Abstract
This study introduces a flexible format for tolerogenic vaccination that incorporates IFN-β and neuroantigen (NAg) in the Alum adjuvant. Tolerogenic vaccination required all three components; IFN-β, NAg, and Alum for inhibition of experimental autoimmune encephalomyelitis (EAE) and induction of tolerance. Vaccination with “IFN-β+NAg in Alum” ameliorated NAg-specific sensitization and inhibited EAE in C57BL/6 (B6) mice in both pre-treatment and therapeutic regimens. Tolerance induction was specific for the tolerogenic vaccine antigen PLP178-191 or MOG35-55 in PLP and MOG-induced models of EAE, respectively, and was abrogated by pretreatment with a depleting anti-CD25 mAb. IFN-β/Alum-based vaccination exhibited hallmarks of infectious tolerance, because “IFN-β+OVA in Alum”-specific vaccination inhibited EAE elicited by “OVA+MOG in CFA” but not by “MOG in CFA”. “IFN-β+NAg in Alum” vaccination elicited elevated numbers and percentages of FOXP3+ T cells in blood and secondary lymphoid organs in 2D2 MOG-specific transgenic mice, and repeated boosters facilitated generation of activated CD44high CD25+ Treg populations. IFN-β and MOG35-55 elicited suppressive FOXP3+ Tregs in vitro in the absence of Alum via a mechanism that was neutralized by anti-TGF-β and that resulted in the induction of an ‘effector’ CD69+ CTLA-4+ IFNAR+ FOXP3+ Treg subset. “In vitro IFN-β+MOG-induced Tregs” inhibited EAE when transferred into actively-challenged recipients. Unlike “IFN-β+NAg in Alum” vaccines, vaccination with “TGF-β+MOG35-55 in Alum” did not elevate Treg percentages in vivo. Overall, this study indicates that “IFN-β+NAg in Alum” vaccination elicits NAg-specific, suppressive CD25+ Tregs that inhibit CNS autoimmune disease. Thus, IFN-β has the activity spectrum that drives selective responses of suppressive FOXP3+ Tregs.
Keywords: Rodent, FOXP3, Regulatory T cells, Interferon-beta, EAE/MS, Tolerance, Neuroimmunology
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) with a presumed autoimmune etiology (1–3). MS is a leading cause of non-traumatic neurological disability of young adults in the western world, including an estimated 350,000 individuals in the USA. Cell types that infiltrate the CNS in MS include CD4+ T cells which are thought to be the primary instigators of disease together with CD8+ T cells and monocytes/ macrophages. B cells may also mediate important effector functions in MS as reflected by the presence of ectopic intrathecal B cell follicles in the CNS of some MS patients and the presence of oligoclonal immunoglobulin species in the cerebrospinal fluid. First-line therapeutics for MS include the immunomodulatory IFN-β drugs which have been used since 1993 (4–9). Disadvantages of contemporary IFN-β therapy for MS include a requirement for chronic treatment, limited efficacy, and a loss of treatment benefit upon discontinuation of therapy. IFN-β efficacy is marked by substantial inter-patient variability with the implication that IFN-β therapy is optimal when MS pathogenesis is mediated by a spectrum of Th1-like effector cells (10, 11). The underlying mechanisms of IFN-β therapy are unresolved and are thought to involve rebalancing of pro-inflammatory versus anti-inflammatory cytokines such as IL-12 and IL-10 and a reset of effector and regulatory CD4+ T cell subsets. New insight into the underlying mechanisms of IFN-β action in T cell regulation and autoimmune disease may reveal new approaches to exploit the beneficial actions of IFN-β in MS.
EAE is widely used as an animal model of MS, in part due to commonalities in T cell regulatory strategies (12, 13). In rodent models of EAE, CD4+ T-helper cells are the primary instigators of disease, particularly T cells of the Th1 IFN-γ producing subset and the Th17 IL-17 producing subset. In select models of EAE, B cells and antibody mediate important pathogenic roles in CNS inflammation (14, 15). EAE has been successfully controlled by different strategies of tolerogenic vaccination (16–19), and several of these tolerogenic vaccine strategies have been advanced in MS as a means to specifically target pathogenic myelin-specific T cells. Tolerogenic vaccine approaches that emerged from studies of EAE into clinical testing in MS have been based on subcutaneous injection (20) or oral delivery (21) of various myelin basic protein preparations, direct administration of myelin basic protein peptide (MBP8298, Dirucotide) (22–24), or administration of a fusion protein comprised of myelin basic protein and proteolipid protein (PLP) epitopes (MP4) (25, 26). Although these approaches had success in EAE, attempts to translate these myelin-specific vaccine strategies in MS did not show robust clinical efficacy. Indeed, use of altered peptide variants of the myelin basic protein (MBP)83–99 peptide resulted in treatment-induced exacerbations of MS (27). Additional tolerogenic vaccine strategies that have been advanced from EAE to MS include the use of a pooled set of naked myelin peptide antigens (28), DNA vaccines that encode myelin basic protein (29), transdermal application of myelin peptides (30, 31), leukocyte-coupled peptides (32–34), and fusion proteins incorporating myelin peptides linked to a dendritic cell targeting domain (35, 36).
A novel tolerogenic vaccine strategy designed to improve therapeutic efficacy is based on the use cytokine-antigen fusion proteins that incorporate a regulatory cytokine such as IFN-β, GM-CSF, IL-2, or IL-16 with a dominant encephalitogenic epitope of a myelin autoantigen within a single-chain recombinant protein (37–44). Two previous studies (38, 41) provided evidence that IFN-β could be repurposed from an inhibitory cytokine to a tolerogenic vaccine as a single-chain IFNβ-NAg fusion protein and thereby used to enable immunological tolerance to a myelin NAg. The IFNβ-NAg vaccine immunotherapy has particular promise due to widespread use of IFN-β as a well-tolerated, effective therapy for MS. A tolerogenic, vaccine-induced memory would decrease the clinical need for high-frequency, high-dose administration of IFN-β and would thereby mitigate the antigenic stimulus responsible for generation of neutralizing anti-IFN-β Ab. Overall, an IFN-β-based tolerogenic vaccine may have qualitative advantages compared to IFN-β monotherapy or tolerogenic vaccination with naked myelin peptides.
Tolerogenic vaccination with cytokine-NAg fusion proteins generally required physical linkage of cytokine and NAg for optimal tolerance induction (37–40, 43, 44). This study is based on the concept that physical linkage of IFN-β and NAg could also be achieved in a substantially more flexible format by use of an intermediate that facilitated indirect, noncovalent bonds between IFN-β and NAg. The solution was provided by the Alum adjuvant which is in common clinical use as an adjuvant in vaccine formulations and is well-known to bind and immobilize proteins onto a common matrix. IFN-β and NAg peptides mixed in the Alum adjuvant were predicted to have the required physical linkage in that both IFN-β and NAg would noncovalently bind a common substrate. This study provides evidence that IFN-β and NAg, when mixed and administered in the Alum adjuvant, elicited active tolerogenic responses that inhibited EAE in B6 mice by a mechanism dependent upon IFN-β, NAg, and Alum. The tolerogenic mechanisms reflected induction of a major FOXP3+ regulatory T cell (Treg) population because culture with IFN-β and MOG35-55 induced MOG-specific FOXP3+ T cells in vitro and vaccination of mice with the “IFN-β + MOG35-55 in Alum” vaccine directly elicited FOXP3+ Tregs in vivo. Tolerogenic vaccination with “IFN-β + MOG35-55 in Alum” inhibited MOG-induced EAE but not PLP-induced EAE, and vice versa, vaccination with “IFN-β + PLP178-191 in Alum” inhibited PLP-induced EAE but not MOG-induced EAE. In both cases, antibody-mediated depletion of CD25+ Tregs in vivo blocked tolerogenic vaccination. These data reveal IFN-β as a key cytokine controlling specification of a FOXP3+ Treg lineage in vitro and in vivo. IFN-β-based tolerogenic vaccines represent a new class of tolerogenic vaccines that could be exploited as a therapy for MS.
Materials and Methods
Animals and Reagents
B6 mice, Foxp3-IRES-GFP knock-in (FIG) mice (B6.Cg-Foxp3tm2Tch/J, Stock Number 006772), and MOG35-55 specific TCR transgenic 2D2 mice (B6-Tg(Tcra2D2,Tcrb2D2)1Kuch/J) (Stock Number 006912) were obtained from the Jackson Laboratory (Bar Harbor, ME) and were housed and bred in the Department of Comparative Medicine at East Carolina University Brody School of Medicine. 2D2-FIG mice were obtained through intercross breeding. 2D2 mice have a myelin oligodendrocyte glycoprotein (MOG)-specific, self-reactive T cell repertoire. Routine screening of 2D2 mice was performed by FACS analysis of PBMC by use of antibodies specific for TCR Vβ11 and/ or Vα3.2. The FIG genotype was screened by use of forward (CAC CTA TGC CAC CCT TAT CC) and reverse (ATT GTG GGT CAA GGG GAA G) primers. The FIG knock-in product was 390 base-pairs, and the wt product was 341 base-pairs. Animal care and use was performed in accordance with approved animal use protocols and guidelines of the East Carolina University Institutional Animal Care and Use Committee.
Antigens and IFNβ-NAg fusion proteins
Synthetic MOG35-55 (M-E-V-G-W-Y-R-S-P-F-S-R-V-V-H-L-Y-R-N-G-K) or PLP178-191 (N-T-W-T-T-C-Q-S-I-A-F-P-S-K) was obtained from Genscript (Piscataway, NJ). The initial preparation of recombinant TGFβ1 used in this project was a generous gift from Dr. Peter Sun (NIH). Subsequently, a rat TGFβ1 sequence was cloned into the pIRES2-AcGFP1 vector (Clontech, Mountain View, CA) and expressed via stable transfection of human embryonic kidney (HEK) cells. TGFβ1 was designed as described (45). This expression vector encoded a rat serum albumin leader sequence, an 8-histidine purification tag, the latency-associated peptide (LAP), the native RHRR cleavage site, and the C-terminal TGF-β1 sequence. A C32S substitution in the LAP domain enabled high level expression. The protein was expressed in HEK supernatants, purified on Ni-NTA affinity columns, and was activated by 10 minutes of exposure to 70°C. The quantitative bioactivity of each TGF-β1 preparation was verified by induction of FOXP3 in cultures of MOG-stimulated 2D2-FIG splenocytes.
Derivation, expression, purification, and bioassay of the murine cytokine-NAg fusion proteins were described in previous studies (38, 42). Murine IFN-β and the N-terminal domain of IFNβ-MOG are comprised of the murine IFN-β sequence (accession number NP_034640, http://www.ncbi.nlm.nih.gov/gene) except that a non-native alanine residue was added as the second amino acid to encode an optimal Kozak translation-initiation site (GCCGCCACC-ATG-GCC-). The C-terminus of the IFNβ-MOG fusion protein included an enterokinase linker cleavage site, the MOG35-55 sequence, and an 8-histidine affinity chromatography purification tag. IFN-β also had a C-terminal enterokinase linker and an 8-histidine purification tag. Expression supernatants were concentrated on YM10 ultrafiltration membranes and were directly applied to consecutive Ni-NTA Agarose columns (Qiagen, Chatsworth, CA) followed by extensive washing of the resin (50 mM NaH2PO4, 500 mM NaCl, 10 mM imidazole, pH 8.0). IFNβ-MOG or IFN-β was eluted by acid elution (pH 4.0) or with 250 mM imidazole (pH 8.0) and was concentrated and diafiltrated in Amicon Ultra-15 centrifugal filter devices (EMD Millipore, Billerica, MA). Protein quantity was assessed by absorbance at 280 nm, and purity was assessed by SDS-PAGE. The bioactivities of murine IFN-β recombinant proteins were confirmed in vitro by inhibition of IL-2 dependent T cell proliferation as shown by inhibitory constants (Ki/ IC50) in the low picomolar range (i.e., half-maximal inhibition in the 1–10 pM range) and by induction of FOXP3+ T cells in cultures of MOG-stimulated 2D2-FIG splenocytes.
Generation, purification, and administration of mAb
The PC61-5.3 anti-CD25 rat IgG1(λ) hybridoma (46), the Y13-259 anti-v-H-Ras rat IgG1(κ) hybridoma (47), and the 1D11.16.8 anti-mouse-TGF-β1/2/3 mouse IgG1 hybridoma (48, 49) were obtained from ATCC and were subcloned twice to ensure stability. The LRTC1 anti-rat LFA-1 mouse IgG1 hybridoma was originally derived in our laboratory (50–52) and had no crossreactivity with mouse LFA-1. PC61 and Y13 were used as sources of CD25+ Treg depleting mAb and the isotype control mAb, respectively. 1D11 and LRTC1 were used as sources of anti-TGF-β mAb and the isotype control mAb, respectively. For all 4 hybridomas, cells were cultured in supplemented DMEM in C2011 hollow fiber cartridges (FiberCell Systems, Inc., Frederick, MD). Hybridoma supernatants were clarified at 7,200 × g, precipitated with 50% ammonium sulfate, and dissolved in PBS. MAb preparations were purified on protein G agarose columns. Antibody was eluted with 200 mM glycine at pH 3.0 and immediately neutralized by 1M Tris buffer of pH 9.0. The purity of these mAb was verified by SDS-PAGE. Specific activities of all PC61 preparations were determined by staining of murine CD25+ T cells with serial ½ log dilutions of the mAb. After washing, PC61-stained T cells were labeled with a PE-conjugated goat anti-rat IgG(H+L) secondary antibody followed by flow cytometric analysis. As designated for pretreatment experiments, purified mAb were administered i.p. at a dose of 250 µg/ injection to mice on days −5 and −3 (or days −4 and −2) unless designated otherwise. Depletion of specific lymphoid subsets was confirmed by flow cytometric analysis of PBMC on days −1 or 0. Active immunization with MOG35-55 in CFA was initiated on day 0.
Flow cytometric analyses of splenocytes and PBMC
Cells were washed in HBSS with 2% heat-inactivated FBS and stained with designated cocktails of fluorochrome-conjugated antibodies for 1 hr at 4°C in the dark. After staining PBMC, erythrocytes were lysed with 1:10 HBSS for 20 seconds at 4°C followed by addition of 2X PBS. Cells were then washed 3 times with HBSS/ 2% FBS. Data were collected by use of a Becton-Dickinson LSRII flow cytometer (San Jose, CA) and analyzed by use of FlowJo software. In designated experiments, reference ‘counting’ beads were added to samples immediately before flow cytometric analysis (AccuCheck Counting Beads, Life Technologies, Frederick, MD or APC-conjugated CaliBRITE™ beads, BD Biosciences). The use of reference beads enabled comparisons of cell yield or absolute cell numbers. Pairwise comparisons were analyzed by two-tailed t-tests for data that passed Normality (Shapiro-Wilk) and Equal Variance (Brown-Forsythe) Tests. Otherwise, data were assessed with a Mann-Whitney Rank Sum Test. Fluorochrome-conjugated mAb were obtained from BioLegend or BD Biosciences and included CD19 (6D5), CD25 (PC61 or 3C7), CD28 (E18), CD3 (17A2 or 145-2C11), CD4 (GK1.5), CD44 (IM7), CD45 (30-F11), CD5 (53–7.3), CD69 (H1.2F3), CD8 (53-6.7), CD80 (16-10A1), CD86 (GL-1), CTLA-4 (UC10-4B9), GARP (F011-5), I-Ab (AF6-120.1), IFNAR-1 (MAR1-5A3), Ly-6G (Gr1) (1A8), PD1 (CD279) (29F.1A12), PDL1 (CD274) (10F.9G2), PDL2 (CD273) (TY25), TCR-Va3.2 (RR3-16), TCR-Vβ11 (KT11). Multicolor panels were designed in the figure legends.
