Abstract
Background
Virologic and immunologic responses to antiretroviral treatment (ART) in infants may differ from older children due to immunologic, clinical or epidemiologic characteristics.
Methods
Longitudinal ART responses were modeled and compared in HIV-infected infants and children enrolled in cohorts in Nairobi, Kenya. Participants were enrolled soon after HIV diagnosis, started on ART, and followed for two years. Viral load decline was compared between infant and child cohorts using a nonlinear mixed effects model and CD4% reconstitution using a linear mixed effects model.
Results
Among 121 infants, median age at ART was 3.9 months; among 124 children, median age was 4.8 years. At baseline, viral load (VL) was higher among infant than children (6.47 vs. 5.91 log10 copies/ml, p<0.001). Infants were less likely than children to suppress viral load to <250 copies/ml following 6 months of ART (32% infants vs. 73% children p<0.0001). CD4% was higher at baseline in infants than children (19% vs. 7.3%, p<0.001). Older children had more rapid CD4% reconstitution than infants, but failed to catch up to infant CD4%,
Conclusion
Despite substantially higher CD4% at ART initiation, viral suppression was significantly slower among infants than older children. New strategies are needed to optimize infant outcomes on ART.
Keywords: HIV-infected infants, HIV-infected children, Pediatric HIV, Antiretroviral therapy, HIV viral load suppression, CD4 reconstitution
BACKGROUND
Infant HIV-1 viral loads are substantially greater than adult levels[1, 2], and as many as 50% of untreated HIV-1 infected African infants die by two years of age[3]. In contrast to acutely infected adults, vertically infected infants are slow to suppress virus[4] and many do not attain a discernable viral load set-point[2]. Older untreated HIV-1 infected children, the vast majority of whom represent vertically infected survivors, have lower viral loads than infants[5], either due to lower viral setpoint achieved post-infancy or to high early mortality[6] in infants, which results in culling of unfavorable viral or host characteristics among survivors.
Response to antiretroviral therapy (ART) may also differ between infants, children, and adults. In adults, ART typically results in up to 95% achieving viral suppression within 6 months of ART initiation, varying by regimen, the population under study and the definition of suppression used[7–9]. Similar rates of viral suppression have been shown in pediatric populations that include older children[9, 10]. However, infants have slower viral suppression[11, 12] and higher incidence of virologic failure[13]. While failure of ART viral suppression in infants may be due to higher baseline viral loads or challenges with ART adherence or dosing[14], it is also possible that aspects of the immune response that cause poor viral control in untreated infants may contribute to slower viral decline with ART.
Immune recovery on ART usually occurs in tandem with viral decline[15, 16]. In contrast to poor viral suppression with ART, infants generally have better immune status on ART than older children[17–19]; this may be due to their high thymic output[20] and higher age-specific CD4 counts and CD4%[21–23], or to greater damage to the CD4 T-cell population among older children who have lived with untreated HIV for an extended period[24–25]. As ART response is primarily assessed using CD4 monitoring in developing country settings[26–29], treatment failure among infants in particular may be masked by their comparatively good CD4 counts and percentages[26,29,30].
Although drastically improved survival is achievable if ART is initiated early in infancy[31], in practice, infant diagnosis is often delayed[32] and early mortality on ART remains high[33]. In order to understand whether impaired virologic and immunologic response to ART could explain high mortality post-ART in this population, we compared virologic and immunologic responses between infants starting ART in the first year of life versus children starting ART between the ages of 18 months to 12 years and conducted sensitivity analyses to address the potential contribution of chronic non-adherence to any differences found. Our hypothesis was that infants would have slower rates of viral suppression but comparable immune reconstitution to older children.
METHODS
Cohorts
Data from two pediatric ART studies conducted in Nairobi, Kenya were used for these analyses. Both studies were approved by the University of Washington Institutional Review Board and the Kenyatta National Hospital Ethics and Research Committee. Each study has extended follow-up up to >5 years; the current study utilized data on immunologic and virologic markers obtained during the first 2 years of ART. Recruitment and enrollment procedures have been described in detail elsewhere[33, 34], but in brief:.in the infant cohort [Optimizing Pediatric HIV Therapy (OPH), NCT00428116], HIV-infected, ART-naive infants less than 13 months old were identified at routine HIV-1 testing in prevention of mother-to-child transmission of HIV (PMTCT) clinics and pediatric wards between 2007–2010. All infants were started on ART and followed monthly for growth and clinical outcomes for two years before being randomized to treatment interruption or continued treatment[35]; for this analysis, only pre-randomization data were used. HIV viral load was assessed at baseline and every three months throughout follow-up, while CD4 counts and CD4% were assessed every six months.
In the child cohort [Pediatric Adherence (PAD) study, NCT00194545], ART-naive children aged 15 months to 12 years who were ART eligible due to moderate to severe HIV-1 disease (WHO disease stage III-IV and/or CD4<15%) were enrolled from Kenyatta National Hospital HIV clinic and pediatric wards between 2004–2007. Children were started on ART and randomized to adherence counseling alone or with a medication diary, then followed monthly for growth, clinical indicators and self-reported adherence. HIV-1 log10 viral loads were assessed every three months throughout follow-up, while CD4 counts and CD4% were assessed at the 0, 3, 6, 15, 21 and 27 month visits.
ART regimens
Participants were treated according to contemporaneous Kenya national guidelines[27,36] and antiretrovirals were provided by the PEPFAR-supported youth HIV-1 treatment program at the Kenyatta National Hospital Comprehensive Care Clinic (CCC). For the infant cohort, first-line treatment consisted of zidovudine (AZT), lamivudine (3TC) and either nevirapine (NVP) or lopinavir-boosted ritonavir. For the child cohort, first line treatment consisted of AZT and 3TC and either NVP or efavirenz.
