Abstract
IMPORTANCE
The use of acid suppression has been associated with an increased risk of upper and lower respiratory tract infections in the outpatient setting but the mechanism behind this increased risk is unknown. We hypothesize that this infection risk results from gastric bacterial overgrowth with subsequent seeding of the lungs.
OBJECTIVES
To determine if acid-suppression use results in gastric bacterial overgrowth, if there are changes in lung microflora associated with the use of acid suppression, and if changes in lung microflora are related to full-column nonacid gastroesophageal reflux.
DESIGN, SETTING, AND PARTICIPANTS
A 5-year prospective cohort study at a tertiary care center where children ages 1 to 18 years were undergoing bronchoscopy and endoscopy for the evaluation of chronic cough. Acid-suppression use was assessed through questionnaires with confirmation using an electronic medical record review.
MAIN OUTCOMES AND MEASURES
Our primary outcome was to compare differences in concentration and prevalence of gastric and lung bacteria between patients who were and were not receiving acid-suppression therapy. We compared medians using the Wilcoxon signed rank test and determined prevalence ratios using asymptotic standard errors and 95% confidence intervals. We determined correlations between continuous variables using Pearson correlation coefficients and compared categorical variables using the Fisher exact test.
RESULTS
Forty-six percent of patients taking acid-suppression medication had gastric bacterial growth compared with 18% of untreated patients (P = .003). Staphylococcus (prevalence ratio, 12.75 [95% CI, 1.72–94.36]), Streptococcus (prevalence ratio, 6.91 [95% CI, 1.64–29.02]), Veillonella (prevalence ratio, 9.56 [95% CI, 1.26–72.67]), Dermabacter (prevalence ratio, 4.78 [95% CI, 1.09–21.02]), and Rothia (prevalence ratio, 6.38 [95% CI, 1.50–27.02]) were found more commonly in the gastric fluid of treated patients. The median bacterial concentration was higher in treated patients than in untreated patients (P = .001). There was no difference in the prevalence (P > .23) of different bacterial genera or the median concentration of total bacteria (P = .85) in the lungs between treated and untreated patients. There were significant positive correlations between proximal nonacid reflux burden and lung concentrations of Bacillus (r = 0.47, P = .005), Dermabacter (r = 0.37, P = .008), Lactobacillus (r = 0.45, P = .001), Peptostreptococcus (r = 0.37, P = .008), and Capnocytophagia (r = 0.37, P = .008).
CONCLUSIONS AND RELEVANCE
Acid-suppression use results in gastric bacterial overgrowth of genera including Staphylococcus and Streptococcus. Full-column nonacid reflux is associated with greater concentrations of bacteria in the lung. Additional studies are needed to determine if acid suppression–related microflora changes predict clinical infection risk; these results suggest that acid suppression use may need to be limited in patients at risk for infections.
Acid-suppression medications, including proton pump inhibitors (PPIs), are some of the most prescribed medications in adult and pediatric medicine. Prescriptions have risen by more than 400% in some age groups.1 As the use of these medications has increased, the risks from use have become more apparent, with a rise in respiratory tract infections, pharyngitis, gastroenteritis, and Clostridium difficile colitis.2–4 The mechanism behind the infections is not known but a change in the diversity or abundance of bacteria in the gastric fluid has been proposed with associated downstream effects on intestinal microflora.5,6 It is unknown whether acid suppression in children results through nonacid reflux in upstream effects on oropharyngeal and lung microflora that may be responsible for respiratory complications. The goal of this study was to determine the impact of acid-suppression therapy on the abundance and diversity of gastric and lung microflora in children presenting with chronic cough. We hypothesize that acid suppression will increase the abundance and diversity of gastric and lung microflora and that full-column nonacid reflux, which is increased in acid-suppressed patients, changes lung microflora.
Methods
At Boston Children’s Hospital, we conducted a prospective cross-sectional study of children 1 to 18 years of age undergoing esophagogastroduodenoscopy and bronchoscopy for evaluation of cough. To be recruited for participation, patients needed to have a cough at least 3 times a week for at least 1 month of the year. All patients were referred for bronchoscopy by their primary pulmonologist. All patients scheduled for combined procedures between January 1, 2008, and June 1, 2012, were approached for participation. Children younger than 1 year were excluded because of the high rates of physiologic reflux. Patients were considered to be receiving acid-suppression therapy if they had taken a dose of acid-suppression medication within 24 hours of endoscopy and had been taking acid-suppression medication for a minimum of 4 weeks. Patients were considered to be not receiving acid-suppression therapy if the acid suppression was stopped more than 48 hours prior to their procedure. Because bacterial death can occur within 10 minutes of acid exposure, 48 hours off therapy was considered as not receiving therapy.7 The study was approved by the institutional review board at the Boston Children’s Hospital and written informed consent was obtained from all patients.