Preparation of tolerogenic vaccines
A phosphate buffered saline solution was prepared that contained designated doses of IFN-β, TGF-β, or peptide NAg. Aluminum Hydroxide Gel colloidal suspension (A8222 Alum, Sigma Aldrich or Alhydrogel adjuvant # vac-alu-250, Invivogen) was mixed thoroughly. Equal volumes of Alum and a solution containing IFN-β and NAg were combined for a total injection volume of 100 µl per mouse. IFN-β and NAg were given in matched equimolar doses (e.g., a 2 nmole vaccine dose included 2 nmoles IFN-β and 2 nmoles NAg, or a 5 nmole vaccine dose included 5 nmoles IFN-β and 5 nmoles NAg) unless designated otherwise. The mixture was incubated for 1 hr on ice with continuous agitation to allow the protein/ peptide attachment to the Alum gel precipitate. The vaccine was administered subcutaneously by two injections of 50 µl each. No signs of inflammation were noted at the injection site.
Induction and assessment of EAE
CFA (Incomplete Freund’s Adjuvant with 4 mg/ ml heat-killed Mycobacterium tuberculosis H37Ra, BD Biosciences, Franklin Lakes, NJ) was mixed 1:1 with MOG35-55 or PLP178-191 in phosphate-buffered saline. The CFA/ antigen mixture was emulsified by sonication. EAE was elicited in B6 mice by injection of 200 µg MOG35-55 or PLP178-191 in a total volume of 100 µl emulsion via three subcutaneous injections of 33 µl across the lower back. Each mouse received separate injections (200 nanograms i.p.) of Pertussis toxin on days 0 and 2. All immunizations were performed under isoflurane anesthesia (Abbott Laboratories, Chicago, IL). Mice were assessed daily for clinical score and body weight. The following scale was used to score the clinical signs of EAE: 0, no disease; 0.5, partial paralysis of tail without ataxia; 1.0, flaccid paralysis of tail or ataxia but not both; 2.0, flaccid paralysis of tail with ataxia or impaired righting reflex; 3.0, partial hind limb paralysis marked by inability to walk upright but with ambulatory rhythm in both legs; 3.5, same as above but with full paralysis of one leg; 4.0, full hindlimb paralysis; 5.0, total hindlimb paralysis with forelimb involvement or moribund. A score of 5.0 was a humane endpoint for euthanasia.
The incidence of EAE reflected the number of mice afflicted with EAE compared to the total group size. Cumulative EAE scores were calculated by summing daily scores for each mouse across the designated time course of disease. Maximal scores were calculated as the most severe EAE score for each mouse. Mice that did not exhibit EAE had a score of zero for the cumulative and maximal scores, and these scores were included in the group average. Attrition reflected the number of mice that reached clinical endpoints (e.g., score of 5.0). Mice that exhibited humane endpoints as assessed by body weight loss, body score, or clinical score of 5.0 were subjected to humane euthanasia and were omitted from scoring thereafter. Thus, groups of mice exhibiting substantial attrition had artificially depressed mean cumulative scores, but attrition did not affect mean maximal scores. Time-course graphs portrayed daily mean maximal scores. Cumulative and maximal EAE scores were converted to ranked scores and analyzed by nonparametric ANOVA. To calculate percent maximal weight loss, 100% body weight was assigned as the maximal body weight obtained from day 1 through day 10, and daily body weights were calculated for each day after normalization to this 100% value. The minimum body weight was defined as the lowest body weight after normalization to the 100% value during the span of day 11 until the end of the experiment. Maximal weight loss was calculated by subtraction of the normalized minimum value from the 100% value. Negative weight loss values represented weight gain. Weight loss was analyzed by parametric ANOVA. Nonparametric and parametric ANOVA were assessed with a Bonferroni Post Hoc test unless noted otherwise. Incidence of EAE was analyzed pair-wise by Fisher’s Exact Test. Mean EAE and weight loss data were shown with the standard error of the mean.
Results
Vaccination with an IFNβ-NAg fusion protein was a therapeutic intervention in EAE
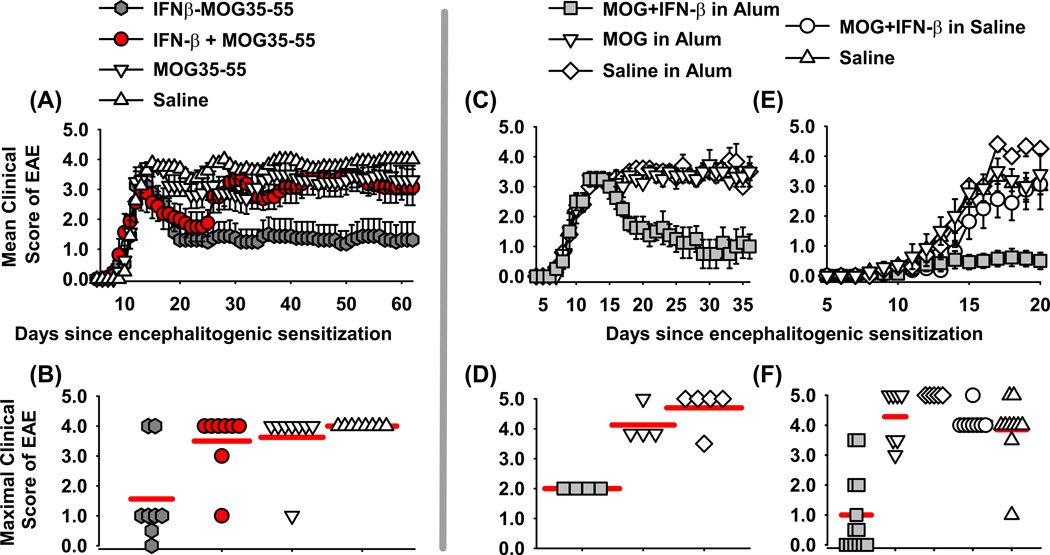
Previous studies revealed that covalent linkage of murine IFN-β and PLP139-151 was essential for tolerance induction in the SJL relapsing remitting model of EAE (38). To assess the generality of these findings, experiments were performed to assess whether covalent linkage was needed for tolerance induction in the B6 model of chronic EAE (Figure 1A–B). A treatment model in B6 mice was chosen because chronic EAE in this model is typically resistant to antigen-specific interventions. To address the requirement of cytokine-NAg covalent linkage, B6 mice were immunized to elicit paralytic EAE on day 0 (Figure 1A–B and Table 1). On day 13, mice were matched for severity of EAE and were treated with 2 nmoles of murine IFNβ-MOG fusion protein, a mixture of IFN-β and MOG, MOG alone, or saline. Treatments were given in saline on day 13, and again on days 15 and 17 with a final treatment on day 19. Mice treated with either the IFNβ-MOG fusion protein or the combination of “IFN-β and MOG35-55” in saline exhibited a partial recovery marked by a nadir in paralytic signs from days 22–24 (Figure 1A). Mice treated with IFNβ-MOG remained stable with mild EAE thereafter and did not relapse whereas mice treated with the combination of IFN-β and MOG relapsed by day 30 and exhibited severe paralytic EAE during the remainder of the experiment. Mice treated with MOG35-55 or saline exhibited a sustained course of paralytic EAE. These data revealed that the covalent linkage of IFN-β and MOG35-55 is critical for a prolonged beneficial activity of IFNβ-MOG in the B6 model of EAE.
Figure 1. IFN-β is a tolerogenic vaccine adjuvant in EAE.
EAE was induced in B6 mice on day 0 by injection of 200 µg of MOG35-55 in CFA with i.p. injections of Pertussis toxin on days 0 and 2 (A–F). Shown are EAE timecourses (A, C, E) and density dotplots (B, D, F) for treatment (A–D) and pretreatment experiments (E–F). (A–B) On day 13, groups were matched for severity of EAE (mean maximal scores of 3.1–3.3, mean cumulative scores of 25.0–26.5). MOG/ CFA immunized mice were treated (2 nmoles each in saline) with IFNβ-MOG, a combination of IFN-β and MOG35-55, MOG35-55, or saline by subcutaneous injections in saline on days 13, 15, 17, and 19. (B) Shown are maximal scores of EAE during days 20–62. (C–D) MOG/ CFA immunized mice were matched for severity of EAE on day 14 (mean maximal scores of 3.2–3.4, mean cumulative scores of 16.5–17.0). Matched groups were given one subcutaneous injection (5 nmoles) of “Saline in Alum”, “MOG35-55 in Alum”, or “IFN-β + MOG in Alum” on day 15. (D) Shown are maximal scores of EAE during days 18–36. (E–F) Designated vaccines were given at a dose of 2 nmoles on days −21, −14, and −7. The EAE timecourse is shown through day 20 and the mean maximal scores are shown based on data collected through day 32. (A, C, E) Error bars represent standard error of the mean. (B, D, F) Red horizontal bars represent the average of the values in each group. Tabular data and statistical analysis for these experiments are shown in Table 1.
Table 1.
IFN-β is a tolerogenic adjuvant.
| Figurea | Group | Incidence | Attrition | Mean (± SE) cumulative scores |
P value | Mean (± SE) maximal scores |
P value | % maximal weight loss |
P value |
|---|---|---|---|---|---|---|---|---|---|
| 1A, B | IFNβ-MOG | 7 of 8 | 0 of 8 | 57.9 ± 21.6 | * | 1.6 ± 0.5 | * | 17.0 ± 3.5% | * |
| 1A, B | IFNβ + MOG | 8 of 8 | 1 of 8 | 125.9 ± 15.2 | ns | 3.5 ± 0.4 | 0.023 | 22.0 ± 3.5% | ns |
| 1A, B | MOG35-55 | 8 of 8 | 0 of 8 | 138.3 ± 19.9 | 0.024 | 3.6 ± 0.4 | 0.006 | 23.9 ± 2.4% | ns |
| 1A, B | Saline | 8 of 8 | 0 of 8 | 163.3 ± 3.8 | 0.001 | 4.0 ± 0.0 | 0.001 | 27.7 ± 1.6% | ns |
| 1C, D | IFN-β + MOG in Alum | 4 of 4 | 0 of 4 | 23.6 ± 7.8 | * | 2.0 ± 0.0 | * | 15.3 ± 3.4% | * |
| 1C, D | MOG in Alum | 4 of 4 | 1 of 4 | 61.8 ± 3.9 | 0.014 | 4.1 ± 0.3 | 0.010 | 30.6 ± 4.3% | 0.037 |
| 1C, D | Saline in Alum | 5 of 5 | 4 of 5 | 57.9 ± 5.3 | 0.034 | 4.7 ± 0.3 | 0.001 | 28.7 ± 3.1% | 0.054 |
| 1E, F | IFN-β + MOG in Alum | 7 of 13 | 0 of 13 | 9.1 ± 3.9 | * | 1.0 ± 0.4 | * | 8.5 ± 1.6% | * |
| 1E, F | IFN-β + MOG in Saline | 8 of 8 | 5 of 8 | 31.0 ± 5.1 | 0.022 | 4.1 ± 0.1 | < 0.001 | 20.3 ± 1.5% | 0.002 |
| 1E, F | MOG in Alum | 7 of 7 | 4 of 7 | 30.5 ± 3.5 | 0.013 | 4.3 ± 0.3 | < 0.001 | 16.4 ± 2.7% | ns |
| 1E, F | Saline in Alum | 5 of 5 | 5 of 5 | 23.1 ± 3.0 | ns | 5.0 ± 0.0 | < 0.001 | 21.2 ± 1.5% | 0.005 |
| 1E, F | Saline | 10 of 10 | 8 of 10 | 21.7 ± 5.5 | ns | 3.9 ± 0.4 | < 0.001 | 22.2 ± 2.6% | < 0.001 |
| 3A–B | MOG-MOG | 6 of 7 | 0 of 7 | 16.5 ± 4.8 | * | 2.4 ± 0.6 | * | 4.7 ± 2.9% | * |
| 3A–B | MOG-MOG (Anti-CD25) | 7 of 7 | 3 of 7 | 46.9 ± 2.5 | 0.002 | 4.1 ± 0.2 | 0.001 | 19.2 ± 2.3% | 0.001 |
| 3A–B | PLP-MOG | 5 of 5 | 3 of 5 | 45.3 ± 5.4 | 0.002 | 4.2 ± 0.2 | < 0.001 | 21.1 ± 3.6% | 0.001 |
| 3A–B | PLP-MOG (Anti-CD25) | 6 of 6 | 3 of 6 | 46.9 ± 2.7 | 0.003 | 4.1 ± 0.2 | 0.001 | 20.9 ± 1.5% | 0.001 |
| 3C–D | MOG-PLP | 5 of 5 | 2 of 5 | 81.1 ± 2.5 | 0.000 | 4.3 ± 0.3 | 0.004 | 17.0 ± 1.8% | 0.009 |
| 3C–D | MOG-PLP (Anti-CD25) | 5 of 5 | 3 of 5 | 65.0 ± 4.1 | ns | 4.4 ± 0.4 | 0.003 | 17.1 ± 3.3% | 0.009 |
| 3C–D | PLP-PLP | 3 of 5 | 0 of 5 | 11.1 ± 10.6 | * | 0.8 ± 0.6 | * | 3.8 ± 0.6% | * |
| 3C–D | PLP-PLP (Anti-CD25) | 5 of 5 | 1 of 5 | 68.2 ± 7.8 | 0.016 | 3.9 ± 0.3 | 0.028 | 15.3 ± 3.1% | 0.026 |
| 5A–B | IFNβ-Tregs | 2 of 8 | 0 of 8 | 1.8 ± 1.4 | * | 0.2 ± 0.1 | * | 1.0 ± 0.8% | * |
| 5A–B | Control T cells | 10 of 10 | 0 of 10 | 30.0 ± 4.6 | < 0.001 | 2.7 ± 0.2 | < 0.001 | 11.3 ± 2.5% | 0.030 |
| 5A–B | Saline | 11 of 11 | 1 of 11 | 24.4 ± 3.5 | < 0.001 | 2.9 ± 0.4 | < 0.001 | 11.9 ± 2.5% | 0.016 |
These data are portrayed graphically in Figures 1, 3, and 5A–B. The experimental approach is described in the respective figure legend. For Figure 3, group designations MOG-MOG, PLP-MOG, MOG-PLP, PLP-PLP refers first to the peptide (MOG35-55 or PLP178-191) in the Alum + IFN-β tolerogenic vaccine and second to the peptide in the CFA challenge. Nonparametric ANOVA based on ranked scores was used to assess group differences in mean cumulative scores and mean maximal scores, and parametric ANOVA was used to assess group differences in percent maximal weight loss relative to the comparator group
(Bonferroni Post-Hoc test).
“IFN-β + MOG in Alum” was therapeutic and tolerogenic in EAE
Given that physical linkage is needed for optimal tolerogenic activity, we predicted that such linkage could be non-covalent and indirect rather than covalent and direct. We reasoned that the widely used Alum adjuvant may fulfill the requirement for indirect noncovalent linkage because the precipitated aluminum hydroxide matrix is well known to bind protein and peptide antigens. The prediction was that IFN-β and NAg peptides would be bound, immobilized, and crosslinked by the Alum adjuvant matrix and thereby achieve the requisite linkage needed for IFN-β mediated tolerogenic activity. To test this prediction, vaccines comprised of “IFN-β + MOG35-55 in Alum”, “MOG35-55 in Alum”, and “Saline in Alum” were administered once after onset of paralytic EAE on day 15 in groups that had been matched for mean cumulative and maximal disease scores (Figure 1C–D and Table 1). A single administration of “5 nmoles IFN-β + 5 nmoles MOG35-55 in Alum” reversed the course of paralytic EAE, facilitated clinical recovery, and ameliorated EAE-associated weight loss. The vaccines “MOG35-55 in Alum”, and “Saline in Alum” had no effect in that the respective mice continued to exhibit severe paralytic EAE throughout the experiment and several mice progressed to a score of 5.0 (humane endpoint, Table 1). These data indicated that the “IFN-β + MOG35-55 in Alum” vaccine had robust efficacy as a therapeutic intervention.