Laboratory methods
HIV-1 viral loads were measured using the Gen Probe assay, which accurately quantifies the HIV-1 subtypes circulating in Kenya[37]. The limit of detection was determined based on amount of plasma tested and was set as the higher value between the two cohorts, 250 copies/mL (2.39 log10 copies / mL). Viral load data were not available to clinicians in real time. CD4 counts and CD4% were measured in real time at Kenyatta National Hospital throughout each study using flow cytometry.
Statistical methods
Unless otherwise noted, all analyses were conducted in Stata 14.0 (StataCorp, College Station, Texas). The two cohorts were compared for characteristics at baseline using Mann-Whitney U-tests for continuous variables and Chi square tests for categorical variables.
Viral suppression
The probability of viral suppression at 3-monthly intervals among infants and children was estimated using life tables; viral load suppression was defined as partial (HIV-1 viral load <1000 copies/mL) and complete (HIV-1 viral load<250 copies/mL). Time to first achievement of partial and full suppression post ART initiation was compared among infants and children using a log-rank test for survival functions. Time to first achievement of partial suppression by regimen (PI-containing vs. NNRTI-containing) among infants was compared using a log-rank test. In order to estimate the durability of viral suppression among infants compared to children, risk of viral rebound to >1000 copies/mL was compared using Cox proportional hazards regression. This analysis was restricted to those who ever achieved full suppression, and time at risk began at the first suppressed visit.
The viral load trajectory in each cohort was estimated to follow the same pattern: high viral load pre-ART, followed by a period of exponential decline to a stabilized long-term cohort mean post-ART, where the post-ART stabilized level reflects how completely viral load was suppressed in the cohort as a whole; while the slope reflects how rapidly the stabilized level was achieved. This pattern was modeled for each cohort using nonlinear mixed effects models, which were fitted using the lme4 package in R (R Foundation for Statistical Computing, Vienna, Austria), and are described by the following nonlinear mixed effects model:
where VL(t) is HIV-1 log10 viral load at t days post-ART, VL0 is HIV-1 log10 viral load at baseline pre-ART, VLpost is the long-term population average stabilized HIV-1 log10 viral load post ART and r is rate of decline. r may be interpreted as the log of the proportion of suppression, −log(VL(1) − VLpost)/(VL0 − VLpost)), achieved in one time unit. Individual random effects are incorporated for VL0 and VLpost. Confidence intervals for mean model estimates were simulated using bootstrap with 1000 repetitions and the bootstrap distributions were compared using the Kolmogorov-Smirnov test.
CD4% reconstitution
CD4% reconstitution on ART was estimated to occur in two phases: a sharp increase from baseline to 6 months (≤ 210 days on ART to account for late visits) followed by a more gradual continued increase from 6 months to the end of follow-up at two years (≤ 750 days on ART). The difference in slope between the infant and child cohorts in each phase was assessed using interaction terms. CD4% at each time-point post-ART is modeled by the following linear mixed effects model:
where CD4%ij is the CD4% for subject j at a given time i post-ART; I indicates whether subject j is an infant, CD4%6j is the CD4% for subject j at 210 days, T1ART indicates time before 210 days and is 0 thereafter, and T2ART indicates time after 210 days and is 0 before. εij is an error term. Individual random effects were incorporated for intercepts and slopes, using an exchangeable correlation structure. CD4% was log-transformed to improve normality of the data.
CD4-for-age reconstitution
CD4 counts and CD4% in healthy children are highest in infancy and decline with age, so that age-specific expected or “healthy” CD4 counts and percentages vary throughout childhood. As such, infants are more immunocompromised at a given CD4% than older children. To correct for this difference, a published model of CD4 count decline with age in HIV-uninfected children[38] was used to predict age-specific expected CD4 counts at each time-point and calculate the ratio of observed CD4 count to expected CD4 count; the ratio is hereafter called “CD4-for-age”, with normal CD4-for-age equal to 1. The increase in CD4-for-age over time on ART was compared between the two cohorts. Reconstitution to stabilized long-term CD4-for-age has been described in pediatric ART cohorts previously[17, 19]; however, the asymptotic model used in these studies was a poor fit for our data, likely because two years of follow-up are not sufficient to achieve a stabilized CD4-for-age. Instead, we modeled log-transformed CD4-for-age using a model similar to the linear mixed effects model described above.
We conducted sensitivity analyses to assess the impact of survival bias and adherence on our analyses. To directly compare treatment response among acutely-infected infants and chronically infected children who had survived the highest-risk period without ART, sensitivity analyses were conducted excluding infants over six months old and children under two years old. In order to remove the effect of chronic non-adherence on estimates of rate of CD4% reconstitution and viral load suppression, sensitivity analyses were conducted excluding participants who never achieved viral load suppression in the first two years on ART.
RESULTS
Study population characteristics
Of 121 infants, 75 completed two years of followup. Median age at enrollment was 3.9 months (IQR 3.3–5.0, range 1.1–13) (Table 1). Of 124 children, 76 completed two years of followup. Median age at enrollment was 59 months or 4.9 years (IQR 32–76, range 16–152). Thirty-eight percent of infants started a protease-inhibitor (PI)-based regimen as first-line treatment, whereas none of the older children did. Older children had more advanced disease at ART initiation; 89% met WHO criteria for stage III or IV compared to 36% of infants (p<0.0001). Most children were cared for by biological parents, although 26% of children’s and 2.5% of infants’ biological mothers were deceased (p<0.0001). A greater proportion of caregivers in the infant cohort lived in one-room houses (p=0.002), suggesting that socio-economic status might have been higher in the child cohort; however, this difference was partly explained by the greater proportion of orphaned children, more of whom lived in larger households.