Procedures
Patients were enrolled at the time of endoscopic procedures. Bronchoscopy was performed first through an endotracheal tube. One milliliter per kilogram of normal saline was lavaged into the right middle lobe of the lung or in the lobe with the most visible secretions. A minimum of 2 mL of bronchoalveolar lavage (BAL) fluid was transferred from the sterile leukitrap to a sterile microtainer. Following the bronchoscopy, an esophagogastroduodenoscopy was performed. The endoscope was advanced through the mouth into the stomach without suctioning. Once in the stomach, the endoscopist suctioned a minimum of 1 mL of gastric fluid into a sterile leukitrap, which was transferred to a sterile microtainer. Gastric and BAL samples were kept on ice from the time of suctioning (for no more than 10 minutes) until being transferred to a −80°C freezer.
Multichannel Intraluminal Impedance with pH
Multichannel intraluminal impedance with pH (pH-MII) testing was performed in 50 patients undergoing esophagogastroduodenoscopy and bronchoscopy. The decision to perform pH-MII testing was at the discretion of the primary physicians rather than mandated by study protocol because of the invasive and prolonged nature of the test. Definitions of reflux episodes (acid, nonacid, and pH-only episodes) by pH-MII are published else where.8 The percentage of time that reflux was in the proximal esophagus was calculated by dividing the sum of the bolus clearance times in the proximal esophagus by the total study duration. The pH portion of the study was considered abnormal if the pH level was less than 4 for more than 6% of the study time.9 The MII portion of the study was considered abnormal if there were more than 73 reflux episodes during the study time.10
Cultures
Gastric and lung fluid was cultured in the Anaerobe Research Laboratory at Brigham and Women’s Hospital. Expanded cultures were performed on specialized media and individual colony types were selected for identification based on colony morphology. Bacterial identification was performed using long-chain fatty acid analysis using the Microbial Identification System ([MIS]; MIDI Inc). Enterobacteriaceae were identified using the MIS or the API 20E system (bioMerieux, Inc). In addition to identification using the MIS, obligate anaerobes were also identified using the IDS RapID ANA II System (Remel Inc). The stored long-chain fatty acid analysis data from the MIS system were used where applicable for developing similarity indexes and dendrograms for groups of phenotypically similar isolates. All counts were recorded as log10 colony-forming unit per milliliter.
Statistics
Distributions of continuous variables were summarized as mean (SD) if normally distributed or otherwise as median with interquartile range. Patient characteristics were compared for those taking and not taking medication using t tests or Fisher exact tests. Prevalence ratios were calculated with asymptotic standard errors and 95% confidence intervals. Correlations between continuous variables were assessed using Pearson correlation coefficients. Wilcoxon signed rank tests were used to compare continuous variables. Receiver operating characteristics were used to determine a cut point for a PPI dose that would predict gastric and lung growth. The nonparametric Jonckheere-Terpstra trend test was performed to investigate a dose-response relation between presence of gastric or lung growth and PPI doses 0.1 to 1.0 mg/kg, 1.1 to 2.0 mg/kg, and more than 2.0 mg/kg. All tests were 2-sided with P < .05 to indicate statistical significance. Data analysis was conducted using SAS (version 9.3), R, and MATLAB (MathWorks; http://www.mathworks.com).
Results
One hundred one patients were recruited but 2 patients had inadequate volume of lung fluid retrieved. Therefore, 99 patients were included in the analysis. Forty-eight children received acid-suppression therapy and 51 patients did not receive acid-suppression therapy. Of the patients receiving therapy, 45 children received PPI therapy and 3 patients received histamine-2 antagonist therapy. Patient demographics are shown in Table 1.
Table 1.