Treatment regimens are used to measure the clinically-significant modality of therapeutic efficacy. Conversely, pretreatment regimens are used to measure tolerogenic activity because vaccine-mediated inhibitory activity must be remembered by the immune system to impact a subsequent encephalitogenic challenge. To test pretreatment efficacy, the “IFN-β + MOG35-55 in Alum” vaccine along with control vaccine formulations were administered at a dose of 2 nmoles on days −21, −14, and −7 followed by an encephalitogenic challenge on day 0 (Figure 1E–F and Table 1). The “IFN-β + MOG35-55 in Alum” vaccine attenuated the subsequent induction of EAE whereas vaccines comprised of “MOG in Alum”, “Saline in Alum”, “MOG + IFN-β in saline”, or saline had no impact on the course of severe chronic EAE. The EAE timecourse is shown through day 20 and not beyond due to attrition of mice that reached a score of 5.0 in the four control groups (Table 1). These data support the hypothesis that NAg in the context of an “IFN-β in Alum” adjuvant specifies a strong NAg-specific tolerogenic response.
Pretreatment with the “IFN-β + MOG in Alum” vaccine altered MOG-specific sensitization in MOG/CFA sensitized mice
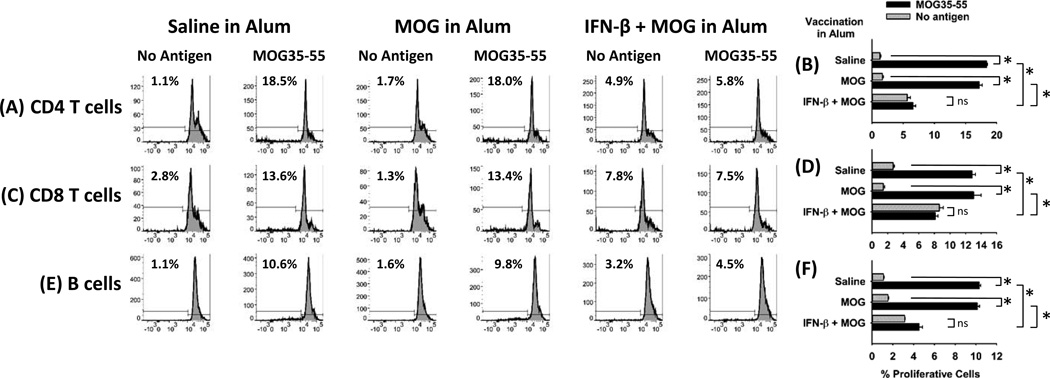
To address whether this vaccine approach modulated MOG/ CFA-induced sensitization, B6 mice were vaccinated with 5 nmoles of “IFN-β + MOG in Alum”, “MOG in Alum”, or “saline in Alum” on day −7 and were immunized with 200 µg MOG in CFA on day 0. After 13 days, draining lymph node cells were labeled with CTV and were cultured with or without 1 µM MOG35-55 for 4 days. Lymph node cells from mice vaccinated with either “MOG in Alum”, or “saline in Alum” exhibited robust, MOG-specific proliferative responses. Responsive cell types included CD4+ T cells (Figure 2A–B), CD8+ T cells (Figure 2C–D), and B cells (Figure 2E–F). In contrast, mice vaccinated with “IFN-β + MOG in Alum” did not exhibit MOG-specific responses and instead exhibited proliferative responses that were ‘autologous’ (i.e., independent of exogenous MOG35-55). Mice vaccinated with “IFN-β + MOG in Alum” lacked MOG-specific proliferative responses and instead exhibited autologous proliferative responses when assayed 10 or 18 days after MOG/ CFA sensitization (data not shown). These data indicate that the IFN-β adjuvant qualitatively alters MOG-specific sensitization in the draining lymph nodes.
Figure 2. Pretreatment with the “IFN-β + MOG in Alum” vaccine impairs MOG-specific sensitization in MOG/CFA sensitized mice.
B6 mice were vaccinated with 5 nmoles of “IFN-β + MOG in Alum”, “MOG in Alum”, or “saline in Alum” on day −7 and were immunized with 200 µg MOG in CFA on day 0. Draining lymph nodes were isolated on day 13, were labeled with CTV, and were cultured in triplicate wells with or without 1 µM MOG35-55 for 4 days. Cells were then analyzed for CD45-BV785, CD3-PE, CD4-APC, CD8-FITC, and CTV. Alternatively, T cells were analyzed for CD45-BV785, CD3-PE.594, CD19-PE, I-Ab-APC, and CTV. Proliferative CD4+ T cells (A, B), CD8+ T cells (C, D), and B cells (E, F) were measured by CTV dye dilution and were defined by the threshold denoted in the histograms. These data are representative of four experiments. (B, D, F: * p ≤ 0.001; ns, not significant)
Depletion of CD25+ Tregs impairs tolerogenic vaccination
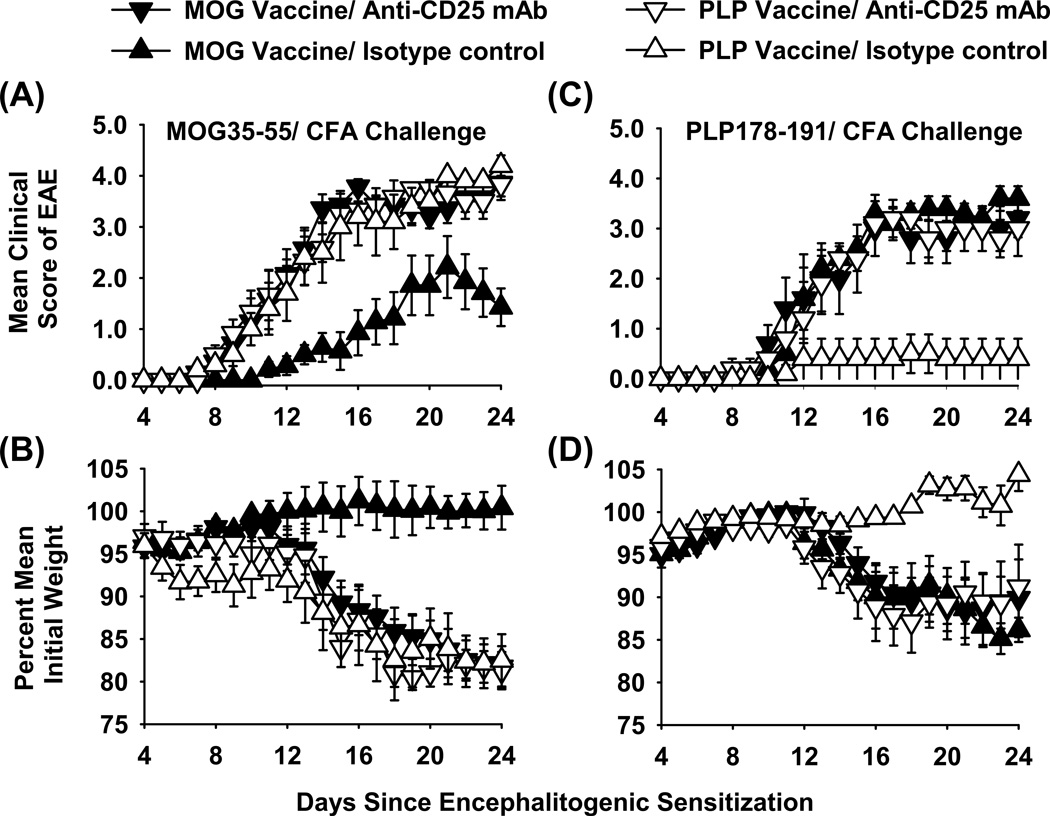
Vaccine-induced tolerogenic memory, as shown in Figure 1, suggested the potential involvement of MOG-specific CD25+ Tregs as an underlying mechanism. To test this possibility, B6 mice were vaccinated with two distinct vaccines (“IFN-β + MOG35-55 in Alum” versus “IFN-β + PLP178-191 in Alum”) on days −21, −14, and −7 (Figure 3 and Table 1). Vaccines were administered subcutaneously at a dose of 2 nmoles (A–B) or 5 nmoles (C–D). Mice were treated with the anti-CD25 PC61 mAb (rat IgG1, 250 µg i.p.) or an isotype control (Y13-259 rat IgG1) on days −4 and −2 to deplete CD25+ Tregs. These mice were then subjected to induction of EAE with MOG35-55/ CFA (A–B) or PLP178-191/ CFA (C–D) on day 0. The results showed that tolerogenic vaccination was NAg-specific. The MOG-specific vaccine inhibited MOG-induced EAE but lacked suppressive activity in PLP/ CFA-challenged mice. Vice versa, the PLP-specific vaccine inhibited PLP-induced EAE but lacked suppressive activity in MOG/ CFA-challenged mice. Pretreatment with the anti-CD25 PC61 mAb but not the isotype control antibody eliminated circulating CD25+ Tregs (data not shown). Pretreatment with the anti-CD25 PC61 mAb reversed the suppressive action of the respective tolerogenic vaccine such that the PC61-treated mice showed a chronic course of paralytic EAE (Figure 3A, C) and weight loss (Figure 3B, D) equivalent to those of the control groups. That is, PC61 pre-treatment restored full EAE susceptibility in MOG-vaccinated mice challenged with MOG/ CFA. Likewise, PC61 pre-treatment restored full EAE susceptibility in PLP-vaccinated mice challenged with PLP/ CFA. Notably, PC61-mediated depletion of Tregs had no impact on EAE in groups not subjected to NAg-specific tolerance induction. In conclusion, these data indicate that “IFN-β + NAg in Alum” tolerogenic vaccination elicited CD25+ NAg-specific Tregs that inhibited EAE via a mechanism of active NAg-specific tolerance.
Figure 3. Depletion of CD25+ Tregs reversed the suppressive action of tolerogenic vaccination.
B6 mice were vaccinated with “IFN-β + MOG35-55 in Alum” or “IFN-β + PLP178-191 in Alum” on days −21, −14, and −7 (2 nmoles in A-B & 5 nmoles in C-D). Mice were treated with the anti-CD25 PC61 mAb or the Y13 rat IgG1 isotype control (250 µg i.p.) on days −4 and −2 and were challenged with 200 µg MOG35-55 in CFA (A–B) or 200 µg PLP178-191 in CFA (C–D) on day 0 (Pertussis toxin was given on days 0 and 2). Shown are the timecourse data through day 24 for EAE (A, C) and weight loss (B, D). Tabular data and statistical analysis are shown in Table 1.
IFN-β and MOG elicited FOXP3 expression in vitro
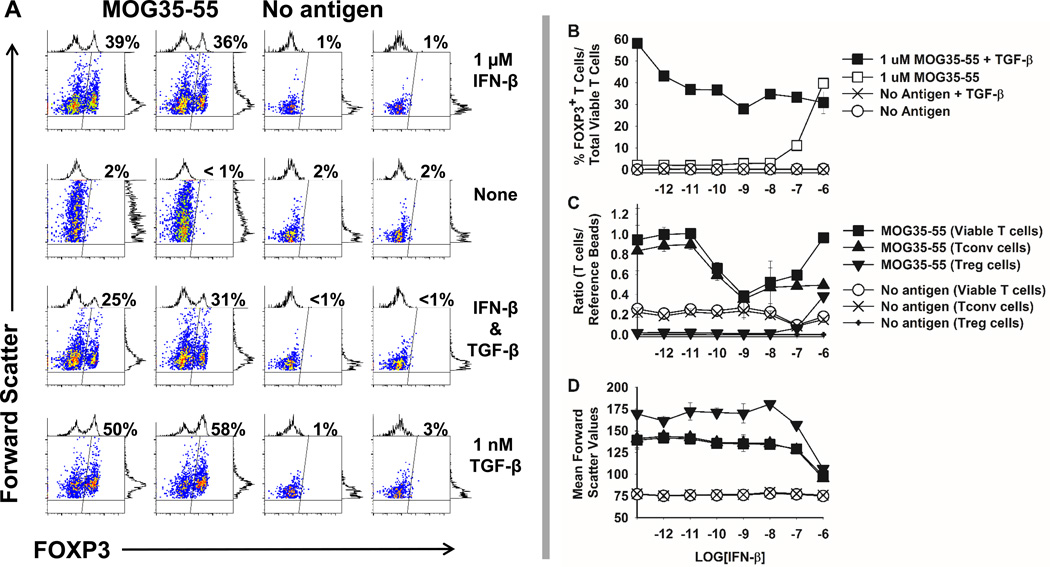
PC61-mediated reversal of tolerance suggested that IFN-β may support induction of CD25+ FOXP3+ Treg cells. Hence, “2D2-FIG” mice were used to test whether IFN-β induced FOXP3 during T cell antigen recognition of MOG35-55 in vitro (Figure 4A). 2D2-FIG mice have a transgenic T cell repertoire specific for MOG35-55 and express a GFP reporter of FOXP3 expression. Naïve 2D2-FIG splenic T cells were cultured in duplicate for 7 days with or without 1 µM MOG35-55 in the presence or absence of 1 µM IFN-β. TGF-β (1 nM) was used as a positive control for the antigen-dependent induction of FOXP3. IFN-β (1 µM) elicited FOXP3 expression in approximately 30–40% of all 2D2-FIG T cells in the presence of MOG35-55. Induction of FOXP3 was NAg-dependent because only 1–2% of T cells expressed FOXP3 in the absence of MOG35-55 despite the presence or absence of IFN-β, TGF-β, or both cytokines. These data showed that IFN-β facilitated induction of FOXP3 in MOG-stimulated naïve T cells. Given that Alum was absent from this in vitro system, one can conclude that IFN-β has Treg biasing activities that are independent from Alum.
Figure 4. IFN-β elicited MOG-dependent induction of FOXP3 in naïve 2D2-FIG T cells.
(A) Naïve 2D2-FIG splenocytes (100,000/ well) were cultured in duplicate with or without 1 µM MOG35-55 in the presence or absence of 1 µM IFN-β and/ or 1 nM TGF-β. After 7 days of culture, CD4+ T cells were assessed for expression of GFP as an indicator of FOXP3, which is indicated as a percentage in the upper right of each dotplot. Shown are duplicate samples for each condition. (B–D) 2D2-FIG splenocytes were cultured in the presence or absence of 1 µM MOG35-55, 1 nM TGF-β, or IFN-β (x-axis, 1 pM to 1 µM) for 7 days. (B) Shown are FOXP3+ T cells as a percentage of total viable cells. (C) When cultured without TGF-β, total viable T cells, FOXP3(neg) conventional T cells (Tconv), and FOXP3+ Treg cells were assessed after a 7-day culture as a ratio relative to a fixed number of fluorescent reference beads (50,000 beads / well) or (D) as a function of cell size (forward scatter). Error bars represent standard deviations. These data are representative of four independent experiments.