Table 1. Baseline pre-ART characteristics of infants and older children included in the analysis.
Data are presented with two significant digits. Unless otherwise specified, missing data were <5% per cohort and n (%) refers to number and proportion per category among those with non-missing data. P-values are from Mann-Whitney U-tests for continuous variables and Chi square tests for categorical variables.
| Infant cohort N=121 n (%) or median (IQR) |
Child cohort N=124 n (%) or median (IQR) |
p-value | |
|---|---|---|---|
|
| |||
| Child characteristics | |||
| Age at enrollment (months) | 3.9 (3.3, 5.0) | 59 (32, 78) | — |
| First line treatment | |||
| NRTI/NNRTI | 75 (62) | 124 (100) | — |
| NRTI/PI | 46 (38) | 0 (0) | |
| Female | 61 (50) | 64 (52) | 0.49 |
| WHO disease stage | <0.0001 | ||
| I/II | 65 (54) | 12 (9.7) | |
| III | 37 (31) | 94 (76) | |
| IV | 7 (5.8) | 16 (13) | |
| Unknown | 12 (9.9) | 2 (1.6) | |
| CD4 count (cells/μL) | 1400 (800, 2100) | 280 (96, 550) | <0.0001 |
| CD4% | 21 (16, 29) | 6.3 (3.2,11) | <0.0001 |
| Log10 HIV viral load | 6.6 (6.1, 7.0) | 6.0 (5.4, 6.5) | <0.0001 |
| Height-for-age / length-for-age Z-score | −2.1 (−3.2, −0.97) | −2.4 (−3.4, −1.3) | 0.32 |
| Weight-for-age Z-score | −2.4 (−3.8, −1.0) | −2.3 (−3.1, −1.4) | 0.31 |
| Weight-for-length Z-score | −1.0 (−2.4, −0.06) | −1.1 (−2.0, −0.16) | 0.64 |
| Caregiver characteristics | |||
| Biological mother deceased | 3 (2.5) | 32 (26) | <0.0001 |
| Mother received ART for PMTCT1 | 50 (45) | N/A | — |
| Parental history of ART for own health2 | 20 (17) | 12 (12)3 | — |
| Caregiver’s home has one room | 86 (71) | 70 (56) | 0.002 |
| Two or more rooms | 27 (22) | 52 (42) | |
| Missing | 8 (6.6) | 2 (1.6) | |
Information on PMTCT was not available in the child cohort.
In the infant cohort, maternal history of ART was collected; in the child cohort, the question referred to either parent. Due to this difference, significance testing was not conducted.
Data were missing for 24 children (19%).
Viral suppression
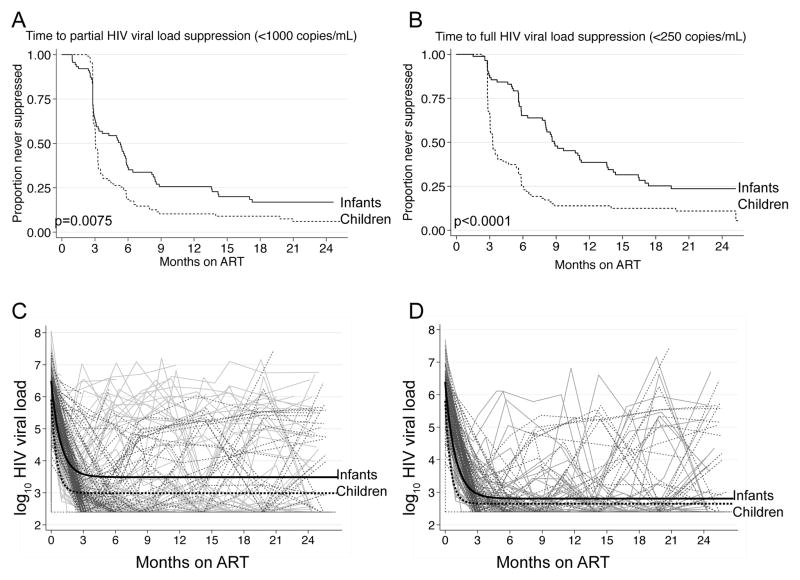
During the two years following ART, a greater proportion of older children than infants ever achieved partial (<1000 copies/mL, Figure 1A) and full (<250 copies/mL, Figure 1B) HIV-1 viral load suppression. Infants took longer to achieve partial suppression (median 159 days vs. 91 days among children, p=0.0075), and to achieve full suppression (median 265 days vs. 98 days among children, p<0.0001). Following 6 months of ART, 73% of children and 32% of infants fully suppressed virus (p<0.0001). By 24 months on ART, 93% of children and 81% of infants had ever suppressed to <1000 copies/mL (p=0.009) and 89% of children and 75% of infants had suppressed to <250 copies/mL (p=0.007). Infants were at increased risk of viral rebound to >1000 copies/mL following full suppression (HR=2.1 (95% CI 1.2–3.5), p=0.005). There was no difference in time to partial suppression among infants by PI vs. NNRTI regimen (p=0.37, data not shown).
Figure 1.
Viral suppression post-ART in the infant (solid) and child (dashed) cohorts. Panel A shows time to first partial HIV viral load suppression (<1000 copies/mL) and Panel B shows time to first full HIV viral load suppression (<250 copies/mL) after ART initiation in each cohort. P-values are from the log-rank test for equality of survival functions. Panel C shows individual trajectories of log10 HIV viral load in the infant and child cohorts over two years of follow-up, overlaid with the population mean for each cohort. Panel D shows individual trajectories of log10 HIV viral load in the infant and child cohorts, restricted to those who achieved full suppression (<250 copies/mL) at one or more visits, overlaid with the population mean for each cohort.
