Abstract
Delayed sleep and meal times promote metabolic dysregulation and obesity. Altered coordination of sleeping and eating times may impact food reward valuation and interoception in the brain, yet the independent and collective contributions of sleep and meal times are unknown. This randomized, inpatient crossover study experimentally manipulates sleep and meal times while preserving sleep duration (7.05±0.44h for 5 nights). Resting-state functional MRI scans (2×5-minute runs) were obtained for 4 participants (3 males; 25.3±4.6 y), each completing all study phases (normal sleep/normal meal; late sleep/normal meal; normal sleep/late meal; late sleep/late meal). Normal mealtimes were 1, 5, 11, and 12.5h after awakening; late mealtimes were 4.5, 8.5, 14.5 and 16h after awakening. Seed-based resting-state functional connectivity (RSFC) was computed for a priori regions-of-interest (seeds) and contrasted across conditions. Statistically significant (p<0.05, whole-brain corrected) regionally-specific effects were found for multiple seeds. The strongest effects were linked to the amygdala: increased RSFC for late versus normal mealtimes (equivalent to skipping breakfast). A main effect of sleep and interaction with mealtime were also observed. Preliminary findings support the feasibility of examining the effects of sleep and meal time misalignment, independent of sleep duration, on RSFC in regions relevant to food reward and interoception.
Keywords: sleep, circadian misalignment, functional connectivity, breakfast skipping
INTRODUCTION
Sleep patterns, particularly delayed sleep timing, impact metabolism and obesity risk.1 Late bedtimes and late rise times are associated with delayed eating times1 and increased caloric intake.2 Metabolic dysregulation and obesity are more prevalent in individuals with circadian misalignments, e.g., shift workers,3 breakfast skippers,4 and late night eaters.5 These detrimental effects of voluntarily delaying sleep and eating episodes are typically confounded with reduced sleep duration1 and have not been disentangled in human studies. Furthermore, the neural mechanisms underlying the well-documented behavioral and metabolic effects of circadian misalignment are understudied.
The neurobiological substrates of food reward valuation, a process pivotal to appetite control and caloric intake, have been recently mapped.6 Complementary evidence from primate neuronal recordings and human neuroimaging studies has highlighted key players in primary, secondary and association areas processing gustatory, olfactory, visual and somatosensory information (e.g., amygdala and insula).6 Viewing food vs. non-food images engages reward valuation, cognitive control, and interoception circuits.4,7 Moreover, food-elicited activations are modulated by sleep restriction.8,9 Beyond stimulus-driven brain responses, a handful of functional magnetic resonance imaging (fMRI) studies without an explicit task (known as resting-state)10 also support the notion that resting-state functional connectivity (RSFC) patterns are affected by sleep restriction11 and differ between obese and non-obese individuals.12,13 Altered coordination of sleeping and eating likely affects food reward valuation and interoception. Yet the independent and collective contribution of sleep and meal times on RSFC is unknown.
This pilot in-patient randomized crossover study experimentally manipulates both sleep and meal times while preserving normal sleep duration (8h in bed for 5 nights) to assess the feasibility of determining whether this laboratory-induced misalignment of sleeping and eating behaviors affects RSFC across reward and interoception-related brain circuitry.
MATERIALS AND METHODS
Participants
Six participants were enrolled: 5 completed all 4 study phases; another, 3 study phases. For one study completer the scanner was unavailable for one phase, resulting in 4 participants with complete 4-phase data (3 males; 25.3±4.6 years, BMI=29.2±2.7 kg/m2). Prescreening verified adequate sleep duration (7–9 h/night over a 2-week period) using wrist actigraphy, and excluded extreme chronotypes and individuals with sleep and psychiatric disorders. Participants were non-smokers, regular breakfast eaters (within 1h of awakening at least 5 times/week) and inactive (performing <150 min/week of moderate or <75 min/week of vigorous aerobic physical activity).
The Institutional Review Boards of Columbia University Medical Center and NYU School of Medicine granted ethical approval and all participants provided written informed consent.