The ability of IFN-β to induce FOXP3 (30–40% FOXP3+ Tregs) however was less than that achieved with TGF-β (50–60% FOXP3+ Tregs) (Figure 4A–B). IFN-β and TGF-β were not synergistic or additive. Rather, the interaction was non-additive or antagonistic in that the induction of FOXP3 by TGF-β was reduced in the presence of IFN-β to that observed in cultures with IFN-β alone (Figure 4B). To standardize culture-to-culture comparisons, 50,000 fluorochrome-conjugated reference CaliBRITE™ beads (BD Biosciences) were added to each well to enable assessment of T cell numbers relative to control numbers of reference beads (Figure 4C). In the absence of IFN-β, activation of T cells with MOG35-55 increased T cell numbers by approximately 4-fold. IFN-β concentrations in the range of 10 pM to 1 nM progressively reduced T cell numbers consistent with the known pro-apoptotic action of this cytokine. However, the number of viable T cells increased in the range of 1 nM to 1 µM IFN-β to the maximal levels obtained in activation cultures without IFN-β, giving the appearance of a U-shaped concentration curve (Figure 4C, MOG35-55 Viable T cells). This paradoxical increase in T cell numbers at high IFN-β concentrations was driven by selective increases in the percentages of FOXP3+ Treg cells rather than FOXP3null conventional T cells. Indeed, frequencies of conventional T cells remained essentially equal in the high IFN-β concentration range. The FOXP3+ T cell population had higher levels of CD25 (data not shown) and size (mean forward scatter values, Figure 4D) than conventional T cells or total viable T cells. However, at high concentrations of IFN-β (100 nM to 1 µM), the size of FOXP3+ Tregs decreased and were comparable with that of conventional T cells. Overall, these data provided evidence that high IFN-β concentrations (100 nM to 1 µM) selectively favored the emergence of a FOXP3+ Treg subset.
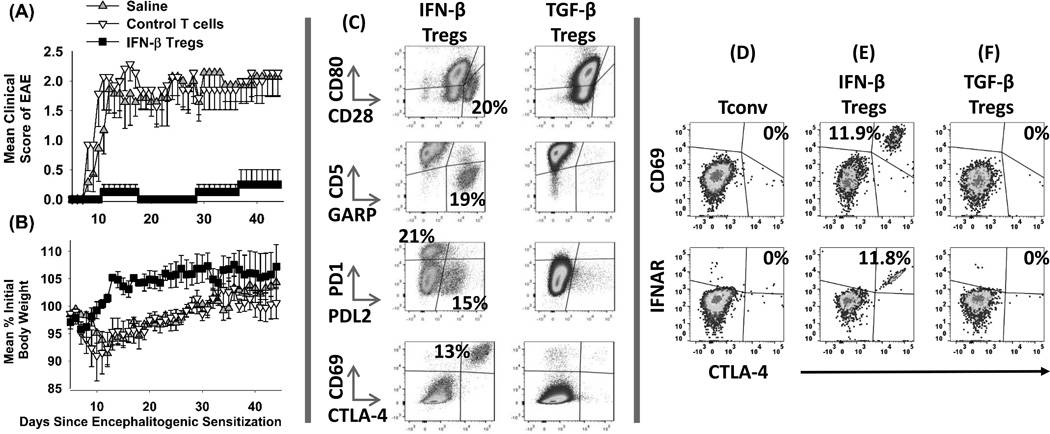
Function and phenotype of IFN-β induced Tregs
A central question was whether IFN-β-dependent expression of FOXP3 in MOG-specific T cells concurred with the induction of Treg-mediated suppressive activity. To address this question, splenic 2D2-FIG IFN-β-induced Tregs were assessed for suppressive activity in adoptive transfer experiments (Figure 5A–B, Table 1). Donor IFNβ-Tregs were compared to control FOXP3null 2D2 conventional T cells that were cultured without IFN-β. T cells were extensively washed and injected 4 days after encephalitogenic challenge. Recipients of IFN-β Tregs were protected from EAE as measured by clinical grade or weight loss (Figures 5A–B) compared to recipients of control T cells or to saline treated mice. These data indicated that IFN-β supported the MOG-dependent expression of FOXP3 (Figure 4) and elicited the acquisition of suppressive activity in adoptive transfer models (Figures 5A–B). The implication was that IFN-β represented a gateway for the differentiation of suppressive MOG-specific FOXP3+ Tregs. IFN-β-induced Tregs were also more heterogeneous than TGF-β-induced Tregs (Figure 5C). For example, IFN-β-Tregs had a CD28high population, a CD5low, GARP+ population, a PD-1high population, a PD-1(neg), PDL2+ population, and a CD69+, CTLA-4+ population that were largely absent in the TGF-β-Treg population. The CD69+, CTLA-4+ IFN-β Treg population also had high levels of the Type I Interferon Receptor (IFNAR-1) (Figure 5E). The IFN-β-mediated induction of CTLA-4 and IFNAR was enriched in the FOXP3+ subset but was not exclusive to FOXP3+ Tregs because IFN-β also induced a CTLA-4+ IFNAR+ Tconv subset (data not shown). Thus, IFN-β induced the expression of these markers on both IFN-β cultivated Tconv and Treg subsets. In the absence of IFN-β, T cells cultured with MOG alone or with “MOG and TGF-β” did not exhibit CTLA-4, CD69, or IFNAR (Figure 5D, F). These data revealed a potential IFN-β regulatory loop in which culture with IFN-β elicited expression of the Type I Interferon receptor IFNAR on a subset of IFN-β-induced T cells.
Figure 5. Function and phenotype of IFN-β-induced Tregs.
2D2-FIG splenocytes were cultured with 1 µM MOG35-55 and IL-2 in the presence (IFNβ-Tregs) or absence (Control T cells) of 1 µM IFN-β for 7 days. Donor IFNβ-Tregs and control T cells were extensively washed after the 7-day culture and injected at a dose of 107 total T cells on day 4 into recipients that were previously challenged with MOG35-55/ CFA (day 0) and Pertussis toxin (days 0, 2). Error bars represent standard error of the mean. Shown are 1 of 2 experiments that were compiled in Table 1. (C–F) 2D2-FIG T cells were activated with 1 µM IFN-β + MOG35-55 (C & E, FOXP3+ Treg gate), 1 nM TGF-β + 1 µM MOG35-55 (C & F, FOXP3+ Treg gate), or 1 µM MOG35-55 alone (D, Tconv cell gate) for 7 days. T cells were analyzed for the designated surface markers. These data are representative of three independent experiments. Cells were stained with CD45-BV785 and CD3-PE.CF594 within different panels that included; [CD86-BV421, CD80-PE, and CD28-APC], [CD69-BV421, CD5-PE, GARP-AF647, and PDL1-PE.Cy7], [PD1-BV421, PDL2-PE, and PDL1-APC], and [CD69-BV421, CTLA4-PE, and IFNAR1-APC].
IFN-β and MOG elicited FOXP3 expression in vivo
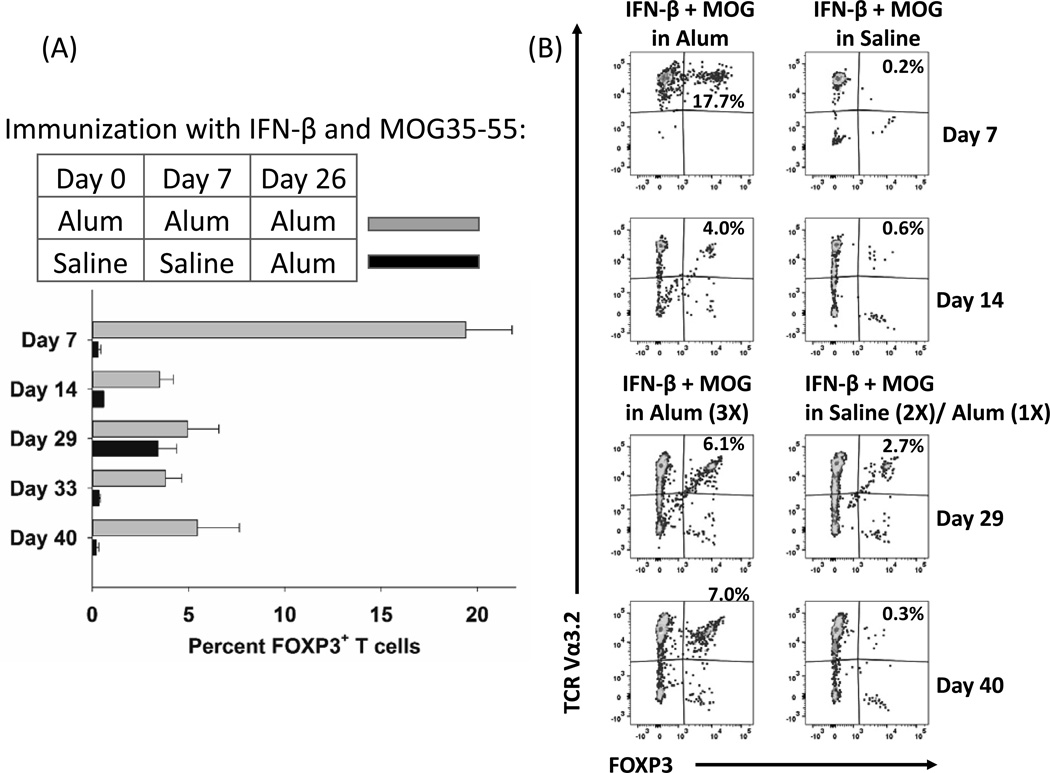
Just as the combination of IFN-β and MOG elicited differentiation of Tregs in vitro (Figures 4–5), immunization of 2D2-FIG mice with “IFN-β + MOG in Alum” also elicited FOXP3+ Tregs in 2D2-FIG mice in vivo (Figure 6). On days 0 and 7, 2D2-FIG mice were injected subcutaneously (5 nmole dose) with “IFN-β + MOG in Alum” or “IFN-β + MOG in saline” (shown) or “IFN-β in saline”, “MOG35-55 in saline”, saline, or were untreated (not shown). On day 26, booster injections were repeated for all groups, but Alum rather than saline was used in all 5 groups. On days 7, 14, 29, 33, and 40, mice were bled via the submandibular vein, and circulating CD45+ CD3+ T cells were assessed for FOXP3+ expression. In mice vaccinated with “IFN-β + MOG in Alum”, over 17% of all circulating 2D2-FIG T cells on day 7 expressed FOXP3 (Figure 6A–B). In mice vaccinated with “IFN-β + MOG in saline”, only 0.2–0.4% of T cells expressed FOXP3. Mice that were vaccinated with “IFN-β + MOG in Alum” 3 times (days 0, 7, 26) maintained a steady population of circulating FOXP3+ T cells through the last day of analysis on day 40 (Fig 6A–B). In contrast, mice that received “IFN-β + MOG in Saline” on days 0 and 7 plus a boost of “IFN-β + MOG in Alum” on day 26 had a temporary presence of FOXP3+ T cells on day 29 but not thereafter. Like mice that were untreated, mice that received “IFN-β in saline”, “MOG35-55 in saline”, or saline along with a booster of the same in Alum on day 26 did not express FOXP3 beyond background levels (< 1%) at any point during the experiment. These data indicate that IFN-β, when combined with NAg and the Alum adjuvant, constitutes a vehicle for tolerogenic vaccination via induction of NAg-specific CD25+ FOXP3+ T cells.
Figure 6. The “IFN-β + MOG in Alum” vaccine elicited FOXP3+ Tregs in vivo.
On days 0, 7, and 26, 2D2-FIG mice were injected subcutaneously (5 nmole dose) with “IFN-β + MOG in Alum”. Another group was injected with 5 nmoles of “IFN-β + MOG in saline” on days 0 and 7 followed by one injection of “IFN-β + MOG in Alum” on day 26 (n = 2). On days 7, 14, 29, 33, and 40, mice were bled via the submandibular vein, and circulating CD45+ CD3+ T cells were assessed for expression of the 2D2 TCR transgenic Vα3.2 receptor and GFP (FOXP3+) expression. (B) The total combined percentages of transgenic Vα3.2+ and nontransgenic Vα3.2 FOXP3+ Tregs is given in the upper right of each dotplot. Analysis panels included CD25-BV421, TCR-Vα3.2-PE, CD3-PE.CF594, TCR-Vβ11-AF647, and CD45-BV785.
The “IFN-β + NAg in Alum” vaccine elicited tolerance in 2D2-FIG mice
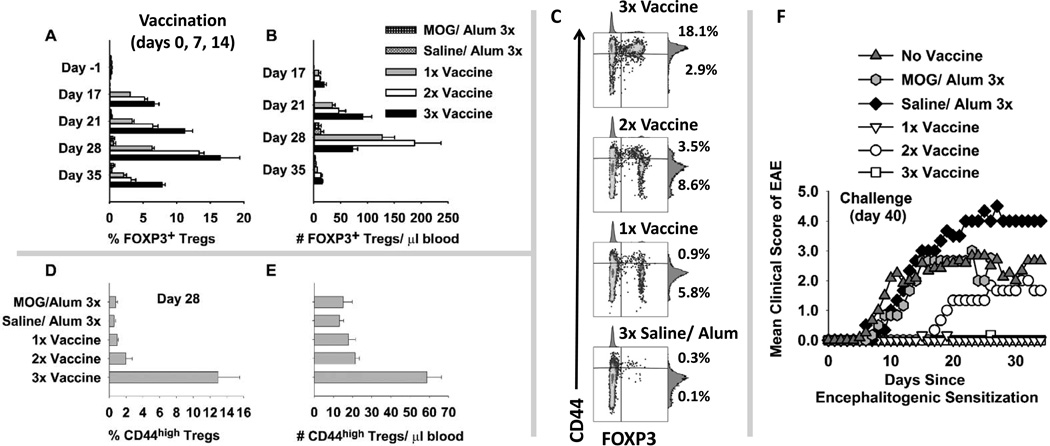
Given that one can use an IFN-β-based vaccine to elicit Treg responses (Figures 6), an important question was whether one could use repeated ‘booster’ vaccinations to amplify effector/ memory Treg responses. 2D2-FIG mice were given three (3x) injections (days 0, 7, 14), two (2x) injections (days 7 and 14), or one (1x) injection (day 14) of 5 nmoles of the “IFN-β + MOG in Alum” vaccine (1x, 2x, 3x vaccine, Figure 7). Control groups were given three (3x) injections (days 0, 7, 14) of 5 nmoles of “MOG in Alum” or “Saline in Alum”. Tolerogenic vaccination with “IFN-β + MOG in Alum” (3x, 2x, and 1x) elicited FOXP3+ Tregs in PBMC when assayed on days 17, 21, 28, and 35 (Figure 7A–B). Repeated boosters generally resulted in higher FOXP3+ Treg percentages (A) and numbers (B). By day 28, mice that received 3 vaccinations also had circulating Tregs that expressed high levels of CD44 whereas Tregs from the 2x, 1x, or control groups expressed intermediate levels of CD44 (Figure 7C–E). These data indicated that multiple tolerogenic boosters increased the abundance and memory phenotype of circulating FOXP3+ Tregs.
Figure 7. The “IFN-β + MOG in Alum” vaccine elicited FOXP3+ Tregs and tolerance in 2D2 TCR transgenic mice.
2D2-FIG mice were given three (3x) injections (days 0, 7, 14), two (2x) injections (days 7 and 14), or one (1x) injection (day 14) of 5 nmoles of the “IFN-β + MOG in Alum” vaccine (n = 3). When not receiving tolerogenic vaccination, mice received a control injection of Saline in Alum. Control mice were given three (3x) injections (days 0, 7, 14) of “Saline in Alum” or 5 nmoles of “MOG in Alum” (n = 3). Mice were assessed for percentages (A) (relative to 2D2 T cell population) and absolute numbers (B) (cells/ µl blood) of circulating FOXP3+ Tregs on days 17, 21, 28, and 35. (A) 3x or 2x versus MOG or Saline, p < 0.010 for days 17–35; 1x versus MOG or Saline, p < 0.05 for days 17–28. (B) 3x versus MOG or Saline, p < 0.006 for days 17–35; 2x versus MOG or Saline, p < 0.006 for days 17–28. (C) On day 28, CD3+ T cells were analyzed for expression of CD44 (y-axis) and FOXP3+ (x-axis). Percentages of CD44high Tregs and CD44low Tregs (upper and lower right quadrants) are given for each representative dotplot. Mean percentages (D) and numbers (E) of CD44high FOXP3+ Tregs are shown (3x versus the other 4 groups, p ≤ 0.001). (A-B, D-E) Shown are means and standard error of the mean. Analysis panels included CD25-BV421, TCR-Vα3.2-PE, CD3-PE.CF594, CD44-APC, and CD45-BV785. (F) Mice were challenged with 100 µg MOG35-55 in CFA on day 40, were given Pertussis toxin on days 40 and 42, and were weighed/ scored for EAE daily for the next 34 days. The compiled clinical data and statistical analysis of EAE are provided in Table 2.