From the nonlinear mixed effects model, estimated mean infant viral load (VL) prior to ART was >0.5 log10 higher than the pre-ART VL in the child cohort (6.47 versus 5.91 log10 copies/ml in infants vs. children, Figure 1C, Table 2). Population-stabilized viral load post-ART (VLpost) was higher among infants than children (3.49 log10 vs. 2.86 log10 copies/mL, p<0.001). The rate of decline parameter r was larger in older children than infants (p<0.001), such that among infants viral load declined 50% of the way toward the population-stabilized post treatment viral load in 17 days, and 90% in 55 days; stabilized post-ART viral load was reached at >110 days. In the child cohort, viral load declined 50% of the way toward stabilized viral load in 11 days and 90% of the way in 36 days; stabilized post-ART viral load was reached at >71 days. In sensitivity analyses, exclusion of infants older than six months and children under 24 months did not substantially alter these estimates (Table 2). When analysis was limited to children who ever achieved full suppression, rate of viral suppression remained slower in the infant cohort (Figure 1D).
Table 2.
Nonlinear mixed effects model estimates comparing mean rate of HIV viral load suppression post ART initiation among children vs. infants.
| VL0 (95% CI) | VLpost (95% CI) | r (95% CI) | Days to % VL0-VLpost | ||||
|---|---|---|---|---|---|---|---|
| 50 | 75 | 90 | 99 | ||||
|
Including all children
| |||||||
| Child | 5.91 (5.79, 6.05) | 2.86 (2.66, 2.92) | 0.065 (0.037, 0.083) | 11 | 21 | 36 | 71 |
| Infant | 6.47 (6.37, 6.64) | 3.49 (3.15, 3.66) | 0.042 (0.038, 0.059) | 17 | 33 | 55 | 110 |
|
|
|||||||
| p-value | <0.001 | <0.001 | <0.001 | ||||
|
| |||||||
|
Excluding infants >6 months old and children < 2 years old
| |||||||
| Child | 5.83 (5.67, 5.99) | 2.82 (2.64, 2.90) | 0.053 (0.038, 0.079) | 13 | 26 | 43 | 86 |
| Infant | 6.51 (6.38, 6.71) | 3.53 (3.17, 3.78) | 0.039 (0.035, 0.055) | 18 | 35 | 59 | 118 |
|
|
|||||||
| p-value | <0.001 | <0.001 | <0.001 | ||||
|
| |||||||
|
Excluding children who never suppressed HIV viral load
| |||||||
| Child | 5.81 (5.64, 6.00) | 2.65 (2.54, 2.75) | 0.068 (0.046, 0.080) | 10 | 20 | 34 | 68 |
| Infant | 6.37 (6.20, 6.56) | 2.81 (2.69, 2.97) | 0.035 (0.032, 0.041) | 19 | 39 | 64 | 129 |
|
|
|||||||
| p-value | <0.001 | <0.001 | <0.001 | ||||
CD4% reconstitution
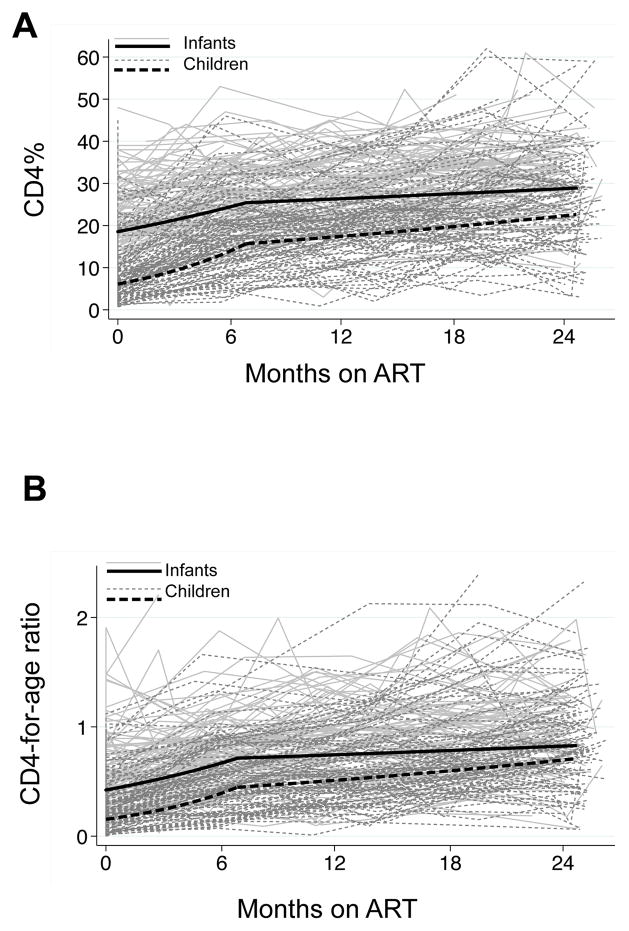
Children had substantially lower CD4% than infants pre-ART (geometric mean 7.3% vs. 19.0%, p<0.001, Figure 2A). Over two years of ART, 50% of children and 90% of infants ever reconstituted CD4 to 25% (data not shown). From the linear mixed effects model, children had significantly more rapid CD4 reconstitution in the first six months than infants (Table 3). For an average child, CD4% increased 1.45-fold/100 days in the first 6 months on ART, compared to 1.15-fold/100 days for an average infant. By 6 months, geometric mean CD4 was 15.7% for an average child and 25.4% for an average infant. From 6 months to two years, both cohorts had a slower rate of increase, and only the child slope remained statistically significant (1.07-fold/100 days for an average child vs. 1.02-fold /100 days for an average infant). The difference in slopes was strongly significant in the first phase (p<0.001) but not significant in the second phase (p=0.11). After two years of ART, the difference in CD4% between cohorts had decreased but geometric mean CD4% remained lower in the child than infant cohort (22% vs. 29%, p=0.04). Sensitivity analyses did not differ substantially from the main analysis (Table 3).