Study design
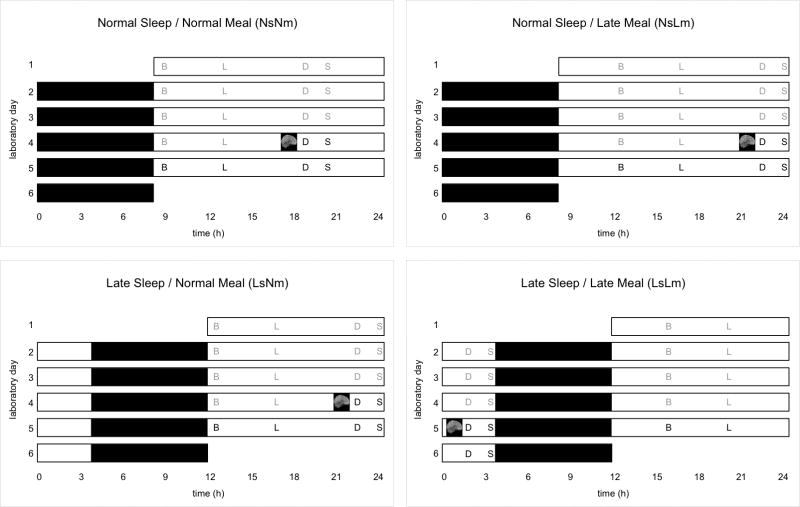
The randomized (reduced Latin square), crossover design (Figure 1) comprised 4 phases: normal sleep/normal meal (NsNm); late sleep/normal meal (LsNm); normal sleep/late meal (NsLm); late sleep/late meal (LsLm). Normal times in bed (Ns) were midnight to 8:00AM; late times in bed (Ls) were 3:30AM–11:30AM. These times reflected habitual sleep times of normal and late sleepers in a convenience sample.1 Sleep duration is reported in supplementary information. Normal mealtimes (Nm) were 1, 5, 11, and 12.5h after awakening (breakfast, lunch, dinner, and snack); late mealtimes (Lm) were 4.5, 8.5, 14.5 and 16h after awakening. Each phase entailed a 5-day in-patient stay at Columbia University Medical Center. Neuroimaging took place on day 4 with scan midpoint at 62±11min before scheduled dinner mealtime.
Figure 1.
Schematic of the 5-day inpatient randomized, crossover research design. Four participants completed all four experimental conditions (Normal Sleep/Normal Meal, Normal Sleep/Late Meal, Late Sleep/Normal Meal, Late Sleep/Late Meal) including neuroimaging. Black bars represent sleep opportunities (8 hours in bed; 0000 to 0800h for Normal Sleep, 0330–1130h for Late Sleep). Meal times are indicated with initial (B, breakfast; L, lunch; D, dinner; S, snack; gray font denotes weight-maintenance meals; bold font denotes ad libitum meals). Normal mealtimes occur 1, 5, 11 and 12.5 hours after awakening; late mealtimes occur 4.5, 8.5, 14.5, and 16 hours after awakening. The timing of the fMRI scan visit during which resting-state neuroimaging data were collected was approximately 1 hour before the 4th dinnertime and is represented by a gray brain schematic inside a black box.
FMRI Data Acquisition
Neuroimaging data were acquired using a 3T Siemens Skyra scanner (32-channel head coil) at the NYU Center for Biomedical Imaging. High-resolution anatomical T1-weighted images were obtained (3D-MPRAGE: TR=1900ms, TE=2.52ms, TI=900ms, flip angle=9°, isotropic 1mm3 voxels). Two ~5-min resting-state eyes-open scans (echo planar images: 122 time-points, TR=2500ms, TE=30ms, flip angle=80°, voxel size=3×3×3mm) were collected consecutively. Resting-state scan instructions were identical for all 4 phases.
Data preprocessing and seed-based correlation analysis
The Configurable Pipeline for the Analysis of Connectomes v0.3.9 (http://fcp-indi.github.com) was used for data preprocessing with steps detailed in supplementary information. Notably, to control for head motion effects and attenuate irrelevant signals, preprocessed data were regressed on 24 head motion parameters and on the mean time-courses of white matter and cerebrospinal fluid using subject-specific masks,14 while unbiased pairwise image registration15 served to increase robustness of registration. Nine region-of-interest seeds (4mm-radius spheres, coordinates provided in Table S1) were selected based on prior literature;12 left ventral striatum was also included.16 Each seed’s RSFC was calculated as the correlation between its time-series and that of every other voxel in the brain and then contrasted between conditions in a 2×2 ANOVA. Non-parametric statistical inference was performed with threshold-free cluster enhancement17 implemented in FSL v5.0.8 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise18).