Demographic Data
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Not Receiving Acid Suppression (n = 51) | Receiving Acid Suppression (n = 48) | ||
| Age, mean (SD), y | 6.6 (4.2) | 8.9 (5.6) | .02 |
| History of proton pump inhibitor use in infancy | 14 (27) | 8 (17) | .19 |
| History of histamine-2 antagonist use in infancy | 13 (26) | 11 (23) | .78 |
| Asthma in last 6 mo | 39 (77) | 27 (56) | .03 |
| Croup in last 6 mo | 8 (16) | 4 (8) | .26 |
| Cough in last 6 mo | 31 (61) | 25 (52) | .48 |
| Abdominal pain in last 6 mo | 20 (39) | 20 (42) | .80 |
| Feels food coming into the mouth in last 6 mo | 17 (33) | 15 (31) | .82 |
| Impedance performed | 31 (61) | 19 (40) | .006 |
| Abnormal impedance | 14 (45) | 7 (40) | .56 |
| Esophagitis | 16 (31) | 10 (21) | .23 |
| Gastric growth | 9 (18) | 22 (46) | .003 |
| Lung growth | 34 (67) | 33 (69) | .83 |
Gastric Growth
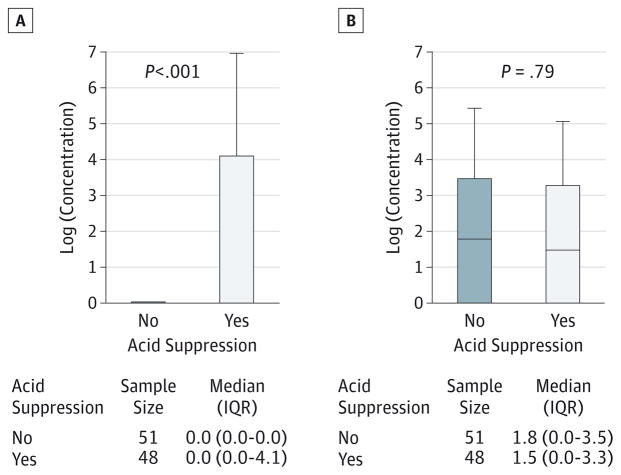
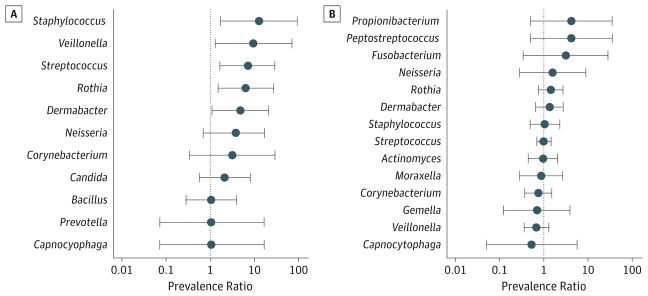
Thirty-one patients (31%) had growth from gastric fluid; there was a significant difference in the presence of bacterial growth between patients not receiving therapy (18%) and those receiving therapy (46%; P = .003). Patients receiving acid-suppression therapy had a higher median total bacterial concentration than untreated patients, as shown in Figure 1A. Acid-suppression use was not only associated with positive gastric growth but more specifically with an increased prevalence of potential pathogens including Staphylococcus (prevalence ratio, 12.75 [95% CI, 1.72–94.36]), Streptococcus (prevalence ratio, 6.91 [95% CI, 1.64–29.02]), and other bacteria such as Veillonella (prevalence ratio, 9.56 [95% CI, 1.26–72.67]), Dermabacter (prevalence ratio, 4.78 [95% CI, 1.09–21.02]), and Rothia (prevalence ratio, 6.38 [95% CI, 1.50–27.02]) (Figure 2A). As shown in Table 2, patients receiving acid-suppression therapy not only had a higher prevalence but also had higher concentrations of the bacteria mentioned above.
Figure 1. Difference in Total Median Concentrations.
A, Patients receiving and not receiving acid-suppression therapy in gastric samples. B, Patients receiving and not receiving acid-suppression therapy in lung samples. IQR indicates interquartile range.
Figure 2. Differences in Bacterial Prevalence Ratios.
A, Patients receiving and not receiving acid-suppression therapy in the gastric flora. B, Patients receiving and not receiving acid-suppression therapy in the lung flora. Genera with prevalence ratios to the right of 1 are more common in patients taking acid-suppression therapy while genera to the left of 1 are more common in patients not receiving therapy.
Table 2.