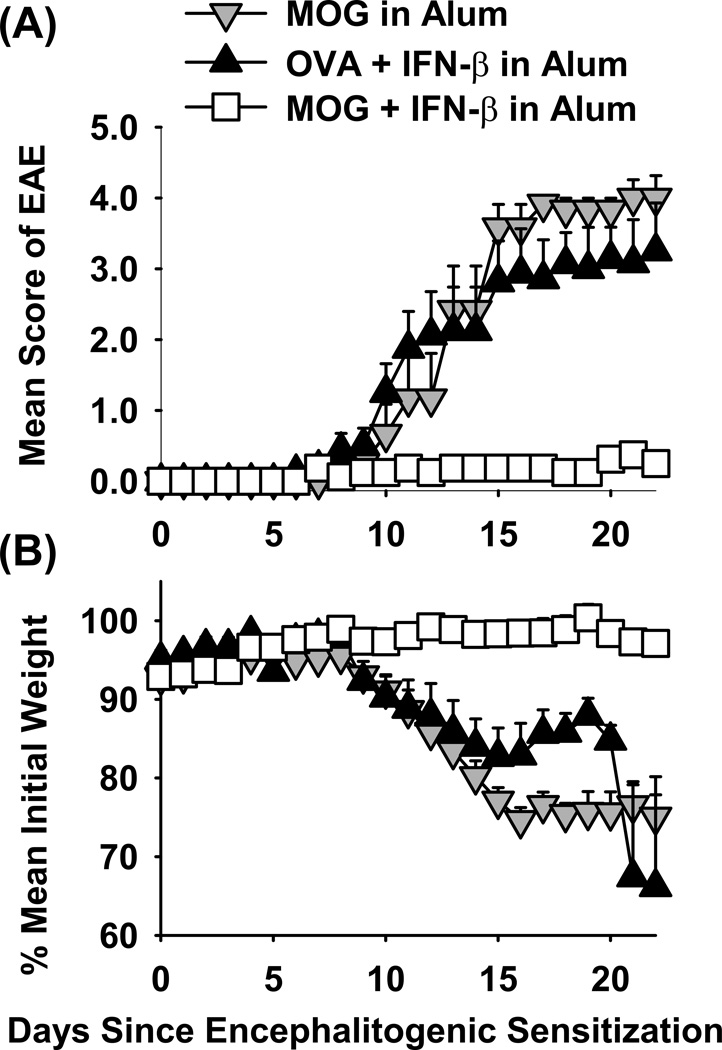
By day 35, percentages of Tregs waned in that only low levels of FOXP3+ Tregs remained in circulation. An important question was whether waning Treg levels in the blood correlated with waning resistance to EAE, but this was not the case (Figure 7F and Table 2). When challenged to induce EAE on day 40, 2D2-FIG mice previously vaccinated with “IFN-β + MOG in Alum” once (1x) or three (3x) times exhibited profound resistance to EAE compared to controls. Two mice in the ‘2x vaccine’ group exhibited ‘late-breaking’ paralytic EAE whereas one mouse remained disease-free throughout the observation period. EAE ‘break-through’ in the 2x group most likely represented stochastic events. We therefore refrain from any conclusions regarding comparison of the 2x group to the 1x or 3x groups of mice. When the 1x, 2x, and 3x groups were analyzed in aggregate, mice that received tolerogenic vaccination (Table 2a–c) were significantly more resistant to EAE than mice in the three pooled control groups (Table 2d–f). These data indicate that tolerogenic vaccination with “IFN-β + MOG in Alum” elicits an enduring tolerance in 2D2 TCR transgenic mice. Induction of tolerance in TCR transgenic mice is a stringent test of tolerogenic vaccine efficacy because the vast majority of T cells bear the transgenic MOG-specific TCR, and the vaccine must control this expanded MOG-specific T cell population. The persistence of tolerance despite the gradual disappearance of FOXP3+ Tregs from the blood is consistent with the possibility that circulating Tregs may emigrate from the blood into the peripheral tissues to maintain tolerance. To confirm induction of tolerance in the 2D2-FIG model, vaccines comprised of “IFN-β + MOG35-55 in Alum”, “IFN-β + OVA323-339 in Alum”, or “MOG35-55 in Alum” (5 nmoles) were given on days −21, −14, and −7 followed by active challenge with MOG35-55/ CFA on day 0. As shown in Figure 8 and Table 2, the “IFN-β + MOG in Alum” prevented the subsequent induction of EAE and EAE-associated weight loss whereas the control vaccines had no effect on induction of severe paralytic EAE.
Table 2.
Tolerogenic vaccination with “IFN-β + MOG in Alum” elicits tolerance in 2D2 TCR transgenic mice.
| Figure | Groupa | Incidence | Attrition | Cumulative EAE |
P value | P value | Maximal EAE |
P value | P value | Maximum Weight Loss |
P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7F | (a) IFN-β + MOG in Alum (3x) | 1/3 | 0/3 | 0.2 ± 0.2 | 0.017 | 0.000 | 0.2 ± 0.2 | 0.020 | 0.000 | 1.2 ± 1.3% | 0.019 |
| 7F | (b) IFN-β + MOG in Alum (2x) | 2/3 | 0/3 | 25.8 ± 13.1 | ns | 2.2 ± 1.1 | ns | 13.3 ± 4.5% | |||
| 7F | (c) IFN-β + MOG in Alum (1x) | 1/3 | 0/3 | 0.3 ± 0.3 | 0.022 | 0.2 ± 0.2 | 0.020 | 2.6 ± 1.1% | |||
| 7F | (d) Saline in Alum (3x) | 3/3 | 2/3 | 64.5 ± 11.9 | ns | * | 4.7 ± 0.3 | * | * | 25.6 ± 1.6% | * |
| 7F | (e) MOG in Alum (3x) | 3/3 | 2/3 | 41.2 ± 17.4 | ns | 3.7 ± 1.3 | ns | 20.4 ± 6.7% | |||
| 7F | (f) Naive | 6/6 | 3/6 | 56.3 ± 11.9 | * | 3.5 ± 0.6 | ns | 10.0 ± 5.0% | |||
| 8 | (g) IFN-β + MOG in Alum (3x) | 8/8 | 0/8 | 2.9 ± 0.9 | 0.002 | 0.005 | 0.6 ± 0.1 | 0.003 | 0.003 | 4.5 ± 1.2% | 0.000 |
| 8 | (h) IFN-β + OVA in Alum (3x) | 8/8 | 2/8 | 31.8 ± 5.4 | * | 3.9 ± 0.3 | * | 24.7 ± 3.5% | * | ||
| 8 | (i) MOG in Alum (3x) | 6/6 | 1/6 | 38.2 ± 4.3 | * | 4.3 ± 0.3 | * | 29.9 ± 2.7% | * |
These data are portrayed graphically in Figures 7F and 8A–B. The experimental approach is described in the figure legends. Nonparametric ANOVA based on ranked scores was used to assess group differences in mean cumulative scores and mean maximal scores relative to the comparator group
whereas parametric ANOVA was used to assess weight loss (Bonferroni Post-Hoc test; a–e versus f; g versus h and i). An Independent Samples T-test was used to analyze differences in data compiled for tolerogenic vaccination (a–c) versus control groups (d–f).
Figure 8. Pretreatment with the “IFN-β + MOG in Alum” vaccine inhibited the subsequent induction of EAE in MOG35-55 TCR transgenic mice.
Vaccines comprised of “MOG35-55 in Alum”, “IFN-β + OVA323-339 in Alum”, or “IFN-β + MOG35-55 in Alum” were given on days −21, −14, and −7 to 2D2-FIG mice at dosages of 5 nmoles for both IFN-β and antigen. Mice were challenged with 100 µg MOG35-55 in CFA on day 0, were given Pertussis toxin on days 0 and 2. The timecourses for EAE (A) and body weight (B) are shown through day 22. Error bars represent standard error of the mean. The statistical analysis is given in Table 2.
Does IFN-β adjuvant promote infectious tolerance
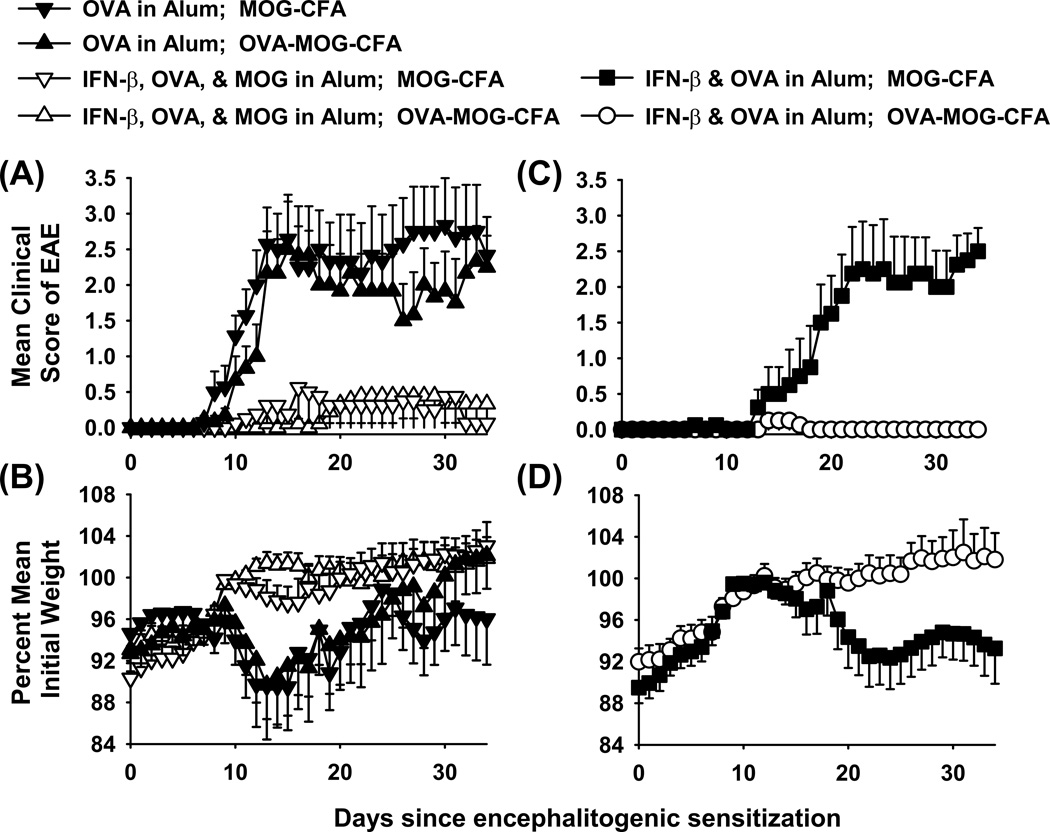
An important question is whether the “IFN-β in Alum” adjuvant fosters infectious tolerance via the spread to antigens beyond those epitopes directly included in the vaccine formulation. To address this question (Figure 9, Table 3), B6 mice were vaccinated with “OVA in Alum”, “IFN-β + OVA + MOG in Alum”, or “IFN-β + OVA in Alum” on day −8 and then were challenged with either “OVA + MOG in CFA” or “MOG in CFA” on day 0. The results showed that the “OVA in Alum” vaccine had no activity whereas the “IFN-β + OVA + MOG in Alum” inhibited the subsequent induction of EAE (Figure 9A–B). The key observation was that the “IFN-β + OVA in Alum” vaccine was or was not effective depending on whether OVA was or was not included in the MOG/ CFA emulsion, respectively (Figure 9C–D). That is, the “IFN-β + OVA in Alum” vaccine inhibited EAE upon immunization with “OVA+MOG”/ CFA but did not impact EAE upon immunization with MOG/ CFA. These data were consistent with the possibility that vaccine-induced, OVA-specific Tregs modulated EAE only when OVA was included in the encephalitogenic emulsion. These data revealed the potential cross-regulation of encephalitogenic T cell precursors by OVA-specific Tregs when the respective epitopes were presented in the same localized environment or on the same APC.
Figure 9. Does IFN-β adjuvant promote infectious tolerance?
Mice were vaccinated once with three different vaccines including “IFN-β + OVA + MOG in Alum”, “IFN-β + OVA in Alum”, and “OVA in Alum” (5 nmole dose for all reagents) on day −8. Mice were challenged with two different emulsions to induce EAE, including “OVA + MOG in CFA” or “MOG in CFA” (100 µg dose for each peptide) on day 0. Pertussis toxin was given on days 0 and 2. Shown are the timecourse data through day 34 for EAE (A, C) and weight loss (B, D). Statistical analyses are given in Table 3.
Table 3.
IFN-β is an adjuvant that fosters ‘infectious tolerance’.
| Vaccine in Alum | Challenge in CFA | Incidence | Mean (± SE) cumulative scores |
P value | Mean (± SE) maximal scores |
P value | % maximal weight loss |
P value |
|---|---|---|---|---|---|---|---|---|
| IFN-β + OVA + MOG | OVA + MOG | 4 of 9 | 6.5 ±6.2 | ns | 0.6 ±0.4 | ns | 2.7 ±1.5 | ns |
| IFN-β + OVA + MOG | MOG | 3 of 8 | 7.2 ±5.2 | ns | 0.8 ±0.5 | ns | 5.6 ±1.9 | ns |
| IFN-β + OVA | OVA + MOG | 2 of 8 | 0.4 ±0.4 | * | 0.2 ±0.1 | * | 2.9 ±1.1 | * |
| IFN-β + OVA | MOG | 8 of 8 | 37.3 ±9.9 | p < 0.001 | 3.0 ±0.4 | p ≤ 0.001 | 11.7 ±2.9 | ns |
| OVA | OVA + MOG | 6 of 6 | 47.6 ±11.2 | p < 0.001 | 3.0 ±0.5 | p ≤ 0.001 | 13.1 ±4.8 | ns |
| OVA | MOG | 7 of 7 | 54.5 ±13.2 | p < 0.001 | 3.4 ±0.5 | p ≤ 0.001 | 15.7 ±3.7 | p = 0.027 |
These data are portrayed graphically in Figure 9. Mice were vaccinated once with three different vaccines including “IFN-β + OVA + MOG in Alum”, “IFN-β + OVA in Alum”, and “OVA in Alum” (5 nmole dose for all reagents) on day −8. Mice were then challenged with two different emulsions to induce EAE, including “OVA + MOG in CFA” and “MOG in CFA” (100 µg dose for each peptide) on day 0. Pertussis toxin was given on days 0 and 2. Nonparametric ANOVA based on ranked scores was used to assess group differences in mean cumulative scores and mean maximal scores, and parametric ANOVA was used to assess group differences in percent maximal weight loss relative to the comparator group
Bonferroni Post-Hoc test).
