016/j.virol.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuvim MJ, Gilbert BE, Dickey BF, Evans SE. Synergistic TLR2/6 and TLR9 activation protects mice against lethal influenza pneumonia. PLoS One. 2012;7:e30596. doi: 10.1371/journal.pone.0030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinya K, Okamura T, Sueta S, Kasai N, Tanaka M, Ginting TE, Makino A, Eisfeld AJ, Kawaoka Y. Toll-like receptor pre-stimulation protects mice against lethal infection with highly pathogenic influenza viruses. Virol J. 2011;8:97. doi: 10.1186/1743-422X-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norton EB, Clements JD, Voss TG, Cardenas-Freytag L. Prophylactic administration of bacterially derived immunomodulators improves the outcome of influenza virus infection in a murine model. J Virol. 2010;84:2983–2995. doi: 10.1128/JVI.01805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo SU, Kwon HJ, Song JH, Byun YH, Seong BL, Kawai T, Akira S, Kweon MN. MyD88 signaling is indispensable for primary influenza A virus infection but dispensable for secondary infection. J Virol. 2010;84:12713–12722. doi: 10.1128/JVI.01675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzman Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J Immunol. 2011;186:5916–5926. doi: 10.4049/jimmunol.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 10.Ayala VI, Teijaro JR, Farber DL, Dorsey SG, Carbonetti NH. Bordetella pertussis infection exacerbates influenza virus infection through pertussis toxin-mediated suppression of innate immunity. PLoS One. 2011;6:e19016. doi: 10.1371/journal.pone.0019016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, Teuscher C, Haynes L, Rincon M. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dienz O, Rud JG, Eaton SM, Lanthier PA, Burg E, Drew A, Bunn J, Suratt BT, Haynes L, Rincon M. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauder SN, Jones E, Smart K, Bloom A, Williams AS, Hindley JP, Ondondo B, Taylor PR, Clement M, Fielding C, Godkin AJ, Jones SA, Gallimore AM. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol. 2013 doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Peper RL, Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog. 1995;19:175–183. doi: 10.1006/mpat.1995.0056. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Matsuoka M, Cantor H, Homer R, Enelow RI. Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J Immunol. 2008;180:25–29. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Damjanovic D, Divangahi M, Kugathasan K, Small CL, Zganiacz A, Brown EG, Hogaboam CM, Gauldie J, Xing Z. Negative regulation of lung inflammation and immunopathology by TNF-alpha during acute influenza infection. Am J Pathol. 2011;179:2963–2976. doi: 10.1016/j.ajpath.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinstry KK, Strutt TM, Swain SL. Hallmarks of CD4 T cell immunity against influenza. J Intern Med. 2011;269:507–518. doi: 10.1111/j.1365-2796.2011.02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, Swain SL, Dutton RW. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–1038. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 22.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16:558–564. 551–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman TJ, Lambert K, Topham DJ. Rapid reactivation of extralymphoid CD4 T cells during secondary infection. PLoS ONE. 2011;6:e20493. doi: 10.1371/journal.pone.0020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strutt TM, McKinstry KK, Swain SL. Control of innate immunity by memory CD4 T cells. Adv Exp Med Biol. 2011;780:57–68. doi: 10.1007/978-1-4419-5632-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamada H, Bassity E, Flies A, Strutt TM, de Garcia-Hernandez LM, McKinstry KK, Zou T, Swain SL, Dutton RW. Multiple redundant effector mechanisms of CD8+ T cells protect against influenza infection. J Immunol. 2013;190:296–306. doi: 10.4049/jimmunol.1200571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy BR, Chanock RM, Clements ML, Anthony WC, Sear AJ, Cisneros LA, Rennels MB, Miller EH, Black RE, Levine MM, Betts RF, Douglas RG, Jr, Maassab HF, Cox NJ, Kendal AP. Evaluation of A/Alaska/6/77 (H3N2) cold-adapted recombinant viruses derived from A/Ann Arbor/6/60 cold-adapted donor virus in adult seronegative volunteers. Infect Immun. 1981;32:693–697. doi: 10.1128/iai.32.2.693-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuoka Y, Lamirande EW, Subbarao K. Current Protocols in Microbiology. John Wiley & Sons, Inc; 2005. The Mouse Model for Influenza. [DOI] [PubMed] [Google Scholar]
- 30.Sanders CJ, Vogel P, McClaren JL, Bajracharya R, Doherty PC, Thomas PG. Compromised respiratory function in lethal influenza infection is characterized by the depletion of type I alveolar epithelial cells beyond threshold levels. Am J Physiol Lung Cell Mol Physiol. 2013;304:L481–488. doi: 10.1152/ajplung.00343.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marois I, Cloutier A, Garneau E, Richter MV. Initial infectious dose dictates the innate, adaptive, and memory responses to influenza in the respiratory tract. J Leukoc Biol. 2012;92:107–121. doi: 10.1189/jlb.1011490. [DOI] [PubMed] [Google Scholar]