Significant Differences in Gastric and Lung Bacteria Concentrations in Patients Receiving and Not Receiving Acid-Suppression Therapy
| Bacteria Concentrations | Not Receiving Therapy | Receiving Therapy | P Value |
|---|---|---|---|
| Gastric, log10 CFU/mL | |||
| Streptococcus | 0.2 | 1.4 | .001 |
| Staphylococcus | 0.04 | 0.85 | .001 |
| Veillonella | 0.09 | 0.87 | .006 |
| Rothia | 0.19 | 1.1 | .003 |
| Dermabacter | 0.18 | 0.8 | .02 |
| Lung, log10 CFU/mL | |||
| Bacillus | 0.07 | 0 | .05 |
| Lactobacillus | 0.23 | 0 | .03 |
Abbreviation: CFU, colony-forming unit.
BAL Growth
Seventy-seven patients (76%) had growth of bacteria in the lung. There was no difference in the median total bacteria concentration between treated and untreated patients (Figure 1B). Acid-suppression use was not associated with a positive lung culture (P = .23) or with the presence of specific types of bacteria (Figure 2B). Differences in concentrations between individual genera between patients taking and not taking medications are shown in Table 2.
Overlap Between Gastric and Lung Microflora
Correlation matrices were performed to determine the relationship between microflora in the stomach and the lung. In treated patients, there was a significant correlation between the abundance of Corynebacterium (r = 0.34, P = .01) in the gastric and lung fluid and the abundance of Propionibacterium (r = 0.4, P = .006) in the gastric and lung fluid of patients receiving acid-suppression therapy, suggesting overlap between the sites.
Influence of Gastroesophageal Reflux on Lung Bacterial Growth
Fifty patients had pH-MII testing. The mean (SD) number of acid, nonacid, pH only, and total number of events are 25 (20), 21 (18), 16 (14), and 45 (31), respectively. In the 50 patients with impedance testing, we found a significant correlation between proximal nonacid reflux burden and lung concentrations of Bacillus (r = 0.47, P = .005), Dermabacter (r = 0.37, P = .008), Lactobacillus (r = 0.45, P = .001), Peptostreptococcus (r = 0.37, P = .008), and Capnocytophagia (r = 0.37, P = .008). There was no significant relationship between an abnormal MII study or an abnormal percentage of time pH less than 4 and lung growth (P > .20).
Dose of Acid Suppression and Growth
Using a receiver operating characteristic analysis, the optimal PPI dose per kilogram predictive cut point for gastric growth was 1.0 milligram per kilogram, which has a sensitivity of 64%, a specificity of 60%, and an area under the curve of 0.62. The optimal PPI dose per kilogram predictive cut point for lung growth was 0.89 mg/kg with a sensitivity of 66%, a specificity of 53%, and an area under the curve of 0.5. Based on the low sensitivities, these results suggest that there is no clear milligram per kilogram dose predicting which patients will have gastric or lung growth. We then performed a χ2 analysis to determine if a low dose (<1 mg/kg) or high dose (>1 mg/kg) predicted gastric growth or lung growth and found no significant difference between the 2 dosing groups and gastric (P = .20) or lung (P = .80) growth.
Discussion
This is the first study, to our knowledge, to determine in noncritically ill pediatric patients the differences in gastric and lung microflora between patients who are and are not receiving acid-suppression therapy and the impact of gastroesophageal reflux on lung microflora. We found a significant increase in the abundance and diversity of bacteria in the gastric fluid of patients receiving acid-suppression therapy as well as a correlation with full-column nonacid reflux and the concentrations of certain types of bacteria in the lungs.