Interplay of TGF-β and IFN-β in mechanisms of Treg induction
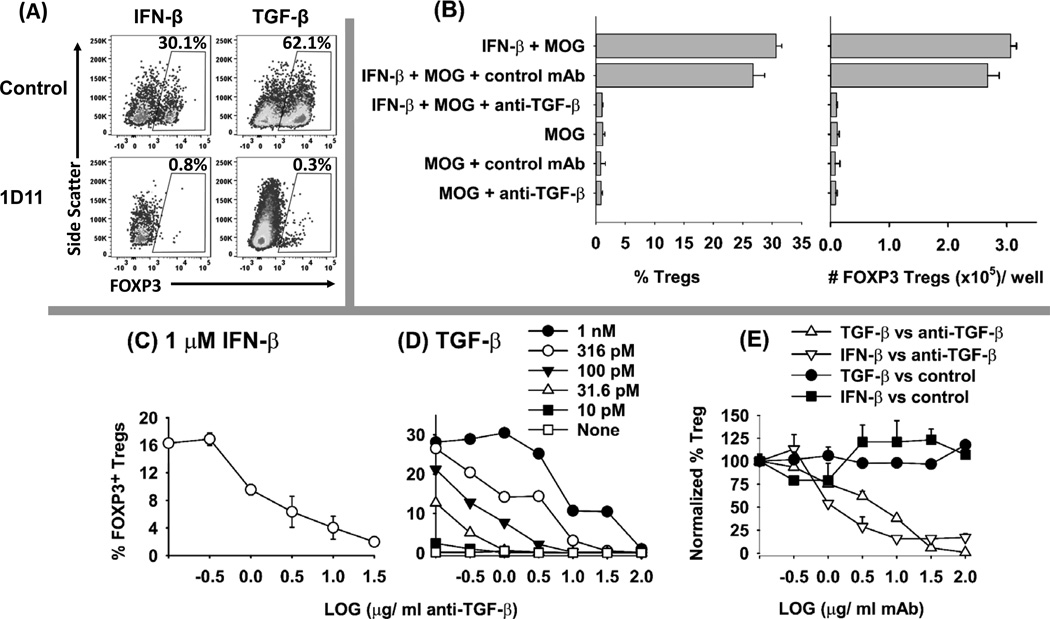
Both IFN-β and TGF-β shared overlapping functionality for antigen-dependent induction of CD25+ FOXP3+ Tregs. However, IFN-β was less efficient and required substantially higher concentrations than TGF-β (e.g., 1 µM IFN-β versus 1 nM TGF-β, Figure 4). This observation suggested the possibility that IFN-β-mediated Treg induction may be TGF-β-dependent. To assess this issue, naïve 2D2-FIG splenocytes were cultured in the presence or absence of 1 µM IFN-β, 100 pM TGF-β, 31.6 µg/ ml anti-mouse-TGF-β (1D11.16.8, mouse IgG1), or an isotype control anti-rat-LFA-1 (LRTC 1, mouse IgG1) with 1 µM MOG35-55 (Figure 10) or without antigen (not shown). The results showed that induction of IFN-β-Tregs was fully blocked by the anti-TGF-β mAb (Figure 10A, B, C, E). Control cultures showed that TGF-β activity was also blocked by anti-TGF-β (Figure 10 A, D, E). IFN-β-mediated induction of Tregs as measured by T cell percentage or absolute numbers was inhibited by anti-TGF-β but not by the isotype control mAb (Figure 10B, E). The inhibitory activity of the 1D11 anti-TGF-β mAb reflected a competitive interaction (Figure 10D). In cultures induced with 1 µM IFN-β or 100–316 pM TGF-β, 50% inhibition was evident at 1D11 concentrations of approximately 1 µg/ ml (Figure 10C–D). Overall, these data indicate the IFN-β elicits FOXP3+ Treg differentiation in vitro, at least in part, through the action of TGF-β. Although IFN-β elicited FOXP3+ Tregs via a TGF-β dependent mechanism, these data did not preclude the possibility that unique IFN-β activities apart from TGF-β shaped Treg differentiation and function.
Figure 10. The anti-TGF-β mAb 1D11 inhibits IFN-β dependent induction of FOXP3+ Tregs in vitro.
(A–E) Naïve 2D2-FIG splenocytes (200,000/well) were cultured in triplicate with 1 µM MOG35-55 and either 1 µM IFN-β or 100 pM TGF-β (or as designated in D). Cultures also included either anti-mouse-TGF-β (1D11) or the isotype control mAb (LRTC 1) (31.6 µg/ ml) (A–B) or as designated on the x-axis (C–E). After 7 days of culture, CD4+ T cells were assessed for GFP expression. (A) Shown are representative dot plots of side scatter (y-axis) and FOXP3 expression (x-axis) together with (B) percentages and absolute numbers of FOXP3+ Tregs after culture with MOG35-55 in the presence or absence of IFN-β and mAb. The quantitative neutralization profiles are shown for the anti-TGF-β 1D11 mAb in cultures of IFN-β-induced Tregs (C, E) and TGF-β-induced Tregs (D, E). Error bars represent standard error of the mean. These data are representative of three independent experiments.
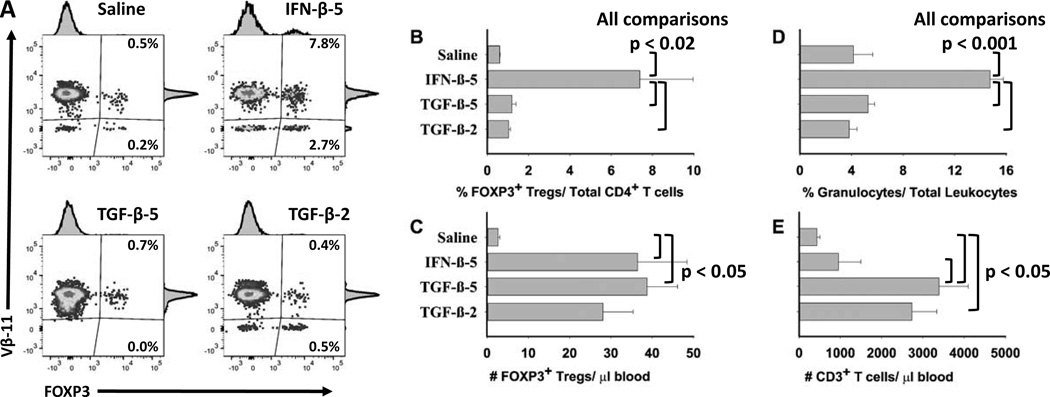
Given the possibility that IFN-β may act indirectly via the induction of TGF-β, a relevant question was whether TGF-β, like IFN-β, may exhibit activity as a tolerogenic adjuvant in Alum. Thus, we assessed the relative activity of IFN-β versus TGF-β for induction of FOXP3+ T cells upon tolerogenic vaccination of 2D2-FIG mice (Figure 11). The full biological potency of both IFN-β and TGF-β preparations were confirmed by in vitro bioassay. Administration of “IFN-β + MOG35-55 in Alum” elicited increased percentages (Figure 11A–B) and numbers (Figure 11C) of Tregs in PBMC compared to vaccination with “Saline in Alum”. The “IFN-β + MOG35-55 in Alum” vaccine also increased percentages (Figure 11D) and numbers (not shown) of circulating granulocytes. The latter observation may reflect IFN-β-mediated activation of an innate immune response. Administration of TGF-β at a dose of either 5 nmoles or 2 nmoles with MOG35-55 (5 nmoles) in Alum also increased the total number of FOXP3+ Tregs per µl of blood (Figure 11C), but this increase reflected a proportional increase in the number of CD3+ T cells (Figure 11E) and total leukocytes (not shown). Overall, TGF-β-based vaccination did not elicit increased FOXP3+ Tregs as a percentage of the CD4+ T cell pool (Figure 11A–B) or the total leukocyte pool. Cytokine adsorption to Alum was evident for both IFN-β and TGF-β because both were completely bound during incubation with Alum (data not shown). These data revealed differential activities of IFN-β and TGF-β for Treg induction in vivo, with the implication that IFN-β may have a more dedicated alignment with the FOXP3 T cell lineage compared to TGF-β.
Figure 11. When combined with Alum and MOG35-55, IFN-β uniquely increased the percentages of FOXP3+ Tregs relative to the total pool of CD4+ T cells.
On day 0, 2D2-FIG mice (n = 5) were injected with “Saline in Alum”, “5 nmoles IFN-β + 5 nmoles MOG35-55 in Alum” (IFN-β-5), “5 nmoles TGF-β + 5 nmoles MOG35-55 in Alum” (TGF-β-5), or “2 nmoles TGF-β + 5 nmoles MOG35-55 in Alum” (TGF-β-2). On day 13, PBMC were assessed for FOXP3+ T cells as a percentage of the CD45+, CD3+ CD4+ T cell population (A–B), the total numbers of FOXP3+ Tregs per µl of blood (C), the percentages of granulocytes as a percentage of total leukocytes (D), and the total numbers of CD3+ T cells per µl of blood (E). Analysis panels included CD3-BV421, CD4-PE, Vβ11-AF647, and CD45-BV785.
Discussion
IFN-β is a gateway cytokine for the immunosuppressive FOXP3+ Treg lineage
IFN-β has been a front-line therapeutic for MS for nearly two decades and is uniformly inhibitory in wildtype models of EAE (11, 53–72) but underlying suppressive mechanisms of IFN-β remain unresolved. This study provides evidence that IFN-β is a gateway cytokine that polarizes T cell differentiation toward the immunosuppressive FOXP3+ Treg lineage. Furthermore, this study reveals that the regulatory activities of IFN-β can be exploited to impose tolerogenic memory for specific myelin NAg.
Several lines of evidence support a role of Type I IFNs in Treg development and homeostasis. In MS therapy, IFN-β treatment has been associated with the restoration of Treg frequency and suppressive function (73–79). Indeed, 3–12 months of IFN-β therapy increased the frequencies and suppressive activity of peripheral CD4+ CD25+ FOXP3+ T cells ex vivo. Collectively, these studies support the idea that IFN-β alleviates MS in part via induction of Treg numbers and function. A similar relationship between IFN-β treatment and Treg induction has been noted in EAE. That is, IFN-β administration induced the expression of various costimulatory ligands on dendritic cells that in turn enhanced Treg expansion and function (78). Type I IFN administration was also required for maintenance of FOXP3 expression and suppressive Treg activity in a mouse model of colitis by a mechanism dependent upon the expression of IFN-α/β receptors (80). In competitive environments, IFNAR signaling in T cells, and particularly in FOXP3+ Tregs, was needed for homeostasis and functional differentiation (81). Type I IFNs also facilitated alternative Treg lineages. Type I IFNs were implicated as pivotal cytokines for the differentiation of the IL-10-producing, Tr1 regulatory T cell subset (82–85) and the recently discovered FOXA1+ FOXP3(neg) regulatory T cell subset (86). FOXA1 drives the Pdl1 promoter in this Treg subset to confer expression of programmed cell death ligand-1 (PDL1) to enable Treg killing of activated conventional effector T cells. Adoptive transfer experiments showed that FOXA1+ Tregs suppressed EAE by a mechanism that required T cell–intrinsic IFN-α/β receptor signaling. In relapsing-remitting MS, IFN-β treatment increased the frequencies of suppressive FOXA1+ Treg cells in blood. In summary, Type I IFNs are implicated as potential lineage specification/ polarization factors for three unique Treg subsets including FOXP3+, Tr1, and FOXA1+ T cells.
The requirement for linked recognition of IFN-β and NAg
Previous studies of cytokine-NAg fusion proteins established the general concept that cytokine action and T cell antigen recognition must be tightly linked to achieve tolerance induction. In this regard, the covalent linkage of cytokine and NAg imposed tight linkage of cytokine and NAg recognition, and this linkage was required for tolerance induction. The only exception was noted in studies of the rat IFNβ-NAg tolerogenic vaccine which was comprised of the rat IFN-β fused to the dominant 73–87 encephalitogenic epitope of myelin basic protein (MBP) (41). Unlike all other fusion proteins tested to date (37–40, 42–44, 87), the covalent attachment of IFN-β and NAg was not necessary, because separate injections of IFN-β and NAg at adjacent sites were as effective as injection of the IFNβ-NAg fusion protein for prevention of disease. When treatment was initiated after disease onset, the rat IFNβ-NAg fusion protein blunted disease progression and elicited an accelerated recovery. Although not as effective as the fusion protein, treatment with the mixture of IFN-β plus NAg had suppressive efficacy that was superior to either IFN-β or NAg alone. These findings presented the unexpected prospect that IFN-β and NAg could induce tolerance without the constraints of engineering single chain fusion proteins.
Murine models of EAE, including the B6 chronic model and the SJL relapsing-remitting model, were used to confirm that IFNβ-NAg fusion proteins had tolerogenic activity and to test the requirement for covalent cytokine-NAg linkage (38). Unlike the rat EAE system however, covalent linkage of the murine IFN-β domain with PLP139-151 was necessary for tolerance induction because equimolar doses of IFN-β and PLP139-151 as a mix of separate molecules had limited tolerogenic activity (38). Likewise, the IFNβ-MOG fusion protein facilitated clinical recovery in the chronic B6 model of EAE whereas the combination of IFN-β and MOG had limited beneficial action (Figure 1A–B). Thus, in two murine EAE systems, attachment of IFN-β to the NAg was required for long-lasting tolerance induction.
Why the rat and murine IFNβ-NAg had differential requirements for cytokine-NAg linkage is not known. One possibility is that the rat and mouse EAE systems had intrinsic differences in regulatory mechanisms, given that Lewis rats exhibited a brief monophasic disease whereas both mouse models exhibit chronic EAE. Perhaps mixtures of IFN-β + NAg may exhibit a temporary inhibitory action that might be sufficient to suppress EAE in Lewis rats but might not be sufficient to control the sustained inflammatory insult in chronic murine EAE. For example, IFN-β + MOG exhibited a temporary suppression of EAE in B6 mice from approximately day 20–25 (Figure 1A), but this inhibitory action was not sustained beyond day 30. Perhaps such a temporary suppressive activity would be sufficient to control a monophasic course of disease, because the rodents would exhibit spontaneous recovery before the erosion of vaccine-mediated suppression. Thus, the short-term inhibitory action of IFN-β + NAg and the long-term tolerogenic activity of IFNβ-NAg simply may not be distinguished in monophasic rat EAE. As another possibility to explain the lack of requirement for covalent linkage in the rat model, rat IFN-β and the MBP peptide may exhibit a direct or indirect binding interaction (perhaps via a third party molecule) by which the IFN-β became non-covalently associated with NAg peptide. If so, then the requirement for physical linkage of IFN-β and NAg may be due to APC targeting. That is, the IFN-β domain of IFNβ-NAg complex may interact with Type I Interferon Receptors (IFNAR) on dendritic cells (DC) to evoke DC-mediated tolerogenic activity while simultaneously targeting the physically-associated NAg into the MHCII antigen processing pathway. The net effect would be the concentrated presentation of NAg on tolerogenic IFN-β-conditioned DC and the consequent induction of NAg-specific Tregs.
The prospect of an effective tolerogenic vaccine containing non-linked IFN-β and NAg moieties would entail many advantages. Multivalent tolerogenic vaccines could be formulated by including selected NAg peptides in the IFN-β tolerogenic vaccine. This advantage would accommodate the prospect that MS may be mediated by a polyclonal repertoire of myelin-reactive T cells. Such vaccines could be adapted to include NAg epitopes to match myelin-specificities on a per patient basis and could be adjusted based on emerging pathogenic epitopes due to epitope spread and disease evolution. A major advantage would also be realized in manufacturing and quality control. Such vaccines would obviate the potential difficulty that attachment of some NAg peptides to the IFN-β C-terminus may impair the biological activity of IFN-β or create immunogenic neoantigens. Given the development and use of IFN-β over the past 2 decades as a front-line MS therapeutic, the main manufacturing challenge would encompass synthetic NAg peptides rather than recombinant fusion proteins.