RESULTS
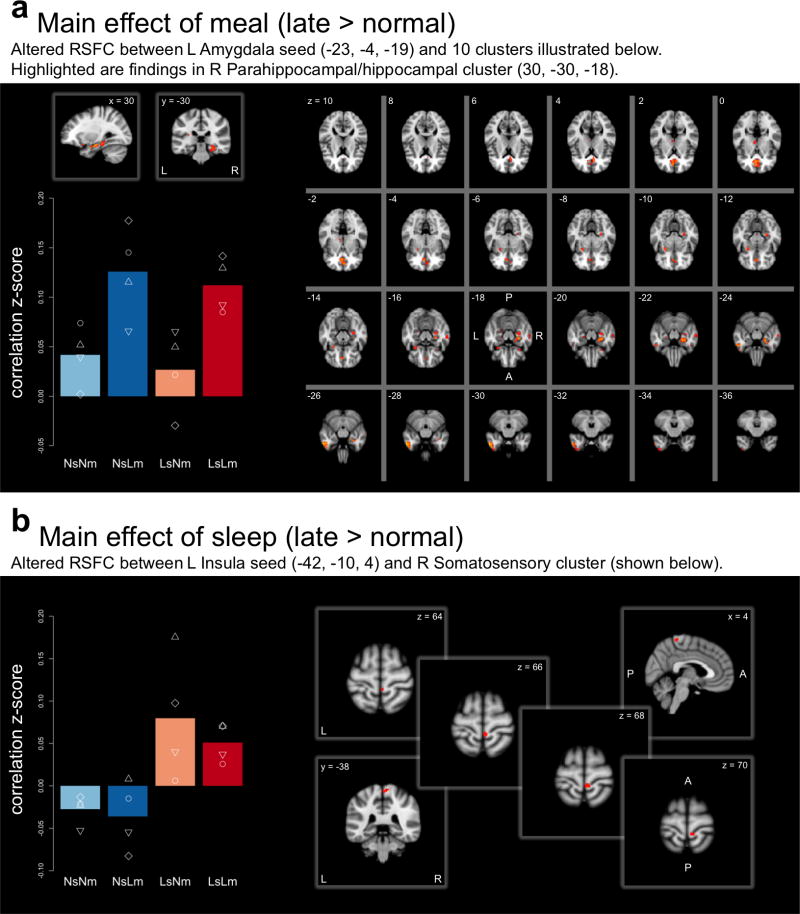
Statistically significant (p<0.05, whole-brain corrected) regionally specific effects of meal and/or sleep timing were found across multiple seeds (see complete list of significant effects in Table S1). For the meal timing main effect, 7 of the 10 seeds showed Lm>Nm RSFC, while only one seed yielded the reverse, Lm<Nm effect (Fisher’s exact p=0.009 for directionality of effect). The strongest Lm>Nm effects on RSFC were found in left and right amygdala (surviving Bonferroni correction for number of seeds). Left amygdala RSFC with ten clusters, including right paracingulate gyrus, hippocampal/parahippocampal gyri, fusiform gyrus, right amygdala, left temporal pole, putamen and thalamus, was increased in Lm (Figure 2A). Right amygdala seed RSFC in Lm broadly echoed its homologous seed connectivity patterns of increased RSFC with 8 clusters, including right hippocampal/parahippocampal gyri, cerebellum, temporal areas and lingual gyrus (see Figure S1). Across all seeds and respective clusters (Figure S2), the main effect of meal was driven by increased RSFC in Lm relative to Nm, in which RSFC was negligible.
Figure 2.
Seed-based RSFC for A. Left Amygdala seed. Regions showing significant (P<0.005, controlling for FWE) RSFC increase in late > normal meal overlaid on the MNI152 template. Bar graph plots the mean Fisher z-transformed correlation (overlaid white shapes are individual values) between the left Amygdala seed and the cluster peaking at [30, −30, −18; MNI] for each of the 4 conditions normal sleep/normal meal (NsNm), normal sleep/late meal (NsLm), late sleep/normal meal (LsNm), and late sleep/late meal (LsLm). B. Left Insula seed. Regions showing significant (P<0.05, controlling for FWE) RSFC increase in late > normal sleep overlaid on the MNI152 template. Bar graph plots the mean Fisher z-transformed correlation (overlaid white shapes are individual values) between the left Insula seed and the cluster peaking at [4, −38, 64; MNI] for each of the 4 conditions. A = anterior; FEW = family-wise error; L = left; MNI = Montreal Neurological Institute; P = posterior; RSFC = resting-state functional connectivity; R = right
Main effects of sleep (Ls>Ns) were found for two seeds; for example, RSFC between left insula and right somatosensory, pre-/post-central gyrus was greater for Ls relative to Ns (Figure 2B). Two seeds showed significant interactions between meal and sleep timing. As illustrated in Figure S2, RSFC between the right frontal pole and eight clusters (right occipital, fusiform regions, lingual gyrus, and extensive middle and superior temporal regions) reflected increased RSFC when meal and sleep times were misaligned (NsLm or LsNm), by contrast to when both meals and sleep were normal or late.