These findings are of direct clinical importance. We found not only that there was an increase in gastric bacterial concentrations with acid-suppression use but that acid-sensitive bacteria including Streptococcus and Staphylococcus were more prevalent and more abundant in the gastric fluid of patients receiving acid-suppression therapy. Because both of these genera may serve as potential pathogens in pharyngitis, upper respiratory tract disorders, and pneumonia, this finding may provide for the first time, to our knowledge, a link between acid-suppression use and lung infections in the noncritically ill patient. In a study of 37 adults undergoing endoscopy after treatment with histamine-2 antagonists and PPIs, Thorens et al5 found 53% of patients had evidence of gastric bacterial overgrowth compared with 8% prior to starting acid suppression. Similar to our results, they found that there was overgrowth of Staphylococcus and Streptococcus. While the Thorens et al study is limited by its small size and comorbidities of patients with gastrointestinal symptoms, the rates of gastric bacterial growth in 53% of adults are similar to our rate of 46%, suggesting that the effects are truly a result of the acid suppression and not a result of the underlying disease (gastrointestinal vs respiratory disease). Also, Thorens et al found an increased abundance of Corynebacterium with acid suppression. This is similar to our study in that we found a correlation between the concentrations of Corynebacterium in the gastric and BAL fluid. This may have clinical importance, as the presence of Corynebacterium in the oropharynx has been significantly associated with wheezing in children.11
While acid suppression has been associated with changes in small-intestinal microflora, it is unknown if there are upstream bacterial effects.12 Our data show that there is an exchange of bacteria between the stomach and the lungs; we found 8 genera that were found in both the gastric and bronchial fluid and 2 genera whose concentrations in the stomach and lungs were highly correlated. In the only other study, to our knowledge, that addresses the potential exchange between gastrointestinal and extra-esophageal sites, Segal et al,13 who studied gastric and oral cultures in 52 hospitalized geriatric patients with nasogastric tubes, found 9 genera in common between the gastric fluid and oropharyngeal secretions, also suggesting communication between the sites. In our study, we have overcome the 3 main limitations of the Segal et al study, namely that their findings may not represent what is seen in otherwise healthy patients including children, may be heavily influenced by the presence of indwelling tubes that alter flora and serve as a conduit between sites, and may not represent what is seen in the lung.14
We have shown that gastric growth is significantly increased in patients receiving acid-suppression therapy while acid-suppression therapy alone does not seem to change lung microflora on a population level. There may be 2 reasons for this. First, because we studied children with chronic cough, these patients may have greater rates of lung culture positivity, which mask any smaller acid-suppression effect. In particular, some of the bacterial genera seen such as Staphylococcus and Streptococcus are highly prevalent in the lung even in healthy control participants so finding an acid-suppression effect may be difficult.15 Second, the length of time needed to change lung microflora once acid-suppression therapy has begun is not known. Because lungs are not continually bathed in gastric fluid, we may not have captured the acid-suppression effect in all patients, whereas the immediate gastric effect was more clear. While we did not see any concentration effects when we pooled all of the lung samples, we did find a clear relationship between higher full-column nonacid reflux burden and specific bacterial concentrations. For example, not only was Dermabacter more common and more abundant in the gastric fluid of patients receiving acid-suppression therapy but its concentrations in the lung were correlated with full-column nonacid reflux burden; this directly supports our hypothesis that gastric microflora can exert an influence over lung flora through nonacid gastroesophageal reflux in the acid-suppressed patient. These findings also support our prior studies showing that full-column nonacid reflux has been associated with increased BAL culture positivity in a hospital-based clinical microbiology laboratory and a culture positivity of Pseudomonas in patients with cystic fibrosis.16,17 Therefore, our results provide for the first time, to our knowledge, a plausible mechanism behind increased rates of clinical infections in patients receiving acid-suppression therapy.
Some of the bacteria seen in increased amounts or prevalence are not typical pathogens and so one might ask if the microflora changes are clinically relevant. First, there is increasing evidence suggesting changes in commensal bacteria may alter the microbial environment such that pathogens are more able to invade. The best examples of this are the interrelationship between commensal bacterial and viral infections such as respiratory syncytial virus and influenza, which manifest clinically with increased otitis media and upper and lower respiratory tract infections.18–21 Second, in the immunocompromised host, even commensal bacteria can result in clinical infection, which may change the risk-benefit ratio of acid-suppression use in the immunocompromised host.22,23 Finally, while some of the bacteria present in both the gastric and BAL fluid are not pathogens, we have now proposed that aerodigestive disorders could result from exchanges in microflora, a novel mechanism beyond just acid damage. This study will serve as a springboard for additional studies that shift the focus away from only the acid effect on the lung.