Although physical linkage is needed for optimal tolerogenic activity in both SJL and B6 models of EAE, one would predict that such linkage could be non-covalent and indirect rather than covalent and direct. To test this possibility, we reasoned that the Alum adjuvant may fulfill the requirement for indirect noncovalent linkage because the aluminum hydroxide gel is well known to bind protein and peptide antigens. The prediction is that IFN-β and NAg peptides would be crosslinked and immobilized by the Alum gel and thereby achieve stable physical linkage of IFN-β and NAg that is requisite for APC targeting and IFN-β mediated tolerogenic activity. Aluminum based adjuvants have been extensively used in vaccines over the past century and remain the only adjuvant approved and licensed for routine use in human vaccines and represent the most common adjuvants used in veterinary vaccines. These Alum adjuvants are comprised of aluminum phosphate (AlPO4) or aluminum hydroxide (Al(OH)3). Despite intensive research into alternative adjuvants, Alum adjuvants continue to dominant the adjuvant landscape due to significant advantages in safety, manufacturing, quality control, and adjuvanticity for humoral (but not cellular) vaccine responses. Alum is thought to bind vaccine antigens via electrostatic interactions to form a concentrated tissue depot that promotes recognition, phagocytosis, antigen processing, and presentation by dendritic cells. In this regard, the prediction is that the “IFN-β + MOG in Alum” vaccine forms a concentrated depot at the inoculation site and within the associated lymphatic drainage. This antigenic depot may attract dendritic cells that engage high concentrations of IFN-β and NAg to provide the stimuli for NAg-specific FOXP3+ Treg responses.
IFN-β-based tolerogenic vaccines and the directed specification of FOXP3+ Tregs
The tolerogenic vaccine had three components necessary for inhibition of EAE, including; (a) IFN-β as the key Treg specification adjuvant, (b) NAg as the clonotypic specification antigen, and (c) Alum as the requisite cohesive adjuvant (Figures 1, 3, 7–9). The “IFN-β + NAg in Alum” vaccine also inhibited MOG-induced sensitization (Figure 2). This is an effective and practical approach for tolerogenic vaccination based on inductive suppressive responses by NAg-specific FOXP3+ Tregs. Although tolerogenic vaccination in vivo was entirely contingent upon Alum, IFN-β and NAg elicited Treg differentiation in vitro in the absence of Alum (Figures 4–5). These observations indicate that IFN-β was key for the specification of the Treg lineage, whereas Alum was a passive adjuvant that was compatible but not directive for Treg differentiation. Alum appeared to have three main attributes. We postulate that (a) Alum provided a stable antigen depot required for persistent antigenic exposure, (b) Alum provided the physical matrix that enabled IFN-β/ NAg crosslinking requisite for effective vaccination, and (c) Alum had the compatibility needed for induction of tolerogenic FOXP3+ Treg responses because the adjuvant’s intrinsic activities on innate immunity did not interfere or preempt Treg responses.
Tolerogenic vaccination with “IFN-β+MOG in Alum” appeared contingent upon CD25+ FOXP3+ Tregs. Pretreatment with the anti-CD25 PC61 mAb purged circulating CD25+ FOXP3+ Tregs (88) and fully reversed the suppressive activity of the “IFN-β + MOG in Alum” vaccine (Figure 3). The suppressive activity of the Tregs was NAg-specific because the “IFN-β + MOG in Alum” vaccine inhibited MOG-induced EAE but not PLP-induced EAE. Conversely, the “IFN-β + PLP in Alum” vaccine inhibited PLP-induced EAE but not MOG-induced EAE. Thus, prophylactic vaccination induced Treg-mediated suppressive activity by a mechanism restricted to the TCR-specific Treg clonotype. Although strict clonotypic specificity was noted in this paradigm, tolerogenic clonotypes nonetheless controlled disparate specificities if both epitopes were included in the encephalitogenic emulsion. That is, an “IFN-β + OVA in Alum” vaccine inhibited EAE when mice were subsequently challenged with “MOG + OVA in CFA” emulsion whereas the same vaccine had no impact on mice challenged with “MOG in CFA” (Figure 9). These data revealed that clonotypic Tregs have the capacity to impact clonotypically distinct conventional T cells if the two clonotypes are bridged by proximal APC recognition events. Thus, “IFN-β + NAg in Alum” vaccines may be useful for establishing broad CNS-based tolerogenic coverage of epitopes that are unknown and not directly included in the vaccine.
Repeated tolerogenic vaccinations with “IFN-β + MOG in Alum” appeared to boost Treg abundance and elicit high-level expression of the CD44 glycoprotein on FOXP3+ Tregs (Figure 7). Given that CD44 is a marker of effector or memory status, repeated vaccine boosters may elicit more efficient functional responses. Three repeated vaccinations with “IFN-β + MOG in Alum” uniquely resulted in the accumulation of CD44high Treg subset. In contrast, one or two vaccinations elicited Tregs that mostly expressed intermediate levels of CD44. These findings provided evidence that repeated boosters of tolerogenic vaccines may not simply elicit more Tregs but may also drive memory/ effector Treg populations. In support, IFN-β-induced Tregs exhibited diverse phenotypic subsets not observed among TGF-β-induced Tregs (Figure 5C–F). Of interest, IFN-β uniquely evoked a FOXP3+ T cell subset that expressed high levels of CD69, CTLA-4, and IFNAR. Overall, these findings support the concept that IFN-β facilitates nascent NAg-specific Treg differentiation and maturation of Treg effector function.
A central question pertained to the potential interplay between IFN-β and TGF-β in that both cytokines had overlapping activities that fostered expression of FOXP3 and tolerogenic Treg-mediated pathways. TGF-β was more effective than IFN-β in vitro (Figure 4) in that low concentrations of TGF-β (1 nM) stimulated FOXP3 expression in over 50% of MOG-stimulated 2D2-FIG T cells whereas relatively high concentrations of IFN-β (1 µM) were required to approximate this response. Importantly, the 1D11 anti-TGF-β mAb blocked the ability of IFN-β to elicit FOXP3+ Tregs in vitro (Figure 10). These data indicate that the Treg-inductive properties of IFN-β, at least in part, stem from an IFN-β-induced, TGF-β-dependent response pathway possibly involving enhanced production of TGF-β during culture. The interaction of IFN-β and TGF-β however was nonadditive or antagonistic (Figure 4), but this observation may simply reflect a bell-shaped concentration curve because elevating TGF-β concentrations above the optimal 1 nM concentration diminished Treg induction (data not shown). The paradox was that IFN-β was more efficient than TGF-β as a vaccine for the selective induction of FOXP3+ Tregs in vivo (Figure 11). Unlike IFN-β, vaccination with “TGF-β + MOG in Alum” did not result in increased percentages of FOXP3+ Tregs in the circulation. TGF-β-based vaccination however was not inert as evidenced by increased numbers of T cells and total leukocytes in the blood but without alteration of Treg/ CD3+ T cell ratios. That is, TGF-β-based vaccines increased total Treg numbers along with proportional increases in CD3+ T cells and total leukocytes, such the frequencies of Tregs did not differ from control mice. One possibility is that TGF-β is pluripotent and supports differentiation of multiple T cells subsets, including Th17 T cells, among others. TGF-β may not be strictly dedicated or restricted to the specification of the FOXP3 lineage, such that a TGF-β-based vaccination strategy may lack the singular focus needed to drive this Treg subset. Based on this argument, the efficacious activity of IFN-β-based vaccination in vivo may reflect a close alignment of IFN-β activity with a focused action on FOXP3+ T cell differentiation and bystander suppression of alternative T cell subsets.
What is the physiological rationale for IFN-β involvement as a differentiation factor for the FOXP3+ Treg subset?
The paradox is that IFN-β has a critical role in innate immune responses against pathogenic viruses and other infectious pathogens yet facilitates tolerance during adaptive T cell responses. Why would IFN-β serve as a linchpin for innate immunity but function as an inhibitor of adaptive immunity? A potential answer dovetails with the strategic role of IFN-β in viral immunity. IFN-β is a major impediment to viral spread by ensuring that the neighboring surround of uninfected cells lack the metabolic capacity necessary to support viral replication, propagation, and transmission to adjacent cells. This containment strategy provides time for B cells to mount anti-viral antibody responses that prevent further viral dissemination. However, this containment strategy may be thwarted by infiltrating anti-viral T cells if these T cells themselves become infected and spread the viral infection by migration throughout the lymphatics and other peripheral tissues. A conceivable solution is that IFN-β may coordinate with IFN-β-induced Tregs to inhibit the activity and migration of highly mobile conventional CD4+ T cells as a strategy to limit viral spread. The subset of IFN-β-induced Tregs may be relatively immobile and may be specialized to be the dominant T cell subset in areas replete with high concentrations of IFN-β. As shown in this study, FOXP3+ Tregs exhibited dominant survival and growth compared to conventional T cell subsets in cultures supplemented with high concentrations of IFN-β (Figure 4C). In this regard, the regulatory activities of Tregs may reflect inhibitory cross-regulatory circuits that preempt other conventional subsets of effector T cells (89). In support, a subset of IFN-β-induced Tregs expressed high levels of CD69, IFNAR, and CTLA-4 (Figure 5). Aside from enforcing ‘regional tolerance’ and suppression of conventional T cell subsets, IFN-β-induced Tregs may also mediate specialized anti-viral cytotoxic activity and thereby may directly resolve viral infection. Although Tregs are often considered to be exclusively immunosuppressive, one must consider the possibility that Tregs instead have specialized effector activities (89), particularly in that Tregs have a phenotype most consistent with activated effector T cells. Accordingly, previous studies have shown that Treg ablation during mucosal herpes simplex virus infection in mice resulted in an accelerated fatal infection marked by elevated viral loads and deficient Type I IFN production at the site of infection (90). These data denote a direct effector action of Tregs in anti-viral immunity and a connection with local protective IFN-β production. Thus, IFN-β-induced Tregs may be an extension of the IFN-β-mediated strategy of viral containment and may mediate T cell-mediated anti-viral immunity together with robust cross-inhibitory suppression of competing conventional T cell subsets.
Conclusion
This study shows that IFN-β and a myelin NAg synergistically caused the emergence of a suppressive, myelin-specific, FOXP3+ Treg subset. Thus, these data revealed the existence of an IFN-β/FOXP3 Treg axis and have implicated this axis as an important mechanism of suppression in EAE. This IFN-β/FOXP3 Treg axis may serve as the experimental foundation for effective tolerogenic vaccination in CNS autoimmune disease. Thus, this study revealed a novel and exquisitely simple approach by which IFN-β and a myelin NAg can be formulated in Alum as an effective tolerogenic vaccine.
Abbreviations
- CTV
CellTrace Violet
- EAE
experimental autoimmune encephalomyelitis
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NAg
neuroantigen(s)
- PLP
proteolipid protein
- Treg
regulatory T cell(s)
Footnotes
This study was supported by the National Institute of Neurological Disorders and Stroke (R15-NS075830 and R01-NS072150, M.D.M.), the Harriet and John Wooten Laboratory for Alzheimer’s and Neurodegenerative Disease Research (M.D.M), and by a research grant from AlzNC Alzheimer’s North Carolina, Inc.
Conflict of interest: The authors have no financial or commercial conflicts of interest in regard to this work.
References
- 1.Nylander A, Hafler DA. Multiple sclerosis. J. Clin. Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comabella M, Khoury SJ. Immunopathogenesis of multiple sclerosis. Clin. Immunol. 2012;142:2–8. doi: 10.1016/j.clim.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L. The gray aspects of white matter disease in multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8083–8084. doi: 10.1073/pnas.0903377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGraw CA, Lublin FD. Interferon beta and glatiramer acetate therapy. Neurotherapeutics. 2013;10:2–18. doi: 10.1007/s13311-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killestein J, Polman CH. Determinants of interferon beta efficacy in patients with multiple sclerosis. Nat. Rev. Neurol. 2011;7:221–228. doi: 10.1038/nrneurol.2011.22. [DOI] [PubMed] [Google Scholar]
- 6.Derwenskus J. Current disease-modifying treatment of multiple sclerosis. Mt. Sinai J. Med. 2011;78:161–175. doi: 10.1002/msj.20239. [DOI] [PubMed] [Google Scholar]
- 7.Kieseier CB. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS drugs. 2011;25:491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Rudick RA, Goelz SE. Beta-interferon for multiple sclerosis. Exp. Cell Res. 2011;317:1301–1311. doi: 10.1016/j.yexcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axtell RC, Raman C, Steinman L. Type I interferons: beneficial in Th1 and detrimental in Th17 autoimmunity. Clin. Rev. Allergy Immunol. 2013;44:114–120. doi: 10.1007/s12016-011-8296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, Shinohara ML. Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Science signaling. 2012;5:ra38. doi: 10.1126/scisignal.2002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 2013;34:410–422. doi: 10.1016/j.it.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangachari M, Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. J. Autoimmun. 2013;45:31–39. doi: 10.1016/j.jaut.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marta CB, Oliver AR, Sweet RA, Pfeiffer SE, Ruddle NH. Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13992–13997. doi: 10.1073/pnas.0504979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J. Immunol. 2003;171:462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- 16.Swanborg RH. Antigen-induced inhibition of experimental allergic encephalomyelitis. II. Studies in guinea pigs with the small rat myelin basic protein. J. Immunol. 1973;111:1067–1070. [PubMed] [Google Scholar]
- 17.Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J. Immunol. 1988;140:440–445. [PubMed] [Google Scholar]
- 18.Brod SA, al-Sabbagh A, Sobel RA, Hafler DA, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin antigens: IV. Suppression of chronic relapsing disease in the Lewis rat and strain 13 guinea pig. Ann. Neurol. 1991;29:615–622. doi: 10.1002/ana.410290608. [DOI] [PubMed] [Google Scholar]
- 19.Javed NH, Gienapp IE, Cox KL, Whitacre CC. Exquisite peptide specificity of oral tolerance in experimental autoimmune encephalomyelitis. J. Immunol. 1995;155:1599–1605. [PubMed] [Google Scholar]
- 20.Campbell B, Vogel PJ, Fisher E, Lorenz R. Myelin basic protein administration in multiple sclerosis. Arch. Neurol. 1973;29:10–15. doi: 10.1001/archneur.1973.00490250028003. [DOI] [PubMed] [Google Scholar]
- 21.Faria AM, Weiner HL. Oral tolerance. Immunol. Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loo EW, Krantz MJ, Agrawal B. High dose antigen treatment with a peptide epitope of myelin basic protein modulates T cells in multiple sclerosis patients. Cell. Immunol. 2012;280:10–15. doi: 10.1016/j.cellimm.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Freedman MS, Bar-Or A, Oger J, Traboulsee A, Patry D, Young C, Olsson T, Li D, Hartung HP, Krantz M, Ferenczi L, Verco T. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology. 2011;77:1551–1560. doi: 10.1212/WNL.0b013e318233b240. [DOI] [PubMed] [Google Scholar]
- 24.Darlington C. MBP-8298, a synthetic peptide analog of myelin basic protein for the treatment of multiple sclerosis. Curr. Opin. Mol. Ther. 2007;9:398–402. [PubMed] [Google Scholar]