DISCUSSION
This pilot investigation specifically targeted the impact of sleep and meal misalignment on resting state functional connectivity, independent of sleep duration (total sleep time did not differ across phases). Despite only obtaining full datasets from four of the six enrolled participants, we observed significant effects, corrected for whole-brain multiple comparisons. The strongest effects (bilateral amygdala seeds) also survived Bonferroni correction for testing 10 separate seeds. The consistency of observed patterns across seeds and conditions suggests that the repeated measures within-subject design and high quality data (supplementary information) generated reliable signal in our small sample size. Specifically, head motion, which is known to produce RSFC artifacts, was low (mean frame-wise displacement=0.07mm)19 with no significant differences across conditions or sessions. Further, we leveraged non-parametric inference to empirically derive p-values, thereby avoiding the tendency of standard parametric statistics to inflate false positives.
Given the small sample size, our significant effects are admittedly preliminary. Nonetheless, comparing the current patterns of RSFC changes with related reports yields some general convergence. Primate amygdala contains neurons responsive to taste and interfaces with regions central to gustatory and olfactory processing.6 The substantial increase in RSFC between amygdala and hippocampal and parahippocampal regions under late relative to normal meals, a contrast that parallels breakfast skipping, is consistent with reduced activation in response to food images in these regions when participants consumed breakfast rather than having skipped breakfast.4 We observed RSFC modulation by sleep time shift of posterior insula which is more directly involved in interoceptive functions than primary gustatory cortex.6 The increase in insula connectivity under late relative to normal sleep timing dovetails with findings of increased insula activation when viewing food items following sleep restriction.9 The interaction between sleep and meal timing modulated RSFC between frontal pole and visual areas implicated in food cue processing.6,7
The current study demonstrates the feasibility of obtaining RSFC as an index sensitive to neural effects of delaying meal and sleep timings, independent of sleep duration. These pilot findings that misalignment of sleep and food timing alters RSFC in regions relevant to food reward and interoception motivate examination in a larger sample. Studies employing similar crossover within-subject designs should inform mechanisms mediating the detrimental effects of circadian dysregulation on metabolism and obesity risk, an issue with increasing public health ramifications.
Circadian misalignment plagues not only shift workers (~30% of US workforce) but also the growing number of individuals who voluntarily experience ‘social jetlag,’20 i.e., shift in the midpoint of sleep between work and non-work days. Therefore, insight into mechanisms underlying the sequelae of mistimed sleep and meal occasions can rapidly translate to clinical recommendations. Specifically, we find that breakfast skipping, independent of sleep timing, evokes robust, consistent alterations in RSFC of left and right amygdala. Since meal timing is under greater individual control than sleep timing (e.g., for shift workers), our preliminary findings suggest that encouraging earlier meal timing, relative to the sleeping period, may ameliorate obesity risk.
Supplementary Material
Acknowledgments
We thank Clare Kelly, PhD and Mariana Lazar, PhD for helpful discussions of scan sequence selection and analysis approaches, and Krishna Somandepalli, MS for assistance in data processing.
Funding: NIH grant R56HL119945, New York Obesity Nutrition Research Center DK26687, and National Center for Advancing Translational Sciences UL1 TR000040 (formerly the National Center for Research Resources, UL1 RR024156).
Footnotes
Disclosure: The authors declare no conflicts of interest.
Supplementary information is available at the International Journal of Obesity website (http://www.nature.com/ijo).
References
- 1.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19:1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 2.St-Onge M-P, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsson B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leidy HJ, Lepping RJ, Savage CR, Harris CT. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: A pilot fMRI study. Obesity. 2011;19:2019–2025. doi: 10.1038/oby.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, et al. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8:e56519. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolls ET. Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol. 2015;127–128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.van Meer F, van der Laan LN, Adan RAH, Viergever MA, Smeets PAM. What you see is what you eat: An ALE meta-analysis of the neural correlates of food viewing in children and adolescents. NeuroImage. 2015;104:35–43. doi: 10.1016/j.neuroimage.2014.09.069. [DOI] [PubMed] [Google Scholar]
- 8.St-Onge M-P, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes. 2014;38:411–416. doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St-Onge M-P, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–824. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly C, Castellanos FX. Strengthening connections: Functional connectivity and brain plasticity. Neuropsychol Rev. 2014;24:63–76. doi: 10.1007/s11065-014-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Z, Spaeth AM, Ma N, Zhu S, Hu S, Goel N, et al. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci Rep. 2015;5:8215. doi: 10.1038/srep08215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wijngaarden MA, Veer IM, Rombouts SARB, van Buchem MA, Willems van Dijk K, Pijl H, et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res. 2015;287:127–134. doi: 10.1016/j.bbr.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, et al. Altered default network activity in obesity. Obesity. 2011;19:2316–2321. doi: 10.1038/oby.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Martino A, Scheres A, Margulies DS, Kelly C, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 18.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 20.Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes. 2015;39:842–848. doi: 10.1038/ijo.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.