- 32.McKinstry KK, Dutton RW, Swain SL, Strutt TM. Memory CD4 T cell-mediated immunity against influenza A virus: more than a little helpful. Arch Immunol Ther Exp (Warsz) 2013;61:341–353. doi: 10.1007/s00005-013-0236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretschmer U, Bonhagen K, Debes GF, Mittrucker HW, Erb KJ, Liesenfeld O, Zaiss D, Kamradt T, Syrbe U, Hamann A. Expression of selectin ligands on murine effector and IL-10-producing CD4+ T cells from non-infected and infected tissues. Eur J Immunol. 2004;34:3070–3081. doi: 10.1002/eji.200424972. [DOI] [PubMed] [Google Scholar]
- 34.McKinstry KK, Strutt TM, Bautista B, Zhang W, Kuang Y, Cooper AM, Swain SL. Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat Commun. 2014;5:5377. doi: 10.1038/ncomms6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 36.McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J Clin Invest. 2012;122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1205894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, Jones SA, Gallimore AM. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog. 2008;4:e1000006. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman TJ, Topham DJ. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J Immunol. 2010;184:3841–3849. doi: 10.4049/jimmunol.0902281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci U S A. 2012;109:E2551–2560. doi: 10.1073/pnas.1205894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strutt TM, McKinstry KK, Marshall NB, Vong AM, Dutton RW, Swain SL. Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol Rev. 2013;255:149–164. doi: 10.1111/imr.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein SL, Stack A, Misplon JA, Lo CY, Mostowski H, Bennink J, Subbarao K. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8(+) cytotoxic T lymphocytes: either CD4(+) or CD8(+) T cells can promote survival and recovery after challenge. Int Immunol. 2000;12:91–101. doi: 10.1093/intimm/12.1.91. [DOI] [PubMed] [Google Scholar]
- 45.Alexander J, Bilsel P, del Guercio MF, Stewart S, Marinkovic-Petrovic A, Southwood S, Crimi C, Vang L, Walker L, Ishioka G, Chitnis V, Sette A, Assarsson E, Hannaman D, Botten J, Newman MJ. Universal influenza DNA vaccine encoding conserved CD4+ T cell epitopes protects against lethal viral challenge in HLA-DR transgenic mice. Vaccine. 2010;28:664–672. doi: 10.1016/j.vaccine.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol. 2011;85:448–455. doi: 10.1128/JVI.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, Staples KJ, Dong T, Douek DC, McMichael AJ, Xu XN. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 48.Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis. 2013;207:297–305. doi: 10.1093/infdis/jis684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol. 2005;174:4761–4767. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 51.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 52.Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J Exp Med. 2000;191:915–926. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones SC, Brahmakshatriya V, Huston G, Dibble J, Swain SL. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. J Immunol. 2010;185:6783–6794. doi: 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang R, Lirussi D, Thornton TM, Jelley-Gibbs DM, Diehl SA, Case LK, Madesh M, Taatjes DJ, Teuscher C, Haynes L, Rincon M. Mitochondrial Ca(2)(+) and membrane potential, an alternative pathway for Interleukin 6 to regulate CD4 cell effector function. eLife. 2015:4. doi: 10.7554/eLife.06376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin B, Zhou Z, He J, Yan C, Ding S. IL-6 Inhibits Starvation-induced Autophagy via the STAT3/Bcl-2 Signaling Pathway. Sci Rep. 2015;5:15701. doi: 10.1038/srep15701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuzawa Y, Oshima S, Takahara M, Maeyashiki C, Nemoto Y, Kobayashi M, Nibe Y, Nozaki K, Nagaishi T, Okamoto R, Tsuchiya K, Nakamura T, Ma A, Watanabe M. TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy. Autophagy. 2015;11:1052–1062. doi: 10.1080/15548627.2015.1055439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nish SA, Schenten D, Wunderlich FT, Pope SD, Gao Y, Hoshi N, Yu S, Yan X, Lee HK, Pasman L, Brodsky I, Yordy B, Zhao H, Bruning J, Medzhitov R. T cell-intrinsic role of IL-6 signaling in primary and memory responses. eLife. 2014;3:e01949. doi: 10.7554/eLife.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brincks EL, Roberts AD, Cookenham T, Sell S, Kohlmeier JE, Blackman MA, Woodland DL. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J Immunol. 2013;190:3438–3446. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 61.Hussell T, Cavanagh MM. The innate immune rheostat: influence on lung inflammatory disease and secondary bacterial pneumonia. Biochemical Society transactions. 2009;37:811–813. doi: 10.1042/BST0370811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.