- 25.McFarland HI, Lobito AA, Johnson MM, Palardy GR, Yee CS, Jordan EK, Frank JA, Tresser N, Genain CP, Mueller JP, Matis LA, Lenardo MJ. Effective antigen-specific immunotherapy in the marmoset model of multiple sclerosis. J. Immunol. 2001;166:2116–2121. doi: 10.4049/jimmunol.166.3.2116. [DOI] [PubMed] [Google Scholar]
- 26.Elliott EA, McFarland HI, Nye SH, Cofiell R, Wilson TM, Wilkins JA, Squinto SP, Matis LA, Mueller JP. Treatment of experimental encephalomyelitis with a novel chimeric fusion protein of myelin basic protein and proteolipid protein. J. Clin. Invest. 1996;98:1602–1612. doi: 10.1172/JCI118954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 28.Wraith DC. Therapeutic peptide vaccines for treatment of autoimmune diseases. Immunol. Lett. 2009;122:134–136. doi: 10.1016/j.imlet.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garren H. DNA vaccines for autoimmune diseases. Expert review of vaccines. 2009;8:1195–1203. doi: 10.1586/erv.09.83. [DOI] [PubMed] [Google Scholar]
- 30.Walczak A, Siger M, Ciach A, Szczepanik M, Selmaj K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA neurology. 2013:1–6. doi: 10.1001/jamaneurol.2013.3022. [DOI] [PubMed] [Google Scholar]
- 31.Jurynczyk M, Walczak A, Jurewicz A, Jesionek-Kupnicka D, Szczepanik M, Selmaj K. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann. Neurol. 2010;68:593–601. doi: 10.1002/ana.22219. [DOI] [PubMed] [Google Scholar]
- 32.Lutterotti A, Yousef S, Sputtek A, Sturner KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, Schippling S, Miller SD, Sospedra M, Martin R. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci. Transl. Med. 2013;5:188ra175. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J. Immunol. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Stern JN, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, von Boehmer H, Kretschmer K, Strominger JL. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17280–17285. doi: 10.1073/pnas.1010263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mannie MD, Curtis AD., 2nd Tolerogenic vaccines for Multiple sclerosis. Hum. Vaccin. Immunother. 2013;9:1032–1038. doi: 10.4161/hv.23685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mannie MD, Blanchfield JL, Islam SM, Abbott DJ. Cytokine-neuroantigen fusion proteins as a new class of tolerogenic, therapeutic vaccines for treatment of inflammatory demyelinating disease in rodent models of multiple sclerosis. Front. Immunol. 2012;3:255. doi: 10.3389/fimmu.2012.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott DJ, Blanchfield JL, Martinson DA, Russell SC, Taslim N, Curtis AD, Mannie MD. Neuroantigen-specific, tolerogenic vaccines: GM-CSF is a fusion partner that facilitates tolerance rather than immunity to dominant self-epitopes of myelin in murine models of experimental autoimmune encephalomyelitis (EAE) BMC Immunol. 2011;12:72. doi: 10.1186/1471-2172-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchfield JL, Mannie MD. A GMCSF-neuroantigen fusion protein is a potent tolerogen in experimental autoimmune encephalomyelitis (EAE) that is associated with efficient targeting of neuroantigen to APC. J. Leukoc. Biol. 2010;87:509–521. doi: 10.1189/jlb.0709520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mannie MD, Abbott DJ, Blanchfield JL. Experimental autoimmune encephalomyelitis in Lewis rats: IFN-beta acts as a tolerogenic adjuvant for induction of neuroantigen-dependent tolerance. J. Immunol. 2009;182:5331–5341. doi: 10.4049/jimmunol.0803756. [DOI] [PubMed] [Google Scholar]
- 42.Mannie MD, Devine JL, Clayson BA, Lewis LT, Abbott DJ. Cytokine-neuroantigen fusion proteins: new tools for modulation of myelin basic protein (MBP)-specific T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. Methods. 2007;319:118–132. doi: 10.1016/j.jim.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Mannie MD, Clayson BA, Buskirk EJ, DeVine JL, Hernandez JJ, Abbott DJ. IL-2/neuroantigen fusion proteins as antigen-specific tolerogens in experimental autoimmune encephalomyelitis (EAE): correlation of T cell-mediated antigen presentation and tolerance induction. J. Immunol. 2007;178:2835–2843. doi: 10.4049/jimmunol.178.5.2835. [DOI] [PubMed] [Google Scholar]
- 44.Mannie MD, Abbott DJ. A fusion protein consisting of IL-16 and the encephalitogenic peptide of myelin basic protein constitutes an antigen-specific tolerogenic vaccine that inhibits experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:1458–1465. doi: 10.4049/jimmunol.179.3.1458. [DOI] [PubMed] [Google Scholar]
- 45.Zou Z, Sun PD. Overexpression of human transforming growth factor-beta1 using a recombinant CHO cell expression system. Protein Expr. Purif. 2004;37:265–272. doi: 10.1016/j.pep.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur. J. Immunol. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 47.Lacal JC, Aaronson SA. Monoclonal antibody Y13-259 recognizes an epitope of the p21 ras molecule not directly involved in the GTP-binding activity of the protein. Mol. Cell. Biol. 1986;6:1002–1009. doi: 10.1128/mcb.6.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J. Immunol. 1989;142:1536–1541. [PubMed] [Google Scholar]
- 49.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J. Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 50.Mannie MD, Morrison-Plummer J, Torres-Garcia B, Hannaway C, Jones C, Smith AM. Parallel costimulatory pathways promote myelin basic protein-stimulated proliferation of encephalitogenic rat T cells. Cell. Immunol. 1994;153:312–328. doi: 10.1006/cimm.1994.1031. [DOI] [PubMed] [Google Scholar]
- 51.Arnold PY, Kearse KP, Marinakis CA, Mannie MD. A novel monoclonal antibody against rat LFA-1: blockade of LFA-1 and CD4 augments class II MHC expression on T cells. Hybridoma. 1998;17:331–338. doi: 10.1089/hyb.1998.17.331. [DOI] [PubMed] [Google Scholar]
- 52.Mannie MD, Nardella JP, White GA, Arnold PY, Davidian DK. Class II MHC/peptide complexes on T cell antigen-presenting cells: agonistic antigen recognition inhibits subsequent antigen presentation. Cell. Immunol. 1998;186:111–120. doi: 10.1006/cimm.1998.1301. [DOI] [PubMed] [Google Scholar]
- 53.Abreu SL. Suppression of experimental allergic encephalomyelitis by interferon. Immunol. Commun. 1982;11:1–7. doi: 10.3109/08820138209050718. [DOI] [PubMed] [Google Scholar]
- 54.Hertz F, Deghenghi R. Effect of rat and beta-human interferons on hyperacute experimental allergic encephalomyelitis in rats. Agents Actions. 1985;16:397–403. doi: 10.1007/BF01982879. [DOI] [PubMed] [Google Scholar]
- 55.Brod SA, Khan M, Kerman RH, Pappolla M. Oral administration of human or murine interferon alpha suppresses relapses and modifies adoptive transfer in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1995;58:61–69. doi: 10.1016/0165-5728(94)00188-t. [DOI] [PubMed] [Google Scholar]
- 56.Brod SA, Scott M, Burns DK, Phillips JT. Modification of acute experimental autoimmune encephalomyelitis in the Lewis rat by oral administration of type 1 interferons. J. Interferon Cytokine Res. 1995;15:115–122. doi: 10.1089/jir.1995.15.115. [DOI] [PubMed] [Google Scholar]
- 57.Brod SA, Khan M. Oral administration of IFN-alpha is superior to subcutaneous administration of IFN-alpha in the suppression of chronic relapsing experimental autoimmune encephalomyelitis. J. Autoimmun. 1996;9:11–20. doi: 10.1006/jaut.1996.0003. [DOI] [PubMed] [Google Scholar]
- 58.Vriesendorp FJ, Flynn RE, Khan M, Pappolla MA, Brod SA. Oral administration of type I interferon modulates the course of experimental allergic neuritis. Autoimmunity. 1996;24:157–165. doi: 10.3109/08916939608995361. [DOI] [PubMed] [Google Scholar]
- 59.Yu M, Nishiyama A, Trapp BD, Tuohy VK. Interferon-beta inhibits progression of relapsing-remitting experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1996;64:91–100. doi: 10.1016/0165-5728(95)00160-3. [DOI] [PubMed] [Google Scholar]
- 60.Yasuda CL, Al-Sabbagh A, Oliveira EC, Diaz-Bardales BM, Garcia AA, Santos LM. Interferon beta modulates experimental autoimmune encephalomyelitis by altering the pattern of cytokine secretion. Immunol. Invest. 1999;28:115–126. doi: 10.3109/08820139909061141. [DOI] [PubMed] [Google Scholar]
- 61.Tuohy VK, Yu M, Yin L, Mathisen PM, Johnson JM, Kawczak JA. Modulation of the IL-10/IL-12 cytokine circuit by interferon-beta inhibits the development of epitope spreading and disease progression in murine autoimmune encephalomyelitis. J. Neuroimmunol. 2000;111:55–63. doi: 10.1016/s0165-5728(00)00384-2. [DOI] [PubMed] [Google Scholar]
- 62.Floris S, Ruuls SR, Wierinckx A, van der Pol SM, Dopp E, van der Meide PH, Dijkstra CD, De Vries HE. Interferon-beta directly influences monocyte infiltration into the central nervous system. J. Neuroimmunol. 2002;127:69–79. doi: 10.1016/s0165-5728(02)00098-x. [DOI] [PubMed] [Google Scholar]
- 63.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galligan CL, Pennell LM, Murooka TT, Baig E, Majchrzak-Kita B, Rahbar R, Fish EN. Interferon-beta is a key regulator of proinflammatory events in experimental autoimmune encephalomyelitis. Mult. Scler. 2010;16:1458–1473. doi: 10.1177/1352458510381259. [DOI] [PubMed] [Google Scholar]
- 65.Kalinke U, Prinz M. Endogenous, or therapeutically induced, type I interferon responses differentially modulate Th1/Th17-mediated autoimmunity in the CNS. Immunol. Cell Biol. 2012;90:505–509. doi: 10.1038/icb.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fitzgerald DC, Fonseca-Kelly Z, Cullimore ML, Safabakhsh P, Saris CJ, Zhang GX, Rostami A. Independent and interdependent immunoregulatory effects of IL-27, IFN-beta, and IL-10 in the suppression of human Th17 cells and murine experimental autoimmune encephalomyelitis. J. Immunol. 2013;190:3225–3234. doi: 10.4049/jimmunol.1200141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inoue M, Shinohara ML. The role of interferon-beta in the treatment of multiple sclerosis and experimental autoimmune encephalomyelitis - in the perspective of inflammasomes. Immunology. 2013;139:11–18. doi: 10.1111/imm.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou Y, Heon Ryu C, Jun JA, Kim SM, Jeong CH, Jeun SS. Interferon beta-secreting mesenchymal stem cells combined with minocycline attenuate experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014;274:20–27. doi: 10.1016/j.jneuroim.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Boivin N, Baillargeon J, Doss PM, Roy AP, Rangachari M. Interferon-beta suppresses murine Th1 cell function in the absence of antigen-presenting cells. PLoS One. 2015;10:e0124802. doi: 10.1371/journal.pone.0124802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng W, Zhao Q, Xi Y, Li C, Xu Y, Wang L, Niu X, Wang Z, Chen G. IFN-beta inhibits T cells accumulation in the central nervous system by reducing the expression and activity of chemokines in experimental autoimmune encephalomyelitis. Mol. Immunol. 2015;64:152–162. doi: 10.1016/j.molimm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Khorooshi R, Morch MT, Holm TH, Berg CT, Dieu RT, Draeby D, Issazadeh-Navikas S, Weiss S, Lienenklaus S, Owens T. Induction of endogenous Type I interferon within the central nervous system plays a protective role in experimental autoimmune encephalomyelitis. Acta Neuropathol. 2015;130:107–118. doi: 10.1007/s00401-015-1418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhornitsky S, Johnson TA, Metz LM, Weiss S, Yong VW. Prolactin in combination with interferon-beta reduces disease severity in an animal model of multiple sclerosis. J. Neuroinflammation. 2015;12:55. doi: 10.1186/s12974-015-0278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Andres C, Aristimuno C, de Las Heras V, Martinez-Gines ML, Bartolome M, Arroyo R, Navarro J, Gimenez-Roldan S, Fernandez-Cruz E, Sanchez-Ramon S. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2007;182:204–211. doi: 10.1016/j.jneuroim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Korporal M, Haas J, Balint B, Fritzsching B, Schwarz A, Moeller S, Fritz B, Suri-Payer E, Wildemann B. Interferon beta-induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naive regulatory T cells. Arch. Neurol. 2008;65:1434–1439. doi: 10.1001/archneur.65.11.1434. [DOI] [PubMed] [Google Scholar]
- 75.Vandenbark AA, Huan J, Agotsch M, La Tocha D, Goelz S, Offner H, Lanker S, Bourdette D. Interferon-beta-1a treatment increases CD56bright natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. J. Neuroimmunol. 2009;215:125–128. doi: 10.1016/j.jneuroim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Aristimuno C, de Andres C, Bartolome M, de las Heras V, Martinez-Gines ML, Arroyo R, Fernandez-Cruz E, Sanchez-Ramon S. IFNbeta-1a therapy for multiple sclerosis expands regulatory CD8+ T cells and decreases memory CD8+ subset: a longitudinal 1-year study. Clin. Immunol. 2010;134:148–157. doi: 10.1016/j.clim.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Namdar A, Nikbin B, Ghabaee M, Bayati A, Izad M. Effect of IFN-beta therapy on the frequency and function of CD4(+)CD25(+) regulatory T cells and Foxp3 gene expression in relapsing-remitting multiple sclerosis (RRMS): a preliminary study. J. Neuroimmunol. 2010;218:120–124. doi: 10.1016/j.jneuroim.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 78.Chen M, Chen G, Deng S, Liu X, Hutton GJ, Hong J. IFN-beta induces the proliferation of CD4+CD25+Foxp3+ regulatory T cells through upregulation of GITRL on dendritic cells in the treatment of multiple sclerosis. J. Neuroimmunol. 2012;242:39–46. doi: 10.1016/j.jneuroim.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 79.Piconese S, Pacella I, Timperi E, Barnaba V. Divergent effects of type-I interferons on regulatory T cells. Cytokine Growth Factor Rev. 2014;26:133–141. doi: 10.1016/j.cytogfr.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Lee SE, Li X, Kim JC, Lee J, Gonzalez-Navajas JM, Hong SH, Park IK, Rhee JH, Raz E. Type I interferons maintain Foxp3 expression and T-regulatory cell functions under inflammatory conditions in mice. Gastroenterology. 2012;143:145–154. doi: 10.1053/j.gastro.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metidji A, Rieder SA, Glass DD, Cremer I, Punkosdy GA, Shevach EM. IFN-alpha/beta receptor signaling promotes regulatory T cell development and function under stress conditions. J. Immunol. 2015;194:4265–4276. doi: 10.4049/jimmunol.1500036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 83.Ziegler-Heitbrock L, Lotzerich M, Schaefer A, Werner T, Frankenberger M, Benkhart E. IFN-alpha induces the human IL-10 gene by recruiting both IFN regulatory factor 1 and Stat3. J. Immunol. 2003;171:285–290. doi: 10.4049/jimmunol.171.1.285. [DOI] [PubMed] [Google Scholar]
- 84.Dikopoulos N, Bertoletti A, Kroger A, Hauser H, Schirmbeck R, Reimann J. Type I IFN negatively regulates CD8+ T cell responses through IL-10-producing CD4+ T regulatory 1 cells. J. Immunol. 2005;174:99–109. doi: 10.4049/jimmunol.174.1.99. [DOI] [PubMed] [Google Scholar]
- 85.Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, Leighty RM, Roers A, Karp CL, Muller W, Trinchieri G. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J. Clin. Invest. 2013;123:4859–4874. doi: 10.1172/JCI65180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, Carlsson R, Comabella M, Wang J, Kosicki M, Carrion B, Hasan M, Wu X, Montalban X, Dziegiel MH, Sellebjerg F, Sorensen PS, Helin K, Issazadeh-Navikas S. FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat. Med. 2014;20:272–282. doi: 10.1038/nm.3485. [DOI] [PubMed] [Google Scholar]
- 87.Islam SM, Curtis AD, 2nd, Taslim N, Wilkinson DS, Mannie MD. GM-CSF-neuroantigen fusion proteins reverse experimental autoimmune encephalomyelitis and mediate tolerogenic activity in adjuvant-primed environments: association with inflammation-dependent, inhibitory antigen presentation. J. Immunol. 2014;193:2317–2329. doi: 10.4049/jimmunol.1303223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghosh D, Curtis AD, 2nd, Wilkinson DS, Mannie MD. Depletion of CD4+ CD25+ regulatory T cells confers susceptibility to experimental autoimmune encephalomyelitis (EAE) in GM-CSF-deficient Csf2−/− mice. J. Leukoc. Biol. 2016 doi: 10.1189/jlb.3A0815-359R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corthay A. How do regulatory T cells work? Scand. J. Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]