Figure 2.
Panel A shows individual CD4% trajectories in the infant and child cohorts over two years of follow-up. Black lines show the mean CD4% trajectories in each cohort estimated using a linear mixed effects model. Panel B shows individual trajectories of CD4-for-age, overlaid with the mean CD4-for-age trajectories for each cohort estimated using a linear mixed effects model.
Table 3.
Linear mixed effects model estimates comparing mean rate of CD4% reconstitution on ART among children vs. infants. The model contained two slopes, one from baseline to the 6 month measurement (days on ART ≤ 210 to account for late visits) and the other from the 6 month visit to the end of follow-up at two years (days on ART ≤ 750).
| Child (95% CI) | Infant (95% CI) | p-value | |
|---|---|---|---|
| Including all children | |||
| log(CD4%) at six months | 2.75 (2.67, 2.84) | 3.24 (3.16, 3.31) | <0.001 |
| CD4% at six months | 15.7 (14.4, 17.1) | 25.4 (23.6, 27.4) | |
| First six months slope (/100 days) | 0.45 (0.39, 0.51) | 0.15 (0.10, 0.20) | <0.001 |
| Six months - 2 years slope (/100 days) | 0.07 (0.03, 0.11) | 0.02 (−0.02, 0.06) | 0.11 |
|
| |||
|
Excluding infants >6 months old and children < 2 years old
| |||
| log(CD4%) at six months | 2.75 (2.66, 2.85) | 3.25 (3.14, 3.36) | <0.001 |
| CD4% at six months | 15.7 (14.3, 17.3) | 25.8 (23.1, 28.8) | |
| First six months slope (/100 days) | 0.48 (0.41, 0.54) | 0.20 (0.13, 0.28) | <0.001 |
| Six months - 2 years slope (/100 days) | 0.06 (0.01, 0.10) | 0.02 (−0.04, 0.08) | 0.33 |
|
| |||
|
Excluding children who never suppressed HIV viral load
| |||
| log(CD4%) at 6 months | 2.78 (2.70, 2.87) | 3.33 (3.23, 3.42) | <0.001 |
| CD4% at six months | 16.1 (14.9, 17.6) | 27.8 (25.3, 30.6) | |
| First six months slope (/100 days) | 0.43 (0.36–0.49) | 0.17 (0.09, 0.25) | <0.001 |
| Six months - 2 years slope (/100 days) | 0.07 (0.03–0.11) | 0.02 (−0.03, 0.07) | 0.13 |
CD4-for-age reconstitution
Infants had significantly higher pre-ART CD4-for-age than children (geometric mean 0.45 vs. 0.19, p<0.001, Figure 2B). Older children showed more rapid reconstitution in both the first phase from 0–6 months (1.51-fold vs. 1.25-fold increase /100 days, p<0.001) and the second phase from 6–24 months (1.09-fold vs. 1.03-fold increase /100 days, p=0.19), although the difference was statistically significant only in the first six months. Throughout the two years of ART, infants had consistently higher CD4-forage; 24-month values were 0.68 among children compared to 0.82 among infants (p=0.04).
DISCUSSION
Response to ART differed substantially among infants and children in our study. Despite their less advanced clinical disease at baseline, infants had higher viral loads and substantially worse virologic response to ART than children. In contrast, children had lower CD4% and failed to catch up to infant CD4% or CD4-for-age throughout two years of ART.
We found that rate of viral suppression was markedly slower in infants than children. Fewer infants than children achieved viral suppression at any time during follow-up, and infants who did fully suppress were at higher risk of viral rebound. The population-mean stabilized viral load post-ART was also higher in infants than children, reflecting the continued prevalence of detectable virus in the infant cohort at all time-points throughout follow-up; this was due to both the smaller proportion of infants ever achieving full suppression and the larger proportion with viral rebound after initial suppression. Virologic failure in pediatric populations occurs frequently[13, 39] particularly in infancy and adolescence[25], and is of particular concern in developing countries, owing to the limited availability of second-line treatment regimens.
Various factors may explain poor viral suppression in infants. Ideal antiretroviral dosage and adherence may be particularly challenging in pediatric populations[14,40,41] and doses may be hard for caregivers to calibrate,[42] particularly among infants due to frequent regurgitation, emesis, or spillage while administering the drug. The relative ease of administering medication to older children compared to infants may partly explain the differences we observed, as may differences in the bioavailability of different formulations[43]. To exclude differential non-adherence as a cause of differing rates of viral suppression among infants and children, we conducted a sensitivity analysis restricted to those who achieved viral suppression. In this analysis, rates of viral load suppression remained slower among infants, suggesting that adherence alone did not explain different rates of viral suppression.