This study has some limitations. First, bacteria in gastric fluid may represent contamination from oropharyngeal contents that were brought into the stomach during endoscopy. While this is theoretically possible, we would expect if gastric bacteria were an endoscopic artifact the degree of oropharyngeal contamination should be similar in treated and untreated patients alike and gastric growth should be nearly universal because bacteria would be brought into the stomach with every endoscopy. Instead, we found a clear difference in the concentration of gastric bacteria in patients receiving and not receiving therapy and there were a significant number of samples that did not grow bacteria. Furthermore, a study by Donatsky et al24 determined the change in bacteria concentrations in gastric fluid in adults who did and did not receive an oral decontaminant prior to endoscopy. They found that oral decontamination had no effect on gastric growth, suggesting that oral flora does not have an appreciable effect on gastric fluid flora obtained endoscopically.24 Another limitation is that the channel of the scope was not sterile. However, Thorens et al, who collected gastric samples using a sterile tube with a distal rubber cap that was pierced at the time of endoscopy, identified a similar bacterial burden as we did despite different methods of collection.5 And again, if growth was a result of contamination, we would not expect to see differences between the treated and untreated groups.
A second limitation is that the patient’s primary clinician determined if the patient was receiving acid-suppression therapy and if pH-MII testing was needed. Therefore, treated patients may have more reflux symptoms and might be more likely to take medications and undergo pH-MII testing. However, baseline symptoms, rates of abnormal impedance testing, and rates of esophagitis between the 2 groups were not different between treated and untreated patients, suggesting that the differences in acid-suppression prescribing or diagnostic testing reflected the primary physician’s bias, not differences in the patient populations.
Third, the rate of colonization with bacteria from BAL fluid was high, with 76% of samples yielding bacteria by culture. This rate is higher than reported for normal control participants in children and adults and may reflect the fact that all of the patients had pulmonary symptoms.25,26 While the ideal study would be to perform bronchoscopies in healthy children without lung disease, this would be an ethically impossible study to perform.
Finally, we chose bacterial culture methods as a way to identify and quantify bacterial genera because we were interested in live potentially pathogenic bacteria. Using this method, we may have missed unculturable bacteria detected by 16S deep sequencing. While no studies, to our knowledge, have been done to compare 16S sequencing to culture in the pediatric stomach or lung, pediatric studies of other sites suggest that of all clinical samples sent to a pediatric microbiology laboratory, 18% of 16S sequencing negative samples were culture positive.27 A follow-up study to our current study would be to reanalyze the samples using 16S sequencing to identify unculturable bacteria.
Conclusions
Acid-suppression therapy clearly alters gastric microflora and this may impact lung microflora through full-column reflux. Future studies need to determine what degree of acid suppression will reduce gastric growth while still controlling symptoms and whether gastric growth predicts clinical infection risk. Our results highlight that any benefit of acid suppression should be weighed against potential risk, particularly in the immunocompromised host.
Acknowledgments
Funding/Support: This work was supported by grants K23 DK073713, 1R03DK089146, and K24DK082792A from the National Institutes of Health and a junior investigator award from the Translational Research Program at Boston Children’s Hospital.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Dr Rosen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rosen, Amirault, Onderdonk, Nurko.
Acquisition, analysis, or interpretation of data: Rosen, Amirault, Liu, Mitchell, Hu, Khatwa, Onderdonk.
Drafting of the manuscript: Rosen, Liu.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Rosen, Liu, Mitchell, Hu, Onderdonk.
Obtained funding: Rosen.
Administrative, technical, or material support: Amirault.
Study supervision: Rosen, Khatwa.