Untreated infants have less viral control than older children and adults[2], and the mediators of poor viral control in the absence of ART may contribute to poor viral suppression with ART in infants. Higher baseline viral levels in infants would not necessarily lead to slower rate of viral decline but could have contributed to longer time to suppression[44]. It is unclear why infants control virus more poorly than adults in the absence of treatment. It is possible that higher infant CD4 counts contribute more target cells for viral infection and replication; in addition, infants share maternal HLA-types and may be infected with viruses that escaped maternal HLA-restricted immune responses[45]. Infants infected later postpartum have lower viral loads and mortality than those infected before the first month of life[1], possibly due to either a lower infective dose or to the ability to mount a more effective immune response. It is possible that older children, being a select group of survivors, represented those who were able to mount a more effective immune response in infancy and were more able to synergistically control virus concurrent with ART. Complementary strategies such as a therapeutic HIV vaccine or provision of HIV-specific antibodies to infants during early ART may be useful to accelerate suppression in this critical period.
Compared to infants, children had more rapid rates of CD4% and CD4-for-age recovery on ART, although differences were significant in the first six months only. Despite their greater rate of recovery, children failed to catch up to infant CD4% and CD4-for-age following two years of ART. This is consistent with previous studies[17,18,25,46,47]. A recent study from Uganda and Zimbabwe found lower CD4-for-age at ART initiation was associated with both older age at ART initiation and more rapid initial slope in CD4-for-age reconstitution; long-term predicted CD4 count was higher among children who initiated ART at a younger age in that study[19]. While some studies have suggested that most children can restore normal CD4 with treatment[48], others have concluded that those who initiate ART at an older age[17,19] or when more immunocompromised[24] are unlikely ever to normalize CD4 count. Immune recovery despite lack of viral suppression has been described in many pediatric cohorts[15,16,49,50]. In these studies, viral responders generally reconstitute CD4 more rapidly than non-responders[16,50]. Similarly, we saw a trend toward greater CD4% increase among those with viral suppression in our study, although the difference in slopes was not significant.
Our study had several strengths and limitations. Mixed effects models took advantage of longitudinal data available in both cohorts to describe immunologic and virologic response in detail. We conducted several sensitivity analyses, and were able to establish that differences between infants and children persisted when restricted to those who eventually achieved viral suppression, and were slightly more pronounced when infants under six months and children older than two years were compared. Limitations include some regimen differences between cohorts, with some infants but no children receiving PI-containing first-line regimens. However, differences in regimen were not associated with time to viral suppression in the infant cohort. We were unable to directly account for the effect of differential adherence owing to the lack of a reliable adherence measurements, such as drug levels, in our cohorts. Exclusion of children at WHO stage I from the child study was also a limitation; however, as older children are often symptomatic[51] and have low CD4%[52] at diagnosis, the study population is fairly representative of children presenting to care in developing country settings. Finally, longitudinal comparison of clinical outcomes in these cohorts is of interest, but was outside the scope of this manuscript.
CONCLUSIONS
In our infant cohort, despite ART initiation by a median of four months of age, viral suppression was slow and many failed to suppress throughout the first two years of ART. Combined with the high early mortality among infants on ART[33], our results suggest that intensification of early ART regimens to achieve rates of suppression more comparable to those in older populations could improve prognosis and achieve lower stabilized viral loads early in infection. New formulations specifically for infants are needed to address dosage and adherence challenges; complementary immune strategies also may enhance early ART responses in HIV-infected infants. Monitoring of CD4% is insufficient to identify children at risk of viral failure, and regular viral load monitoring is particularly important in infant populations among whom CD4 may appear healthy even in the presence of very high viral loads.
Acknowledgments
Source of Funding: The authors gratefully acknowledge the study participants and their families, as well as the clinical, laboratory and data teams in Nairobi who made these studies possible. The authors also acknowledge the University of Nairobi and Kenyatta National Hospital, and the Center for AIDS Research (CFAR) and the Global Center for Integrated Health of Women, Adolescents and Children (Global WACh) at the University of Washington. This work was supported by National Institutes of Health [R01 HD-23412 to GJS for the Optimizing Pediatric HAART study and R01 TW-007632 to DW for the Pediatric Adherence Study] and by the Fogarty International Center Global Research Initiative Program for New Foreign Investigators (to DW). KHA was supported by an Interdisciplinary Training Grant in Cancer Research from the National Institutes of Health [T32 CA080416].
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Author contributions
G.J.-S. and D.W. conceived and acquired funding for the infant and child studies, respectively. G.J.-S., D.W., S.B.N. and K.T. developed the primary cohorts and designed study protocols. A.L. and E.M.-O. participated in the design of clinical protocols, collected clinical data and interpreted clinical results. H.M.O. managed the infant study and data collection. J.O. oversaw assessment of cohort viral loads. K.H.A., J.P.H., J.A.S., A.R.R., and G.J.-S. participated in the design and interpretation of statistical analyses. The final manuscript was written by K.H.A. with comments and input from all authors.