References
- 1.Nelson SP, Kothari S, Wu EQ, Beaulieu N, McHale JM, Dabbous OH. Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12(4):348–355. doi: 10.3111/13696990903378680. [DOI] [PubMed] [Google Scholar]
- 2.Canani RB, Cirillo P, Roggero P, et al. Working Group on Intestinal Infections of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP) Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117(5):e817–e820. doi: 10.1542/peds.2005-1655. [DOI] [PubMed] [Google Scholar]
- 3.Turco R, Martinelli M, Miele E, et al. Proton pump inhibitors as a risk factor for paediatric Clostridium difficile infection. Aliment Pharmacol Ther. 2010;31(7):754–759. doi: 10.1111/j.1365-2036.2009.04229.x. [DOI] [PubMed] [Google Scholar]
- 4.Holbrook JT, Wise RA, Gold BD, et al. Writing Committee for the American Lung Association Asthma Clinical Research Centers. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307(4):373–381. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorens J, Froehlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39(1):54–59. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres A, El-Ebiary M, Soler N, Montón C, Fàbregas N, Hernández C. Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator-associated pneumonia. Eur Respir J. 1996;9(8):1729–1735. doi: 10.1183/09031936.96.09081729. [DOI] [PubMed] [Google Scholar]
- 7.Hill M. Normal and pathological microbial flora of the upper gastrointestinal tract. Scand J Gastroenterol Suppl. 1985;111:1–6. doi: 10.3109/00365528509093747. [DOI] [PubMed] [Google Scholar]
- 8.Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol. 2004;99(12):2452–2458. doi: 10.1111/j.1572-0241.2004.40268.x. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph CD, Mazur LJ, Liptak GS, et al. North American Society for Pediatric Gastroenterology and Nutrition. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32(suppl 2):S1–S31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 10.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99(6):1037–1043. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 11.Cardenas PA, Cooper PJ, Cox MJ, et al. Upper airways microbiota in antibiotic-naïve wheezing and healthy infants from the tropics of rural Ecuador. PLoS One. 2012;7(10):e46803. doi: 10.1371/journal.pone.0046803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(5):483–490. doi: 10.1016/j.cgh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Segal R, Pogoreliuk I, Dan M, Baumoehl Y, Leibovitz A. Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods. J Hosp Infect. 2006;63(1):79–83. doi: 10.1016/j.jhin.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Leibovitz A, Plotnikov G, Habot B, Rosenberg M, Segal R. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med Sci. 2003;58(1):52–55. doi: 10.1093/gerona/58.1.m52. [DOI] [PubMed] [Google Scholar]
- 15.Morris A, Beck JM, Schloss PD, et al. Lung HIV Microbiome Project. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187(10):1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen R, Johnston N, Hart K, Khatwa U, Katz E, Nurko S. Higher rate of bronchoalveolar lavage culture positivity in children with nonacid reflux and respiratory disorders. J Pediatr. 2011;159(3):504–506. doi: 10.1016/j.jpeds.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palm K, Sawicki G, Rosen R. The impact of reflux burden on Pseudomonas positivity in children with cystic fibrosis. Pediatr Pulmonol. 2012;47(6):582–587. doi: 10.1002/ppul.21598. [DOI] [PubMed] [Google Scholar]
- 18.Ruohola A, Pettigrew MM, Lindholm L, et al. Bacterial and viral interactions within the nasopharynx contribute to the risk of acute otitis media. J Infect. 2013;66(3):247–254. doi: 10.1016/j.jinf.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. MBio. 2011;2(1):e00245–e10. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mühlemann K, Uehlinger DE, Büchi W, Gorgievski M, Aebi C. The prevalence of penicillin-non-susceptible Streptococcus pneumoniae among children aged < 5 years correlates with the biannual epidemic activity of respiratory syncytial virus. Clin Microbiol Infect. 2006;12(9):873–879. doi: 10.1111/j.1469-0691.2006.1472_1.x. [DOI] [PubMed] [Google Scholar]
- 22.Sakwinska O, Bastic Schmid V, Berger B, et al. Nasopharyngeal microbiota in healthy children and pneumonia patients. J Clin Microbiol. 2014;52(5):1590–1594. doi: 10.1128/JCM.03280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambotte O, Timsit JF, Garrouste-Orgeas M, Misset B, Benali A, Carlet J. The significance of distal bronchial samples with commensals in ventilator-associated pneumonia: colonizer or pathogen? Chest. 2002;122(4):1389–1399. doi: 10.1378/chest.122.4.1389. [DOI] [PubMed] [Google Scholar]
- 24.Donatsky AM, Holzknecht BJ, Arpi M, et al. Oral chlorhexidine and microbial contamination during endoscopy: possible implications for transgastric surgery. A randomized, clinical trial. Surg Endosc. 2013;27(6):1914–1922. doi: 10.1007/s00464-012-2686-5. [DOI] [PubMed] [Google Scholar]
- 25.Riedler J, Grigg J, Stone C, Tauro G, Robertson CF. Bronchoalveolar lavage cellularity in healthy children. Am J Respir Crit Care Med. 1995;152(1):163–168. doi: 10.1164/ajrccm.152.1.7599817. [DOI] [PubMed] [Google Scholar]
- 26.Cabello H, Torres A, Celis R, et al. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J. 1997;10(5):1137–1144. doi: 10.1183/09031936.97.10051137. [DOI] [PubMed] [Google Scholar]
- 27.Harris KA, Hartley JC. Development of broad-range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service. J Med Microbiol. 2003;52(Pt 8):685–691. doi: 10.1099/jmm.0.05213-0. [DOI] [PubMed] [Google Scholar]