References
- 1.Obimbo EM, Wamalwa D, Richardson B, Mbori-Ngacha D, Overbaugh J, Emery S, et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr. 2009;51:209–215. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson BA, Mbori-Ngacha D, Lavreys L, John-Stewart GC, Nduati R, Panteleeff DD, et al. Comparison of human immunodeficiency virus type 1 viral loads in Kenyan women, men, and infants during primary and early infection. J Virol. 2003;77:7120–7123. doi: 10.1128/JVI.77.12.7120-7123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.Shearer WT, Quinn TC, LaRussa P, Lew JF, Mofenson L, Almy S, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. N Engl J Med. 1997;336:1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo PE, Raskino C, Fiscus S, Pahwa S, Fowler MG, Spector SA, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–761. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 6.Natural history of vertically acquired human immunodeficiency virus-1 infection. The European Collaborative Study. Pediatrics. 1994;94:815–819. [PubMed] [Google Scholar]
- 7.El-Khatib Z, Ekstrom AM, Coovadia A, Abrams EJ, Petzold M, Katzenstein D, et al. Adherence and virologic suppression during the first 24 weeks on antiretroviral therapy among women in Johannesburg, South Africa - a prospective cohort study. BMC Public Health. 2011;11:88. doi: 10.1186/1471-2458-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reepalu A, Balcha TT, Skogmar S, Jemal ZH, Sturegard E, Medstrand P, et al. High rates of viral suppression in a cohort of HIV-positive adults receiving ART in Ethiopian health centers irrespective of concomitant tuberculosis. J Int AIDS Soc. 2014;17:19612. doi: 10.7448/IAS.17.4.19612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulu A, Liebert UG, Maier M. Virological efficacy and immunological recovery among Ethiopian HIV-1 infected adults and children. BMC Infect Dis. 2014;14:28. doi: 10.1186/1471-2334-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruel TD, Kakuru A, Ikilezi G, Mwangwa F, Dorsey G, Rosenthal PJ, et al. Virologic and immunologic outcomes of HIV-infected Ugandan children randomized to lopinavir/ritonavir or nonnucleoside reverse transcriptase inhibitor therapy. J Acquir Immune Defic Syndr. 2014;65:535–541. doi: 10.1097/QAI.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J. 2011;30:974–979. doi: 10.1097/INF.0b013e31822539f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk JH, Sutcliffe CG, Munsanje B, Sinywimaanzi P, Hamangaba F, Thuma PE, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS One. 2011;6:e19006. doi: 10.1371/journal.pone.0019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aboulker JP, Babiker A, Chaix ML, Compagnucci A, Darbyshire J, Debre M, et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. AIDS. 2004;18:237–245. doi: 10.1097/00002030-200401230-00013. [DOI] [PubMed] [Google Scholar]
- 14.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6:194–200. doi: 10.1007/s11904-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghaffari G, Passalacqua DJ, Caicedo JL, Goodenow MM, Sleasman JW. Two-year clinical and immune outcomes in human immunodeficiency virus-infected children who reconstitute CD4 T cells without control of viral replication after combination antiretroviral therapy. Pediatrics. 2004;114:e604–611. doi: 10.1542/peds.2004-0274. [DOI] [PubMed] [Google Scholar]
- 16.Anselmi A, Vendrame D, Rampon O, Giaquinto C, Zanchetta M, De Rossi A. Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to anti-retroviral therapy. Clin Exp Immunol. 2007;150:442–450. doi: 10.1111/j.1365-2249.2007.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis J, Walker AS, Castro H, De Rossi A, Gibb DM, Giaquinto C, et al. Age and CD4 count at initiation of antiretroviral therapy in HIV-infected children: effects on long-term T-cell reconstitution. J Infect Dis. 2012;205:548–556. doi: 10.1093/infdis/jir787. [DOI] [PubMed] [Google Scholar]
- 18.Newell ML, Patel D, Goetghebuer T, Thorne C European Collaborative S. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: is it associated with age at initiation? J Infect Dis. 2006;193:954–962. doi: 10.1086/500842. [DOI] [PubMed] [Google Scholar]
- 19.Picat MQ, Lewis J, Musiime V, Prendergast A, Nathoo K, Kekitiinwa A, et al. Predicting patterns of long-term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in sub-Saharan Africa: a cohort-based modelling study. PLoS Med. 2013;10:e1001542. doi: 10.1371/journal.pmed.1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rossi A, Walker AS, Klein N, De Forni D, King D, Gibb DM. Increased thymic output after initiation of antiretroviral therapy in human immunodeficiency virus type 1-infected children in the Paediatric European Network for Treatment of AIDS (PENTA) 5 Trial. J Infect Dis. 2002;186:312–320. doi: 10.1086/341657. [DOI] [PubMed] [Google Scholar]
- 21.Lisse IM, Aaby P, Whittle H, Jensen H, Engelmann M, Christensen LB. T-lymphocyte subsets in West African children: impact of age, sex, and season. J Pediatr. 1997;130:77–85. doi: 10.1016/s0022-3476(97)70313-5. [DOI] [PubMed] [Google Scholar]
- 22.Embree J, Bwayo J, Nagelkerke N, Njenga S, Nyange P, Ndinya-Achola J, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr Infect Dis J. 2001;20:397–403. doi: 10.1097/00006454-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Dyke RB, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008;46:1751–1760. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin DE, Warshaw MG, Miller WC, Castro H, Fiscus SA, Harper LM, et al. Using CD4 percentage and age to optimize pediatric antiretroviral therapy initiation. Pediatrics. 2014;134:e1104–1116. doi: 10.1542/peds.2014-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar-Vizcaya L, Keiser O, Karl T, Davies MA, Haas AD, Blaser N, et al. Viral load versus CD4(+) monitoring and 5-year outcomes of antiretroviral therapy in HIV-positive children in Southern Africa: a cohort-based modelling study. AIDS. 2014;28:2451–2460. doi: 10.1097/qad.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NASCOP K. Guidelines for Antiretroviral Therapy in Kenya. 4. Nairobi, Kenya: Ministry of Health; 2011. [Google Scholar]
- 28.Tucker JD, Bien CH, Easterbrook PJ, Doherty MC, Penazzato M, Vitoria M, et al. Optimal strategies for monitoring response to antiretroviral therapy in HIV-infected adults, adolescents, children and pregnant women: a systematic review. AIDS. 2014;28(Suppl 2):S151–160. [PubMed] [Google Scholar]
- 29.Rutherford GW, Anglemyer A, Easterbrook PJ, Horvath T, Vitoria M, Penazzato M, et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS. 2014;28(Suppl 2):S161–169. doi: 10.1097/QAD.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 30.Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankale JL, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53:1283–1290. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Innes S, Lazarus E, Otwombe K, Liberty A, Germanus R, Van Rensburg AJ, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late? J Int AIDS Soc. 2014;17:18914. doi: 10.7448/IAS.17.1.18914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wamalwa D, Benki-Nugent S, Langat A, Tapia K, Ngugi E, Slyker JA, et al. Survival benefit of early infant antiretroviral therapy is compromised when diagnosis is delayed. Pediatr Infect Dis J. 2012;31:729–731. doi: 10.1097/INF.0b013e3182587796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wamalwa DC, Farquhar C, Obimbo EM, Selig S, Mbori-Ngacha DA, Richardson BA, et al. Medication diaries do not improve outcomes with highly active antiretroviral therapy in Kenyan children: a randomized clinical trial. J Int AIDS Soc. 2009;12:8. doi: 10.1186/1758-2652-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wamalwa D, Benki-Nugent S, Langat A, Tapia K, Ngugi E, Moraa H, et al. Treatment interruption after 2-year antiretroviral treatment (ART) initiated during acute/early HIV in infancy: a randomized trial. AIDS. 2016 doi: 10.1097/QAD.0000000000001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NASCOP K. Guidelins for Antiretroviral Therapy in Kenya. 3. Nairobi, Kenya: Ministry of Health; 2005. [Google Scholar]
- 37.Emery S, Bodrug S, Richardson BA, Giachetti C, Bott MA, Panteleeff D, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huenecke S, Behl M, Fadler C, Zimmermann SY, Bochennek K, Tramsen L, et al. Age-matched lymphocyte subpopulation reference values in childhood and adolescence: application of exponential regression analysis. Eur J Haematol. 2008;80:532–539. doi: 10.1111/j.1600-0609.2008.01052.x. [DOI] [PubMed] [Google Scholar]
- 39.Penazzato M, Prendergast AJ, Muhe LM, Tindyebwa D, Abrams EJ. Optimization of antiretroviral therapy in HIV-infected children under 3 years of age: a systematic review. AIDS. 2014;28(Suppl 2):S137–146. doi: 10.1097/QAD.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 40.Vreeman RC, Wiehe SE, Pearce EC, Nyandiko WM. A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. Pediatr Infect Dis J. 2008;27:686–691. doi: 10.1097/INF.0b013e31816dd325. [DOI] [PubMed] [Google Scholar]
- 41.Turkova A, Webb RH, Lyall H. When to start, what to start and other treatment controversies in pediatric HIV infection. Paediatr Drugs. 2012;14:361–376. doi: 10.2165/11599640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Gogtay JA, Malhotra G. Reformulation of existing antiretroviral drugs. Curr Opin HIV AIDS. 2013;8:550–555. doi: 10.1097/COH.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 43.Ivanovska V, Rademaker CM, van Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134:361–372. doi: 10.1542/peds.2013-3225. [DOI] [PubMed] [Google Scholar]
- 44.Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22:1333–1343. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn L, Abrams EJ, Palumbo P, Bulterys M, Aga R, Louie L, et al. Maternal versus paternal inheritance of HLA class I alleles among HIV-infected children: consequences for clinical disease progression. AIDS. 2004;18:1281–1289. doi: 10.1097/00002030-200406180-00006. [DOI] [PubMed] [Google Scholar]
- 46.Rosenblatt HM, Stanley KE, Song LY, Johnson GM, Wiznia AA, Nachman SA, et al. Immunological response to highly active antiretroviral therapy in children with clinically stable HIV-1 infection. J Infect Dis. 2005;192:445–455. doi: 10.1086/431597. [DOI] [PubMed] [Google Scholar]
- 47.Puthanakit T, Kerr S, Ananworanich J, Bunupuradah T, Boonrak P, Sirisanthana V. Pattern and predictors of immunologic recovery in human immunodeficiency virus-infected children receiving non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy. Pediatr Infect Dis J. 2009;28:488–492. doi: 10.1097/inf.0b013e318194eea6. [DOI] [PubMed] [Google Scholar]
- 48.Vrisekoop N, van Gent R, de Boer AB, Otto SA, Borleffs JC, Steingrover R, et al. Restoration of the CD4 T cell compartment after long-term highly active antiretroviral therapy without phenotypical signs of accelerated immunological aging. J Immunol. 2008;181:1573–1581. doi: 10.4049/jimmunol.181.2.1573. [DOI] [PubMed] [Google Scholar]
- 49.Chiappini E, Galli L, Zazzi M, de Martino M. Immunological recovery despite virological failure is independent of human immunodeficiency virus-type 1 resistant mutants in children receiving highly active antiretroviral therapy. J Med Virol. 2003;70:506–512. doi: 10.1002/jmv.10424. [DOI] [PubMed] [Google Scholar]
- 50.Marconi VC, Grandits G, Okulicz JF, Wortmann G, Ganesan A, Crum-Cianflone N, et al. Cumulative viral load and virologic decay patterns after antiretroviral therapy in HIV-infected subjects influence CD4 recovery and AIDS. PLoS One. 2011;6:e17956. doi: 10.1371/journal.pone.0017956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prendergast A, Tudor-Williams G, Jeena P, Burchett S, Goulder P. International perspectives, progress, and future challenges of paediatric HIV infection. Lancet. 2007;370:68–80. doi: 10.1016/S0140-6736(07)61051-4. [DOI] [PubMed] [Google Scholar]
- 52.Koller M, Patel K, Chi BH, Wools-Kaloustian K, Dicko F, Chokephaibulkit K, et al. Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2015;68:62–72. doi: 10.1097/QAI.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]